|
1
|
Al-Snafi AE: Medical importance of
Cichorium intybus-A review. IOSR J Of Pharm. 6:41–56.
2016.
|
|
2
|
Bais HP and Ravishankar GA: Cichorium
intybus L- cultivation, processing, utility, value addition and
biotechnology, with an emphasis on current status and future
prospects. J Sci Food Agric. 81:467–484. 2001. View Article : Google Scholar
|
|
3
|
Street RA, Sidana J and Prinsloo G:
Cichorium intybus: Traditional uses, phytochemistry,
pharmacology, and toxicology. Evid Based Complement Alternat Med.
2013:5793192013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberfroid MB: Inulin-type fructans:
Functional food ingredients. J Nutr. 137 (Suppl 11):S2493–S2502.
2007. View Article : Google Scholar
|
|
5
|
Carazzone C, Mascherpa D, Gazzani G and
Papetti A: Identification of phenolic constituents in red chicory
salads (Cichorium intybus) by high-performance liquid
chromatography with diode array detection and electrospray
ionisation tandem mass spectrometry. Food Chem. 138:1062–1071.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malik B, Pirzadah TB, Tahir I and Rehman
RU: Chemo-profiling, antioxidant potential and ionomic analysis of
Cichorium intybus L. Pharmacogn J. 9:917–928. 2017.
View Article : Google Scholar
|
|
7
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Zhang H, Zhou HJ, Ji W and Min W:
Mitochondrial redox signaling and tumor progression. Cancers
(Basel). 8:402016. View Article : Google Scholar :
|
|
11
|
Saikolappan S, Kumar B, Shishodia G, Koul
S and Koul HK: Reactive oxygen species and cancer: A complex
interaction. Cancer Lett. 452:132–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conforti F, Ioele G, Statti GA, Marrelli
M, Ragno G and Menichini F: Antiproliferative activity against
human tumor cell lines and toxicity test on Mediterranean dietary
plants. Food Chem Toxicol. 46:3325–3332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hafez ESE, Badr EA, Mabrouk YM, Seehy MA
and Aggag SA: Expression of tumor-markers and cytokines in response
to Cichorium endivia L. in cancerous mice. Int J Life Sci
Biotech Pharma Res. 3:1–7. 2014.
|
|
14
|
Alshehri A and Elsayed HE: Molecular and
biochemical evaluation of anti-proliferative effect of
(Cichorium endivia L.) phenolic extracts on breast cancer
cell line: MCF7. J Biotechnol Pharma Res. 3:74–82. 2012.
|
|
15
|
Hafez EE, Badr E, Mabrouk Y, El-Seehy M
and Aggag S: Molecular genetic evaluation of Cichorium
endivia L. as an anticancer agent against colorectal cancer.
Int J Phytomed. 8:551–557. 2016. View Article : Google Scholar
|
|
16
|
Hazra B, Sarkar R, Bhattacharyya S and Roy
P: Tumour inhibitory activity of chicory root extract against
Ehrlich ascites carcinoma in mice. Fitoterapia. 73:730–733. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nawab A, Yunus M, Mahdi AA and Gupta S:
Evaluation of anticancer properties of medicinal plants from the
Indiansub-continent. Mol Cell Pharmacol. 3:21–29. 2011.
|
|
18
|
Mehrandish R, Awsat Mellati A, Rahimipour
A and Dehghan Nayeri N: Anti-cancer activity of methanol extracts
of Cichorium intybus on human breast cancer SKBR3 cell line.
Razavi Int J Med. 5:e383692017.
|
|
19
|
Saleem M, Abbas K, Naseer F, Mobasher A,
Syed NH, Fatima J, Hussain K and Samia A: Anticancer activity of
n-hexane extract of Cichorium intybus on lymphoblastic
leukemia cells (Jurkat cells). Asian J Plant Sci. 8:315–319.
2014.
|
|
20
|
Esmaeilbeig M, Kouhpayeh SA and
Amirghofran Z: An investigation of the growth inhibitory capacity
of several medicinal plants from Iran on tumor cell lines. Iran J
Cancer Prev. 8:e40322015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leclercq E: Determination of lactucin in
roots of chicory (Cichorium intybus L.) by high-performance
liquid chromatography. J Chromatogr A. 283:441–444. 1984.
View Article : Google Scholar
|
|
22
|
Bischoff TA, Kelley CJ, Karchesy Y,
Laurantos M, Nguyen-Dinh P and Arefi AG: Antimalarial activity of
lactucin and lactucopicrin: Sesquiterpene lactones isolated from
Cichorium intybus L. J Ethnopharmacol. 95:455–457. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kisiel W and Zielińska K: Guaianolides
from Cichorium intybus and structure revision of Cichorium
sesquiterpene lactones. Phytochemistry. 57:523–527. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malarz J, Stojakowska A, Szneler E and
Kisiel W: A new neolignan glucoside from hairy roots of
Cichorium intybus. Phytochem Lett. 6:59–61. 2013. View Article : Google Scholar
|
|
25
|
Pyrek JS: Sesquiterpene lactones of
Cichorium intybus and Leontodon autumnalis.
