Introduction
Prostate cancer is the most commonly diagnosed
cancer in males and is also the leading cause of cancer-associated
mortality among males (1,2). Even though remarkable progress has been
made in the treatment of prostate cancer, most patients are
diagnosed with middle- or late-stage disease, which leads to
disease progression and a poor prognosis (3). Currently, androgen-deprivation therapy
is widely used in the treatment of prostate cancer (4–6); however,
many patients develop drug resistance which contributes to a high
male mortality rate. Therefore, there is an urgent need to identify
novel regulators involved in modulating the progression of prostate
cancer and to design more effective therapeutic strategies.
Long non-coding RNAs (lncRNAs) are a class of RNAs
processing more than 200 nucleotides without protein-coding
capacity (7). Increasing evidence
suggests that lncRNAs play important roles in a variety of
biological processes via diverse mechanisms, including chromatin
regulation, alternative splicing and epigenetic control (8–10).
Notably, previous studies have uncovered that lncRNAs act as
competing endogenous RNA (ceRNAs) to sponge the function of
microRNAs (miRNAs) (11,12). miRNAs are characterized as a group of
small (~18–22 nt), single-stranded, non-coding RNAs (13,14). It is
well documented that miRNAs negatively regulate the gene expression
via binding the 3′-untranslated region (3′-UTR) of target mRNAs,
which results in the degradation or translation inhibition of mRNAs
(15,16). As important regulators of gene
expression, both lncRNAs and miRNAs are widely involved in
tumorigenesis (17–19). For example, lncRNA PVT1 was found to
function as a ceRNA to regulate the expression of HIF1α via
sponging miR-186 in gastric cancer (20). In prostate cancer, lncRNA HOTTIP was
found to promote the proliferation and migration of prostate caner
cells via sponging miR-216a-5p (21).
Additionally, lncRNA RNCR3 was demonstrated to enhance the
progression of prostate cancer by targeting miR-185-5p (22). These results highlight the critical
roles of lncRNAs in regulating the growth of cancer cells by acting
as ceRNAs.
lncRNA histocompatibility leukocyte antigen (HLA)
complex P5 (HCP5) is primarily detected in immune cells, including
spleen, blood and thymus (23). In
addition to its function in autoimmunity, aberrant expression of
HCP5 has been found in human cancers (24). A recent study reported that HCP5 is
upregulated in glioma tissues (25).
HCP5 was found to modulate the proliferation, migration and
invasion of glioma cells via binding to miR-139 and to increase the
expression of Runt1 (25).
Additionally, HCP5 was found to promote the progression of cervical
cancer by regulating MACC1 by suppressing miR-15a (24). The HCP5 region was also identified as
the susceptibility locus for HCV-related hepatocellular carcinoma
(26). However, the function of HCP5
in prostate cancer remains uncharacterized.
In the present study, HCP5 was found to be highly
expressed in prostate cancer. Mechanistically, HCP5 was found to
promote the proliferation of prostate cancer cells via sponging
miR-4656 to upregulate the expression of CEMIP. Our results provide
novel insight into clarifying the critical function of HCP5 in
regulating the progression of prostate cancer.
Materials and methods
Tissue samples
Fifty paired prostate cancer tissues and
corresponding normal tissues were collected from patients (47–78
years of age) who were diagnosed with prostate cancer and underwent
surgery at The People's Hospital of Hanchuan City from August 2012
to May 2014. The samples were reviewed by three pathologists
independently. None of the patients were administered local or
systemic treatment prior to surgery. Informed consent was provided
by all of the enrolled patients, and the study was approved by the
Ethics Committee of The People's Hospital of Hanchuan City
(accession no. LL2017014).
Cells and transfection
The prostate cancer cell lines PC3, Du145, PPC-1 and
C4-2 were purchased from the Institute of Biochemistry (Shanghai,
China). The PPC-1 cell line was authenticated with the STR profile.
Cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 mg/ml streptomycin and
200 U/ml penicillin. The nontumorigenic human prostate epithelial
cell line 9 (NHP9) was maintained in PrEBM medium (Clonetics)
containing insulin, hydrocortisone, epidermal growth factor and
bovine pituitary extract. Cells were maintained in an incubator at
37°C with 5% CO2. For the overexpression of HCP5, the
full-length of HCP5 was constructed into the pcDNA vector. miR-4656
mimics (UGGGCUGAGGGCAGGAGGCCUGU; HMI1647) and control-miRNA
(GGUUCGUACGUACACUGUUCA; HMC0002) (both from Sigma-Aldrich; Merck
KGaA) were transfected (10,000 cells) at the concentration of 25 µM
with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) for 15 min at room temperature (RT). After transfection for
48 h, the cells were harvested for further analysis. shRNA-HCP5
(0.5 µg)
(CCGGGATCTATTACCTGTGCCTGGACTCGAGTCCAGGCACAGGTAATAGATCTTTTTTG;
SHCNV-NM_006674; Sigma-Aldrich; Merck KGaA) or control-shRNA
(CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG; #1864, Addgene,
USA) were transfected into cells (10,000) with Lipofectamine 2000
for 48 h.
RNA extraction and RT-qPCR
analysis
Total RNA from the tissue samples or cells was
isolated using Trizol reagent (Takara, Dalian, China). RNA (1 µg)
from each sample was used to synthesize the cDNA using the
PrimeScript RT Master Mix (Takara, Dalian, China). Quantitative PCR
was performed to detect the expression of HCP5 with the One-step
SYBR PrimeScript RT-PCR kit (Takara) on the Applied Biosystem 7900
Fast Real-Time PCR System (Thermo Fisher Scientific, Inc.). The
level of HCP5 was normalized to that of GAPDH. Primers used in this
assay were listed as below: HCP5, forward,
5′-CCGCTGGTCTCTGGACACATACT-3′ and reverse,
5′-CTCACCTGTCGTGGGATTTTGC-3′; GAPDH, forward,
5′-GTCGGTGTGAACGGATTTG-3′ and reverse, 5′-AAGATGGTGATGGGCTTCC-3′.
The PCR protocol was set as 95°C for 10 min; 40 cycles at 95°C for
10 sec and 60°C for 1 min. Relative gene expression of HCP5 was
calculated using the 2−ΔΔCq method (27).
Western blot analysis
Prostate cancer cells transfected with the
corresponding expression vector were harvested and lysed with RIPA
buffer (Beyotime, Shanghai, China). Samples were certificated at
10,000 × g for 10 min at 4°C. The supernatant was collected and the
protein concentration was quantified with the BCA kit (Bio-Rad).
