Introduction
Prostate cancer is one of the most common malignant
tumors and is ranked as the second cause of cancer-associated
deaths in male patients in developed countries (1). Prostate cancer mainly develops due to a
switch from androgen-dependent to androgen-independent growth
(2). Nicotinic acetylcholine
receptors (nAChRs) comprise ligand-gated ion channels, which
consist of 5 subunits with 4 transmembrane domains in each subunit.
The nicotinic acetylcholine receptor (nAChR) signaling pathway
affects various cancer-associated pathways that are important in
promoting tumor cell proliferation, metastasis and angiogenesis
(3–8).
It has been revealed that the α5 subunit of the
nAChR (α5-nAChR), a member of the nAChR subunit family, is closely
associated with the incidence of lung cancer. The activation of
α5-nAChR is involved in tumor growth and metastasis and is
important in the formation, metastasis and recurrence of lung
cancer (9,10). Previous studies have demonstrated that
α5-nAChR is associated with gastric cancer (11–17). For
example, exposure of nicotine to gastric cancer cells inhibits the
cisplatin-induced apoptosis via the α5-nAChR/AKT signaling pathway
(11). However, it is unknown whether
α5-nAchR is involved in the progression of prostate cancer. Based
on the aforementioned findings, the involvement of α5-nAChR in the
incidence and progression of prostate cancer was examined.
Therefore, in the present study, the expression levels of α5-nAChR
were examined in prostate cancer and normal prostate tissues. The
expression levels of α5-nAChR were upregulated in prostate cancer
tissues. Furthermore, silencing of α5-nAChR expression
significantly inhibited prostate cancer cell invasion and migration
in vitro. In addition, previous studies have identified the
downstream signaling pathways of α5-nAchR, which are involved in
prostate cancer progression.
Materials and methods
Cell culture
The human normal prostate epithelial cell line
RWPE-1 and the androgen-independent prostate cancer cell lines PC3
and DU145 were used in the present study. The metastatic potential
of DU145 is higher than that of the PC3 cell line. 293 cells were
obtained from the Type Culture Collection of the Chinese Academy of
Sciences. DU145 cells were maintained in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with a combination of
1% penicillin and streptomycin in the presence of 10% fetal bovine
serum (Gibco). Ham's F-12K (Kaighn's) Medium (Gibco; Thermo Fisher
Scientific, Inc.) was used to culture PC3 cells with 1% penicillin
and streptomycin and 10% fetal bovine serum in the culture medium.
293 cells were cultured in Dulbecco's Modified Eagle's Medium
(DMEM) (Gibco; Thermo Fisher Scientific, Inc.) with a combination
of 1% penicillin and streptomycin and 10% fetal bovine serum. All
the cells were incubated in standard cell culture conditions with
5% CO2 and 95% humidity at 37°C.
Cell transfection
A siRNA oligonucleotide targeting α5-nAChR
mRNA was synthesized by Shanghai Genepharma Co. Ltd. The sequence
was as follows: 5′-CCCGCAAACUACAAAAGUUTT-3′. A pair of scrambled
control siRNAs with sequences different from those of the
siRNA-α5-nAChR was designed. The pair of sequences was not
homologous to any sequences found in GeneBank. When the cells
reached 70–80% confluence, the transfection was conducted according
to the transfection instructions. The cells were subsequently
cultured under normal conditions for 36 h at 37°C.
Tissue samples from patients and
immunohistochemistry (IHC)
IHC was performed in 8 normal prostate tissues (all
male, aged 66.37±4.53) and the results were compared with the
analysis performed in 36 prostate cancer samples (Table I). The clinicopathological variables
of these patients were collected. Written informed consent forms
were obtained from the subjects. The study protocol was approved by
the Research Ethics Committee of the Second Hospital of Hebei
Medical University. IHC was performed to determine α5-nAChR
expression. Paraffin-embedded tissue sections (5-µm thick) were
deparaffinized with xylene, followed by rehydration using a graded
series of 100, 90, 80 and 70% ethanol. Then, intrinsic peroxidase
was deactivated with 0.3% H2O2 and intrinsic
biotin was deactivated with skim milk. The sections were reacted
with the primary (cat. no. ab166718; Abcam) and secondary (cat. no.
ab97048; Abcam) antibodies. Finally, H2O2 was
added to DAB to undergo reaction. Sections were then stained with
methyl green, and the target proteins were observed under a light
microscope (magnification, ×100 and ×400).
 | Table I.Expression of α5-nAChR in prostate
tissue from prostate cancer patients. |
Table I.
Expression of α5-nAChR in prostate
tissue from prostate cancer patients.
| Clinical
characteristics | Case no. (n) | α5-nAChR low
expression (n) | α5-nAChR high
expression (n) | P-value |
|---|
| Age |
|
|
|
|
|
≤60 | 15 | 8 | 7 | 0.5348 |
|
>60 | 21 | 9 | 12 |
|
| Tumor diameter
(cm) |
|
|
|
|
|
≤3.0 | 19 | 7 | 4 | 0.1907 |
|
>3.0 | 17 | 10 | 15 |
|
| Lymph-node
metastasis |
|
|
|
|
|
Yes | 4 | 2 | 2 | 0.906 |
| No | 32 | 15 | 17 |
|
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (CWBIO) was used to isolate total RNA
as determined by the manufacturer's instructions. A total of 500 ng
of RNA was extracted from each sample and reverse-transcribed using
the Reverse Transcription Reaction Kit (CWBIO). The relative mRNA
levels of α5-nAChR, and β-actin (internal control)
were determined by RT-qPCR using an FTC-3000 Real-Time PCR System.
All real-time PCR assays used the SYBR Green Supermix. The cycles
used for RNA amplification included a pre-denaturing step at 95°C
for a duration of 10 sec, followed by 40 PCR cycles consisting of 5
sec at 95°C, 30 sec at 60°C, and 10 min at 72°C. All samples were
repeatedly assayed in triplicate in each experiment. The relative
amount of mRNA was determined by the comparative ΔΔCq method
(18) and then normalized to the
β-actin mRNA levels. The sequences of α5-nAChR
primers were as follows: Forward, GACTCCACCGGCAAACTACA and reverse,
TTTGCTCCCTGTTGCACTCA.