Phytochemistry. 24:186–188. 1985. View Article : Google Scholar
|
|
26
|
Satmbekova D, Srivedavyasasri R, Orazbekov
Y, Omarova R, Datkhayev U and Ross SA: Chemical and biological
studies on Cichorium intybus L. Nat Prod Res. 32:1343–1347.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Beek TA, Maas P, King BM, Leclercq E,
Voragen AGJ and De Groot A: Bitter sesquiterpene lactones from
chicory roots. J Agric Food Chem. 38:1035–1038. 1990. View Article : Google Scholar
|
|
28
|
Nwafor IC, Shale K and Achilonu MC:
Chemical composition and nutritive benefits of chicory
(Cichorium intybus) as an ideal complementary and/or
alternative livestock feed supplement. ScientificWorldJournal.
2017:73439282017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aisa HA and Xue-Lei X: Cichorium
glandulosum Bioss. Et Huet (Juju, Chicory)Dietary Chinese
Herbs. Springer, Vienna Pharmacology and Clinical Evidence; pp.
711–720. 2015, View Article : Google Scholar
|
|
30
|
Malarz J, Stojakowska A and Kisiel W:
Long-term cultured hairy roots of chicory-a rich source of
hydroxycinnamates and 8-deoxylactucin glucoside. Appl Biochem
Biotechnol. 171:1589–1601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malarz J, Stojakowska A and Kisiel W:
Sesquiterpene lactones in a hairy root culture of Cichorium
intybus. Z Naturforsch C. 57:994–997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Monde K, Oya T, Takasugi M and Shirata A:
A guaianolide phytoalexin, cichoralexin, from Cichorium
intybus. Phytochemistry. 29:3449–3451. 1990. View Article : Google Scholar
|
|
33
|
Seto M, Miyase T, Umehara K, Ueno A,
Hirano Y and Otani N: Sesquiterpene lactones from Cichorium endivia
L. and C. intybus L. and cytotoxic activity. Chem Pharm Bull
(Tokyo). 36:2423–2429. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Zhao M, Bai L, Hasegawa T, Wang
J, Wang L, Xue H, Deng Q, Xing F, Bai Y, et al: Bioactive
guaianolides from siyekucai (Ixeris chinensis). J Nat Prod.
69:1425–1428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren Y, Zhou Y, Chen X and Ye Y: Discovery,
structural determination and anticancer activities of Lactucin like
guaianolides. Lett Drug Des Discov. 2:444–450. 2005. View Article : Google Scholar
|
|
36
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rüngeler P, Castro V, Mora G, Gören N,
Vichnewski W, Pahl HL, Merfort I and Schmidt TJ: Inhibition of
transcription factor NF-kappaB by sesquiterpene lactones: A
proposed molecular mechanism of action. Bioorg Med Chem.
7:2343–2352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
García-Piñeres AJ, Castro V, Mora G,
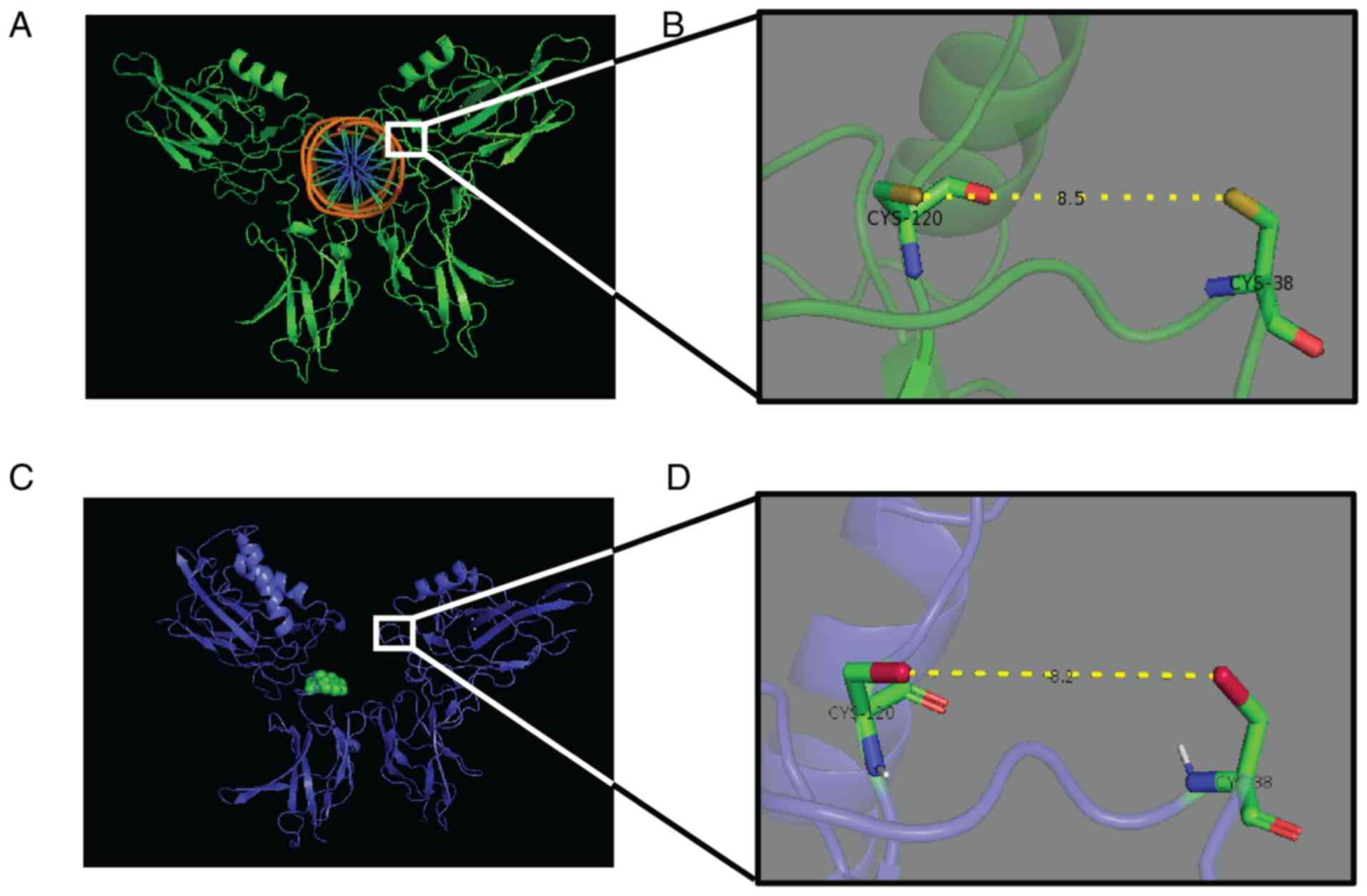
Schmidt TJ, Strunck E, Pahl HL and Merfort I: Cysteine 38 in
p65/NF-kappaB plays a crucial role in DNA binding inhibition by
