Introduction
Cervical cancer is one of the most common malignant
carcinomas amongst women worldwide. There are an estimated 530,000
new cases of cervical cancer annually with 270,000 cancer-related
deaths (1,2). Infection with high-risk human
papillomaviruses (HPVs), such as HPV16 and HPV18, is the primary
cause of cervical cancer, amongst multiple factors (3). Understanding that HPV infection is the
primary inducer of development of cervical cancer has improved
prevention of cervical cancer (4). At
present, the strategies for treatment of cervical cancer include
surgery, radiation and chemotherapy. However, the severe systemic
toxicity caused by radiation and chemotherapy affect prognosis
(4–7).
Therefore, developing a safe therapeutic strategy for cervical
cancer treatment is desirable.
Isolating components of traditional medicines with
antineoplastic activities has been demonstrated as a promising
alternative strategy for treating patients with cancer (8,9).
Tanshinone II A (Tan IIA) is the primary lipophilic component
isolated form the traditional Chinese medicine Salvia
miltiorrhiza. Numerous animal and clinical studies have
demonstrated that Tan IIA is an effective reagent commonly used for
the prevention and treatment of cardiovascular diseases, including
atherosclerosis via its antioxidant and anti-inflammatory
activities (10–12). Tan IIA has recently attracted
considerable attention in cancer therapy due to its potential
antitumor activities. The antitumor effect of Tan IIA has been
verified in various cancer cell lines, including glioma,
osteosarcoma, leukemia, prostate, breast, colon, lung, liver,
stomach, pancreas, bile duct, kidney, ovarian, cervical and
nasopharyngeal cells (13–16). Studies have demonstrated that Tan IIA
may suppress the growth and induce apoptosis in certain types of
cancer cells through multiple mechanisms, including cell cycle
arrest and caspase-dependent apoptosis (17,18).
However, the antitumor mechanisms of Tan IIA remain unknown.
Over the last decade, advances in understanding of
the metabolism in cancer cells has revealed that cancer cells
predominantly rely on aerobic glycolysis to maintain cell
proliferation (19,20). Inhibition of aerobic glycolysis in
tumors has been identified as an effective strategy to aid in
overcoming obstacles in cancer treatment, and the effectiveness of
this approach has been validated in pre-clinical studies (21). Inhibiting glycolysis in a tumor may be
an effective treatment strategy for potential future treatments
(22–24).
Several studies have reported that Tan IIA inhibits
proliferation in cervical cancer cells by inducing apoptosis in
these cells (25,26). However, previous research has only
focused on the use of Tan IIA to induce tumor cell apoptosis. The
role of Tan IIA on inhibition of glycolysis in cervical cancer has
not been studied. The aim of the present study was to examine the
effect of Tan IIA on cell growth in cervical cancer and determine
the underlying mechanism, by assessing change in metabolic
phenotype and apoptosis. The present study provides insight into
the molecular mechanism through which Tan IIA induces apoptosis in
cervical cancer and highlights the potential use of Tan IIA as a
novel therapeutic agent for treating patients with cervical
cancer.
Materials and methods
Materials
Human cervical cancer cell lines SiHa (HPV
16-positive cells), HeLa (HPV 18-positive cells) and C33a (HPV
negative) were provided by the Chinese Academy of Sciences
(Shanghai, China). Dulbecco's Modified Eagle's Medium (DMEM) and
fetal bovine serum (FBS) were purchased from Thermo Fisher
Scientific, Inc. Dimethyl sulfoxide (DMSO, analytical grade) was
obtained from Beijing Chemical Reagent Factory. Tan IIA was
obtained from PhytoMyco, Inc. The mouse anti-human Bcl-2 antibody
(cat. no. sc-23960), mouse anti-human Bax antibody (cat. no.
sc-20067), rabbit ant-human cleaved caspase-3 antibody (cat. no.
sc-7148), mouse anti-human cleaved caspase-9 antibody (cat. no.
sc-17784), mouse anti-human cytochrome c antibody (cyto
c; cat. no. sc-13561) were obtained from Santa Cruz
Biotechnology, Inc. Rabbit ant-human p-AKT antibody (ID product
code ab18206), rabbit ant-human AKT antibody (ID product code
ab8805), rabbit ant-human p-mTOR antibody (ID product code
ab84400), rabbit ant-human mTOR antibody (ID product code ab2732)
were obtained from Abcam, Inc. Rabbit ant-human GLUT1 antibody
(cat. no. PA5-16793), rabbit ant-human PKM2 antibody (cat. no.
PA5-23034), rabbit ant-human HK2 antibodies (cat. no. PA5-29326)
were purchased from Invitrogen; Thermo Fsiher Scientific, Inc.
Cell culture
SiHa, HeLa and C33a cells were maintained in DMEM
supplemented with 10% FBS, 100 units/ml of penicillin and 100
units/ml of streptomycin at 37°C in a humidified atmosphere of 5%
carbon dioxide (CO2) and 95% air. Cells were
sub-cultured every three days with 0.25% trypsin.
Cytotoxicity assay
The cytotoxicity of Tan IIA on tumor cells was
detected by MTT assay (27). SiHa,
HeLa and C33a cells were seeded into a 96-well assay plate with a
concentration 1.0×105 cells/ml (200 µl/well). After 24 h
of incubation, the cells were treated with Tan IIA with doses of
0–8 mg/l in the growth medium. Each concentration was assessed five
times. The cells were cultured for another 24, 48 and 72 h.
Subsequently, 20 µl of MTT (5 mg/ml) was added to each well and
incubated for another 4 h. Then, supernatant was discarded and 150
µl DMSO was loaded to each well. The optical density (OD) was
measured at 490 nm using a Bio-assay reader (Bio-Rad 550; Bio-Rad
Laboratories, Inc.).
Quantitative reverse transcription PCR
(qRT-PCR)
The total RNA form Tan IIA-treated and untreated
SiHa cells were extracted to detect the expression of HPV16-E6/E7
gene. Total cellular RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) as per the
manufacturer's protocol. A high-capacity cDNA reverse-transcription
kit was used to prepare cDNA (Thermo Fisher Scientific, Inc).
qRT-PCR was carried out using gene-specific oligonucleotide primers
(Table I), an iScript One-Step RT-PCR
kit with SYBR-Green and an ABI 7500 Fast real-time PCR system. The
PCR conditions were set as 95°C for 5 min, followed by 40 cycles at
95°C for 30 sec, 60°C for 45 sec and 72°C for 30 min. All values
were normalized to β-actin and then standardized to the control
condition. Error bars represent the standard error of the mean
(SEM), and the statistical significance was assessed using the
Student's t-test. After completion of the RT-PCR, Cq values were
obtained using the ABI 7500 fast v2.0.1 software. The ΔΔCq method
was used to represent mRNA fold change (28).
 | Table I.Sequences of primers for qRT-PCR. |
Table I.
Sequences of primers for qRT-PCR.
| Gene | Forward primer | Reverse primer |
|---|
| E6 |
GAGCGACCCAGAAAGTTACCA |
AAATCCCGAAAAGCAAAGTCA |
| E7 |
CATGGAGATACACCTACATTGC |
CACAACCGAAGCGTAGAGTC |
Fluorescence microscope-detected
apoptosis
SiHa cells were cultured on slides at a density of
5×104/ml in 24-well plates. After Tan IIA treatment, the
medium was removed and the cells were washed with PBS twice. Then,
the cells were fixed with 4% paraformaldehyde for 10 min at 25°C.
Subsequently, the cells were stained with Hoechst 33258 for 5 min
at 25°C. The apoptosis morphology was observed using a fluorescence
microscope. The images were captured at magnifications of ×200.
Flow cytometric evaluation of
apoptosis
After Tan IIA treatment, SiHa cells were harvested
by centrifugation at 1,000 × g for 3 min and washed with PBS twice.
