Introduction
Nasopharyngeal carcinoma (NPC) is a cancer that is
distinct from other epithelial tumors of the head and neck region
(1). NPC has a peculiar geographical
distribution, and its incidence is higher in Southern China and
South-Central Asia, representing the most frequent carcinoma of the
head and neck region in these geographical regions (1,2). Efficient
imaging techniques are crucial for staging and planning the correct
radiotherapy treatment for patients with NPC (1). Integrated positron emission
tomography-computed tomography (PET/CT) using fluorine-18
fludeoxyglucose (18F-FDG), which provides metabolic
parameters such as the standardized uptake value (SUV) and total
lesion glycolysis, is a widely used techniques in cancer staging
assessment and for predicting clinical outcomes (3,4). PET/CT is
sensitive and accurate for the detection of nodal metastases and
distant metastases, but lacks the soft tissue resolution compared
to the magnetic resonance imaging (MRI) when analyzing primary
tumor (5–7). Similarly, MRI provides a higher
resolution than CT in terms of assessing parapharyngeal spaces,
marrow infiltration of the skull base, intracranial disease and
deep cervical nodes in NPC (1).
Additionally, the development of the MRI technology, including
diffusion and perfusion MRI, has added the potential to analyze
biological features in addition to the structural analysis
(1).
PET and MR both provide functional parameters:
Cellular glucose metabolism as expressed by SUV can be identified
by PET, and the random Brownian motion of water molecules expressed
by the apparent diffusion coefficient (ADC) can be analyzed by MRI
(8). Many malignant tumors show a
significantly increased glucose uptake, and malignant tumor with
high cellularity exhibit higher diffusions restrictions, which are
indicated by a low ADC (9–14). Therefore, based on the hypothesis that
SUV and ADC provide similar information about tumors and show a
negative correlation, many previous studies reported the
correlation between SUV and ADC for various tumors, but the results
are not homogenous (8–19). An inverse correlation was demonstrated
in non-small cell lung cancer (9),
breast cancer (10–12), gastrointestinal stromal tumor
(13) and cervical cancer (14). However, data from patients with
lymphoma suggested that SUV and ADC are independent biomarkers
(15,16). For head and neck squamous cell
carcinoma (HNSCC), it is unclear whether there is a significant
correlation between SUV and ADC (8,17–19).
The movement of water molecules contributing to the
signal in diffusion-weighted imaging (DWI) are caused by different
factors: Extracellular space diffusion, intracellular space
diffusion and intravascular space diffusion (or perfusion)
(20–22). Accordingly, the signal attenuation on
conventional mono-exponential DWI does not always present a linear
relationship, and it is difficult to calculate an accurate ADC
value (23). In order to separate the
motion of water molecules due to perfusion in the microcirculation
from that due to diffusion in the extravascular space, the
intravoxel incoherent motion (IVIM) imaging method, first described
by Le et al (22) may be used.
IVIM is a powerful imaging technique that uses DWI with multiple b
values followed by bi-exponential curve fitting. IVIM can provide
the true diffusion coefficient (D), the pseudo-diffusion
coefficient or diffusion within the microcirculation (D*), and the
perfusion fraction or the contribution of water moving in the
capillaries (f). Several studies have reported the application of
IVIM in NPC, and these previous reports have shown that IVIM is a
feasible technique (24,25). In addition, IVIM parameters have been
associated with clinical stage (26)
and dynamic contrast enhanced-MRI parameters (25). IVIM can be applied to differentiating
lymphoma (27) and enlarged adenoids
(24), and can predict the early
treatment response of patients with NPC (28,29).
PET/MR, a new medical imaging tool, combines the
metabolic information of PET with the anatomical detail of MR, thus
exhibiting good potential for clinical application (30). There are two types of PET/MR systems:
Sequential PET/MR, in which PET and MR data are acquired
sequentially, either in a single room or using a tri-modality
PET/CT-MRI system, and simultaneous PET/MR, in which PET and MR are
integrated systems (30,31). Several previous studies have
investigated the diagnostic performance of PET/MR compared with
PET/CT in head and neck cancer (32–38).
Certain previous studies examined the clinical potential of PET/MR
in NPC using simultaneous PET/MR, and no significant correlation
between SUV and ADC in NPC was found by conventional
mono-exponential DWI (39,40). However, the feasibility of a routine
application of PET/CT-MRI and the correlation between the IVIM
parameters and SUV need to be further explored in NPC. Therefore,
the aim of the present study was to assess the performance of
PET/MR for the visualization and characterization of lesions and to
further explore the correlation between SUV and ADC value, between
SUV and D value, between SUV and D* value, and between SUV and f
value using sequential PET/MR in NPC.
Materials and methods
Patients
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Jinan University, and
written informed consent was obtained from each participant.
Inclusion criteria were as follows: All patients were diagnosed
with NPC using histology, all underwent whole body
18F-FDG PET/CT, and all patients were examined with
regular head and neck using a PET/CT-MRI system, and none received
anti-tumor treatment before the PET/CT-MRI scan. Exclusion criteria
were the following: Patients under 18 years of age, patients with
claustrophobia and patients with MRI-incompatible medical devices,
including cardiac pacemakers, cochlear implants and insulin
pumps.
Between June 2015 and October 2017, 35 patients (7
women and 28 men; age, 50.4±11.7 years; age range, 24–77 years)
were included. All patients presented non-keratinizing
undifferentiated carcinoma. There were two patients at stage T1,
four at T2, 14 at T3 and 15 at T4 primary tumors (41). Nodal metastases and distant metastases
were found in eight and 31 patients, respectively. All of the
patients underwent additional multiple b-value DWI scans. However,
two patients were excluded from further evaluation of the
correlation between the SUV and ADC, and D*, D and f values,
because of image distortions in the DW images.
Imaging modalities
All of the patients underwent a tri-modality
PET/CT-MRI (full ring; time-of-flight Discovery PET/CT 690; 3T
Discovery MR 750; GE Healthcare) comprising a sequential whole-body
18F-FDG PET/CT and a regular head and neck MRI. The
patients were positioned on a dedicated shuttle board, facilitating
their transport from the PET/CT to the MRI system without
movement.
PET/CT imaging
The patients fasted for at >6 h before scanning
and their blood glucose levels were measured immediately before the
tracer injection. The patients were intravenously administered with
18F-FDG with an activity range of 0.08–0.10 mCi/kg body
weight, and the data acquisition was performed after an uptake time
of 50–70 min. The data acquisition was performed from the skull
base to the mid-thigh, which were used for both the PET and CT
modalities. The CT data were acquired with an automatic dose
modulation at 120 kV, a range of 80–160 mA, a 512×512 image matrix,
a field of view (FOV) of 50 cm, a noise index of 30, reconstructed
slice thickness of 3.75 mm, and a slice interval of 3.27 mm. The
PET protocol encompassed seven bed positions with a scan duration
of 120 sec per bed position. The PET data was acquired in 3D
time-of-flight mode using the adaptive statistical iterative
reconstruction (42) with an axial
FOV of 70 cm, reconstructed slice thickness of 3.27 mm, a slice
interval of 3.75 mm and a 192×192 image matrix. The attenuation
correction was based on the whole-body CT.
MRI
After PET/CT procedure, the patients were
transferred to the MRI via a dedicated shuttle board. The MRI scans
were acquired using the dedicated RF-covered nasopharynx coil. No
contrast medium was applied during MRI. The imaging acquisition
parameters for the applied MR sequences are presented in Table I.
 | Table I.Acquisition parameters for the
applied MR sequences. |
Table I.
Acquisition parameters for the
applied MR sequences.
|
| FSE T1WI | FRFSE T2WI | SS-EPI DWI |
|---|
|
|
|
|
|
|---|
| Parameter | Axial | Sagittal | Axial | Coronal | Axial |
|---|
| TR/TE, ms | 466/14.1 | 350/13.8 | 3,877/81.3 | 3,023/108.2 | 3,000/80 |
| FOV, cm | 20×20 | 26×26 | 20×20 | 26×26 | 24×24 |
| Thickness, mm | 3.5 | 4.0 | 3.5 | 4.0 | 3.5 |
| Slice space,
mm | 0.5 | 1.5 | 0.5 | 0.5 | 0.5 |
| Matrix | 288×224 | 320×256 | 288×256 | 288×256 | 128×128 |
| Acceleration
factor | 2 | 2 | 2 | 2 | 2 |
| Bandwidth, kHz | 31.2 | 31.2 | 41.7 | 41.7 | 250 |
| Flip angle, ° | 111 | 111 | 111 | 111 | 90 |
| B Values,
s/mm2 | NA | NA | NA | NA | 0, 50, 200, 500,
800, 1,000, 1,500, 2,000, 3,000 |
| NEX | 2 | 1 | 3 | 3 | 1, 1, 1, 1, 2, 2,
4, 4, 6 |
Image analysis
All the images were analyzed by two investigators
who evaluated both data sets. One investigator was an experienced
nuclear medicine physician with 4 years of experience in MRI, and
the second was a radiologist with additional experience in PET/CT.
