Introduction
Lung cancer was the leading cause of cancer-related
deaths worldwide in 2016 (1,2). Lung adenocarcinoma (LUAD), a
histological type of non-small-cell lung cancer, accounts for a
major proportion of this disease (3).
In the last decade, the development of targeted therapy using
tyrosine kinase inhibitors and crizotinib has led to improved
clinical outcomes in LUAD patients with alterations in the
epidermal growth factor receptor gene or fusion of echinoderm
microtubule-associated protein-like 4 and anaplastic lymphoma
kinase, respectively (4–7). More recently, immunotherapy has been
developed and increasingly used in patients with lung cancer,
including immune checkpoint inhibitors that target programmed cell
death 1 ligand 1 (PD-L1)-expressing tumor cells by blocking
PD-L1/PD-1 signaling (8,9). Despite recent advances in targeted
therapy and immunotherapy, the prognosis of LUAD remains poor
(10). It has become a research trend
to explore novel molecular biomarkers or therapeutic targets in the
era of precision medicine (11). In
fact, databases based on large-scale, genome-wide association
studies have facilitated the discovery of new biomarkers for cancer
management (12).
The minichromosome maintenance (MCM) family consists
of 8 highly conserved members, including MCM2-7, MCM8 and MCM10
(13). MCM2-7 form the MCM complex, a
hexamer that binds to DNA and functions in the initiation of DNA
replication (14,15). MCM8 is unique in that it serves as a
DNA helicase during replication elongation, but not initiation
(16). MCM10 helps to regulate DNA
replication elongation (17). In line
with their essential roles in DNA replication, MCM genes have
become valuable biomarkers for cancer diagnosis and prognosis
prediction (18–21). Previous studies have shown that MCM2,
MCM4 and MCM7 regulate cell proliferation in non-small cell lung
cancer (NSCLC) (22–24). However, the functions of other MCM
family members remain unclear, and a comprehensive mRNA profiling
of MCM family members in lung cancer has not been performed. In the
present study, database research and bioinformatic analysis were
performed to determine the prognostic significance of MCM mRNA
expression in patients with lung cancer.
Materials and methods
Ethics statement
This study was performed in accordance with standard
guidelines, and was approved by the Ethics Committee of the Second
Affiliated Hospital, Zhejiang University School of Medicine
(Zhejiang, China). The datasets were retrieved from published
literature in which informed consent was obtained from
patients.
Oncomine analysis
The Oncomine database (www.oncomine.org) is a bioinformatics tool for
collecting, standardizing, analyzing and delivering cancer
transcriptome data to the biomedical research community. It was
used to compare the transcription levels of MCMs between cancer
specimens and paracarcinoma tissue. In Oncomine, Student's t-test
is generated for two class differential expression analyses
(25). In the present study,
P<0.01 and an absolute fold-change ≥1.5 were selected as the cut
off values to analyze the gene expression chart of each MCM family
member.
Gene expression profiling interactive
analysis (GEPIA) database
GEPIA (http://gepia.cancer-pku.cn/) is a web tool that
provides fast and customizable functionalities based on data from
The Cancer Genome Atlas (TCGA; http://tcga-data.nci.nih.gov/tcga/) and the
Genotype-Tissue Expression project (GTEx; http://www.gtexportal.org/home/index.html).
Differential analysis was performed using one-way ANOVA, using
disease state or tumor stage as the variable for calculating
differential expression (26). In the
current study, GEPIA was used to represent the differential
expression of MCMs graphically between LUAD and paracarcinoma
tissues and the association between the expression of MCMs and
tumor stages in patients with LUAD (27).
The human protein atlas database
The Human Protein Atlas (https://www.proteinatlas.org/) is a database of
immunohistochemistry (IHC)-based protein expression profiles in
normal tissue, cancer and cell lines (28). IHC images of MCM protein expression in
clinical specimens of patients with LUAD and paracarcinoma tissues
were obtained from the Human Protein Atlas database.
Kaplan-meier plotter
The Kaplan-Meier Plotter tool (www.kmplot.com) includes survival information of 866
patients with LUAD. The prognostic value of MCM expression was
assessed by overall survival (OS), progression-free survival (PFS)
and post-progression survival (PPS), using the hazard ratio (HR),
95% confidence intervals (CI) and log-rank P-value. In the
analysis, patient samples were split into high expression group and
low expression group based on the median mRNA levels of the MCMs.
The prognostic value of a gene was assessed by univariate Cox
regression analysis (29). JetSet
scores were used to select a single representative probe set for
each gene (30). In the current
study, only the probe sets with best JetSet scores for MCMs were
selected to produce Kaplan-Meier plots. The one-to-one matches
between MCM genes and probe sets, identified by Affymetrix IDs,
were as follows: MCM2 and 272107_s_at; MCM3 and 201555_at; MCM4 and
222036_s_at; MCM5 and 216237_s_at; MCM6 and 238977_at; MCM7 and
208795_s_at; MCM8 and 224320_s_at; and MCM10 and 223570_at. The
relevant concepts are defined as follows: OS, time from diagnosis
to death; PFS, time from diagnosis to tumor progression; PPS, time
from progression to death; HR>1, worse survival prognosis for
the group with high mRNA expression; HR<1, unfavorable survival
prognosis in the low mRNA expression group; 95% CI does not cross
1, mRNA expression is associated with survival rate. As not all
gene expression levels were available in all patients and only the
JetSet probes were included in the study, the sample sizes vary for
each survival analysis.
cBioPortal for cancer genomics
(cBioPortal) dataset
cBioPortal (http://cbioportal.org) is based on other authoritative
databases, including the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and TCGA
database. cBioPortal is a web resource for exploring, visualizing
and analyzing multidimensional cancer genomics data. The genomic
profile of each gene includes mutations, putative copy-number
alterations and mRNA expression z-scores. The z score for each gene
is the normalized expression of mRNA using RNA-Seq by expectation
maximization count estimates method. The co-expression of each gene
pair was performed by Fisher's exact test (31) and the network was constructed
according to the correlation.
Cancer SEA
CancerSEA (http://biocc.hrbmu.edu.cn/CancerSEA/) is a dedicated
database for comprehensively exploring distinct functional states
of cancer cells at the single-cell level. The cancer-related single
cell RNA-seq (scRNA-seq) datasets for human samples in CancerSEA
were collected from the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra), the GEO
database and ArrayExpress (https://www.ebi.ac.uk/arrayexpress/). For each
cancer-related scRNA-seq dataset, the original paper was read and
the corresponding metadata was extracted, including the cancer
types and sources, including patient-derived xenograft (PDX) and
circulating tumor cell (CTC). Thus, CancerSEA contained the cancer
single-cell functional state atlas of 41,900 cancer single cells
from 25 cancer types. LUAD chips and PDX are numbered with Exp and
LC-PT as the headers, respectively. For each single-cell dataset
derived from PDX and CTC tumor tissue, significant correlations
between gene expression and functional state activities were
analyzed using Spearman's rank correlation test with false
discovery rate correction for multiple comparisons (32). In the present study, CancerSEA was
used for the functional analysis of MCMs.
Results
Transcriptional levels of MCMs in
patients with LUAD
The MCM transcriptional levels in cancers were
compared with those in paracarcinoma tissues using the Oncomine
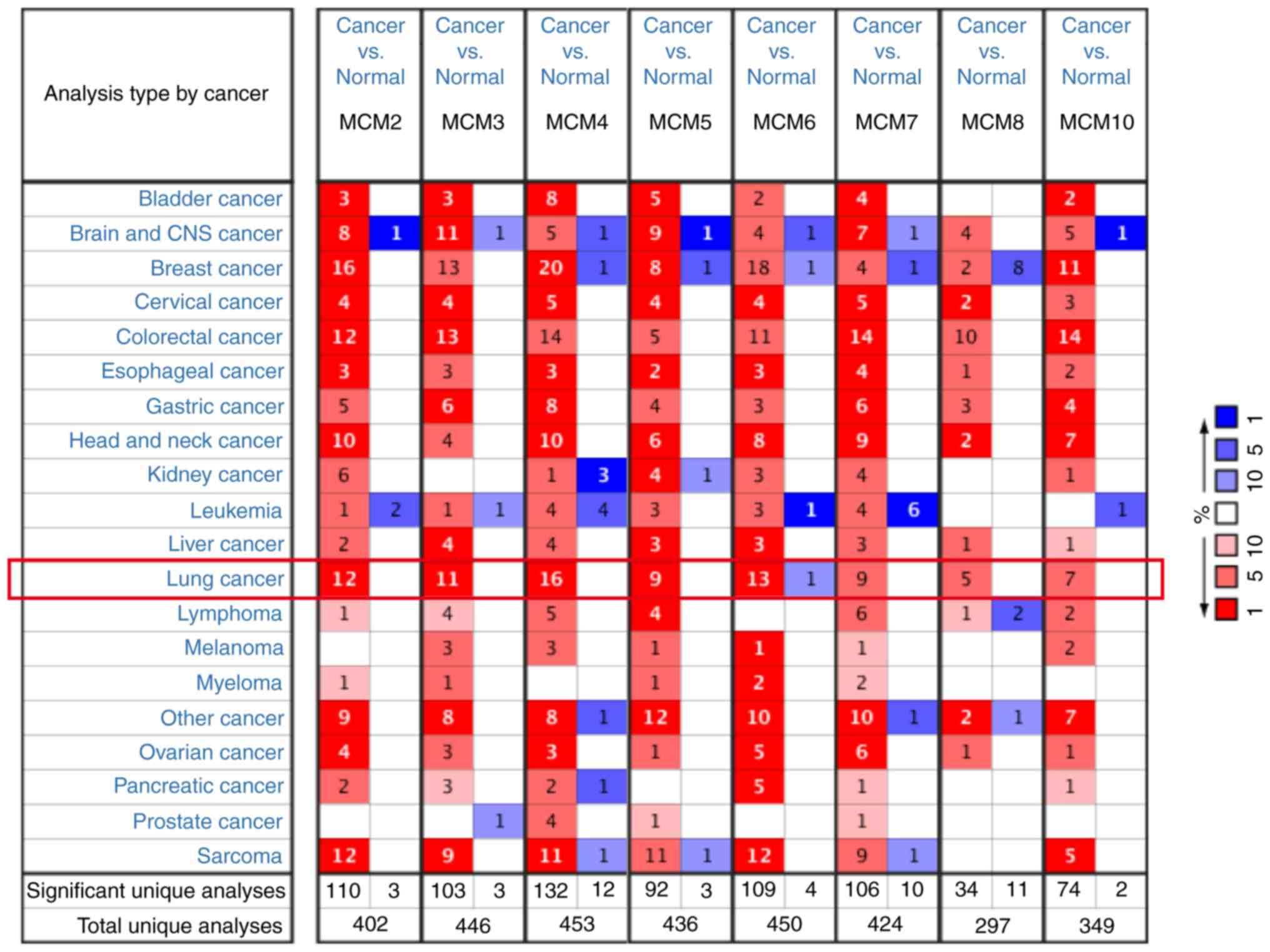
database. As presented in Fig. 1,
MCMs were generally overexpressed in most tumors. In lung cancer,
all MCM members were upregulated in cancer tissues, except MCM6,
which was downregulated in one dataset, which may be due to the
limited numbers of samples. MCM mRNA expression in LUAD and
paracarcinoma tissues are summarized in Table I. The mRNA levels of all MCM members
were significantly increased in LUAD tissues. MCM2 overexpression
was present in 8 databases (33–39),
followed by MCM4 in 7 datasets (33–37,39,40).
