Introduction
Breast cancer is the leading cause of cancer-related
mortality amongst women worldwide (1), with a mortality rate of ~627,000
annually estimated in 2018 (2).
Uncontrolled proliferative growth and angiogenesis are two basic
cancer hallmarks governing the critical transitions towards
malignancy during carcinogenesis (3).
PI3K/PTEN signaling, frequently altered in breast carcinoma
(4), confers a survival advantage to
tumor cells (5). Anillin, encoded by
anillin actin-binding protein (ANLN), is an actin-binding
protein, which has been identified as being involved in the
PI3K/PTEN pathway (6,7). It is an F-actin binding protein, which
maintains podocyte cytoskeletal dynamics, cell motility and
signaling through its interaction with CD2-associated protein,
which stimulates the phosphorylation of AKT at serine 473 (6,8). The
inhibition of PI3K/AKT activity in non-small cell lung cancer cells
decreased ANLN stability and reduced nuclear levels, suggesting the
critical involvement of ANLN in PI3K/AKT signaling (7). ANLN also serves a significant role in
pulmonary carcinogenesis through PI3K/AKT pathway-dependent nuclear
function (7). The nuclear expression
of ANLN in tumor cells is independently prognostic of a poor
outcome in patients with breast cancer (9,10), and
ANLN mutations are suggestive of estrogen receptor-positive
breast cancer tumorigenesis and endocrine therapy resistance
(11) due to the hyperactivation of
PI3K/PTEN (6). VEGFR-2, also known as
kinase insert domain receptor (KDR), promotes angiogenesis
(12,13). PI3K/PTEN activation enhances VEGF
signaling, forming a positive feedback loop leading to uncontrolled
progressive signaling in tumor cells (4,5). This body
of evidence is indicative of the potential synergy between
ANLN and KDR influencing breast cancer prognosis.
The majority of breast malignancies are caused by
acquired and uncorrected genetic adjustments in somatic genes due
to inherited gene shuffling (14).
Single nucleotide polymorphisms (SNPs) represent a predominant
genetic variation in the human genome (15), a large number of which are associated
with various types of cancer (16).
ANLN undergoes genetic changes, including amplification,
deletion and SNP mutations, in patients with several types of
cancer; mutation rates vary between 0.2% in clear cell renal cell
carcinoma and 19.6% in prostate cancer (17,18). A
total of 27 mutations, including 12 amplifications, two deletions
and 13 SNPs, were identified in lung adenocarcinoma (19). The potential functional genetic
variant rs10013228 in KDR is a prognostic marker of resected
colorectal cancer (20) and renal
cell carcinoma (21). SNPs
rs10020464, rs11941492 and rs12498529 of KDR are associated
with KRAS2-mutated tumors, which are also microsatellite
instable and CpG island methylator phenotype-positive (22), and rs11941492 is significantly
associated with the early onset of esophageal adenocarcinoma
(23). The genetic variant rs1870377
of KDR is associated with sunitinib-mediated overall
survival (OS) rate (24). However, no
SNPs of KDR with clinical implications have been reported
for breast cancer (25). In the
present study, the potential synergy between ANLN and
KDR and its effect on breast cancer outcome were
investigated, and relevant SNPs driving this synergy at the genetic
level were identified.
Materials and methods
Datasets
A total of 14,481 SNPs for ANLN and 11,704
SNPs for KDR were retrieved from the dbSNP NCBI database
(26). Among these, 20 SNPs of
ANLN and 13 SNPs of KDR were mapped to the Affymetrix
SNP6.0 Array, which was used in The Cancer Genome Atlas (TCGA;
http://cancergenome.nih.gov). Genotype
data of the 33 SNPs covering 501 samples were retrieved from the
TCGA. The gene expression and clinical data were retrieved from the
TCGA bioportal (http://www.cbioportal.org/), containing 20,440 genes
and 1,102 samples.
Pairwise SNP survival analysis
Breast cancer OS analysis was conducted on
interactions between SNPs of ANLN and KDR using the
Cox proportional hazard model. The recessive and dominant models
were tested in the pairwise SNP association analysis. In the
recessive model, the heterozygote is combined with the common
homozygote, assuming that the disease-associated phenotype is
caused by the concomitant presence of both rare alleles; the
dominant model combines the heterozygote with the rare homozygote,
assuming that the disease-associating phenotype is caused by the
presence of the rare allele. A 10-year breast cancer OS analysis
was performed utilizing the ‘survival’ package (27) in R software (28) and a log-rank test was used to assess
the statistical significance of the association between SNPs and
the clinical outcome. An SNP pair was considered interactive if the
P-values of the Cos regression model and the interaction term were
both <0.05 and the number of iterations was <10.
Expression quantitative trait loci
analysis (eQTL) and function predictions
To identify those genes for which expression was
significantly affected by the identified disease-associating SNPs,
eQTL analysis was performed using a linear model in R to identify
genes associated with complex phenotypes (29). Whether the allele status (rare
homozygote, heterozygote or common homozygote) of a given SNP was
linearly associated with the expression of a given gene was
assessed. The top 25 percentile of the SNPs with a P-value in the
linear model at P<0.05 were considered eQTLs of a gene.
The combined impact of ANLN and KDR on
genes identified from the eQTL analysis was investigated by
stratifying the expression of the genes of interest by the combined
expression of ANLN and KDR. One-way ANOVA and a Least
Significant Difference (LSD) post hoc test were applied using R
(version 3.5.2) to assess the statistical significance, with
P<0.05 used as the threshold suggestive of a significant
trilateral correlation.
The PredictSNP version 2.1 interface (30) was used to predict the functional
interaction network of the SNPs of interest, which uses a series of
tools and databases for SNP functional prediction. PredictSNP2
provides easy access to binary predictions and uniform confidence
values for the five best-performing prediction tools CADD, DANN,
FATHMM, FunSeq2 and GWAVA, and the results obtained from these
tools are combined into a consensus score (31). CADD (32) estimates the relative pathogenicity of
human genetic variants, DANN (33)
uses a deep learning approach for annotating the pathogenicity of
genetic variants, GWAVA (34) is
designed for the analysis of regulatory variants, and the FunSeq2
(31) framework annotates and
prioritizes non-coding regulatory variants in cancer.
Pathway analysis and network
construction
In order to investigate the biologically functional
consequences introduced by SNPs, pathway enrichment analysis was
performed using genes affected by SNPs with statistical
significance. Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway (http://www. genome.jp/kegg/) (35) and Gene Ontology (GO) term enrichment
analyses were performed using the packages clusterProfiler
(36) and org.Hs.eg.db (37) in R, and Chi-square and Fisher's exact
tests were used for statistical assessment.
The genes identified from the enriched pathways were
collected for gene regulatory network construction using GeneMANIA
(http://www.genemania.org) (38), which uses the label propagation
algorithm to predict gene-gene interactions at seven levels
(co-expression, co-localization, genetic interaction, physical
interaction, shared protein domain, pathway and predicted). The
interactions at the co-localization, genetic interaction, physical
interaction, shared protein domain and pathway levels were used for
network construction. The output comprises a regulatory network
that uses the user-defined gene list based on databases and
publications from multiple resources (38,39). The
‘max resultant genes’ was set as five, the ‘max resultant
attributes’ was set as 10, and the GO weighting system was used,
which uses biological process (BP)-based, molecular function
(MF)-based and cellular component (CC)-based approaches. The
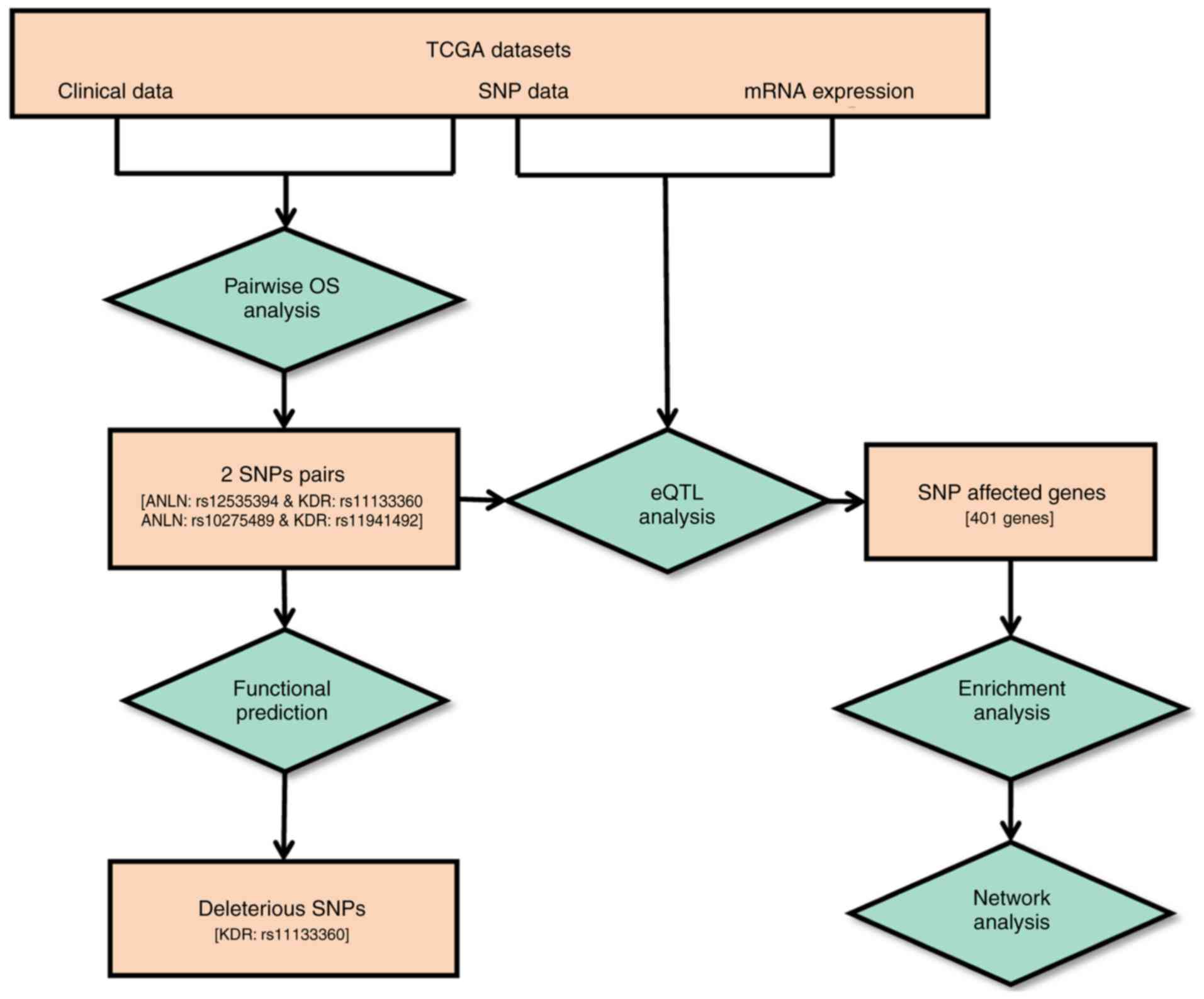
complete workflow is illustrated in Fig.
