|
1
|
Restifo NP, Dudley ME and Rosenberg SA:
Adoptive immunotherapy for cancer: Harnessing the T cell response.
Nat Rev Immunol. 12:269–281. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galluzzi L and Martin P: CARs on a highway
with roadblocks. Oncoimmunology. 6:e13884862017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perales MA, Kebriaei P, Kean LS and
Sadelain M: Building a safer and faster CAR: Seatbelts, airbags,
and CRISPR. Biol Blood Marrow Transplant. 24:27–31. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharpe M and Mount N: Genetically modified
T cells in cancer therapy: Opportunities and challenges. Dis Model
Mech. 8:337–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McGuirk J, Waller EK, Qayed M, Abhyankar
S, Ericson S, Holman P, Keir C and Myers GD: Building blocks for
institutional preparation of CTL019 delivery. Cytotherapy.
19:1015–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad V: Immunotherapy:
Tisagenlecleucel-the first approved CAR-T-cell therapy:
Implications for payers and policy makers. Nat Rev Clin Oncol.
15:11–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neelapu SS, Tummala S, Kebriaei P, Wierda
W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et
al: Chimeric antigen receptor T-cell therapy-assessment and
management of toxicities. Nat Rev Clin Oncol. 15:47–62. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miliotou AN and Papadopoulou LC: CAR
T-cell therapy: A new era in cancer immunotherapy. Curr Pharm
Biotechnol. 19:5–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mirzaei HR, Jamali A, Jafarzadeh L,
Masoumi E, Alishah K, Fallah Mehrjardi K, Emami SAH, Noorbakhsh F,
Till BG and Hadjati J: Construction and functional characterization
of a fully human anti-CD19 chimeric antigen receptor