Western blotting
The cells were treated with PBS and subsequently
lysed in RIPA buffer (Beyotime Institute of Biotechnology). Then
the protein concentration was determined by the Pierce BCA Protein
Assay (Thermo Fisher Scientific, Inc.). In each sample, 44 µg
protein was resolved by gel electrophoresis using 15% sodium
dodecyl sulfate (SDS)-polyacrylamide gels. The proteins were
transferred to a PVDF membrane, and finally analyzed by western
blotting. The primary antibodies against α-tubulin (cat. no.
ab18251) and anti-α5-nAChR (cat. no. ab166718) (rabbit polyclonal
antibody) and the secondary alkaline phosphatase-coupled
anti-rabbit IgG antibody (cat. no. ab97048) were obtained from
Abcam. The membranes were blocked in 5% fat-free milk in TBS
containing 0.1% Tween-20 at room temperature for 1 h and
subsequently incubated with primary antibodies against tubulin
(1:10,000 dilution) and α5-nAChR (1:1,000 dilution) for 2 h. The
membranes were further incubated with secondary antibodies
(1:5,000) for 1 h. The western blot assay was repeated 3 times in
order to evaluate the repeatability of the procedure. Finally, the
labeled proteins were detected by chemiluminescence (ECLPlus;
Amersham Pharmacia Biotech; GE Healthcare) and analyzed using
ImageJ software (v1.43; National Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
PC3 and DU145 cells were cultured in a 96-well plate
at a density of 1,000 cells/well. The cells were divided into the
control (si-RNA-NC, scramble sequence) and the test groups
(si-α5-nAChR). The cell proliferation assay was performed as
follows: A total of 10 µl CCK-8 solution (Beijing Solarbio Science
& Technology Co., Ltd.) was added to each well and the plate
was incubated at 37°C for 2 h. A microplate reader was used to
measure the absorbance at 450 nm.
Transwell assay
The cell migration assay was conducted using
Transwell chambers. The Transwell inserts were placed in 24 well
plates (Corning, Inc.). Following treatment with either full-length
α5-nAChR plasmid or α5-nAChR-specific siRNA, 5×104
cells/well were placed in the upper chamber with 200 µl culture
medium (RPMI-1640 medium for DU145 and Ham's F-12K for PC3). The
lower chamber was filled with 600 µl of 10% FBS culture medium
(RPMI-1640 medium for DU145 and Ham's F-12K for PC3). The cells
were then cultured for 24 h. The outer surface was subsequently
washed three times with PBS, fixed with methanol for 20 min and
stained with 0.2% crystal violet for 15 min at room temperature.
The images were obtained immediately after drying. The number of
migrated cells were counted in five randomly microscopic fields of
view (Olympus Corp.) at ×400 magnification. The experiments were
repeated three times and the data were summarized.
TUNEL assay
One-Step TUNEL kit (Beyotime Institute of
Biotechnology) was used following the manufacturer's
recommendations. Briefly, PC3 and DU145 cells were exposed to
si-RNA-NC (scramble sequence) or si-α5-nAChR for 24 h and then
fixed in 4% paraformaldehyde for 10 min at room temperature.
Subsequently, the cells were washed with PBS three times and
permeabilized for 2 min on ice and then the cells were resuspended
in TUNEL working solution. Following incubation for 1 h in a
humidified atmosphere at 37°C in the dark, the cells were
counterstained with DAPI staining solution for 5 min at room
temperature, DAPI was used for the nuclear staining and afterwards
washed with PBS. The staining solution consisted of a mixture of
methanol and DAPI as follows: 1 ml methanol and 2 µl DAPI (from a
stock solution of 1 mg/ml). The mounting medium was S2100 (Solarbio
Science and Technology Co., Ltd.). The number of TUNEL-positive
cells were counted in five randomly fluorescence microscopic fields
of view. The experiments were repeated three times and the data
were summarized.
Wound healing assay
The cells were transfected by siRNA targeting
α5-nAChR mRNA using NanoFectin Transfection Reagent.
Following 24 h of incubation, a scratch was made to the bottom of
the culture dish by the tip of a glass micropipette in order to
establish a clean wound area. The cultured cells were maintained in
their original culture medium. The culture dish with the scratched
wound was photographed at the following time-points: 0, 12 and 24 h
after the wound was made.
Statistical analysis
The data were expressed as the mean ± SD and were
analyzed using SPSS v17 (SPSS, Inc.). Pearson's chi-squared test
was performed to examine the correlation of α5-nAChR expression
with the various clinical factors. One-way ANOVA was performed to
determine the differences between the experimental groups, and the
least significant difference (LSD) post hoc test was used. A
P-value <0.05 (P<0.05) was considered to indicate a
statistically significant difference.
