Introduction
Cichorium intybus L. is a perennial
herbaceous plant and is one of the six members of the genus
Cichorium under family Asteraceae. Although it is a native
weed from Europe, C. intybus has been naturalized in
different parts of the world including Africa, temperate and
tropical Asia, Europe, Australia, North and South America (1,2). Its leaf
is commonly used as a vegetable and powdered root as a coffee
substitute; the whole plant is also used for animal forage
(3). C. intybus L. has been
used as a common medicinal herb worldwide in different
civilizations. Ancient Egyptians (3)
and Romans (mentioned by ‘Horace’ in ‘Odes’) used C. intybus
as a medicinal plant. However, local nomenclature has vast
differences, and so do the medicinal uses. Ethnomedicinal use of
C. intybus covers diarrhea, liver diseases, prostate and
reproductive organ disorder, pulmonary disease, cough, malaria and
cancer (1). The recent use of C.
intybus as an alternate for coffee has led to its commercial
cultivation. C. intybus is also currently cultivated as a
major source of inulin, a dietary fiber fructan (4).
As reviewed in the following sections, several
studies have shown that phytochemicals may be a reliable source of
compounds with therapeutic benefits. For example, many drugs
derived from phytochemicals and their derivatives have shown
promise and utility in tumor treatment. C. intybus contains
diverse types of phytochemicals such as guaianolides,
6-methoxyflavone, eudesmanolides, germacranolides, polyacetylene,
sterol, anthocyanin, delphinidin, 3,4-dihydroxyphenethyl and other
novel compounds in different quantities. Fractionated and purified
extracts of C. intybus have been the subject of many
studies. Many researchers have reported the therapeutic properties
of C. intybus compounds, on in vitro as well as in
vivo models of tumors.
C. intybus phytochemicals have shown tissue-
and tumor type-specific antitumor activity, which indicates against
indiscriminate cytotoxicity and instead suggests the presence of a
well-regulated mechanism. In this review, we summarize C.
intybus derived phytochemicals with reported antitumor
properties from traditional and pharmacological trials. We also
review their cell specificities and antitumor mechanisms.
Literature search method
We used PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Google Scholar
(https://scholar.google.com), and Baidu
Xueshu (http://xueshu.baidu.com) scientific
article search engines as well as PubChem (https://pubchem.ncbi.nlm.nih.gov/), and U.S. National
Library of Medicine (https://clinicaltrials.gov/) for the preliminary
search. We searched for articles published between 1975 to 2018.
Keywords such as chicory, Cichorium intybus, phytochemicals,
phytometabolites, anticancer, antitumor, cytotoxic, and clinical
trial were used individually or in combination to search for
antitumor phytometabolites of C. intybus. Articles for each
metabolite and its activity were searched individually within the
same time frame. Chemical structures of metabolites were sourced
primarily from research articles and using ChemDraw search and then
confirmed from PubChem and Chem Spider databases. Chemical
structures were drawn with ChemDraw according to journal
guidelines. Illustrations were drawn in combination with ChemDraw
and Adobe Illustrator.
Antitumor activity of the
phytochemicals
The medicinal properties of C. intybus L. led
to many successful investigations to identify its phytochemical
constituents. C. intybus is used in a variety of illnesses,
and the phytochemicals derived from it are also diverse. Overall,
our analysis revealed 87 reported phytochemicals, of which
Carazzone et al (5) identified
63; Street et al (3) reported
84; and Malik et al (6) found
78. Several other studies also reported some of these
phytochemicals. Most of these compounds show bioactivity, and some
have multiple pharmacological properties. However, in the present
review, we focus on the phytochemicals with reported antitumor
properties, as listed in Table I.
 | Table I.Cichorium intybus L.-derived
phytochemicals that exert antitumor properties. |
Table I.
Cichorium intybus L.-derived
phytochemicals that exert antitumor properties.
| Phytochemicals | Chemical
structure | Cell lines | (Refs.) |
|---|
| Jacquilenin |  | Lung cancer WI38,
VA13, A549, HepG2 cells | (34) |
|
11,13-Dihydrolactucopicrin |  | Nasopharyngeal
cancer KB cell, liver cancer Bel 7402 cell | (35) |
| Putrescine |  | Breast cancer
MDA-MB-231 and T-47D cells | (43–45) |
| Spermidine |  | Bone cancer U-2 OS
cell, cervical cancer HeLa cell, skin cancer Malme-3M cell,
prostate cancer PC-3 and 293 cells | (41,42) |
| Caffeic acid |  | Mammary duct
carcinoma T-47D cell, promyelocytic leukemia HL-60 cell, liver
carcinoma Hep-3B cell | (54–56,58) |
| Chlorogenic
acid |  | Hepatoblastoma
Hep-G2/2.2.15 cell | (61) |
|
| Mouse preadipocyte:
3T3-L1 cell | (62,63) |
|
| Rat insulinoma
INS-1E cell | (64) |
| 5-Caffeoylquinic
acid |  | Lung cancer
(non-small cell): H1299 cells | (66) |
| Chicoric acid |  | Mouse preadipocyte
3T3-L1 cells | (71) |
|
| Cervical cancer
HeLa cell line, breast cancer MCF-7 cells | (72) |
|
Trans-caftaric acid |  | Liver cancer HepG2
cells, cervical cancer HeLa cells | (67) |
| 5-Caffeoylshikimic
acid |  | Rat skeletal
myoblasts L6 cells | (68) |
| Quercetin
3-O-β-D-glucoside |  | Liver cancer HepG2
cells, colon cancer Caco-2 and 293 cells | (76) |
|
| Gastric BGC-82
cells | (75) |
|
1,3-Dicaffeoylquinic acid |  | Colon cancer DLD-1
cells | (53,147) |
|
3,4-Dicaffeoylquinic acid |  | Stomach cancer:
Kato III cells, colon cancer DLD-1 cells, promyelocytic leukemia
HL-60 cells | (53) |
|
Quercetin-7-O-galactoside |  | Liver cancer HepG2
cells | (73) |
|
| Mouse neuroblastoma
N2a cells | (79) |
|
Quercetin-3-O-(6-O-malonyl)-glucoside |  | Not determined |
|
|
Cyanidin-3-O-galactoside |  | Vulva carcinoma
A431 cells | (84) |
|
Cyanidin-3-O-glucoside |  | Lung carcinoma LLC
cells | (81) |
|
| Pancreatic β-cell
MIN6 | (82,83) |
|
| Breast cancer
HS578T and MDA-MB-453 cells | (83) |
|
Apigenin-7-O-glucoside |  | Colon cancer HCT116
cells | (85) |
|
| Prostate cancer
PC-3 cells | (86) |
|
Kaempferol-7-O-glucoside |  | Cervical cancer
HeLa cells | (87) |
| Delphinidin
3,5-di-O-(6-O-malonyl-β-D-glucoside) |  | Breast epithelial
MCF10A cells | (90) |
|
Malvidin-3-O-glucoside |  | Breast cancer MCF-7
cells | (106) |
|
| Vulva carcinoma
A431 cells | (84) |
|
Pelargonidin-3-O-monoglucuronide |  | Human monocytic
leukemia THP-1 cells | (107) |
|
Artesin/Artemisinin |  | Colon cancer HCT116
and SW480 cells, leukemia HL-60 cells, breast cancer MCF-7 cells,
melanoma KM, MJT3 cells, lung cancer (non-small cell) NSCLC cells,
pancreas PANC-1 and MIAPaCa cells, glioma cancer U87MG and A172
cells | (113–115) |
| β-sitosterol |  | Cervical cancer
HeLa cells | (99) |
|
| Intrahepatic
cholangiocarcinoma KKU-M213 and RMCCA-1 cells, immortalized normal
cholangiocytes MMNK-1 | (93) |
|
| Breast cancer MCF-7
cells | (100,101) |
|
β-sitosterol-3-O-glucoside |  | Breast cancer MCF-7
cells | (91) |
|
| Leukemia HL-60
cells, liver cancer Hep G2 cells | (103) |
| Campesterol |  | Liver cancer HepG2
cells, breast cancer MCF-7 cells | (91) |
| Stigmasterol |  | Intrahepatic
cholangiocarcinoma KKU-M213 and RMCCA-1 cells, immortalized normal
cholangiocytes MMNK-1 cells Breast cancer MCF-7 cells,
cervical | (93) |
|
| carcinoma Ca Ski
cells, colon cancer HCT-116 cells | (94) |
|
| Chronic myelogenous
leukemia K-562 cells | (95) |
| 3-O-p-Coumaroyl
quinic acid |  | Prostate cancer
PC-3 cells, mouse preadipocyte 3T3L1 cells | (116) |
| 4-O-feruloylquinic
acid |  | Colon cancer HT-29
cells | (69) |
| Usnic acid |  | Breast cancer MCF-7
and MDA-MB-231 cells, lung cancer H1299 cells, prostate cancer
LNCaP cells | (117,118) |
| Inulin |  | Transplantable
liver tumor TLT cells, mouse mammary carcinoma EMT6 cells | (121–123) |
Reactive oxygen species (ROS) are highly reactive
radicals, ions or molecules with a single unpaired electron in
their outermost shell. Recent studies have shown that ROS
contribute to several diseases and disorders (6) including chronic inflammation and a wide
variety of different cancers (7). ROS
are categorized into two broad types: Free oxygen radicals such as
superoxide, hydroxyl radical, nitric oxide, organic radicals,
peroxyl radicals, alkoxyl radicals, thiyl radicals, disulfides,
sulfonyl radicals, and thiyl peroxyl radicals; non-radical ROS such
as hydrogen peroxide, singlet oxygen, ozone/trioxygen, organic
hydroperoxides, hypochlorite, peroxynitrite, nitrosoperoxycarbonate
anion, nitrocarbonate anion, dinitrogen dioxide, nitronium, and
highly reactive lipid- or carbohydrate-derived carbonyl compounds
(8). ROS are produced as an
inevitable byproduct of cellular processes such as mitochondrial
oxidative phosphorylation (8) and
play vital roles in the stimulation of cell signaling pathways in
response to intracellular and extracellular changes (7). In almost all cancers, the ROS
concentration is elevated. However, to survive, cancer cells
produce antioxidant proteins to detoxify ROS (8,9). In cancer
cells, the ROS elevation can be caused by mitochondrial
dysfunction, peroxisome activity, oncogene activity, increased
metabolism, increased cellular receptor signaling, increased
activity of oxidases, cyclooxygenases, lipoxygenases, and thymidine
phosphorylase, as well as through crosstalk with infiltrating
immune cells (8,10). The difference between ROS and
antioxidant levels creates oxidative stress. Free radicals produced
by oxidative stress alter macromolecules such as DNA, proteins, and
lipids, and thus play a significant role in inducing carcinogenesis
(7,10).
These altered macromolecules, unable to perform
their function, can hinder cell growth and even cause death.
However, a dysfunctional cell divisional mechanism causes
uncontrolled cell division. For example, ROS can upregulate cyclin
mRNA levels including cyclin B2, cyclin D3, cyclin E1, and cyclin
E2; resulting in fast transition from the G1 to S phase of the cell
cycle (8). The uncontrolled cell
growth results in a cell mass called a tumor. Afterward, these
tumors, unable to support their growth, create new blood vessels
around them, a process known as angiogenesis. These blood vessels
facilitate growth of the tumors to a point after which cancerous
cells start to detach from the malignant tumor and migrate through
blood vessels to other tissues of the body known as metastasis
(11). ROS can promote tumor cell
metastasis by decreasing extracellular matrix anchorage or
increasing vascular permeability (8).
These transformed cell-containing organs are unable to function
properly leading to organ failure and death (11). To be considered as an antitumor drug,
the therapeutic agent should have the following qualities of
counteracting tumorigenesis: i) counteract ROS; ii) counteract the
oxidative stress caused by ROS; iii) prevent angiogenesis; iv)
prevent the metastasis of cancerous cells; and v) selective
cytotoxicity towards cancerous cells to induce apoptosis.
To be considered as a therapeutic agent, the
compound must also show fewer side effects compared with existing
antitumor drugs. The present review also describes the possibility
of using C. intybus phytochemicals and their derivatives as
potent tissue-specific antitumor agents.