Protein (20 µg) was separately by 15% SDS-PAGE and then transferred
onto PVDF membranes (EMD Millipore). After blocking with 5% nonfat
milk, the membranes were incubated with the primary antibody
against CEMIP (1:2,000 dilution; XY2112901; XYbscience, Shanghai,
China), GAPDH (1:3,000 dilution; ab9485; Abcam, Shanghai, China),
Snail (1:1,000 dilution; ab229701; Abcam, Shanghai, China), VEGF
(1:1,000 dilution; AV202; Beyotime, Shanghai, China), E-cadherin
(1:5,000 dilution; 20874-1-AP; Proteintech Group, Wuhan, China)
overnight at 4°C, followed by HRP-conjugated secondary antibody
[rabbit anti-mouse IgG H&L (HRP), 1:5,000 dilution; ab6728;
Abcam; Shanghai, China; Goat anti-Rabbit IgG H&L (HRP), 1:5,000
dilution; ab6721; Abcam; Shanghai, China] for 2 h at RT. The
protein signal was visualized with enhanced chemiluminescence
reagent (Bio-Rad).
Cell counting Kit-8 (CCK-8) assay
Cell proliferation was measured with the CCK-8 assay
(Dojindo, Tokyo, China) according to the manufacturer's
instructions. Briefly, cells transfected with the corresponding
expression vector were seeded in a 96-well plate in triplicate at
the density of 1,000 cells per well. CCK-8 reagent (10 µl) was
added into the cells at the time point of 1, 2, 3, 4 and 5 days and
incubated at 37°C for a further 3 h. The absorbance of each well at
450 nm was measured using a microplate reader (Bio-Rad, IQ,
USA).
Targes prediction
The binding between HCP5 and miR-4656 was predicted
with the RNA22 version 2.0 (https://cm.jefferson.edu/rna22). The targets of
miR-4656 were predicted using the miRDB database (28).
Luciferase reporter assay
To detect the binding between HCP5 and miR-4656, the
wild-type (WT) or mutant (Mut) HCP5 sequences harboring the
predicted binding site of miR-4656 were constructed into the pGL3
reporter vector (Promega). Similarly, to examine the binding for
miR-4656 and CEMIP, the WT or Mut 3′-UTR of CEMIP was inserted into
the pGL3 reporter vector. Cells cultured in a 96-well plate were
transfected with the indicated expressing vectors with
Lipofectamine 2000. After transfection for 48 h, cells were
harvested and the luciferase activity was measured using the
Dual-Luciferase Reporter Assay Kit (Promega) according to the
manufacturer's instructions. The assay was performed in
triplicate.
Cell apoptosis
PC-3 and PPC-1 cells were transfected with
shRNA-control or shRNA-HCP5 for 48 h. The percentage of apoptotic
cells was determined using the Annexin V-
fluorescein-5-isothiocyanate (FITC) Apoptosis Detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Cells were collected and washed twice
with pre-cold PBS. The cell pellets were resuspended with Annexin V
binding buffer. Cell resuspension (100 µl) (~10,000 cells) was used
and stained with FITC-Annexin V and propidium iodide for 15 min at
RT in darkness. The cell apoptosis rate was analyzed with the
Becton Dickinson FACScan instrument (BD Pharmingen™, USA).
Xenograft mouse model
Both PC3 and PPC-1 cells (1×106)
harboring the lentivirus vector of the shRNA-control or shRNA-HCP5
were injected subcutaneously into the flank of nude mice (BALB/c,
5–6 weeks of age, female, 23–26 g; N=6 per group) and left to grow
for three weeks. All mice were maintained in a specific
pathogen-free (SPF) barrier condition with controlled temperature
(25±2°C), relative humidity (60±5%), and 12-h light/dark cycle with
free access to food and water. The tumor size was measured every
three days according to the ethical IACUC guidelines. After that,
mice were sacrificed by cervical dislocation and the tumor weight
was measured. This experiment was approved by the Animal Research
Ethics Committee of The People's Hospital of Hanchuan City. All
animals were handled following the ‘Guide for the Care and Use of
Laboratory Animals’ and the ‘Principles for the Utilization and
Care of Vertebrate Animals’ (29).
Statistical analysis
Data are presented as mean ± standard deviation (SD)
and were analyzed with SPSS 13.0 (SPSS, Inc.). The comparison
between two groups was analyzed by the Student's t-test.
Comparisons among multiple groups were analyzed with ANOVA followed
by a post hoc test. Spearman's rank-order correlation was applied
to the correlation analysis. The r-value indicates the strength and
direction of the correlation between the two variables. P<0.05
was considered as indicative of a statistically significant
difference.