After that the harvested cells pellet was resuspend in 100 µl DMEM
medium. Then 100 µl Muse Annexin V and Dead Cell reagents were
added and incubated at room temperature for 20 min. The percentage
of apoptosis was detected and analyzed using a Muse Cell Analyzer
(EMD Millipore).
Caspase-3/7 activation detected
The caspase-3/7 activation change was detected by
Muse Cell Analyzer following the manufacturer's instructions. After
Tan IIA treatment, SiHa cells were harvested by centrifugation at
850 × g and the cell pellet was resuspended in 50 µl PBS. Muse
Caspase-3/7 reagent working solution (5 µl) was added to the cell
suspension, and it was mixed thoroughly and incubated at 37°C for
30 min. After incubation, 150 µl of Muse™ Caspase 7-AAD working
solution was loaded to each sample, and incubated at room
temperature for 5 min. Then, the caspase-3/7 activation was
detected by Muse Cell Analyzer.
Measurements of glucose uptake levels
and lactate production
The SiHa cells were seeded in 6-well plates at a
density of 1×104 cells/well. After treatment with Tan
IIA for 48 h, the cell culture media was collected to detect the
glucose uptake and lactate production. For assessment of lactate
production, the collected culture media was diluted 1:50 by using
lactate assay buffer. The amount of lactate present in the media
was then estimated using the Lactate Assay kit (Sigma-Aldrich;
Merck KGaA) according to the manufacturer's instructions. The
content of glucose in the collected culture media was detected
immediately using a Glucose Assay kit according to the
manufacturer's instructions. Glucose uptake and lactate production
were all normalized by cell number (29).
Determination of ATP content
SiHa cells were seeded in 6-well plates
(1×105 cells/ml) and then incubated with Tan IIA for 48
h. Then, the cells were harvested and lysed on ice with somatic
cell ATP releasing reagent. The ATP content was detected by ATP
assay kits (Genmed Scientifics, Inc.) according to the
manufacturer's instructions. ATP content was normalized by cell
number (29).
Western blot analysis
After Tan IIA treatment, 5×105 SiHa cells
were harvested and lysed in RIPA buffer (Sigma-Aldrich; Merck KGaA)
to extract the total protein. The extract total protein content was
determined by Bio-Rad BCA protein assay kit and 50 µg protein/lane
was loaded on 12% SDS-PAGE. After SDS-PAGE, the separated proteins
were transferred to nitrocellulose membranes. Non-specific binding
was blocked by incubating nitrocellulose membranes in 5% non-fat
dry milk in TBS/0.1% Tween for 120 min. After blocking, the blots
were incubated with primary antibody: Bcl-2 antibody (dilution
1:2,000), Bax (dilution 1:2,000), cleaved caspase-3 (dilution
1:2,000), cleaved caspase-9 (dilution 1:2,000), cyto c
antibody (dilution 1:2,000), p-AKT (dilution 1:1,000), AKT
(dilution 1:1,000), p-mTOR (dilution 1:1,000), mTOR (dilution
1:1,000), GLUT1 (dilution 1:1,000), PKM2 (dilution 1:1,000), HK2
(dilution 1:1,000) at 4°C overnight. Then, the appropriate
secondary antibody (cat. nos. sc-2357 and sc-516132; Santa Cruz
Biotechnology) at a 1:5,000 dilution was added and incubation
followed for 1 h at room temperature. Immunoreactive protein bands
were detected by chemiluminescence using enhanced chemilumunescence
reagents (ECL) was obtained from Santa Cruz Biotechnology, Inc.
Blots were also stained with anti-β-actin antibody (cat. no.
60008-1-Ig; Proteintech Group) as an internal control for the
amounts of target proteins. The films were then subjected to
densitometry analysis using a Gel Doc 2000 system (Bio-Rad
Laboratories, Inc.).
In vivo antitumor test
All animal experiments were performed in compliance
with the guidelines of the Ethics Committee for Animal Use and Care
of Beihua University. This committee approved the experiments in
the present study. Kunming (KM) female mice (n=25) were purchased
from Jilin University (8 weeks old; 20–25 g). The animals were
maintained under a dedicated room with a 12-h light/dark cycle at a
constant temperature of 22°C.
First, U14 cervical cancer cells obtained from The
Chinese Academy of Sciences (Shanghai, China) were harvested by
trypsin digestion, washed by PBS, and re-suspended in serum-free
RPMI-1640 medium. U14 cervical cancer cell suspension (0.2 ml) with
a density of 5×106 was injected into the right
infra-axillary dermis of the mice. When the size of the tumor
reached 150 mm3, the mice were randomly assigned to 5
groups with 5 mice in each group: Control, cyclophosphamide (CTX;
25 mg/kg/day, i.g.), and three doses of Tan IIA (40 mg/kg/day; 20
mg/kg/day; and 10 mg/kg/day). The tumor volume and body weight were
measured every two days for 14 days. The relative tumor volume
Vt/V0 as a function of time was used to
investigate the inhibitory effect. All groups were treated every
two days for 20 days, and 24 h after the last administration, the
mice of the four groups were sacrificed. The tumors were excised to
evaluate tumor inhibition. The tumor inhibition rate was calculated
by the formula: Inhibitory rate (IR) = (the tumor weight of the
control group - the tumor weight of the treated group/the tumor
weight of the control group) ×100%. For euthanasia, animals were
anesthetized with 5% isoflurane vaporized in oxygen and thereafter
euthanatized with a 20% container volume/min gas replacement rate
of carbon dioxide. Euthanasia was confirmed when the heartbeat
stopped and the pupils were enlarged.
Histopathological and morphological
examination
The tumor tissues were excised and fixed at 25°C for
one week in 4% formalin, embedded in paraffin, and cut into 4-mm
sections for histological study. Hematoxylin and eosin (H&E)
purchased form Sigma-Aldrich (Merck KGaA) was used to stain the
tumor for histological analysis. Histopathological analysis of the
tumor tissue sections was performed using a light microscope at an
×200, magnification.
Statistical analysis
Results are presented as the mean ± SEM which are
derived from three or more independent experiments. All data were
analyzed by one-way ANOVA using SPSS version 13 software (SPSS,
Inc.). Tukey's post hoc test was used to determine the significance
for all pairwise comparisons of interest. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Tan IIA inhibits the cell viability
against human cervical cancer cells
An MTT assay was performed to determine the
cytotoxicity of Tan IIA in SiHa, HeLa, and C33a cells after 48 h of
treatment. The results revealed that Tan IIA significantly
inhibited cell viability in SiHa, HeLa and C33a cells in a
dose-dependent manner (Fig. 1A). Tan
IIA had a greater inhibitory effect on cell viability in SiHa and
HeLa cells compared with the HPV-negative C33a cells. Thus, SiHa
cells were used for all subsequent experiments.