In vitro studies have revealed the antitumor
activities of whole and fractionated extracts of C. intybus
and its parts with different solvents. A 100 µg/ml hydroalcoholic
extract of C. intybus leaf was found to be significantly
effective against a prostate cancer cell line (LNCaP; percent of
inhibition: 3.67±0.12) and a root extract was found to be
significantly effective against breast cancer cells (MCF-7),
amelanotic melanoma cells (C32) and renal adenocarcinoma cells
(ACHN) (percent of inhibition: 12.65±0.26, 30.78±0.75, 14.93±0.29,
respectively) (12). A whole plant
extract fed to a mouse carcinoma model (dimethyl hydrazine-induced)
showed lower expression of natural interferon α (INF-α), and B-cell
lymphoma 2 (Bcl-2) and higher expression of interleukin (IL-12 and
IL-4), confirming the antitumor property (13). A methanol extract of root from C.
endivia, a related plant, was found to inhibit the growth of
breast cancer cells (MCF-7; IC50: 401 µg/ml) in
vitro (14), and a whole plant
water extract inhibited tumor growth in a colorectal cancer mouse
model in situ (200 mg/kg body weight) (15). The antitumor activity of a whole
ethanolic extract of C. intybus root was demonstrated in an
Ehrlich ascites carcinoma (EAC) mouse model, resulting in a 70%
increase in lifespan with 500 mg/kg/day treatment (16). A comparative study between different
plants showed that C. intybus seed water extract moderately
reduced the development and colony formation in PC-3 prostate
cancer cells (2–30%), T47D breast carcinoma cells (2–21%), and RKO
colon cancer cells (6–26%) in vitro (17). A methanolic extract of C.
intybus decreased the viability of breast cancer cells (SKBR3)
in a time-dependent manner with an IC50 of 800, 400 and
300 µg/ml at 24, 48, and 72 h treatment, respectively (18). The n-hexane extract of the aerial part
demonstrated significant antiproliferative (70% at 100 µg/ml) as
well as cytotoxic activity (50.3% at 100 µg/ml) against lymphocytic
leukemia Jurkat cells (19). In
another study, comparing the aerial part's methanolic extract of
different plants on five cancer cell lines, C. intybus
efficiently inhibited Jurkat cell growth (IC50 of 138
µg/ml), and moderately inhibited bladder carcinoma cell (Fen), and
cervical epithelioid carcinoma cell (HeLa) growth (25% decrease at
200 µg/ml), but had no inhibitory effect on myelogenous leukemia
cells (K562) (20). Here we describe
C. intybus-derived phytochemicals with the reported
antitumor, anticancer or antiproliferative properties.
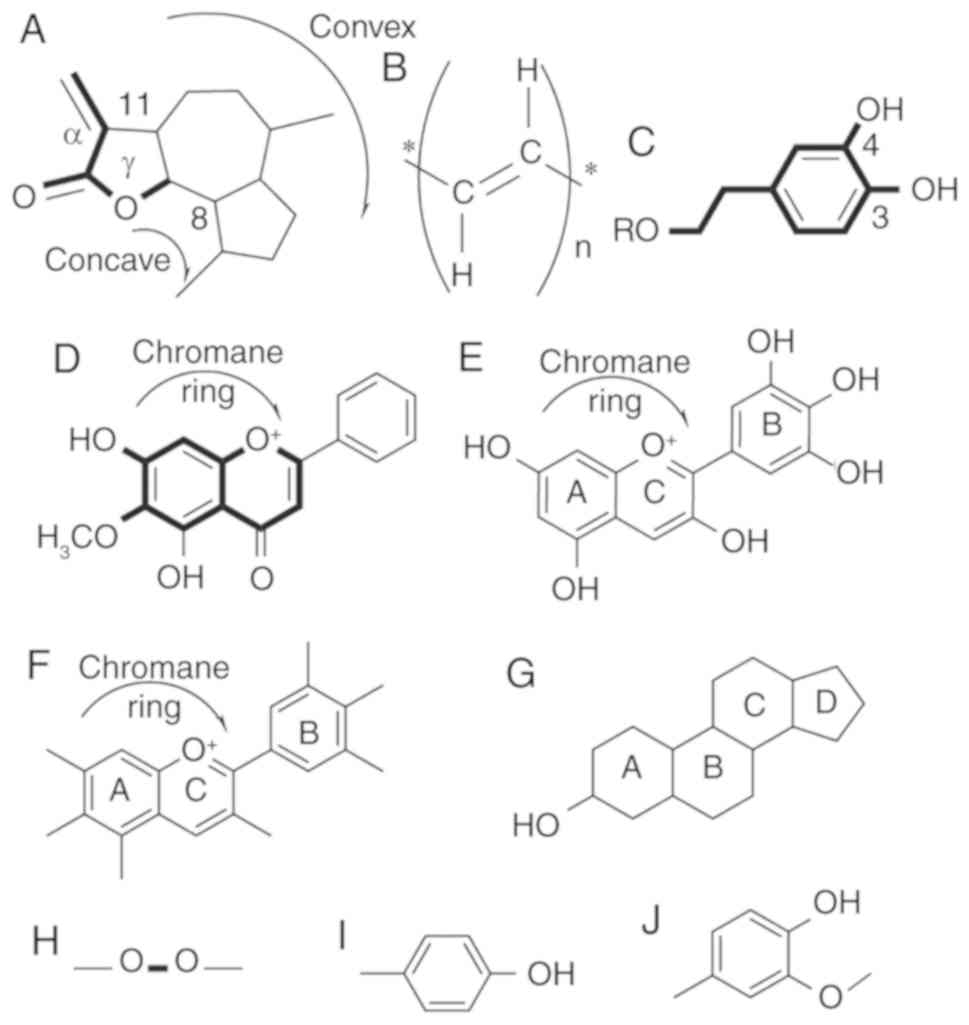
Guaianolides
Sixteen guaianolides have been identified in C.
intybus L. (Fig. 1A) (21–33) of
which only two, 13-dihydro-8-deoxylactucin (jacquilenin) and
11,13-dihydrolactucopicrin, have chemotherapeutic properties. Both
were isolated from a fractionated ethanol extract of leaves through
a combination of column, thin layer chromatography, and HPLC
(23). Leclercq (21) and Van Beek et al (27) also purified 11,13-dihydrolactucopicrin
from C. intybus root methanolic extract. Jacquilenin showed
inhibitory activity on the induction of ICAM-1 induced by IL-1α and
TNF-α in alveolar basal epithelial adenocarcinoma cells (A549;
IC50 values of 16.1 and 20.1 µM for IL-1α and TNF-α,
respectively) and cytotoxicity against human lung fibroblast cells
(WI38 and VA13; IC50 values of 2.7 and 8.5 µM,
respectively) and hepatocellular carcinoma cells (HepG2;
IC50 of 25 µM) in vitro (34). Isolated 11,13-dihydrolactucopicrin
from Mulgedium tatarica by Ren et al (35) demonstrated antitumor activity in human
nasopharyngeal cancer cells (KB; IC50 of 22 µM) and
human liver cancer cells (Bel 7402; IC50 of 30 µM).
Structural activity relationship (SAR) studies by
Ren et al (35) revealed that
the position 8 ester group (γ-butyrolactone) and the methylene
group at exocyclic position 11 (α) (Fig.
1A) play a major role in antitumor activities of lactucin-like
guaianolides (35).
α-methylene-γ-lactone, the ‘-enone’ or unsaturated carbonyl
(O=C-C=CH2) system was found to increase the toxicity
towards tumor cells (36). Both
reported chemoprotective guaianolides in C. intybus
(Jacquilenin and 11,13-dihydrolactucopicrin) share these features,
confirming the structural basis of the activity. Further studies
have shown that this structural motif works as a monofunctional
alkylate and selectively deactivates the p65 dimer of NF-κB
preventing NF-κB transcription in treated cells (37,38). This
targeting of specific signaling pathways is also observed with
other metabolites.
Polyacetylenes. C. intybus contains five
polyacetylenes (Fig. 1B) (21,23,26,31,39,40).
Putrescine and spermidine extracted from leaves by Krebsky et
al (39) show potency as
antitumor agents (41–45). Putrescine is the precursor of
spermidine, and both are ubiquitous constituents of eukaryotic
cells (41). Their cellular
concentration is associated with the effectiveness of many
anticancer therapeutics. The in vitro antitumor activity of
putrescine has been demonstrated in breast cancer cell line
MDA-MB-231 (IC50 of 110 µg/ml) (43,45), and
it showed antiproliferative effect on T-47D breast cancer cells
(0.1 mM) (44). Inhibition of
proliferation of human alveolar basal epithelial adenocarcinoma
cells (A549; IC50 of 4.54 µg/ml), prostate
adenocarcinoma cells (LNCaP; IC50 of 1.1 µg/ml), breast
epithelial cancer cells (T47D; IC50 of 0.97 µg/ml), bone
osteosarcoma cells (U2-OS, IC50 of 4.47 µg/ml), HeLa
cells, skin fibroblast cells (Malme-3M), prostate cancer cells
(PC-3) and embryonic kidney cells (HEK-293) was demonstrated by
Cheng et al (42). Putrescine
and spermidine were found to be able to cross the cell membrane by
a single unique channel or by separate ones (41). The higher lipophilicity of
polyacetylene compounds facilitates plasma membrane penetration but
often lowers their bioavailability in vivo (36). A comparison between different
plant-derived polyacetylenes by Kinjo et al (46) suggested an inverse relationship of the
presence of bulky side chains (hydroxy, methoxy, amino or other
groups) with activity and effectiveness. The authors also found
that C. intybus polyacetylenes were more effective against
MK-1 cells than HeLa and B16F10 cells, and their antiproliferative
effects were more profound than other chemoprotective
characteristics (46). In
estrogen-responsive breast cancer cells (ZR-75-1), both putrescine
and spermidine were internalized via the same or similar
transporter and both Vmax was rapidly upregulated by
estrogens and insulin (47). The
presence of positively charged primary and secondary amino groups
(at physiologic pH) and hydrophobic methylene bridging groups
indicate their capability of acting as ligands at multiple
locations of DNA, RNA, proteins, phospholipids and nucleotide
triphosphate. Few of these connections are electrostatic and
replaced easily by inorganic cations. The rest are specific to the
extent of the aliphatic carbon chain (Fig. 1B) (48,49).
Although polyamines are critical for cell proliferation, their high
concentration can lead to interruption in transcriptions and
protein-protein interactions, and thus inhibition of tumor
growth.
3,4-Dihydroxyphenethyl
Phytometabolites containing a 3,4-dihydroxyphenethyl
group (Fig. 1C) are the most frequent
type of metabolites found in C. intybus. Furthermore, 19 of
the 28 phytometabolites (5,24,50–52) were
found to exhibit antitumor properties. Major members of this group
include hydroxycinnamic acids, quercetins, kaempferols, and
cyanidins, among others.
Among the hydroxycinnamic acids, chlorogenic acid
and caffeic acid have both been extracted from C. intybus
hairy root (Agrobacterium rhizogenes-induced) culture
(24) and leaves [MeOH-HCOOH (99:1,
v/v) extract] (5,24,51).
Caffeic acid is highly effective against human promyelocytic
leukemia cells (HL-60; 90% inhibition at 10 µM) (53,54),
mammary duct carcinoma cells (T-47D; IC50 of
2.17×10−9 M) (55) and
liver carcinoma cells (Hep3B) (56).
It also demonstrated high antioxidant as well as anti-inflammatory
activity (57). Caffeic acid was
found to be moderately effective against epidermal DNA synthesis,
epidermal ornithine decarboxylase activity, and skin tumors induced
by 12-O-tetradecanoylphorbol-13-acetate (TPA;
IC50 of 72.3 µM) (58).
Chlorogenic acid showed in vivo inhibitory effects on
8-hydroxydeoxyguanosine (8-OH-dG) formation induced by lipid
peroxides in 4-nitroquinoline-1-oxide (an oxygen radical-forming
carcinogen)-treated animal tongue, but not the endogenous 8-OH-dG
(59). Chlorogenic acid was found to
protect against environmental carcinogen-induced carcinogenesis
(60). An in vitro study also
demonstrated the antiviral potency of chlorogenic acid in human
hepatoblastoma cells (Hep-G2.2.15; IC50 >1,000 µM)
(61), antitumor potency against
mouse preadipocyte cells (3T3-L1; IC50 of 72.3 µM)
(62,63), and increased insulin secretion in rat
insulinoma cells (INS-1E) (64).
Caffeic acid contains both phenolic (Fig.
1I) and acrylic functional groups. The amount of absorption in
the small intestine (most of the caffeic acid and one-third of the
chlorogenic acid) indicates that most chlorogenic acid reaches the
colon and only a fraction enters the blood circulation (65).
5-Caffeoylquinic acid, 5-caffeoylshikimic acid,
di-caffeoyl tartaric acid (also called chicoric acid),
trans-caftaric acid and 4-O-feruloylquinic acid have been
purified from C. intybus leave MeOH-HCOOH (99:1, v/v)
extract (5). A previous study showed
that 5-caffeoylquinic acid inhibited non-small cell lung cancer
cell (H1299) invasion (66).
Trans-caftaric acid has antioxidant properties and protects
against DNA damage caused by ROS. A trans-caftaric acid rich
extract was found to demonstrate cytotoxicity in HepG2 (liver
cancer cells; IC50 of 50±12 µg/ml) and HeLa cells
(IC50 of 32±16 µg/ml) (67). 5-Caffeoylshikimic acid displayed
antioxidant and antitumor properties on rat skeletal myoblast (L6;
IC50 of 90 µg/ml) cells (68). A 4-O-feruloylquinic acid rich extract
from Oplopanax horridus (Sm.) Miq. exhibited
anti-proliferative effects against human colon adenocarcinoma cells
(HT-29; 56.5% inhibition with a 0.2 mg/ml extract) (69). Chicoric acid, in synergy with luteolin
was found to act as an anti-oxidant and anti-inflammatory agent in
mouse macrophage cells (RAW 264.7; luteolin:chicoric acid =1:1,
1:2, 1:4 where IC50 of luteolin was 11.6, 9.8 and 9.8
µM, respectively) (70). Chicoric
acid caused the apoptosis of mouse 3T3-L1 preadipocytes (71) and displayed antiproliferative activity
in MCF-7 cells but promoted the proliferation of the HeLa cell line
(72).