Results
HCP5 is upregulated in prostate cancer
tissues
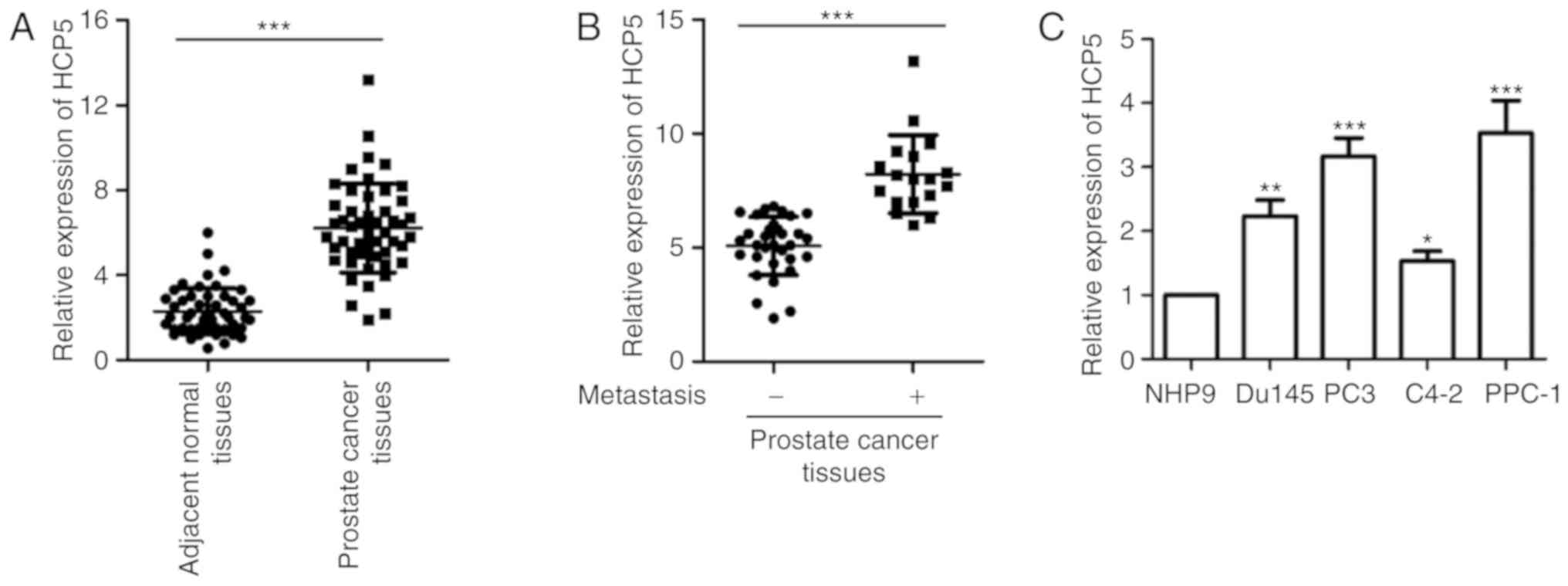
To validate the expression of HCP5 in prostate
cancer, the level of HCP5 was analyzed in paired prostate cancer
tissues and non-tumor tissues by RT-qPCR. The result showed that
HCP5 was significantly upregulated in prostate cancer tissues
compared to that noted in the adjacent normal tissues (Fig. 1A). Notably, increased expression of
HCP5 was positively correlated with the metastasis of prostate
cancer patients (Fig. 1B). The result
showed that HCP overexpression was linked to relative poor
progression of prostate cancer patients. To support these data, the
expression of HCP5 in prostate cancer cell lines including PC3,
Du145, PPC-1 and C4-2 and normal control cell line NHP9 was
detected. As presented in Fig. 1C,
overexpression of HCP was observed in all the prostate cancer cell
lines when compared with the expression noted in the normal cells.
These results demonstrate the high expression of HCP5 in prostate
cancer.
Downregulation of HCP5 inhibits the
proliferation and colony formation and induces the apoptosis of
prostate cancer cells
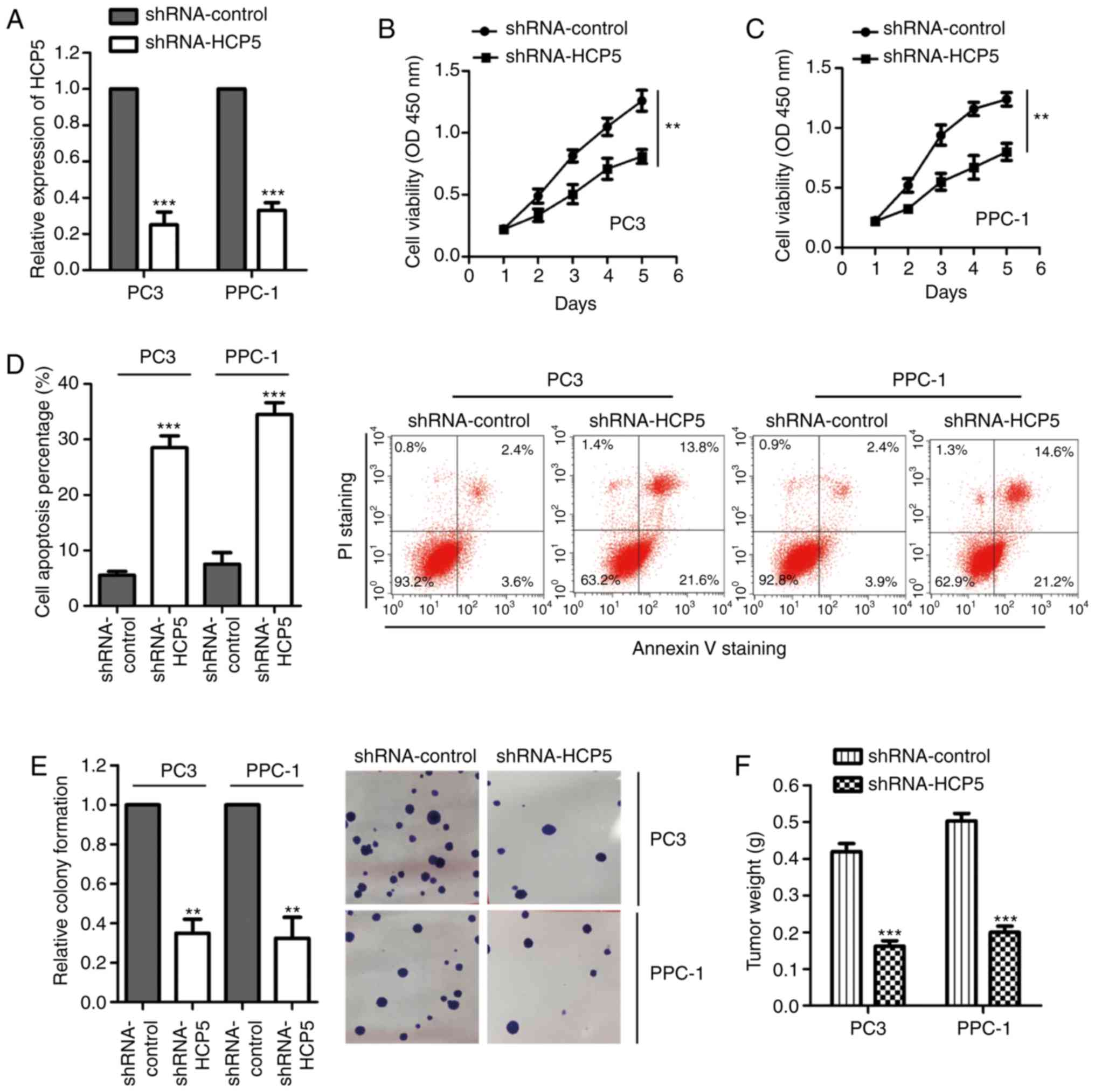
To investigate the effect of HCP5 on the growth of
prostate cancer cells, HCP5 was downregulated by transfection of
shRNA-HCP5 into PPC-1 and PC3 cells. RT-qPCR assay was performed to
confirm the knockdown efficiency, which showed that HCP5 expression
was significantly reduced after the transfection of shRNA-HCP5
(Fig. 2A). The influence of HCP5
silencing on the growth of prostate cancer cells was evaluated with
the CCK-8 assay. As indicated in Fig. 2B
and C, the OD values were significantly decreased with the
depletion of HCP5 in both PPC-1 and PC3 cell lines, demonstrating
that knockdown of HCP5 inhibited prostate cancer cell
proliferation. Consistently, the apoptotic rate (early and late
apoptosis) of both PPC-1 and PC3 cell lines was significantly
increased with the downregulation of HCP5 in comparison with that
of the control group (Fig. 2D).