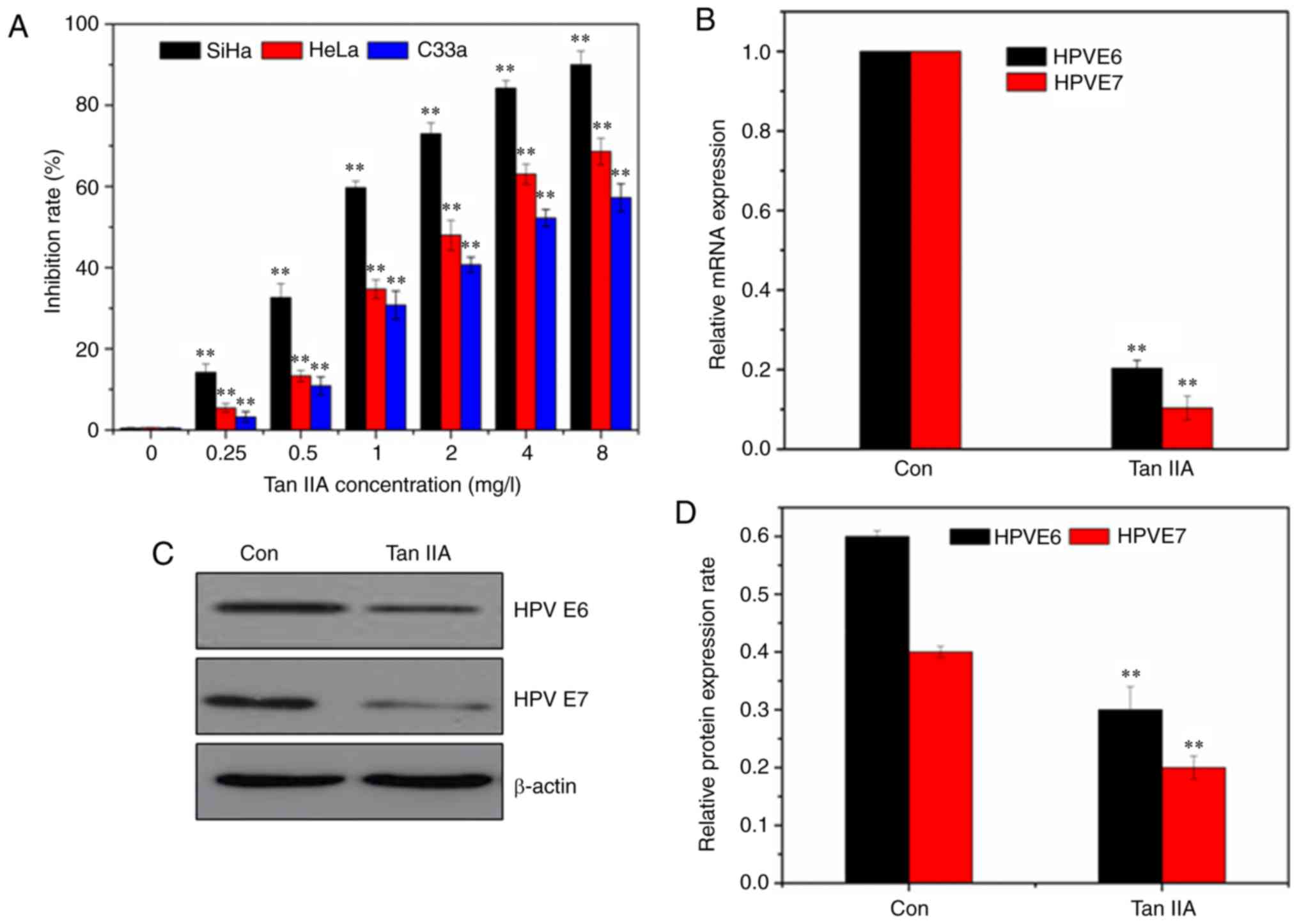 | Figure 1.Tan IIA inhibits the cell viability
of human cervical cancer cells and decreases the expression of HPV
oncogenes. (A) Tan IIA inhibited the cell viability of SiHa, HeLa,
and C33a cells in a dose-dependent manner. Cells were treated with
increasing concentrations (0, 0.25, 0.5, 1, 2, 4 and 8 mg/l) of Tan
IIA for 48 h. Cell viability was assessed using an MTT assay. (B)
Effect of Tan IIA on the mRNA expression levels of HPV16 E6 and E7
in SiHA cells. (C) Western blotting revealed the expression of HPV
oncogenes in SiHa cells treated with Tan IIA. (D) Densitometry
analysis of the blots presented in C. Data are presented as the
mean ± standard deviation (SD), n=5. **P<0.01 compared with the
control. Tan IIA, tanshinone IIA; HPV, human papillomavirus. |
Tan IIA suppresses HPV16-E6/E7 gene
expression
The effect of Tan IIA expression on mRNA expression
of HPV16-E6/E7 in SiHa cells was determined using reverse
transcription-quantitative (RT-q)PCR (Fig. 1B). Treatment with Tan IIA (1.5 mg/l)
resulted in a significant reduction in the mRNA expression of HPV
E6 and HPV E7. Western blot analysis also confirmed the results of
the RT-qPCR (Fig. 1C).
Tan IIA induces SiHa cell
apoptosis
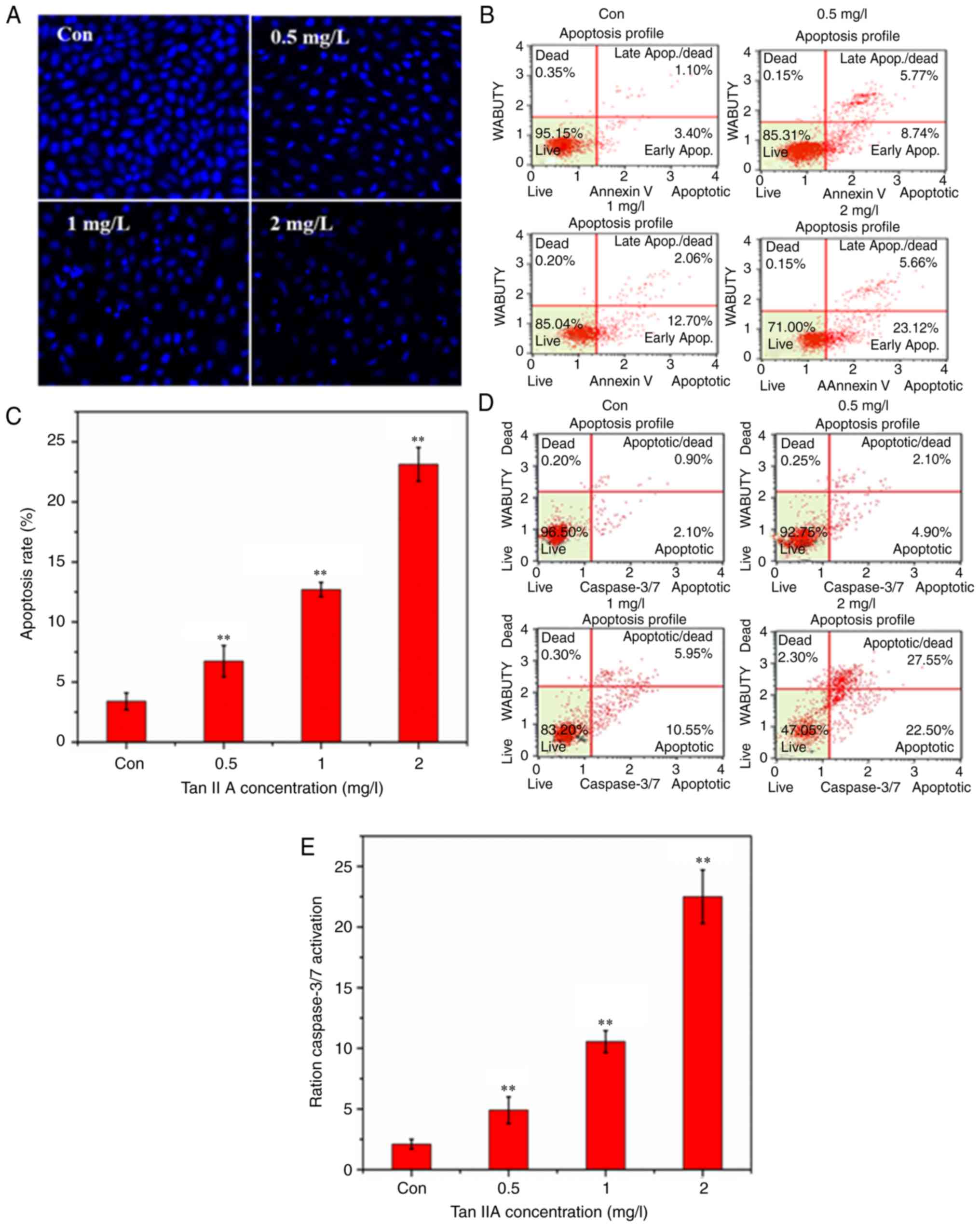
Hoechst 333258 was used to stain apoptotic nuclei.