The PET, CT, and MRI data were sent to a dedicated review
workstation (GE Advantage workstation, version 4.6; GE Healthcare
Life Sciences) that allowed the simultaneous review of PET, CT and
MR images side by side or in fused/overlay mode (PET/CT, T1w PET/MR
and T2w PET/MR imaging).
Visualization and characterization of
lesions
Each PET examination was evaluated for the presence
of PET-positive lesions within the body area that was covered by
the head and neck MR imaging. NPC cases were considered
PET-positive primary tumors if their maximum standardized uptake
value (SUVmax) was markedly higher than the surrounding background
activity. Cervical lymph node metastases were defined based on both
functional and morphological criteria. The functional criteria used
for the PET compound were: i) Visually detectable metabolic
activity higher than normality; or ii) asymmetric metabolic
activity greater than that of normal-appearing lymph nodes at the
same level in the contralateral neck (43). The morphological criteria for lymph
node metastases on CT and MRI included (44): i) A shortest axial diameter of >5
mm in the retropharyngeal region and >10 mm in other regions of
the neck; ii) any nodes with necrosis or extracapsular spread; and
iii) a group with >2 nodes with a shortest axial diameter of ~5
mm in the retropharyngeal region and ~10 mm in other regions of the
neck.
Image quality
A 3-point scale was used for the assessment of image
quality: i) 1=substantial artefacts with insufficient image quality
for further assessment; ii) 2=mild artefacts with sufficient image
quality for lesion assessment; and iii) 3=absence of relevant
artefacts.
Lesion conspicuity
Lesion conspicuity was graded on a 4-point scale
according to the lesion delineation: i) 1, not detectable or poorly
delineated (less than 25% of lesion borders definable); ii) 2,
moderately delineated lesion borders (25–50% of borders definable);
iii) 3, well delineated lesion borders (50–75% of borders
definable); and iv) 4, excellently delineated (>75% of lesion
borders definable).
Diagnostic confidence
To identify whether CT imaging and MR imaging can
increase the diagnostic confidence for PET-positive primary tumors
when characterizing tumor lesions, three scores were assigned: A
grade of 1 indicated that MR imaging provided more relevant
information than CT imaging, including infiltration of the adjacent
structures such as the skull-base bone and prevertebral muscle. A
grade of −1 indicated that CT imaging provided more information
than MR imaging, including subtle bone destruction not visible on
MR imaging. A grade of 0 indicated that both CT imaging and MR
imaging provided the same information.
SUV, ADC, and D*, D and f values
measurements
Using the dedicated review workstation (GE Advantage
workstation; version 4.6; GE Healthcare Life Sciences), SUV of each
voxel was calculated according to the following equation:
SUV=measuredradioactivityconcentration[Bq/mL]injectedradioactivity[Bq]/(leanbodymass[kg]x1000)
The SUVmax and mean SUV (SUVmean) were calculated on
the basis of activity values in regions of interest manually placed
on the area of the tumor containing the highest-SUV pixel. The
margin of the tumor was determined by visual inspection.
Subsequently, each investigator measured the IVIM
parameters (D*, D and f values) using the standard software on the
workstation. The bi-exponential model from an IVIM sequence was
expressed using the following equation, as described by Le et
al (45): Sb/S0=(1-f) exp (-b D)
+ f exp [-b (D* + D)]. Where Sb is the signal intensity with
diffusion gradient b; S0 is the signal intensity for a b value of 0
s/mm2; D is the true diffusion coefficient as reflected
by pure molecular diffusion; f is the fractional perfusion related
to microcirculation; and D* is the pseudo-diffusion coefficient
related to perfusion.
In addition, each investigator extracted the DW
image with b values of 0 and 1,000 s/mm2 to calculate
the ADC using a mono-exponential model with the following equation:
Sb/S0=exp (-b ADC).
The regions of interest were manually drawn to
delineate the borders of the primary tumor, in order to cover as
much of the tumor as possible on each slice of the axial DW images
that corresponded to the high-signal area on T2-weighted imaging
with fat suppression, and avoiding apparent cystic changes,
necrosis and adjacent anatomic structures (Fig. 1). The IVIM parameters obtained for
each tumor were calculated on a pixel-by-pixel basis and expressed
as the mean values of all pixels within the volume of interest. The
minimum ADC (ADCmin) and mean ADC (ADCmean) were calculated for all
primary tumors, with the ADCmin defined as the lowest ADC value
within the whole tumor on the ADC map. Therefore, for each primary
tumor, the ADCmean was defined as the mean value of all the tumor
pixels on the ADC map.
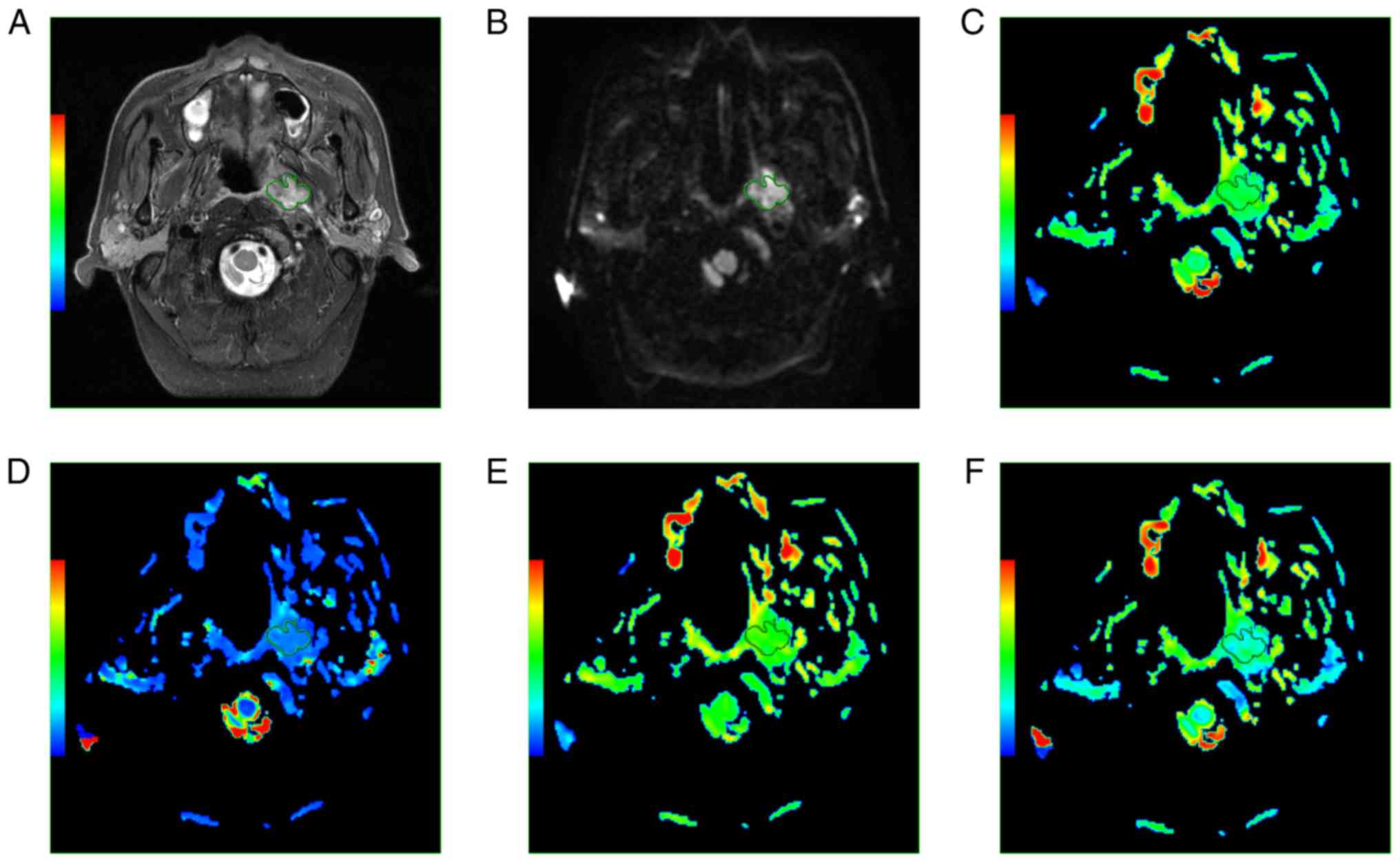 | Figure 1.A 62-year-old male patient with NPC.