Moreover, the colony formation assay was performed to evaluate the
effect of HCP5 on the growth of prostate cancer cells. As presented
in Fig. 2E, silencing of HCP5
significantly suppressed the colony formation ability of the PPC-1
and PC3 cells. To further confirm the suppression of growth of
prostate cancer tissues with downregulation of HCP5, an in
vivo xenograft mouse model was established by injecting PC3 or
PPC-1 cells harboring shRNA-control or shRNA-HCP5 into the flank of
nude mice. The tumor weight was measured after three weeks and the
result showed that depletion of HCP5 significantly decreased the
tumor formation (Fig. 2F).
Consistently, the tumor diameter and volumes were also decreased
upon the silencing of HCP5 (Table
SI). These results indicated that the downregulation of HCP5
suppressed the tumorigenesis of prostate cancer.
miR-4656 is identified as a target of
HCP5
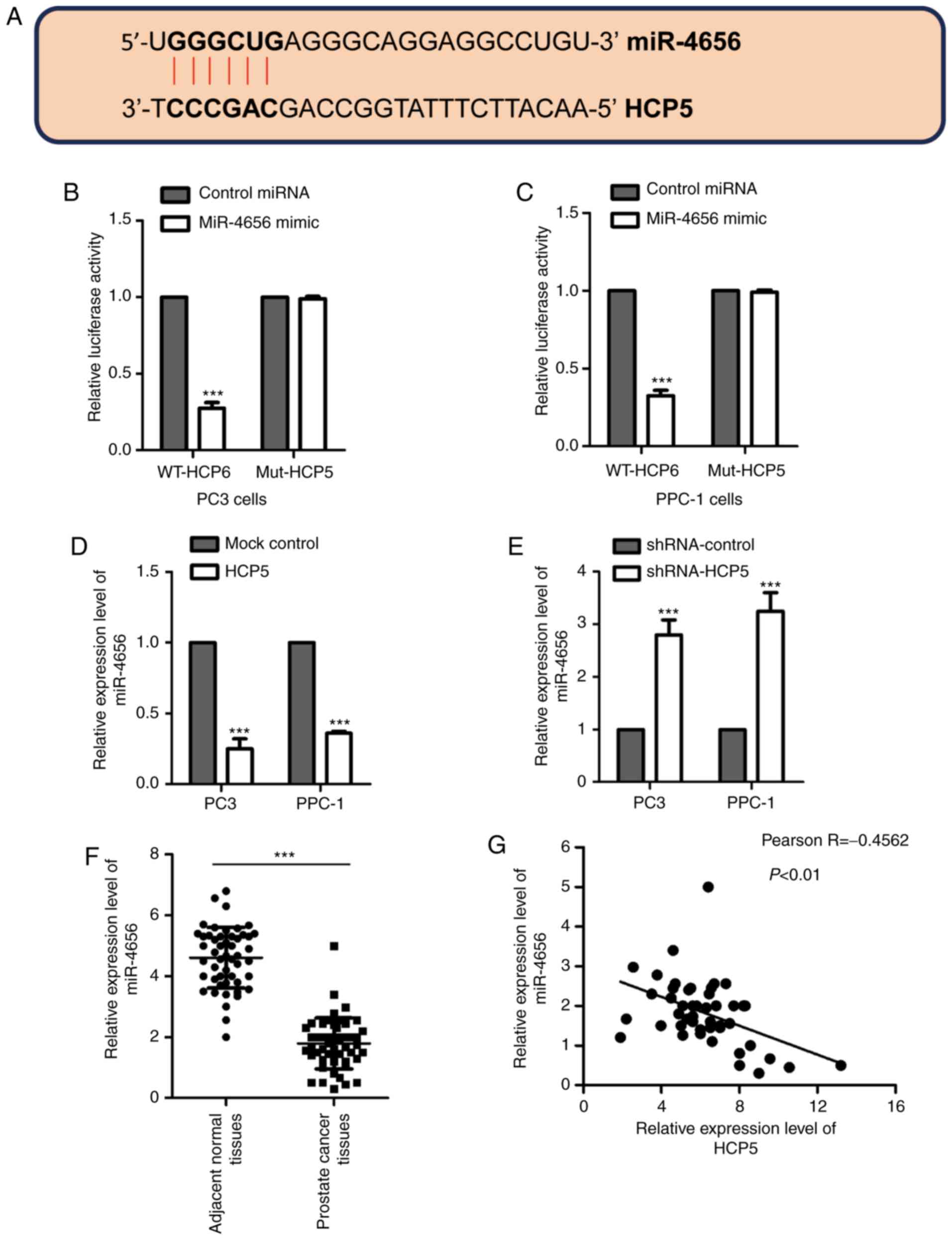
Mounting evidence suggests that lncRNAs act as
competing endogenous RNA to modulate the expression of miRNAs. To
further understand the function of HCP5 in regulating the growth of
prostate cancer cells, the putative binding partners of HCP5 were
predicted. We found that there was a complementary binding sequence
to the miR-4656 seed region in HCP5 (Fig.
3A). To confirm whether HCP5 binds to miR-4656, luciferase
reporter assay was performed by transfecting the luciferase vector
harboring the wild-type (WT) or mutant (Mut) sequence of HCP5,
which was the seeding region of miR-4656. The results showed that
overexpression of miR-4656 significantly reduced the luciferase
reporter activity of WT but not mutant HCP5 (Fig. 3B and C). This observation suggested
the interaction between HCP5 and miR-4656 in prostate cancer
cells.
To ascertain whether the binding of HCP5 to miR4656
affects the stability of miR-4656, PPC-1 and PC3 cells were
transfected with control lncRNA or HCP5 and the level of miR-4656
was detected using the RT-qPCR assay. The data showed that ectopic
expression of HCP5 significantly reduced the level of miR-4656 in
both PPC-1 and PC3 cell lines (Fig.
