Introduction
Colorectal cancer is a worldwide problem, and among
solid cancers, it has a significantly high incidence and mortality
rate (1). In the wake of learning
about the increasing importance of risk factors such as an
unbalanced diet, the incidence and mortality rate have been
decreasing for decades (2).
Nevertheless, as the fifth most diagnosed cancer, colorectal cancer
is one of the leading causes of cancer-related deaths in China
(3). The 5-year survival rate for
colorectal cancer patients can increase to 90% if diagnosed at an
early stage. However, this rate will decrease to 70.4% with lymph
node metastasis or invasion to adjacent organs, and it will further
decrease to 12.5% when the cancer cells invade distant organs
(4). As a result, the search for
new genes that promote the progression of colorectal cancer may
contribute to anticancer therapies.
The matrix metalloproteinases (MMPs) are a family of
zinc-containing endopeptidases composed of 25 members (including
collagenases, gelatinases and stromelysins) that contribute greatly
to the physiological and pathological extracellular matrix
remodelling (5). Their roles in the
progression of cancer have been explained by the degradation of the
extracellular matrix (6,7). However, increasing evidence has
revealed that the function of MMPs is not confined to breaking down
the ECM; they can also degrade many other biomacromolecules
(8). Matrix metalloproteinase-1
(MMP-1), also known as collagenase-1, has been revealed to play a
significant role in the pathological progression of many cancers.
The upregulation of MMP-1 has been revealed to be involved in the
incidence or invasion of diverse cancers, including bladder
(9), prostate (10), gastric (11), pancreatic (12) and melanoma (13). A recent study indicated that the
significance of MMP-1 could make it a potential therapeutic target
in lung adenocarcinoma (14). Some
previous studies have suggested that MMP-1 may be expressed at a
high level in colorectal cancer (15,16).
However, the precise mechanism by which MMP-1 regulates the
progression of colorectal cancer is still unexplored.
Epithelial-mesenchymal transformation (EMT) is a
common transversion by which the cells with an epithelial-like
phenotype change to slender cells with a mesenchymal-like phenotype
(17). EMT occurs in the
progression of many pathologies, and it is followed by the
attenuated capability of adhesion and absence of apico-basal
polarity (18). The abated
adherence function contributes to the invasion and metastasis of
solid tumors (19), and it is
accompanied by the weakened expression of E-cadherin and reinforced
expression of vimentin (17). The
latest investigations demonstrated that MMPs (MMP-2,
MMP-7 and MMP-9) play a significant role in EMT in
tumors (20–24). However, the relationship between
MMP-1 and EMT is still unexplored.
In the present study, the relationship between the
expression levels of MMP-1 and the prognosis of colorectal cancer
was investigated. The underlying molecular mechanism by which MMP-1
promotes the advancement of colorectal cancer was further
explored.
Materials and methods
Data source
A first dataset with access number NM_002421 was
obtained from the UCSC database (https://genome.ucsc.edu/). The dataset contained 24
samples of normal colorectal tissues and 81 samples of colorectal
cancer tissues. The next dataset with the access number X05231
contained 18 samples of colorectal cancer and 18 samples of matched
adjacent non-tumor tissues. Then, the last dataset, which included
22 normal colorectal tissues and 215 colorectal sample tissues, was
obtained using the Cancer Genome Atlas (TCGA) database (http://tcga-data.nci.nih.gov/tcga/). The
expression levels of MMP-1 in these datasets were statistically
analyzed.
Cell culture
The following human colorectal cancer cell lines
were obtained from Sangon Biotech Co., Ltd.: Lovo, HT-29, SW-480,
HCT-116, Caco-2 and SW-620. All of these cells were cultured at
37°C and 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 100
units/ml penicillin, 100 units/ml streptomycin and 10% foetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences); the FBS
was inactivated by incubating at a constant 56°C for 30 min.
Patients and specimens
We collected 49 paraffin-embedded colorectal cancer
tissues and matched adjacent noncancerous tissues from patients
with colorectal cancer from the First Affiliated Hospital of Xi'an
Jiaotong University from January 2014 to December 2015. All
patients were diagnosed with primary colorectal cancer without
distant metastases. All patients underwent surgery for the first
time for the treatment of colorectal cancer. No patients received
radiotherapy or chemotherapy before the operation. All patients
provided consent for the use of their samples, and this use was
approved by the Institute Research Medical Ethics Committee of the
First Affiliated Hospital of Xi'an Jiaotong University.
Transfection with shRNA
An RNA interference lentivirus containing a
puromycin marker from GeneChem, Inc. was obtained; the vector can
steadily downregulate the expression of MMP-1. The targeted
sequence of the shRNA was UGAACAUCACCACUGAAGGUGUAGC (25), and its efficiency was demonstrated
by western blotting. A control lentivirus that did not target
anything, but carried a puromycin marker as well, was also
obtained. In the present study, HT-29 and SW-480 cells were
infected with shRNA lentiviruses targeting MMP-1. Successfully
transfected cells were selected in the aforementioned DMEM
containing 1 µg/ml puromycin and were then expanded for the
subsequent experiments.
Cell proliferative assay
Cell proliferation was assessed via a Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology). A total of
2,000 cells in 200 µl of medium were placed in each well of 96-well
plates and were cultured in the abovementioned complete medium. At
the instructed time-point, 20 µl of CCK-8 was added to each well,
which was followed by 2 h of incubation. Then, the OD 450
absorbance was detected by a microplate reader (BioTek Instruments,
Inc.).
Colony formation assay
Cell colony formation was examined by a colony
formation assay. A total of 2,000 cells in complete medium were
inoculated in 60-mm plates and were then incubated at 37°C in a 5%
CO2 environment for two weeks. Cells were dyed with 0.2%
dissolved crystal violet after fixation with methanol for half an
hour. The images were obtained using a chemiluminescence imager
(Bio-Rad Raboratories, Inc.). Then, the number of colonies that
contained >50 cells were counted. Each assay was repeated three
times.