1.
Experimental validation
Cell culture
One human normal mammary epithelial cell line
(MCF10A) and one breast cancer cell line (SUM149PT) were purchased
from ATCC and used in the present study. The MCF10A cells were
cultured in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 5% charcoal-stripped horse serum (Gibco; Thermo
Fisher Scientific, Inc.), 10 µg/ml insulin (PeproTech, Inc.), 20
ng/ml epithelial growth factor (PeproTech, Inc.) and
1.4×10−6 mol/l hydrocortisone (PeproTech, Inc.). The
SUM149PT cells were cultured in F12 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 5% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 20 µg/ml insulin (PeproTech,
Inc.), 1% HEPES (PeproTech, Inc. and 2.8×10−6 mol/l
hydrocortisone (PeproTech, Inc.). Assay-ready cells were prepared
by culturing the cells in a large batch and aliquoting them into
ampules that were kept in liquid nitrogen in solution containing
90% FBS and 10% DMSO. Immediately prior to transfection, the cells
were thawed and washed with culture medium and the cell number was
counted using a hemocytometer (Thermo Fisher Scientific, Inc.).
SgRNA preparation
pLenti-U6-sgRNA-PGK-Neo (cat. no. K019),
piLenti-EF1a-dCas9-SAM (cat. no. K015) and piLenti-EF1a-dCas9-KRAB
(cat. no. K203) were purchased from Applied Biological Materials,
Inc. The sgRNAs were designed using the publicly available software
CHOPCHOP (version 3, http://chopchop.cbu.uib.no/) (40) (Table
SI), supplemented with the BbsI restriction site, and
synthesized from Applied Biological Materials (ABM), Inc. Each
sgRNA was added with a sequence complementary to the sticky ends of
BbsI, following ligation with the pLenti-U6-sgRNA-PGK-Neo
vector digested using BbsI, and the recombinant plasmid was
amplified in DH5 Escherichia coli (Sigma-Aldrich; Merck
KGaA). The plasmids containing sgRNAs were validated using PCR,
enzyme digestion and sequencing, and transfected together with
piLenti-EF1a-dCas9-SAM or piLenti-EF1a-dCas9-KRAB into cells. The
controls were designed as cells concomitantly transfected with all
sgRNAs modulating the target gene alone, without
piLenti-EF1a-dCas9-SAM or piLenti-EF1a-dCas9-KRAB.
Cell transfection
A total of 1×106 cells per well were
added in 2 ml of culture medium and transferred to 6-well plates
(Nalgene, cat. no. 167018). The cells were incubated overnight and
were at 70–80% confluence prior to transfection. The medium was
replaced with 2 ml serum-free medium prior to transfection.
Subsequently, 100 µl Opti-MEM (Gibco; Thermo Fisher Scientific,
Inc.) containing 1 µg sgRNA (ABM, Inc.) and 1 µg CRISPR/dCas9 (ABM,
Inc.) plasmids were added to 100 µl Opti-MEM containing 6 µl
lipo2000 transfection reagent per well and mixed for 15–20 min
prior to transfection. The mixture was transferred to 6-well plate
and incubated at 37°C for 5–8 h in the presence of 5%
CO2 (HERA Cell 150i, Thermo Fisher Scientific, Inc.).
The serum-free medium was replaced with 2 ml medium containing 10%
serum. The cells were incubated at 37°C for 24 h, followed by the
addition of G418 and puromycin and incubation for 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Following transfection, the cells were collected and
extracted for total RNA using TRIzol reagent (Tiangen Biotech Co.,
Ltd.) 3 days after transfection. The cDNA was synthesized using
PrimeScript RT reverse transcriptase as per the manufacturer's
protocol (Takara Bio, Inc.). The primers used for RT-qPCR analysis
are listed in Table SII. The qPCR
sample consisted of 5 µl 2X SYBR premix ex Taq, 0.4 µl, 10 µm
forward and reverse primers, 0.2 µl ROX reference dye, 2 µl cDNA
and 2 µl H2O. The detailed procedure for RT-qPCR was as
follows: Initial denaturation at 95°C for 5 min, 45 cycles of
denaturation at 95°C for 5 sec, annealing at 57°C for 30 sec,
extension at 72°C for 15 sec. The absorbance value was recorded at
the extension stage. The relative expression level was calculated
using the 2−∆∆Cq method (41). All RT-qPCR experiments were performed
using the ABI StepOnePlus Real-Time PCR system (ABI; Thermo Fisher
Scientific, Inc.).
Statistical analysis
One-way ANOVA coupled with Scheffe's post hoc test
were conducted using R software (version 3.5.2) to evaluate the
significance of changes in the expression level of ANLN or
KDR following genetic modulations compared with each
corresponding control where the P-value threshold was set as
0.05.
Results
SNPs of ANLN and KDR synergistically
affect breast cancer survival rates
Pairwise SNP survival analysis was performed using
multivariate Cox regression models and statistical significance was
assessed using the likelihood ratio test. Of all 260 SNP pairs, two
pairs were identified with significant synergistic effects on
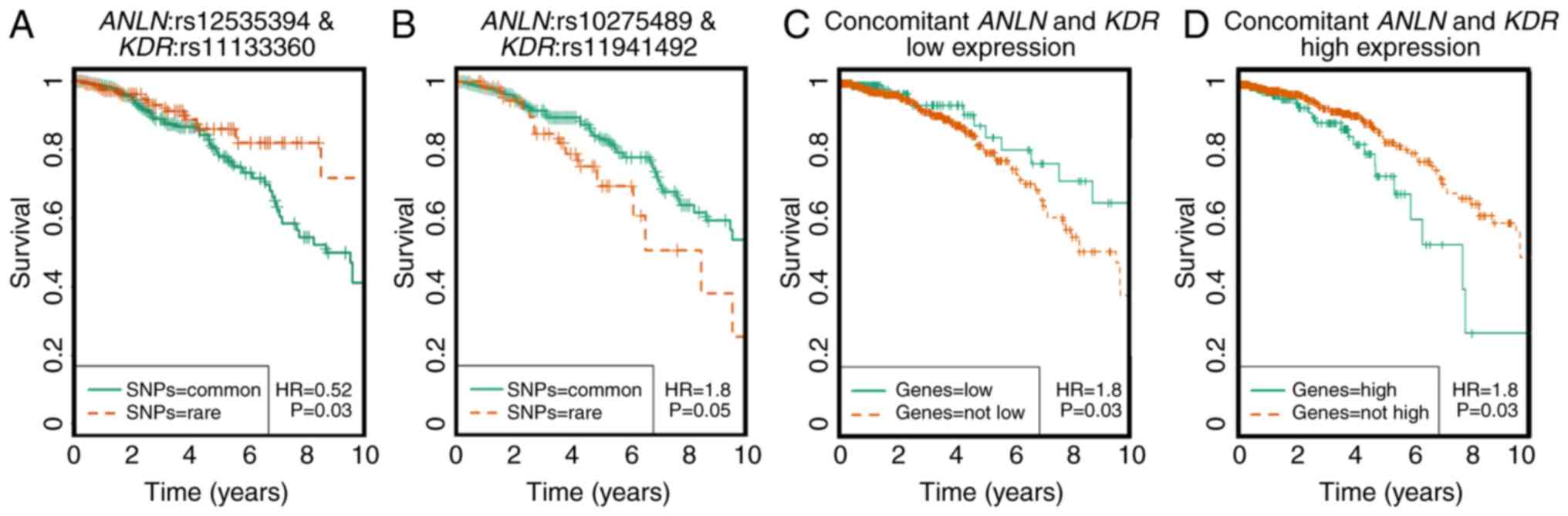
breast cancer survival rate from the dominant model (Fig. 2A-D). The two pairs were
ANLN:rs12535394 and KDR:rs11133360 and
ANLN:rs10275489 and KDR:rs11941492. The concurrent
presence of both rare homozygotes in the ANLN:rs12535394 and
KDR:rs11133360 SNP pair is associated with favorable
clinical outcomes (HR=0.52, P=0.03), whereas the
ANLN:rs10275489 and KDR:rs11941492 SNP pair was
associated with poor breast cancer prognosis (HR=1.8, P=0.05).