(A) Axial T2w imaging and (B) axial DW imaging showing a region of
interest around the margin of NPC. (C) ADC, (D) D*, (E) D and (F) f
maps. The ADC, D*, D, and f values were 8.43×10−4
mm2/s, 4.27×10−3 mm2/s,
5.59×10−4 mm2/s, and 0.291, respectively.
NPC, nasopharyngeal carcinoma; ADC, apparent diffusion coefficient;
D, true diffusion coefficient; D*, pseudo-diffusion
coefficient. |
Statistical analysis
The data are presented as the mean ± SD. The
differences between image quality and lesion conspicuity in PET/CT,
T1w PET/MR, and T2w PET/MR imaging were analyzed using Friedman M
test. The Nemenyi post hoc test was used to further evaluate the
differences between two methods. The inter-observer agreement for
the ADC, D*, D and f values was evaluated using intraclass
correlation coefficients (ICC). Pearson's correlation coefficient
analysis was used to evaluate the correlation between the ADC
(ADCmin and ADCmean), D*, D and f values and the SUV (SUVmax and
SUVmean). P<0.05 was considered to indicate a statistically
significant difference.
Results
Lesion detection
A total of 99 PET-positive lesions were evaluated,
including 35 primary tumors and 64 lymph nodes. Among the 64 lymph
nodes, there were 25 in the retropharyngeal space and 39 in other
regions of the neck. All of the lesions had increased
18F-FDG uptake and all of the primary tumors could be