Among the eight quercetins (a polyphenolic flavonoid
compound) found in C. intybus, six were found to inhibit the
growth of several malignant tumors (73). Both leaf and flower extracts of C.
intybus contain quercetin 3-O-β-D-glucoside (52,74), which
promoted the apoptosis of human gastric carcinoma cells (BGC-823;
12.1±0.03% inhibition at 100 µM) (75), HepG2 (IC50 of 150 µg/ml),
Caco-2 (IC50 of 79 µg/ml), HEK-293 (IC50 of
186 µg/ml) cells (76).
Quercetin-7-O-galactoside, quercetin-3-O-(6″-O-malonyl)-glucoside,
quercetin-7-O-glucoside, quercetin-7-O-glucuronide,
quercetin-7-O-(6″-O-acetyl)-glucoside,
quercetin-7-O-p-coumaroylglucoside, and
quercetin-3-O-glucuronide-7-O-(6″-O-malonyl)-glucoside have been
extracted from the leaf (5). Most of
the compounds containing 3,4-dihydroxyphenethyl are potent
antioxidant and specific inhibitors of NF-κB and Akt. The
functionality of quercetin depends largely on the positioning of
glycosylation and derivatization of a sugar molecule (77). With the different glycation sites,
glucoside derivatives of quercetin show ex-vivo
2,2-diphenyl-1-picrylhydrazyl (DPPH) and
3-ethylbenzothiazoline-6-sulphonic acid (ABTS) free radical
scavenging capacity at a varying degree (77). Quercetin isomers with a pentyl group
in 7 positions were found to significantly inhibit CT26 cell
proliferation (78). Quercetin
hydrate demonstrated cytotoxicity against a human liver cancer cell
line (HepG2) (73) and demonstrated
antitumor activity against N2a, a mouse neuroblastoma cell line
whereas C3 quercetin showed excellent antioxidant property in
ex-vivo trials (77,79).
Leaf extract from C. intybus also yields
phytochemicals such as 1,3-dicaffeoylquinic acid (5), 3,4-dicaffeoylquinic acid (5), cyanidin-3-O-galactoside (5) and cyanidin-3-O-glucoside (50). 1,3-Dicaffeoylquinic acid was found to
exhibit antioxidant properties and inhibit oxidative damage created
by FeSO4 and AAPH [2,20-azobis(2-amidinopropane)
dihydrochloride] and scavenge ROS. 1,3-Dicaffeoylquinic acid is
required in low concentrations than other known antioxidants
(80). 3,4-Dicaffeoylquinic acid
inhibited the growth of Kato III (human stomach cancer; 20–40%
inhibition at 100–500 µM), DLD-1 (20–40% inhibition at 1,000 µM)
(53) and HL-60 (40–90% inhibition at
1,000 µM) cells in vitro (53). Cyanidin-3-O-glucoside inhibited tumor
cell growth, induced apoptosis in vitro, and suppressed
tumor growth in vivo (81). It
protected MIN6 (pancreatic β-cells; cell viability 86.6% at 200
µg/ml) cells against apoptosis induced by oxidative stress
(82), and showed dose-dependent
growth inhibition on tumors derived from HS578T, LLC (Lewis lung
carcinoma) (81) and MDA-MB-453
(human breast cancer) cells in a xenografted animal model (83). Cyanidin-3-O-galactoside inhibited the
development of the A431 cell line (human vulva carcinoma;
IC50 >100 µM) (84).
6-Methoxyflavone
The second largest group of phytochemicals is the
6-methoxyflavone (also known as 6-hydroxyflavone) group (Fig. 1D), with 18 members (5,52). Six
have shown antitumor properties. Among them,
apigenin-7-O-glucoside, kaempferol-7-O-glucoside,
isorhamnetin-7-O-glucoside, and isorhamnetin-7-O-glucuronide have
been isolated from leaf extract (5).
Apigenin-7-O-glucoside was found to be cytotoxic against colon
cancer cells (HCT116) (85),
androgen-refractory PC-3 cells, and other cancer cell lines
(86). Interestingly, there are 11
known isomers of kaempferols in C. intybus, but only
kaempferol-7-O-glucoside was found to induce G2/M mitotic phase
arrest and cell death in a p53-independent manner in HeLa cells
(87). Among the three derivatives of
isorhamnetin (a 6-O-methylated flavanol) (5), only C7 glucoside and glucuronide isomers
were demonstrated to exert antitumor properties (88,89). In
lipopolysaccharide-challenged mouse Abelson murine leukemia
virus-transformed macrophage cells (RAW264.7),
isorhamnetin-3-O-glucuronide increased heme-oxygenase-1 but
suppressed p38 and c-Jun N-terminal kinase (JNK) activation
(89). This indicates that
derivatives of isorhamnetin from C. intybus can be modified
to generate chemoprotective agents. Among four delphinidins
isolated by Nørbaek et al (52) from C. intybus flower extract,
only delphinidin 3,5-di-O-(6-O-malonyl-β-D-glucoside) exerted an
antitumor effect, and the mono sugar substitute was effective
against breast epithelial cells (MCF10A) (90).
Another study showed that 6-methoxyflavone (Fig. 1D) was associated with HeLa cell growth
inhibition (46). However, the
precise mechanism is not yet known.
Phytosterol. C. intybus contains four
phytosterols (Fig. 1G), all with
chemoprotective properties. Campesterol and stigmasterol are two of
the major phytosterols found in the leaves (39). Campesterol shows antitumor and
antiproliferative activity against HepG2 and MCF-7 cells (91). Stigmasterol was found to exhibit
antitumor activity against EAC in swiss albino mice (92). The cytotoxicity of stigmasterol was
demonstrated by Kangsamaksin et al (93) in KKU-M2123, RMCCA-1 and MMNK-1 cell
lines; by Syed Abdul Rahman et al (94) in MCF-7, CaSki (cervical carcinoma),
and HCT-116 cell lines; and by Dutra et al (95) in a chronic myelogenous leukemia cell
line (K-562). In vivo, stigmasterol showed antitumor
efficacy against a 1,3-dimethylbutylamine-induced skin carcinoma
mouse model (96) and antioxidant
activity against EAC in swiss albino mice (92). β-sitosterol is the most abundant
phytosterol, present in leaves (39),
roots (97), and total areal part
extract (26). β-sitosterol increased
the activity of antioxidant enzymes, glutathione peroxidase, and
superoxide dismutase in cultured macrophage cells with phorbol
12-myristate 13-acetate-induced oxidative stress. This indicates
that phytosterols can protect cells from ROS induced damage
(98). In in vitro
experiments, β-sitosterol was found to be cytotoxic to HeLa
(99), intrahepatic
cholangiocarcinoma (KKU-M213, RMCCA-1) (93), immortalized normal cholangiocyte
(MMNK-1) (93), and breast cancer
cells (MCF-7) (100,101). In in vivo experiments,
β-sitosterol reduced tumor growth in 17 β-estradiol-treated mice
(98). A nonmalignant enlargement of
the prostate known as benign prostatic hyperplasia (BPH) was
reduced by β-sitosterol treatment (102). β-sitosterol was also found to
inhibit the proliferation and thus reduce the viability of mouse
fibrosarcoma (98).
β-sitosterol-3-O-glucoside from the areal part of C. intybus
isolated by Satmbekova et al (26) exerted an anticancer effect against
three cancer cell lines, MCF-7, HL-60 and HepG2 (91,103).
The phytosterols containing an unsaturated ring
structure (Fig. 1G) are susceptible
to oxidation under certain conditions. Comparison of the
corresponding phytosterol and cholesterol oxidation-products (POP)
in four cell lines demonstrated that phytosterol induces
oxidation-independent apoptosis (104), which is in contrast to a previous
report by O'Callaghan et al (105). These authors suggested that POP
induced apoptosis by high oxidative stress, glutathione reduction,
mitochondrial dysregulation and elevated caspase activity (105). From Table
I, we can see that phytosterols show specificity towards fatty
tissue-related cancer lines such as those derived from breast,
cholangiocyte, and cervix cancer.
Delphinidin
All four delphinidins (Fig. 1E) found in C. intybus are
di-glucoside isomers, and none has antitumor properties. However,
mono-glucoside substitutes of delphinidins are cytotoxic (88). Delphinidin-3-glucoside was found to be
chemopreventive against breast epithelial cells (MCF10A) (90). Derivatives of C. intybus
derived delphinidins can serve as potent chemoprotective
agents.
Anthocyanins
The aglycons are the richest anthocyanins (Fig. 1F) found in food. Cyanidin and
delphinidin were found to inhibit the growth of human tumor cells
in vitro in small quantities (84). C. intybus fresh leaf extract
[MeOH-HCOOH (99:1, v/v)] contains malvidin-3-O-glucoside and
pelargonidin-3-O-monoglucuronide (5).
Malvidin-3-O-glucoside was demonstrated to have antitumor activity
against MCF-7 (106) and A431
(84) cell lines.
Pelargonidin-3-O-monoglucuronide increased the concentration of
IL-6 and monocytes in vitro, affecting tumor prognosis of
THP-1 cells (human monocytic leukemia) (107). Its glucoside derivative,
pelargonidin-3-O-glucoside, acts as an anti-inflammatory agent
(108).
Seong et al (109) demonstrated that delphinidin
treatment induced hypoacetylation of histone acetyltransferase
(HAT) and inhibited p65 acetylation in a human rheumatoid
fibroblast-like synoviocyte cell line (MH7A) (Fig. 4). TNF-α stimulation increases NF-κB
expression, and thus promotes the functions of NF-κB target genes
(109). Delphinidin also inhibited
the release of pro-inflammatory cytokines IL-6 and TNF-α in
lipopolysaccharide-treated Jurkat T lymphocytes (109). The chemotherapeutic property of
delphinidin-3-glucoside against MCF10A cells is related to
downregulation of non-coding RNA (lncRNA) and HOX transcript
antisense RNA (HOTAIR) expression (90). Pelargonidin-3-O-monoglucuronide
treatment was found to increase the IL-6 and monocytes
concentration (107). Bioactivity of
these compounds is related to the presence of hydroxyl group in
position 3 of the C ring and 3, 4, 5 in the B ring (Fig. 1E and F) which corresponds to the
report by Wang and Stoner (110),
showing that methylation on these positions decreases the
activity.
4-Hydroxy-3-methoxyphenyl
4-O-Feruloylquinic acid is one of the two
4-hydroxy-3-methoxyphenyl groups (Fig.
1J) containing phytochemicals found in C. intybus. It
was demonstrated to scavenge oxygen radical absorbance capacity
(ORAC) and DPPH radical in an in vitro experiment (69).
Other phytochemicals
Researchers also identified several other types of
phytochemicals in C. intybus. Artesin (also known as
artemisinin, an antimalarial agent) was identified in the root
extract fraction by Kisiel and Zielińska (23). Its antitumor property has been well
studied (111–115). The derivatives of artesin have in
vivo chemosensitizing effects in breast, lung, pancreas, and
glioma cancer cells (112). Artesin
and its derivatives activated by heme [Fe (II)] showed selective
inhibition of colon (HCT116, SE480), leukemia (HL-60), breast
(MCF-7), melanoma (KM, MJT3), lung (NSCLC), pancreas (PANC-1,
MIAPaCa), and glioma (U87MG, A172) cancer cell lines (113–115).