RNA-isolation and real-time PCR
TRIzol reagent (Sigma-Aldrich; Merck KGaA) was used
to purify the total RNA from cells according to the manufacturer's
instructions. Then, the total RNA was reverse-transcribed using
SYBR Green (Takara Biotechnology Co., Ltd.) and an ABI 7500
instrument (Thermo Fisher Scientific, Inc.). Primers were designed
according to the reported sequences (26): MMP-1 forward,
5′-AAATGCAGGAATTCTTTGGG-3′ and reverse, 5′-ATGGTCCACATCTGCTCTTG-3′;
β-actin forward, 5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. The real-time PCR data were
normalized to endogenous levels and were carried out in
triplicate.
Immunohistochemistry (IHC)
Tissue samples were formalin-fixed and
paraffin-embedded, sliced into 4-µm sections and mounted on slides.
Graded ethanol was used to rehydrate the sections after
deparaffinization in xylene at room temperature. After washing with
phosphate-buffered saline (PBS), the sections were placed in 3%
hydrogen peroxide for 20 min to inhibit endogenous peroxidase.
Antigen retrieval was carried out by soaking the slides in 0.01 M
citrate buffer (pH 6.0) and heating them for 30 min in a microwave.
After incubation with a primary MMP-1 antibody (dilution 1:500;
ab137332; Abcam) at 4°C overnight, the slides were washed with PBS
and incubated with biotinylated secondary antibody for half an hour
at 37°C. 3,′3-Diaminobenzidine tetrahydrochloride (DAB) was applied
as a chromogen, and the sections were counterstained with
hematoxylin. PBS was substituted for the primary antibody and used
as a negative control.
The intensity degrees of positive signals were
determined as 0, none; 1, weak; 2, moderate; 3, intense; and 4,
strongly intense; and the percentage degrees of the number of
positive cells were recorded as 0, 0%; 1, 1–25%; 2, 26–50%; 3,
51–75%; and 4, 76–100%. The present study defined the outcome by
multiplying the aforementioned two scores to achieve the final
score of IHC staining. A score of <3 was categorized as
‘negative’, and a score of ≥3 was categorized as ‘positive’ in the
following statistical analysis. Two pathologists evaluated the
scores separately to exclude subjectivity.
Western blot analysis
Cells were harvested, washed with PBS and lysed with
radioimmunoprecipitation assay buffer (RIPA; Beyotime Institute of
Biotechnology) to extract the total proteins. After determining the
protein concentrations using a BCA assay kit (Thermo Fisher
Scientific, Inc.), an equal amount of 20 µg protein from each
sample was resolved via 10% SDS-PAGE and then the proteins were
transferred to polyvinylidene difluoride membranes (EMD Millipore).
After submerging the membranes in 5% non-fat dry milk for 2 h to
block non-specific binding, they were incubated with a primary
antibody in TBST at 4°C overnight. The primary antibodies used were
as follows: MMP-1 (dilution 1:500; ab6721), p-Akt (dilution
1:1,000; ab64148), c-myc (dilution 1:1,000; ab32072), E-cadherin
(dilution 1:1,000; ab40772), N-cadherin (dilution 1:1,000;
ab76057), vimentin (dilution 1:1,000; ab92547), Twist1 (dilution
1:1,000; ab50581), and GAPDH (dilution 1:1,000; ab9485; all from
Abcam). The PVDF membranes were subsequently washed with PBS and
were then incubated with a Goat Anti-Rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (dilution 1:2,000; ab6721;
Abcam). The proteins were developed with a chemiluminescent
substrate (Thermo Fisher Scientific, Inc.), and the expression was
normalized to GAPDH.
Wound healing assay
Approximately 5×105 cells were seeded
into each well of 6-well plates containing complete medium and the
cells were cultured overnight in an incubator set at 37°C and 5%
CO2. When the cell monolayers reached 100% confluence, a
yellow pipette tip was used to scrape them. After washing with 1X
PBS, the cells were placed back in the incubator. The breadth of
the scratched areas was measured at 0, 24, 48 and 72 h under a
fluorescence microscope (CKX41; Olympus Corp.).
Transwell assay
To estimate the migration ability of the cells,
individual groups of cells (approximately 1×105
cells/well) suspended in 200 µl of serum-free medium were placed
into the upper chambers of an 8-µm-pore Transwell (Corning Inc.)
coated with Matrigel matrix. All the upper chambers were placed in
24-well plates after the lower chambers were filled with 500 µl of
medium containing 20% FBS. Next, the chambers were incubated at
37°C in a 5% CO2 atmosphere for 24 h, swabbing the upper
chambers with cotton swabs in succession. The cells located on the
underside of the upper chambers were fixed using 4%
paraformaldehyde and stained using 0.1% crystal violet at 25°C for
15 min. The migrated cells were counted in five randomly selected
visual fields at an ×200 magnification. The same procedures were
applied in the migration assay, however, the upper chambers were
replaced with uncoated chambers. All assays were carried out in
triplicate.
Tumorigenesis assay in vivo
A total of six female BALB/c nude mice (age 6 weeks,
weight 18–22 g) were purchased from the SLAC Laboratory Animal
Center and divided into two groups with three mice in each group.
The use of animals was approved by the Institute Research Medical
Ethics Committee of The First Affiliated Hospital of Xi'an Jiaotong
University.
The SW-480 cells treated with a negative control or
with an MMP-1 knockdown were harvested and inoculated into the
right forelimb of nude mice (1×106 cells/mouse). Every
week, the tumor was measured using Vernier calipers and the volume
was computed by the following formula: Tumor volume
(mm3)=length (mm) × width2(mm2)/2.
All assays were carried out in triplicate. All animals were
euthanized by cervical dislocation at the 3rd week. Then, the
tumors were resected, weighed and fixed with paraffin for
subsequent experiments.
Statistical analysis
All data were statistically analyzed using GraphPad
Prism 6.0 (GraphPad Software) and SPSS 18.0 software. All in
vitro assays were repeated three times, and the data are
presented as the mean ± SD. Differences between experimental groups
were evaluated with Student's t-tests or one-way analysis of
variance (ANOVA) followed by Fishers' least significant difference
test (LSD). Statistical significance was defined as P<0.05.