3D). In contrast, downregulation of HCP5 promoted the
expression of miR-4656 (Fig. 3E).
Supporting this observation, the expression of miR-4656 was
significantly lower in prostate cancer tissues in comparison with
that noted in the normal tissues (Fig.
3F), and expression of miR-4656 was inversely correlated with
the level of HCP5 (Fig. 3G). These
results indicate that HCP5 acts as the sponge of miR-4656 and
reduced the expression of miR-4656 in prostate cancer cells.
miR-4656 targets CEMIP in prostate
cancer cells
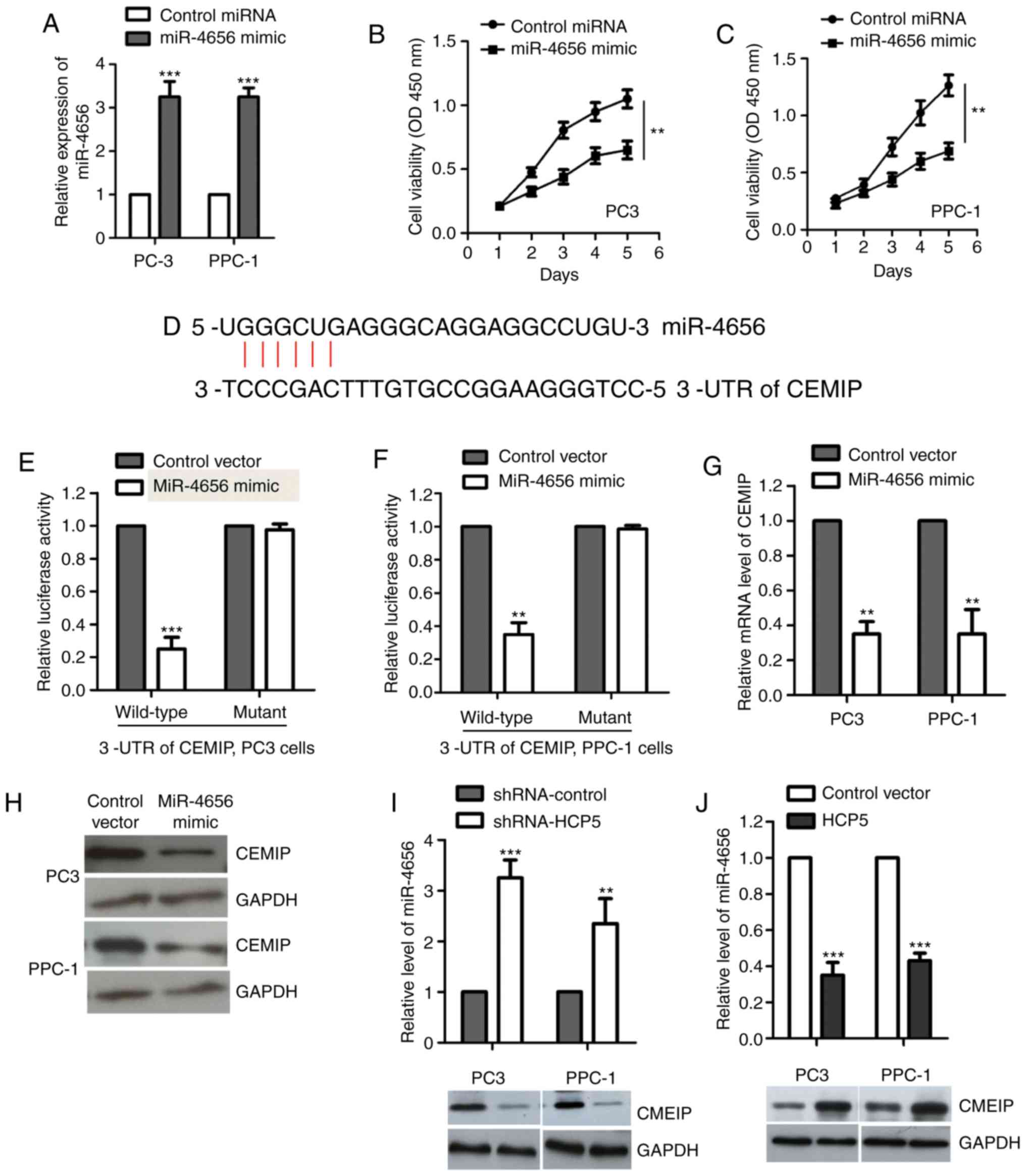
To detect the effect of miR-4656 on the growth of
prostate cancer cells, both PC-3 and PPC-1 cell lines were
transfected with miRNA control or miR-4656 mimic to upregulate the
expression of miR-4656 (Fig. 4A). The
CCK-8 assay showed that overexpression of miR-4656 significantly
decreased the proliferation of both PC-3 and PPC-1 cell lines
(Fig. 4B and C). To further
understand the molecular mechanisms by which miR-4656 regulates the
proliferation of prostate cancer cells, the possible targets of
miR-4656 were predicted using the miRDB database. The prediction
revealed that CEMIP is a potential binding candidate of miR-4656
(Fig. 4D). Increasing evidence has
demonstrated the oncogenic function of CEMIP in promoting
tumorigenesis by modulating cancer-related signaling pathways
(30–33). The overexpression of CEMIP in tumors
provides a novel target for individualized therapy (34). However, the involvement of CEMIP in
the malignancy of prostate cancer has not been fully understood.
There were three target sites of miR-4656 in the 3′-UTR of CEMIP
that were predicted and the first one (seed location 121) was
examined for further analysis. To confirm this, luciferase reporter
assay was performed by constructing the WT or mutant 3′-UTR which
contained the binding sites of miR-4656 into the luciferase
reporter vector. The results showed that overexpression of miR-4656
significantly reduced the WT but not the mutant 3′-UTR luciferase
activity (Fig. 4E and F). RT-qPCR
assay showed that high expression of miR-4656 decreased both the
mRNA and protein levels of CEMIP in the PPC-1 and PC3 cell lines
(Fig. 4G and H). To check the
co-expression of HCP5/miR-4656/CEMIP, cells were transfected with
HCP-5 or shRNA-HCP5. The results showed that depletion of HCP5
significantly upregulated the level of miR-4656 and decreased the
expression of CEMIP (Fig. 4I).