Results
α5-nAChR is overexpressed in prostate
cancer
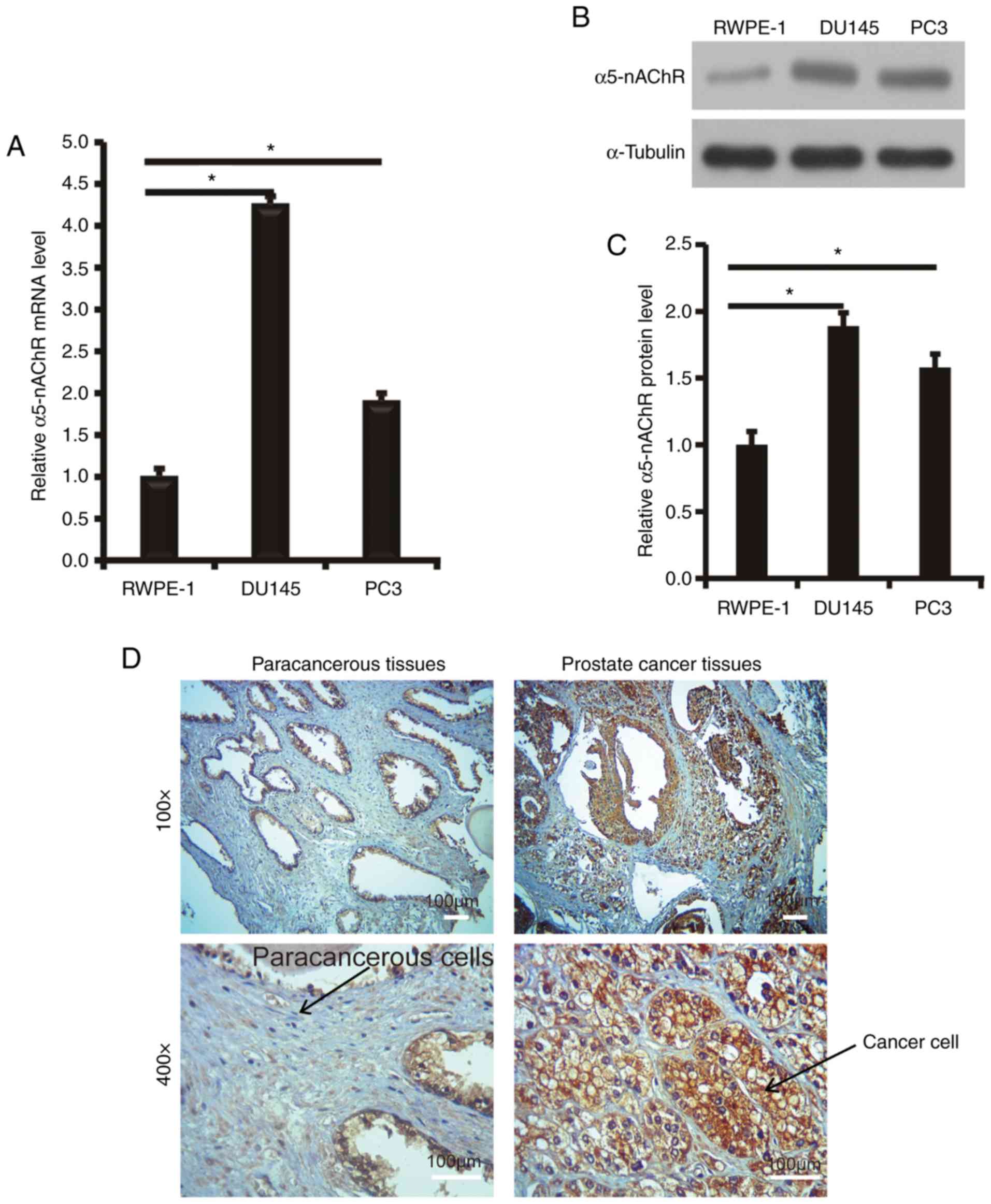
To investigate the expression levels of
α5-nAChR in prostate cancer tissues, RT-PCR was initially
used and the α5-nAChR mRNA levels were analyzed in 2 human
prostate cancer cell lines. The results were compared with the
human prostate epithelial cell line RWPE-1. The mRNA levels of
α5-nAChR in the prostate cancer cell lines (PC3 and DU145)
were higher than those noted in the normal prostate epithelial cell
line (RWPE-1) (Fig. 1A). α5-nAChR
protein expression was further assessed in the PC3 and DU145 cancer
cell lines and in the normal prostate epithelial cell line RWPE-1
by western blotting. A similar expression pattern was noted to that
observed for the mRNA expression (Fig. 1B
and C).
Subsequently, IHC analysis was performed to examine
the α5-nAChR expression levels in a tissue array consisting of 8
normal prostate tissues compared with those noted in 36 prostate
cancer samples (Fig. 1D). The
expression levels of α5-nAChR in prostate cancer tissues were
increased (P=0.0384). Subsequently, the correlation between the
levels of α5-nAChR expression and the clinicopathological variables
of prostate cancer patients was evaluated. The expression levels of
α5-nAChR were not associated with the parameters age, lymph-node
metastasis and tumor diameter (Table
I).
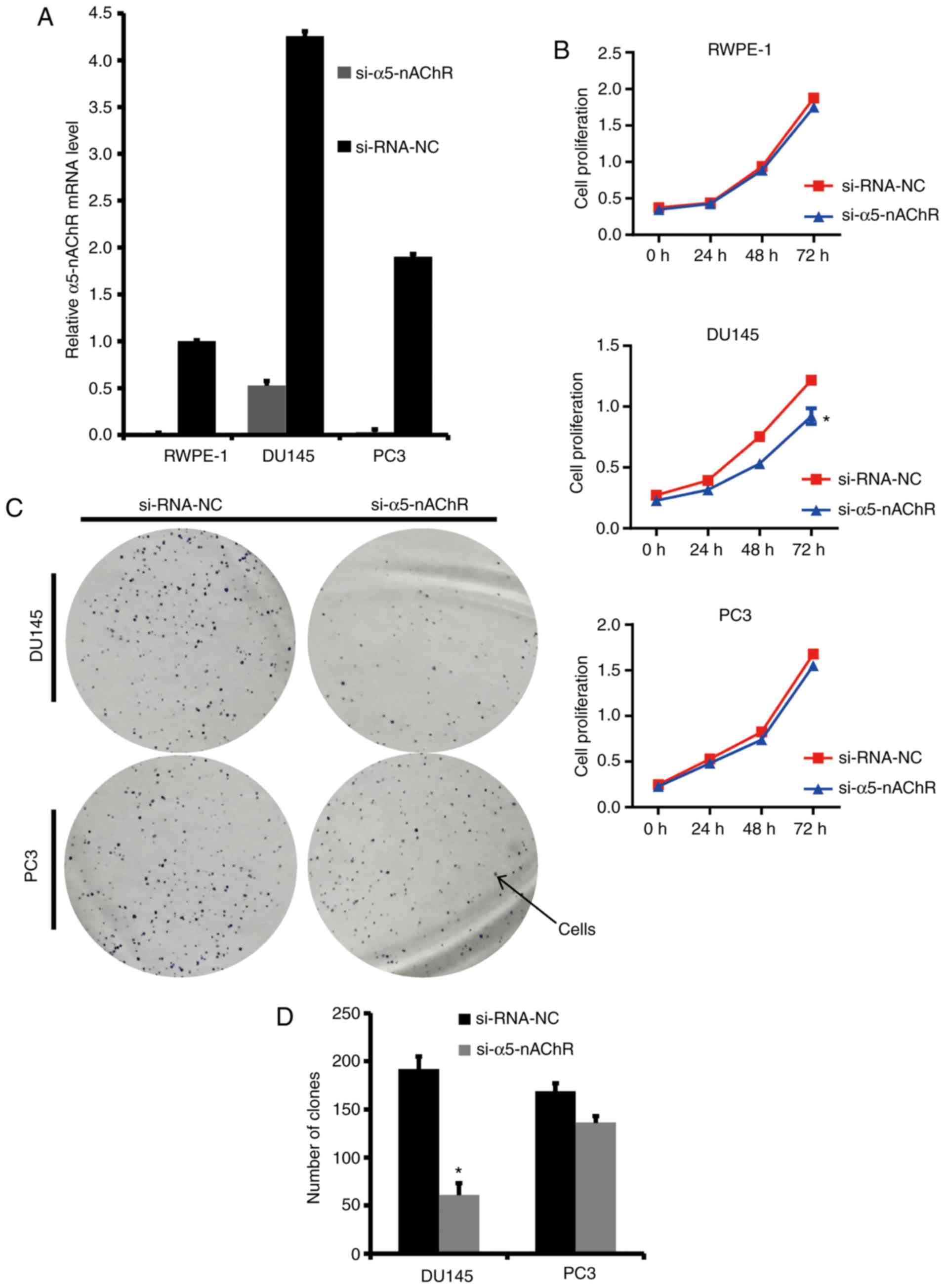
α5-nAChR promotes proliferation and
migration of human prostate cancer cells
The initial experiments demonstrated that the
α5-nAChR expression levels were different between prostate cancer
and normal tissues. Additional experiments focused on identifying
whether α5-nAChR could promote the proliferation and migration of
prostate cancer cells and therefore contribute to the progression
of prostate cancer. To determine the role of α5-nAChR in the
biological behavior of prostate cancer cells, siRNA sequences of
α5-nAChR were transfected into 3 cell lines (PC3, DU145 and
RWPE-1). RT-qPCR was used to detect the transfection efficiency.