sesquiterpene lactones. J Biol Chem. 276:39713–39720. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krebsky EO, Geuns JMC and De Proft M:
Polyamines and sterols in Cichorium heads. Phytochemistry.
50:549–553. 1999. View Article : Google Scholar
|
|
40
|
Papetti A, Mascherpa D, Carazzone C,
Stauder M, Spratt DA, Wilson M, Pratten J, Ciric L, Lingström P,
Zaura E, et al: Identification of organic acids in Cichorium
intybus inhibiting virulence-related properties of oral
pathogenic bacteria. Food Chem. 138:1706–1712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amendola R, Cervelli M, Fratini E,
Polticelli F, Sallustio DE and Mariottini P: Spermine metabolism
and anticancer therapy. Curr Cancer Drug Targets. 9:118–130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng B, Bux R and Cheng D:
Spermidine/spermine N1-acetyltransferase antibodies as anti-cancer
drug compounds. US Patent 2016/0017054 A1. Filed January 30 2014;
issued January 21 2016.
|
|
43
|
Kremmer T, Pälyi I, Daubner D, Boldizsár
M, Vincze B, Paulik E, Sugár J, Pokorny E and Túry E: Comparative
studies on the polyamine metabolism and DFMO treatment of MCF-7 and
MDA-MB-231 breast cancer cell lines and xenografts. Anticancer Res.
11:1807–1813. 1991.PubMed/NCBI
|
|
44
|
Lima G and Shiu RP: Role of polyamines in
estradiol-induced growth of human breast cancer cells. Cancer Res.
45:2466–2470. 1985.PubMed/NCBI
|
|
45
|
Pályi I, Kremmer T, Kálnay A, Turi G,
Mihalik R, Bencsik K and Boldizsár M: Effects of methylacetylenic
putrescine, an ornithine decarboxylase inhibitor and potential
novel anticancer agent, on human and mouse cancer cell lines.
Anticancer Drugs. 10:103–111. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kinjo J, Nakano D, Fujioka T and Okabe H:
Screening of promising chemotherapeutic candidates from plants
extracts. J Nat Med. 70:335–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lessard M, Zhao C, Singh SM and Poulin R:
Hormonal and feedback regulation of putrescine and spermidine
transport in human breast cancer cells. J Biol Chem. 270:1685–1694.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pegg AE and McCann PP: Polyamine
metabolism and function. Am J Physiol. 243:C212–C221. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thomas T and Thomas TJ: Polyamines in cell
growth and cell death: Molecular mechanisms and therapeutic
applications. Cell Mol Life Sci. 58:244–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bridle P, Thomas Loeffler RS, Timberlake
CF and Self R: Cyanidin 3-malonylglucoside in Cichorium
intybus. Phytochemistry. 23:2968–2969. 1984. View Article : Google Scholar
|
|
51
|
Tousch D, Lajoix AD, Hosy E, Azay-Milhau
J, Ferrare K, Jahannault C, Cros G and Petit P: Chicoric acid, a
new compound able to enhance insulin release and glucose uptake.