Consistently, overexpression of HCP5 significantly suppressed
miR-4656 and increased the abundance of CEMIP (Fig. 4J). Our results identified CEMIP as a
target of the HCP5/miR-4656 axis in prostate cancer cells.
HCP5 regulates the miR-4656/CEMIP
pathway
To determine whether HCP5 regulates CEMIP via
suppression of miR-4656, miR-4656 was overexpressed and the
expression of CEMIP was detected. The data showed that rescue of
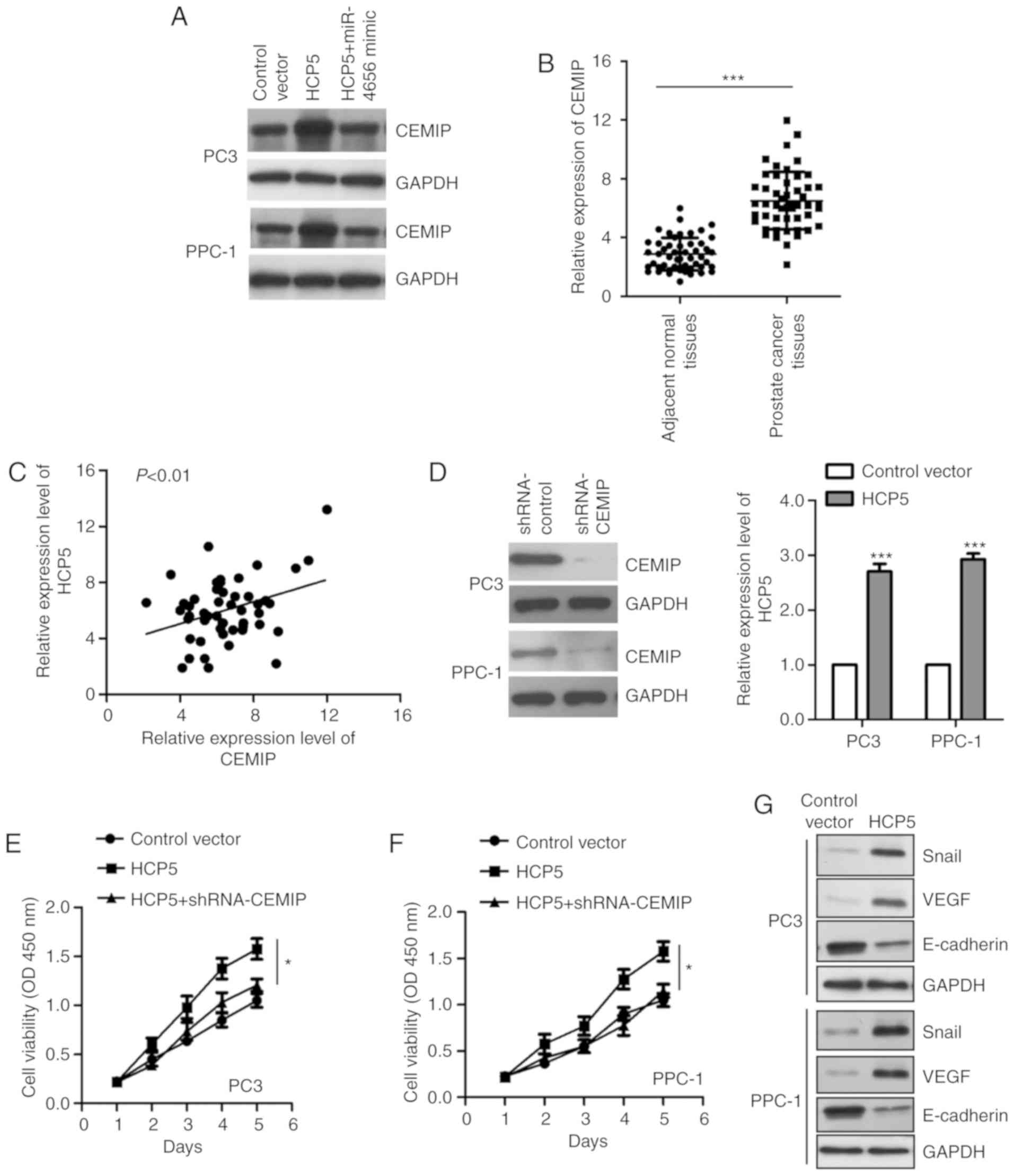
miR-4656 reversed the upregulation of CEMIP by HCP5 (Fig. 5A). To further support the correlation
between HCP5 and CEMIP, the mRNA level of CEMIP in paired prostate
cancer tissues and adjacent normal tissues was compared via
RT-qPCR. The data revealed significantly increased expression of
CEMIP in the prostate cancer tissues (Fig. 5B). Meanwhile, the correlation between
the abundance of HCP5 and CEMIP was analyzed by Spearman test. As
indicated in Fig. 5C, the expression
of CEMIP was positively correlated with that of HCP5 in prostate
cancer tissues. To verify whether CEMIP mediates the function of
HCP5 in prostate cancer, CEMIP was downregulated and HCP5 was
overexpressed in both PC-3 and PPC-1 cell lines (Fig. 5D). The CCK-8 assay showed that
knockdown of CEMIP reduced the HCP5-promoted proliferation of PC-3
and PPC-1 cells (Fig. 5E and F).
These data indicate that CEMIP is regulated by HCP5 and modulates
the growth of prostate cancer cells. Increasing studies have
demonstrated that CEMIP orchestrates tumorigenesis via regulating
Wnt/β-catenin signaling (33). To
further demonstrate the functional mechanism of CEMIP in prostate
cancer, we evaluated the expression of several targets of the
Wnt/β-catenin pathway including Snail, E-cadherin, and VEGF. The
results showed that overexpression of HCP5 upregulated Snail and
VEGF, and inhibited the level of E-cadherin in both PC3 and PPC1
cell lines (Fig. 5G). These data
provide the possible mechanism underlying the regulatory function
of CEMIP in prostate cancer.
Discussion
Accumulating evidence indicates the important roles
of long noncoding RNAs (lncRNAs) in the initiation and development
of human cancers (9,35). Considering the high occurrence of
prostate cancer in the male population and the high mortality rate
of prostate cancer patients, understanding the molecular mechanism
of lncRNAs in regulating the growth of prostate cancer cells may
provide new therapeutic strategies for the treatment of prostate
cancer. In the present study, we identified that lncRNA
histocompatibility leukocyte antigen (HLA) complex P5 (HCP5) is
overexpressed in prostate cancer tissues and cell lines.