These four SNPs are non-linkage disequilibrium (LD) linked and were
defined as disease-associated SNPs. Of these four
disease-associated SNPs, KDR:rs11133360 was identified as
intronic (chr4:55116585) and its rare allele was predicted as
deleterious by the FunSeq2 and GWAVA tools (Table I).
 | Table I.Effects of the identified
disease-associated single nucleotide polymorphisms predicted using
PredictSNP2. |
Table I.
Effects of the identified
disease-associated single nucleotide polymorphisms predicted using
PredictSNP2.
|
| KDR: rs11133360
(position: chr4:55116585) | KDR: rs11941492
(position: chr4:55112043) |
|---|
|
|
|
|
|---|
| Function
predictor | Prediction | Score | Expected
accuracy | Prediction | Score | Expected
accuracy |
|---|
| PredictSNP2 | Neutral | −1 | 0.88 | Neutral | −1 | 0.88 |
| CADD | Neutral | 8.242 | 0.76 | Neutral | 3.493 | 0.82 |
| DANN | Neutral | 0.3796 | 0.8 | Neutral | 0.5371 | 0.82 |
| FATHMM | Neutral | 0.1461 | 0.91 | Neutral | 0.0614 | 0.95 |
| FunSeq2 | Deleterious | 2.3903 | 0.67 | – | 0.7762 | 0.45 |
| GWAVA | Deleterious | 0.49 | 0.64 | Neutral | 0.15 | 0.79 |
Genes affected by disease-associated
SNPs
The eQTL analysis of the four disease-associated
SNPs resulted in 401 genes with KEGG annotations (Tables SIII and SIV; Figs. 3
and 4). Amongst these genes, the
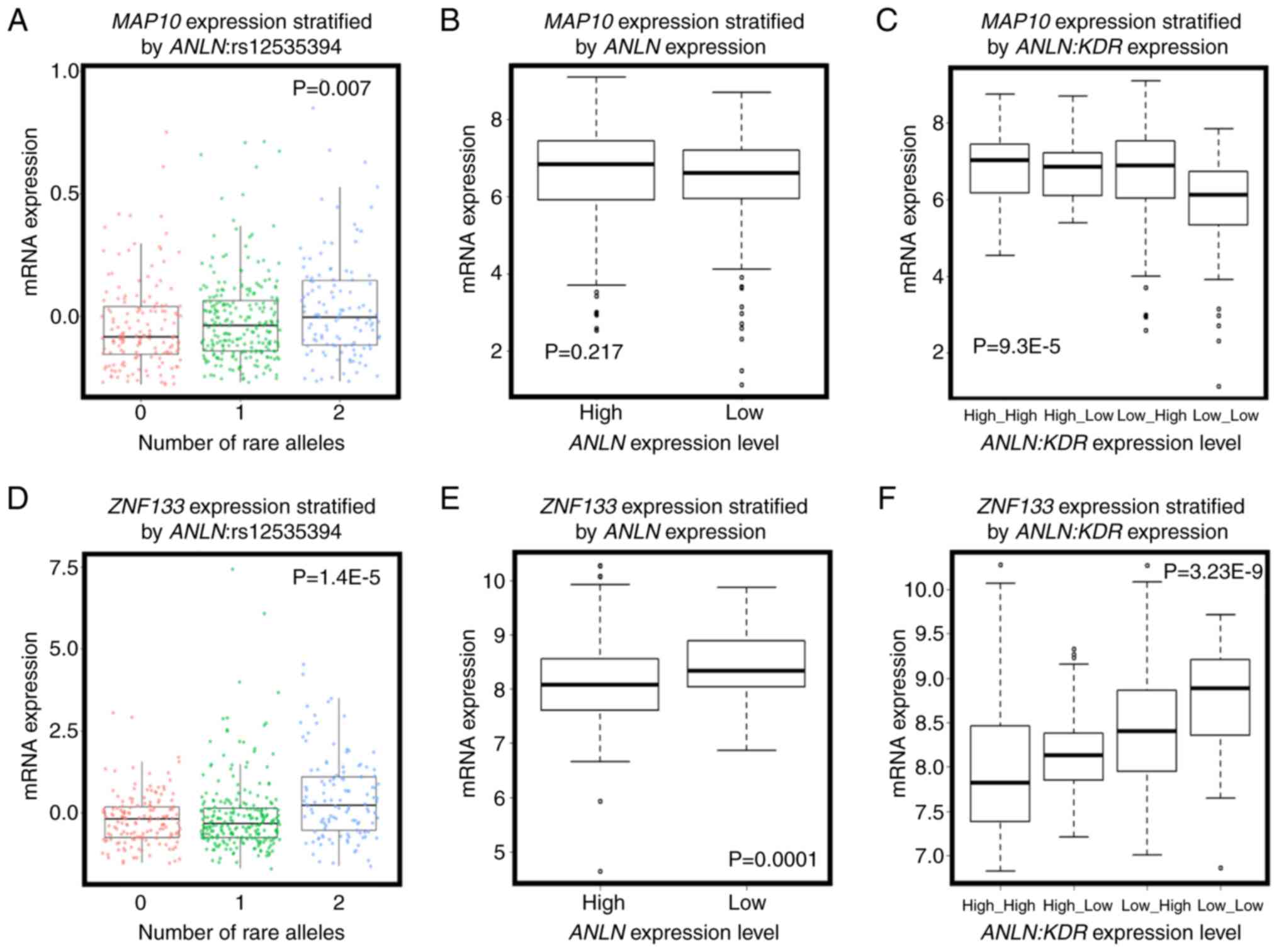
expression of microtubule-associated protein 10 [MAP10;
correlation (cor)=0.554, P=0.001] was significantly positively
correlated with the number of rare alleles of
ANLN:rs12535394 (P=0.007, Fig.
3A), and interacted with ANLN at the transcriptional
level to predict breast cancer OS (Fig.
4C and D). Furthermore, the overexpression of MAP10
conveyed favorable clinical outcomes (HR=0.73, P=9.5E-5, Fig. 4A), but was associated with poor breast
cancer OS when the expression of ANLN was concomitantly low
(HR=2.66, P=0.0062, Fig. 4C). The
expression of MAP10 was not directly associated with that of
ANLN (cor=0.04, P=0.198, Fig.
3B), but was expressed at a low level under the concomitant low
expression of both ANLN and KDR (P=9.3E-5, Fig. 3C), further suggesting the involvement
of MAP10 in the synergy created between ANLN and
KDR at the transcriptional level. Zinc finger protein 133
(ZNF133) was the top gene whose expression was significantly
associated with the allele status of ANLN:rs12535394. The
SNP rare allele was associated with a high and statistically
significant expression of ZNF133 (P=1.4E-5). The rare allele
status of this SNP was positively correlated with the expression of
ZNF133 (cor=0.14, P=0.3.8E-6, Fig.
3D) and negatively correlated with the expression of
ANLN. The expression of ZNF133 was stratified into
distinct expression levels by the expression of ANLN
(P=0.0001); the expression of ZNF133 and expression of
ANLN were negatively correlated (cor=−0.22, P=1.6E-12,
Fig. 3E), suggesting a negative
correlation between the rare allele of ANLN:rs12535394 and
the expression of ANLN. Such a negative association was
exemplified by the low expression of KDR, i.e., the
concomitant underexpression of ANLN and KDR was
associated with the overexpression of ZNF133 (P=3.23E-9,
Fig. 3F). Given the favorable
clinical outcome associated with the high expression of
ZNF133 (HR=0.76, P=7.8E-7, Fig.
4E), it was reasoned that the ANLN rare allele is
associated with desirable breast cancer relapse-free survival.