Results
MMP-1 is overexpressed in colorectal
carcinomas and is related to poor prognosis in colorectal
patients
Bioinformatics analyses revealed that the expression
of MMP-1 was significantly increased in colorectal carcinoma
samples (Fig. 1A-C). The results of
immunohistochemistry revealed that the expression levels of MMP-1
protein were significantly increased in 28/49 colorectal cancer
tissues compared with 11/49 adjacent non-tumor tissues (Fig. 1D and E). To further investigate the
association between the expression level of MMP-1 protein and
clinicopathological features, a chi-square test and a two
independent samples t-test were performed to assess the
relationship between MMP-1 and the clinical characteristics of
colorectal cancer patients. The P-values revealed that high
expression of MMP-1 was associated with the TNM stage (P<0.01)
as well as with lymphatic metastasis (P<0.01; Table I). These results demonstrated that
increased MMP-1 expression was related to poor diagnosis in
colorectal carcinoma.
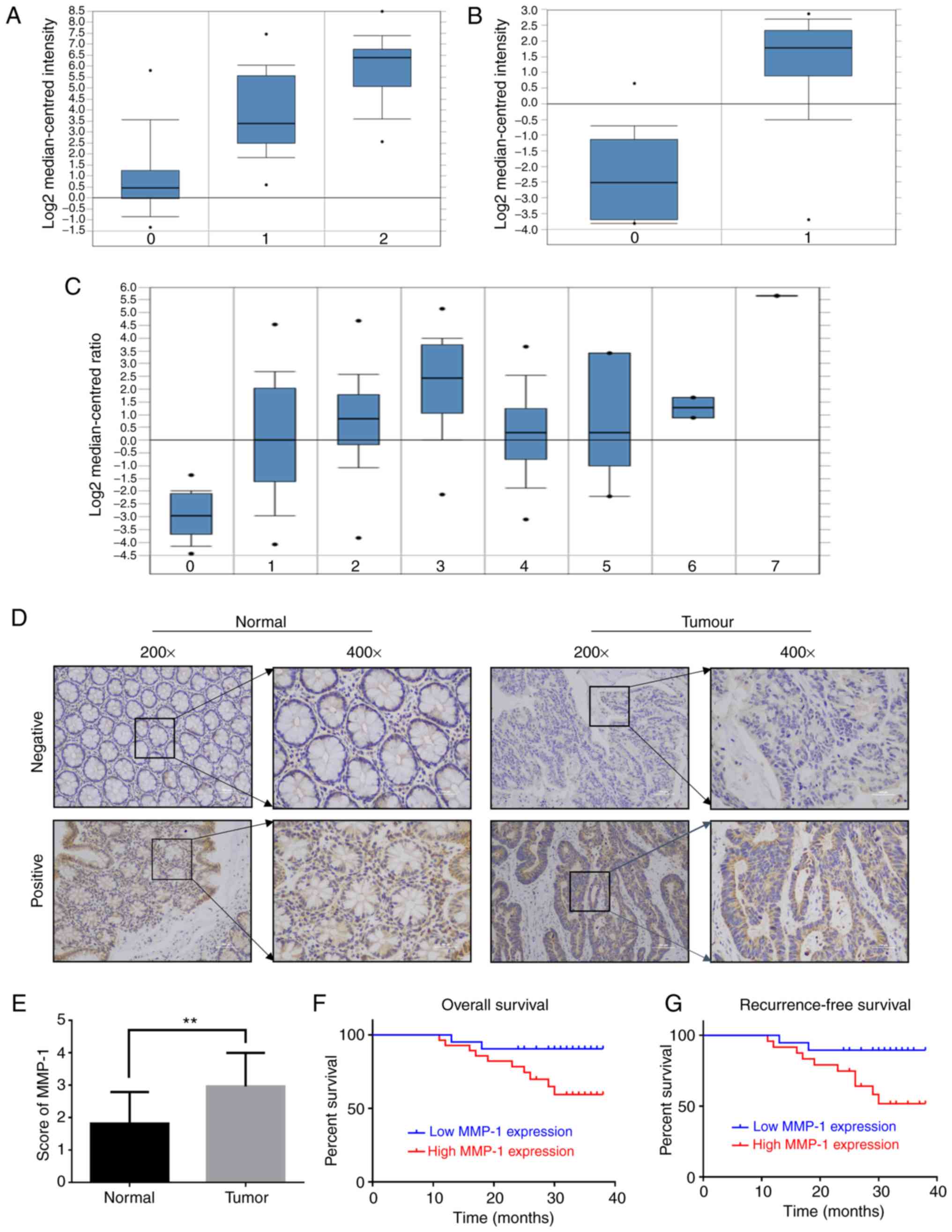 | Figure 1.Expression of MMP-1 in patient
samples and colorectal cancer cell lines. (A) The expression levels
of MMP-1 in a first dataset, 0 represents normal tissues (n=24), 1
represents colorectal carcinoma (n=36) and 2 represents colorectal
adenocarcinoma (n=45). (B) The expression levels of MMP-1 in a
second dataset, 0 represents colon adenocarcinoma (n=18), and 1
represents the adjacent non-tumor tissues (n=18). (C) The
expression levels of MMP-1 in a third dataset, 0–7 represents
respectively the normal tissues (n=22), cecum adenocarcinoma
(n=22), colon adenocarcinoma (n=101), colon mucinous adenocarcinoma
(n=22), rectal adenocarcinoma (n=60), rectal mucinous
adenocarcinoma (n=6), rectosigmoid adenocarcinoma (n=3),
rectosigmoid mucinous adenocarcinoma (n=1). (D) Expression of MMP-1
in 49 colorectal cancer samples were assessed by IHC. Typical scans
of low and high expression of MMP-1 are presented. (E) Comparison
of MMP-1 expression in tumor and normal tissues by IHC score
(**P<0.01). (F) Kaplan-Meier analysis of the relationship
between the expression level of MMP-1 and overall survival time in
colorectal cancer patients. (G) Kaplan-Meier analysis of the
relationship between the expression level of MMP-1 and
recurrence-free survival time in colorectal cancer patients. IHC,
immunohistochemistry. MMP-1, matrix metalloproteinase-1. |
 | Table I.Relationship between MMP-1 and
clinicopathological parameters in colorectal tumors. |
Table I.