MCM10 is the most upregulated member with a fold increase of 6.446
in the dataset from a study by Hou et al (33).
 | Table I.Comparison of mRNA expression of MCMs
in lung adenocarcinoma and normal lung tissues from the Oncomine
database. |
Table I.
Comparison of mRNA expression of MCMs
in lung adenocarcinoma and normal lung tissues from the Oncomine
database.
| Gene | Cases, n | Fold change | P-value | (Refs.) |
|---|
| MCM2 | 110 | 3.251 |
3.46×10−13 | (33) |
|
| 116 | 2.436 |
3.20×10−17 | (34) |
|
| 66 | 2.411 |
2.36×10−6 | (35) |
|
| 246 | 2.120 |
3.44×10−11 | (36) |
|
| 107 | 1.993 |
7.61×10−11 | (37) |
|
| 39 | 1.846 |
6.36×10−5 | (38) |
|
| 96 | 1.668 |
4.35×10−6 | (39) |
| MCM3 | 46 | 1.992 |
5.27×10−5 | (40) |
|
| 116 | 1.617 |
4.13×10−16 | (34) |
|
| 110 | 1.591 |
1.34×10−7 | (33) |
| MCM4 | 110 | 3.390 |
3.60×10−15 | (33) |
|
| 66 | 2.649 |
7.09×10−10 | (35) |
|
| 116 | 2.618 |
1.36×10−18 | (34) |
|
| 107 | 2.403 |
8.50×10−19 | (37) |
|
| 96 | 1.982 |
3.80×10−20 | (39) |
|
| 46 | 1.923 |
1.02×10−4 | (40) |
|
| 246 | 1.668 |
6.17×10−12 | (36) |
| MCM5 | 46 | 1.810 |
4.58×10−6 | (40) |
|
| 110 | 1.544 |
1.09×10−8 | (33) |
| MCM6 | 46 | 2.932 |
1.15×10−4 | (40) |
|
| 110 | 2.114 |
1.69×10−12 | (33) |
|
| 39 | 2.012 |
4.04×10−6 | (38) |
|
| 107 | 1.830 |
2.25×10−15 | (37) |
|
| 116 | 1.797 |
2.63×10−12 | (34) |
|
| 66 | 1.760 |
3.20×10−6 | (35) |
| MCM7 | 110 | 4.547 |
4.11×10−8 | (33) |
|
| 107 | 1.628 |
2.08×10−10 | (37) |
|
| 66 | 1.579 |
1.72×10−5 | (35) |
|
| 116 | 1.551 |
1.24×10−10 | (34) |
|
| 39 | 1.513 |
1.60×10−4 | (38) |
| MCM8 | 110 | 1.987 |
1.37×10−8 | (33) |
| MCM10 | 110 | 6.446 |
7.96×10−8 | (33) |
|
| 246 | 1.792 |
1.92×10−9 | (36) |
|
| 116 | 1.733 |
5.36×10−14 | (34) |
|
| 107 | 1.509 |
1.22×10−9 | (37) |
MCM mRNA expression is associated with
pathological stages of LUAD
As with Oncomine, GEPIA analysis indicated that
expression of MCM2-7, MCM8 and MCM10 was higher in LUAD than in
lung tissues (Fig. 2), although
statistically significant differences were observed for MCM2
(Fig. 2A), MCM4 (Fig. 2C) and MCM10 (Fig. 2H) only. The association between MCM
expression and LUAD pathological stage was then investigated. As