Mechanistically, HCP5 was found to promote the proliferation of
prostate cancer cells via sponging miR-4656 to upregulate the
expression of CEMIP.
HCP5 is located on chromosome 6p21.3, and the
function of HCP5 is not fully understood (36). A recent study demonstrated that HCP5
is overexpressed in glioma tissues and is positively correlated
with the histopathological grade of glioma cancer patients
(25). Knockdown of HCP5 was found to
inhibit the malignancy of glioma cells by upregulating miR-139.
These data suggest the oncogenic function of HCP5 in glioma.
Increased expression of HCP5 was also identified in follicular
thyroid carcinoma (FTC). HCP5 was found to promote the
tumorigenesis of FTC (37).
Mechanistic experiments indicated that HCP5 functions as a ceRNA to
sponge miR-22-3p, miR-186-5p and miR-216-5p, which triggers
activity of alpha-2, 6-siallyltransferase 2 in FTC cells (37). Consistently, our results showed the
upregulation of the expression of HCP5 in prostate cancer, which
was associated with the worse prognosis of prostate cancer
patients. Functional experiments showed that downregulation of HCP5
suppressed the proliferation and induced the apoptosis of prostate
cancer cell lines. To further explore the molecular mechanism by
which HCP5 regulates the tumorigenesis of prostate cancer, the
miRNA targets of HCP5 were predicted with the miRDB database. The
results identified miR-4656 as a potential target of HCP5.
Overexpression of HCP5 significantly decreased the abundance of
miR-4656 in prostate cancer cells. Our data uncovered the critical
involvement of the HCP5/miR-4656 axis in prostate cancer.
The function of miRNAs in regulating the growth of
cancer cells occurs mainly by inhibiting the expression of
downstream target genes. The involvement of miR-4656 in cancer is
not fully understood. Recent research showed that miR-4656 is
aberrantly expressed in glioma tissues and is correlated with the
prognosis of glioma cancer patients (38). In the present study, to further
understand the functional mechanism of miR-4656 in prostate cancer,
downstream targets of miR-4656 were predicted. The data showed that
miR-4656 bound the 3′-UTR of CEMIP and suppressed the expression of
CEMIP. CEMIP was primary identified as a hearing loss-related gene
(39). Interestingly, increased
expression of CEMIP has been identified in a variety of human
cancer (31,32,39–41). For
example, upregulated expression of CEMIP may be a valuable
biomarker for the detection of pancreatic cancer at an early stage
(39). A recent study showed that
overexpression of CEMIP is triggered by the AMPK/GSK3β/β-catenin
cascade and promotes the invasion and migration of prostate cancer
cells via elevating metabolic reprogramming. These results
demonstrated that the targeting of CEMIP may be a promising
strategy for the treatment of advanced prostate cancer patients
(42). The oncogenic function of
CEMIP was also found in colorectal cancer (31,40,41). In
this study, overexpression of HCP5 enhanced the abundance of CEMIP.
These results provided the possible mechanism by which HCP5
regulates the growth of prostate cancer cells.
In conclusion, our data revealed that HCP5 is
overexpressed in prostate cancer tissues, and is correlated with a
worse prognosis of cancer patients. For further study,
immunostaining of prostate cancer tissues is necessary to evaluate
the cancer grade and neovascularization using fresh samples.
Functional mechanistic investigation uncovered that HCP5 promotes
the growth of prostate cancer cells via acting as the sponge of
miR-4656 and consequently upregulated the expression of CEMIP. HCP5
may be a potential target for designing a novel therapeutic
strategy for prostate cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding authors upon reasonable
request.
Authors' contributions
RH and ZL designed the experiments. RH performed the
experiments. RH and ZL analyzed the data and wrote the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The People's Hospital of Hanchuan City (accession no. LL2017014).
Informed consents were received from all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamamoto S, Kawakami S, Yonese J, Fujii Y,
Urakami S, Masuda H, Numao N, Ishikawa Y, Kohno A and Fukui I:
Long-term oncological outcome and risk stratification in men with
high-risk prostate cancer treated with radical prostatectomy. Jpn J
Clin Oncol. 42:541–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoang DT, Iczkowski KA, Kilari D, See W
and Nevalainen MT: Androgen receptor-dependent and -independent
mechanisms driving prostate cancer progression: Opportunities for
therapeutic targeting from multiple angles. Oncotarget.
8:3724–3745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong CW, Kang M, Il Jung S, Kim TH, Park
SW, Joung JY, Jeon SS, Hong JH, Lee JY, Chung BH, et al: Importance
of androgen-deprivation therapy during enzalutamide treatment in
men with metastatic castration-resistant prostate cancer following
chemotherapy: Results from retrospective, multicenter data.
Prostate Cancer Prostatic Dis. 22:150–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onozawa M, Akaza H, Hinotsu S, Oya M,
Ogawa O, Kitamura T, Suzuki K, Naito S, Namiki M, Nishimura K, et
al: Combined androgen blockade achieved better oncological outcome
in androgen deprivation therapy for prostate cancer: Analysis of
community-based multi-institutional database across Japan using
propensity score matching. Cancer Med. 7:4893–4902. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siddiqui ZA and Krauss DJ: Adjuvant
androgen deprivation therapy for prostate cancer treated with
radiation therapy. Transl Androl Urol. 7:378–389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Z, Liu X, Liu L, Deng H, Zhang J, Xu Q,
Cen B and Ji A: Regulation of lncRNA expression. Cell Mol Biol
Lett. 19:561–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khorkova O, Hsiao J and Wahlestedt C:
Basic biology and therapeutic implications of lncRNA. Adv Drug
Deliv Rev. 87:15–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balas MM and Johnson AM: Exploring the
mechanisms behind long noncoding RNAs and cancer. Noncoding RNA
Res. 3:108–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang S, Sun Z, Zhou Q, Wang W, Wang G,
Song J, Li Z, Zhang Z, Chang Y, Xia K, et al: MicroRNAs, long
noncoding RNAs, and circular RNAs: Potential tumor biomarkers and
targets for colorectal cancer. Cancer Manag Res. 10:2249–2257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olgun G, Sahin O and Tastan O: Discovering
lncRNA mediated sponge interactions in breast cancer molecular
subtypes. BMC Genomics. 19:6502018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paraskevopoulou MD and Hatzigeorgiou AG:
Analyzing MiRNA-LncRNA Interactions. Methods Mol Biol.