Relationship between MMP-1 and
clinicopathological parameters in colorectal tumors.
|
| Low expression | High
expression |
|---|
|
|
|
|
|---|
|
Characteristics | N=21 | N=28 | t-test or
χ2 | P-value |
|---|
| Age (x±s) |
56.47±14.63 |
59.53±11.02 | 0.835 | 0.408 |
| Sex |
|
| 0.062 | 0.804 |
|
Male | 12 | 15 |
|
|
|
Female | 9 | 13 |
|
|
| BMI
(kg/m2, x±s) | 22.26±2.60 | 23.18±3.04 | 1.113 | 0.271 |
| Tumor location |
|
| 0.340 | 0.560 |
|
Colon | 11 | 17 |
|
|
|
Rectum | 10 | 11 |
|
|
| Tumor size (cm,
x±s) |
4.21±1.53 |
4.95±2.07 | 1.371 | 0.177 |
| Stage |
|
| 8.041 | 0.018 |
| I | 6 | 3 |
|
|
| II | 10 | 7 |
|
|
|
III | 5 | 18 |
|
|
| pT status |
|
| 0.331 | 0.565 |
|
1/2 | 6 | 6 |
|
|
|
3/4 | 15 | 22 |
|
|
|
Differentiation |
|
|
|
0.625a |
|
Well | 1 | 3 |
|
|
|
Moderate/Poor | 20 | 25 |
|
|
| Vascular cancer
embolus |
|
| 0.526 | 0.468 |
|
Positive | 2 | 6 |
|
|
|
Negative | 19 | 22 |
|
|
| Lymphatic
metastasis |
|
| 7.894 | 0.005 |
|
Positive | 5 | 18 |
|
|
|
Negative | 16 | 10 |
|
|
In the next experiment, it was explored whether the
high expression of MMP-1 influenced the prognosis of colorectal
cancer patients. The outcome of Kaplan-Meier analyses revealed that
a high expression level of MMP-1 was related to poor prognosis in
both overall survival (P<0.01) and recurrence-free survival
(P<0.01; Fig. 1F and G).
Downregulation of MMP-1 inhibits cell
proliferation in vitro
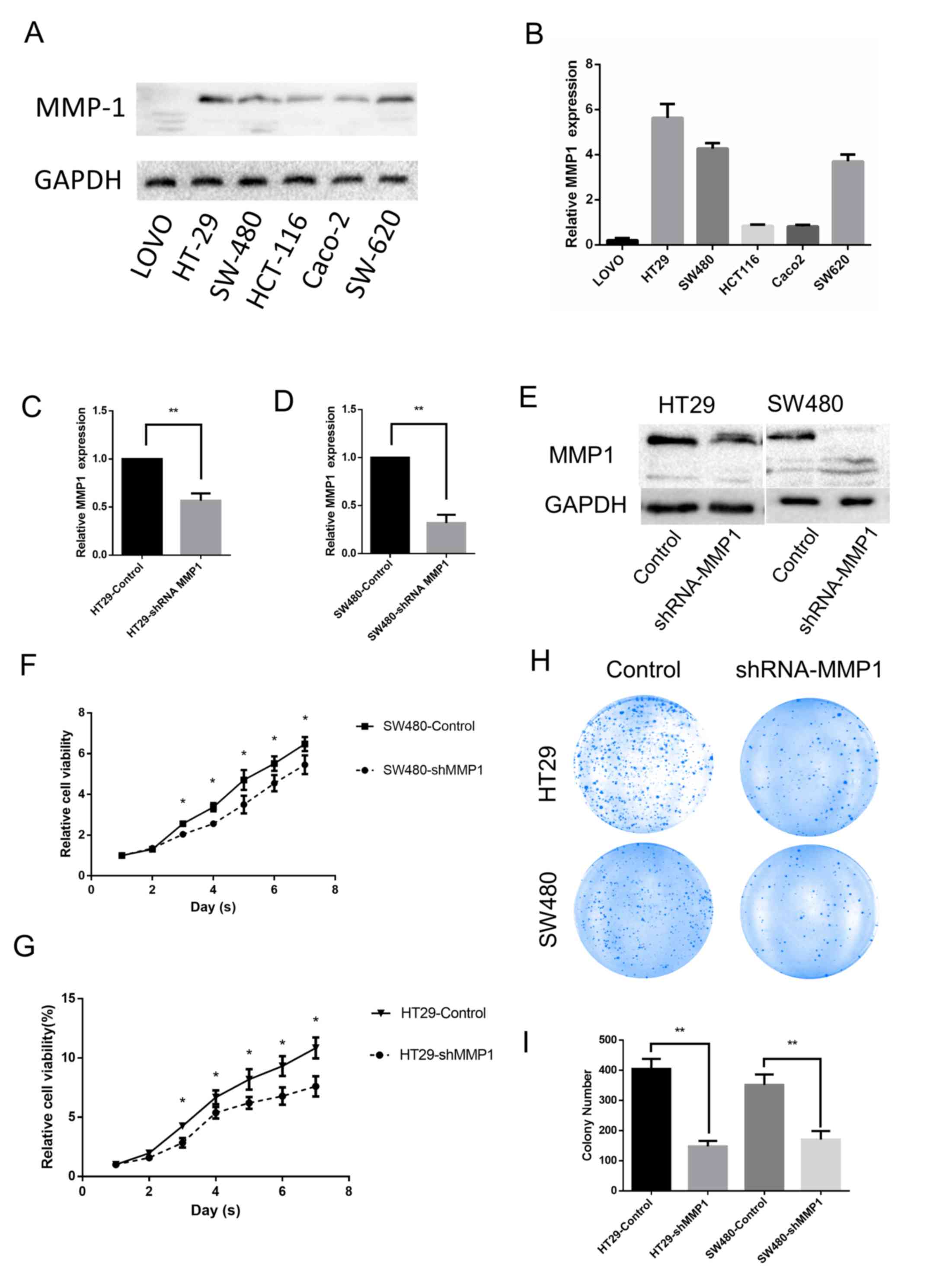
This study examined the expression levels of MMP-1
protein in various colorectal cancer cell lines via western
blotting. Although the expression levels were variable in these
cell lines, MMP-1 was expressed in most of them (Fig. 2A and B). A similar expression
pattern was also revealed when measuring the mRNA of MMP-1 by means
of real-time PCR. According to the aforementioned results, the
HT-29 and SW-480 cell lines were selected for the next
experiments.