(huCAR)-expressing primary human T cells. J Cell Physiol.
234:9207–9215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vormittag P, Gunn R, Ghorashian S and
Veraitch FS: A guide to manufacturing CAR T cell therapies. Curr
Opin Biotechnol. 53:164–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
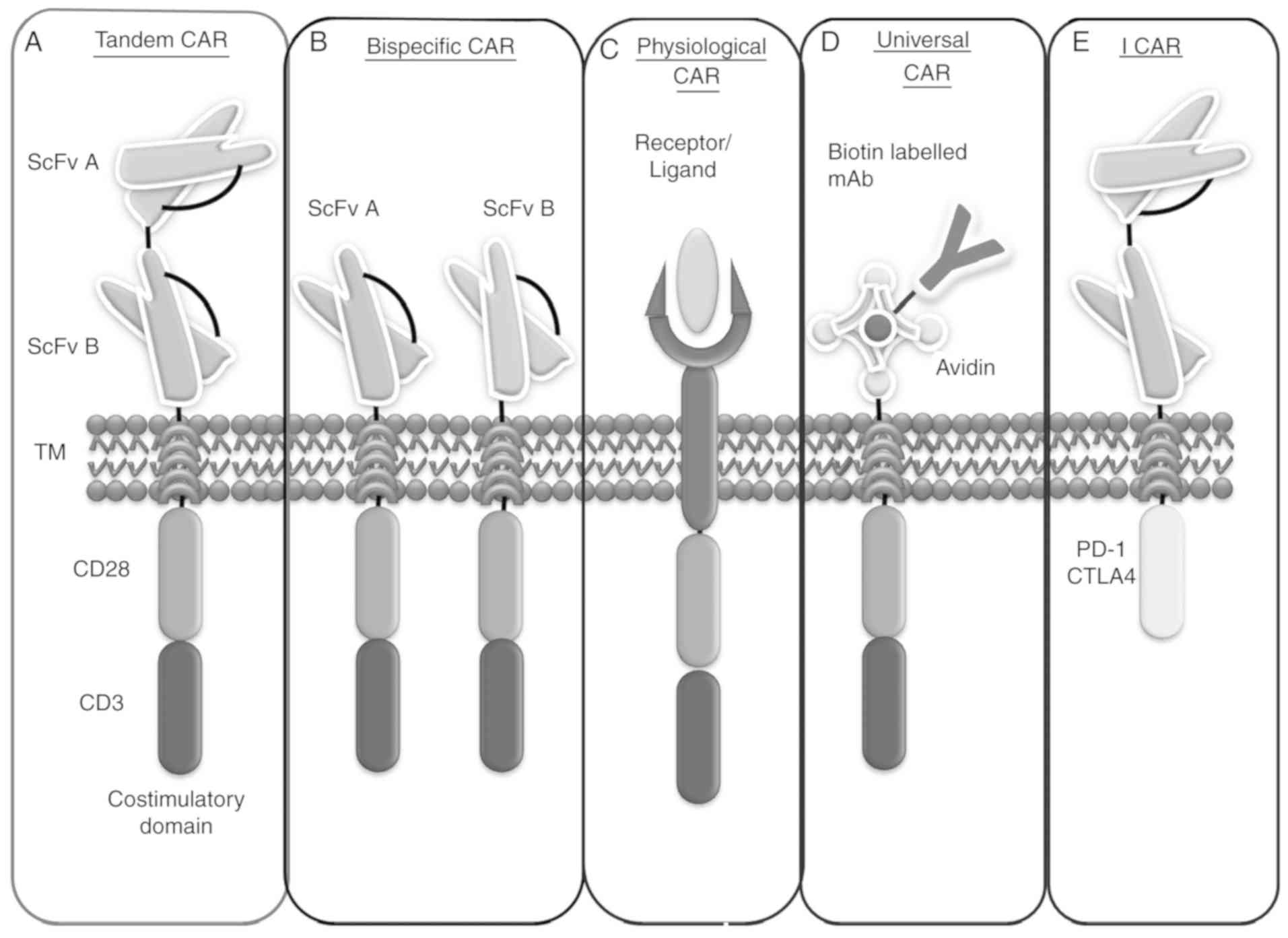
|
11
|
Abate-Daga D and Davila ML: CAR models:
Next-generation CAR modifications for enhanced T-cell function. Mol
Ther Oncolytics. 3:160142016. View Article : Google Scholar : PubMed/NCBI
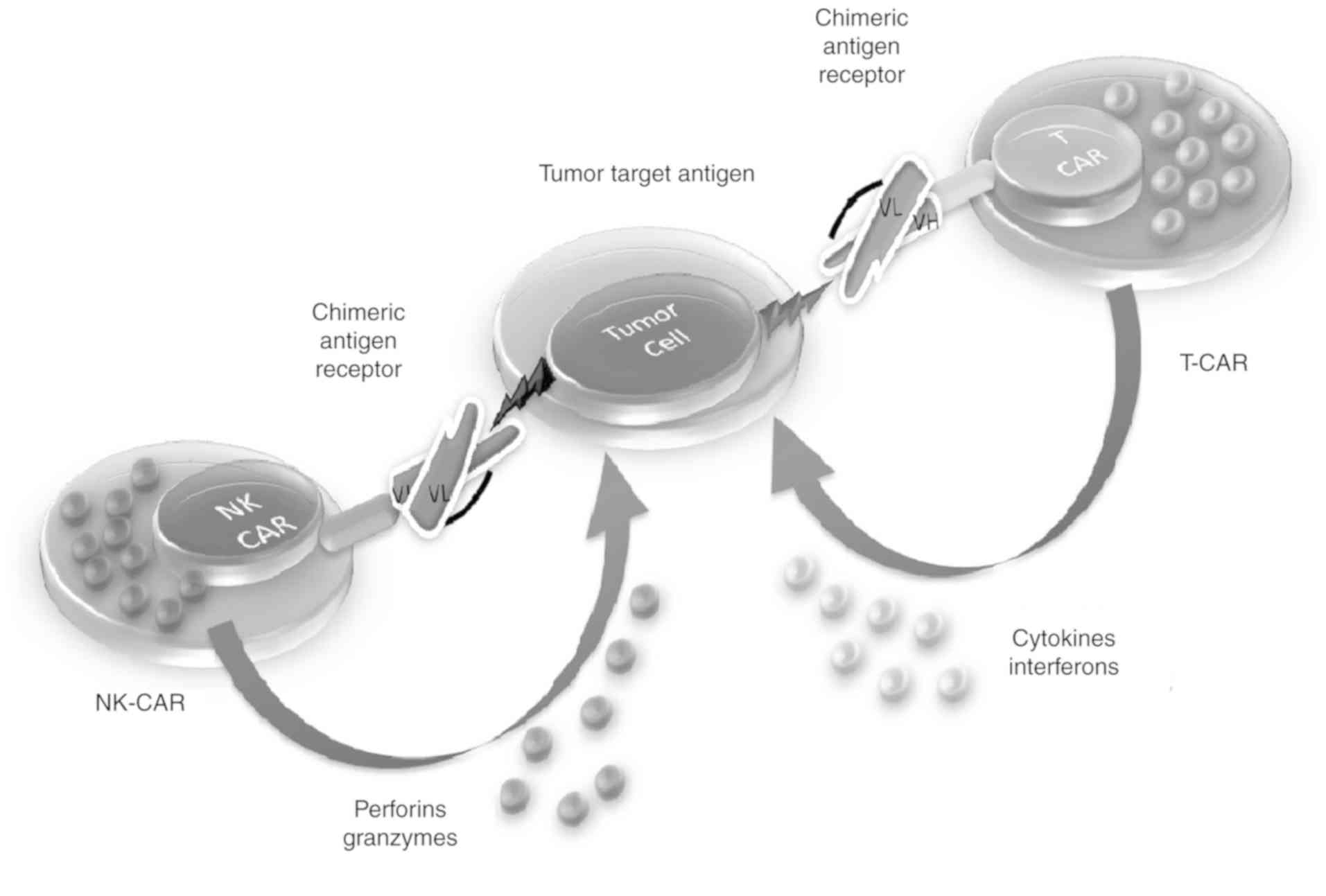
|
|
12
|
Yang QY, Yang JD and Wang YS: Current
strategies to improve the safety of chimeric antigen receptor (CAR)
modified T cells. Immunol Lett. 190:201–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mirzaei HR, Mirzaei H, Namdar A, Rahmati
M, Till BG and Hadjati J: Predictive and therapeutic biomarkers in
chimeric antigen receptor T-cell therapy: A clinical perspective. J
Cell Physiol. 234:5827–5841. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai H, Wang Y, Lu X and Han W: Chimeric
antigen receptors modified T-cells for cancer therapy. J Natl
Cancer Inst. 108(pii): djv4392016.PubMed/NCBI
|
|
15
|
Mirzaei HR, Mirzaei H, Lee SY, Hadjati J
and Till BG: Prospects for chimeric antigen receptor (CAR) γδ T
cells: A potential game changer for adoptive T cell cancer
immunotherapy. Cancer Lett. 380:413–423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgan RA: Chapter 17-Adoptive T-cell
therapy: Engineering T-cell receptors. Cancer Immunother (Sec Ed).
261–272. 2013. View Article : Google Scholar
|
|
17
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor
activity and long-term fate of chimeric antigen receptor-positive T
cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duong CP, Yong CS, Kershaw MH, Slaney CY
and Darcy PK: Cancer immunotherapy utilizing gene-modified T cells:
From the bench to the clinic. Mol Immunol. 67:46–57. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sadelain M: CAR therapy: The CD19
paradigm. J Clin Invest. 125:3392–3400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eshhar Z: The T-body approach: Redirecting
T cells with antibody specificity. Handb Exp Pharmacol. 329–342.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brentjens R, Yeh R, Bernal Y, Riviere I
and Sadelain M: Treatment of chronic lymphocytic leukemia with
genetically targeted autologous T cells: Case report of an
unforeseen adverse event in a phase I clinical trial. Mol Ther.
18:666–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Till BG, Jensen MC, Wang J, Qian X, Gopal
AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE, et al:
CD20-specific adoptive immunotherapy for lymphoma using a chimeric
antigen receptor with both CD28 and 4-1BB domains: Pilot clinical
trial results. Blood. 119:3940–3950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH and Brentjens RJ: Are all chimeric
antigen receptors created equal? J Clin Oncol. 33:651–653. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian L, Li D, Ma L, He T, Qi F, Shen J and
Lu XA: The novel anti-CD19 chimeric antigen receptors with
humanized scFv (single-chain variable fragment) trigger leukemia
cell killing. Cell Immunol. 304-305:49–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lock D, Mockel-Tenbrinck N, Drechsel K,
Barth C, Mauer D, Schaser T, Kolbe C, Al Rawashdeh W, Brauner J,
Hardt O, et al: Automated manufacturing of potent CD20-directed
chimeric antigen receptor T cells for clinical use. Hum Gene Ther.