detected by PET/CT, T1w PET/MR, and T2w PET/MR imaging. For the
lymph nodes, PET/CT and T1w PET/MR imaging detected 61 lymph nodes,
and the three other retropharyngeal lymph nodes were not detected
by either PET/CT or T1w PET/MR imaging. However, T2w PET/MR imaging
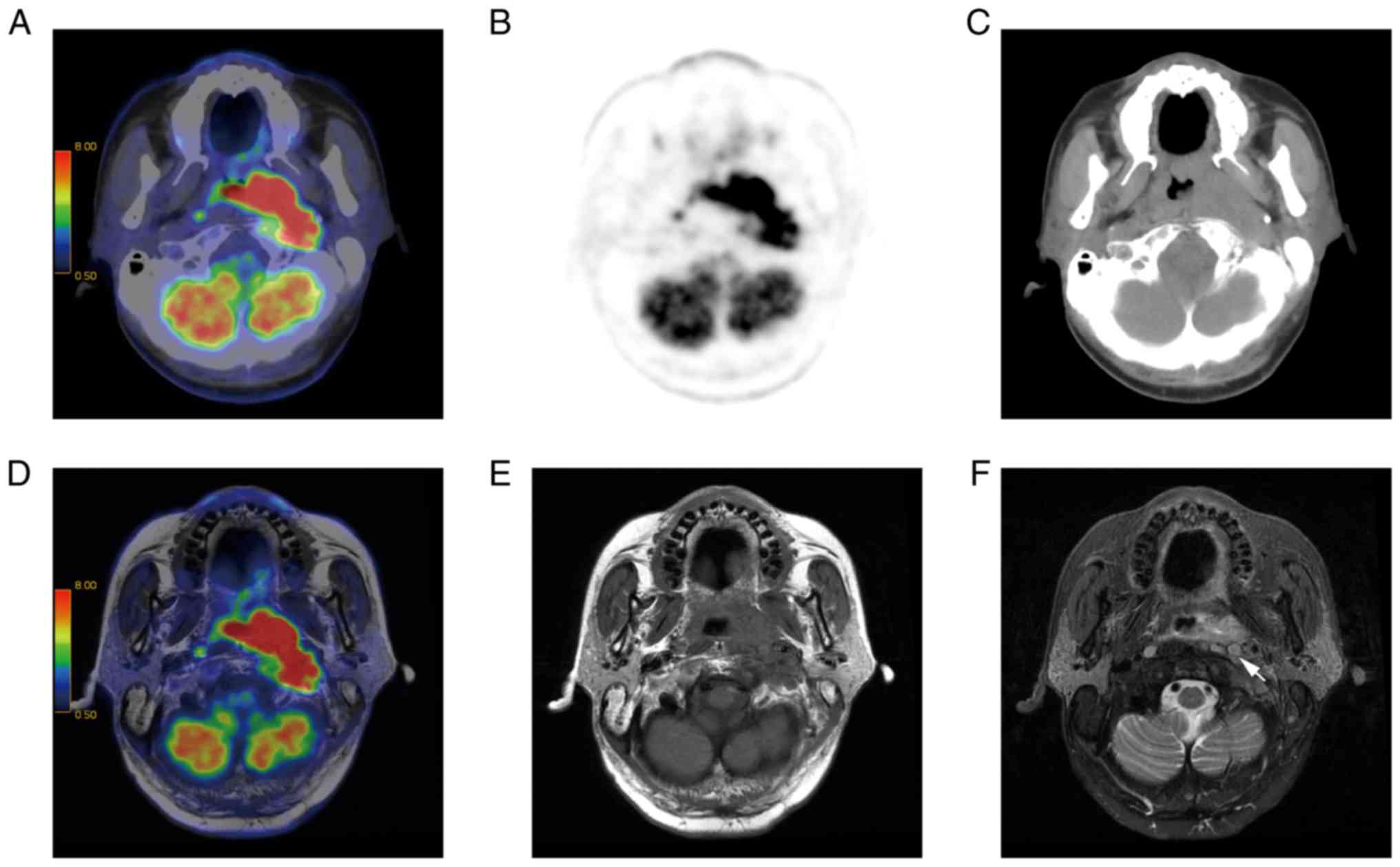
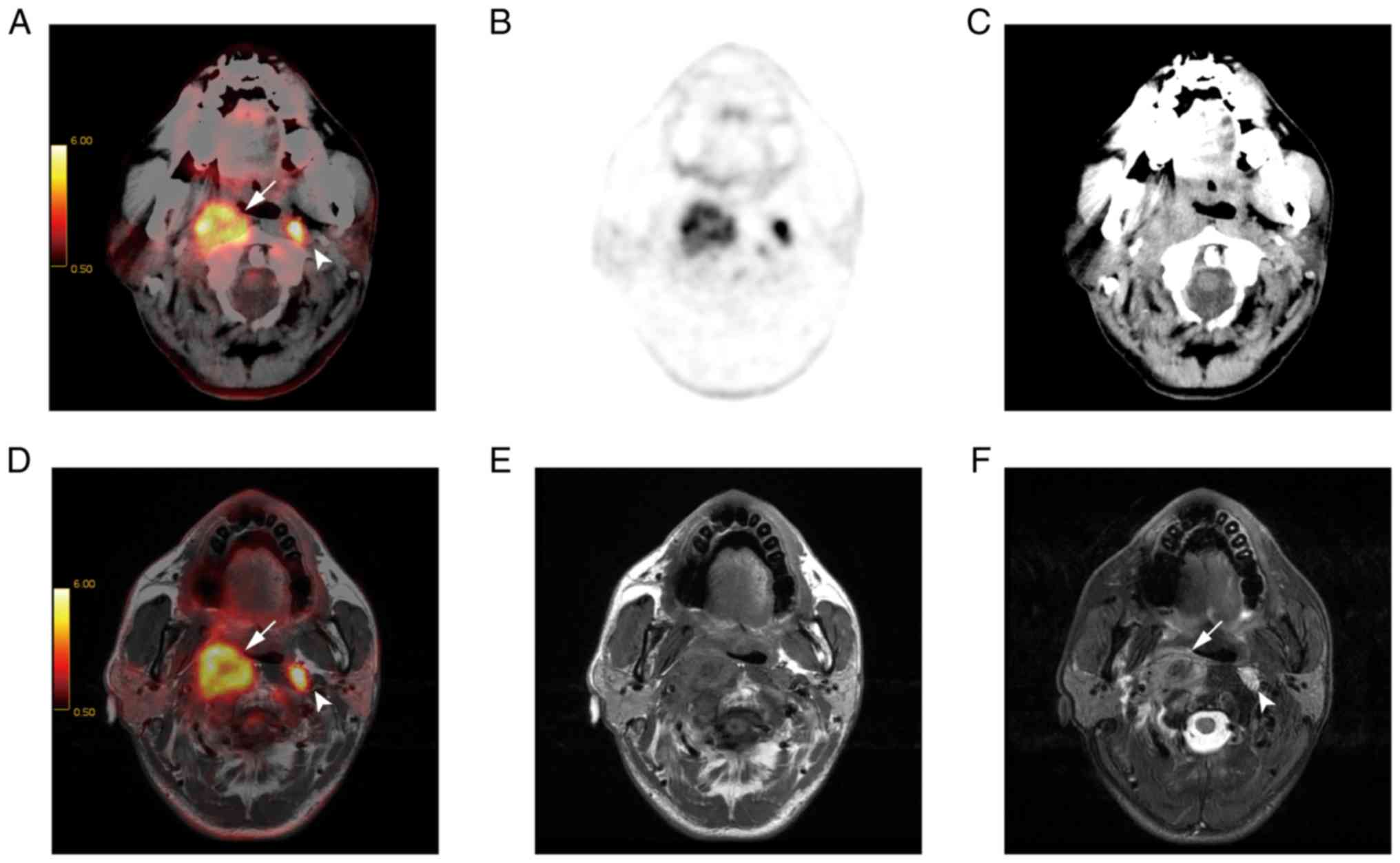
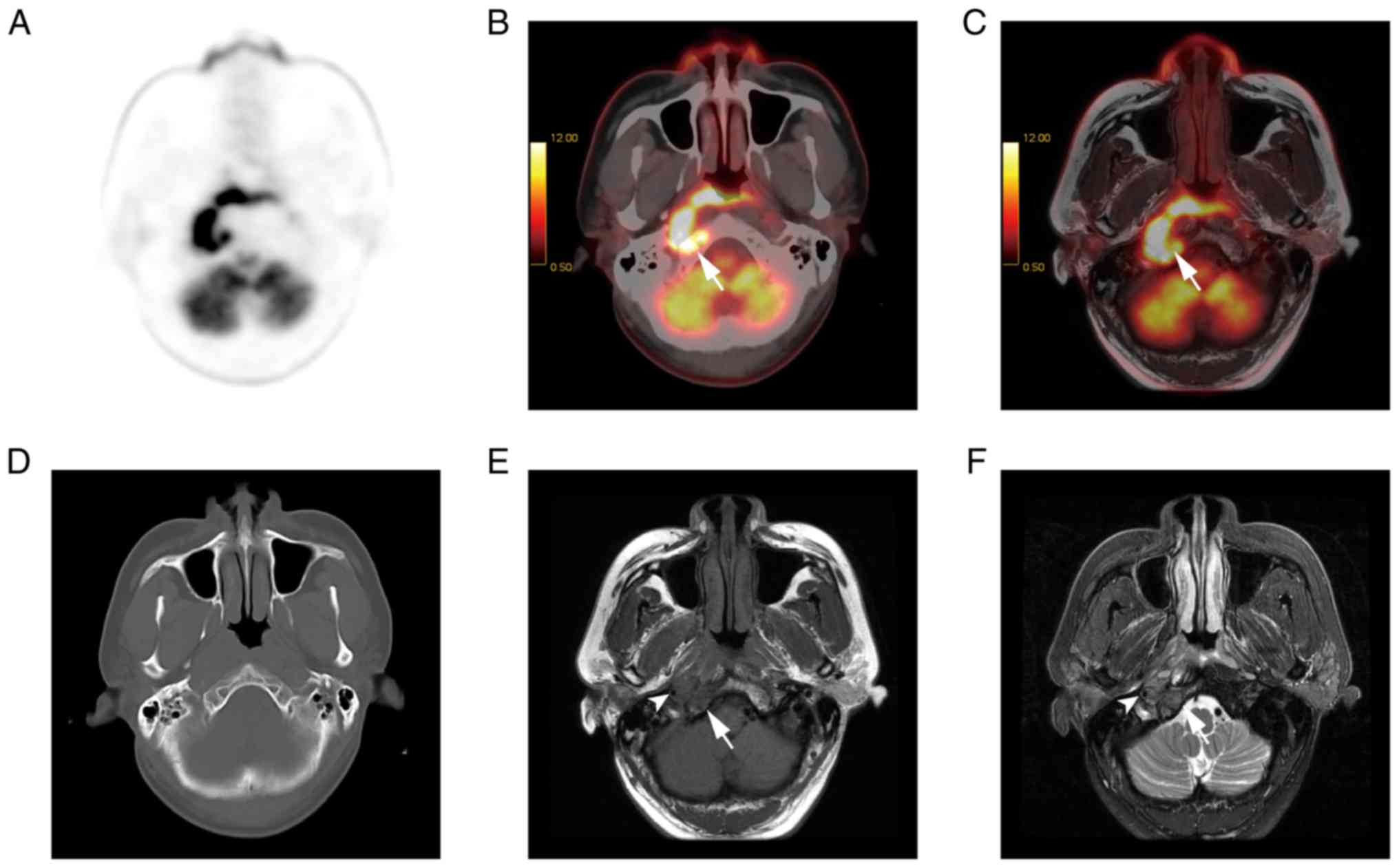
detected all of the lymph nodes. T2w PET/MR imaging is presented in
Fig. 2.
Image quality
No statistically significant differences were found
between the artefact grading, an indicator of image quality, in T1w
PET/MR, T2w PET/MR imaging and PET/CT (Tables II and III). The artefacts did not originate in
the nasopharynx but in the oral cavity, and these artefacts were
caused by dental implants and, in one case, by eyeball motion. All
of the dental implant artefacts were found in T1w PET/MR, T2w
PET/MR and PET/CT, whereas the eyeball motion artefacts were found
only in T2w PET/MR imaging (Table
II).
 | Table II.Artefact graded for the three
techniques. |
Table II.
Artefact graded for the three
techniques.
| Grade | PET/CT (%) | T1w PET/MR (%) | T2w PET/MR (%) |
|---|
| No | 88.6 | 88.6 | 80.0 |
| Mild |
8.6 | 11.4 | 20.0 |
| Substantial |
2.8 | 0 | 0 |
 | Table III.Image quality and lesion conspicuity
in the three imaging techniques. |
Table III.
Image quality and lesion conspicuity
in the three imaging techniques.