The HT-29 and SW-480 cell lines were stably
transfected with an shMMP-1 lentivirus and an empty vector as a
control. To verify the efficiency of infection, real-time PCR was
performed after transfection (Fig. 2C
and D). To further determine the effect of transfection, the
expression levels were assessed by western blotting (Fig. 2E). All of the aforementioned results
revealed that the expression levels of MMP-1 protein decreased
after the cells were transfected with lentivirus.
Having knocked down the expression of MMP-1 in the
HT-29 and SW480 cell lines, the role of MMP-1 in the progression of
colorectal carcinoma was investigated. CCK-8 assays revealed that
downregulation of MMP-1 expression attenuated the proliferative
capability of colorectal cell lines (Fig. 2F and G). In addition, the number of
HT-29 and SW-480 colonies was significantly decreased after the
expression of MMP-1 was knocked down, indicating that MMP-1
enhances the colony formation capability of these cell lines
(Fig. 2H and I).
Downregulation of MMP-1 attenuates the
migration and invasion of colorectal cancer cells
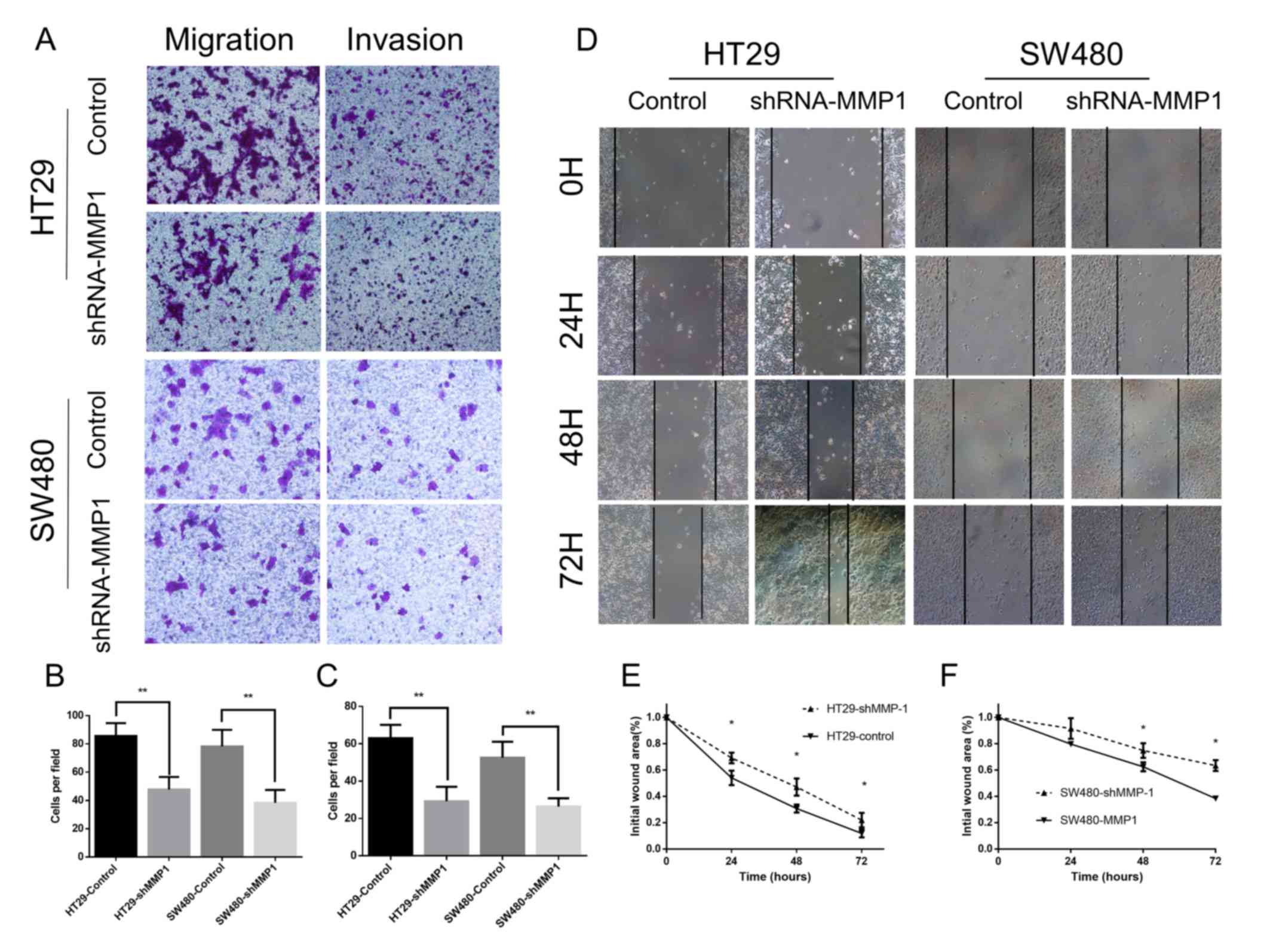
Subsequently, Transwell assays were performed to
evaluate the influence of MMP-1 on the invasive ability of
colorectal cells. The migration and invasion experiments
demonstrated that downregulated expression of MMP-1 attenuated the
migratory and invasive capabilities of colorectal cell lines
(Fig. 3A-C). Then, wound healing
assays were carried out to measure the influence of MMP-1 on the
migration capability of the cells (Fig.