28:914–925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang XY, Sun Y, Zhang A, Hu GL, Cao W,
Wang DH, Zhang B and Chen H: Third-generation CD28/4-1BB chimeric
antigen receptor T cells for chemotherapy relapsed or refractory
acute lymphoblastic leukaemia: A non-randomised, open-label phase I
trial protocol. BMJ Open. 6:e0139042016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yeku OO and Brentjens RJ: Armored CAR
T-cells: Utilizing cytokines and pro-inflammatory ligands to
enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans.
44:412–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chmielewski M and Abken H: TRUCKs: The
fourth generation of CARs. Expert Opin Biol Ther. 15:1145–1154.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kueberuwa G, Kalaitsidou M, Cheadle E,
Hawkins RE and Gilham DE: CD19 CAR T cells expressing IL-12
eradicate lymphoma in fully lymphoreplete mice through induction of
host immunity. Mol Ther Oncolytics. 8:41–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kerkar SP, Muranski P, Kaiser A, Boni A,
Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, et
al: Tumor-specific CD8+ T cells expressing interleukin-12 eradicate
established cancers in lymphodepleted hosts. Cancer Res.
70:6725–6734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giacca M and Zacchigna S: Virus-mediated
gene delivery for human gene therapy. J Control Release.
161:377–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi Y, Noh MJ and Lee KH: Current Advances
in retroviral gene therapy. Curr Gene Ther. 11:218–228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kidd ME, Shin S and Shea LD: Fibrin
hydrogels for lentiviral gene delivery in vitro and in vivo. J
Control Release. 157:80–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Munñoz-López M and García-Perez JL: DNA
transposons: Nature and applications in genomics. Curr Genomics.
11:115–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morita D, Nishio N, Saito S, Tanaka M,
Kawashima N, Okuno Y, Suzuki S, Matsuda K, Maeda Y, Wilson MH, et
al: Enhanced expression of Anti-CD19 chimeric antigen receptor in
piggyBac transposon-engineered T cells. Mol Ther Methods Clin Dev.
8:131–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kebriaei P, Singh H, Huls MH, Figliola MJ,
Bassett R, Olivares S, Jena B, Dawson MJ, Kumaresan PR, Su S, et
al: Phase I trials using sleeping beauty to generate CD19-specific
CAR T cells. J Clin Invest. 126:3363–3376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manuri PV, Wilson MH, Maiti SN, Mi T,
Singh H, Olivares S, Dawson MJ, Huls H, Lee DA, Rao PH, et al:
piggyBac Transposon/Transposase system to generate CD19-specific T
cells for the treatment of B-lineage malignancies. Hum Gene Ther.
21:427–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yarmush ML, Golberg A, Serša G, Kotnik T
and Miklavčič D: Electroporation-based technologies for medicine:
Principles, applications, and challenges. Annu Rev Biomed Eng.
16:295–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chicaybam L, Sodre AL, Curzio BA and
Bonamino MH: An efficient low cost method for gene transfer to T
lymphocytes. PLoS One. 8:e602982013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chicaybam L, Barcelos C, Peixoto B,
Carneiro M, Limia CG, Redondo P, Lira C, Paraguassú-Braga F,
Vasconcelos ZF, Barros L and Bonamino MH: An efficient
electroporation protocol for the genetic modification of mammalian
cells. Front Bioeng Biotechnol. 4:992017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kotnik T, Frey W, Sack M, Haberl Meglič S,
Peterka M and Miklavčič D: Electroporation-based applications in
biotechnology. Trends Biotechnol. 33:480–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ramamoorth M and Narvekar A: Non viral
vectors in gene therapy-an overview. J Clin Diagn Res. 9:GE01–GE06.
2015.PubMed/NCBI
|
|
43
|
Yin H, Kauffman KJ and Anderson DG:
Delivery technologies for genome editing. Nat Rev Drug Discov.
16:387–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smith TT, Stephan SB, Moffett HF, McKnight
LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS and
Stephan MT: In situ programming of leukaemia-specific T cells using
synthetic DNA nanocarriers. Nat Nanotechnol. 12:813–820. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miller MA: Nanoparticles improve economic
mileage for CARs. Sci Transl Med. 9(pii): eaan27842017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Smith TT, Moffett HF, Stephan SB, Opel CF,
Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD and
Stephan MT: Biopolymers codelivering engineered T cells and STING
agonists can eliminate heterogeneous tumors. J Clin Invest.