| Parameter
examined | PET/CT | T1w PET/MR | T2w PET/MR | P-value |
|---|
| Image quality,
median (range) | 3 (1–3) | 3 (2–3) | 3 (2–3) | 0.174 |
| Lesion
conspicuity |
|
|
|
|
| Primary
tumors, median (range) | 2 (1–3) | 3 (2–4) | 4 (3–4) | <0.001 |
| Lymph
nodes, median (range) | 3 (1–4) | 4 (1–4) | 4 (3–4) | <0.01 |
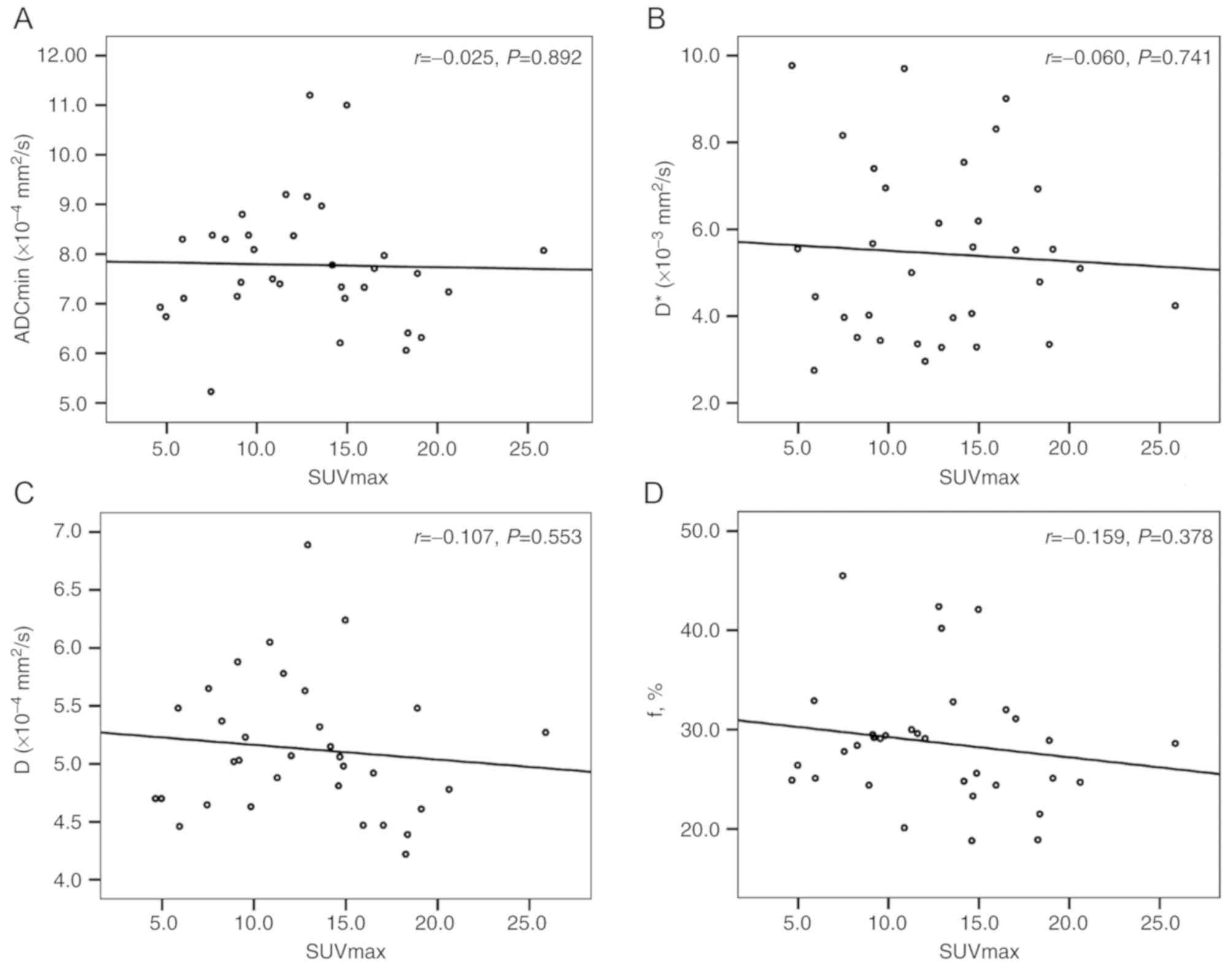
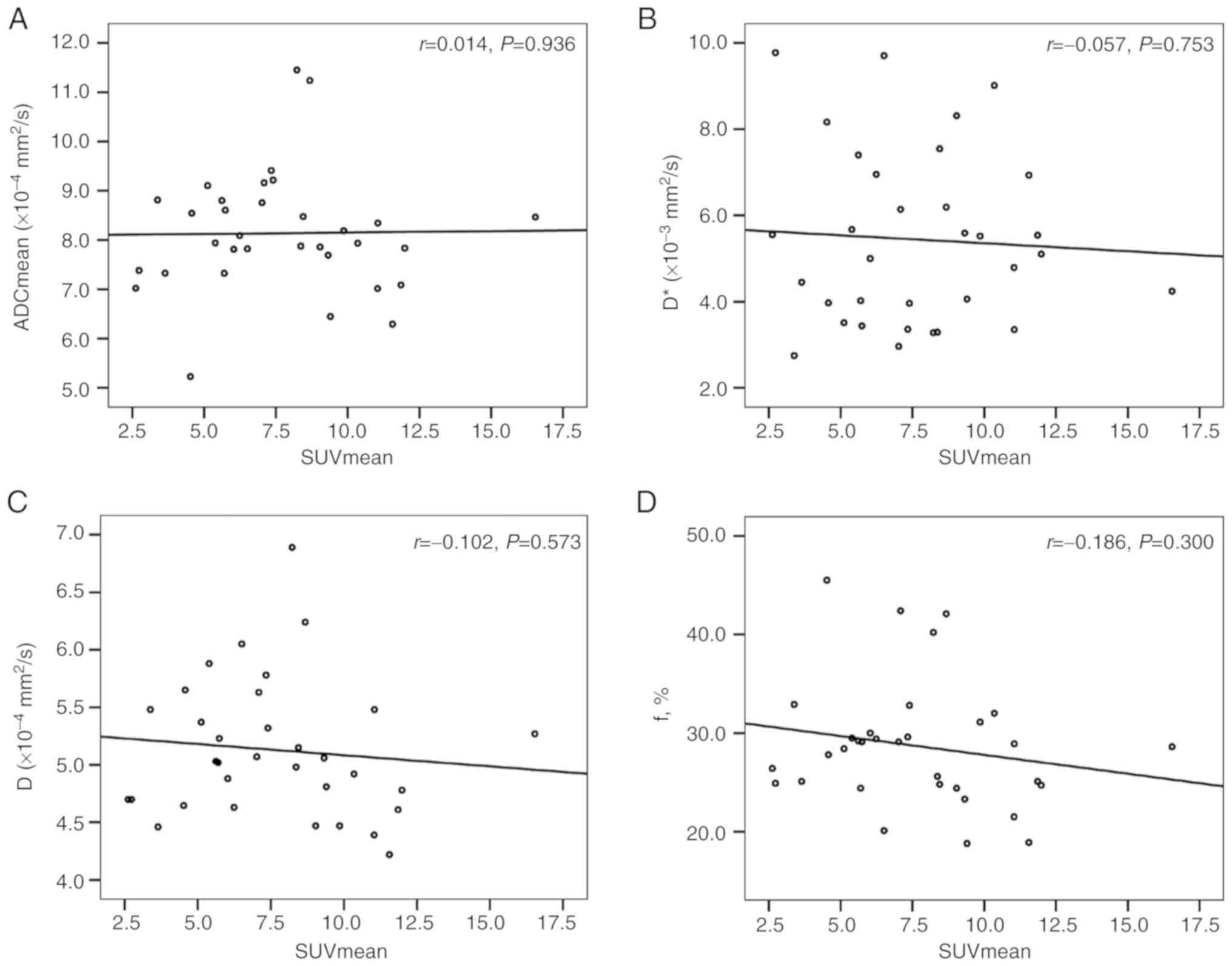
Correlations between the SUV and ADC,
and D, D* and f values
There was no correlation between the ADC (ADCmin and
ADCmean), D, D* and f values and the SUV (SUVmax and SUVmean) for
the primary tumors (Table IV;
Figs. 3 and 4).
 | Table IV.Correlations between the SUV and ADC,
and D, D* and f values. |
Table IV.
Correlations between the SUV and ADC,
and D, D* and f values.
| Correlation | r | P-value |
|---|
| SUVmax and
ADCmin | −0.025 | 0.892 |
| SUVmax and D | −0.107 | 0.553 |
| SUVmax and D* | −0.060 | 0.741 |
| SUVmax and f | −0.159 | 0.378 |
| SUVmean and
ADCmean | 0.014 | 0.936 |
| SUVmean and D | −0.102 | 0.573 |
| SUVmean and D* | −0.057 | 0.753 |
| SUVmean and f | −0.186 | 0.300 |
Lesion conspicuity
Regarding the conspicuity of the primary tumors and
lymph nodes, there were significant differences between PET/CT, T1w
PET/MR imaging and T2w PET/MR imaging (Tables III and V). Further comparison indicated that T1w
PET/MR imaging and T2w PET/MR imaging were significantly more
efficient than PET/CT in primary tumors (both P<0.01) and in
lymph nodes (both P<0.01; Fig. 5).
T2w PET/MR imaging was more efficient than T1w PET/MR imaging in
primary tumors and in lymph nodes (data not shown).
 | Table V.Lesion conspicuity in the three
imaging techniques. |
Table V.