presented in Fig. 3, the mRNA levels
of MCM2 (P=0.0407; Fig. 3A), MCM4
(P=0.00101; Fig. 3C), MCM6 (P=0.0096;
Fig. 3E), MCM7 (P=0.00595; Fig. 3F) and MCM 10 (P=0.00598; Fig. 3H) significantly different between the
tumor stages I to IV. Similar trends occurred for MCM3 (P=0.0605;
Fig. 3B) and MCM5 (P=0.0957; Fig. 3D). However, there was no association
between MCM8 (P=0.231; Fig. 3G) and
tumor stage.
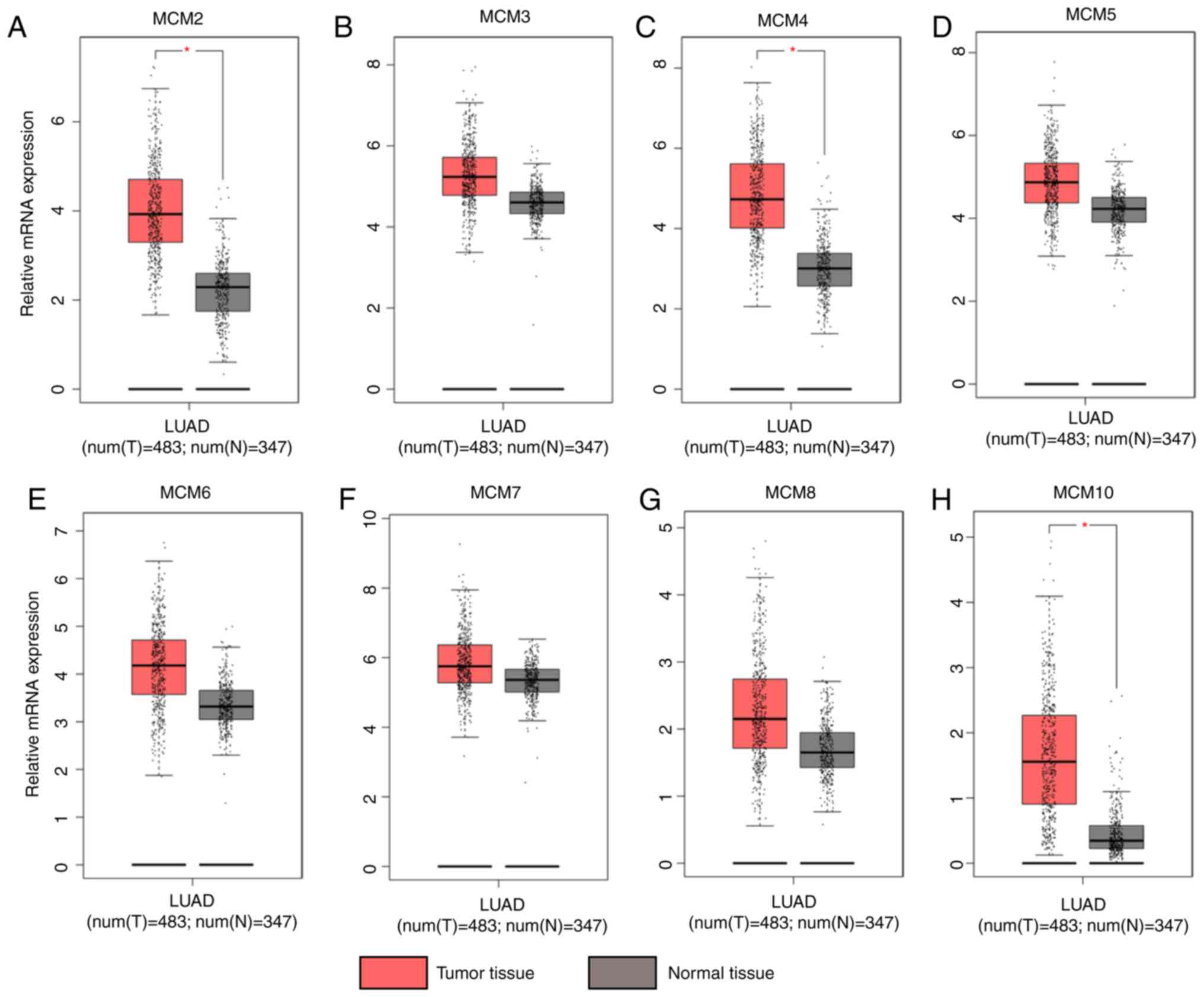 | Figure 2.Expression of MCMs in lung
adenocarcinoma and normal tissues analyzed using GEPIA. (A) MCM2,
(B) MCM3, (C) MCM4, (D) MCM5, (E) MCM6, (F) MCM7, (G) MCM8 and (H)
MCM10. In the box plots, the thick line in the middle represents
the median, and the upper and lower limits of the box represent the
third and first quartile respectively. The top and bottom of the
error bars represent the maximum and minimum values of data,
respectively; outliers were considered to be >1.5 quartile
spacing, and were excluded. *P<0.05. MCM, minichromosome
maintenance; T, tumor; N, normal; num, number. |
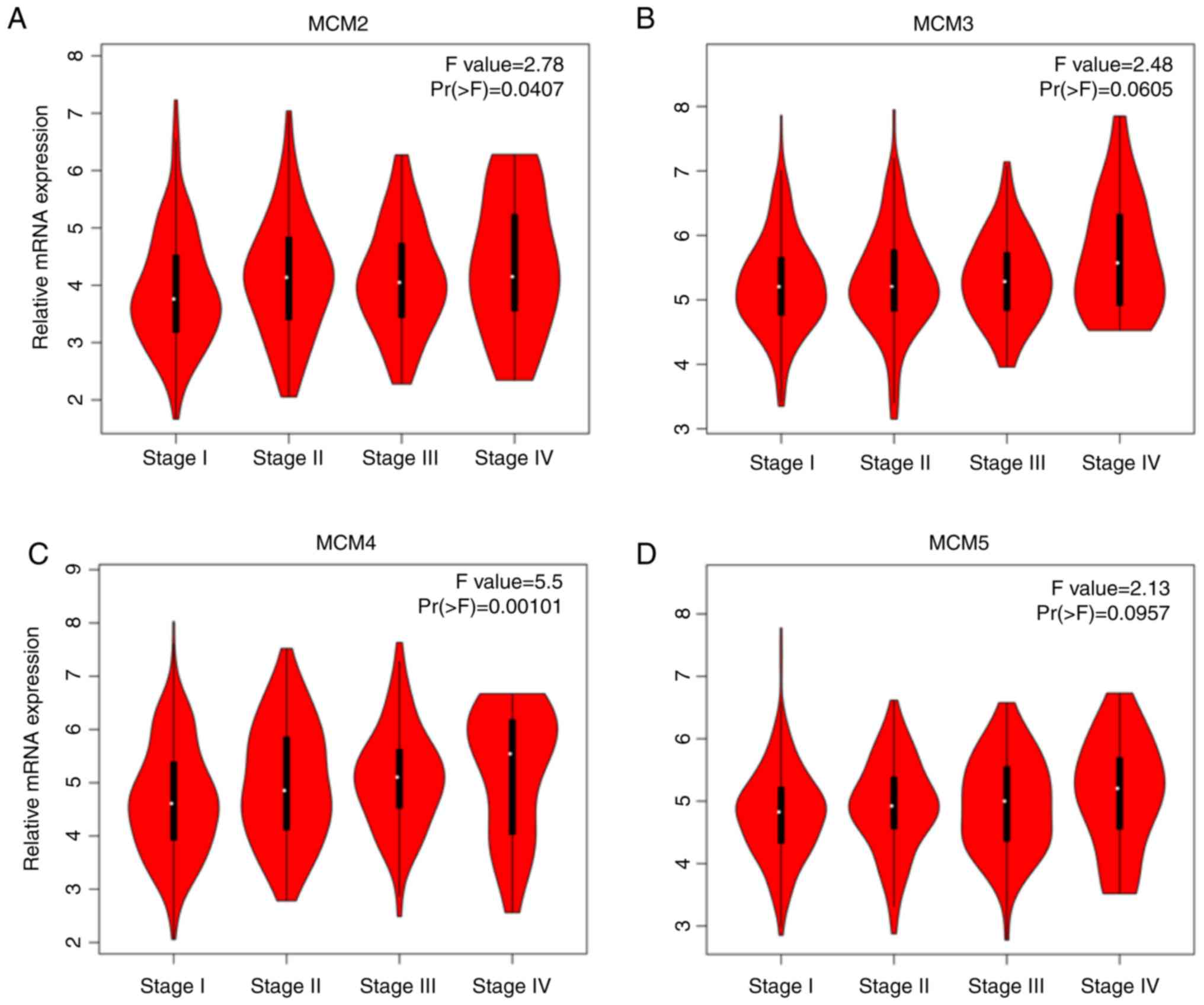 | Figure 3.Association of mRNA expression of
MCMs and tumor stages in patients with lung adenocarcinoma analyzed
using GEPIA. (A) MCM2, (B) MCM3, (C) MCM4, (D) MCM5, (E) MCM6, (F)
MCM7, (G) MCM8 and (H) MCM10. In the violin plots, the white dots
represent the median; the black bars represent the 95% confidence
intervals; the black lines represent the interquartile range; and
the width of the red shapes represent the density of distribution.
MCM, minichromosome maintenance. F-value, the statistical value of
F test; Pr (>F), P-value. |
Protein expression levels of MCMs in
patients with LUAD
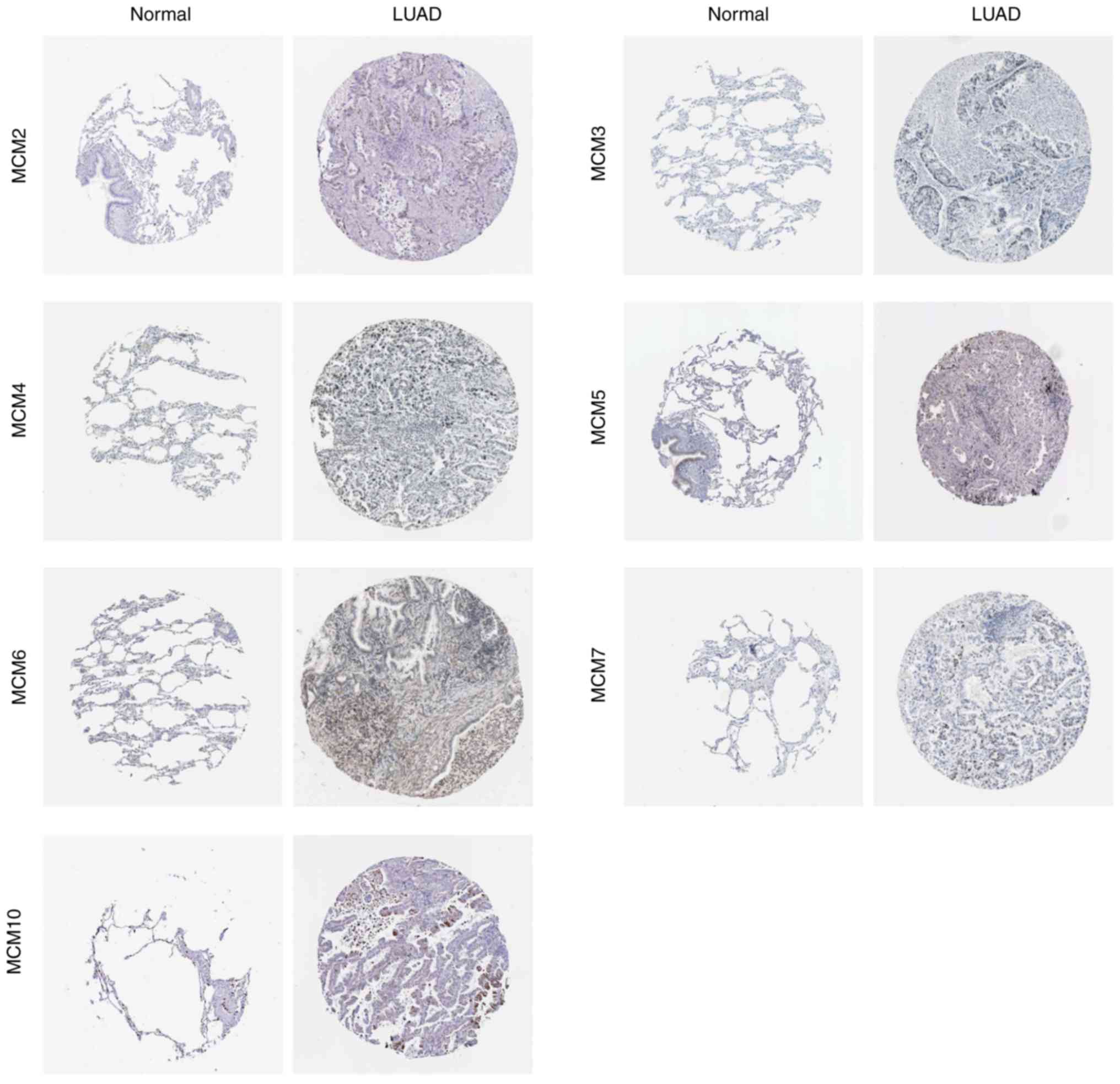
To examine whether MCM protein was also
differentially expressed in LUAD tissues, immunohistochemical
staining images for the MCM proteins in LUAD and paracarcinoma
tissues were obtained from the Human Protein Atlas database
(Fig. 4). Consistent with RNA
expression data, the results demonstrated that MCM2, MCM5, MCM6 and
MCM7 protein levels were higher in LUAD tissue compared with normal
tissue, whereas MCM3, MCM4 and MCM10 proteins were only slightly
increased in LUAD tissue.