Biochem Biophys Res Commun. 377:131–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nørbaek R, Nielsen K and Kondo T:
Anthocyanins from flowers of Cichorium intybus.
Phytochemistry. 60:357–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kurata R, Adachi M, Yamakawa O and
Yoshimoto M: Growth suppression of human cancer cells by
polyphenolics from sweetpotato (Ipomoea batatas L.) leaves.
J Agric Food Chem. 55:185–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen YJ, Shiao MS, Hsu ML, Tsai TH and
Wang SY: Effect of caffeic acid phenethyl ester, an antioxidant
from propolis, on inducing apoptosis in human leukemic HL-60 cells.
J Agric Food Chem. 49:5615–5619. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kampa M, Alexaki VI, Notas G, Nifli AP,
Nistikaki A, Hatzoglou A, Bakogeorgou E, Kouimtzoglou E, Blekas G,
Boskou D, et al: Antiproliferative and apoptotic effects of
selective phenolic acids on T47D human breast cancer cells:
Potential mechanisms of action. Breast Cancer Res. 6:R63–R74. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dilshara MG, Jayasooriya RG, Park SR, Choi
YH, Choi IW and Kim GY: Caffeic acid phenethyl ester enhances
TRAIL-mediated apoptosis via CHOP-induced death receptor 5
upregulation in hepatocarcinoma Hep3B cells. Mol Cell Biochem.
418:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Búfalo MC, Ferreira I, Costa G, Francisco
V, Liberal J, Cruz MT, Lopes MC, Batista MT and Sforcin JM:
Propolis and its constituent caffeic acid suppress LPS-stimulated
pro-inflammatory response by blocking NF-κB and MAPK activation in
macrophages. J Ethnopharmacol. 149:84–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang MT, Smart RC, Wong CQ and Conney AH:
Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and
ferulic acid on tumor promotion in mouse skin by
12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 48:5941–5946.
1988.PubMed/NCBI
|
|
59
|
Kasai H, Fukada S, Yamaizumi Z, Sugie S
and Mori H: Action of chlorogenic acid in vegetables and fruits as
an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a
rat carcinogenesis model. Food Chem Toxicol. 38:467–471. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Feng R, Lu Y, Bowman LL, Qian Y,
Castranova V and Ding M: Inhibition of activator protein-1,
NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme
activity by chlorogenic acid. J Biol Chem. 280:27888–27895. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang GF, Shi LP, Ren YD, Liu QF, Liu HF,
Zhang RJ, Li Z, Zhu FH, He PL, Tang W, et al: Anti-hepatitis B
virus activity of chlorogenic acid, quinic acid and caffeic acid in
vivo and in vitro. Antiviral Res. 83:186–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hsu CL, Huang SL and Yen GC: Inhibitory
effect of phenolic acids on the proliferation of 3T3-L1
preadipocytes in relation to their antioxidant activity. J Agric
Food Chem. 54:4191–4197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Maki C, Funakoshi-Tago M, Aoyagi R, Ueda
F, Kimura M, Kobata K, Tago K and Tamura H: Coffee extract inhibits
adipogenesis in 3T3-L1 preadipocyes by interrupting insulin
signaling through the downregulation of IRS1. PLoS One.
12:e01732642017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Meng S, Cao J, Feng Q, Peng J and Hu Y:
Roles of chlorogenic acid on regulating glucose and lipids
metabolism: A review. Evid Based Complement Alternat Med.
2013:8014572013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Olthof MR, Hollman PCH and Katan MB:
Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr.
131:66–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
In JK, Kim JK, Oh JS and Seo DW:
5-Caffeoylquinic acid inhibits invasion of non-small cell lung
cancer cells through the inactivation of p70S6K and Akt activity:
Involvement of p53 in differential regulation of signaling
pathways. Int J Oncol. 48:1907–1912. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Apostolou A, Stagos D, Galitsiou E, Spyrou
A, Haroutounian S, Portesis N, Trizoglou I, Wallace Hayes A,
Tsatsakis AM and Kouretas D: Assessment of polyphenolic content,
antioxidant activity, protection against ROS-induced DNA damage and
anticancer activity of Vitis vinifera stem extracts. Food
Chem Toxicol. 61:60–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kirmizibekmez H, Calis I, Perozzo R, Brun
R, Dönmez AA, Linden A, Rüedi P and Tasdemir D: Inhibiting
activities of the secondary metabolites of Phlomis
brunneogaleata against parasitic protozoa and plasmodial
enoyl-ACP Reductase, a crucial enzyme in fatty acid biosynthesis.