Hoechst 333258 staining revealed that the nuclei appeared as
uniform blue with organized chromatin structure in the control
group (Fig. 2A). Following treatment
with Tan IIA at a range of concentrations (0.5, 1 and 2 mg/l) for
48 h, the SiHa cells were decreased in number and presented with
smaller nuclei, strong fluorescent spots, pyknotic nuclei and
extensive blebbing. Condensed chromatin and apoptotic bodies were
observed under a fluorescence microscope in the treated cells
suggesting that the Tan IIA treatment had induced apoptosis in the
SiHa cells. Similar results were obtained from staining with Muse
Annexin V and Dead Cell reagents. As revealed in Fig. 2B and C the percentage of early
apoptotic cells was increased as the dose of Tan IIA increased
compared with the untreated group (P<0.01).
Detection of caspase-3/7
activation
Muse caspase-3/7 assay kits were used to detect
caspase-3/7 activation following treatment with Tan IIA in the SiHa
cells. Stained cells were quantified using a Muse cell analyzer.
The percentage of cells in early apoptosis was 4.9, 10.55, and
22.55% when treated with 0.5, 1 and 2 mg/l Tan IIA, respectively
(Fig. 2D and E).
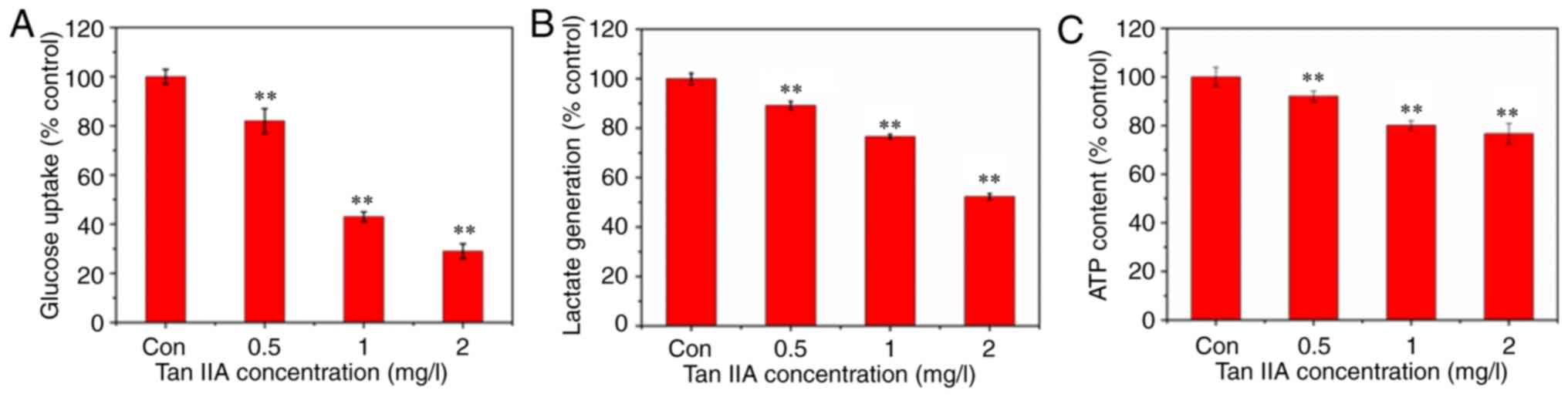
Inhibitory effect of Tan IIA on
glucose uptake, lactate generation, and adenosine triphosphate
(ATP) production
Tumor cells use glucose as a substrate for ATP
synthesis and production. Glucose uptake in SiHa cells following
treatment with Tan IIA for 48 h was measured. The results revealed
that glucose uptake in Tan IIA-treated SiHa cells was decreased
(Fig. 3A). The primary product of
aerobic glycolysis is lactic acid and ATP. Thus, the ATP and lactic
acid content were measured using reagent kits to investigate
whether glycolysis inhibition was associated with Tan IIA-mediated
SiHa cell apoptosis. The results revealed that the levels of
intracellular ATP and extracellular lactic acid were decreased in
SiHa cells treated with Tan IIA (0.5, 1 and 2 mg/l) compared with
the control group (Fig. 3B and
C).
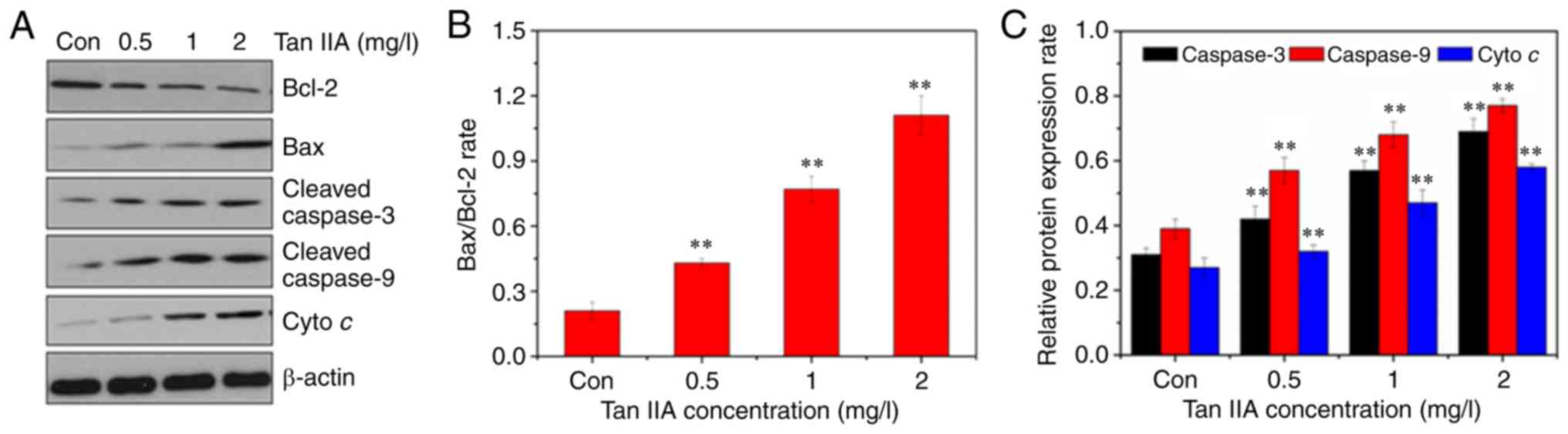
Tan IIA induces SiHa cell apoptosis
through the mitochondrial apoptosis pathway
Western blotting was used to assess the expression
of apoptosis-associated proteins in Tan IIA-treated SiHa cells to
determine which apoptosis pathway underlied Tan IIA activity in
apoptosis of cervical cancer cells. As revealed in Fig. 4, after treatment with increasing doses
of Tan IIA for 48 h, the expression levels of Bax, cleaved
caspase-3 and cleaved caspase-9 were significantly increased. Bcl-2
expression signficantly decreased. Thus, there was a dose-dependent
increase in the expression ratio of Bax/Bcl-2. In the Tan
IIA-treated SiHa cells, cyto c was released from the
mitochondria to the cytosol in a dose dependent manner.