si-α5-nAChR caused a significant inhibition in the expression
levels of α5-nAChR in PC3, DU145 and RWPE-1 cells (Fig. 2A).
The effect of α5-nAChR on the growth of prostate
cancer cells was determined using a CCK-8 assay. The proliferation
of DU145 cells transfected with α5-nAChR-specific siRNA was
signficantly reduced (Fig. 2B). The
effects of α5-nAChR-siRNA on DU145 cell proliferation indicated
successful transfection of α5-nAChR siRNA in prostate cancer cells
in vitro. Clone formation assays of PC3 and DU145 cells
indicated that the clone number of the si-α5-nAChR-treated cells
decreased significantly compared with that of the NC cells
(Fig. 2C and D).
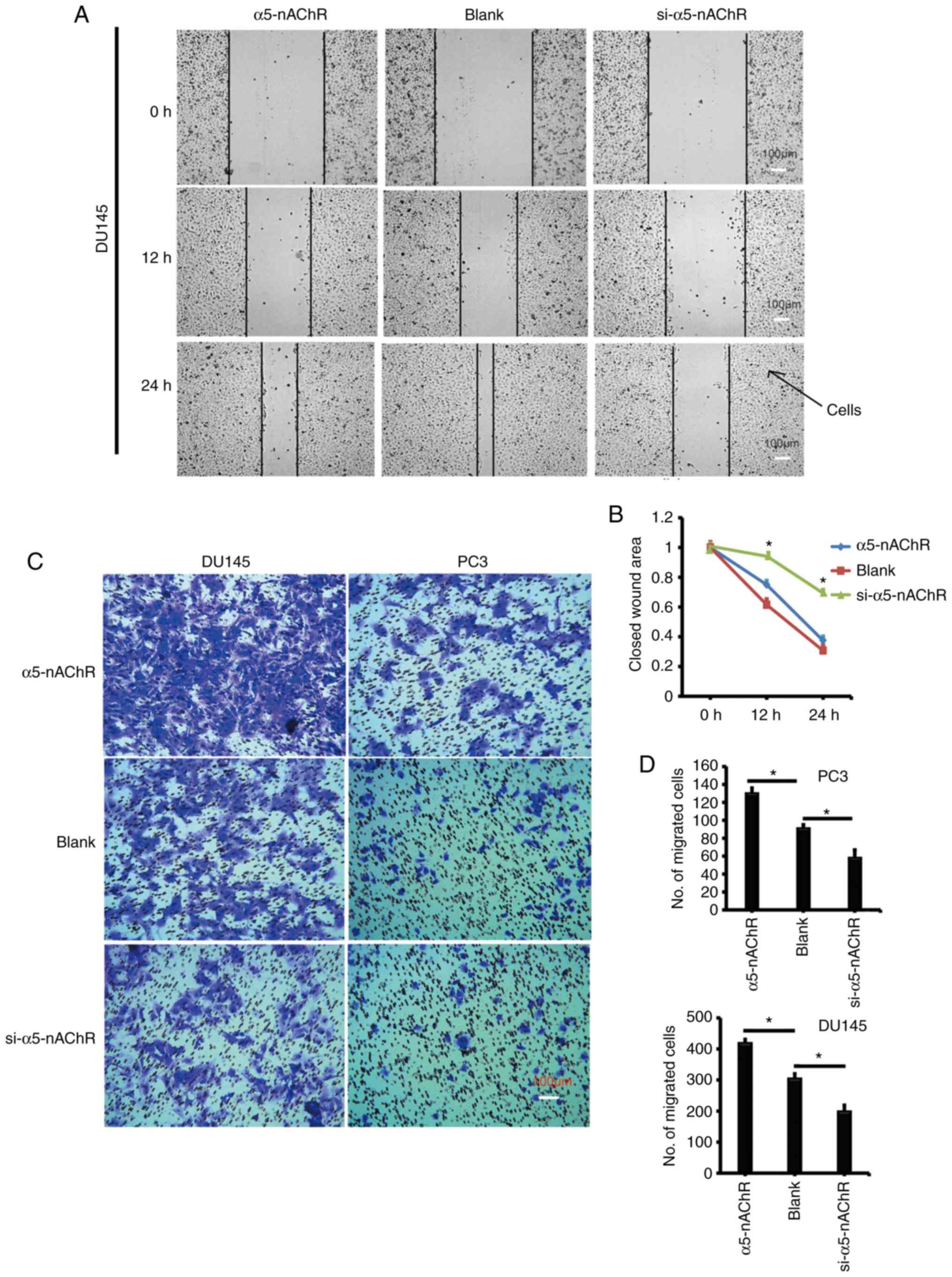
Subsequently, the wound healing assay indicated that
overexpression of α5-nAChR markedly promoted cell migration into
the wound areas compared with that of the negative control (NC)
cells. Downregulation of α5-nAChR by si-RNA significantly inhibited
the migratory activities of DU145 cells that expressed high levels
of α5-nAChR (Fig. 3A and B).