3D-F). The outcomes demonstrated that silencing of MMP-1
attenuated the migration potential of colorectal cells.
Downregulation of MMP-1 inhibits cell
proliferation in vivo
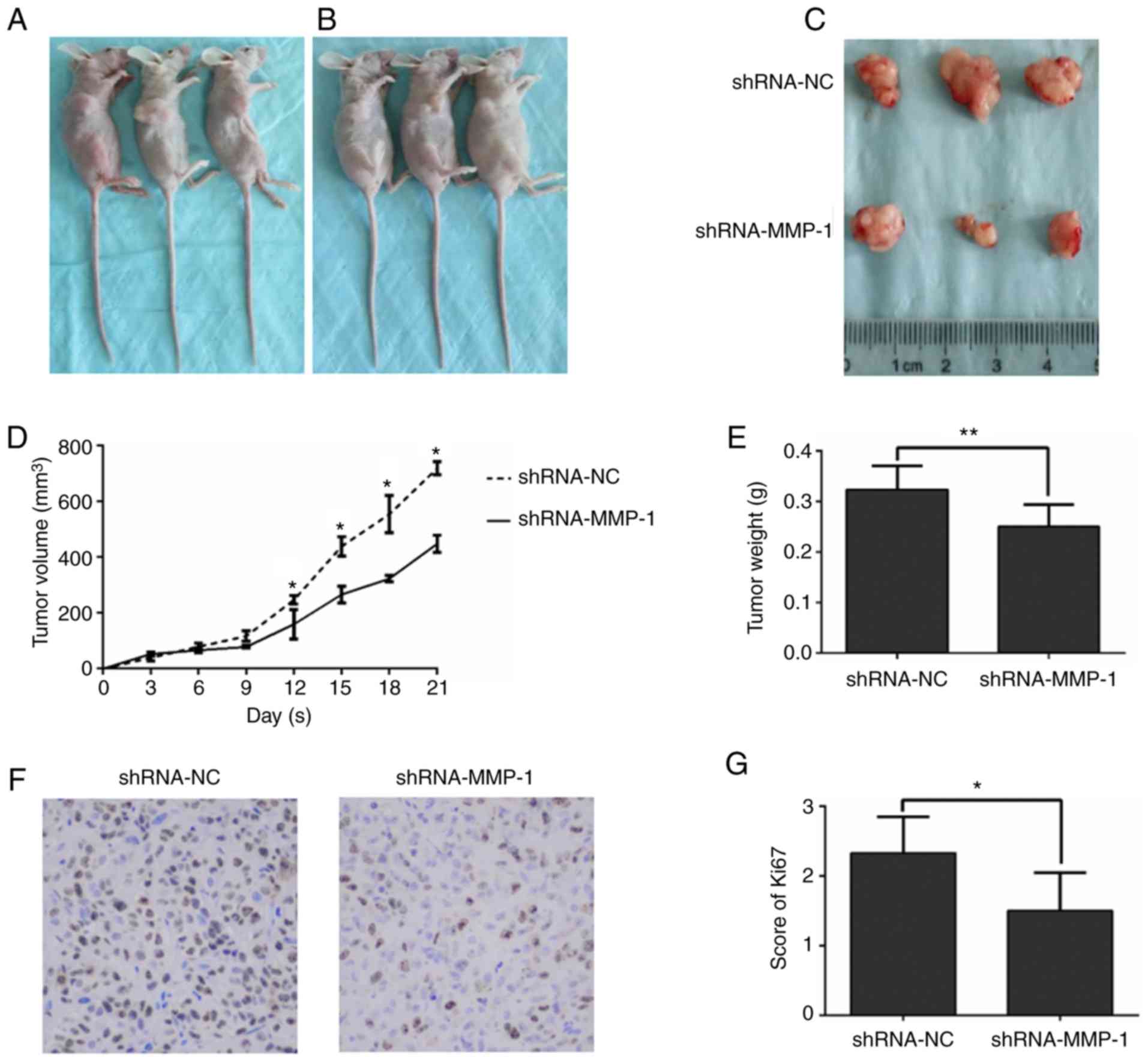
To further verify the tumorigenic ability of MMP-1
in vivo, mice were subcutaneously inoculated with
shMMP-1-treated HT-29 cells and control HT-29 cells. The
subcutaneous tumor nodules resected from the mice with
shMMP-1-treated HT-29 cells grew slower than those resected from
the empty vector group (Fig. 4A-E).
In addition to tumor volume and weight, the common proliferation
marker, Ki-67, was assessed via western blotting. The results
demonstrated the suppressed expression of Ki-67 in tumor nodules
derived from shMMP-1-treated cells compared to tumor nodules
derived from control cells (Fig. 4F and
G). All of the evidence revealed in Fig. 4 demonstrated that interfering with
the expression of MMP-1 in colorectal cancer can inhibit the growth
of tumors in vivo.