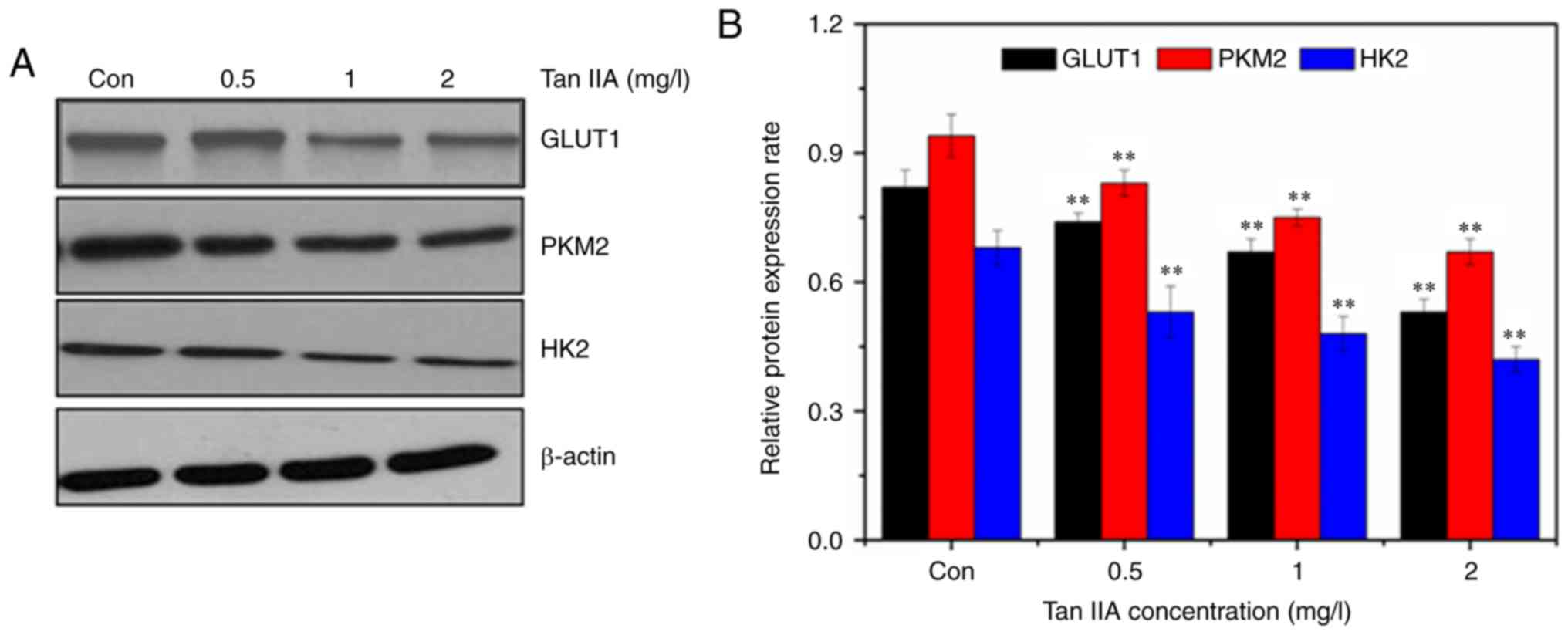
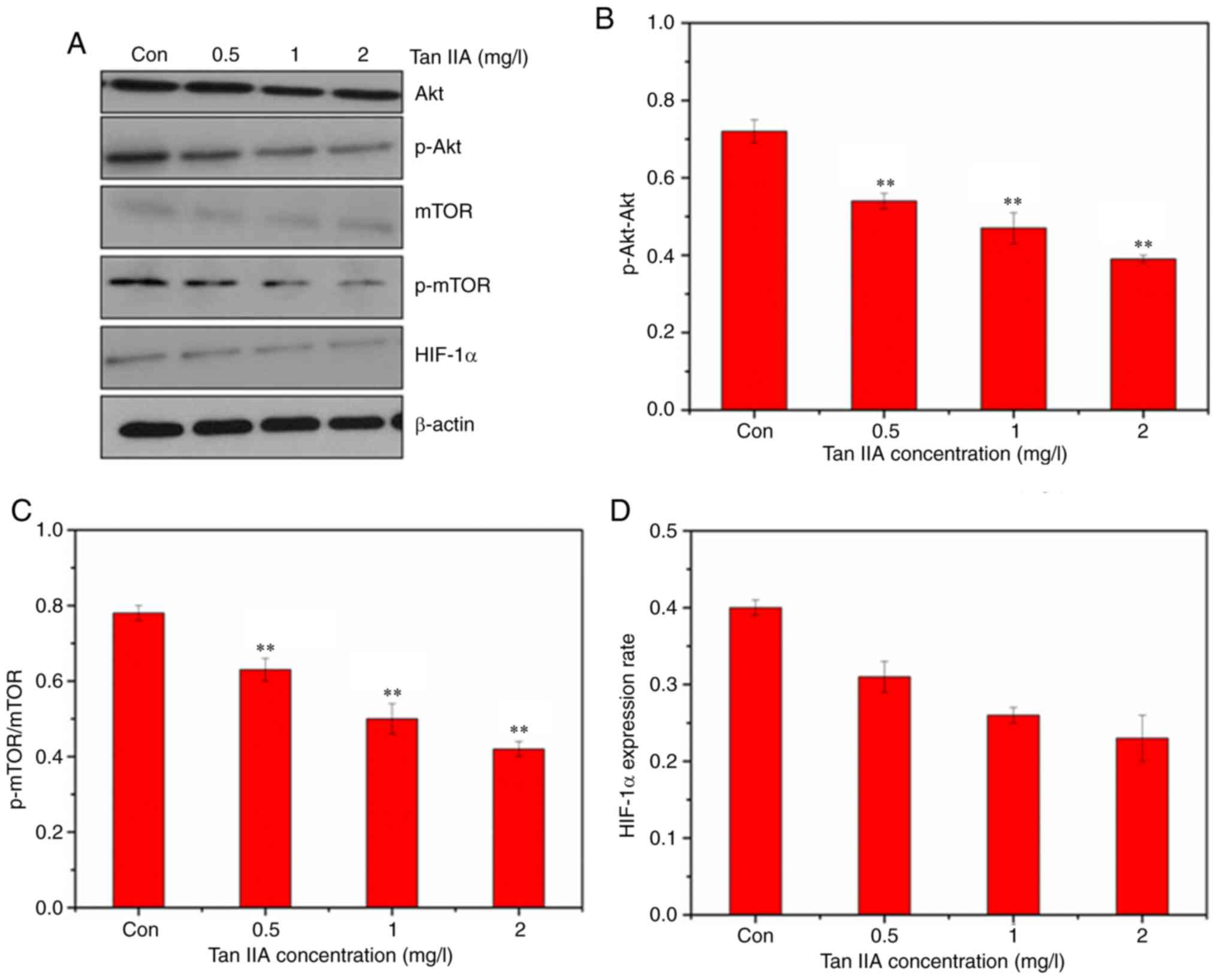
Tan IIA downregulates the Akt/mTOR
signaling pathway and inhibits glycolysis
The expression of GLUT1 was detected by western
blotting. The western blot results revealed that Tan IIA decreased
the expression of GLUT1, PKM2, and HK2 (Fig. 5), confirming inhibition of glycolysis
in SiHa cells treated with Tan IIA. The AKT/mTOR pathway serves a
major role in the activation of aerobic glycolysis in tumors.
Therefore, it was determined whether the AKT/mTOR signaling pathway
was involved in regulation of aerobic glycolysis in the Tan
IIA-treated SiHa cells. Treatment with Tan IIA resulted in a
dose-dependent decrease of the phosphorylation of Akt and mTOR
(Fig. 6). HIF-1α mediates
transformation of cell metabolism, whereas the PI3K/AKT pathways
are involved in the regulation of HIF-1α. As revealed in Fig. 6, Tan IIA inhibited the expression of
HIF-1α in SiHa cells.