The migratory activity of the cells transfected with
either full-length α5-nAChR plasmid or α5-nAChR-specific siRNA was
evaluated. When the cells were transfected with the α5-nAChR
vector, their migratory activity into the lower chamber was
increased (Fig. 3C and D). The cells
transfected with α5-nAChR-specific siRNA demonstrated reduced
migratory activity into the lower chamber. This finding suggested
that α5-nAChR could promote the migratory activity of the DU145
prostate cancer cells.
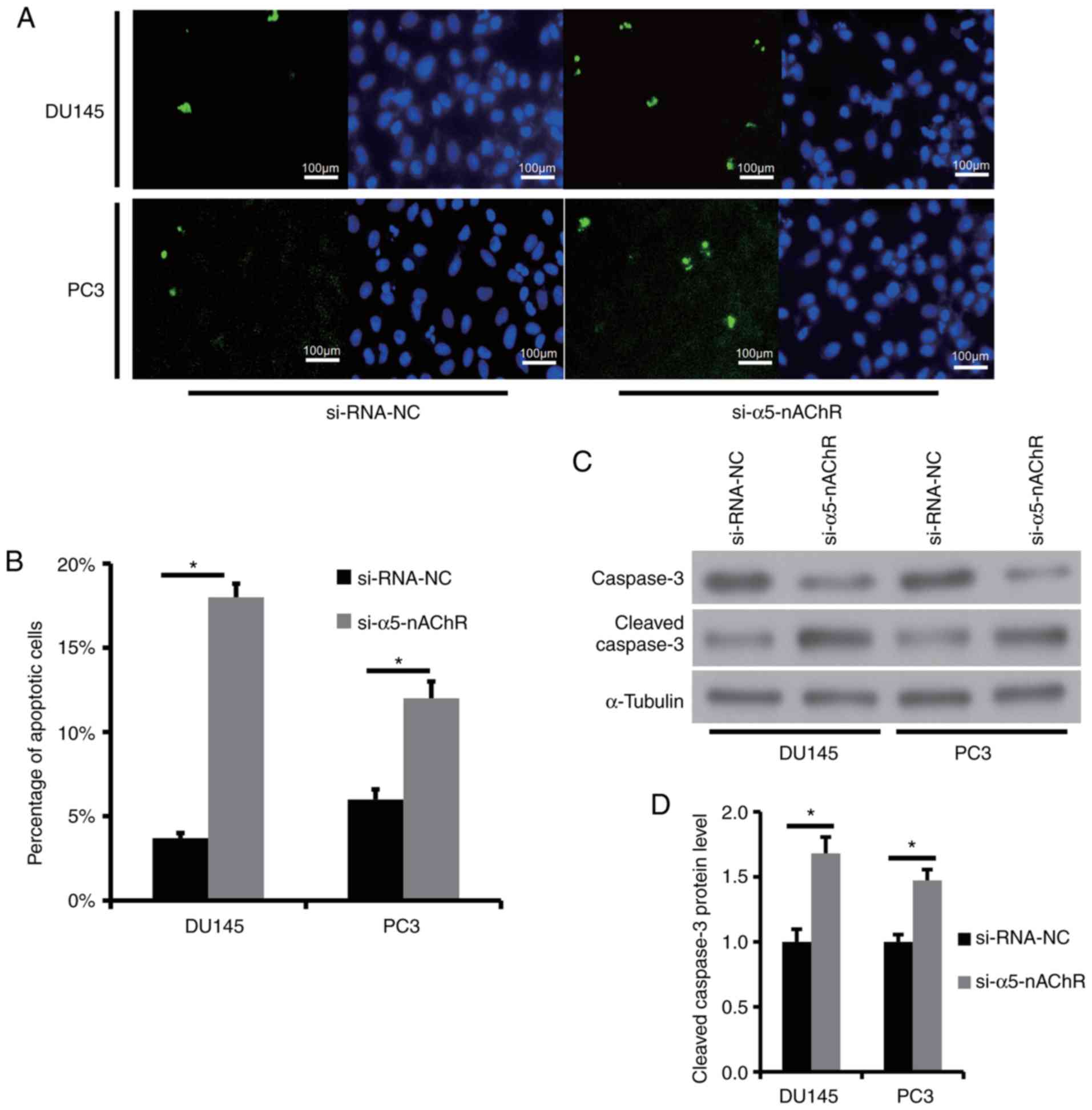
α5-nAChR inhibits the induction of
apoptosis of human DU145 and PC3 cells
The induction of apoptosis was detected by the TUNEL
assay. The experimental results indicated that downregulation of
α5-nAChR with siRNA-α5-nAChR increased the number of apoptotic
cells compared with that of the control group (P<0.05, Fig. 4A and B).
Since cleaved caspase-3 expression is positively
associated with cell apoptosis, the role of α5-nAChR in cell
apoptosis was detected by western blot analysis of caspase-3 and
cleaved caspase-3. The expression levels of total caspase-3 and
cleaved caspase-3 were determined in DU145 and PC3 cells treated
with control si-RNA-NC and siRNA-α5-nAChR. Silencing of α5-nAChR
expression by siRNA-α5-nAChR decreased the expression levels of
total caspase-3 and increased the expression levels of cleaved
caspase-3 (P<0.05) (Fig. 4C and
D).