Furthermore, ZNF133 interacted with ANLN to influence
breast cancer OS at the transcriptional level (Fig. 4G and H), providing further evidence of
the association between the allele status of ANLN:rs12535394
and the expression and prognostic value of ANLN. Similar to
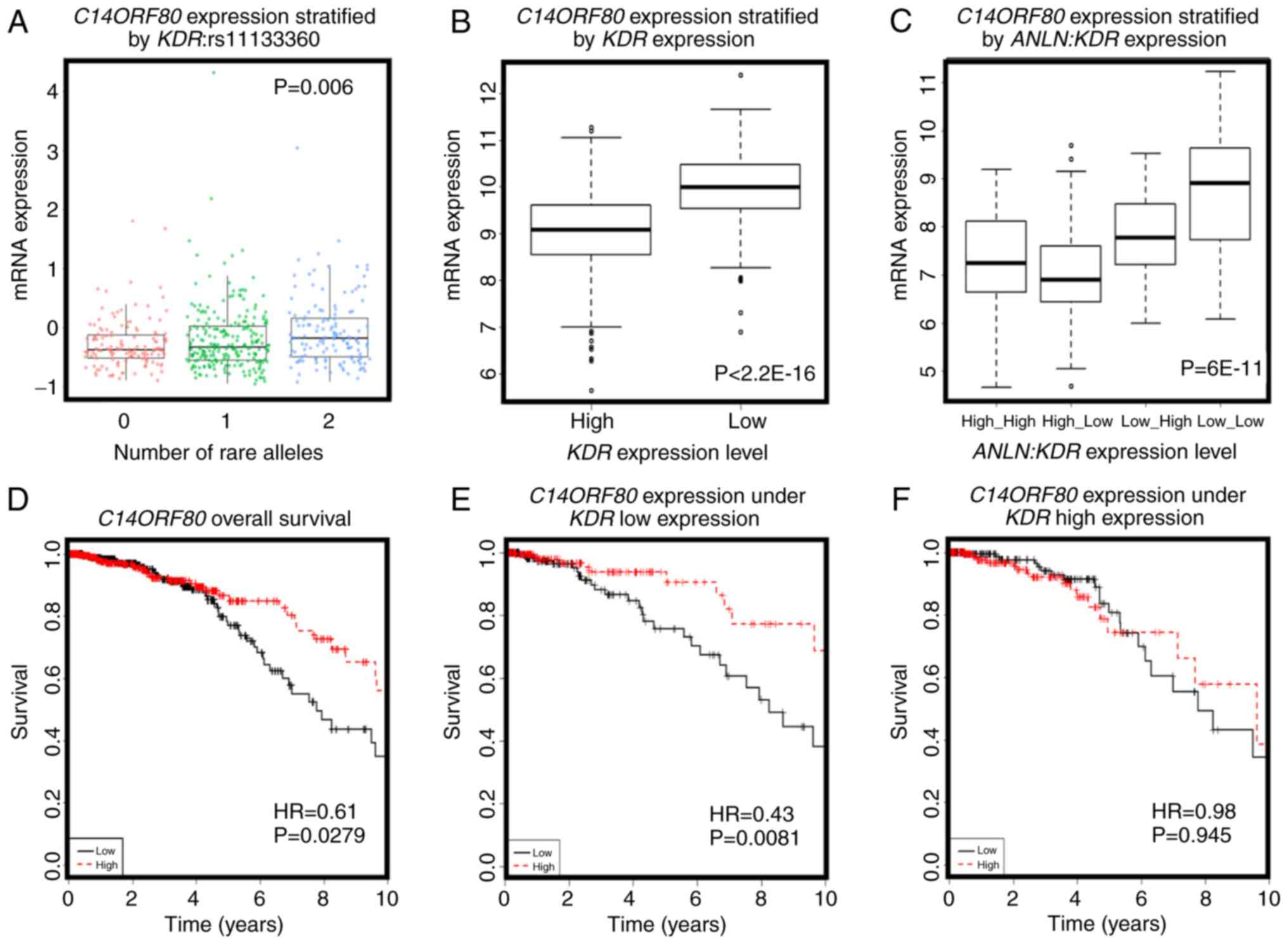
ZNF133, C14ORF80 was the top gene whose expression was
significantly positively correlated with the rare allele expression
of KDR:rs11133360 (cor=0.2, P=0.001). The SNP rare allele
was significantly associated with a high expression of
C14ORF80 (P=0.006, Fig. 5A)
and negatively correlated with the expression of KDR
(cor=−0.4, P<2.2E-16). The expression of C14ORF80 was
stratified into distinct expression levels by the expression of
KDR (P<2.2E-16, Fig. 5B),
indicating a negative correlation between the rare allele of
KDR:rs11133360 and expression of KDR. In addition,
the concomitant high expression of ANLN and KDR was
associated with a high expression of C14ORF80 (P=6E-11,
Fig. 5C), which was prognostic of
favorable breast cancer OS (HR=0.61, P=0.0279, Fig. 5D), suggesting the favorable prognostic
value of KDR:rs11133360. Furthermore, C14ORF80 was
shown to interact with KDR to influence breast cancer OS at
the transcriptional level (Fig. 5E and
F), supporting the association between the allele status of
KDR:rs11133360 and the expression and prognostic value of
KDR. The rare alleles of ANLN:rs12535394 and
KDR:rs11133360 have been implicated with favorable clinical
outcomes, which is consistent with the observations at the SNP
level in the present study (HR=0.52, P=0.0313, Fig. 2A), and have been associated with a low
expression of ANLN and KDR at the transcriptional
level, which was in accordance with what was observed at the
transcriptional level in the present study (Fig. 2C).
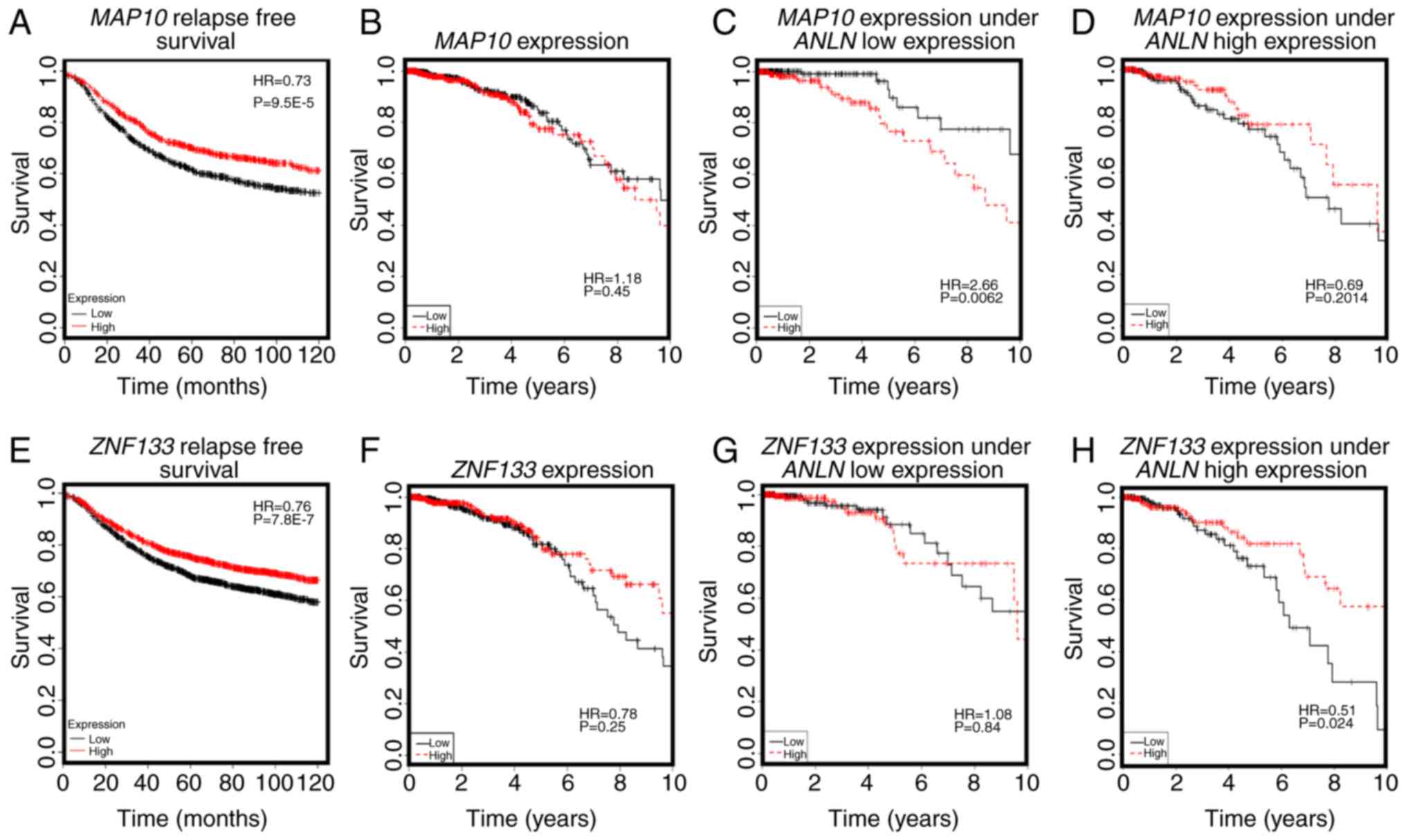 | Figure 4.Prognostic value of key genes
associated with ANLN:rs12535394 and their impact on the
interactions between ANLN and KDR on breast cancer survival. (A)
Prognostic value of MAP10 on breast cancer relapse-free survival,
drawn using Kaplan Meier Plotter. (B) Prognostic value of MAP10 on
breast cancer overall survival drawn using TCGA data. (C)
Expression of MAP10 on breast cancer survival under ANLN low
expression, drawn using TCGA data. (D) Expression of MAP10 on
breast cancer survival under high expression of ANLN, drawn using
TCGA data. (E) Prognostic value of ZNF133 on breast cancer
relapse-free survival, drawn using Kaplan Meier Plotter. (F)
Prognostic value of ZNF133 on breast cancer overall survival, drawn
using TCGA data. (G) Expression of ZNF133 on breast cancer survival
under low expression of ANLN, drawn using TCGA data. (H) Expression
of ZNF133 on breast cancer survival under high expression of ANLN,
drawn using TCGA data. ANLN, anillin actin-binding protein; KDR,
kinase insert domain receptor; MAP10, microtubule-associated
protein 10; ZNF133, zinc finger protein 133; TCGA, The Cancer
Genome Atlas. |
No genes were found to be significantly associated
with the ANLN:rs10275489 allele status and expression of
ANLN, or with the KDR:rs11941492 allele status and
expression of KDR.