MMP-1 is involved in EMT and
activation of the Akt signaling pathway in colorectal
carcinoma
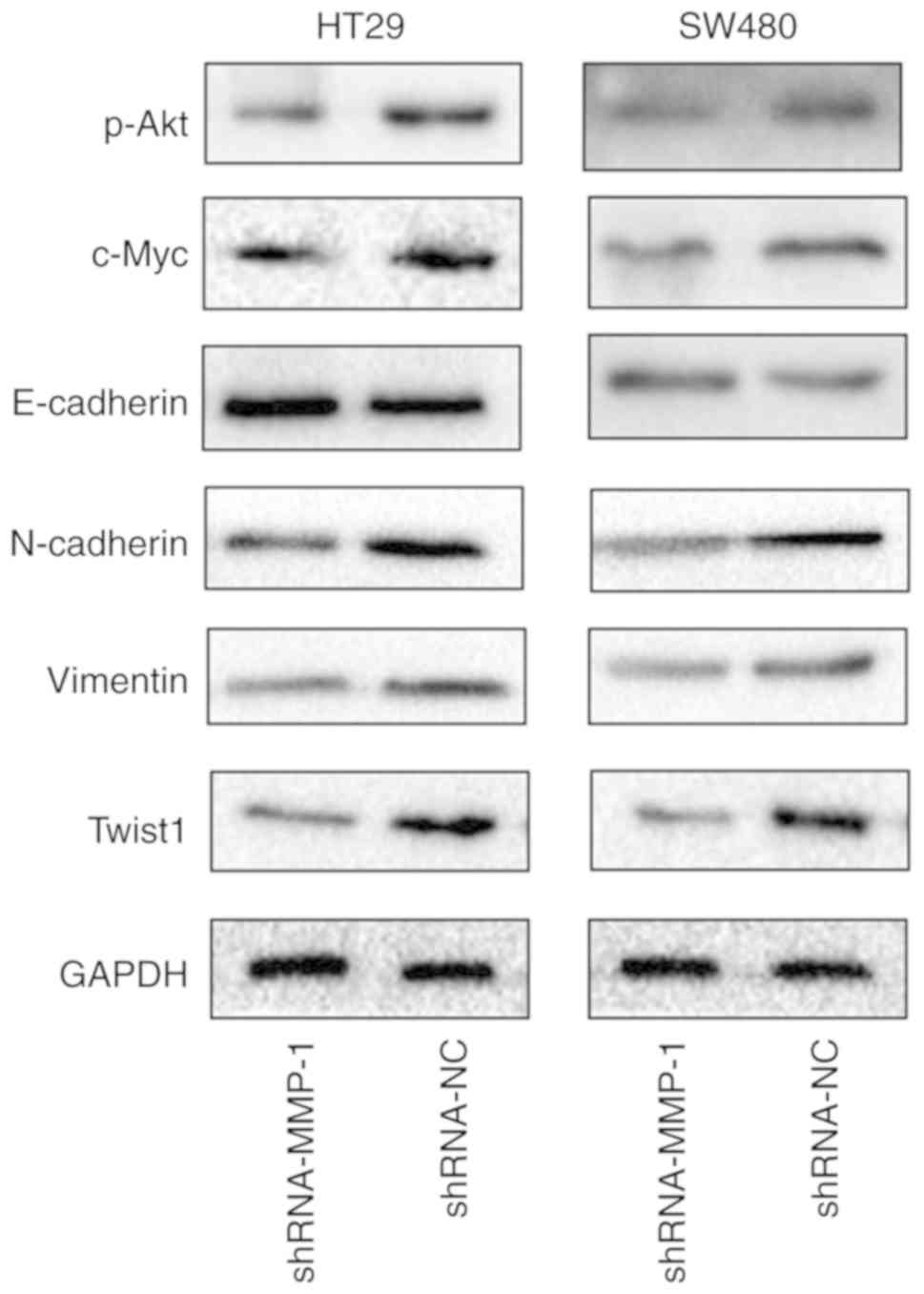
To reveal the latent mechanism through which MMP-1
enhances the progression of colorectal carcinoma, western blotting
was used to identify the potential signaling pathway associated
with the downregulation of MMP-1. This study revealed that the Akt
signaling pathway was involved in both the HT-29 and SW-480 cell
lines transfected with shMMP-1. The western blot results revealed
that the expression of phosphorylated Akt as well as c-Myc was
suppressed after the downregulation of MMP-1 (Fig. 5), while the expression of total Akt
was not significantly different (data not shown). Furthermore, the
knockdown of MMP-1 suppressed EMT in colorectal carcinoma.
In HT-29 and SW-480 cells, the downregulation of
MMP-1 induced a significant decrease in N-cadherin, vimentin and
Twist1 expression and a simultaneous increase in E-cadherin
expression compared to cells transfected with the empty vector
(Fig. 5).
Collectively, MMP-1 promoted activation of the Akt
signaling pathway and EMT in colorectal carcinoma.
Discussion
At the beginning of our study, the expression of
MMP-1 was analyzed in colorectal cancer patients based on datasets
from the UCSC and TCGA databases, and the results revealed that
MMP-1 was upregulated in colorectal cancer. Next, it was confirmed
that MMP-1 was expressed in all assessed colorectal cell lines by
western blotting. The immunohistochemical assessment indicated that
the expression of MMP-1 had a negative relationship with prognosis
in colorectal carcinoma. This result corresponded to a study by
Langenskiöld et al, which demonstrated that MMP-1 predicted
poor diagnosis and short-term survival in a cohort of 136 patients
(27). Accumulating evidence has
indicated that high expression MMP-1 predicts poor diagnosis and
short-time survival not only in colorectal cancer but also in other
human cancers (26,28,29).
Moreover, the clinicopathological parameters demonstrated that high
expression of MMP-1 was related to lymphatic metastasis, which was
in accordance with studies on MMP-1 in breast cancer (30), esophageal adenocarcinomas (31), oral tongue cancer (32), and oropharyngeal cancer (33).
In the present study, in vitro and in
vivo experiments revealed that downregulated expression of
MMP-1 attenuated the proliferation, migration and invasion
capabilities of colorectal cancer cells, implicating the oncogenic
significance of MMP-1 in colorectal carcinogenesis. These results
indicated that MMP-1 plays a significant role in the progression of
colorectal cancer. Some recent studies also indicated that MMP-1
was able to promote the proliferation, migration and invasion of
cancer in vitro and in vivo. Weiss et al
reported that MMP-1 participated in the proliferation of melanoma
in coordination with TWIST1 in vitro (34). A study by Saito et al
revealed that MMP-1 contributed to the migration of lung carcinoma
via the mTOR signaling pathway in vitro (14). Furthermore, research by Fuhrman-Luck
et al demonstrated that MMP-1 promoted prostate cancer bone
metastases in a humanized in vivo bone metastasis model
related to KLK4 (35). Evidence has
revealed that increased expression of MMP-1 may serve as an
oncogene in esophageal cancer, promoting the proliferation and
migration of esophageal squamous cell carcinoma (26). In addition to functions in the
incidence and invasion of the aforementioned cancers, MMP-1 may
also regulate the early perineural invasiveness of pancreatic
cancer via a PAR1/SP/NK1R paracrine loop (36). As an agonist of the
protease-activated receptor-1 (PAR1), MMP-1 is propitious to the
angiogenesis and permeability of blood vessels in breast cancer
(37). Moreover, MMP-1 was revealed
to facilitate resistance to chemotherapy as well as invasive
progression of glioblastoma (38).