The prognostic significance of MCMs in
patients with LUAD
Using the Kaplan-Meier plotter, the prognostic
significance of the mRNA expression of MCMs in patients with LUAD
was determined. Seven MCM members were significantly associated
with reduced OS in patients with LUAD (Fig. 5A). Survival curves are presented in
Fig. 5B-I. High expression of MCM2
(Fig. 5B; HR, 1.33; 95% CI,
1.05–1.67; P=0.018), MCM3 (Fig. 5C;
HR, 2.20; 95% CI, 1.72–2.82; P=1.1×10−10), MCM4
(Fig. 5D; HR, 2.27; 95% CI,
1.78–2.90; P=1.1×10−11), MCM5 (Fig. 5E; HR, 1.34; 95% CI, 1.06–1.79;
P=0.014), MCM7 (Fig. 5G; HR, 1.29;
95% CI, 1.02–1.63; P=0.031), MCM8 (Fig.
5H; HR, 1.34; 95% CI, 1.05–1.71; P=0.019) and MCM10 (Fig. 5I; HR, 1.29; 95% CI, 1.02–1.63;
P=0.031) were associated with worse OS, while MCM6 was not
associated with altered OS (Fig. 5F;
HR, 1.08; 95% CI, 0.85–1.37; P=0.530).
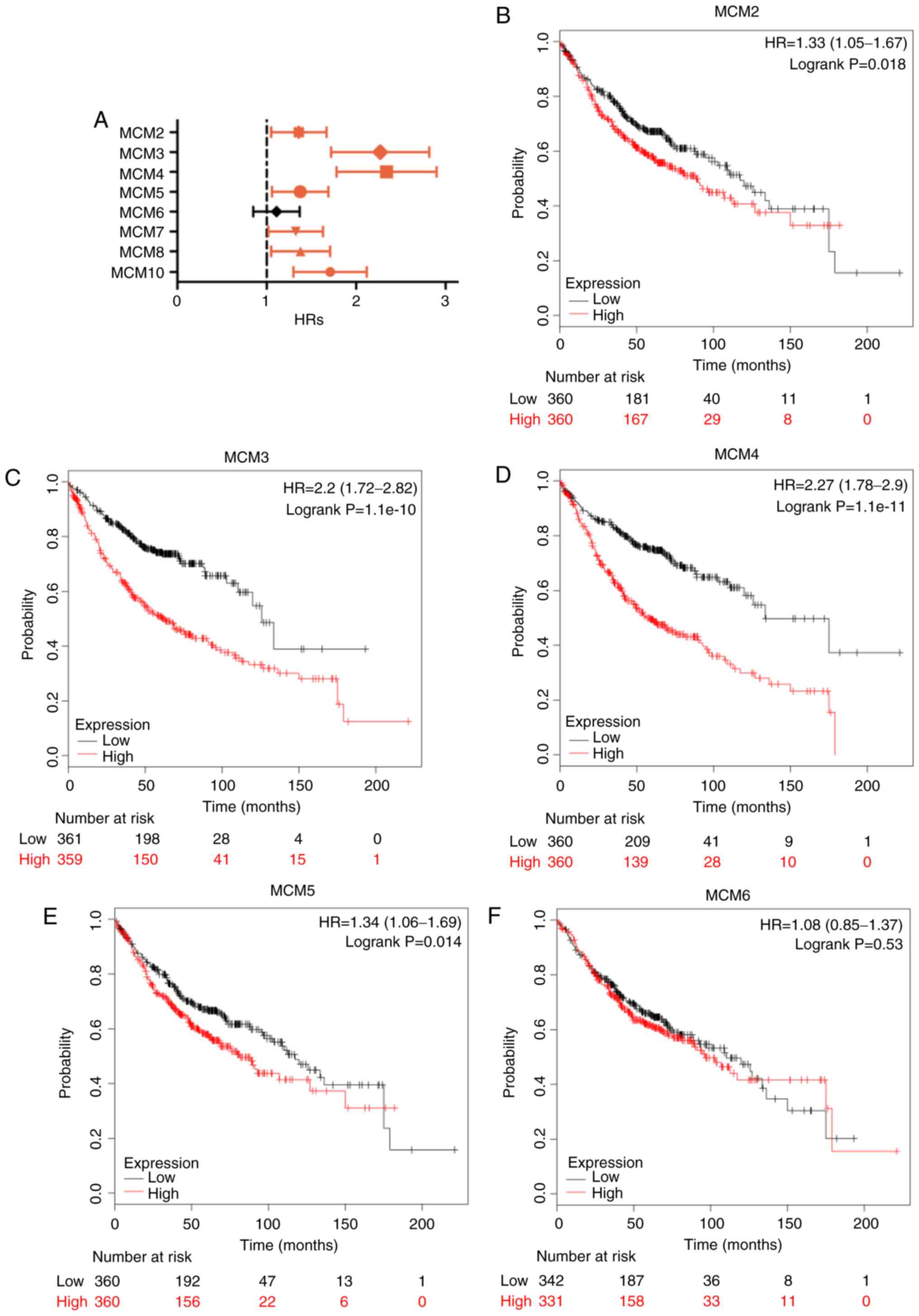 | Figure 5.OS of LUAD patients with high and low
mRNA expression of MCM, analyzed using the Kaplan-Meier Plotter
tool. (A) Prognostic HRs of individual MCM members in LUAD; error
bars represent the 95% confidence intervals. (B-I) OS curves of
MCM2, MCM3, MCM4, MCM5, MCM6, MCM7, MCM8 and MCM10 plotted for all
patients (n=720). OS, overall survival; HR, hazard ratio; MCM,
minichromosome maintenance; LUAD, lung adenocarcinoma. |
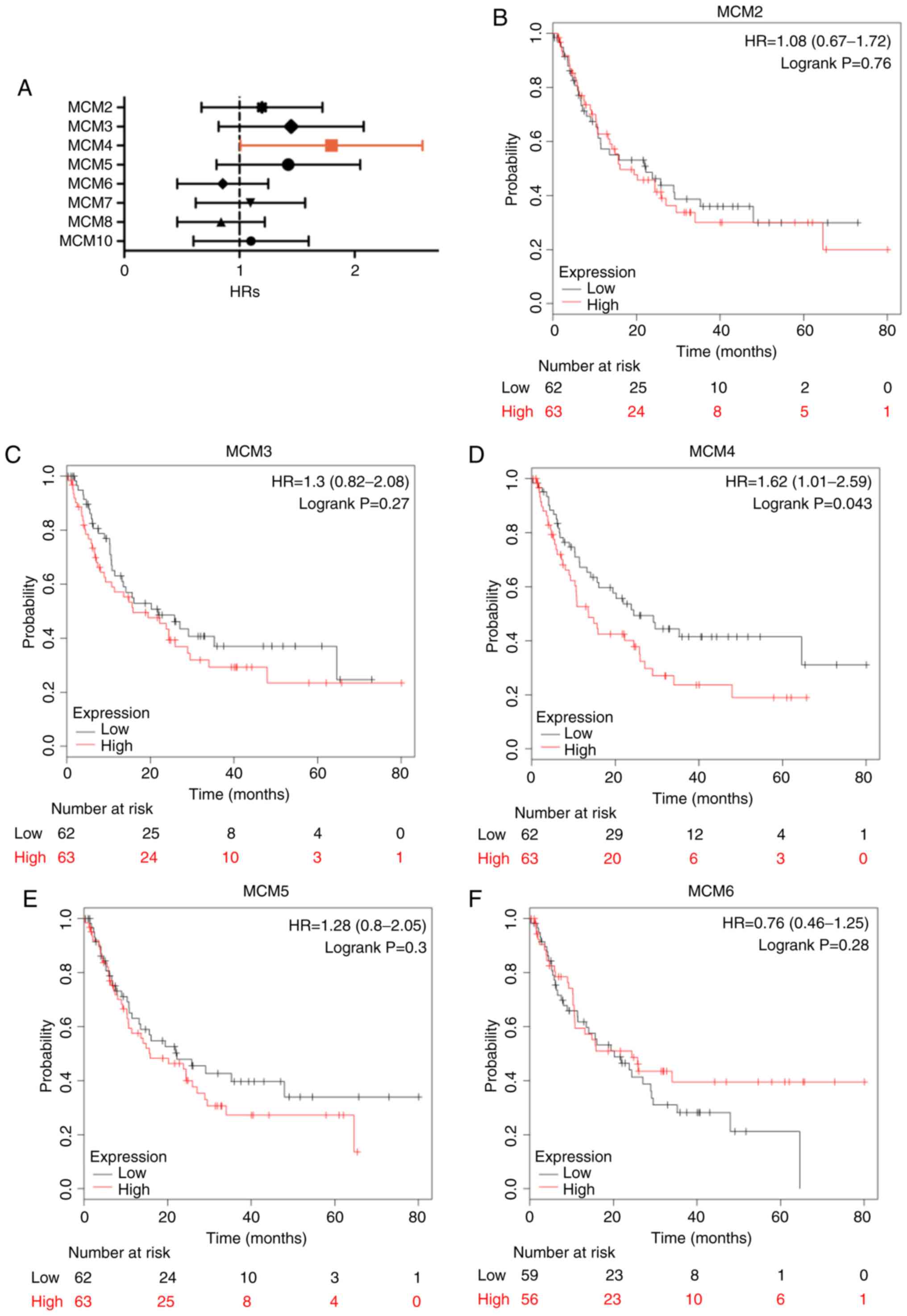
Similarly, high expression of MCM2 (Fig. 6B; HR, 1.62; 95% CI, 1.18–2.22;
P=0.003), MCM3 (Fig. 6C; HR, 1.39;
95% CI, 1.02–1.90; P=0.037), MCM4 (Fig.