In primary cancer culture, cell lines and xenograft models, artesin
inhibited tumor proliferation, metastasis, and angiogenesis
(114). Several antitumor mechanisms
of artesin have been proposed, including apoptosis, cell cycle
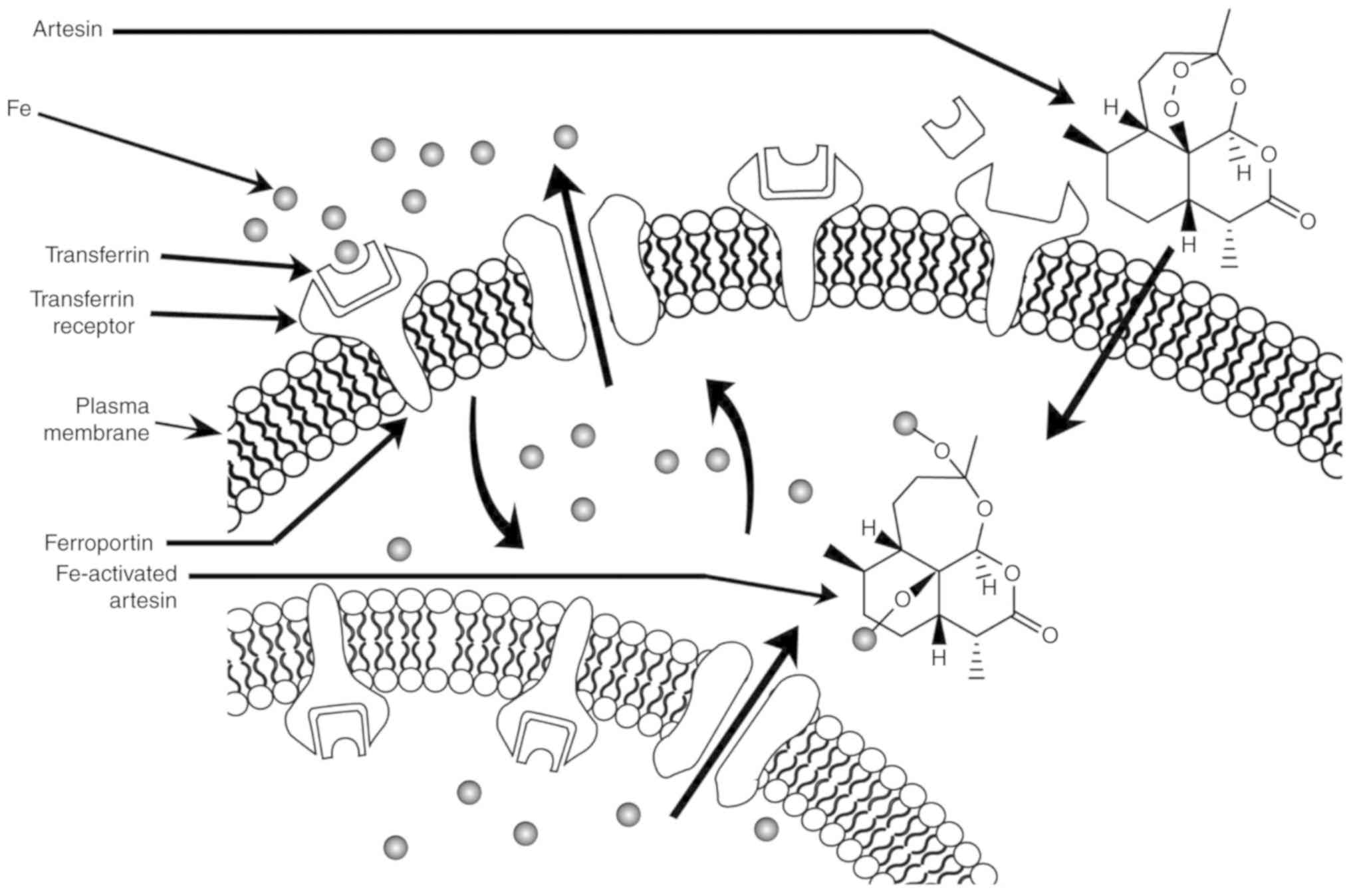
arrest at G0/G1, and oxidative stress (111). Rapidly proliferating cancer cells
express more transferrin receptors on their cell surface, leading
to higher iron uptake. Artesin/artemisinin is the only
phytochemical found in C. intybus with a peroxide bridge
(Fig. 1H). When artesin binds to Fe
(II), the endoperoxide bridge (Fig.
1H) is disrupted, resulting in the production of toxic C-4 and
seco-C-4 free radicals that destroy tumor cells (Fig. 2) (36).
3-O-p-Coumaroyl quinic acid was isolated by Nørbaek
et al (52) from an extract of
C. intybus flower. 3-O-p-Coumaroyl quinic acid was
demonstrated to show antiproliferative activity against PC-3 and
undifferentiated non-cancerous 3T3L1 fibroblast cells (116). Usnic acid purified from the areal
part extracts (26) was reported to
exhibit chemoprotective effects against the wild-type p53 MCF-7
cell line along with a breast cancer cell line with non-functional
p53 (MDA-MB-231), lung cancer cell line (H1299) (117) and prostate cancer cell line (LNCaP)
(118). Mechanistically, (+)-usnic
acid treatment dose-dependently decreases β-catenin-mediated
transfection grade T-cell factor reporter plasmid activity and KAI1
COOH-terminal interacting tetraspanin-mediated AP-1 activity. In
addition, (+)-usnic acid decreases the mRNA levels of CD44 (a
cell-surface glycoprotein), cyclin D1 (a mitotic regulatory
protein) and c-myc (a transcription factor). These are the
downstream target genes of both β-catenin/LEF and c-jun/AP-1.
Furthermore, (+)-usnic acid treatment was found to decrease the
functionality of Rac1 and RhoA. Interestingly, cotreatment of
(+)-usnic acid and cetuximab showed higher inhibition of cell
proliferation then the single cetuximab treatment. These results
indicate the potential antitumor activity and metastasis inhibitory
quality of (+)-usnic acid and suggest (+)-usnic acid can be used
for anticancer therapy with distinct mechanisms of action (119).
C. intybus root is a major source of inulin,
a heterogeneous collection of fructose polymers primarily used as a
prebiotic (120). The
chemoprotective property of inulin has been confirmed in colon
cancer colonic preneoplastic aberrant crypt foci inhibition
(121). Its antitumor property was
also demonstrated on transplantable liver tumor cells (TLT) and
mouse mammary carcinoma cells (EMT6) (121–123).
Low fermentation of inulin indicates its possibility to reach the
distal part of the intestine (124).
Inulin extracted from Cichorium endivia L. (a related
species) was found to reduce the occurrence of intestinal tumors in
an APCMIN mouse model (121).
Molecular mechanisms
SARs
Most of the phytochemicals identified in C.
intybus appear to show potent activity as inhibitors in
specific tumor cell lines; only a few phytochemicals work on all
cell lines. A screening study of anti-proliferative activity
revealed the specificity of purified C. intybus
phytochemicals against different cancerous cell lines. Kinjo et
al (46) and other researchers
showed that groups of phytochemicals had specificity towards
specific cancer cell lines (listed in Table II). In general, polyacetylenes are
potent antiproliferative agents against MK-1 cells, and compounds
with 3,4-dihydroxyphenethyl against B16F10 cells, and some
6-methoxyflavone derivatives and 8-hydroxy furanocoumarins against
HeLa cells are potent anti-proliferative agents (46). This indicates that the structural
features of these phytochemicals directly interact with the
cytochemistry of specific cancerous cells. The biological
activities of the guaianolides, 6-hydroxyflavone, and anthocyanin
depend broadly on the following: i) reactivity of alkylation
center; ii) lipophilicity of the side chain; iii) electronic
features and molecular genomics (36).
 | Table II.Chemical groups of Cichorium
intybus L.-derived phytochemicals with cell-specific
activity. |
Table II.
Chemical groups of Cichorium
intybus L.-derived phytochemicals with cell-specific
activity.
| Chemical
groups/structural motifs | Cell lines | Activity | (Refs.) |
|---|
| Polyacetylene | MK-1 | Cytotoxic | (46) |
|
3,4-Dihydroxyphenethyl | B16F10 |
Antiproliferative | (46) |
|
| HSC-2, HSG | Cytotoxic | (138) |
|
6-Methoxyflavone/6-hydroxyflavone | HeLa | Inhibitory | (46) |
| Delphinidin | Jurkat, MH7A |
Anti-inflammatory | (109) |
|
Phytosterol/sterol | U937 | Apoptosis | (104) |
|
| CaCo-2, HepG2 | Necrosis |
|
| Usnic acid | LNCaP | Apoptosis | (118) |
Similarly, every phytochemical has its own
conserved structural features responsible for bioactivity. The
antitumor property of flavonoids is partly due to their ability to
counteract fatty acid synthase (FASN). Cancerous cells have an
elevated metabolic rate compared with healthy cells, which enables
cancer cells to grow and proliferate at a faster rate (18). Rapid cell division often leaves trails
of both biochemically and structurally irregular cells. FASN is
overexpressed in cancerous tissues of the breast, prostate, and
colon (18), and is also related to
angiogenesis and metastasis (125);
therefore, FASN is recognized as an important antitumor target.
Previous studies have reported that phytochemicals
of C. intybus exert antitumor effects by affecting many
critical overactive signaling pathways in cancerous cells, such as
NF-κB, p53-associated cell cycle, and CYP-mediated inactivation.
The various effects of these phytochemicals confer the advantages
to effectively target more than one cell type, which is often
encountered during metastasis.
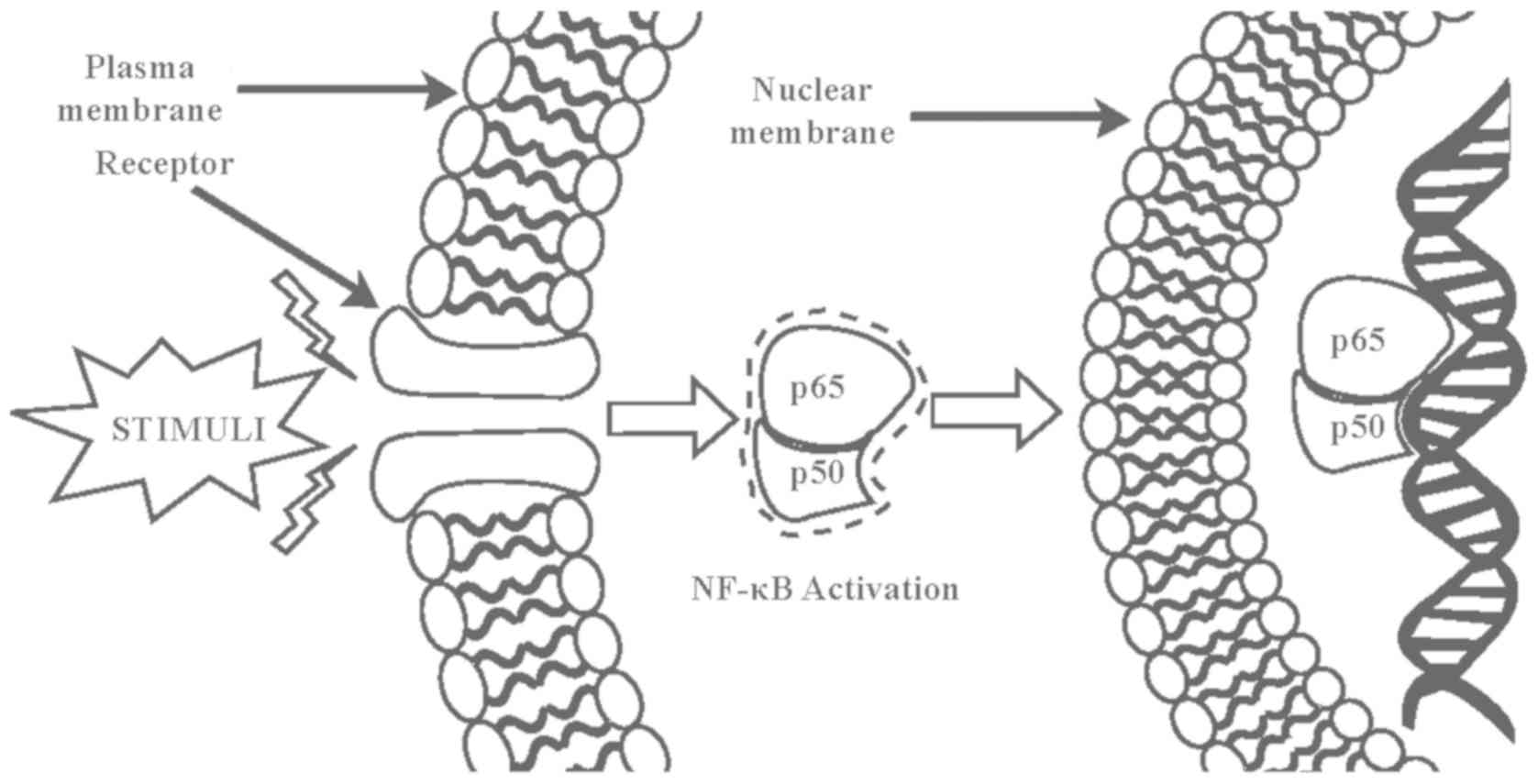
Inhibition of NF-κB
The nuclear factor-κB (NF-κB) transcription factor
plays a critical role in cell development, growth, and survival as
well as various biological processes, including immune response and
inflammation. Numerous inflammatory stimuli such as growth factors
and infectious microbes lead to NF-κB activation (126). Activated NF-κB in turn regulates the
expression of genes governing cell growth, proliferation, survival,
and apoptosis as well as immune responses, stress responses,
embryogenesis, and development of a variety of stimuli (127). Abnormal NF-κB activation causes
various autoimmune, inflammatory, and malignant disorders such as
rheumatoid arthritis, atherosclerosis, inflammatory bowel diseases,
multiple sclerosis, and malignant tumors. Thus, inhibition of NF-κB
signaling is a key target in the treatment of tumors and
inflammatory diseases (127). The
mammalian NF-κB family is composed of five members that form
various dimeric complexes. Among these complexes, the p50/65
heterodimer is most abundant. Overexpression of this complex in
cancer cells leads to the aberrant levels of cell cycle control
factors. Several phytochemicals of C. intybus disrupt
different stages of NF-κB activation and NF-κB-DNA complex
formation. Guaianolides, germacranolides, heliangolides, pseudo
guaianolides, hypocretenolides, and eudesmanolides are collectively