Planta Med. 70:711–717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
You Q, Chen F, Ni H, Wang X, Jiang Y and
McCoy JA: HPLC-MS analyses and bioactivities of novel chemicals in
Devil's club (Oplopanax horridus (Sm.) Miq.). Food Chem.
135:199–207. 2012. View Article : Google Scholar
|
|
70
|
Park CM, Jin KS, Lee YW and Song YS:
Luteolin and chicoric acid synergistically inhibited inflammatory
responses via inactivation of PI3K-Akt pathway and impairment of
NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J
Pharmacol. 660:454–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiao H, Wang J, Yuan L, Xiao C, Wang Y and
Liu X: Chicoric acid induces apoptosis in 3T3-L1 preadipocytes
through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric
Food Chem. 61:1509–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huntimer ED, Halaweish FT and Chase CCL:
Proliferative activity of Echinacea angustifolia root
extracts on cancer cells: Interference with doxorubicin
cytotoxicity. Chem Biodivers. 3:695–703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou J, Fang L, Liao J, Li L, Yao W, Xiong
Z and Zhou X: Investigation of the anti-cancer effect of quercetin
on HepG2 cells in vivo. PLoS One. 12:e01728382017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Saleh MR, Metwally AM and Amer MM:
Isolation of a flavonoidal substance from Cichorium pumilum
jacq. Pharmazie. 30:4041975.PubMed/NCBI
|
|
75
|
Chen Z, Liu YM, Yang S, Song BA, Xu GF,
Bhadury PS, Jin LH, Hu DY, Liu F, Xue W and Zhou X: Studies on the
chemical constituents and anticancer activity of Saxifraga
stolonifera (L) Meeb. Bioorg Med Chem. 16:1337–1344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Maiyo FC, Moodley R and Singh M:
Cytotoxicity, antioxidant and apoptosis studies of quercetin-3-O
glucoside and
4-(β-D-glucopyranosyl-1→4-α-L-rhamnopyranosyloxy)-benzyl
isothiocyanate from Moringa oleifera. Anticancer Agents Med Chem.
16:648–656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Panat NA, Amrute KB, Battu S, Haram S,
Sharma G and Ghaskadbi S: Antioxidant profiling of C3 quercetin
glycosides: Quercitrin, quercetin 3-β-D-glucoside and quercetin
3-O-(6″-O-malonyl)-β-D-glucoside in cell free environment. Free Rad
Antiox. 5:90–100. 2015. View Article : Google Scholar
|
|
78
|
Sassi N, Mattarei A, Espina V, Liotta L,
Zoratti M, Paradisi C and Biasutto L: Potential anti-cancer
activity of 7-O-pentyl quercetin: Efficient, membrane-targeted
kinase inhibition and pro-oxidant effect. Pharmacol Res. 124:9–19.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sugantha Priya E, Selvakumar Benny K,
Bavithra S, Elumalai P, Arunkumar R, Raja Singh P, Brindha Mercy A
and Arunakaran J: Anti-cancer activity of quercetin in
neuroblastoma: An in vitro approach. Neurol Sci. 35:163–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Danino O, Gottlieb HE, Grossman S and
Bergman M: Antioxidant activity of 1,3-dicaffeoylquinic acid
isolated from Inula viscosa. Food Res Int. 42:1273–1280.
2009. View Article : Google Scholar
|
|
81
|
Chen PN, Chu SC, Chiou HL, Chiang CL, Yang
SF and Hsieh YS: Cyanidin 3-glucoside and peonidin 3-glucoside
inhibit tumor cell growth and induce apoptosis in vitro and
suppress tumor growth in vivo. Nutr Cancer. 53:232–243. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cho E, Chung EY, Jang HY, Hong OY, Chae
HS, Jeong YJ, Kim SY, Kim BS, Yoo DJ, Kim JS and Park KH:
Anti-cancer effect of cyanidin-3-glucoside from mulberry via
caspase-3 cleavage and DNA fragmentation in vitro and in vivo.
Anticancer Agents Med Chem. 17:1519–1525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lee JS, Kim YR, Song IG, Ha SJ, Kim YE,
Baek NI and Hong EK: Cyanidin-3-glucoside isolated from mulberry
fruit protects pancreatic β-cells against oxidative stress-induced
apoptosis. Int J Mol Med. 35:405–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Meiers S, Kemény M, Weyand U, Gastpar R,
von Angerer E and Marko D: The anthocyanidins cyanidin and
delphinidin are potent inhibitors of the epidermal growth-factor
receptor. J Agric Food Chem. 49:958–962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Srivastava JK and Gupta S: Extraction,
characterization, stability and biological activity of flavonoids
isolated from Chamomile flowers. Mol Cell Pharmacol. 1:1382009.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Smiljkovic M, Stanisavljevic D, Stojkovic
D, Petrovic I, Marjanovic Vicentic J, Popovic J, Golic Grdadolnik
S, Markovic D, Sankovic-Babice S, Glamoclija J, et al:
Apigenin-7-O-glucoside versus apigenin: Insight into the modes of
anticandidal and cytotoxic actions. EXCLI J. 16:795–807.