6D; HR, 2.38; 95% CI, 1.72–3.31; P=8.3×10−8), MCM5
(Fig. 6E; HR, 1.42; 95% CI,
1.04–1.95; P=0.027), MCM8 (Fig. 6H;
HR, 1.44; 95% CI, 1.04–1.99; P=0.027) and MCM10 (Fig. 6I; HR, 1.94; 95% CI, 1.40–2.71;
P=6.5×10−5) was significantly associated with reduced
PFS. Additionally, high MCM4 mRNA expression also indicated adverse
PPS (Fig. 6D; HR, 1.62; 95% CI,
1.01–2.59; P=0.043). Other MCMs were not associated with PFS or PPS
(Figs. 6 and 7).
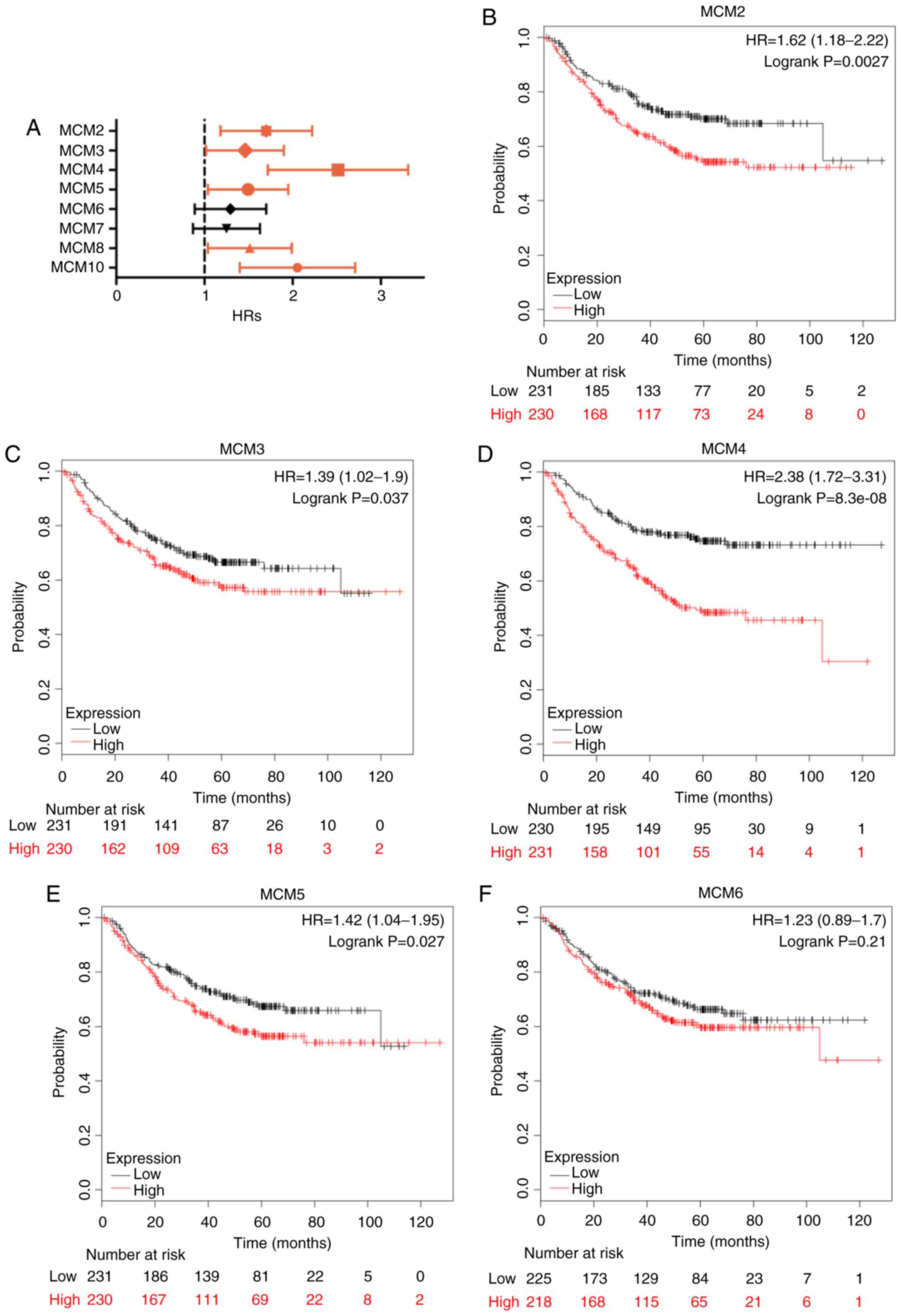 | Figure 6.PFS in LUAD patients with high and
low mRNA expression of MCM, analyzed using the Kaplan-Meier Plotter
tool. (A) Prognostic HRs of individual MCM members in LUAD; error
bars represent the 95% confidence intervals. (B-I) PFS curves of
MCM2, MCM3, MCM4, MCM5, MCM6, MCM7, MCM8 and MCM10 plotted for all
patients (n=461). PFS, progression-free survival; HR, hazard ratio;
MCM, minichromosome maintenance; LUAD, lung adenocarcinoma. |
 | Figure 7.PPS of LUAD patients with high and
low mRNA expression of MCM, analyzed using the Kaplan-Meier Plotter
tool. (A) Prognostic HRs of individual MCM members in LUAD; error
bars represent the 95% confidence intervals. (B-I) PPS curves of
MCM2, MCM3, MCM4, MCM5, MCM6, MCM7, MCM8 and MCM10 plotted for all
patients (n=125). PPS, post-progression survival; HR, hazard ratio;
MCM, minichromosome maintenance; LUAD, lung adenocarcinoma. |
MCM expression changes in LUAD and the
network within each MCM or with other genes
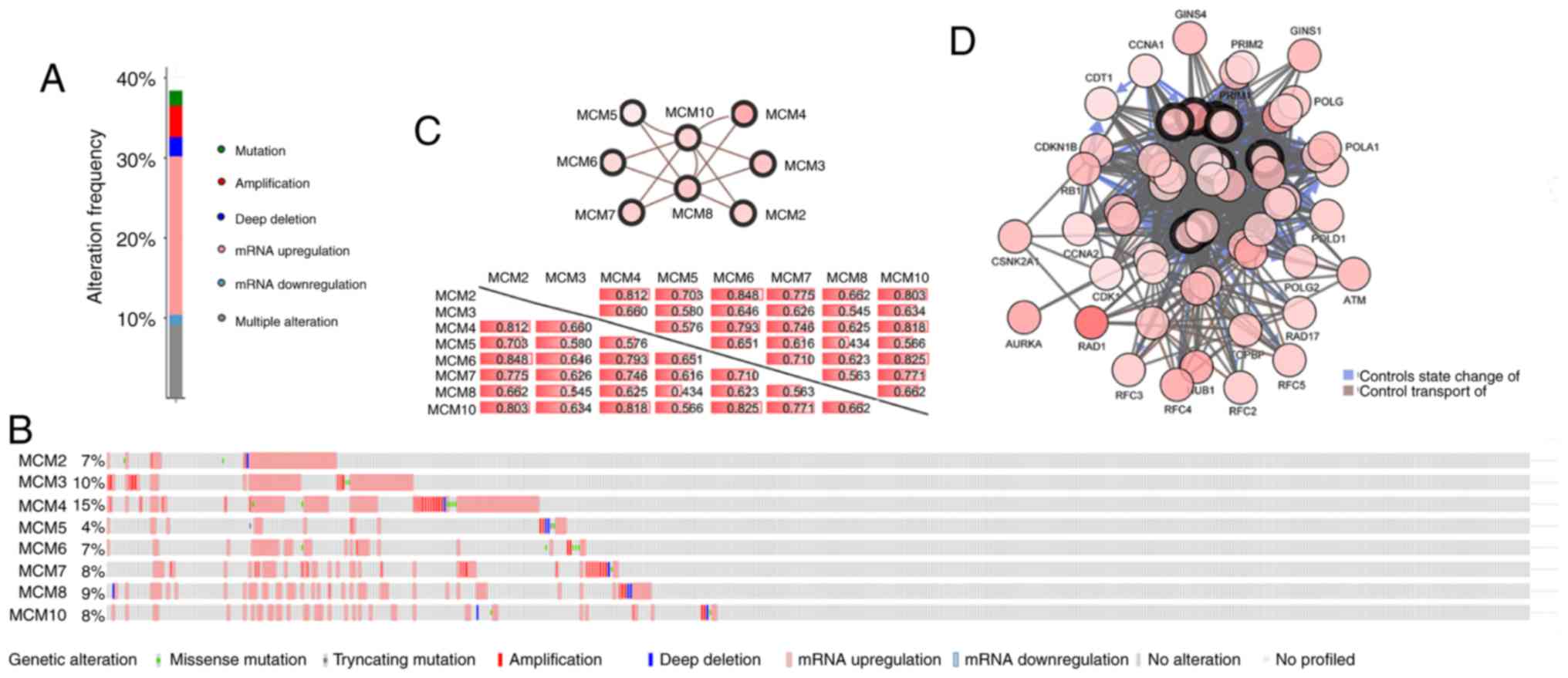
With the cBioPortal online tool, the alterations,
correlations and networks of MCMs in LUAD were analyzed. Of the
LUAD samples, 39% had altered MCMs, and the most common genetic
change was gene amplification (Fig.