Pathway and network construction using
eQTL genes influenced by disease-associated SNPs
Pathway enrichment analysis showed that these genes
were significantly enriched in the primary immunodeficiency
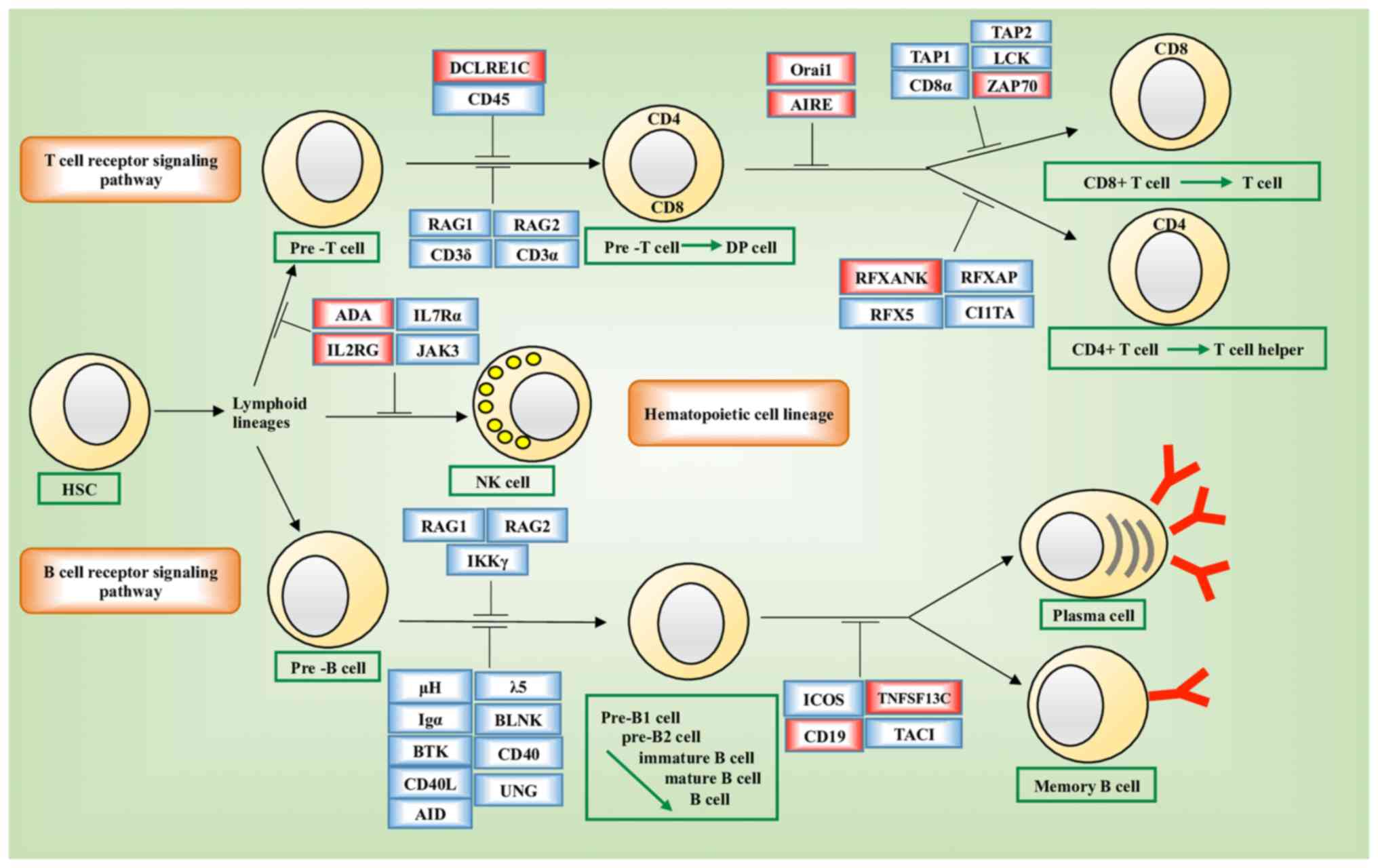
disorder (PID) pathways (hsa05340, P=10−4; Fig. 6). Genes enriched in hsa05340 included
ORAI1, DCLRE1C, IL2RG, RFXANK, ADA, AIRE, CD19, TNFRSF13C
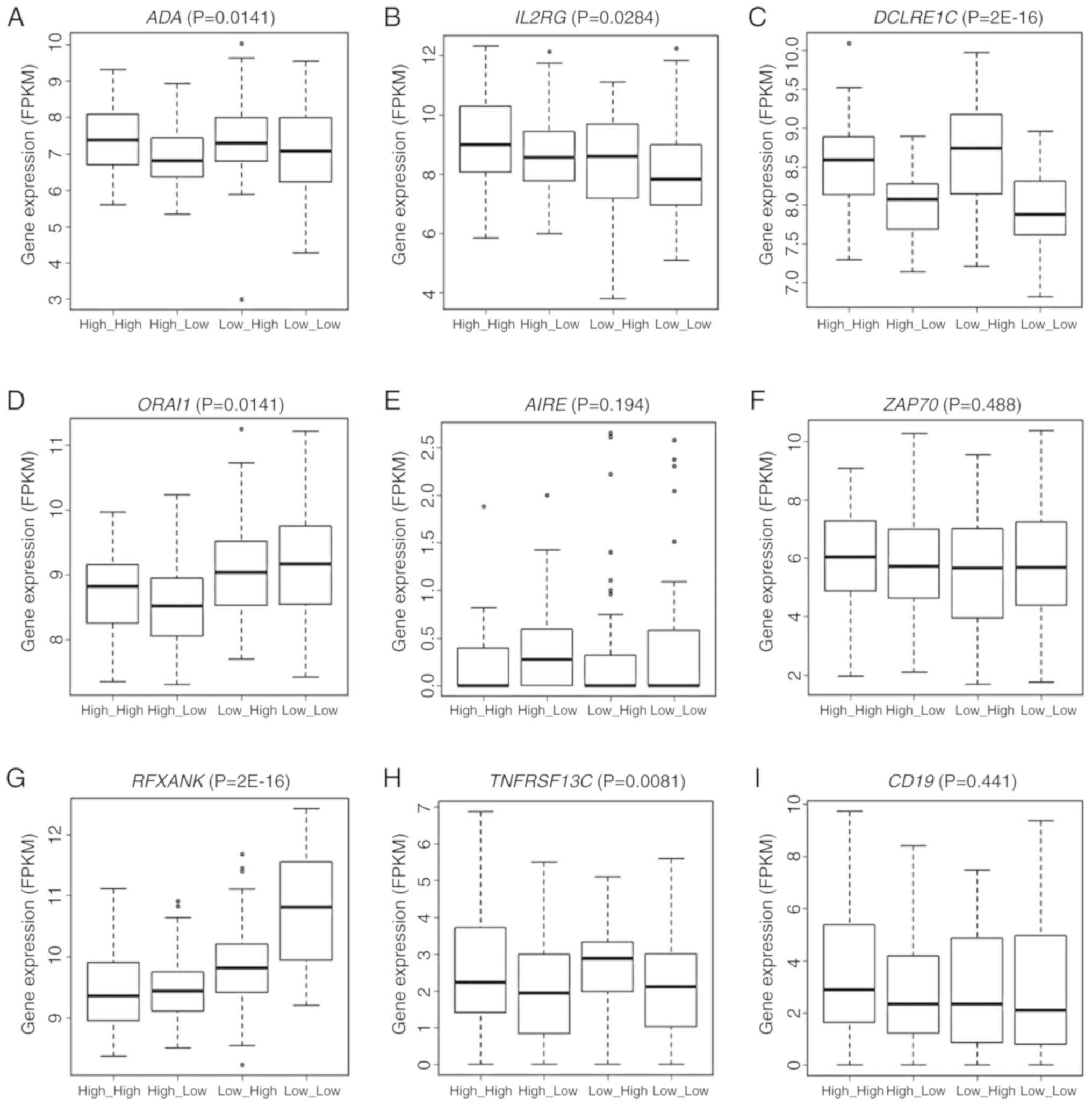
and ZAP70. The expression of six of these nine genes, i.e.,
ADA (P=0.0141), IL2RG (P=0.0284), DCLRE1C
(P=2E-16), ORAI1 (P=0.0141), RFXANK (P=2E-16) and
TNFRSF13C (P=0.0081) varied significantly across the groups
stratified by the joint assessment of ANLN and KDR
expression (Fig. 7A-I). Significant
pairs were ‘high_high vs. low_high’, ‘high_low vs. low_high’,
‘high_low vs. low_low’ in ADA, ‘high_high vs. low_high’ and
‘high_high vs. low_low’ in IL2RG, ‘high_high vs. low_high’,
‘high_low vs. low_high’, ‘high_high vs. low_low’, ‘high_low vs.
low_low’ in DCLRE1C, ‘high_high vs. high_low’, ‘high_high
vs. low_high’, ‘high_high vs. low_low’, ‘high_low vs. low_high’,
‘low_high vs. low_low’ in ORAI1, ‘high_high vs. high_low’,
‘high_low vs. low_high’, ‘high_high vs. low_low’, ‘high_low vs.
low_low’, ‘low_high vs. low_low’ in RFXANK, and ‘high_low
vs. low_high’ and ‘high_low vs. low_low’ in TNFRSF13C. All
statistical P-values from ANOVA and the LSD test for pairwise
comparisons are listed in Table
SV.