2017.PubMed/NCBI
|
|
87
|
Xu W, Liu J, Li C, Wu HZ and Liu YW:
Kaempferol-7-O-beta-D-glucoside (KG) isolated from Smilax
china L. rhizome induces G2/M phase arrest and apoptosis on
HeLa cells in a p53-independent manner. Cancer Lett. 264:229–240.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nasri I, Chawech R, Girardi C, Mas E,
Ferrand A, Vergnolle N, Fabre N, Mezghani-Jarraya R and
Racaud-Sultan C: Anti-inflammatory and anticancer effects of
flavonol glycosides from Diplotaxis harra through GSK3β
regulation in intestinal cells. Pharm Biol. 55:124–131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park JY, Kim SI, Lee HJ, Kim SS, Kwon YS
and Chun W: Isorhamnetin-3-O-glucuronide suppresses JNK and p38
activation and increases heme-oxygenase-1 in
lipopolysaccharide-challenged RAW264.7 cells. Drug Dev Res.
77:143–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang X, Luo E, Liu X, Han B, Yu X and Peng
X: Delphinidin-3-glucoside suppresses breast carcinogenesis by
inactivating the Akt/HOTAIR signaling pathway. BMC Cancer.
16:4232016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
He X and Liu RH: Cranberry phytochemicals:
Isolation, structure elucidation, and their antiproliferative and
antioxidant activities. J Agric Food Chem. 54:7069–7074. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ghosh T, Maity T and Singh J: Evaluation
of antitumor activity of stigmasterol, a constituent isolated from
Bacopa monnieri Linn aerial parts against ehrlich ascites
carcinoma in mice. Orient Pharm Exp Med. 11:41–49. 2011. View Article : Google Scholar
|
|
93
|
Kangsamaksin T, Chaithongyot S,
Wootthichairangsan C, Hanchaina R, Tangshewinsirikul C and Svasti
J: Lupeol and stigmasterol suppress tumor angiogenesis and inhibit
cholangiocarcinoma growth in mice via downregulation of tumor
necrosis factor-α. PLoS One. 12:e01896282017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Syed Abdul Rahman SN, Abdul Wahab N and
Abd Malek SN: In vitro morphological assessment of apoptosis
induced by antiproliferative constituents from the rhizomes of
Curcuma zedoaria. Evid Based Complement Alternat Med.
2013:2571082013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Dutra LM, Bomfim LM, Rocha SL, Nepel A,
Soares MB, Barison A, Costa EV and Bezerra DP: ent-Kaurane
diterpenes from the stem bark of Annona vepretorum
(Annonaceae) and cytotoxic evaluation. Bioorg Med Chem Lett.
24:3315–3320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ali H, Dixit S, Ali D, Alqahtani SM,
Alkahtani S and Alarifi S: Isolation and evaluation of anticancer
efficacy of stigmasterol in a mouse model of DMBA-induced skin
carcinoma. Drug Des Devel Ther. 9:2793–2800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Süntar I, Küpeli Akkol E, Keles H,
Yesilada E, Sarker SD and Baykal T: Comparative evaluation of
traditional prescriptions from Cichorium intybus L. for
wound healing: Stepwise isolation of an active component by in vivo
bioassay and its mode of activity. J Ethnopharmacol. 143:299–309.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Woyengo TA, Ramprasath VR and Jones PJ:
Anticancer effects of phytosterols. Eur J Clin Nutr. 63:813–820.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cheng D, Guo Z and Zhang S: Effect of
β-sitosterol on the expression of HPV E6 and p53 in cervical
carcinoma cells. Contemp Oncol (Pozn). 19:36–42. 2015.PubMed/NCBI
|
|
100
|
Awad AB, Chinnam M, Fink CS and Bradford
PG: β-Sitosterol activates Fas signaling in human breast cancer
cells. Phytomedicine. 14:747–754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chai JW, Kuppusamy UR and Kanthimathi MS:
Beta-sitosterol induces apoptosis in MCF7 cells. Malays J Biochem
Mol Biol. 16:28–30. 2008.
|
|
102
|
Wilt TJ, Ishani A, MacDonald R, Stark G,
Mulrow CD and Lau J: Beta-sitosterols for benign prostatic
hyperplasia. Cochrane Database Syst Rev. CD0010432000.PubMed/NCBI
|
|
103
|
Nibret E, Youns M, Krauth-Siegel RL and
Wink M: Biological activities of xanthatin from Xanthium
strumarium leaves. Phytother Res. 25:1883–1890. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ryan E, Chopra J, McCarthy F, Maguire AR
and O'Brien NM: Qualitative and quantitative comparison of the
cytotoxic and apoptotic potential of phytosterol oxidation products
with their corresponding cholesterol oxidation products. Br J Nutr.