127:2176–2191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dowaidar M, Abdelhamid HN, Hällbrink M,
Freimann K, Kurrikoff K, Zou X and Langel Ü: Magnetic nanoparticle
assisted Self-assembly of cell penetrating
peptides-oligonucleotides complexes for gene delivery. Sci Rep.
7:91592017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kebriaei P: CAR T-cell therapies:
Overcoming the challenges and new strategies. Clin Lymphoma Myeloma
Leuk. 17 (Suppl 2):S74–S78. 2017. View Article : Google Scholar
|
|
49
|
Bonifant CL, Jackson HJ, Brentjens RJ and
Curran KJ: Toxicity and management in CAR T-cell therapy. Mol Ther
Oncolytics. 3:160112016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brudno JN and Kochenderfer JN: Toxicities
of chimeric antigen receptor T cells: Recognition and management.
Blood. 127:3321–3330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene Ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Paietta E: Immunobiology of acute
leukemia. In: Neoplastic diseases of the bloodSpringer; Cham: pp.
237–279. 2018
|
|
53
|
Zah E, Lin MY, Jensen MC, Silva-Benedict A
and Chen YY: Abstract IA12: Combating antigen escape with CD19/CD20
bispecific CAR-T cell therapy. Cancer Immunol Res 5 (3 Suppl).
IA122017.
|
|
54
|
Majzner RG and Mackall CL: Tumor antigen
escape from CAR T-cell therapy. Cancer Discov. 8:1219–1226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu M, Wu B, Brandl C, Johnson J, Wolf A,
Chow A and Doshi S: Blinatumomab, a Bispecific T-cell Engager
(BiTE(®)) for CD-19 targeted cancer immunotherapy:
Clinical pharmacology and its implications. Clin Pharmacokinet.
55:1271–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Martyniszyn A, Krahl AC, André MC, Hombach
AA and Abken H: CD20-CD19 Bispecific CAR T cells for the treatment
of B-cell malignancies. Hum Gene Ther. 28:1147–1157. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu F, Shah N, Xu H, Schneider D, Orentas
R, Dropulic B, Hari P and Keever-Taylor CA: Closed-system
manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen
receptor T cells using the CliniMACS prodigy device at an academic
medical center. Cytotherapy. 20:394–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun LL, Ellerman D, Mathieu M,
Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E,
et al: Anti-CD20/CD3 T cell-dependent bispecific antibody for the
treatment of B cell malignancies. Sci Transl Med. 7:287ra702015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang W, Liu Y, Wang Y, Wang C, Yang QM,
Zhu HL and Han WD: Long-term safety and efficacy of CART-20 cells
in patients with refractory or relapsed B-cell non-Hodgkin
lymphoma: 5-years follow-up results of the phase I and IIa trials.
Signal Transduct Target Ther. 2:170542017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hegde M, Mukherjee M, Grada Z, Pignata A,
Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK,
et al: Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor
antigen escape. J Clin Invest. 126:3036–3052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Schneider D, Xiong Y, Wu D, Nӧlle V,
Schmitz S, Haso W, Kaiser A, Dropulic B and Orentas RJ: A tandem
CD19/CD20 CAR lentiviral vector drives on-target and off-target
antigen modulation in leukemia cell lines. J Immunother Cancer.
5:422017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Grada Z, Hegde M, Byrd T, Shaffer DR,
Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, et al:
TanCAR: A novel bispecific chimeric antigen receptor for cancer
immunotherapy. Mol Ther Nucleic Acids. 2:e1052013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ahmed N, Brawley V, Hegde M, Bielamowicz
K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et
al: HER2-specific chimeric antigen receptor-modified virus-specific
T cells for progressive glioblastoma: A phase 1 dose-escalation
trial. JAMA Oncol. 3:1094–1101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bielamowicz K, Fousek K, Byrd TT, Samaha
H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, et
al: Trivalent CAR T cells overcome interpatient antigenic
variability in glioblastoma. Neuro Oncol. 20:506–518. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ott PA, Hodi FS and Robert C: CTLA-4 and
PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable
clinical benefit in melanoma patients. Clin Cancer Res.