classified as sesquiterpene lactones. Sesquiterpene lactones bind
to the p65 dimer in the NF-κB transcription factor to prevent
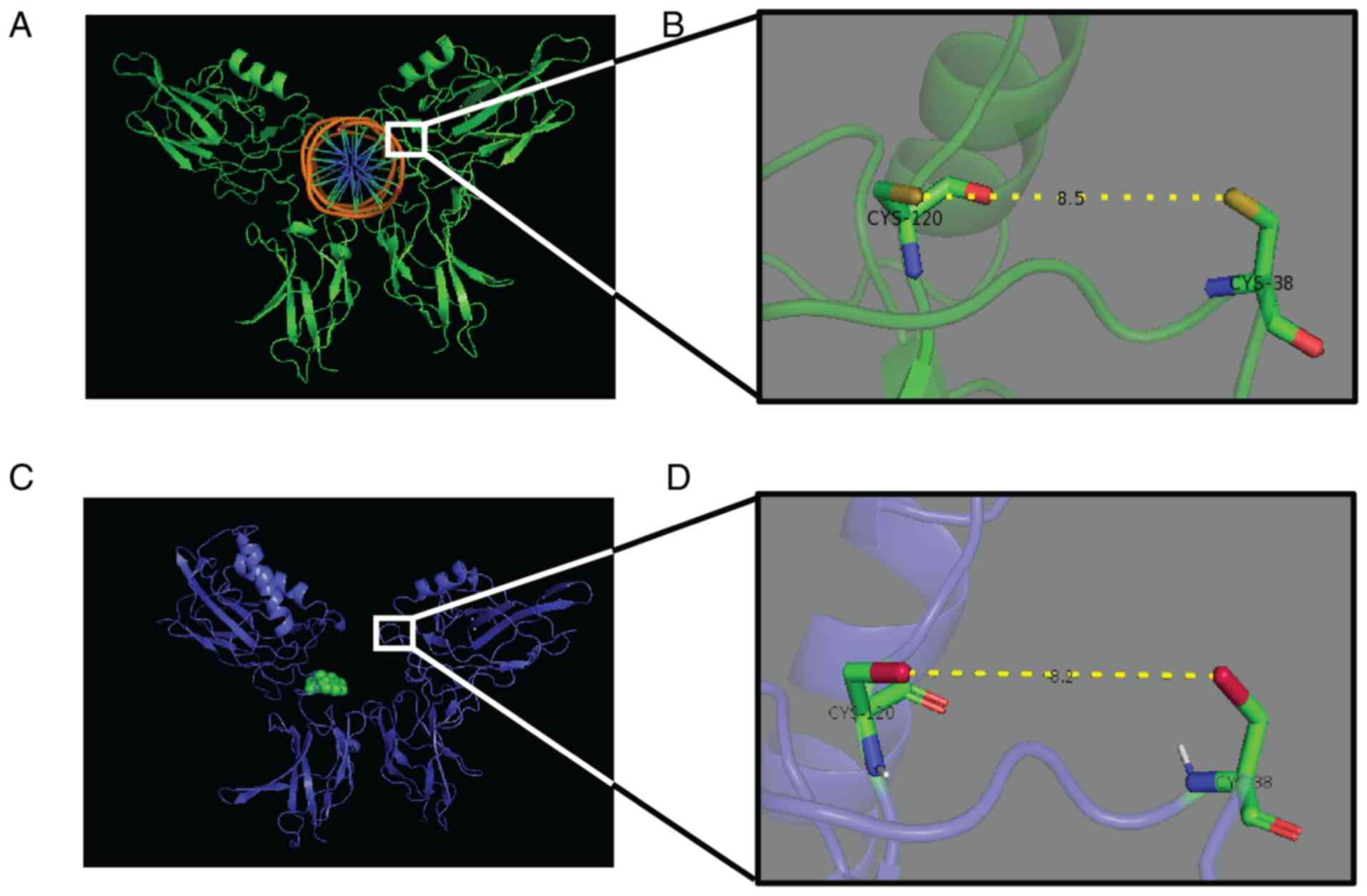
NF-κB-DNA binding (Fig. 3). The
three-dimensional structure created by Arg 33, Arg 35, Tyr 36, Cys
38, Glu 39 and Arg 187 is crucial for DNA binding of the p65 dimer.
Rüngeler et al (37) proposed
that lactucin creates cross linkage of Cys 38 to Cys 120 in the p65
molecule (Fig. 3) that changes the
DNA binding motif structure and affects subsequent transcription,
eventually leading to apoptosis. This mechanism was further
demonstrated in a computer-generated model by García-Piñeres et
al (38). The authors showed the
change in native confirmation of p65 caused by a sesquiterpene
lactone that led to its inability of NF-κB-DNA complex
formation.
Búfalo et al (57) demonstrated that caffeic acid mediated
cell viability independent anti-inflammatory activity and proposed
that caffeic acid exhibited an inhibitory effect in
lipopolysaccharide (LPS)-induced NF-κB activity. The
chemoprotective effect of chlorogenic acid may be accomplished
through its increase of cellular antioxidant enzymes and
suppression of ROS-mediated NF-κB, activator protein 1 (AP-1), and
mitogen-activated protein kinase (MAPK) activation (60). 5-Caffeoylquinic acid inactivates
ribosomal protein S6 kinase (p70S6K) and protein kinase B (PKB/Akt)
activity and thus affects multiple cellular processes and signal
transduction pathways in cancerous cells. Another study showed that
1,3-dicaffeoylquinic acid scavenged hydroxyl radical and superoxide
radicals as measured by electron spin resonance (ESR) (80). Chicoric acid was found to exert its
anti-inflammatory function by halting the phosphorylation of NF-κB
(70). Upon co-treatment with
luteolin, chicoric acid simultaneously reduced the concentration of
nitric oxide and prostaglandin E2 (PGE2) in cells and also
inhibited inducible nitric oxide synthase (iNOS) and
cyclooxygenase-2 (COX-2) expression (70).
p53 associated cell cycle
inhibition
Genomic instability is one of the fundamental cause
of tumor development. p53, commonly known as TP53 or tumor protein
53, is a cell cycle regulatory protein that functions as a tumor
suppressor. p53 responds to DNA damage and other types of genotoxic
stress and functions to maintain genomic stability. The close
involvement of p53 in maintaining genomic stability is why nearly
half of human cancers lack functional p53. In the other half of
cancers, the p53-independent regulatory mechanism is absent or the
p53-dependent pathway gets disabled at different key points. For
instance, the p53 inhibitor MDM2 is overexpressed in tumors that
lack p53 gene mutation. p53 is a crucial component of a complex
network of signaling pathways. However, other components of this
pathway can be alternately targeted for inactivation in cancer.
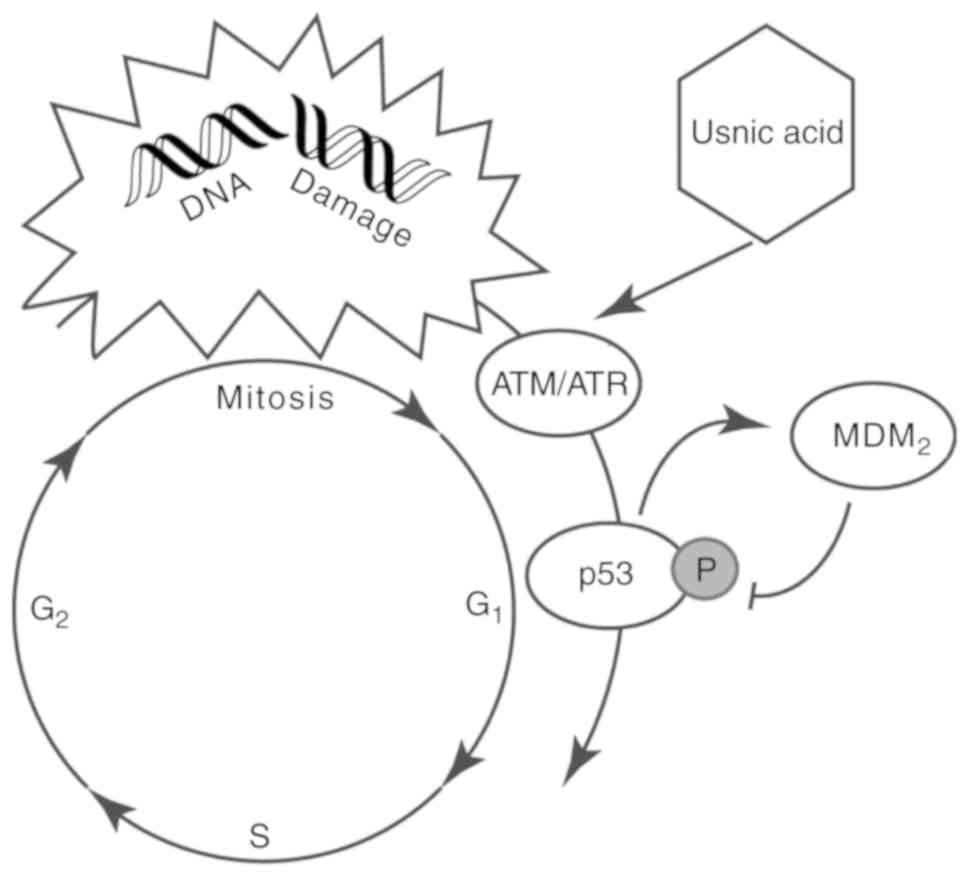
C. intybus extract, in particular, usnic acid, was found to
induce levels of factors such as ataxia telangiectasia mutase (ATM)
or reductase (ATR) that phosphorylates p53 at the MDM2 binding site
(Fig. 5) (117). However, kaempferol-7-O-glucoside
arrests cell division in a p53-independent manner (87).
CYP-mediated inactivation
Cytochrome P450 enzymes (CYPs) are a superfamily of
enzymes that are important for the metabolism of endobiotics and
xenobiotics (128). Rodriguez-Antona
and Ingelman-Sundberg (129)
described the pharmacogenetics of CYPs in cancer formation and
treatment. CYPs are linked to the metabolic activation of numerous
pre-carcinogens and participate in the activation and inactivation
of antitumor drugs (129). Hepatic
CYP expression and activity can be upregulated or downregulated by
bioactive phytochemicals (130).
Direct foddering of dried C. intybus roots to pigs resulted
in increased activities of CYP1A2 and CYP2A, which in turn reduced
the skatole concentration in plasma and fat (128), reducing the chances for colon cancer
occurrence (131). CYP1A2 is one of
the class I CYPs, which are distinguished by a well-conserved
sequence and lack of functional polymorph. CYP1A2 activity has
interindividual (genetic) difference, and this polymorphism is
triggered by external factors such as smoking (129). Although downregulated by total
extract, artemisinin upregulates the mRNA expression of CYP1A2,
CYP2C33, CYP2D25 and CYP3A29 in porcine hepatocyte culture
(132), suggesting a shared
regulatory mechanism of CYP transcription and an inverse agonist
effect of C. intybus.
Toxicological studies
A toxicological study in a 28-day sub-chronic
toxicity study of C. intybus root extract in male and female
Sprague-Dawley rats revealed no adverse effects at 1,000 mg/kg/day
dose (133). The C. intybus
seed chloroform extract inhibited 50% cell growth of human
colorectal adenocarcinoma cells (HCT-15) at 1,411.37 µg/ml
concentration (134). The inhibition
of mouse embryo-derived teratocarcinoma cells (P-19) by a
methanolic extract of C. intybus was least bio-toxic as well
as concentration and duration dependent (135). The cytotoxic activity of chlorogenic
acid (CGA) at a millimolar concentration was higher in human oral
squamous cell carcinoma cells (HSC-2) and a salivary gland tumor
cell line (HSG) than in human gingival fibroblast cells (HGF)
(136).
Clinical trials
The number of completed cancer or tumor-related
clinical trials of C. intybus whole plant or crude or
purified extract is inadequate. A phase 1, placebo-controlled,
double-blind, dose-escalating trial was performed in patients with
osteoarthritis and showed the potential of C. intybus root
extract in the management of osteoarthritis (137). A second clinical trial reported that
daily consumption of chicory coffee reduced the risk of
cardiovascular disorder by lowering whole blood and plasma
viscosity as well as serum MIF level but had a variable effect on
platelet aggregation (138). A
multi-herbal liver tonic formula called Liv-52 that contains C.
intybus as one of the ingredients was tested in a randomized,
double-blind, placebo-controlled clinical trial in cirrhotic
patients. Liv-52 showed a hepatoprotective effect in cirrhotic
patients due to the diuretic, anti-inflammatory, anti-oxidative,
and immunomodulating properties of the component herbs (139). A spermidine-rich diet has been
linked to increased survival in an animal model (140). Spermidine also reduced the overall
(141) and cancer-related (142) mortality in a human clinical trial.
In a difluoromethylornithine (DFMO) + sulindac colorectal adenoma
prevention trial involving dietary putrescine, spermine and
spermidine administration exogenous putrescine effectively
increased cellular polyamine concentration and decreased the risk
and reoccurrence of metachronous adenomas and advanced adenomas
(143). Clinical evidence has shown
that artesin derivatives (artemether and artesunate) substantially
reduce tumor size, and metastasis and increase the survival of
patients with laryngeal carcinoma, uveal melanomas, and pituitary
macroadenomas. These derivatives are in phase I-II-III clinical
trials for lupus nephritis and breast, colorectal (NCT03093129) and
non-small cell lung cancer (NCT02786589) (36,111).