1402:271–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye S, Yang L, Zhao X, Song W, Wang W and
Zheng S: Bioinformatics method to predict two regulation mechanism:
TF-miRNA-mRNA and lncRNA-miRNA-mRNA in pancreatic cancer. Cell
Biochem Biophys. 70:1849–1858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao NB, He YF, Li XQ, Wang K and Wang RL:
The role of miRNA and lncRNA in gastric cancer. Oncotarget.
8:81572–81582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang G, Pian C, Chen Z, Zhang J, Xu M,
Zhang L and Chen Y: Identification of cancer-related miRNA-lncRNA
biomarkers using a basic miRNA-lncRNA network. PLoS One.
13:e01966812018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang T, Liu HW, Chen JQ, Wang SH, Hao LQ,
Liu M and Wang B: The long noncoding RNA PVT1 functions as a
competing endogenous RNA by sponging miR-186 in gastric cancer.
Biomed Pharmacother. 88:302–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang B, Gao G, Wang Z, Sun D, Wei X, Ma Y
and Ding Y: Long non-coding RNA HOTTIP promotes prostate cancer
cells proliferation and migration by sponging miR-216a-5p. Biosci
Rep. 38(pii): BSR201805662018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian C, Deng Y, Jin Y, Shi S and Bi H:
Long non-coding RNA RNCR3 promotes prostate cancer progression
through targeting miR-185-5p. Am J Transl Res. 10:1562–1570.
2018.PubMed/NCBI
|
|
23
|
Liu Y, Helms C, Liao W, Zaba LC, Duan S,
Gardner J, Wise C, Miner A, Malloy MJ, Pullinger CR, et al: A
genome-wide association study of psoriasis and psoriatic arthritis
identifies new disease loci. PLoS Genet. 4:e10000412008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Shen HM, Fang DM, Meng QJ and Xin
YH: LncRNA HCP5 promotes the development of cervical cancer by
regulating MACC1 via suppression of microRNA-15a. Eur Rev Med
Pharmacol Sci. 22:4812–4819. 2018.PubMed/NCBI
|
|
25
|
Teng H, Wang P, Xue Y, Liu X, Ma J, Cai H,
Xi Z, Li Z and Liu Y: Role of HCP5-miR-139-RUNX1 feedback loop in
regulating malignant behavior of glioma cells. Mol Ther.
24:1806–1822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lange CM, Bibert S, Dufour JF, Cellerai C,
Cerny A, Heim MH, Kaiser L, Malinverni R, Müllhaupt B, Negro F, et
al: Comparative genetic analyses point to HCP5 as susceptibility
locus for HCV-associated hepatocellular carcinoma. J Hepatol.
59:504–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43 (Database Issue):D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
U.S. National Institutes of Health, .
Laboratory animal welfare; proposed U.S. government principles for
the utilization and care of vertebrate animals used in testing,
research and training. Fed Regist. 49:29350–29351. 1984.PubMed/NCBI
|
|
30
|
Shen F, Zong ZH, Liu Y, Chen S, Sheng XJ
and Zhao Y: CEMIP promotes ovarian cancer development and
progression via the PI3K/AKT signaling pathway. Biomed
Pharmacother. 114:1087872019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fink SP, Myeroff LL, Kariv R, Platzer P,
Xin B, Mikkola D, Lawrence E, Morris N, Nosrati A, Willson JK, et
al: Induction of KIAA1199/CEMIP is associated with colon cancer
phenotype and poor patient survival. Oncotarget. 6:30500–30515.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang G, Fang X, Yang Y and Song Y:
Knockdown of CEMIP suppresses proliferation and induces apoptosis
in colorectal cancer cells: Downregulation of GRP78 and attenuation
of unfolded protein response. Biochem Cell Biol. 96:332–341. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang G, Fang X, Yang Y and Song Y:
Silencing of CEMIP suppresses Wnt/β-catenin/Snail signaling
transduction and inhibits EMT program of colorectal cancer cells.
Acta Histochem. 120:56–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Yan LH, Manoj S, Li Y and Lu L:
Central role of CEMIP in tumorigenesis and its potential as
therapeutic target. J Cancer. 8:2238–2246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Corra F, Agnoletto C, Minotti L,
Baldassari F and Volinia S: The network of Non-coding RNAs in
cancer drug resistance. Front Oncol. 8:3272018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Medici M, Porcu E, Pistis G, Teumer A,
Brown SJ, Jensen RA, Rawal R, Roef GL, Plantinga TS, Vermeulen SH,
et al: Identification of novel genetic Loci associated with thyroid
peroxidase antibodies and clinical thyroid disease. PLoS Genet.
10:e10041232014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang L, Xu J, Wang M, Xu G, Zhang N, Wang
G and Zhao Y: LncRNA HCP5 promotes follicular thyroid carcinoma
progression via miRNAs sponge. Cell Death Dis. 9:3722018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shou J, Gu S and Gu W: Identification of
dysregulated miRNAs and their regulatory signature in glioma
patients using the partial least squares method. Exp Ther Med.
9:167–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee HS, Jang CY, Kim SA, Park SB, Jung DE,
Kim BO, Kim HY, Chung MJ, Park JY, Bang S, et al: Combined use of
CEMIP and CA 19-9 enhances diagnostic accuracy for pancreatic
cancer. Sci Rep. 8:33832018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang D, Zhao L, Shen Q, Lv Q, Jin M, Ma
H, Nie X, Zheng X, Huang S, Zhou P, et al: Down-regulation of
KIAA1199/CEMIP by miR-216a suppresses tumor invasion and metastasis
in colorectal cancer. Int J Cancer. 140:2298–2309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Evensen NA, Li Y, Kuscu C, Liu J, Cathcart
J, Banach A, Zhang Q, Li E, Joshi S, Yang J, et al: Hypoxia
promotes colon cancer dissemination through up-regulation of cell
migration-inducing protein (CEMIP). Oncotarget. 6:20723–20739.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang P, Song Y, Sun Y, Li X, Chen L, Yang
L and Xing Y: AMPK/GSK3β/β-catenin cascade-triggered overexpression
of CEMIP promotes migration and invasion in anoikis-resistant
prostate cancer cells by enhancing metabolic reprogramming. FASEB
J. 32:3924–3935. 2018. View Article : Google Scholar : PubMed/NCBI
|