Downregulation of α5-nAChR expression
alters the phosphorylation status of ERK1/2 and AKT
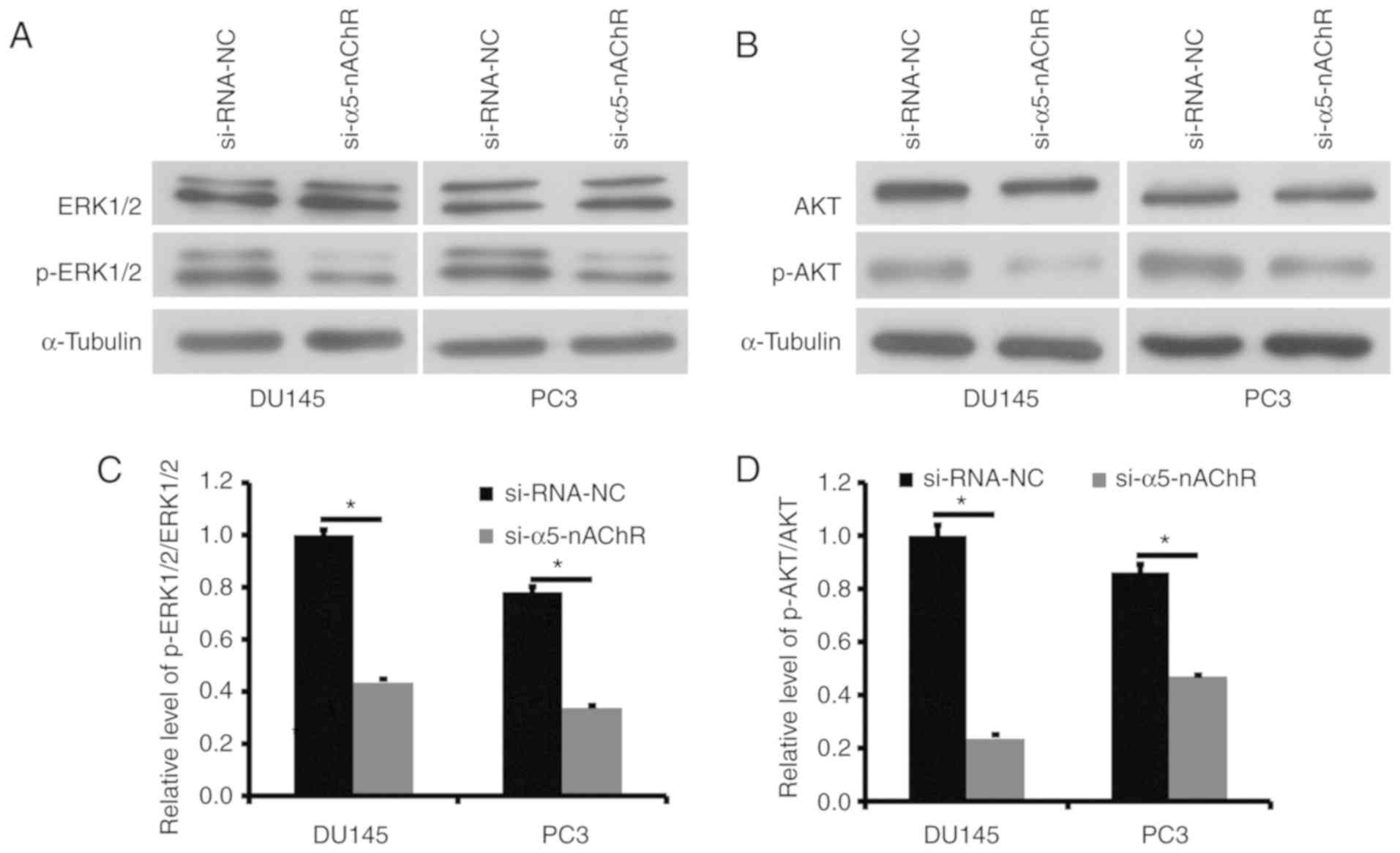
It has been revealed that upon binding to nicotine,
nAChRs activate the ERK and PI3K/AKT signaling pathways in several
human cancer cells. The levels of total ERK1/2 and phosphorylated
ERK1/2 and levels of total AKT and phosphorylated were analyzed
following transfection of DU145 and PC3 cancer cells with
siRNA-α5-nAChR (Fig. 5A and B). The
phosphorylation levels of p-ERK1/2 and p-AKT were significantly
decreased by downregulation of α5-nAChR (Fig. 5C and D). The results indicated that
phosphorylation of ERK and AKT proteins was involved in the
Α5-nAChR-mediated abnormal growth of prostate cancer cells.
Discussion
Smoking is an independent risk factor for several
tumors and has further been studied as a risk factor for prostate
cancer (19–22). However, the results are inconsistent.
Some studies have revealed that the risk of prostate cancer
increases with increasing smoking frequency, which is considered an
independent risk factor for this disease (23–25). In
contrast to these findings, a numerous studies have revealed no
significant correlation between the smoking incidence and the
incidence of prostate cancer, although several studies have
revealed a high mortality percentage due to prostate cancer in
patients who smoke (26–28). A possible explanation may be the
presence of specific compounds in cigarettes that do not
necessarily lead to the cancer, but affect the cellular behavior of
cancer cells and accelerate the progression of cancer. According to
previous studies, it is speculated that nicotine, which is a
carcinogenic compound found in cigarettes, may cause cancer
progression by mimicking the biological function of acetylcholine
and by binding to the nAChR, which subsequently causes a series of
signaling cascades within the cell. α5-nAChR is a regulatory
subunit of nAChR and is involved in the growth and metastasis of
solid tumors, such as lung and gastric cancers. This subunit is a
promising therapeutic target for human tumors. In the present
study, it was speculated that α5-nAChR may be associated with the
progression of prostate cancer.
Initial experiments demonstrated that α5-nAChR
expression was increased in prostate cancer cell lines and cancer
tissues compared with that observed in normal prostate cell lines
and the corresponding adjacent tissues. The molecular mechanism
underlying the upregulation of α5-nAChR in prostate cancer is
complex. The gene cluster encoding nAChR and CHRNA5/A3/B4 is
located on the 15q24-25 region and is co-expressed in a variety of
cell types (29,30). The promoter region contains a GC
structure, which has multiple transcription factor binding sites.
The transcription of these 3 genes may be regulated by various
transcription factors (31). Noatbly,
the transcriptional direction for α5-nAChR is opposite to that of
the A3 and B4 subunits, indicating that in addition to regulating
the entire gene cluster, a distinct transcriptional regulatory
mechanism may exist for α5-nAChR expression. The mechanism of the
regulation of α5-nAChR expression is poorly understood compared
with the evidence presented on the other subunits of nAChR.