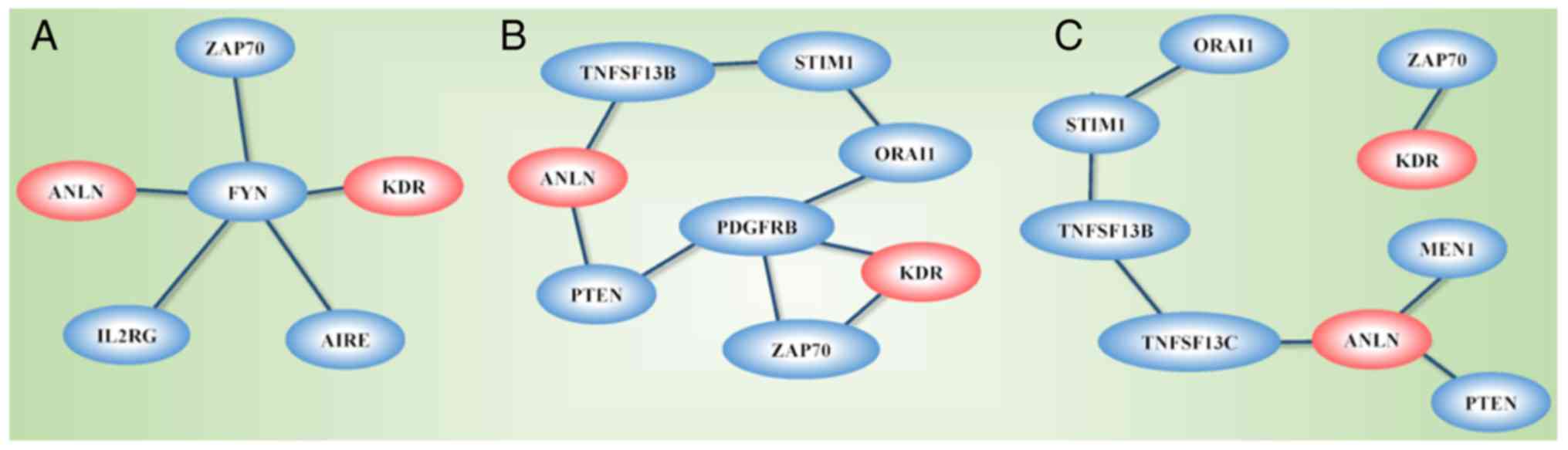
The network constructed from GeneMANIA using KDR,
ANLN and the genes affected by the disease-associated SNPs as
the initial input exhibited different topological structures when
constructed using different GO-weighted approaches (Fig. 8A-C). In addition to ANLN and
KDR, the network included PTEN, PDGFRB, ZAP70, ORAI1,
STIM1, TNFSF13B and TNFRSF13C when BP was used as the
weighting approach, FYN, ZAP70, IL2RG and AIRE when
the weighting criteria was based on MF, and PTEN, MEN1,
TNFRSF13C, TNFSF13B, STIM1, ORAI1 and ZAP70 when CC was
used in weighting. The network was segregated into two areas when
CC was used as the weighting method.
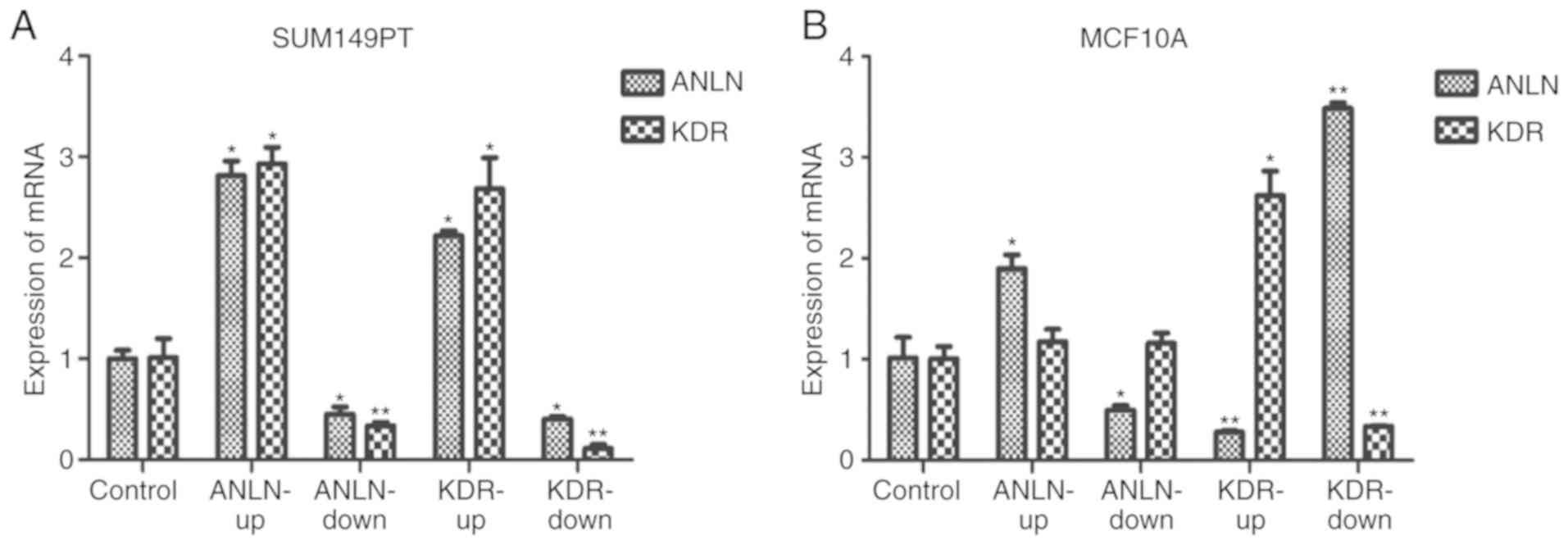
Experimental validation of
interactions between ANLN and KDR
In the in vitro experiments, ANLN did
not affect the expression of KDR in the MCF10A normal breast
epithelial cells (P=0.27 when ANLN was upregulated, P=0.09
when ANLN was downregulated, Fig.
9B), but positively regulated the expression of KDR in
the SUM149PT breast cancer cells (P=2.74E-6 when ANLN was
upregulated, P=1.47E-2 when ANLN was downregulated, Fig. 9A). KDR had an opposing effect
on the expression of ANLN in the MCF10A cells (P=2.82E-4
when KDR was upregulated, P=3.36E-9 when KDR was
downregulated, Fig. 9B), but
positively influenced the expression of ANLN in SUM149PT
cells (P=1.82E-7 when KDR was upregulated, P=1.25E-4 when
KDR was downregulated, Fig.
9A). All statistical P-values from ANOVA with Scheffe's test
for all pairwise comparisons are listed in Table SVI.
Discussion
Through pairwise interactive OS analyses of the
SNPs of ANLN and KDR, the present study identified
four disease-associated SNPs (ANLN:rs12535394,
KDR:rs11133360, ANLN:rs10275489 and
KDR:rs11941492), where ANLN:rs12535394 paired with
KDR:rs11133360, and ANLN:rs10275489 paired with
KDR:rs11941492 to synergistically influence the clinical
outcome of breast cancer. Of the two SNP pairs, the allele status
of ANLN:rs12535394 and KDR:rs11133360 was associated
with the expression of ANLN and KDR, respectively,
via ZNF133 and C14ORF80, and were synergistically
prognostic of a favorable clinical outcome in breast cancer.
The quantity of the rare allele of
ANLN:rs12535394 was positively associated with the
expression of ZNF133 and negatively correlated with the
expression of ANLN with statistical significance, and the
overexpression of ZNF133 was prognostic for a favorable
clinical outcome. These results indicate that the rare allele of
ANLN:rs12535394 is protective and associated with a low
expression of ANLN via ZNF133. Therefore,
ZNF133 interacts with ANLN to affect breast cancer
survival, in which the protective effect of the overexpression of
ZNF133 is amplified under the high expression of ANLN
(Fig. 4F and H). Few ZNF133
functionalities have been reported in cancer, but the
transcriptionally suppressive activity of zinc finger protein has
been reported for its overamplification in neuroblastoma cells
(42) and overexpression in chronic
myeloid leukemia (43) in two
independent high-throughput studies. These findings collectively
suggest the tissue-specific pathological functionalities of
ZNF133, i.e., ZNF133 is tumor suppressive in breast
cancer, particularly in situations under a high expression of
ANLN, which warrants experimental validation.
Furthermore, the quantity of the rare allele of
ANLN:rs12535394 was positively associated with the
expression of MAP10. MAP family proteins regulate
microtubule properties (44) and
serve an important role in an array of cellular processes,
including cell division, cell motility, intracellular trafficking,
microtubule stability and cell morphology maintenance (45). The overexpression and
post-translational modifications of MAPs contribute to the
dysregulation of microtubule dynamics and the development of
serious diseases including human breast cancer (46–50). In
the present study, the overexpression of MAP10 was not
pathologically relevant but conveyed a poor clinical outcome when
the expression of ANLN was low (Fig. 4C), suggesting the conditional
prognostic value of MAP10 in breast cancer, i.e., protective
when the expression of ANLN is high and risky when the
expression of ANLN is low.