Lesion conspicuity in the three
imaging techniques.
|
| Primary tumors | Lymph nodes |
|---|
|
|
|
|
|---|
| Grade | PET/CT (%) | T1w PET/MR (%) | T2w PET/MR (%) | PET/CT (%) | T1w PET/MR (%) | T2w PET/MR (%) |
|---|
| Poor | 28.6 | 0 | 0 | 15.6 |
4.7 | 0 |
| Moderate | 48.6 | 14.3 | 0 | 15.6 |
3.1 | 0 |
| Well | 22.8 | 40.0 | 14.3 | 20.3 | 10.9 | 1.6 |
| Excellent | 0 | 45.7 | 85.7 | 48.5 | 81.3 | 98.4 |
Diagnostic confidence
Regarding primary tumors characterization, T1w
PET/MR imaging was considered superior to PET/CT in 71.4% of cases,
equal to PET/CT in 22.9% of cases, and inferior to PET/CT in 5.7%
of cases. T2w PET/MR imaging was considered superior to PET/CT in
77.2% of cases (Fig. 6), equal to
PET/CT in 17.1% of cases, and inferior to PET/CT in 5.7% of
cases.
SUV and ADC, and D*, D and f
values
The mean SUVmax and mean SUVmean values of all
primary tumors were 12.6±4.9 (range 4.7–25.9) and 7.6±3.0 (range
2.6–16.5), respectively (data not shown). The means of the ADCmin,
ADCmean, D*, D and f values of all primary tumors were (7.78±1.25)
×10−4 mm2/s, (8.14±1.23) ×10−4
mm2/s, (5.44±2.01) ×10−3 mm2/s,
(5.13±0.59) ×10−4 mm2/s and 28.68±6.40%,
respectively.
Inter-observer agreement
The ICCs (95% CI) of the inter-observer
reproducibility for the measurements of the ADC, D*, D and f values
were 0.990 (0.983 to 0.994), 0.970 (0.940 to 0.985), 0.898 (0.801
to 0.948) and 0.969 (0.938 to 0.985), respectively, which suggested
good inter-observer reproducibility and consistency.
Discussion
PET/CT has high sensitivity in diagnosing NPC, and
MR shows a sensitivity of ~100% (46). In the present study, a total of 35 NPC
primary tumors had increased 18F-FDG uptake and were
detected on PET/CT and PET/MR, in line with a previous study
(39). However, Chen et al
(47) reported only 15 patients with
FDG uptake out of 20 newly-diagnosed patients with NPC. This
difference may be due to the pathological subtype of NPC
investigated in the present study. In fact, the NPC examined was a
non-keratinizing undifferentiated carcinoma of a relatively simple
type, which resulted in high 18F-FDG uptake from the
NPC. In terms of tumor conspicuity, PET/CT did not efficiently
delineated the tumor borders, whereas 85.7% of lesion borders could
be efficiently delineated by T2w PET/MR imaging. These present
results showed that tumor conspicuity was significantly higher in
T2w PET/MR imaging than in PET/CT. However, the quality of PET/MR
imaging was enough to delineate lesions relative to the surrounding
soft tissue. Considering the potential infiltration of NPC into the
adjacent structures, such as the prevertebral muscle, internal
carotid artery and skull base, as well as intracranial spread,
PET/MR showed more reliable determination of tumor tissue.
FDG PET/CT has a growing role in the diagnosis and
management of NPC. The clinical value of 18F-FDG PET/CT
for evaluating the T stage of patients with NPC is limited by the
spillover effect of 18F-FDG, low spatial resolution and
imaging misregistration (2,48). The present study showed that the tumor
conspicuity and the diagnostic confidence of PET/MR were
significantly higher than those of PET/CT. These parameters are of
particular importance because the T classification of NPC depends
on the infiltration of adjacent structures. A previous study also
showed that 18F-FDG PET/MR is more accurate than head
and neck MRI and PET/CT in terms of tumor staging (39).
NPC is prone to cervical lymph node metastasis and
the retropharyngeal lymph nodes are considered first echelon nodes
(1). Accurate detection of lymph node
metastasis is important for N staging (41) in NPC. In the present study, a total of
64 suspicious metastatic lymph nodes were detected by T2w PET/MR.
However, PET/CT and T1w PET/MR imaging detected 61 lymph nodes,
apart from three retropharyngeal lymph nodes. Since the three
retropharyngeal lymph nodes were merging with the primary tumors,
PET/CT and T1w PET/MR imaging could not separate the
retropharyngeal lymph nodes from the primary tumors, while a clear
distinction between the primary tumor and the attached
retropharyngeal lymph nodes was achieved using T2w PET/MR
imaging.
Considering the conspicuity of the lymph nodes, the
present results showed that T1w PET/MR imaging and T2w PET/MR
imaging were more efficient than PET/CT. However, a previous study
reported no significant difference between contrast-enhanced
PET/CT, T2w PET/MR imaging, and contrast-enhanced PET/MR in the
detection and conspicuity of cervical lymph nodes (33). This discrepancy may be due to the fact
that 42.1% (32/76) of the lymph nodes were retropharyngeal in the
present study, and CT without contrast medium does not define the
margins in retropharyngeal lymph nodes. Since MR has high soft
tissue contrast, especially T2w MR imaging with fat suppression, it
is efficient to detect and to delineate lymph nodes, and this
modality is also adequate for the detection of cystic or necrotic
lymph nodes.
Intensity-modulated radiotherapy (IMRT) is the
primary and preferred treatment method for NPC (1), and the accurate delineation of target
volumes and normal organs relies on clear lesion borders. CT, MR
and MR/CT fusion imaging are the basic methods for delineating the
target volumes, and PET/CT facilitates radiation therapy planning
(49). Moreover, in the present
study, it was showed that T1w PET/MR imaging and T2w PET/MR imaging
performed significantly better than PET/CT with respect of the
conspicuity of the primary tumors and lymph nodes. PET/MR
delineated more lesion borders, and it may be possible to determine
and deliver the most appropriate radiation dose level to different
parts of the target volume with IMRT. The volume of interest (VOI)
of DWI and the VOI of FDG PET were not completely overlapping and
the volume defined by cluster analysis might be useful for dose
painting (40), which has been
applied in radiotherapy for NPC.
Whether SUV and ADC calculated by conventional
mono-exponential DWI are statistically correlated or independent
biomarkers in tumors has been investigated by numerous previous
studies (8–19,40). Cao
et al (40) reported that no
significant correlation between SUV and ADC was observed in NPC.
The present results were concordant with these previous findings,
indicating that these two biomarkers are independent in NPC; the
present results are also in line with previous studies in HNSCC
(8,17,19).
However, Nakajo et al (18)
found a significant negative correlation between SUVmax and
ADC800 in 26 patients with HNSCC, while Choi et
al (19) showed that the ADCratio
(ADC2000/ADC1000) was significantly and
positively correlated to SUV. There were some differences in these
previous studies in the interval times between PET and MR scans,
which ranged between 3 days and 2 weeks. In the present study, PET
and MR scanning were performed on the same day using sequential
PET/MR. Therefore, the correlation between SUV and ADC may be made
more reliable by shortening the time interval between PET and MR
analyses.
Traditional DWI via the conventional
mono-exponential model cannot distinguish between true diffusion
and microcirculatory perfusion (27).
It has been confirmed that microcirculation of the blood or
perfusion in capillary networks can substantially affect the
measurement of ADC values (21).
According to the IVIM theory, D represents true molecular
diffusion, while D* is proportional to the average blood velocity
and the mean capillary segment length (50), which indicates that D* is linked to
vascularity and perfusion in tissues. The f value is another
perfusion-related parameter corresponding to the fractional volume
(percentage) of capillary blood flow (27). Therefore, whether IVIM parameters (D*,
D and f values) had any significant correlation with SUV in NPC
were investigated in the present, and IVIM showed the ability to
separately quantitate the diffusion and perfusion effects.
The present preliminary results showed that there
was no significant correlation between the SUV and D*, D and f
values. ADC represents the total diffusion value and D represents
true diffusion, and there was no correlation between these two
factors with SUV regarding cellular glucose metabolism.
Furthermore, no relationship was found between cellular glucose
metabolism and cellular perfusion in the present study. The SUV and
D, D*, and f values are independent biomarkers in NPC. Although the
possible correlation between SUV and ADC remains controversial, and
there was no significant correlation between the SUV and D*, D and
f values in NPC, these parameters are all biomarkers that may
potentially predict early treatment responses. Additional work
should investigate the correlation between the SUV and D*, D and f
values in other tumors.