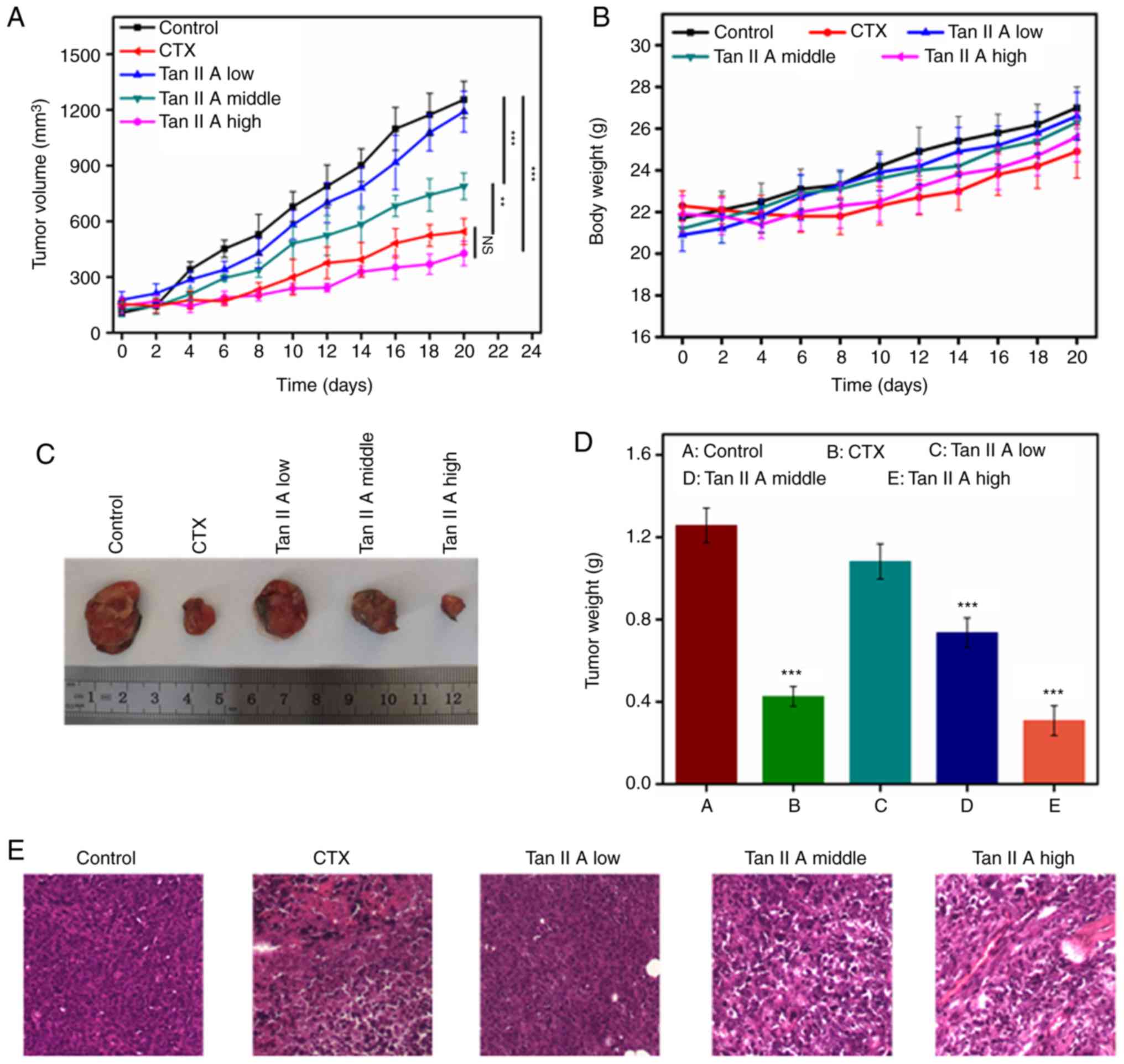
Effect of Tan IIA on tumor growth in
vivo
U14 tumor-bearing Kunming mice were used to
determine the antitumor effects of Tan IIA in vivo. When the
the tumor size of mice reached 150 mm3, they were
randomly divided into 5 groups. Mice were intraperitoneally
administered with CTX (25 mg/kg) and Tan IIA (three doses) for 14
days. As revealed in Fig. 7A, the
tumor in the control group grew rapidly, whereas the tumors in the
mice treated with CTX and Tan IIA grew significantly slower. The
body weight of the mice in the Tan IIA-treated group gradually
increased but did not significantly differ from the control group,
suggesting that Tan IIA did not demonstrate any significant toxic
effect. At the end of the experiment, the mice were sacrificed, and
the size of the tumors and tumor weights were measured. The results
revealed that the tumor weight and volume in the CTX- and Tan
IIA-treated groups decreased compared with the control group, in
which the high dose of Tan IIA resulted in mice with a tumor weight
similar to that in the CTX-treated group. The diameter of the tumor
in each group is revealed in Table
SI. The maximum tumor volume in each group is revealed in
Table SII. The tumor inhibition rate
in the CTX-treated group was 65.1%, whereas the rate in mice
treated with a high dose (40 mg/kg/day) of Tan IIA was 72.7%
(Fig. 7C and D and Table SIII). Pathological analysis of the
tumor tissues revealed that tumors in the U14 model group exhibited
invasive growth and rapid diffusion with high degrees of tumor cell
proliferation. CTX-treated tumor cells exhibited unclear structures
with regional necrosis, clear cell edema and a large number of
disintegrated granular cells. Following treatment with Tan IIA, the
tumor cells demonstrated regional necrosis, with shrunken cell
volumes, nuclear condensation and clear interstitial edema
occasionally infiltrated with inflammatory cells.
Discussion
The energy required for proliferation of cancer
cells is obtained by glycolysis which is less efficient than
aerobic oxidation of glucose (30).
The use of glycolysis as the predominant source of energy is
associated with certain factors, including upregulation of critical
enzymes involved in glycolysis and glucose transporters.
Concurrently, expression of enzymes involved in oxidative
phosphorylation is downregulated. High glycolytic metabolism has
been reported to be associated with resistance to apoptosis in
cancer following treatment with anticancer drugs (31,32). Tan
IIA has been demonstrated to inhibit cell viability of cervical
cancer by decreasing the expression of HPV oncogenes (26). However, the effect of Tan IIA on the
inhibition of glycolysis in cervical cancer is still unknown.
Therefore, in the present study, the effect of Tan IIA on inducing
apoptosis and inhibiting glycolysis in cervical cancer and its
possible mechanism were examined.
There are several subtypes of HPV, including
high-risk subtypes which are associated with cancer development
(33). More than 20 common subtypes
of high-risk HPVs exist, with HPV16 being the most prevalent
(34). The present study demonstrated
that Tan IIA markedly decreased the cell viability of SiHa, HeLa,
and C33a cervical cancer cells. Treatment with Tan IIA resulted in
a greater decrease in cell viability in SiHa cells compared with
HeLa and C33a cells. Therefore, SiHa cells (HPV 16-positive cells)
were selected to determine the mechanism underlying the effects of
Tan IIA. High-risk HPVs, including HPV16 and HPV18 result in the
expression of oncogenes, E6 and E7. E6 and E7 combine to tumor
suppressor p53 and Rb, individually. This causes p53 degradation
and loss of Rb products (35–37). The results of the present study
revealed that following treatment Tan IIA, the mRNA and protein
expression levels of E6 and E7 protein were decreased in SiHa
cells. Abnormal cell proliferation and differentiation, and
apoptotic imbalance are also important causes of tumorigenesis
(38). A number of chemotherapeutic
agents affect cancer cells, inducing apoptosis. Apoptotic cells
display characteristic morphological changes, such as cell
shrinkage, cytoplasmic vacuolation, chromatin condensation, nuclear
fragmentation, and cell blebbing, to produce apoptotic bodies
(39,40). Hoechst 333258 staining results
revealed typical apoptotic morphology in Tan IIA-treated SiHa
cells. Fluorescence correlation spectroscopy revealed that the
number of apoptotic cells increased as the concentration of Tan IIA
was increased. The mechanism through which Tan IIA-induced
apoptosis was examined, and the results revealed a marked decrease
in Bcl-2 expression in the cells treated with Tan IIA,
significantly increased Bax expression levels, and an increased
Bax/Bcl-2 ratio (41). Bcl-2 is an
anti-apoptotic protein involved in the intrinsic apoptotic pathway
which inhibits apoptosis by binding and subsequently inhibiting
proapoptotic molecules, such as Bax and Bak (42). Therefore, changes in the Bax/Bcl-2
ratio may explain the increased rate of apoptosis in the Tan
IIA-treated SiHa cells.
Apoptotic factors, such as cyto c, serve a
crucial role in the mitochondrial apoptotic pathway, activating the
activity of caspases in cancer cells. Chemotherapeutic agents and
cytotoxic drugs frequently induce the release of cyto c from
mitochondria into the cytosol resulting in activation of caspase-9.