Similarly, the quantity of the rare allele of
KDR:rs11133360 was positively associated with the expression
of C14ORF80, which was significantly negatively correlated
with the expression of KDR, and the overexpression of
C14ORF80 was prognostic for favorable breast cancer OS
(Fig. 5); these results indicate that
the rare allele of KDR:rs11133360 is negatively associated
with the expression of KDR via the overexpression of
C14ORF80 and conveys desirable prognostic value on the
clinical outcome of breast cancer. The prognostic value of
C14ORF80 was increased under a low expression of KDR,
suggesting interactions between these genes. C14ORF80, also
termed TEDC1, has not previously been annotated nor shown to
be associated with cancer. There are currently no publications
available on this gene from the Web of Science. The present study
highlights the potential prognostic role of C14ORF80 in
predicting breast cancer survival rates and its involvement in
KDR-mediated tumor angiogenesis.
The synergy created amongst SNPs affecting breast
cancer survival may involve more than two SNPs. However, it is
plausible to include fewer indicators in the diagnostic panel for
the sake of clinical convenience. Therefore, pairwise interaction
models were used initially in the present study to examine the
potential synergy; establishing multivariate models was a
consideration if no specific results were obtained from the
pairwise interaction analysis.
A total of 401 genes were obtained for which the
expression profiles were significantly affected by the
disease-associated SNPs and these were annotated in the KEGG. These
genes were enriched in the PID pathway. PID is a diverse group of
illnesses characterized by defects in the function of one or more
components of the immune system, which predisposes affected
individuals to an increased incidence of infections, autoimmunity
and malignancies (51). Patients with
PID are at increased risk of developing malignancies compared with
healthy individuals in the population (52), and the overall risk for developing
cancer in patients with PID was estimated to be up to 25% (53). The majority of these identified genes
have immune-related functionalities. For example, ADA
encodes an enzyme that increases the rate of hydrolyzation of
adenine to inosine and serves a potential role in the development
of the immune system and maturation of mammalian cells (54). IL2RG encodes a protein that is
an important signaling component of numerous interleukin receptors,
including those of interleukin-2, −4, −7 and −21. Importantly, of
the nine genes involved in the PID pathway and transcriptionally
associated with the rare allele status of these disease-associated
SNPs, six are significantly affected by interactions between
ANLN and KDR at the transcriptional level. Together,
these results suggest the prominent role of the immune response in
the synergies created between ANLN and KDR on their
prognostic value in breast cancer survival, which may be the
driving force behind the life/death control of cells and
angiogenesis/metastasis transition during carcinogenesis.
The network constructed using ANLN, KDR and
genes affected by the identified disease-associated SNPs as the
input revealed differential networks depending on the weighting
approaches used. ANLN and KDR were connected through
PTEN and PDGFRB when BP weighting was used, were
linked through FYN when MF weighting was used, and did not
connect when CC was used as the weighting approach. The
differential topological structures obtained through the use of
different GO weighting approaches suggest that ANLN and
KDR interact and create synergies in BPs and MFs, but do not
share the use or functionalities of CCs. PDGFRB encodes a
typical receptor tyrosine kinase, PDGFRβ, which physically
interacts with PTEN according to an in situ proximity
ligation assay (55). PTEN is
a representative molecule in PI3K/PTEN signaling that shares the
same biological pathway with ANLN (7). FYN physically interacts with ANLN
according to the human interactome generated from quantitative
proteomics (56), and FYN
shares similar oncological roles with ANLN, i.e., the
overexpression of FYN promotes cell proliferation, migration
and invasion in breast cancer cells (57,58).
Experimentally the present study showed that
ANLN and KDR interact in normal breast epithelial
cells and breast cancer cells. ANLN alterations did not
affect the expression of KDR, however, modulating the
expression of KDR led to the inverse regulation of
ANLN in normal breast epithelial cells, suggesting that
KDR is an upstream regulator of ANLN under normal
conditions. When the cells were attracted in the malignant state,
altering either ANLN or KDR led to regulation of the other gene in
the same direction, suggesting the formation of a feed-forward loop
that may lead to oncological signal amplification. This is
clinically plausible as the concomitant low expression of
ANLN and KDR, which is associated with favorable
breast cancer survival, was easily achieved by targeting
ANLN or KDR alone, with ANLN being a more
plausible therapeutic target than KDR as it is a downstream
effector of KDR in non-malignant cells. By contrast, a
change in the interaction mode of the two genes in normal and
cancer cells implicates the importance of the ANLN-KDR
interaction in the transition of cells between normal and cancerous
states and on breast cancer clinical outcomes. Whether
ANLN-KDR interactions constitute to or are the consequence
of carcinogenesis remain to be elucidated.
The present study used a triple-negative breast
cancer cell line to experimentally validate interactions between
ANLN and KDR, as the main effect of the synergy is
driven by that in triple-negative breast cancer (Fig. S1). In addition, the use of SNP/gene
pairs with synergistic prognostic values for triple-negative breast
cancer carriers is more plausible clinically than other subtypes,
as the triple-negative subtype is highly malignant and lacks
targeted therapies (59,60). However, investigating the association
between ANLN-KDR synergy and breast cancer subtyping is
worthwhile and remains the subject of future investigations.
In conclusion, the concurrent presence of both rare
homozygotes in ANLN:rs12535394 and KDR:rs11133360 was
identified as prognostic for favorable survival in breast cancer.
The quantity of the rare allele of ANLN:rs12535394 was
negatively associated with the expression of ANLN, and that
of KDR:rs11133360 was negatively associated with the
expression of KDR, both of which are protective. Novel roles
of genes that bridge the gap between SNPs and corresponding genes
were revealed and merit in-depth investigation, including the
potential tissue-specific tumor suppressive roles of ZNF133
in breast cancer, the conditional effects of MAP10 on breast
cancer survival rates and the possible suppressive role of
C14ORF80 (a gene not being annotated) during tumor
angiogenesis. Pathways controlling cell proliferation/apoptosis and
angiogenesis/migration genetically interact and ultimately
influence immune responses and patient clinical outcomes,
suggesting the intrinsic connection amongst cancer hallmarks and
the prominent role for immunotherapy in cancer state transition.
Experimental validations confirmed the roles of the ANLN-KDR
interaction in the transition of cells between normal and cancerous
states and in breast cancer prognosis, and implicate the
therapeutic potential of ANLN.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81972789), National Science
and Technology Major Project of China (grant. no.
2018ZX10302205-004-002), the Natural Science Foundation of Jiangsu
Province (grant. no. BK20161130), the Six Talent Peaks Project in
Jiangsu Province (grant. no. SWYY-128), the Technology Development
Funding of Wuxi (grant. no. WX18IVJN017), the Postgraduate
Education Reform Project of Jiangsu Province, and Research Funds
for the Medical School of Jiangnan University ESI Special
Cultivation Project (grant. no. 1286010241170320).
Availability of data and materials
The datasets generated and/or analyzed in the
present study were retrieved from TCGA (http://cancergenome.nih.gov) and (http://www.cbioportal.org/).
Authors' contributions
XD designed, supervised and financed the project,
analyzed the results and drafted the manuscript. XC and OH
conducted the computational analysis. YM performed the experiments.
XD, XC and YM prepared the figures and tables. All authors have
read and approved the content of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and Trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Zhang R, Liu Z, Li S and Xu H: The
genetic variants in the PTEN/PI3K/AKT pathway predict
susceptibility and CE(A)F chemotherapy response to breast cancer
and clinical outcomes. Oncotarget. 8:20252–20265. 2017.PubMed/NCBI
|
|
5
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hall G, Lane BM, Khan K, Pediaditakis I,
Xiao J, Wu G, Wang L, Kovalik ME, Chryst-Stangl M, Davis EE, et al:
The Human FSGS-causing ANLN R431C mutation induces dysregulated
PI3K/AKT/mTOR/Rac1 signaling in podocytes. J Am Soc Nephrol.
29:2110–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki C, Daigo Y, Ishikawa N, Kato T,
Hayama S, Ito T, Tsuchiya E and Nakamura Y: ANLN plays a critical
role in human lung carcinogenesis through the activation of RHOA
and by involvement in the phosphoinositide 3-kinase/AKT pathway.
Cancer Res. 65:11314–11325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huber TB, Hartleben B, Kim J, Schmidts M,
Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, et
al: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase
and stimulate AKT-dependent signaling. Mol Cell Biol. 23:4917–4928.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou W, Wang Z, Shen N, Pi W, Jiang W,
Huang J, Hu Y, Li X and Sun L: Knockdown of ANLN by lentivirus
inhibits cell growth and migration in human breast cancer. Mol Cell
Biochem. 398:11–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Magnusson K, Gremel G, Rydén L, Pontén V,
Uhlén M, Dimberg A, Jirström K and Pontén F: ANLN is a prognostic
biomarker independent of Ki-67 and essential for cell cycle
progression in primary breast cancer. BMC Cancer. 16:9042016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ciruelos Gil EM: Targeting the
PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer.