8A). The distribution of genetic alterations for individual
MCMs indicated that nearly 15% of LUAD cases had MCM4 amplification
(Fig. 8B). Significant and positive
correlations were observed between the MCMs (Fig. 8C). Among them, MCM2 and MCM6 had the
highest positive correlation with a Spearman's correlation
coefficient of 0.848. MCM10 exhibited the tightest association with
all other MCMs, with a median Spearman's correlation coefficient of
0.771. Next, the network for MCMs and the genes frequently altered
with MCMs was constructed (Fig. 8D).
The majority of the genes frequently altered with MCMs were cell
cycle-related or involved in DNA damage/repair, such as ATM
serine/threonine kinase, RAD1 checkpoint DNA exonuclease, cyclin
dependent kinase 1, cyclin A1 and replication factor C subunit 5,
suggesting that the MCM family is critical for maintenance of
genome integrity.
The functions of MCMs in single LUAD
cell
Heterogeneity between cancer cells poses a major
challenge for cancer diagnosis and treatment. Single-cell
sequencing technology provides an unprecedented opportunity to
accurately decipher the functional states of cancer cells at a
single-cell resolution.
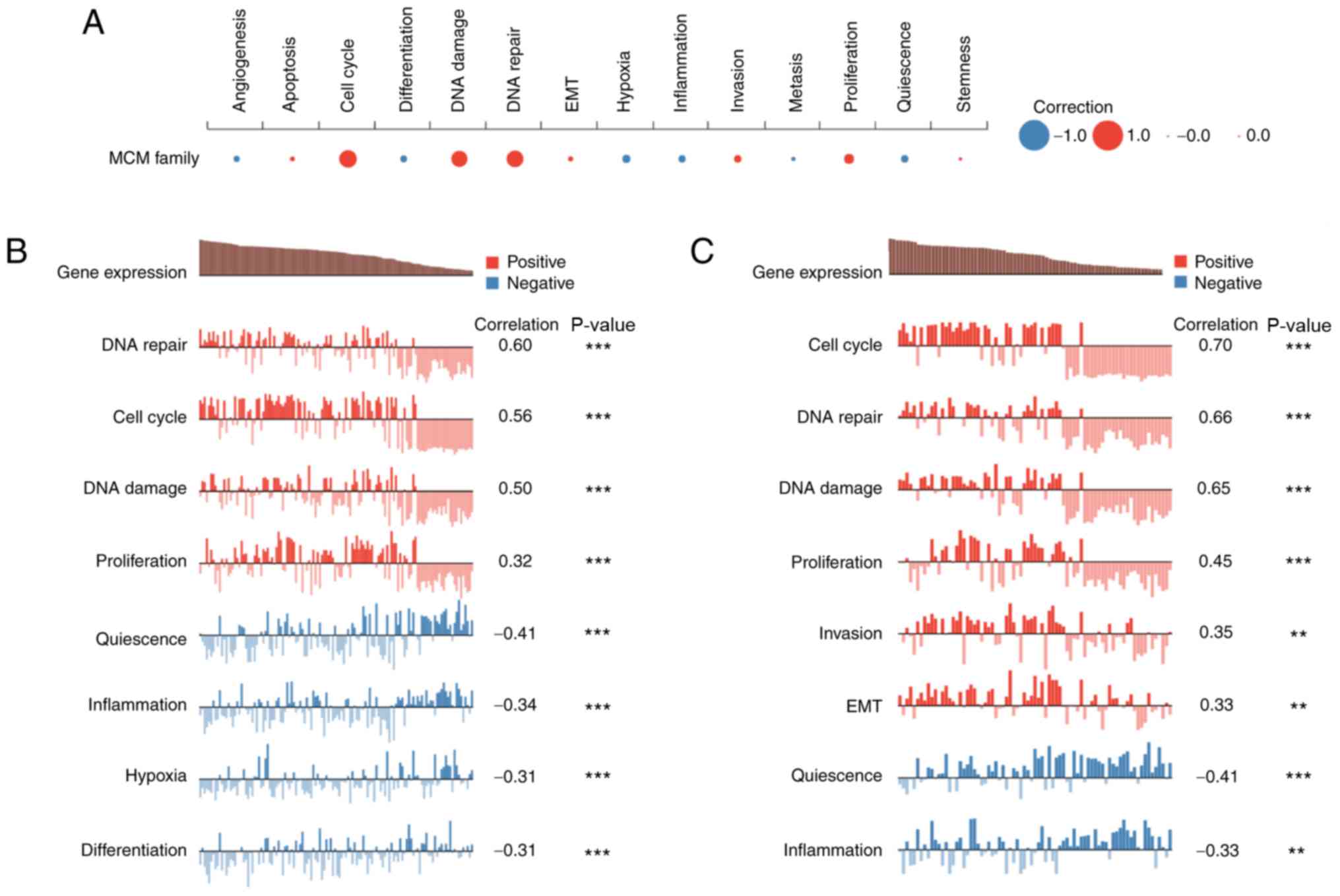
Using CancerSEA, the functions of MCMs in single
LUAD cells were explored. MCM function was found to be mainly
related to cell cycle, DNA damage or DNA repair (Fig. 9A). Kim (Exp0066) showed high
expression of the MCM family was positively correlated with DNA
repair, cell cycle, DNA damage and proliferation (Spearman's
coefficients, 0.60, 0.56, 0.50 and 0.32, respectively; P<0.001)
(41). Correlation analysis also
revealed a negative correlation of MCM expression and quiescence,
inflammation, hypoxia and differentiation (Spearman's coefficient,
−0.41, −0.32, −0.31 and −0.31, respectively; P<0.001) (Fig. 9B). Similar results were observed in
patient-derived xenograft (LC-PT-45) (41) (Fig.
9C).
Discussion
Uncontrolled cancer cell proliferation is usually
accompanied by dysregulated DNA replication, and chemotherapeutic
agents targeting replication machinery have been widely used for
cancer treatment (42). MCMs,
essential molecules in the initiation and elongation of DNA
replication, are considered to be useful indicators of cell
proliferation, and MCM alterations are more frequent in neoplastic
cells than in non-neoplastic cells (43). Moreover, MCM proteins are expressed in
rapidly dividing cells, but not in quiescent, aging or
differentiated cells (44).
Therefore, MCMs may have potential clinical application as markers
for cancer screening. However, a comprehensive bioinformatics
analysis of MCMs in LUAD has yet to be performed. To the best of
our knowledge, this is the first study to explore the mRNA
expression levels of the MCM family and their prognostic relevance
for predicting OS, PFS and PPS in LUAD. Our findings highlight
potential roles for MCMs in diagnosis and risk stratification in
patients with LUAD.
MCM2 is the most studied MCM member that is
upregulated in LUAD, and MCM2 dysregulation is associated with cell
proliferation, cell cycle progression and migration (45). A significant correlation between MCM2
mRNA expression and LUAD stages was previously observed, and MCM2
was demonstrated to be the therapeutic target of lovastatin for
NSCLC treatment (46). Additionally,
Veena et al (47) reported
that the MCM2 was present in lung tissues and in sputum, which is
more accessible for MCM2 detection. However, the association
between MCM2 expression and treatment outcomes of patients with
NSCLC remains controversial (44,48–50). In
the present study, analysis using the Oncomine and GEPIA
bioinformatics tools revealed that MCM2 expression was higher in
LUAD tissues compared with that in normal tissues, and this finding
was consistent with immunohistochemical staining for MCM2 in the
Human Protein Atlas. Using the Kaplan-Meier Plotter, elevated MCM2
mRNA was found to be significantly associated with reduced OS and
PFS in patients with LUAD.
MCM3 had been found to be differentially expressed
in LUAD and adjacent normal tissue samples (51,52);
however, the role of MCM3 in NSCLC was unclear. Zhang et al
(53) found that the PI3K inhibitor,
LY294002, inhibited MCM3 expression in NSCLC cells, indicating that
MCM3 is involved in the PI3K-Akt pathway. In the present study,
MCM3 upregulation was also observed in LUAD tissues, and high MCM3
expression was associated with poor OS and PFS, but not PPS in
patients with LUAD.
MCM4 overexpression is an oncogenic event in LUAD
(54). A study by Kikuchi et
al (55) showed that high MCM4
expression was associated with clinicopathological features of
LUAD, but was not associated with survival. However, in the present
study, high expression of MCM4 was associated with worse OS, PFS
and PPS.
MCM5 is upregulated in lung squamous cell carcinoma,
and patients with high MCM5 expression have reduced OS (43). In the current study, MCM5 was also
found to be elevated in LUAD and was associated with adverse OS and
PFS.
MCM6, together with Ki-67 and HuR, an RNA binding
protein, were associated with poor prognosis in NSCLC (44,56).
However, no association was found between MCM6 expression and
patient outcome in the current study.
MCM7 is involved in proliferation and signal
transduction of the receptor of activated protein C kinase 1/Akt
pathway (57). Moreover, pre-clinical
research based on NSCLC cell lines showed that MCM7 and its
targets, a cluster of miR-25, miR-93 and miR-106b, elicited
oncogenic activity in lung cancer (58). MCM7 expression was also associated
with worse prognosis in patients with NSCLC (59,60). In
the present study, high MCM7 expression was suggested to be a
predictor for poor OS and PFS.