Notably, the present study had several limitations.
No contrast medium was applied during MRI and CT, therefore, it was
not possible to analyze the lesion characterization in cePET/CT and
cePET/MR imaging. In addition, the PET/MR comprised only a head and
neck protocol, so no other body were analyzed by the MR imaging to
evaluate distant metastasis. Moreover, the patient cohort was
relatively small, and all NPC patients presented undifferentiated
carcinomas. Therefore, a larger sample size should be used for
further investigation and the correlation between the SUV and ADC,
D, D* and f values of differentiated carcinomas should be further
analyzed. Further studies are required to analyze whether the
biomarkers of NPC may show a correlation with tumor
differentiation.
The present study suggested that PET/MR was more
efficient in the visualization and characterization of NPC compared
with PET/CT. As a result, PET/MR showed high lesion detection and
good image quality. Additionally, PET/MR offered higher lesion
conspicuity and diagnostic confidence for NPC, which may facilitate
radiotherapy planning and tumor classification. However, no
correlations were identified between the SUV assessed by
18F-FDG PET/CT, and the ADC, D, D* and f values
determined by IVIM with multiple b values in NPC. The present
findings suggested that SUV and ADC, D, D* and f values are
independent biomarkers in NPC, and they may provide different
information, thus facilitating a correct diagnosis.
Acknowledgements
The authors would like to thank Professor Hansheng
Lin (Department of Medical Statistics, School of Medicine, Jinan
University) for his excellent statistics support.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81871383),
the Medical Scientific Research Foundation of Guangdong Province
(grant no. A2018135), the Fundamental Research Funds for the
Central Universities (grant no. 21617311) and the Science and
Technology Program of Guangzhou (grant no. 201804010440).
Availability of data and materials
Additional data are available from the corresponding
author upon reasonable request.
Authors' contributions
YC, LB and HX conceived the present study. YC, LB,
JS and YT collected and analyzed the data. YC, LB, JS, YT, XL, BG,
JG and LW analyzed the data. YC and LB drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The First Affiliated Hospital of Jinan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of nasopharyngeal carcinoma: Current practice and future
perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang KP, Tsang NM, Liao CT, Hsu CL, Chung
MJ, Lo CW, Chan SC, Ng SH, Wang HM and Yen TC: Prognostic
significance of 18F-FDG PET parameters and plasma
Epstein-Barr virus DNA load in patients with nasopharyngeal
carcinoma. J Nucl Med. 53:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Z, Shi Q, Zhang Y, Pan H, Yao Z, Hu
S, Shi W, Zhu B, Zhang Y and Hu C: Pretreatment 18F-FDG
uptake heterogeneity can predict survival in patients with locally
advanced nasopharyngeal carcinoma-a retrospective study. Radiat
Oncol. 10:42015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vellayappan BA, Soon YY, Earnest A, Zhang
Q, Koh WY, Tham IW and Lee KM: Accuracy of
18F-flurodeoxyglucose-positron emission
tomography/computed tomography in the staging of newly diagnosed
nasopharyngeal carcinoma: A systematic review and meta-analysis.
Radiol Oncol. 48:331–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chua ML, Ong SC, Wee JT, Ng DC, Gao F, Tan
TW, Fong KW, Chua ET, Khoo JB and Low JS: Comparison of 4
modalities for distant metastasis staging in endemic nasopharyngeal
carcinoma. Head Neck. 31:346–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang MC, Chen JH, Liang JA, Yang KT,
Cheng KY and Kao CH: Accuracy of whole-body FDG-PET and FDG-PET/CT
in M staging of nasopharyngeal carcinoma: A systematic review and
meta-analysis. Eur J Radiol. 82:366–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varoquaux A, Rager O, Lovblad KO,
Masterson K, Dulguerov P, Ratib O, Becker CD and Becker M:
Functional imaging of head and neck squamous cell carcinoma with
diffusion-weighted MRI and FDG PET/CT: Quantitative analysis of ADC
and SUV. Eur J Nucl Med Mol Imaging. 40:842–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Regier M, Derlin T, Schwarz D, Laqmani A,
Henes FO, Groth M, Buhk JH, Kooijman H and Adam G: Diffusion
weighted MRI and 18F-FDG PET/CT in non-small cell lung
cancer (NSCLC): Does the apparent diffusion coefficient (ADC)
correlate with tracer uptake (SUV). Eur J Radiol. 81:2913–2918.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baba S, Isoda T, Maruoka Y, Kitamura Y,
Sasaki M, Yoshida T and Honda H: Diagnostic and prognostic value of
pretreatment SUV in 18F-FDG/PET in breast cancer:
Comparison with apparent diffusion coefficient from
diffusion-weighted MR imaging. J Nucl Med. 55:736–742. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakajo M, Kajiya Y, Kaneko T, Kaneko Y,
Takasaki T, Tani A, Ueno M, Koriyama C and Nakajo M: FDG PET/CT and
diffusion-weighted imaging for breast cancer: Prognostic value of
maximum standardized uptake values and apparent diffusion
coefficient values of the primary lesion. Eur J Nucl Med Mol
Imagin. 37:2011–2020. 2010. View Article : Google Scholar
|
|
12
|
Kitajima K, Yamano T, Fukushima K, Miyoshi
Y and Hirota S, Kawanaka Y, Miya M, Doi H, Yamakado K and Hirota S:
Correlation of the SUVmax of FDG-PET and ADC values of
diffusion-weighted MR imaging with pathologic prognostic factors in
breast carcinoma. Eur J Radiol. 85:943–949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong CS, Gong N, Chu YC, Anthony MP, Chan
Q, Lee HF, Chu KM and Khong PL: Correlation of measurements from
diffusion weighted MR imaging and FDG PET/CT in GIST patients: ADC
versus SUV. Eur J Radiol. 81:2122–2126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brandmaier P, Purz S, Bremicker K, Höckel
M, Barthel H, Kluge R, Kahn T, Sabri O and Stumpp P: Simultaneous
[18F]FDG-PET/MRI: Correlation of apparent diffusion
coefficient (ADC) and standardized uptake value (SUV) in primary
and recurrent cervical cancer. PLoS One. 10:e01416842015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu X, Korkola P, Pertovaara H, Eskola H,
Järvenpää R and Kellokumpu-Lehtinen PL: No correlation between
glucose metabolism and apparent diffusion coefficient in diffuse
large B-cell lymphoma: A PET/CT and DW-MRI study. Eur J Radiol.
79:e117–e121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giraudo C, Karanikas G, Weber M, Raderer
M, Jaeger U, Simonitsch-Klupp I and Mayerhoefer ME: Correlation
between glycolytic activity on [18F]-FDG-PET and cell density on
diffusion-weighted MRI in lymphoma at staging. J Magn Reson
Imaging. 47:1217–1226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fruehwald-Pallamar J, Czerny C,
Mayerhoefer ME, Halpern BS, Eder-Czembirek C, Brunner M, Schuetz M,
Weber M, Fruehwald L and Herneth AM: Functional imaging in head and
neck squamous cell carcinoma: Correlation of PET/CT and
diffusion-weighted imaging at 3 tesla. Eur J Nucl Med Mol Imaging.
38:1009–1019. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakajo M, Nakajo M, Kajiya Y, Tani A,
Kamiyama T, Yonekura R, Fukukura Y, Matsuzaki T, Nishimoto K,
Nomoto M and Koriyama C: FDG PET/CT and diffusion-weighted imaging
of head and neck squamous cell carcinoma: Comparison of prognostic
significance between primary tumor standardized uptake value and
apparent diffusion coefficient. Clin Nucl Med. 37:475–480. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi SH, Paeng JC, Sohn CH, Pagsisihan JR,
Kim YJ, Kim KG, Jang JY, Yun TJ, Kim JH, Han MH and Chang KH:
Correlation of 18F-FDG uptake with apparent diffusion
coefficient ratio measured on standard and high b value diffusion
MRI in head and neck cancer. J Nucl Med. 52:1056–1062. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dixon WT: Separation of diffusion and
perfusion in intravoxel incoherent motion MR imaging: A modest
proposal with tremendous potential. Radiology. 168:566–567. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le Bihan D, Breton E, Lallemand D, Aubin
ML, Vignaud J and Laval-Jeantet M: Separation of diffusion and
perfusion in intravoxel incoherent motion MR imaging. Radiology.