94:443–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
O'Callaghan Y, McCarthy FO and O'Brien NM:
Recent advances in phytosterol oxidation products. Biochem Biophys
Res Commun. 446:786–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Oliveira H, Wu N, Zhang Q, Wang J,
Oliveira J, de Freitas V, Mateus N, He J and Fernandes I:
Bioavailability studies and anticancer properties of malvidin based
anthocyanins, pyranoanthocyanins and non-oxonium derivatives. Food
Funct. 7:2462–2468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Amini AM, Spencer JPE and Yaqoob P:
Effects of pelargonidin-3-O-glucoside and its metabolites on
lipopolysaccharide-stimulated cytokine production by THP-1
monocytes and macrophages. Cytokine. 103:29–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Duarte LJ, Chaves VC, Nascimento MVPDS,
Calvete E, Li M, Ciraolo E, Ghigo A, Hirsch E, Simões CMO,
Reginatto FH and Dalmarco EM: Molecular mechanism of action of
Pelargonidin-3-O-glucoside, the main anthocyanin responsible for
the anti-inflammatory effect of strawberry fruits. Food Chem.
247:56–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Seong AR, Yoo JY, Choi K, Lee MH, Lee YH,
Lee J, Jun W, Kim S and Yoon HG: Delphinidin, a specific inhibitor
of histone acetyltransferase, suppresses inflammatory signaling via
prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A
cells. Biochem Biophys Res Commun. 410:581–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang LS and Stoner GD: Anthocyanins and
their role in cancer prevention. Cancer Lett. 269:281–290. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Das AK: Anticancer effect of Antimalarial
artemisinin compounds. Ann Med Health Sci Res. 5:93–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Slezakova S and Ruda-Kucerova J:
Anticancer activity of artemisinin and its derivatives. Anticancer
Res. 37:5995–6003. 2017.PubMed/NCBI
|
|
113
|
Wong YK, Xu C, Kalesh KA, He Y, Lin Q,
Wong WSF, Shen HM and Wang J: Artemisinin as an anticancer drug:
Recent advances in target profiling and mechanisms of action. Med
Res Rev. 37:1492–1517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: From a well-known
antimalarial agent to a potential anticancer drug. J Biomed
Biotechnol. 2012:2475972012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gravett AM, Liu WM, Krishna S, Chan WC,
Haynes RK, Wilson NL and Dalgleish AG: In vitro study of the
anti-cancer effects of artemisone alone or in combination with
other chemotherapeutic agents. Cancer Chemother Pharmacol.
67:569–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Alotaibi KS, Li H, Rafi R and Siddiqui RA:
Papaya black seeds have beneficial anticancer effects on PC-3
prostate cancer cells. J Cancer Metastasis Treat. 3:161–168. 2017.
View Article : Google Scholar
|
|
117
|
Mayer M, O'Neill MA, Murray KE,
Santos-Magalhães NS, Carneiro-Leão AM, Thompson AM and Appleyard
VC: Usnic acid: A non-genotoxic compound with anti-cancer
properties. Anticancer Drugs. 16:805–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Eryilmaz IE, Eskiler GG, Yurdacan B, Egeli
Ü, Çeçener G and Tunca B: The cytotoxic and apoptotic effects of
usnic acid on prostate cancer versus normal cells. Proceedings.
1:10272017. View Article : Google Scholar
|
|
119
|
Yang Y, Nguyen TT, Jeong MH, Crişan F, Yu
YH, Ha HH, Choi KH, Jeong HG, Jeong TC, Lee KY, et al: Inhibitory
activity of (+)-usnic acid against non-small cell lung cancer cell
motility. PLoS One. 11:e01465752016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Couri S, Gomes F, Nogueira R and Almeida
DL: Determination of inulin content of chicory roots (Cichorium
intybus L.) cultivated organically in three regions of Rio de
Janeiro state. 2018.
|
|
121
|
Pool-Zobel B, van Loo J, Rowland I and
Roberfroid MB: Experimental evidences on the potential of prebiotic
fructans to reduce the risk of colon cancer. Br J Nutr. 87 (Suppl
2):S273–S281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Taper HS and Roberfroid MB: Possible
adjuvant cancer therapy by two prebiotics-Inulin or oligofructose.
In Vivo. 19:201–204. 2005.PubMed/NCBI
|
|
123
|
Taper HS and Roberfroid M: Influence of
inulin and oligofructose on breast cancer and tumor growth. J Nutr.
129 (Suppl 7):S1488–S1491. 1999. View Article : Google Scholar
|
|
124
|
Reddy BS, Hamid R and Rao CV: Effect of
dietary oligofructose and inulin on colonic preneoplastic aberrant
crypt foci inhibition. Carcinogenesis. 18:1371–1374. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Epstein JI, Carmichael M and Partin AW:
OA-519 (fatty acid synthase) as an independent predictor of
pathologic state in adenocarcinoma of the prostate. Urology.
45:81–86. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Park MH and Hong JT: Roles of NF-κB in
cancer and inflammatory diseases and their therapeutic approaches.