Little is known about the role of MCM8 and MCM10 in
lung cancer. MCM8 is expressed in tissue with a high percentage of
proliferating cells, such as lung and liver (13,16). MCM10
promotes cell proliferation (17), is
associated with poor prognosis and is considered to be a potential
therapeutic target in breast and prostate cancer (61). In the current study, MCM8 mRNA, but
not protein expression was found to be is higher in tumor compared
with normal tissues. Similarly, MCM10 was also found to be
overexpressed in LUAD. However, neither MCM8 nor MCM10 were
associated with the OS of patients with LUAD.
MCMs have frequently been compared with Ki-67, a
well-established marker of cell proliferation (62). In adrenocortical cancer, MCM3 and MCM7
share a similar staining pattern with Ki-67 in benign and malignant
tissues (63). MCM4 and MCM7 are more
sensitive markers than Ki-67 for the detection of esophageal cancer
(64). Furthermore, MCM5 and MCM2,
but not Ki-67, have been associated with poor prognosis in gastric
cancer and NSCLC (65,66). As a marker of G1-phase arrest in
mantle cell lymphoma, MCM6 is superior to other clinical
parameters, including Ki-67 (48).
MCM2 expression is higher than Ki-67 in megakaryocytes, suggesting
that MCM2 may be a more sensitive marker for some hematological
diseases (67). In tissue sections,
MCM proteins are more highly expressed than Ki-67 in quiescent
cells (68,69). Taken together, these results suggest
that the MCM family may be better proliferation markers than Ki-67
for cancer diagnosis and risk stratification.
In the present study, the expression and prognostic
significance of MCMs in LUAD was analyzed. MCM2-6 were found to be
upregulated and associated with LUAD prognosis. Notably, high
expression of MCM2-5 was associated with reduced OS and PFS. MCM7
expression was also associated with poor OS, and MCM4 predicted
worse PPS. As MCM expression was found to be consistent at the mRNA
and protein level, analysis of mRNA in patient samples may be
preferred as it is easier to analyze. Thus, transcriptional
detection of MCM members may provide robust biomarkers to improve
patient survival and prognosis prediction in LUAD. As all data and
results in this study are based on bioinformatic analysis, further
studies are required to determine the role of MCMs in vitro
and in vivo.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81572920), the Natural
Science Foundation of Zhejiang Province of China (grant no.
LY15H160038) and the National Basic Research Program of China
(grant no. 2013CB911303).
Availability of data and materials
The datasets analyzed during the current study are
publicly available from the following online databases: Oncomine
database (www.oncomine.org); GEPIA database
(http://gepia.cancer-pku.cn/);
Kaplan-Meier Plotter (www.kmplot.com); The Human Protein Atlas (https://www.proteinatlas.org/); cBioPortal (http://cbioportal.org); GEO (http://www.ncbi.nlm.nih.gov/geo/); TCGA (https://tcga-data.nci.nih.gov/tcga/); CancerSEA
(http://biocc.hrbmu.edu.cn/CancerSEA/).
Authors' contributions
SL and YX designed the study. SL, ZJ and YL acquired
the data, performed data analysis and interpretation. ZJ and YL
drafted the manuscript, and YX and SL critically revised it for
important intellectual content. All authors gave final approval of
the version to be published, and agreed to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
This study was performed in accordance with standard
guidelines and was approved by the Ethics Committee of the Second
Affiliated Hospital, Zhejiang University School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maruthappu M, Watkins J, Noor AM, Williams
C, Ali R, Sullivan R, Zeltner T and Atun R: Economic downturns,
universal health coverage, and cancer mortality in high-income and
middle-income countries, 1990–2010: A longitudinal analysis.
Lancet. 388:684–695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosell R, Bivona TG and Karachaliou N:
Genetics and biomarkers in personalisation of lung cancer
treatment. Lancet. 382:720–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsao MS, Sakurada A, Cutz JC, Zhu CQ,
Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, et
al: Erlotinib in lung cancer-molecular and clinical predictors of
outcome. N Engl J Med. 353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lastwika KJ, Wilson W III, Li QK, Norris
J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et
al: Control of PD-L1 expression by oncogenic activation of the
AKT-mTOR pathway in non-small cell lung cancer. Cancer Res.
76:227–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Facchinetti F, Marabelle A, Rossi G, Soria
JC, Besse B and Tiseo M: Moving immune checkpoint blockade in
thoracic tumors beyond NSCLC. J Thorac Oncol. 11:1819–1836. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to the 2015 world health
organization classification of tumors of the lung, pleura, thymus,
and heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsao AS, Scagliotti GV, Bunn PA Jr,
Carbone DP, Warren GW, Bai C, de Koning HJ, Yousaf-Khan AU,
McWilliams A, Tsao MS, et al: Scientific advances in lung cancer
2015. J Thorac Oncol. 11:613–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dirks RA, Stunnenberg HG and Marks H:
Genome-wide epigenomic profiling for biomarker discovery. Clin
Epigenetics. 8:1222016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson EM, Kinoshita Y and Daniel DC: A
new member of the MCM protein family encoded by the human MCM8
gene, located contrapodal to GCD10 at chromosome band 20p12.3–13.
Nucleic Acids Res. 31:2915–2925. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bik KT: MCM proteins in DNA replication.
Annu Rev Biochem. 68:649–686. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Remus D, Beuron F, Tolun G, Griffith JD,
Morris EP and Diffley JF: Concerted loading of Mcm2-7 double
hexamers around DNA during DNA replication origin licensing. Cell.
139:719–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maiorano D, Cuvier O, Danis E and Méchali
M: MCM8 is an MCM2-7-related protein that functions as a DNA
helicase during replication elongation and not initiation. Cell.
120:315–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lõoke M, Maloney MF and Bell SP: Mcm10
regulates DNA replication elongation by stimulating the CMG
replicative helicase. Genes Dev. 31:291–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hua C, Zhao G, Li Y and Bie L:
Minichromosome maintenance (MCM) family as potential diagnostic and
prognostic tumor markers for human gliomas. BMC Cancer. 14:5262014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng YP, Zhu Y, Yin LD, Zhang JJ, Guo S,
Fu Y, Miao Y and Wei JS: The expressionand prognostic roles of MCMs
in pancreatic cancer. PLoS One. 11:e01641502016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwok HF, Zhang SD, McCrudden CM, Yuen HF,
Ting KP, Wen Q, Khoo US and Chan KY: Prognostic signifcance of
minichromosome maintenance proteins in breast cancer. Am J Cancer
Res. 5:52–71. 2014.PubMed/NCBI
|
|
21
|
Liao X, Liu X, Yang C, Wang X, Yu T, Han
C, Huang K, Zhu G, Su H, Qin W, et al: Distinct diagnostic and
prognostic values of minichromosome maintenance gene expression in
patients with hepatocellular carcinoma. J Cancer. 9:2357–2373.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Werynska B, Pula B, Muszczynska-Bernhard
B, Piotrowska A, Jethon A, Podhorska-Okolow M, Dziegiel P and
Jankowska R: Correlation between expression of metallothionein and
expression of Ki-67 and MCM-2 proliferation markers in non-small
cell lung cancer. Anticancer Res. 31:2833–2839. 2011.PubMed/NCBI
|
|
23
|
Wu W, Wang X, Shan C, Li Y and Li F:
Minichromosome maintenance protein 2 correlates with the malignant
status and regulates proliferation and cell cycle in lung squamous
cell carcinoma. Onco Targets Ther. 11:5025–5034. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujioka S, Shomori K, Nishihara K, Yamaga
K, Nosaka K, Araki K, Haruki T, Taniguchi Y, Nakamura H and Ito H:
Expression of minichromosome maintenance 7 (MCM7) in small lung
adenocarcinomas (pT1): Prognostic implication. Lung Cancer.
65:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chansky K, Detterbeck FC, Nicholson AG,
Rusch VW, Vallières E, Groome P, Kennedy C, Krasnik M, Peake M,
Shemanski L, et al: The IASLC lung cancer staging project: External
validation of the revision of the TNM stage groupings in the eighth
edition of the TNM classification of lung cancer. J Thorac Oncol.
12:1109–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pontén F, Jirström K and Uhlen M: The
human protein atlas-a tool for pathology. J Pathol. 216:387–393.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q, Birkbak NJ, Gyorffy B, Szallasi Z
and Eklund AC: Jetset: Selecting the optimal microarray probe set
to represent a gene. BMC Bioinformatics. 12:4742011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan H, Yan M, Zhang G, Liu W, Deng C,
Liao G, Xu L, Luo T, Yan H, Long Z, et al: CancerSEA: A cancer
single-cell state atlas. Nucleic Acids Res. 47(D1): D900–D908.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou J, Aerts J, den Hamer B, van Ijcken W,
den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens J
Hoogsteden HC, et al: Gene expression-based classification of
non-small cell lung carcinomas and survival prediction. PLoS One.
5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selamat SA, Chung BS, Girard L, Zhang W,
Zhang Y, Campan M, Siegmund KD, Koss MN, Hagen JA, Lam WL, et al:
Genome-scale analysis of DNA methylation in lung adenocarcinoma and
integration with mRNA expression. Genome Res. 22:1197–1211. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ,
Liang SC, Lin CH, Whang-Peng J, Hsu SL, Chen CH and Huang CY:
Selection of DDX5 as a novel internal control for Q-RT-PCR from
microarray data using a block bootstrap re-sampling scheme. BMC
Genomics. 8:1402017. View Article : Google Scholar
|
|
36
|
Okayama H, Kohno T, Ishii Y, Shimada Y,
Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S,
et al: Identification of genes upregulated in ALK-positive and
EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res.