In the present study, the data confirmed that
α5-nAChR could promote the proliferation and migration of prostate
cancer cells. The opposite effect was noted for the induction of
apoptosis, which was inhibited in prostate cancer cells. The
function of α5-nAChR in tumors is not widely studied compared with
the other subunits of nAChR. To the best of our knowledge, α5-nAChR
is upregulated and can promote tumor growth and metastasis in lung
and gastric cancers. The results from previous studies are
consistent with our findings. In the present study, silencing of
α5-nAChR inhibited only the proliferation of the prostate cancer
cell line DU145 and exhibited little or no effect on the
proliferation of the prostate cancer cell line PC3. Both PC3 and
DU145 cell lines are androgen-independent prostate cancer cell
lines. However, their cellular response to exogenous stimuli may be
distinct. For example, the metastatic potential of DU145 cells is
higher than that of PC3 cells. Knockdown of α5-nAChR did not
influence the proliferation of PC3 cells but inhibited cell
proliferation of DU145 cells. The data indicated that α5-nAChR
played a significant role in promoting cell proliferation of
certain prostate cancer cell types.
The data demonstrated that α5-nAChR promoted the
development of prostate cancer by activating the PI3K/AKT signaling
pathway, which has been revealed to play an important role in
cancer progression by previous studies (32,33).
Activation of AKT is associated with the clinical characteristics
of prostate cancer. A previous study revealed that the activated
AKT levels are higher in poorly differentiated prostate cancer
compared with those observed in highly and moderately
differentiated prostate cancer (34).
The activation of AKT was positively correlated with the prognosis
of prostate cancer (35). Moreover,
AKT activation was correlated with prostate cancer progression and
androgen independence (36). The
transcriptional activity, expression and even stability of the AR
are regulated by the PI3K/AKT signaling pathway (37). The inhibition of the AKT pathway by
siRNA interference or by a PI3K inhibitor leads to the inactivation
of the HER2/neu-activated AR signaling pathway. This process is not
affected by AR expression. In addition, nicotine increases AKT
phosphorylation in a PI3K-dependent manner, suggesting that smoking
causes bladder cancer progression via nicotine-induced activation
of the PI3K/AKT pathway (38). In
addition to the action of the PI3K/AKT pathway, α5-nAChR can act
via the ERK1/2 pathway, which is activated in 30% of all human
cancers. It has been revealed that increased activation of ERK can
inhibit apoptosis induction by endoplasmic reticulum stress in
order to promote cell survival. In the present study,
downregulation of α5-nAChR decreased the phosphorylated levels of
the ERK1/2 protein in the DU145 and PC3 cell lines. Based on these
results, it was speculated that α5-nAChR may also be a target for
nicotine that could promote prostate cancer progression. However,
this hypothesis requires further confirmation.
In conclusion, the data demonstrated for the first
time that α5-nAChR levels were increased in prostate cancer samples
and that they could promote the proliferation and migration of
prostate cancer cells by the activation of the AKT signaling
pathway. This finding provides clinically relevant information on
utilizing α5-nAChR as a novel biomarker in order to improve
prostate cancer prognosis. The present study provides a rational to
develop new therapeutic approaches in order to suppress the
proliferation and invasion of prostate cancer cells.
Acknowledgements
Not applicable.
Funding
Internal funding was received for the present study
from the Second Hospital of the Hebei Medical University.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JCQ designed and performed the experiments, wrote
the manuscript. WYX, YPZ, CBQ, BSL, YWY, KLL, DBW and WL
contributed to experimental work and data analysis. ZMZ conducted
the experiments and wrote the manuscript. All authors have read and
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Research
Ethics Committee of the Second Hospital of Hebei Medical
University. Written informed consent forms were obtained from the
subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reiter RE: Risk stratification of prostate
cancer 2016. Scand J Clin Lab Invest Suppl. 245 (Suppl):S54–S59.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li HZ and Zhang YS: Review of hot topics
in the diagnosis and treatment of prostate cancer in 2016. Zhonghua
Wai Ke Za Zhi. 55:59–62. 2017.(In Chinese). PubMed/NCBI
|
|
3
|
Brown KC, Perry HE, Lau JK, Jones DV,
Pulliam JF, Thornhill BA, Crabtree CM, Luo H, Chen YC and Dasgupta
P: Nicotine induces the up-regulation of the α7-nicotinic receptor
(α7-nAChR) in human squamous cell lung cancer cells via the
Sp1/GATA protein pathway. J Biol Chem. 288:33049–33059. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cesario A, Russo P, Nastrucci C and
Granone P: Is α7-nAChR a possible target for lung cancer and
malignant pleural mesothelioma treatment? Curr Drug Targets.
13:688–694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo L, Wu Z and Zhou Q: Roles of nicotine
and nicotinic acetylcholine receptors (nAChR) in carcinogenesis and
development of lung cancer. Zhongguo Fei Ai Za Zhi. 14:753–757.
2011.(In Chinese). PubMed/NCBI
|
|
6
|
Li H, Wang S, Takayama K, Harada T,
Okamoto I, Iwama E, Fujii A, Ota K, Hidaka N, Kawano Y and
Nakanishi Y: Nicotine induces resistance to erlotinib via
cross-talk between α 1 nAChR and EGFR in the non-small cell lung
cancer xenograft model. Lung Cancer. 88:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raffa RB: Cancer ‘survivor-care’: I. the
α7 nAChR as potential target for chemotherapy-related cognitive
impairment. J Clin Pharm Ther. 36:437–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu CC, Huang CY, Cheng WL, Hung CS, Chang
YJ and Wei PL: Silencing A7-nAChR levels increases the sensitivity
of gastric cancer cells to ixabepilone treatment. Tumour Biol.
37:9493–9501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun H and Ma X: α5-nAChR modulates
nicotine-induced cell migration and invasion in A549 lung cancer
cells. Exp Toxicol Pathol. 67:477–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JC, Cruchaga C, Saccone NL, Bertelsen
S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, et al: Risk
for nicotine dependence and lung cancer is conferred by mRNA
expression levels and amino acid change in CHRNA5. Hum Mol Genet.