168:497–505. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Bihan D, Breton E, Lallemand D, Grenier
P, Cabanis E and Laval-Jeantet M: MR imaging of intravoxel
incoherent motions: Application to diffusion and perfusion in
neurologic disorders. Radiology. 161:401–407. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Liang C, Liu Z, Zhang S and Huang
B: Intravoxel incoherent motion (IVIM) in evaluation of breast
lesions: Comparison with conventional DWI. Eur J Radiol.
82:e782–e789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang SX, Jia QJ, Zhang ZP, Liang CH, Chen
WB, Qiu QH and Li H: Intravoxel incoherent motion MRI: Emerging
applications for nasopharyngeal carcinoma at the primary site. Eur
Radiol. 24:1998–2004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia QJ, Zhang SX, Chen WB, Liang L, Zhou
ZG, Qiu QH, Liu ZY, Zeng QX and Liang CH: Initial experience of
correlating parameters of intravoxel incoherent motion and dynamic
contrast-enhanced magnetic resonance imaging at 3.0 T in
nasopharyngeal carcinoma. Eur Radiol. 24:3076–3087. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai V, Li X, Lee VH, Lam KO, Fong DY,
Huang B, Chan Q and Khong PL: Nasopharyngeal carcinoma: Comparison
of diffusion and perfusion characteristics between different tumour
stages using intravoxel incoherent motion MR imaging. Eur Radiol.
24:176–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu XP, Hou J, Li FP, Wang H, Hu PS, Bi F
and Wang W: Intravoxel incoherent motion diffusion weighted
magnetic resonance imaging for differentiation between
nasopharyngeal carcinoma and lymphoma at the primary site. J Comput
Assist Tomogr. 40:413–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao-Ping Y, Jing H, Fei-ping L, Yin H,
Qiang L, Lanlan W and Wei W: Intravoxel incoherent motion MRI for
predicting early response to induction chemotherapy and
chemoradiotherapy in patients with nasopharyngeal carcinoma. J Magn
Reson Imaging. 43:1179–1190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao Y, Pan J, Chen Y, Chen Y, He Z and
Zheng X: Intravoxel incoherent motion-magnetic resonance imaging as
an early predictor of treatment response to neoadjuvant
chemotherapy in locoregionally advanced nasopharyngeal carcinoma.
Medicine (Baltimore). 94:e9732015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Queiroz MA and Huellner MW: PET/MR in
cancers of the head and neck. Semin Nucl Med. 45:248–265. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spick C, Herrmann K and Czernin J:
18F-FDG PET/CT and PET/MRI perform equally well in
cancer: Evidence from studies on more than 2,300 patients. J Nucl
Med. 57:420–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Queiroz MA, Hüllner M, Kuhn F, Huber G,
Meerwein C, Kollias S, von Schulthess G and Veit-Haibach P: PET/MRI
and PET/CT in follow-up of head and neck cancer patients. Eur J
Nucl Med Mol Imaging. 41:1066–1075. 2014.PubMed/NCBI
|
|
33
|
Kuhn FP, Hüllner M, Mader CE, Kastrinidis
N, Huber GF, von Schulthess GK, Kollias S and Veit-Haibach P:
Contrast-enhanced PET/MR imaging versus contrast-enhanced PET/CT in
head and neck cancer: How much MR information is needed. J Nucl
Med. 55:551–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kubiessa K, Purz S, Gawlitza M, Kühn A,
Fuchs J, Steinhoff KG, Boehm A, Sabri O, Kluge R, Kahn T and Stumpp
P: Initial clinical results of simultaneous 18F-FDG
PET/MRI in comparison to 18F-FDG PET/CT in patients with
head and neck cancer. Eur J Nucl Med Mol Imaging. 41:639–648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Partovi S, Kohan A, Vercher-Conejero JL,
Rubbert C, Margevicius S, Schluchter MD, Gaeta C, Faulhaber P and
Robbin MR: Qualitative and quantitative performance of
18F-FDG-PET/MRI versus 18F-FDG-PET/CT in
patients with head and neck cancer. AJNR Am J Neuroradiol.
35:1970–1975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Varoquaux A, Rager O, Poncet A, Delattre
BM, Ratib O, Becker CD, Dulguerov P, Dulguerov N, Zaidi H and
Becker M: Detection and quantification of focal uptake in head and
neck tumours: 18F-FDG PET/MR versus PET/CT. Eur J Nucl
Med Mol Imaging. 41:462–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Covello M, Cavaliere C, Aiello M, Cianelli
MS, Mesolella M, Iorio B, Rossi A and Nicolai E: Simultaneous
PET/MR head-neck cancer imaging: Preliminary clinical experience
and multiparametric evaluation. Eur J Radiol. 84:1269–1276. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schaarschmidt BM, Heusch P, Buchbender C,
Ruhlmann M, Bergmann C, Ruhlmann V, Schlamann M, Antoch G, Forsting
M and Wetter A: Locoregional tumour evaluation of squamous cell
carcinoma in the head and neck area: A comparison between MRI,
PET/CT and integrated PET/MRI. Eur J Nucl Med Mol Imaging.
43:92–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chan SC, Yeh CH, Yen TC, Ng SH, Chang JT,
Lin CY, Yen-Ming T, Fan KH, Huang BS, Hsu CL, et al: Clinical
utility of simultaneous whole-body 18F-FDG PET/MRI as a single-step
imaging modality in the staging of primary nasopharyngeal
carcinoma. Eur J Nucl Med Mol Imaging. 45:1297–1308. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao C, Yang P, Xu Y, Niu T, Hu Q and Chen
X: Feasibility of multiparametric imaging with PET/MR in
nasopharyngeal carcinoma: A pilot study. Oral Oncol. 93:91–95.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A: AJCC Cancer Staging Manual7th. Springer; New
York, NY: 2010
|
|
42
|
Prakash P, Kalra MK, Digumarthy SR, Hsieh
J, Pien H, Singh S, Gilman MD and Shepard JA: Radiation dose
reduction with chest computed tomography using adaptive statistical
iterative reconstruction technique: Initial experience. J Comput
Assist Tomogr. 34:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rodrigues RS, Bozza FA, Christian PE,
Hoffman JM, Butterfield RI, Christensen CR, Heilbrun M, Wiggins RH
3rd, Hunt JP, Bentz BG, et al: Comparison of whole-body PET/CT,
dedicated high-resolution head and neck PET/CT, and
contrast-enhanced CT in preoperative staging of clinically M0
squamous cell carcinoma of the head and neck. J Nucl Med.
50:1205–1213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdel Khalek Abdel Razek A and King A: MRI
and CT of nasopharyngeal carcinoma. AJR Am J Roentgenol. 198:11–18.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Le Bihan D, Turner R and MacFall JR:
Effects of intravoxel incoherent motions (IVIM) in steady-state
free precession (SSFP) imaging: Application to molecular diffusion
imaging. Magn Reson Med. 10:324–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
King AD, Vlantis AC, Bhatia KS, Zee BC,
Woo JK, Tse GM, Chan AT and Ahuja AT: Primary nasopharyngeal
carcinoma: Diagnostic accuracy of MR imaging versus that of
endoscopy and endoscopic biopsy. Radiology. 258:531–537. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen YK, Su CT, Ding HJ, Chi KH, Liang JA,
Shen YY, Chen LK, Yeh CL, Liao AC and Kao CH: Clinical usefulness
of fused PET/CT compared with PET alone or CT alone in
nasopharyngeal carcinoma patients. Anticancer Res. 26:1471–1477.
2006.PubMed/NCBI
|
|
48
|
Ng SH, Chan SC, Yen TC, Chang JT, Liao CT,
Ko SF, Liu FY, Chin SC, Fan KH and Hsu CL: Staging of untreated
nasopharyngeal carcinoma with PET/CT: Comparison with conventional
imaging work-up. Eur J Nucl Med Mol Imaging. 36:12–22. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mohandas A, Marcus C, Kang H, Truong MT
and Subramaniam RM: FDG PET/CT in the management of nasopharyngeal
carcinoma. AJR Am J Roentgenol. 203:W146–W157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Le Bihan D and Turner R: The capillary
network: A link between IVIM and classical perfusion. Magn Reson
Med. 27:171–178. 1992. View Article : Google Scholar : PubMed/NCBI
|