19:5300–5309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Curran MA, Montalvo W, Yagita H and
Allison JP: PD-1 and CTLA-4 combination blockade expands
infiltrating T cells and reduces regulatory T and myeloid cells
within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:4275–4280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved Survival with Ipilimumab in Patients with
Metastatic Melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Iwai Y, Hamanishi J, Chamoto K and Honjo
T: Cancer immunotherapies targeting the PD-1 signaling pathway. J
Biomed Sci. 24:262017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 Blockade with Nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen R, Zinzani PL, Fanale MA, Armand P,
Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos
TP, et al: Phase II study of the efficacy and safety of
pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J
Clin Oncol. 35:2125–2132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Barbee MS, Ogunniyi A, Horvat TZ and Dang
TO: Current status and future directions of the immune checkpoint
inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology.
Ann Pharmacother. 49:907–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Curran MA, Kim M, Montalvo W, Al-Shamkhani
A and Allison JP: Combination CTLA-4 blockade and 4-1BB activation
enhances tumor rejection by increasing T-cell infiltration,
proliferation, and cytokine production. PLoS One. 6:e194992011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Seidel JA, Otsuka A and Kabashima K:
Anti-PD-1 and Anti-CTLA-4 therapies in cancer: Mechanisms of
action, efficacy, and limitations. Front Oncol. 8:862018.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ren J, Liu X, Fang C, Jiang S, June CH and
Zhao Y: Multiplex genome editing to generate universal CAR T cells
resistant to PD1 inhibition. Clin Cancer Res. 23:2255–2266. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Azijli K, Stelloo E, Peters GJ and Van Den
Eertwegh AJ: New developments in the treatment of metastatic
melanoma: Immune checkpoint inhibitors and targeted therapies.
Anticancer Res. 34:1493–1505. 2014.PubMed/NCBI
|
|
77
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shi H, Sun M, Liu L and Wang Z: Chimeric
antigen receptor for adoptive immunotherapy of cancer: Latest
research and future prospects. Mol Cancer. 13:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu L, Sun M and Wang Z: Adoptive T-cell
therapy of B-cell malignancies: Conventional and physiological
chimeric antigen receptors. Cancer Lett. 316:1–5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Minutolo NG, Hollander EE and Powell DJ
Jr: The emergence of universal immune receptor T cell therapy for
cancer. Front Oncol. 9:1762019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhao J, Lin Q, Song Y and Liu D: Universal
CARs, universal T cells, and universal CAR T cells. J Hematol
Oncol. 11:1322018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lohmueller JJ, Ham JD, Kvorjak M and Finn
OJ: mSA2 affinity-enhanced biotin-binding CAR T cells for universal
tumor targeting. Oncoimmunology. 7:e13686042018. View Article : Google Scholar
|
|
83
|
Qasim W, Zhan H, Samarasinghe S, Adams S,
Amrolia P, Stafford S, Butler K, Rivat C, Wright G, Somana K, et
al: Molecular remission of infant B-ALL after infusion of universal
TALEN gene-edited CAR T cells. Sci Transl Med. 9:eaaj20132017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Glienke W, Esser R, Priesner C, Suerth JD,
Schambach A, Wels WS, Grez M, Kloess S, Arseniev L and Koehl U:
Advantages and applications of CAR-expressing natural killer cells.
Front Pharmacol. 6:212015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Klingemann H: Are natural killer cells
superior CAR drivers? Oncoimmunology. 3:e281472014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jiang H, Zhang W, Shang P, Zhang H, Fu W,
Ye F, Zeng T, Huang H, Zhang X, Sun W, et al: Transfection of
chimeric anti-CD138 gene enhances natural killer cell activation
and killing of multiple myeloma cells. Mol Oncol. 8:297–310. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Boissel L, Betancur-Boissel M, Lu W,
Krause DS, Van Etten RA, Wels WS and Klingemann H: Retargeting
NK-92 cells by means of CD19- and CD20-specific chimeric antigen
receptors compares favorably with antibody-dependent cellular
cytotoxicity. Oncoimmunology. 2:e265272013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Romanski A, Uherek C, Bug G, Seifried E,
Klingemann H, Wels WS, Ottmann OG and Tonn T: CD19-CAR engineered
NK-92 cells are sufficient to overcome NK cell resistance in B-cell
malignancies. J Cell Mol Med. 20:1287–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hermanson DL and Kaufman DS: Utilizing
chimeric antigen receptors to direct natural killer cell activity.