Cells. 5:E152016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Rasmussen MK, Zamaratskaia G and Ekstrand
B: In vivo effect of dried chicory root (Cichorium intybus
L.) on xenobiotica metabolising cytochrome P450 enzymes in porcine
liver. Toxicol Lett. 200:88–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Rodriguez-Antona C and Ingelman-Sundberg
M: Cytochrome P450 pharmacogenetics and cancer. Oncogene.
25:1679–1691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chang TKH: Activation of pregnane X
receptor (PXR) and constitutive androstane receptor (CAR) by herbal
medicines. AAPS J. 11:590–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zuccato E, Venturi M, Di Leo G, Colombo L,
Bertolo C, Doldi SB and Mussini E: Role of bile acids and metabolic
activity of colonic bacteria in increased risk of colon cancer
after cholecystectomy. Dig Dis Sci. 38:514–519. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Rasmussen MK, Klausen CL and Ekstrand B:
Regulation of cytochrome P450 mRNA expression in primary porcine
hepatocytes by selected secondary plant metabolites from chicory
(Cichorium intybus L.). Food Chem. 146:255–263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Schmidt BM, Ilic N, Poulev A and Raskin I:
Toxicological evaluation of a chicory root extract. Food Chem
Toxicol. 45:1131–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Mali P: Cytotoxicity activities of
chloroform extract of Cichorium intybus seed against HCT-15
and Vero cell line. Int J Health Allied Sci. 4:267–270. 2015.
View Article : Google Scholar
|
|
135
|
Soltanian S, Sheikhbahaei M and Mohamadi
N: Cytotoxicity evaluation of methanol extracts of some medicinal
plants on P19 embryonal carcinoma cells. J Appl Pharm Sci.
7:142–149. 2017.
|
|
136
|
Jiang Y, Kusama K, Satoh K, Takayama E,
Watanabe S and Sakagami H: Induction of cytotoxicity by chlorogenic
acid in human oral tumor cell lines. Phytomedicine. 7:483–491.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Schumacher E, Vigh E, Molnár V, Kenyeres
P, Fehér G, Késmárky G, Tóth K and Garai J: Thrombosis preventive
potential of chicory coffee consumption: A clinical study.
Phytother Res. 25:744–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Olsen NJ, Branch VK, Jonnala G, Seskar M
and Cooper M: Phase 1, placebo-controlled, dose escalation trial of
chicory root extract in patients with osteoarthritis of the hip or
knee. BMC Musculoskelet Disord. 11:1562010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Huseini HF, Alavian SM, Heshmat R, Heydari
MR and Abolmaali K: The efficacy of Liv-52 on liver cirrhotic
patients: A randomized, double-blind, placebo-controlled first
approach. Phytomedicine. 12:619–624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Soda K, Dobashi Y, Kano Y, Tsujinaka S and
Konishi F: Polyamine-rich food decreases age-associated pathology
and mortality in aged mice. Exp Gerontol. 44:727–732. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kiechl S, Pechlaner R, Willeit P,
Notdurfter M, Paulweber B, Willeit K, Werner P, Ruckenstuhl C,
Iglseder B, Weger S, et al: Higher spermidine intake is linked to
lower mortality: A prospective population-based study. Am J Clin
Nutr. 108:371–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Pietrocola F, Castoldi F, Kepp O,
Carmona-Gutierrez D, Madeo F and Kroemer G: Spermidine reduces
cancer-related mortality in humans. Autophagy. 15:362–365. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Raj KP, Zell JA, Rock CL, McLaren CE,
Zoumas-Morse C, Gerner EW and Meyskens FL: Role of dietary
polyamines in a phase III clinical trial of difluoromethylornithine
(DFMO) and sulindac for prevention of sporadic colorectal adenomas.
Br J Cancer. 108:512–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Imam KMSU, Azam FMS, Jahan R and
Rahmatullah M: Anticancer properties of anthocyanins: A
reviewNatural Products: Research Reviews. Gupta VK: 4. Daya
Publishing House; pp. 1–20. 2016
|
|
145
|
Klippel KF, Hiltl DM and Schipp B: A
multicentric, placebo- controlled, double-blind clinical trial of
beta-sitosterol (phytosterol) for the treatment of benign prostatic
hyperplasia. German BPH-Phyto Study group. Br J Urol. 80:427–432.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Garcia-Peris P, Velasco C, Hernandez M,
Lozano MA, Paron L, de la Cuerda C, Breton I, Camblor M and Guarner
F: Effect of inulin and fructo-oligosaccharide on the prevention of
acute radiation enteritis in patients with gynecological cancer and
impact on quality-of-life: A randomized, double-blind,
placebo-controlled trial. Eur J Clin Nurtr. 70:170–174. 2016.
View Article : Google Scholar
|
|
147
|
Rosa LS, Silva NJA, Soares NCP, Monteiro
MC and Teodoro AJ: Anticancer properties of phenolic acids in colon
cancer a review. J Nutr Food Sci. 6:4682016.
|