Subsequently, pro-caspase-3 is transformed into its active form
(caspase-3) by caspase-9-mediated cleavage. Poly-ADP ribose
polymerase may be cleaved by caspase-3, causing DNA fragmentation
and apoptosis (43). The results of
the present study revealed that Tan IIA treatment resulted in the
release of cyto c from the mitochondria into the cytosol and
the activation of caspase-9 in SiHa cells. Subsequently, activated
caspase-9 impacted the downstream caspase-3 proenzyme and activated
caspase-3, ultimately inducing apoptosis in SiHa cells.
The researcher and scientist Warburg revealed a
phenomenon in which tumors prefer to utilize the energy which is
supplied by glycolysis instead of aerobic oxidation of glucose even
when there is an ample supply of oxygen. Anaerobic respiration in
cancer cells leads to a considerable increase in the use of glucose
(44,45). The results of the present study
revealed that Tan IIA treatment may suppress glucose uptake and
extracellular lactic acid generation in SiHa cells. GLUT1, PKM2,
and HK2 were all downregulated in the Tan IIA treatment group.
GLUT1 is an important glucose transporter, which is a rate-limiting
enzyme of glucose transport. Increased expression of GLUT1 is
associated with proliferation, migration and invasion (46). HK2 and PKM2 isozyme expression is
increased in a number of malignant types of cancer associated with
a propensity for invasion and metastasis (47). The Akt/mTOR signaling is associated
with hypoxia signaling transduction. At the translational level,
the phosphorylation of Akt and mTOR can increase the
hypoxia-induced expression of HIF-1α, which regulates intracellular
glucose utilization and angiogenesis, and promotes stabilization
and activation, eventually promoting tumor cell growth (48–50). The
results of present study revealed that Tan IIA treatment decreased
the expression of HIF-1α and concurrently decreased the
phosphorylation of Akt and mTOR in SiHa cells. This indicates that
regulation of HIF-1α by the Akt/mTOR pathway was involved in the
inhibition of glycolysis when treated with Tan IIA. In
vitro, Tan IIA demonstrated significant antitumor effects in
SiHa cells, and the possible mechanism was associated with
inhibition of glycolysis. Therefore, the antitumor effect of Tan
IIA in carcinoma-bearing mice was determined. The results of the
present study revealed that carcinoma-bearing mice treated with Tan
IIA presented with a significant reduction in the tumor volume and
weight and there was no notable difference in the toxicity between
the different groups of mice. The present study highlights the
potential of Tan IIA as a therapeutic alternative.
In conclusion, the present study revealed that
treatment with Tan IIA inhibited cell viability of cervical cancer
SiHa cells and induced apoptosis. The possible mechanism was
associated with the expression of the oncogenes E6 and E7,
decreasing glycolysis through inhibition of the intracellular
Akt/mTOR pathway and the associated HIF-1α signaling. Tan IIA
treatment increased apoptosis in SiHa cells, and this effect may be
associated with inhibition of glycolysis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project
Agreement for Science and Technology Development, Jilin Province
(nos. 20180101142JC, 20190701062GH, 20130101157JC) and the Science
and Technology Project of Jilin Provincial Department of Education
(JJKH20190662KJ, JJKH20191062KJ, no. 2011286).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL, WZhu and WZhang performed the experiments and
participated in designing some experiments. XK and XC participated
in the experiment of cell experiment and western blot analysis. XS
and RZ performed the PCR and flow cytometry experiments, and WZhu,
WZhang and RZ performed some of the in vivo experiments.
WZhu wrote the manuscript. All authors have read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were performed in compliance
with the guidelines of the Ethics Committee for Animal Use and Care
of Beihua University. This committee approved the experiments in
the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Akt
|
protein kinase B
|
|
DMEM
|
Dulbecco's Modified Eagle's Medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
FBS
|
fetal bovine serum
|
|
GLUT1
|
glucose transporter 1
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
HPVs
|
human papillomaviruses
|
|
p38
|
p38 mitogen-activated protein
kinases
|
|
PI3K
|
phosphatidylinositol-4,5-bisphosphate
3-kinase
|
|
qRT-PCR
|
quantitative reverse transcription
PCR
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
Tan IIA
|
tanshinone II
|
References
|
1
|
Lecavalier-Barsoum M, Chaudary N, Han K,
Koritzinsky M, Hill R and Milosevic M: Targeting the CXCL12/CXCR4
pathway and myeloid cells to improve radiation treatment of locally
advanced cervical cancer. Int J Cancer. 143:1017–1028. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Testa U, Petrucci E, Pasquini L, Castelli
G and Pelosi E: Ovarian cancers: Genetic abnormalities, tumor
heterogeneity and progression, clonal evolution and cancer stem
cells. Medicines (Basel). 5(pii): E162018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papillomavirus types 16 and 18 promotes the degradation of p53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wardak S: Human papillomavirus (HPV) and
cervical cancer. Med Dosw Mikrobiol. 68:73–84. 2016.PubMed/NCBI
|
|
5
|
Suh DH, Kim M, Kim K, Kim HJ, Lee KH and
Kim JW: Major clinical research advances in gynecologic cancer in
2016: 10-year special edition. J Gynecol Oncol. 28:e452017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Research Network;
Albert Einstein College of Medicine; Analytical Biological
Services; Barretos Cancer Hospital; Baylor College of Medicine;
Beckman Research Institute of City of Hope; Buck Institute for
Research on Aging; Canada's Michael Smith Genome Sciences Centre;
Harvard Medical School; Helen F. Graham Cancer Center &
Research Institute at Christiana Care Health Services, et al, .
Integrated genomic and molecular characterization of cervical
cancer. Nature. 543:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wentzensen N, Schiffman M, Palmer T and
Arbyn M: Triage of HPV positive women in cervical cancer screening.
J Clin Virol. 76 (Suppl 1):S49–S55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fox BW: Medicinal plants in tropical
medicine. 2. Natural products in cancer treatment from bench to the
clinic. Trans R Soc Trop Med Hyg. 85:221991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pokrzywa CJ, Abbott DE, Matkowskyj KA,
Ronnekleiv-Kelly SM, Winslow ER, Weber SM and Fisher AV: Natural
history and treatment trends in pancreatic cancer subtypes. J
Gastrointest Surg. 23:768–778. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo J, Ma X, Cai Y, Ma Y, Zhan Z, Zhou YJ,
Liu W, Guan M, Yang J, Cui G, et al: Cytochrome P450 promiscuity
leads to a bifurcating biosynthetic pathway for tanshinones. New
Phytol. 210:525–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Han X, Hong Z, Wu J and Bao L: The
interplay between autophagy and apoptosis induced by tanshinone IIA
in prostate cancer cells. Tumour Biol. 37:7667–7674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao P, Hemmerlin A, Bach TJ and Chye ML:
The potential of the mevalonate pathway for enhanced isoprenoid
production. Biotechnol Adv. 34:697–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Jiang P, Ye M, Kim SH, Jiang C
and Lü J: Tanshinones: Sources, pharmacokinetics and anti-cancer
activities. Int J Mol Sci. 13:13621–13666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan G, Jiang X, Wu X, Fordjour PA, Miao L,
Zhang H, Zhu Y and Gao X: Anti-inflammatory activity of tanshinone
IIA in LPS-Stimulated RAW264.7 macrophages via miRNAs and
TLR4-NF-κB pathway. Inflammation. 39:375–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Shen L, Wang Z, Jiang HP and Liu LX:
Tanshinone IIA protects against myocardial ischemia reperfusion
injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed
Pharmacother. 84:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Cang S and Liu D: Third-generation
inhibitors targeting EGFR T790M mutation in advanced non-small cell
lung cancer. J Hematol Oncol. 9:342016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui ZT, Liu JP and Wei WL: The effects of
tanshinone IIA on hypoxia/reoxygenation-induced myocardial
microvascular endothelial cell apoptosis in rats via the JAK2/STAT3
signaling pathway. Biomed Pharmacother. 83:1116–1126. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yano S, Takehara K, Ming Z, Tan Y, Han Q,
Li S, Bouvet M, Fujiwara T and Hoffman RM: Tumor-specific
cell-cycle decoy by Salmonella typhimurium A1-R combined with
tumor-selective cell-cycle trap by methioninase overcome tumor
intrinsic chemoresistance as visualized by FUCCI imaging. Cell
Cycle. 15:1715–1723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parr R, Harbottle A, Jakupciak JP and
Singh G: Mitochondria and cancer. Biomed Res Int. 2013:7637032013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao
L, Wei S, Crespo J, Wan S, Vatan L, et al: Cancer mediates effector
T cell dysfunction by targeting microRNAs and EZH2 via glycolysis
restriction. Nat Immunol. 17:95–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wen Z, Zhang SL, Hu X and Tam KY:
Targeting tumor metabolism for cancer treatment: Is pyruvate
dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol
Sci. 11:1390–1400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choe M, Brusgard JL, Chumsri S, Bhandary
L, Zhao XF, Lu S, Goloubeva OG, Polster BM, Fiskum GM, Girnun GD,
et al: The RUNX2 transcription factor negatively regulates SIRT6
expression to alter glucose metabolism in breast cancer cells. J
Cell Biochem. 116:2210–2226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Chen M, Gao S, Yuan J, Zhu Z and Zou
X: LY294002 inhibits the Warburg effect in gastric cancer cells by
downregulating pyruvate kinase M2. Oncol Lett. 15:4358–4364.