Front Immunol. 6:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu
T, Yin J, You F, Zhu M, Shen W, et al: First-in-man clinical trial
of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients
with relapsed and refractory acute myeloid leukemia. Am J Cancer
Res. 8:1083–1089. 2018.PubMed/NCBI
|
|
91
|
Hsu PD, Lander ES and Zhang F: Development
and Applications of CRISPR-Cas9 for genome engineering. Cell.
157:1262–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K
and Doudna JA: Sequence- and Structure-specific RNA processing by a
CRISPR endonuclease. Science. 329:1355–1358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cox DB, Platt RJ and Zhang F: Therapeutic
genome editing: Prospects and challenges. Nat Med. 21:121–131.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shang W, Wang F, Fan G and Wang H: Key
elements for designing and performing a CRISPR/Cas9-based genetic
screen. J Genet Genomics. 44:439–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mirzaei HR, Pourghadamyari H, Rahmati M,
Mohammadi A, Nahand JS, Rezaei A and Hadjati J: Gene-knocked out
chimeric antigen receptor (CAR) T cells: Tuning up for the next
generation cancer immunotherapy. Cancer Lett. 423:95–104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Eyquem J, Mansilla-Soto J, Giavridis T,
van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gönen M and
Sadelain M: Targeting a CAR to the TRAC locus with CRISPR/Cas9
enhances tumour rejection. Nature. 543:113–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mou H, Kennedy Z, Anderson DG, Yin H and
Xue W: Precision cancer mouse models through genome editing with
CRISPR-Cas9. Genome Med. 7:532015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
MacLeod DT, Antony J, Martin AJ, Moser RJ,
Hekele A, Wetzel KJ, Brown AE, Triggiano MA, Hux JA, Pham CD, et
al: Integration of a CD19 CAR into the TCR alpha chain locus
streamlines production of allogeneic gene-edited CAR T cells. Mol
Ther. 25:949–961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ren J, Zhang X, Liu X, Fang C, Jiang S,
June CH and Zhao Y: A versatile system for rapid multiplex
genome-edited CAR T cell generation. Oncotarget. 8:17002–17011.
2017.PubMed/NCBI
|
|
100
|
Grupp SA, Laetsch TW, Buechner J,
Bittencourt H, Maude SL, Verneris MR, Myers GD, Boyer MW, Rives S,
De Moerloose B, et al: Analysis of a global registration trial of
the efficacy and safety of CTL019 in pediatric and young adults
with relapsed/refractory acute lymphoblastic leukemia (ALL). Blood.
128:2212016.
|
|
101
|
Zhao Z, Chen Y, Francisco NM, Zhang Y and
Wu M: The application of CAR-T cell therapy in hematological
malignancies: Advantages and challenges. Acta Pharm Sin B.
8:539–551. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Beatty GL, Haas AR, Maus MV, Torigian DA,
Soulen MC, Plesa G and Kalos M: Mesothelin-specific chimeric
antigen receptor mRNA-engineered T cells induce antitumor activity
in solid malignancies. Cancer Immunol Res. 2:112–120. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mirzaei HR, Rodriguez A, Shepphird J,
Brown CE and Badie B: Chimeric antigen receptors T cell therapy in
solid tumor: Challenges and clinical applications. Front Immunol.
8:18502017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Boardman DA, Philippeos C, Fruhwirth GO,
Ibrahim MA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler
RI, Maher J, et al: Expression of a chimeric antigen receptor
specific for donor HLA class I enhances the potency of human
regulatory T cells in preventing human skin transplant rejection.