72:100–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Landi MT, Dracheva T, Rotunno M, Figueroa
JD, Liu H, Dasgupta A, Mann FE, Fukuoka J, Hames M, Bergen AW, et
al: Gene expression signature of cigarette smoking and its role in
lung adenocarcinoma development and survival. PLoS One.
3:e16512008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stearman RS, Dwyer-Nield L, Zerbe L,
Blaine SA, Chan Z, Bunn PA Jr, Johnson GL, Hirsch FR, Merrick DT,
Franklin WA, et al: Analysis of orthologous gene expression between
human pulmonary adenocarcinoma and a carcinogen-induced murine
model. Am J Pathol. 167:1763–1775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Beer DG, Kardia SL, Huang CC, Giordano TJ,
Levin AM, Misek DE, Lin L, Chen G, Gharib TG, Thomas DG, et al:
Gene-expression profiles predict survival of patients with lung
adenocarcinoma. Nat Med. 8:816–824. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garber ME, Troyanskaya OG, Schluens K,
Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen
GD, Perou CM, Whyte RI, et al: Diversity of gene expression in
adenocarcinoma of the lung. Proc Natl Acad Sci USA. 98:13784–13789.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim KT, Lee HW, Lee HO, Kim SC, Seo YJ,
Chung W, Eum HH, Nam DH, Kim J, Joo KM and Park WY: Single-cell
mRNA sequencing identifies subclonal heterogeneity in anti-cancer
drug responses of lung adenocarcinoma cells. Genome Biol.
16:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Simon NE and Schwacha A: The Mcm2-7
replicative helicase: A promising chemotherapeutic target. Biomed
Res Int. 2014:5497192014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu YZ, Wang BS, Jiang YY, Cao J, Hao JJ,
Zhang Y, Xu X, Cai Y and Wang MR: MCMs expression in lung cancer:
Implication of prognostic significance. J Cancer. 8:3641–3647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tachibana KE, Gonzalez MA and Coleman N:
Cell-cycle-dependent regulation of DNA replication and its
relevance to cancer pathology. J Pathol. 205:123–129. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheung CHY, Hsu CL, Chen KP, Chong ST1, Wu
CH, Huang HC and Juan HF: MCM2-regulated functional networks in
lung cancer by multi-dimensional proteomic approach. Sci Rep.
7:133022017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang X, Teng Y, Yang F, Wang M, Hong X,
Ye LG, Gao YN and Chen GY: MCM2 is a therapeutic target of
lovastatin in human non-small cell lung carcinomas. Oncol Rep.
33:2599–2605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Veena VS, Rajan K, Saritha VN, Preethi
Sara G, Chandramohan K, Jayasree K, Thara S and Sujathan K: DNA
replication licensing proteins for early detection of lung cancer.
Asian Pac J Cancer Prev. 18:3041–3047. 2017.PubMed/NCBI
|
|
48
|
Yang J, Ramnath N, Moysich KB, Asch HL,
Swede H, Alrawi SJ, Huberman J, Geradts J, Brooks JS and Tan D:
Prognostic significance of MCM2, Ki-67 and gelsolin in non-small
cell lung cancer. BMC Cancer. 6:2032006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kadara H, Lacroix L, Behrens C, Solis L,
Gu X, Lee JJ, Tahara E, Lotan D, Hong WK, Wistuba II and Lotan R:
Identification of gene signatures and molecular markers for human
lung cancer prognosis using an in vitro lung carcinogenesis system.
Cancer Prev Res (Phila). 2:702–711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramnath N, Hernandez FJ, Tan DF, Huberman
JA, Natarajan N, Beck AF, Hyland A, Todorov IT, Brooks JS and
Bepler G: MCM2 is an independent predictor of survival in patients
with non-small-cell lung cancer. J Clin Oncol. 19:4259–4266. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ha SA, Shin SM, Namkoong H, Lee H, Cho GW,
Hur SY, Kim TE and Kim JW: Cancer-associated expression of
minichromosome maintenance 3 gene in several human cancers and its
involvement in tumorigenesis. Clin Cancer Res. 10:8386–8395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu H, Ma J, Wu J, Chen L, Sun F, Qu C,
Zheng D and Xu S: Gene expression profiling analysis of lung
adenocarcinoma. Braz J Med Biol Res. 49(pii):
S0100–879X2016000300601. 2016.
|
|
53
|
Zhang C, Elkahloun AG, Liao H, Delaney S,
Saber B, Morrow B, Prendergast GC, Hollander MC, Gills JJ and
Dennis PA: Expression signatures of the lipid-based Akt inhibitors
phosphatidylinositol ether lipid analogues in NSCLC cells. Mol
Cancer Ther. 10:1137–1148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yi J, Wei X, Li X, Wan L, Dong J and Wang
R: A genome-wide comprehensive analysis of alterations in driver
genes in non-small-cell lung cancer. Anticancer Drugs. 29:10–18.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kikuchi J, Kinoshita I, Shimizu Y, Kikuchi
E, Takeda K, Aburatani H, Oizumi S, Konishi J, Kaga K, Matsuno Y,
et al: Minichromosome maintenance (MCM) protein 4 as a marker for
proliferation and its clinical and clinicopathological significance
in non-small cell lung cancer. Lung Cancer. 72:229–237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vigouroux C, Casse JM, Battaglia-Hsu SF,
Brochin L, Luc A, Paris C, Lacomme S, Gueant JL, Vignaud JM and
Gauchotte G: Methyl(R217)HuR and MCM6 are inversely correlated and
are prognostic markers in non small cell lung carcinoma. Lung
Cancer. 89:189–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fei L, Ma Y, Zhang M, Liu X, Luo Y, Wang
C, Zhang H, Zhang W and Han Y: RACK1 promotes lung cancer cell
growth via an MCM7/RACK1/Akt signaling complex. Oncotarget.
8:40501–40513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lo Sardo F, Forcato M, Sacconi A, Capaci
V, Zanconato F, Di Agostino S, Del Sal G, Pandolfi PP, Strano S,
Bicciato S and Blandino G: MCM7 and its hosted miR-25, 93 and 106b
cluster elicit YAP/TAZ oncogenic activity in lung cancer.
Carcinogenesis. 38:64–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Toyokawa G, Masuda K, Daigo Y, Cho HS,
Yoshimatsu M, Takawa M, Hayami S, Maejima K, Chino M, Field HI, et
al: Minichromosome Maintenance protein 7 is a potential therapeutic
target in human cancer and a novel prognostic marker of non-small
cell lung cancer. Mol Cancer. 10:652011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu YZ, Jiang YY, Hao JJ, Lu SS, Zhang TT,
Shang L, Cao J, Song X, Wang BS, Cai Y, et al: Prognostic
significance of MCM7 expression in the bronchial brushings of
patients with non-small cell lung cancer (NSCLC). Lung Cancer.
77:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cui F, Hu J, Ning S, Tan J and Tang H:
Overexpression of MCM10 promotes cell proliferation and predicts
poor prognosis in prostate cancer. Prostate. 78:1299–1310. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Brown DC and Gatter KC: Ki67 protein: The
immaculate deception? Histopathology. 40:2–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Aporowicz M, Czopnik P, Kubicka E,
Piotrowska A, Dziegiel P, Bolanowski M and Domoslawski P:
Minichromosome maintenance proteins MCM-3, MCM-5, MCM-7, and Ki-67
as proliferative markers in adrenocortical tumors. Anticancer Res.
39:1151–1159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Choy B, LaLonde A, Que J, Wu T and Zhou Z:
MCM4 and MCM7, potential novel proliferation markers, significantly
correlated with Ki-67, Bmi1, and cyclin E expression in esophageal
adenocarcinoma, squamous cell carcinoma, and precancerous lesions.
Hum Pathol. 57:126–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Giaginis C, Giagini A, Tsourouflis G,
Gatzidou E, Agapitos E, Kouraklis G and Theocharis S: MCM-2 and
MCM-5 expression in gastric adenocarcinoma: Clinical significance
and comparison with Ki-67 proliferative marker. Dig Dis Sci.
56:777–785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Schrader C, Janssen D, Klapper W, Siebmann
JU, Meusers P, Brittinger G, Kneba M, Tiemann M and Parwaresch R:
Minichromosome maintenance protein 6, a proliferation marker
superior to Ki-67 and independent predictor of survival in patients
with mantle cell lymphoma. Br J Cancer. 93:939–945. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lampert IA, Horncastle D, Dilworth S,
Roberts I, Alison MR and Naresh KN: The expression of
minichromosome maintenance protein-2 in normal and abnormal
megakaryocytes and comparison with the proliferative marker Ki-67.
Br J Haematol. 131:490–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Endl E, Kausch I, Baack M, Knippers R,
Gerdes J and Scholzen T: The expression of Ki-67, MCM3, and p27
defines distinct subsets of proliferating, resting, and
differentiated cells. J Pathol. 195:457–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chatrath P, Scott IS, Morris LS, Davies
RJ, Rushbrook SM, Bird K, Vowler SL, Grant JW, Saeed IT, Howard D,
et al: Aberrant expression of minichromosome maintenance protein-2
and Ki67 in laryngeal squamous epithelial lesions. Br J Cancer.
89:1048–1054. 2003. View Article : Google Scholar : PubMed/NCBI
|