Currently, a phase IV clinical trial in China (NCT02556814) is
investigating caffeic acid combined with high-dose dexamethasone in
the management of immune thrombocytopenia (ITP). Anthocyanin-rich
extracts of different plant species have shown promising result in
phase I clinical trials, but no convincing evidence has been shown
with purified compounds (144).
Several clinical trials are currently underway to investigate
quercetin. One clinical trial is investigating its chemoprevention
activity in squamous cell carcinoma patients (NCT03476330). Two
clinical trials are examining quercetin in prostate cancer
(NCT01538316), and another is studying the effect of quercetin on
green tea polyphenol uptake in prostate tissue from prostate cancer
patients undergoing surgery (NCT01912820). One clinical trial in
Germany was previously proposed to study the synergic effect of
dietary apigenin in combination with epigallocathechin gallate in
colorectal cancer patients (NCT00609310), but this study has been
suspended. A phase III clinical trial is currently investigating
the effect of statins with phytosterol as a dietary intervention in
breast cancer patients (NCT03971019). β-sitosterol was successfully
proven to be effective in treating BPH in a phase II clinical trial
(145). Inulin is used during acute
radiation enteritis to prevent indigestion (146).
Conclusion and perspectives
C. intybus has been the subject of multiple
studies examining its various bioactivities. Here we reviewed
numerous reports in regards to the association of C. intybus
whole, partial, fractionated and purified extracts, with
chemotherapeutic properties. Some of the purified compounds from
C. intybus demonstrated efficacy in in vitro and
in vivo experiments as well as in clinical trials. A few of
their functions are associated with the chemicals' structural
features such as chemical groups and positioning. Structural
activity relationship and molecular mechanisms of toxicity studies
have also revealed the importance of certain chemical groups for
functionality. The specificities of some phytochemicals towards
some specific cell lines (Table II)
also indicate structure-specific inhibition activities. Some
clinical trials and cytotoxicity studies have examined the whole
extract and purified compounds. However, little information is
available regarding the molecular mechanism and even fewer clinical
trials have investigated these properties, which is not adequate to
construct a complete pathway. Further investigation of the
following subject areas is crucial for optimizing the therapeutic
potential of C. intybus. i) Identification of more
phytochemicals and chemical groups or features of existing
phytochemicals that interact with the key control points of tumor
development. ii) From the above information, it is important to
develop a complete model explaining the interaction cascade between
phytochemicals and tumor cells and the associated molecular
pathways for developing precision medicines. iii) It is vital to
investigate the selective cytotoxicity of phytochemicals towards
tumor cells and avoiding healthy cells. It is important to confirm
the reproducibility of these properties in in vitro, in situ, in
vivo and human trials.
All chemotherapeutic products currently available
show various levels of indiscriminate cytotoxicity towards normal
cells, hindering successful recovery. Moreover, the interpersonal
and interorgan difference in metabolic profile makes generic
treatment even less effective. A targeted chemotherapeutic product
that does not interfere with healthy cells is thus required.
Natural compounds usually target cancer cells or their metabolic
pathways at the molecular level; therefore, understanding the
interaction of phytochemicals with normal and cancer cells is
required for designing tumor-specific personalized therapeutics.
However, our review suggests that a complete molecular mechanism
and clinical study information is lacking for natural bioactive
compounds. In regards to the rich historical background of
ethnomedicinal use and the scientific findings reported to date,
C. intybus phytometabolites assuredly show excellent promise
as a source of anticancer compounds. Future research should be
focused on understanding the correlation between structure and cell
specificity, phytochemical isolation and designing derivatives to
formulate targeted and efficient therapeutics and prophylactics as
well as establishing clinical trials to approve their mainstream
use.
Acknowledgements
We thank Xiaoguo Qian (Fengning Ping'an High-tech
Industrial Co., Ltd., Hebei Province, China) for the helpful
discussions.
Funding
This work was supported by the National Key
Research and Development Plan ‘Modern Food Processing and Food
Storage and Transportation Technology and Equipment’ (no.
2017YFD0400204).
Availability of data and materials
Datasets used in this review are summarized and
presented with the publication as tables. Any other relevant
information will be made available by the corresponding author upon
reasonable request.
Authors' contributions
KMSUI and YX wrote the first draft, developed the
figures and tables. YL contributed to the writing and argument
development. FW and FX jointly made critical revisions and approved
the final version. All the authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Authors disclose no potential conflicts of
interests.
References
|
1
|
Al-Snafi AE: Medical importance of
Cichorium intybus-A review. IOSR J Of Pharm. 6:41–56.
2016.
|
|
2
|
Bais HP and Ravishankar GA: Cichorium
intybus L- cultivation, processing, utility, value addition and
biotechnology, with an emphasis on current status and future
prospects. J Sci Food Agric. 81:467–484. 2001. View Article : Google Scholar
|
|
3
|
Street RA, Sidana J and Prinsloo G:
Cichorium intybus: Traditional uses, phytochemistry,
pharmacology, and toxicology. Evid Based Complement Alternat Med.
2013:5793192013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberfroid MB: Inulin-type fructans:
Functional food ingredients. J Nutr. 137 (Suppl 11):S2493–S2502.
2007. View Article : Google Scholar
|
|
5
|
Carazzone C, Mascherpa D, Gazzani G and
Papetti A: Identification of phenolic constituents in red chicory
salads (Cichorium intybus) by high-performance liquid
chromatography with diode array detection and electrospray
ionisation tandem mass spectrometry. Food Chem. 138:1062–1071.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malik B, Pirzadah TB, Tahir I and Rehman
RU: Chemo-profiling, antioxidant potential and ionomic analysis of
Cichorium intybus L. Pharmacogn J. 9:917–928. 2017.
View Article : Google Scholar
|
|
7
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Zhang H, Zhou HJ, Ji W and Min W:
Mitochondrial redox signaling and tumor progression. Cancers
(Basel). 8:402016. View Article : Google Scholar :
|
|
11
|
Saikolappan S, Kumar B, Shishodia G, Koul
S and Koul HK: Reactive oxygen species and cancer: A complex
interaction. Cancer Lett. 452:132–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conforti F, Ioele G, Statti GA, Marrelli
M, Ragno G and Menichini F: Antiproliferative activity against
human tumor cell lines and toxicity test on Mediterranean dietary
plants. Food Chem Toxicol. 46:3325–3332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hafez ESE, Badr EA, Mabrouk YM, Seehy MA
and Aggag SA: Expression of tumor-markers and cytokines in response
to Cichorium endivia L. in cancerous mice. Int J Life Sci
Biotech Pharma Res. 3:1–7. 2014.
|
|
14
|
Alshehri A and Elsayed HE: Molecular and
biochemical evaluation of anti-proliferative effect of
(Cichorium endivia L.) phenolic extracts on breast cancer
cell line: MCF7. J Biotechnol Pharma Res. 3:74–82. 2012.
|
|
15
|
Hafez EE, Badr E, Mabrouk Y, El-Seehy M
and Aggag S: Molecular genetic evaluation of Cichorium
endivia L. as an anticancer agent against colorectal cancer.
Int J Phytomed. 8:551–557. 2016. View Article : Google Scholar
|
|
16
|
Hazra B, Sarkar R, Bhattacharyya S and Roy
P: Tumour inhibitory activity of chicory root extract against
Ehrlich ascites carcinoma in mice. Fitoterapia. 73:730–733. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nawab A, Yunus M, Mahdi AA and Gupta S:
Evaluation of anticancer properties of medicinal plants from the
Indiansub-continent. Mol Cell Pharmacol. 3:21–29. 2011.
|
|
18
|
Mehrandish R, Awsat Mellati A, Rahimipour
A and Dehghan Nayeri N: Anti-cancer activity of methanol extracts
of Cichorium intybus on human breast cancer SKBR3 cell line.
Razavi Int J Med. 5:e383692017.
|
|
19
|
Saleem M, Abbas K, Naseer F, Mobasher A,
Syed NH, Fatima J, Hussain K and Samia A: Anticancer activity of
n-hexane extract of Cichorium intybus on lymphoblastic
leukemia cells (Jurkat cells). Asian J Plant Sci. 8:315–319.
2014.
|
|
20
|
Esmaeilbeig M, Kouhpayeh SA and
Amirghofran Z: An investigation of the growth inhibitory capacity
of several medicinal plants from Iran on tumor cell lines. Iran J
Cancer Prev. 8:e40322015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leclercq E: Determination of lactucin in
roots of chicory (Cichorium intybus L.) by high-performance
liquid chromatography. J Chromatogr A. 283:441–444. 1984.
View Article : Google Scholar
|
|
22
|
Bischoff TA, Kelley CJ, Karchesy Y,
Laurantos M, Nguyen-Dinh P and Arefi AG: Antimalarial activity of
lactucin and lactucopicrin: Sesquiterpene lactones isolated from
Cichorium intybus L. J Ethnopharmacol. 95:455–457. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kisiel W and Zielińska K: Guaianolides
from Cichorium intybus and structure revision of Cichorium
sesquiterpene lactones. Phytochemistry. 57:523–527. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malarz J, Stojakowska A, Szneler E and
Kisiel W: A new neolignan glucoside from hairy roots of
Cichorium intybus. Phytochem Lett. 6:59–61. 2013. View Article : Google Scholar
|
|
25
|
Pyrek JS: Sesquiterpene lactones of
Cichorium intybus and Leontodon autumnalis.
Phytochemistry. 24:186–188. 1985. View Article : Google Scholar
|
|
26
|
Satmbekova D, Srivedavyasasri R, Orazbekov
Y, Omarova R, Datkhayev U and Ross SA: Chemical and biological
studies on Cichorium intybus L. Nat Prod Res. 32:1343–1347.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Beek TA, Maas P, King BM, Leclercq E,
Voragen AGJ and De Groot A: Bitter sesquiterpene lactones from
chicory roots. J Agric Food Chem. 38:1035–1038. 1990. View Article : Google Scholar
|
|
28
|
Nwafor IC, Shale K and Achilonu MC:
Chemical composition and nutritive benefits of chicory
(Cichorium intybus) as an ideal complementary and/or
alternative livestock feed supplement. ScientificWorldJournal.
2017:73439282017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aisa HA and Xue-Lei X: Cichorium
glandulosum Bioss. Et Huet (Juju, Chicory)Dietary Chinese
Herbs. Springer, Vienna Pharmacology and Clinical Evidence; pp.
711–720. 2015, View Article : Google Scholar
|
|
30
|
Malarz J, Stojakowska A and Kisiel W:
Long-term cultured hairy roots of chicory-a rich source of
hydroxycinnamates and 8-deoxylactucin glucoside. Appl Biochem
Biotechnol. 171:1589–1601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malarz J, Stojakowska A and Kisiel W:
Sesquiterpene lactones in a hairy root culture of Cichorium
intybus. Z Naturforsch C. 57:994–997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Monde K, Oya T, Takasugi M and Shirata A:
A guaianolide phytoalexin, cichoralexin, from Cichorium
intybus. Phytochemistry. 29:3449–3451. 1990. View Article : Google Scholar
|
|
33
|
Seto M, Miyase T, Umehara K, Ueno A,
Hirano Y and Otani N: Sesquiterpene lactones from Cichorium endivia
L. and C. intybus L. and cytotoxic activity. Chem Pharm Bull
(Tokyo). 36:2423–2429. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Zhao M, Bai L, Hasegawa T, Wang
J, Wang L, Xue H, Deng Q, Xing F, Bai Y, et al: Bioactive
guaianolides from siyekucai (Ixeris chinensis). J Nat Prod.
69:1425–1428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren Y, Zhou Y, Chen X and Ye Y: Discovery,
structural determination and anticancer activities of Lactucin like
guaianolides. Lett Drug Des Discov. 2:444–450. 2005. View Article : Google Scholar
|
|
36
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rüngeler P, Castro V, Mora G, Gören N,
Vichnewski W, Pahl HL, Merfort I and Schmidt TJ: Inhibition of
transcription factor NF-kappaB by sesquiterpene lactones: A
proposed molecular mechanism of action. Bioorg Med Chem.
7:2343–2352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
García-Piñeres AJ, Castro V, Mora G,
Schmidt TJ, Strunck E, Pahl HL and Merfort I: Cysteine 38 in
p65/NF-kappaB plays a crucial role in DNA binding inhibition by
sesquiterpene lactones. J Biol Chem. 276:39713–39720. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krebsky EO, Geuns JMC and De Proft M:
Polyamines and sterols in Cichorium heads. Phytochemistry.