Am J Transplant. 17:931–943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shen CJ, Yang YX, Han EQ, Cao N, Wang YF,
Wang Y, Zhao YY, Zhao LM, Cui J, Gupta P, et al: Chimeric antigen
receptor containing ICOS signaling domain mediates specific and
efficient antitumor effect of T cells against EGFRvIII expressing
glioma. J Hematol Oncol. 6:332013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Owens GL, Sheard VE, Kalaitsidou M, Blount
D, Lad Y, Cheadle EJ, Edmondson RJ, Kooner G, Gilham DE and Harrop
R: Preclinical assessment of CAR T-cell therapy targeting the tumor
antigen 5T4 in ovarian cancer. J Immunother. 41:130–140. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ellebrecht CT, Bhoj VG, Nace A, Choi EJ,
Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis
G, et al: Reengineering chimeric antigen receptor T cells for
targeted therapy of autoimmune disease. Science. 353:179–184. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
O'Rourke DM, Nasrallah MP, Desai A,
Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem
S, Maloney E, Shen A, et al: A single dose of peripherally infused
EGFRvIII-directed CAR T cells mediates antigen loss and induces
adaptive resistance in patients with recurrent glioblastoma. Sci
Transl Med. 9(pii): eaaa09842017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Posey AD, Schwab RD, Boesteanu AC,
Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K,
Haines KM, et al: Engineered CAR T cells targeting the
cancer-associated Tn-Glycoform of the membrane mucin MUC1 control
adenocarcinoma. Immunity. 44:1444–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Burga RA, Thorn M, Point GR, Guha P,
Nguyen CT, Licata LA, DeMatteo RP, Ayala A, Joseph Espat N,
Junghans RP and Katz SC: Liver myeloid-derived suppressor cells
expand in response to liver metastases in mice and inhibit the
anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother.
64:817–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ahmed N, Brawley VS, Hegde M, Robertson C,
Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al:
Human epidermal growth factor receptor 2 (HER2)-specific chimeric
antigen receptor-modified T cells for the immunotherapy of
HER2-positive sarcoma. J Clin Oncol. 33:1688–1696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Akce M, Zaidi MY, Waller EK, El-Rayes BF
and Lesinski GB: The potential of CAR T cell therapy in pancreatic
cancer. Front Immunol. 9:21662018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bach PB, Giralt SA and Saltz LB: FDA
approval of tisagenlecleucel: Promise and complexities of a $475
000 cancer drug. JAMA. 318:1861–1862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Fala L: Yescarta (Axicabtagene Ciloleucel)
second CAR T-cell therapy approved for patients with certain types
of large B-cell lymphoma. Am Health Drug Benefits. 11:109–111.
2018.
|
|
115
|
Hey SP and Kesselheim AS: The FDA, Juno
therapeutics, and the ethical imperative of transparency. BMJ.
354:i44352016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Rotolo A, Caputo V and Karadimitris A: The
prospects and promise of chimeric antigen receptor immunotherapy in
multiple myeloma. Br J Haematol. 173:350–364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Almond LM, Charalampakis M, Ford SJ,
Gourevitch D and Desai A: Myeloid sarcoma: Presentation, diagnosis,
and treatment. Clin Lymphoma Myeloma Leuk. 17:263–267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Locke FL, Neelapu SS, Bartlett NL, Siddiqi
T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et
al: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19
Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol
Ther. 25:285–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Qasim W, Ciocarlie O, Adams S, Inglott S,
Murphy C, Rivat C, Wright G, Lucchini G, Silva J, Rao K, et al:
Preliminary results of UCART19, an allogeneic anti-CD19 CAR T-cell
product in a first-in-human trial (PALL) in pediatric patients with
CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia.
Blood. 130:12712017.
|
|
120
|
Cai T, Galetto R, Gouble A, Smith J,
Cavazos A, Konoplev S, Lane AA, Guzman ML, Kantarjian HM, Pemmaraju
N, et al: Pre-clinical studies of Anti-CD123 CAR-T cells for the
treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Blood. 128:40392016.
|
|
121
|
Gouble A, Schiffer-Mannioui C, Thomas S,
Gautron AS, Poirot L and Smith J: Abstract 3763: UCART22: Allogenic
adoptive immunotherapy of leukemia by targeting CD22 with CAR
T-cells. Cancer Res. 77:3763. 2017.
|
|
122
|
Galetto R, Chion-Sotinel I, Gouble A and
Smith J: Allogenic TCRa/CS1 double knockout T-cells bearing an
anti-CS1 chimeric antigen receptor: An improved immunotherapy
approach for the treatment of multiple myeloma. Cancer Res. 76 (14
Suppl):Abstract nr 2289. 2016.
|
|
123
|
Drent E, Groen RW, Noort WA, Themeli M,
Lammerts van Bueren JJ, Parren PW, Kuball J, Sebestyen Z, Yuan H,
de Bruijn J, et al: Pre-clinical evaluation of CD38 chimeric
antigen receptor engineered T cells for the treatment of multiple
myeloma. Haematologica. 101:616–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Coulter I: Compositions. US Patent
2018/0228866A1. Filed August 12 2016; issued August 16 2018.
|