Cancer Treat Rev. 40:862–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo S, Colbert LS, Fuller M, Zhang Y and
Gonzalez-Perez RR: Vascular endothelial growth factor receptor-2 in
breast cancer. Biochim Biophys Acta. 1806:108–121. 2010.PubMed/NCBI
|
|
13
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
14
|
Mahdi KM, Nassiri MR and Nasiri K:
Hereditary genes and SNPs associated with breast cancer. Asian Pac
J Cancer Prev. 14:3403–3409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nelson MR, Marnellos G, Kammerer S, Hoyal
CR, Shi MM, Cantor CR and Braun A: Large-scale validation of single
nucleotide polymorphisms in gene regions. Genome Res. 14:1664–1668.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng N, Zhou H, Fan H and Yuan Y: Single
nucleotide polymorphisms and cancer susceptibility. Oncotarget.
8:110635–110649. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beltran H, Prandi D, Mosquera JM, Benelli
M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV,
Varambally S, et al: Divergent clonal evolution of
castration-resistant neuroendocrine prostate cancer. Nat Med.
22:298–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cancer Genome Atlas Research, Network, .
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Long X, Zhou W, Wang Y and Liu S:
Prognostic significance of ANLN in lung adenocarcinoma. Oncol Lett.
16:1835–1840. 2018.PubMed/NCBI
|
|
20
|
Dong G, Guo X, Fu X, Wan S, Zhou F, Myers
RE, Bao G, Burkart A, Yang H and Xing J: Potentially functional
genetic variants in KDR gene as prognostic markers in patients with
resected colorectal cancer. Cancer Sci. 103:561–568. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garrigós C, Espinosa M, Salinas A, Osman
I, Medina R, Taron M, Molina-Pinelo S and Duran I: Single
nucleotide polymorphisms as prognostic and predictive biomarkers in
renal cell carcinoma. Oncotarget. 8:106551–106564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slattery ML, Lundgreen A and Wolff RK:
VEGFA, FLT1, KDR and colorectal cancer: Assessment of disease risk,
tumor molecular phenotype, and survival. Mol Carcinog. 53 (Suppl
1):E140–E150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu IC, Zhao Y, Zhai R, Liu G,
Ter-Minassian M, Asomaning K, Su L, Liu CY, Chen F, Kulke MH, et
al: Association between polymorphisms in cancer-related genes and
early onset of esophageal adenocarcinoma. Neoplasia. 13:386–392.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao C, Cao J, Wang Y, Liu B and Wang Z:
Effects of VEGF and VEGFR polymorphisms on the outcome of patients
with metastatic renal cell carcinoma treated with sunitinib: A
systematic review and meta-analysis. Oncotarget. 8:68854–68862.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beeghly-Fadiel A, Shu XO, Lu W, Long J,
Cai Q, Xiang YB, Zheng Y, Zhao Z, Gu K, Gao YT and Zheng W: Genetic
variation in VEGF family genes and breast cancer risk: A report
from the Shanghai Breast Cancer Genetics Study. Cancer Epidemiol
Biomarkers Prev. 20:33–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sherry ST, Ward MH, Kholodov M, Baker J,
Phan L, Smigielski EM and Sirotkin K: dbSNP: The NCBI database of
genetic variation. Nucleic Acids Res. 29:308–311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Therneau T: A package for survival
analysis in S. version 2.38. https://CRAN.Rproject.org/package=survival2015
|
|
28
|
R Development Core Team, . R: A language
and environment for statistical computing. R Foundation for
Statistical Computing. 2009, https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
|
|
29
|
Nicolae DL, Gamazon E, Zhang W, Duan S,
Dolan ME and Cox NJ: Trait-associated SNPs are more likely to be
eQTLs: Annotation to enhance discovery from GWAS. PLoS Genet.
6:e10008882010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bendl J, Musil M, Štourač J, Zendulka J,
Damborský J and Brezovský J: PredictSNP2: A unified platform for
accurately evaluating SNP effects by exploiting the different
characteristics of variants in distinct genomic regions. PLoS
Comput Biol. 12:e10049622016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu Y, Liu Z, Lou S, Bedford J, Mu XJ, Yip
KY, Khurana E and Gerstein M: FunSeq2: A framework for prioritizing
noncoding regulatory variants in cancer. Genome Biol. 15:4802014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kircher M, Witten DM, Jain P, O'Roak BJ,
Cooper GM and Shendure J: A general framework for estimating the
relative pathogenicity of human genetic variants. Nat Genet.
46:310–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quang D, Chen Y and Xie X: DANN: A deep
learning approach for annotating the pathogenicity of genetic
variants. Bioinformatics. 31:761–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ritchie GR, Dunham I, Zeggini E and Flicek
P: Functional annotation of noncoding sequence variants. Nat
Methods. 11:294–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kanehisa M, Araki M, Goto S, Hattori M,
Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T
and Yamanishi Y: KEGG for linking genomes to life and the
environment. Nucleic Acids Res 36 (Database Issue). D480–D484.
2008.
|
|
36
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carlson M: org.Hs.eg.db: Genome wide
annotation for Human. 2019.
|
|
38
|
Franz M, Rodriguez H, Lopes C, Zuberi K,
Montojo J, Bader GD and Morris Q: GeneMANIA update 2018. Nucleic
Acids Res. 46:W60–W64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38:W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Labun K, Montague TG, Krause M, Torres
Cleuren YN, Tjeldnes H and Valen E: CHOPCHOP v3: Expanding the
CRISPR web toolbox beyond genome editing. Nucleic Acids Res.
47:W171–W174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heiskanen MA, Bittner ML, Chen Y, Khan J,
Adler KE, Trent JM and Meltzer PS: Detection of gene amplification
by genomic hybridization to cDNA microarrays. Cancer Res.
60:799–802. 2000.PubMed/NCBI
|
|
43
|
Li H, Jie S, Zou P and Zou G: CDNA
microarray analysis of chronic myeloid leukemia. Int J Hematol.
75:388–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brouhard GJ and Rice LM: Microtubule
dynamics: An interplay of biochemistry and mechanics. Nat Rev Mol
Cell Biol. 19:451–463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dogra N, Kumar A and Mukhopadhyay T:
Fenbendazole acts as a moderate microtubule destabilizing agent and
causes cancer cell death by modulating multiple cellular pathways.
Sci Rep. 8:119262018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Parker AL, Kavallaris M and McCarroll JA:
Microtubules and their role in cellular stress in cancer. Front
Oncol. 4:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Visochek L, Castiel A, Mittelman L, Elkin
M, Atias D, Golan T, Izraeli S, Peretz T and Cohen-Armon M:
Exclusive destruction of mitotic spindles in human cancer cells.
Oncotarget. 8:20813–20824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dong X, Liu F, Sun L, Liu M, Li D, Su D,
Zhu Z, Dong JT, Fu L and Zhou J: Oncogenic function of microtubule
end-binding protein 1 in breast cancer. J Pathol. 220:361–369.
2010.PubMed/NCBI
|
|
50
|
Liu M, Wang X, Yang Y, Li D, Ren H, Zhu Q,
Chen Q, Han S, Hao J and Zhou J: Ectopic expression of the
microtubule-dependent motor protein Eg5 promotes pancreatic
tumourigenesis. J Pathol. 221:221–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
McCusker C, Upton J and Warrington R:
Primary immunodeficiency. Allergy Asthma Clin Immunol. 14 (Suppl
2):S612018. View Article : Google Scholar
|
|
52
|
Shapiro RS: Malignancies in the setting of
primary immunodeficiency: Implications for
hematologists/oncologists. Am J Hematol. 86:48–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Filipovich AH, Mathur A, Kamat D and
Shapiro RS: Primary immunodeficiencies: Genetic risk factors for
lymphoma. Cancer Res 52 (19 Suppl). 5465S–5467S. 1992.
|
|
54
|
Moriwaki Y, Yamamoto T and Higashino K:
Enzymes involved in purine metabolism-a review of histochemical
localization and functional implications. Histol Histopathol.
14:1321–1340. 1999.PubMed/NCBI
|
|
55
|
Chen TC, Lin KT, Chen CH, Lee SA, Lee PY,
Liu YW, Kuo YL, Wang FS, Lai JM and Huang CY: Using an in situ
proximity ligation assay to systematically profile endogenous
protein-protein interactions in a pathway network. J Proteome Res.
13:5339–5346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hein MY, Hubner NC, Poser I, Cox J,
Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F,
et al: A human interactome in three quantitative dimensions
organized by stoichiometries and abundances. Cell. 163:712–723.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie YG, Yu Y, Hou LK, Wang X, Zhang B and
Cao XC: FYN promotes breast cancer progression through
epithelial-mesenchymal transition. Oncol Rep. 36:1000–1006. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee GH, Yoo KC, An Y, Lee HJ, Lee M, Uddin
N, Kim MJ, Kim IG, Suh Y and Lee SJ: FYN promotes mesenchymal
phenotypes of basal type breast cancer cells through STAT5/NOTCH2
signaling node. Oncogene. 37:1857–1868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J
and Shi B: Breast cancer intrinsic subtype classification, clinical
use and future trends. Am J Cancer Res. 5:2929–2943.
2015.PubMed/NCBI
|
|
60
|
Dai X, Xiang L, Li T and Bai Z: Cancer
hallmarks, biomarkers and breast cancer molecular subtypes. J
Cancer. 7:1281–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|