50:549–553. 1999. View Article : Google Scholar
|
|
40
|
Papetti A, Mascherpa D, Carazzone C,
Stauder M, Spratt DA, Wilson M, Pratten J, Ciric L, Lingström P,
Zaura E, et al: Identification of organic acids in Cichorium
intybus inhibiting virulence-related properties of oral
pathogenic bacteria. Food Chem. 138:1706–1712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amendola R, Cervelli M, Fratini E,
Polticelli F, Sallustio DE and Mariottini P: Spermine metabolism
and anticancer therapy. Curr Cancer Drug Targets. 9:118–130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng B, Bux R and Cheng D:
Spermidine/spermine N1-acetyltransferase antibodies as anti-cancer
drug compounds. US Patent 2016/0017054 A1. Filed January 30 2014;
issued January 21 2016.
|
|
43
|
Kremmer T, Pälyi I, Daubner D, Boldizsár
M, Vincze B, Paulik E, Sugár J, Pokorny E and Túry E: Comparative
studies on the polyamine metabolism and DFMO treatment of MCF-7 and
MDA-MB-231 breast cancer cell lines and xenografts. Anticancer Res.
11:1807–1813. 1991.PubMed/NCBI
|
|
44
|
Lima G and Shiu RP: Role of polyamines in
estradiol-induced growth of human breast cancer cells. Cancer Res.
45:2466–2470. 1985.PubMed/NCBI
|
|
45
|
Pályi I, Kremmer T, Kálnay A, Turi G,
Mihalik R, Bencsik K and Boldizsár M: Effects of methylacetylenic
putrescine, an ornithine decarboxylase inhibitor and potential
novel anticancer agent, on human and mouse cancer cell lines.
Anticancer Drugs. 10:103–111. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kinjo J, Nakano D, Fujioka T and Okabe H:
Screening of promising chemotherapeutic candidates from plants
extracts. J Nat Med. 70:335–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lessard M, Zhao C, Singh SM and Poulin R:
Hormonal and feedback regulation of putrescine and spermidine
transport in human breast cancer cells. J Biol Chem. 270:1685–1694.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pegg AE and McCann PP: Polyamine
metabolism and function. Am J Physiol. 243:C212–C221. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thomas T and Thomas TJ: Polyamines in cell
growth and cell death: Molecular mechanisms and therapeutic
applications. Cell Mol Life Sci. 58:244–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bridle P, Thomas Loeffler RS, Timberlake
CF and Self R: Cyanidin 3-malonylglucoside in Cichorium
intybus. Phytochemistry. 23:2968–2969. 1984. View Article : Google Scholar
|
|
51
|
Tousch D, Lajoix AD, Hosy E, Azay-Milhau
J, Ferrare K, Jahannault C, Cros G and Petit P: Chicoric acid, a
new compound able to enhance insulin release and glucose uptake.
Biochem Biophys Res Commun. 377:131–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nørbaek R, Nielsen K and Kondo T:
Anthocyanins from flowers of Cichorium intybus.
Phytochemistry. 60:357–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kurata R, Adachi M, Yamakawa O and
Yoshimoto M: Growth suppression of human cancer cells by
polyphenolics from sweetpotato (Ipomoea batatas L.) leaves.
J Agric Food Chem. 55:185–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen YJ, Shiao MS, Hsu ML, Tsai TH and
Wang SY: Effect of caffeic acid phenethyl ester, an antioxidant
from propolis, on inducing apoptosis in human leukemic HL-60 cells.
J Agric Food Chem. 49:5615–5619. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kampa M, Alexaki VI, Notas G, Nifli AP,
Nistikaki A, Hatzoglou A, Bakogeorgou E, Kouimtzoglou E, Blekas G,
Boskou D, et al: Antiproliferative and apoptotic effects of
selective phenolic acids on T47D human breast cancer cells:
Potential mechanisms of action. Breast Cancer Res. 6:R63–R74. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dilshara MG, Jayasooriya RG, Park SR, Choi
YH, Choi IW and Kim GY: Caffeic acid phenethyl ester enhances
TRAIL-mediated apoptosis via CHOP-induced death receptor 5
upregulation in hepatocarcinoma Hep3B cells. Mol Cell Biochem.
418:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Búfalo MC, Ferreira I, Costa G, Francisco
V, Liberal J, Cruz MT, Lopes MC, Batista MT and Sforcin JM:
Propolis and its constituent caffeic acid suppress LPS-stimulated
pro-inflammatory response by blocking NF-κB and MAPK activation in
macrophages. J Ethnopharmacol. 149:84–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang MT, Smart RC, Wong CQ and Conney AH:
Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and
ferulic acid on tumor promotion in mouse skin by
12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 48:5941–5946.
1988.PubMed/NCBI
|
|
59
|
Kasai H, Fukada S, Yamaizumi Z, Sugie S
and Mori H: Action of chlorogenic acid in vegetables and fruits as
an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a
rat carcinogenesis model. Food Chem Toxicol. 38:467–471. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Feng R, Lu Y, Bowman LL, Qian Y,
Castranova V and Ding M: Inhibition of activator protein-1,
NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme
activity by chlorogenic acid. J Biol Chem. 280:27888–27895. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang GF, Shi LP, Ren YD, Liu QF, Liu HF,
Zhang RJ, Li Z, Zhu FH, He PL, Tang W, et al: Anti-hepatitis B
virus activity of chlorogenic acid, quinic acid and caffeic acid in
vivo and in vitro. Antiviral Res. 83:186–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hsu CL, Huang SL and Yen GC: Inhibitory
effect of phenolic acids on the proliferation of 3T3-L1
preadipocytes in relation to their antioxidant activity. J Agric
Food Chem. 54:4191–4197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Maki C, Funakoshi-Tago M, Aoyagi R, Ueda
F, Kimura M, Kobata K, Tago K and Tamura H: Coffee extract inhibits
adipogenesis in 3T3-L1 preadipocyes by interrupting insulin
signaling through the downregulation of IRS1. PLoS One.
12:e01732642017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Meng S, Cao J, Feng Q, Peng J and Hu Y:
Roles of chlorogenic acid on regulating glucose and lipids
metabolism: A review. Evid Based Complement Alternat Med.
2013:8014572013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Olthof MR, Hollman PCH and Katan MB:
Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr.
131:66–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
In JK, Kim JK, Oh JS and Seo DW:
5-Caffeoylquinic acid inhibits invasion of non-small cell lung
cancer cells through the inactivation of p70S6K and Akt activity:
Involvement of p53 in differential regulation of signaling
pathways. Int J Oncol. 48:1907–1912. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Apostolou A, Stagos D, Galitsiou E, Spyrou
A, Haroutounian S, Portesis N, Trizoglou I, Wallace Hayes A,
Tsatsakis AM and Kouretas D: Assessment of polyphenolic content,
antioxidant activity, protection against ROS-induced DNA damage and
anticancer activity of Vitis vinifera stem extracts. Food
Chem Toxicol. 61:60–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kirmizibekmez H, Calis I, Perozzo R, Brun
R, Dönmez AA, Linden A, Rüedi P and Tasdemir D: Inhibiting
activities of the secondary metabolites of Phlomis
brunneogaleata against parasitic protozoa and plasmodial
enoyl-ACP Reductase, a crucial enzyme in fatty acid biosynthesis.
Planta Med. 70:711–717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
You Q, Chen F, Ni H, Wang X, Jiang Y and
McCoy JA: HPLC-MS analyses and bioactivities of novel chemicals in
Devil's club (Oplopanax horridus (Sm.) Miq.). Food Chem.
135:199–207. 2012. View Article : Google Scholar
|
|
70
|
Park CM, Jin KS, Lee YW and Song YS:
Luteolin and chicoric acid synergistically inhibited inflammatory
responses via inactivation of PI3K-Akt pathway and impairment of
NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J
Pharmacol. 660:454–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiao H, Wang J, Yuan L, Xiao C, Wang Y and
Liu X: Chicoric acid induces apoptosis in 3T3-L1 preadipocytes
through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric
Food Chem. 61:1509–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huntimer ED, Halaweish FT and Chase CCL:
Proliferative activity of Echinacea angustifolia root
extracts on cancer cells: Interference with doxorubicin
cytotoxicity. Chem Biodivers. 3:695–703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou J, Fang L, Liao J, Li L, Yao W, Xiong
Z and Zhou X: Investigation of the anti-cancer effect of quercetin
on HepG2 cells in vivo. PLoS One. 12:e01728382017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Saleh MR, Metwally AM and Amer MM:
Isolation of a flavonoidal substance from Cichorium pumilum
jacq. Pharmazie. 30:4041975.PubMed/NCBI
|
|
75
|
Chen Z, Liu YM, Yang S, Song BA, Xu GF,
Bhadury PS, Jin LH, Hu DY, Liu F, Xue W and Zhou X: Studies on the
chemical constituents and anticancer activity of Saxifraga
stolonifera (L) Meeb. Bioorg Med Chem. 16:1337–1344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Maiyo FC, Moodley R and Singh M:
Cytotoxicity, antioxidant and apoptosis studies of quercetin-3-O
glucoside and
4-(β-D-glucopyranosyl-1→4-α-L-rhamnopyranosyloxy)-benzyl
isothiocyanate from Moringa oleifera. Anticancer Agents Med Chem.
16:648–656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Panat NA, Amrute KB, Battu S, Haram S,
Sharma G and Ghaskadbi S: Antioxidant profiling of C3 quercetin
glycosides: Quercitrin, quercetin 3-β-D-glucoside and quercetin
3-O-(6″-O-malonyl)-β-D-glucoside in cell free environment. Free Rad
Antiox. 5:90–100. 2015. View Article : Google Scholar
|
|
78
|
Sassi N, Mattarei A, Espina V, Liotta L,
Zoratti M, Paradisi C and Biasutto L: Potential anti-cancer
activity of 7-O-pentyl quercetin: Efficient, membrane-targeted
kinase inhibition and pro-oxidant effect. Pharmacol Res. 124:9–19.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sugantha Priya E, Selvakumar Benny K,
Bavithra S, Elumalai P, Arunkumar R, Raja Singh P, Brindha Mercy A
and Arunakaran J: Anti-cancer activity of quercetin in
neuroblastoma: An in vitro approach. Neurol Sci. 35:163–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Danino O, Gottlieb HE, Grossman S and
Bergman M: Antioxidant activity of 1,3-dicaffeoylquinic acid
isolated from Inula viscosa. Food Res Int. 42:1273–1280.
2009. View Article : Google Scholar
|
|
81
|
Chen PN, Chu SC, Chiou HL, Chiang CL, Yang
SF and Hsieh YS: Cyanidin 3-glucoside and peonidin 3-glucoside
inhibit tumor cell growth and induce apoptosis in vitro and
suppress tumor growth in vivo. Nutr Cancer. 53:232–243. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cho E, Chung EY, Jang HY, Hong OY, Chae
HS, Jeong YJ, Kim SY, Kim BS, Yoo DJ, Kim JS and Park KH:
Anti-cancer effect of cyanidin-3-glucoside from mulberry via
caspase-3 cleavage and DNA fragmentation in vitro and in vivo.
Anticancer Agents Med Chem. 17:1519–1525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lee JS, Kim YR, Song IG, Ha SJ, Kim YE,
Baek NI and Hong EK: Cyanidin-3-glucoside isolated from mulberry
fruit protects pancreatic β-cells against oxidative stress-induced
apoptosis. Int J Mol Med. 35:405–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Meiers S, Kemény M, Weyand U, Gastpar R,
von Angerer E and Marko D: The anthocyanidins cyanidin and
delphinidin are potent inhibitors of the epidermal growth-factor
receptor. J Agric Food Chem. 49:958–962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Srivastava JK and Gupta S: Extraction,
characterization, stability and biological activity of flavonoids
isolated from Chamomile flowers. Mol Cell Pharmacol. 1:1382009.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Smiljkovic M, Stanisavljevic D, Stojkovic
D, Petrovic I, Marjanovic Vicentic J, Popovic J, Golic Grdadolnik
S, Markovic D, Sankovic-Babice S, Glamoclija J, et al:
Apigenin-7-O-glucoside versus apigenin: Insight into the modes of
anticandidal and cytotoxic actions. EXCLI J. 16:795–807.
2017.PubMed/NCBI
|
|
87
|
Xu W, Liu J, Li C, Wu HZ and Liu YW:
Kaempferol-7-O-beta-D-glucoside (KG) isolated from Smilax
china L. rhizome induces G2/M phase arrest and apoptosis on
HeLa cells in a p53-independent manner. Cancer Lett. 264:229–240.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nasri I, Chawech R, Girardi C, Mas E,
Ferrand A, Vergnolle N, Fabre N, Mezghani-Jarraya R and
Racaud-Sultan C: Anti-inflammatory and anticancer effects of
flavonol glycosides from Diplotaxis harra through GSK3β
regulation in intestinal cells. Pharm Biol. 55:124–131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park JY, Kim SI, Lee HJ, Kim SS, Kwon YS
and Chun W: Isorhamnetin-3-O-glucuronide suppresses JNK and p38
activation and increases heme-oxygenase-1 in
lipopolysaccharide-challenged RAW264.7 cells. Drug Dev Res.
77:143–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang X, Luo E, Liu X, Han B, Yu X and Peng
X: Delphinidin-3-glucoside suppresses breast carcinogenesis by
inactivating the Akt/HOTAIR signaling pathway. BMC Cancer.