18:3125–3135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia Y, Sun H, Wu H, Zhang H, Zhang X, Xiao
D, Ma X and Wang Y: Nicotine inhibits cisplatin-induced apoptosis
via regulating α5-nAChR/AKT signaling in human gastric cancer
cells. PLoS One. 11:e01491202016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silva JM, Bulman C and McMahon M:
BRAFV600E cooperates with PI3K signaling, independent of AKT, to
regulate melanoma cell proliferation. Mol Cancer Res. 12:447–463.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang CS, Prieto VG, Diwan AH, Lizee G,
Ellerhorst JA, Ekmekcioglu S, Liu P, Eton O, Kinney SA, Grimm EA,
et al: Changes in pERK1/2 and pAKT expression in melanoma lesions
after imatinib treatment. Melanoma Res. 18:241–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Campagne C, Sundstrom E, Sousa P,
Imran S, Seltenhammer M, Pielberg G, Olsson MJ, Egidy G, Andersson
L and Golovko A: Constitutive activation of the ERK pathway in
melanoma and skin melanocytes in Grey horses. BMC Cancer.
14:8572014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trisciuoglio D, Iervolino A, Zupi G and
Del Bufalo D: Involvement of PI3K and MAPK signaling in
bcl-2-induced vascular endothelial growth factor expression in
melanoma cells. Mol Biol Cell. 16:4153–4162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Yang Z, Wen J, Ma F, Wang F, Yu K,
Tang M, Wu W, Dong Y, Cheng X, et al: SKLB-M8 induces apoptosis
through the AKT/mTOR signaling pathway in melanoma models and
inhibits angiogenesis with decrease of ERK1/2 phosphorylation. J
Pharmacol Sci. 126:198–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jazirehi AR, Wenn PB and Damavand M:
Therapeutic implications of targeting the PI3Kinase/AKT/mTOR
signaling module in melanoma therapy. Am J Cancer Res. 2:178–191.
2012.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu S, Chaudhry MR, Berrebi AA,
Papadimitriou JC, Drachenberg CB, Haririan A and Alexiev BA:
Polyomavirus replication and smoking are independent risk factors
for bladder cancer after renal transplantation. Transplantation.
101:1488–1494. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharp L, Johansson H, Hatschek T and
Bergenmar M: Smoking as an independent risk factor for severe skin
reactions due to adjuvant radiotherapy for breast cancer. Breast.
22:634–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhattacharyya S, Mandal S, Banerjee S,
Mandal GK, Bhowmick AK and Murmu N: Cannabis smoke can be a major
risk factor for early-age laryngeal cancer-a molecular
signaling-based approach. Tumour Biol. 36:6029–6036. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hickey K, Do KA and Green A: Smoking and
prostate cancer. Epidemiol Rev. 23:115–125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kenfield SA, Stampfer MJ, Chan JM and
Giovannucci E: Smoking and prostate cancer survival and recurrence.
JAMA. 305:2548–2555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huncharek M, Haddock KS, Reid R and
Kupelnick B: Smoking as a risk factor for prostate cancer: A
meta-analysis of 24 prospective cohort studies. Am J Public Health.
100:693–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Honda GD, Bernstein L, Ross RK, Greenland
S, Gerkins V and Henderson BE: Vasectomy, cigarette smoking, and
age at first sexual intercourse as risk factors for prostate cancer
in middle-aged men. Br J Cancer. 57:326–331. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giovannucci E, Rimm EB, Ascherio A,
Colditz GA, Spiegelman D, Stampfer MJ and Willett WC: Smoking and
risk of total and fatal prostate cancer in united states health
professionals. Cancer Epidemiol Biomarkers Prev. 8:277–282.
1999.PubMed/NCBI
|
|
27
|
Veierãd MB, Laake P and Thelle DS: Dietary
fat intake and risk of prostate cancer: A prospective study of
25,708 Norwegian men. Int J Cancer. 73:634–638. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heikkilä R, Aho K, Heliövaara M, Hakama M,
Marniemi J, Reunanen A and Knekt P: Serum testosterone and sex
hormone-binding globulin concentrations and the risk of prostate
carcinoma: A longitudinal study. Cancer. 86:312–315. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spitz MR, Amos CI, Dong Q, Lin J and Wu X:
The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both
for nicotine dependence and for lung cancer. J Natl Cancer Inst.
100:1552–1556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duga S, Soldà G, Asselta R, Bonati MT,
Dalprà L, Malcovati M and Tenchini ML: Characterization of the
genomic structure of the human neuronal nicotinic acetylcholine
receptor CHRNA5/A3/B4 gene cluster and identification of novel
intragenic polymorphisms. J Hum Genet. 46:640–648. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valor LM, Campos-Caro A, Carrasco-Serrano
C, Ortiz JA, Ballesta JJ and Criado M: Transcription Factors NF-Y
and Sp1 are important determinants of the promoter activity of the
bovine and human neuronal nicotinic receptor beta 4 subunit genes.
J Biol Chem. 277:8866–8876. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Majumder PK and Sellers WR: Akt-regulated
pathways in prostate cancer. Oncogene. 24:7465–7474. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Malik SN, Brattain M, Ghosh PM, Troyer DA,
Prihoda T, Bedolla R and Kreisberg JI: Immunohistochemical
demonstration of phospho-akt in high gleason grade prostate cancer.
Clin Cancer Res. 8:1168–1171. 2002.PubMed/NCBI
|
|
35
|
Kreisberg JI, Malik SN, Prihoda TJ,
Bedolla RG, Troyer DA, Kreisberg S and Ghosh PM: Phosphorylation of
Akt (Ser473) is an excellent predictor of poor clinical outcome in
prostate cancer. Cancer Res. 64:5232–5236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Graff JR, Konicek BW, McNulty AM, Wang Z,
Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, et al:
Increased AKT activity contributes to prostate cancer progression
by dramatically accelerating prostate tumor growth and diminishing
p27Kip1 expression. J Biol Chem. 275:24500–24505. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin HK, Yeh S, Kang HY and Chang C: Akt
suppresses androgen-induced apoptosis by phosphorylating and
inhibiting androgen receptor. Proc Natl Acad Sci USA. 98:7200–7205.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yuge K, Kikuchi E, Hagiwara M, Yasumizu Y,
Tanaka N, Kosaka T, Miyajima A and Oya M: Nicotine induces tumor
growth and chemoresistance through activation of the PI3K/Akt/mTOR
pathway in bladder cancer. Mol Cancer Ther. 14:2112–2120. 2015.
View Article : Google Scholar : PubMed/NCBI
|