2018.PubMed/NCBI
|
|
24
|
Lu W, Hu Y, Chen G, Chen Z, Zhang H, Wang
F, Feng L, Pelicano H, Wang H, Keating MJ, et al: Novel role of NOX
in supporting aerobic glycolysis in cancer cells with mitochondrial
dysfunction and as a potential target for cancer therapy. PLoS
Biol. 10:e10013262012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan TL, Wang PW, Hung YC, Huang CH and Rau
KM: Proteomic analysis reveals tanshinone IIA enhances apoptosis of
advanced cervix carcinoma CaSki cells through mitochondria
intrinsic and endoplasmic reticulum stress pathways. Proteomics.
13:3411–3423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radha M, Farrukh A, Jeyaprakash J and
Gupta RC: Tanshinone IIA inhibits viral oncogene expression leading
to apoptosis and inhibition of cervical cancer. Cancer Lett.
356:536–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu W, Yang Z, Huang R, Min Z and Ye M:
SIRT6 promotes the Warburg effect of papillary thyroid cancer cell
BCPAP through reactive oxygen species. Onco Targets Ther.
12:2861–2868. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gasparre G, Porcelli AM, Lenaz G and Romeo
G: Relevance of mitochondrial genetics and metabolism in cancer
development. Cold Spring Harb Perspect Biol. 5(pii):
a0114112013.PubMed/NCBI
|
|
31
|
Mathupala SP, Rempel A and Pedersen PL:
Aberrant glycolytic metabolism of cancer cells: A remarkable
coordination of genetic, transcriptional, post-translational, and
mutational events that lead to a critical role for type II
hexokinase. J Bioenerg Biomembr. 29:339–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaz CV, Marques R, Alves MG, Oliveira PF,
Cavaco JE, Maia CJ and Socorro S: Androgens enhance the glycolytic
metabolism and lactate export in prostate cancer cells by
modulating the expression of GLUT1, GLUT3, PFK, LDH and MCT4 genes.
J Cancer Res Clin Oncol. 142:5–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Protzel C, Knoedel J, Zimmermann U,
Woenckhaus C, Poetsch M and Giebel J: Expression of proliferation
marker Ki67 correlates to occurrence of metastasis and prognosis,
histological subtypes and HPV DNA detection in penile carcinomas.
Histol Histopathol. 22:1197–1204. 2007.PubMed/NCBI
|
|
34
|
Varier I, Keeley BR, Krupar R, Patsias A,
Dong J, Gupta N, Parasher AK, Genden EM, Miles BA, Teng M, et al:
Clinical characteristics and outcomes of oropharyngeal carcinoma
related to high-risk non-human papillomavirus16 viral subtypes.
Head Neck. 38:1330–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ristriani T, Fournane S, Orfanoudakis G,
Travé G and Masson M: A single-codon mutation converts HPV16 E6
oncoprotein into a potential tumor suppressor, which induces
p53-dependent senescence of HPV-positive HeLa cervical cancer
cells. Oncogene. 28:762–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Termini L, Boccardo E, Esteves GH, Hirata
R Jr, Martins WK, Colo AE, Neves EJ, Villa LL and Reis LF:
Characterization of global transcription profile of normal and
HPV-immortalized keratinocytes and their response to TNF treatment.
BMC Med Genomics. 1:29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu Y, Liu X, Yang Y, Zhao X, Xue J, Zhang
W and Yang A: Effect of FHIT loss and p53 mutation on HPV-infected
lung carcinoma development. Oncol Lett. 10:392–398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirokawa M, Kawabata Y and Miura AB:
Dysregulation of apoptosis and a novel mechanism of defective
apoptotic signal transduction in human B-cell neoplasms. Leuk
Lymphoma. 43:243–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aschoff AP, Günther E and Jirikowski GF:
Tissue transglutaminase in the small intestine of the mouse as a
marker for apoptotic cells. Colocalization with DNA fragmentation.
Histochem Cell Biol. 113:313–317. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cha Y, Park DW, Lee CH, Baek SH, Kim SY,
Kim JR and Kim JH: Arsenic trioxide induces apoptosis in human
colorectal adenocarcinoma HT-29 cells through ROS. Cancer Res
Treat. 38:54–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu W, Zhang W, Xu N, Li Y, Xu J, Zhang H,
Li Y, Lv S, Liu W and Wang H: Dihydroartemisinin induces apoptosis
and downregulates glucose metabolism in JF-305 pancreatic cancer
cells. Rsc Adv. 8:20692–20700. 2018. View Article : Google Scholar
|
|
42
|
Zhu W, Zhang W, Li Y, et al: Possible
mechanism of apoptosis induced by microwave radiation in human
cervical carcinoma cell HeLa. Radiat Protect. 22:378–2258.
2013.
|
|
43
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hou X, Hongzhi DU and Yuan S: Research
progress of tumor metabolism for antitumor drugs. Cancer Res Prev
Treat. 44:231–235. 2017.
|
|
45
|
Zhivotovsky B and Orrenius S: The Warburg
effect returns to the cancer stage. Semin Cancer Biol. 19:1–3.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
North PE, Waner M, Mizeracki A and Mihm MC
Jr: GLUT1: A newly discovered immunohistochemical marker for
juvenile hemangiomas. Hum Pathol. 31:11–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qiu P, Man S, Yang H, Liu Y, Liu Z, Ma L,
Yu P and Gao W: Metabolic regulatory network alterations reveal
different therapeutic effects of cisplatin and Rhizoma paridis
saponins in Lewis pulmonary adenoma mice. RSC Adv. 6:115029–115038.
2016. View Article : Google Scholar
|
|
48
|
Dayan F, Bilton RL, Laferrière J, Trottier
E, Roux D, Pouyssegur J and Mazure NM: Activation of HIF-1alpha in
exponentially growing cells via hypoxic stimulation is independent
of the Akt/mTOR pathway. J Cell Physiol. 218:167–174. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang F, Zhang W, Guo L, Cheng J, Liu P and
Chen B: Gambogic acid suppresses hypoxia-induced HIF-1α/VEGF
expression via inhibiting PI3K/Akt/mTOR pathway in multiple myeloma
cells. Blood. 124:52302014.
|
|
50
|
Yeh YH, Hsiao HF, Yeh YC, Chen TW and Li
TK: Inflammatory interferon activates HIF-1α-mediated
epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J
Exp Clin Cancer Res. 37:702018. View Article : Google Scholar : PubMed/NCBI
|