16:4232016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
He X and Liu RH: Cranberry phytochemicals:
Isolation, structure elucidation, and their antiproliferative and
antioxidant activities. J Agric Food Chem. 54:7069–7074. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ghosh T, Maity T and Singh J: Evaluation
of antitumor activity of stigmasterol, a constituent isolated from
Bacopa monnieri Linn aerial parts against ehrlich ascites
carcinoma in mice. Orient Pharm Exp Med. 11:41–49. 2011. View Article : Google Scholar
|
|
93
|
Kangsamaksin T, Chaithongyot S,
Wootthichairangsan C, Hanchaina R, Tangshewinsirikul C and Svasti
J: Lupeol and stigmasterol suppress tumor angiogenesis and inhibit
cholangiocarcinoma growth in mice via downregulation of tumor
necrosis factor-α. PLoS One. 12:e01896282017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Syed Abdul Rahman SN, Abdul Wahab N and
Abd Malek SN: In vitro morphological assessment of apoptosis
induced by antiproliferative constituents from the rhizomes of
Curcuma zedoaria. Evid Based Complement Alternat Med.
2013:2571082013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Dutra LM, Bomfim LM, Rocha SL, Nepel A,
Soares MB, Barison A, Costa EV and Bezerra DP: ent-Kaurane
diterpenes from the stem bark of Annona vepretorum
(Annonaceae) and cytotoxic evaluation. Bioorg Med Chem Lett.
24:3315–3320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ali H, Dixit S, Ali D, Alqahtani SM,
Alkahtani S and Alarifi S: Isolation and evaluation of anticancer
efficacy of stigmasterol in a mouse model of DMBA-induced skin
carcinoma. Drug Des Devel Ther. 9:2793–2800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Süntar I, Küpeli Akkol E, Keles H,
Yesilada E, Sarker SD and Baykal T: Comparative evaluation of
traditional prescriptions from Cichorium intybus L. for
wound healing: Stepwise isolation of an active component by in vivo
bioassay and its mode of activity. J Ethnopharmacol. 143:299–309.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Woyengo TA, Ramprasath VR and Jones PJ:
Anticancer effects of phytosterols. Eur J Clin Nutr. 63:813–820.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cheng D, Guo Z and Zhang S: Effect of
β-sitosterol on the expression of HPV E6 and p53 in cervical
carcinoma cells. Contemp Oncol (Pozn). 19:36–42. 2015.PubMed/NCBI
|
|
100
|
Awad AB, Chinnam M, Fink CS and Bradford
PG: β-Sitosterol activates Fas signaling in human breast cancer
cells. Phytomedicine. 14:747–754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chai JW, Kuppusamy UR and Kanthimathi MS:
Beta-sitosterol induces apoptosis in MCF7 cells. Malays J Biochem
Mol Biol. 16:28–30. 2008.
|
|
102
|
Wilt TJ, Ishani A, MacDonald R, Stark G,
Mulrow CD and Lau J: Beta-sitosterols for benign prostatic
hyperplasia. Cochrane Database Syst Rev. CD0010432000.PubMed/NCBI
|
|
103
|
Nibret E, Youns M, Krauth-Siegel RL and
Wink M: Biological activities of xanthatin from Xanthium
strumarium leaves. Phytother Res. 25:1883–1890. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ryan E, Chopra J, McCarthy F, Maguire AR
and O'Brien NM: Qualitative and quantitative comparison of the
cytotoxic and apoptotic potential of phytosterol oxidation products
with their corresponding cholesterol oxidation products. Br J Nutr.
94:443–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
O'Callaghan Y, McCarthy FO and O'Brien NM:
Recent advances in phytosterol oxidation products. Biochem Biophys
Res Commun. 446:786–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Oliveira H, Wu N, Zhang Q, Wang J,
Oliveira J, de Freitas V, Mateus N, He J and Fernandes I:
Bioavailability studies and anticancer properties of malvidin based
anthocyanins, pyranoanthocyanins and non-oxonium derivatives. Food
Funct. 7:2462–2468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Amini AM, Spencer JPE and Yaqoob P:
Effects of pelargonidin-3-O-glucoside and its metabolites on
lipopolysaccharide-stimulated cytokine production by THP-1
monocytes and macrophages. Cytokine. 103:29–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Duarte LJ, Chaves VC, Nascimento MVPDS,
Calvete E, Li M, Ciraolo E, Ghigo A, Hirsch E, Simões CMO,
Reginatto FH and Dalmarco EM: Molecular mechanism of action of
Pelargonidin-3-O-glucoside, the main anthocyanin responsible for
the anti-inflammatory effect of strawberry fruits. Food Chem.
247:56–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Seong AR, Yoo JY, Choi K, Lee MH, Lee YH,
Lee J, Jun W, Kim S and Yoon HG: Delphinidin, a specific inhibitor
of histone acetyltransferase, suppresses inflammatory signaling via
prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A
cells. Biochem Biophys Res Commun. 410:581–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang LS and Stoner GD: Anthocyanins and
their role in cancer prevention. Cancer Lett. 269:281–290. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Das AK: Anticancer effect of Antimalarial
artemisinin compounds. Ann Med Health Sci Res. 5:93–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Slezakova S and Ruda-Kucerova J:
Anticancer activity of artemisinin and its derivatives. Anticancer
Res. 37:5995–6003. 2017.PubMed/NCBI
|
|
113
|
Wong YK, Xu C, Kalesh KA, He Y, Lin Q,
Wong WSF, Shen HM and Wang J: Artemisinin as an anticancer drug:
Recent advances in target profiling and mechanisms of action. Med
Res Rev. 37:1492–1517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: From a well-known
antimalarial agent to a potential anticancer drug. J Biomed
Biotechnol. 2012:2475972012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gravett AM, Liu WM, Krishna S, Chan WC,
Haynes RK, Wilson NL and Dalgleish AG: In vitro study of the
anti-cancer effects of artemisone alone or in combination with
other chemotherapeutic agents. Cancer Chemother Pharmacol.
67:569–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Alotaibi KS, Li H, Rafi R and Siddiqui RA:
Papaya black seeds have beneficial anticancer effects on PC-3
prostate cancer cells. J Cancer Metastasis Treat. 3:161–168. 2017.
View Article : Google Scholar
|
|
117
|
Mayer M, O'Neill MA, Murray KE,
Santos-Magalhães NS, Carneiro-Leão AM, Thompson AM and Appleyard
VC: Usnic acid: A non-genotoxic compound with anti-cancer
properties. Anticancer Drugs. 16:805–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Eryilmaz IE, Eskiler GG, Yurdacan B, Egeli
Ü, Çeçener G and Tunca B: The cytotoxic and apoptotic effects of
usnic acid on prostate cancer versus normal cells. Proceedings.
1:10272017. View Article : Google Scholar
|
|
119
|
Yang Y, Nguyen TT, Jeong MH, Crişan F, Yu
YH, Ha HH, Choi KH, Jeong HG, Jeong TC, Lee KY, et al: Inhibitory
activity of (+)-usnic acid against non-small cell lung cancer cell
motility. PLoS One. 11:e01465752016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Couri S, Gomes F, Nogueira R and Almeida
DL: Determination of inulin content of chicory roots (Cichorium
intybus L.) cultivated organically in three regions of Rio de
Janeiro state. 2018.
|
|
121
|
Pool-Zobel B, van Loo J, Rowland I and
Roberfroid MB: Experimental evidences on the potential of prebiotic
fructans to reduce the risk of colon cancer. Br J Nutr. 87 (Suppl
2):S273–S281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Taper HS and Roberfroid MB: Possible
adjuvant cancer therapy by two prebiotics-Inulin or oligofructose.
In Vivo. 19:201–204. 2005.PubMed/NCBI
|
|
123
|
Taper HS and Roberfroid M: Influence of
inulin and oligofructose on breast cancer and tumor growth. J Nutr.
129 (Suppl 7):S1488–S1491. 1999. View Article : Google Scholar
|
|
124
|
Reddy BS, Hamid R and Rao CV: Effect of
dietary oligofructose and inulin on colonic preneoplastic aberrant
crypt foci inhibition. Carcinogenesis. 18:1371–1374. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Epstein JI, Carmichael M and Partin AW:
OA-519 (fatty acid synthase) as an independent predictor of
pathologic state in adenocarcinoma of the prostate. Urology.
45:81–86. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Park MH and Hong JT: Roles of NF-κB in
cancer and inflammatory diseases and their therapeutic approaches.
Cells. 5:E152016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Rasmussen MK, Zamaratskaia G and Ekstrand
B: In vivo effect of dried chicory root (Cichorium intybus
L.) on xenobiotica metabolising cytochrome P450 enzymes in porcine
liver. Toxicol Lett. 200:88–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Rodriguez-Antona C and Ingelman-Sundberg
M: Cytochrome P450 pharmacogenetics and cancer. Oncogene.
25:1679–1691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chang TKH: Activation of pregnane X
receptor (PXR) and constitutive androstane receptor (CAR) by herbal
medicines. AAPS J. 11:590–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zuccato E, Venturi M, Di Leo G, Colombo L,
Bertolo C, Doldi SB and Mussini E: Role of bile acids and metabolic
activity of colonic bacteria in increased risk of colon cancer
after cholecystectomy. Dig Dis Sci. 38:514–519. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Rasmussen MK, Klausen CL and Ekstrand B:
Regulation of cytochrome P450 mRNA expression in primary porcine
hepatocytes by selected secondary plant metabolites from chicory
(Cichorium intybus L.). Food Chem. 146:255–263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Schmidt BM, Ilic N, Poulev A and Raskin I:
Toxicological evaluation of a chicory root extract. Food Chem
Toxicol. 45:1131–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Mali P: Cytotoxicity activities of
chloroform extract of Cichorium intybus seed against HCT-15
and Vero cell line. Int J Health Allied Sci. 4:267–270. 2015.
View Article : Google Scholar
|
|
135
|
Soltanian S, Sheikhbahaei M and Mohamadi
N: Cytotoxicity evaluation of methanol extracts of some medicinal
plants on P19 embryonal carcinoma cells. J Appl Pharm Sci.
7:142–149. 2017.
|
|
136
|
Jiang Y, Kusama K, Satoh K, Takayama E,
Watanabe S and Sakagami H: Induction of cytotoxicity by chlorogenic
acid in human oral tumor cell lines. Phytomedicine. 7:483–491.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Schumacher E, Vigh E, Molnár V, Kenyeres
P, Fehér G, Késmárky G, Tóth K and Garai J: Thrombosis preventive
potential of chicory coffee consumption: A clinical study.
Phytother Res. 25:744–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Olsen NJ, Branch VK, Jonnala G, Seskar M
and Cooper M: Phase 1, placebo-controlled, dose escalation trial of
chicory root extract in patients with osteoarthritis of the hip or
knee. BMC Musculoskelet Disord. 11:1562010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Huseini HF, Alavian SM, Heshmat R, Heydari
MR and Abolmaali K: The efficacy of Liv-52 on liver cirrhotic
patients: A randomized, double-blind, placebo-controlled first
approach. Phytomedicine. 12:619–624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Soda K, Dobashi Y, Kano Y, Tsujinaka S and
Konishi F: Polyamine-rich food decreases age-associated pathology
and mortality in aged mice. Exp Gerontol. 44:727–732. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kiechl S, Pechlaner R, Willeit P,
Notdurfter M, Paulweber B, Willeit K, Werner P, Ruckenstuhl C,
Iglseder B, Weger S, et al: Higher spermidine intake is linked to
lower mortality: A prospective population-based study. Am J Clin
Nutr. 108:371–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Pietrocola F, Castoldi F, Kepp O,
Carmona-Gutierrez D, Madeo F and Kroemer G: Spermidine reduces
cancer-related mortality in humans. Autophagy. 15:362–365. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Raj KP, Zell JA, Rock CL, McLaren CE,
Zoumas-Morse C, Gerner EW and Meyskens FL: Role of dietary
polyamines in a phase III clinical trial of difluoromethylornithine
(DFMO) and sulindac for prevention of sporadic colorectal adenomas.
Br J Cancer. 108:512–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Imam KMSU, Azam FMS, Jahan R and
Rahmatullah M: Anticancer properties of anthocyanins: A
reviewNatural Products: Research Reviews. Gupta VK: 4. Daya
Publishing House; pp. 1–20. 2016
|
|
145
|
Klippel KF, Hiltl DM and Schipp B: A
multicentric, placebo- controlled, double-blind clinical trial of
beta-sitosterol (phytosterol) for the treatment of benign prostatic
hyperplasia. German BPH-Phyto Study group. Br J Urol. 80:427–432.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Garcia-Peris P, Velasco C, Hernandez M,
Lozano MA, Paron L, de la Cuerda C, Breton I, Camblor M and Guarner
F: Effect of inulin and fructo-oligosaccharide on the prevention of
acute radiation enteritis in patients with gynecological cancer and
impact on quality-of-life: A randomized, double-blind,
placebo-controlled trial. Eur J Clin Nurtr. 70:170–174. 2016.
View Article : Google Scholar
|
|
147
|
Rosa LS, Silva NJA, Soares NCP, Monteiro
MC and Teodoro AJ: Anticancer properties of phenolic acids in colon
cancer a review. J Nutr Food Sci. 6:4682016.
|