Akt, as one of the most famous factors in signaling
pathways, has extensive involvement in the regulatory networks of
cancers. Any protein targeting Akt can benefit research aiming at
cancer therapy by influencing various regulatory signaling
pathways. The present study revealed that the low expression of
MMP-1 weakened the Akt signaling pathway in colorectal carcinoma,
in accordance with what Liu et al reported (26). This result implicated MMP-1 as a
potential therapeutic target in colorectal cancer research.
Furthermore, the present study revealed that the
knockdown of MMP-1 affected the expression of E-cadherin,
N-cadherin, vimentin and Twist1, which was consistent with a
previous study by Hanrahan et al on prostate cancer
(39). Of these genes, E-cadherin
is a well-known anti-oncogene that forms a functional complex with
β-catenin (40). This complex
participates in the process of intercellular adhesion in epithelial
cells, securing the strength of the epithelial layer. In contrast,
N-cadherin is an invasion promoter that mediates the morphology
change and renders epithelial cells more motile (41). Vimentin is an extensively expressed
gene in mesenchymal cells, and it is overexpressed in a variety of
cancers. A study by Satelli and Li provided evidence that vimentin
promotes tumorigenesis via various signaling pathways (42). Twist is a typical gene in EMT, and
its function of promoting the invasion and metastasis of colon
cancer cells has been demonstrated by Wang et al (43). These results indicated that MMP-1
promoted invasion and metastasis not only by degrading the ECM but
also by activating EMT.
In conclusion, the aforementioned results elucidated
that MMP-1 promoted the malignant progression of colorectal cancer,
which could function through EMT and the PI3K/Akt pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available on request from the corresponding author. The data are
not publicly available due to privacy or ethical restrictions.
Authors' contributions
KW performed the experiments, acquired the data and
drafted the manuscript. JY and YW collected colorectal cancer
samples, provided clinicopathological characteristics and assisted
with the experiments. JG and ZX analyzed and interpreted the data.
XS and JZ substantially contributed to the study conception and
design. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research, which
ensures that questions about the accuracy or integrity of any part
of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China). Approval for the use of human samples
was provided by the Institute Research Medical Ethics Committee of
The First Affiliated Hospital of Xi'an Jiaotong University and
written informed consent was obtained from all patients. The use of
animals was approved by the Institute Research Medical Ethics
Committee of The First Affiliated Hospital of Xi'an Jiaotong
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi RE and Corcione F: Worldwide burden of colorectal cancer: A
review. Updates Surg. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh D, Srivastava SK, Chaudhuri TK and
Upadhyay G: Multifaceted role of matrix metalloproteinases (MMPs).
Front Mol Biosci. 2:192015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: They're not just for matrix anymore. Curr Opin
Cell Biol. 13:534–540. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shintani T, Kusuhara Y, Daizumoto K,
Dondoo TO, Yamamoto H, Mori H, Fukawa T, Nakatsuji H, Fukumori T,
Takahashi M and Kanayama H: The involvement of hepatocyte growth
Factor-MET-Matrix metalloproteinase 1 signaling in bladder cancer
invasiveness and proliferation. Effect of the MET inhibitor,
Cabozantinib (XL184), on bladder cancer cells. Urology.
101:169.e7–169.e13. 2017. View Article : Google Scholar
|
|
10
|
Wang J, Liu D, Zhou W, Wang M, Xia W and
Tang Q: Prognostic value of matrix
metalloprotease-1/protease-activated receptor-1 axis in patients
with prostate cancer. Med Oncol. 31:9682014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim M, Kim HJ, Choi BY, Kim JH, Song KS,
Noh SM, Kim JC, Han DS, Kim SY and Kim YS: Identification of
potential serum biomarkers for gastric cancer by a novel
computational method, multiple normal tissues corrected
differential analysis. Clin Chim Acta. 413:428–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto H, Itoh F, Iku S, Adachi Y,
Fukushima H, Sasaki S, Mukaiya M, Hirata K and Imai K: Expression
of matrix metalloproteinases and tissue inhibitors of
metalloproteinases in human pancreatic adenocarcinomas:
Clinicopathologic and prognostic significance of matrilysin
expression. J Clin Oncol. 19:1118–1127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci. 17(pii):
E8682016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito R, Miki Y, Ishida N, Inoue C,
Kobayashi M, Hata S, Yamada-Okabe H, Okada Y and Sasano H: The
significance of MMP-1 in EGFR-TKI-resistant lung adenocarcinoma:
Potential for therapeutic targeting. Int J Mol Sci. 19(pii):
E6092018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bendardaf R, Buhmeida A, Ristamäki R,
Syrjänen K and Pyrhönen S: MMP-1 (collagenase-1) expression in
primary colorectal cancer and its metastases. Scand J
Gastroenterol. 42:1473–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiozawa J, Ito M, Nakayama T, Nakashima
M, Kohno S and Sekine I: Expression of matrix metalloproteinase-1
in human colorectal carcinoma. Mod Pathol. 13:925–933. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gurzu S, Silveanu C, Fetyko A, Butiurca V,
Kovacs Z and Jung I: Systematic review of the old and new concepts
in the epithelial-mesenchymal transition of colorectal cancer.
World J Gastroenterol. 22:6764–675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prieto-García E, Díaz-García CV,
García-Ruiz I and Agulló-Ortuño MT: Epithelial-to-mesenchymal
transition in tumor progression. Med Oncol. 34:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakamoto T, Kawano S, Matsubara R, Goto Y,
Jinno T, Maruse Y, Kaneko N, Hashiguchi Y, Hattori T, Tanaka S, et
al: Critical roles of Wnt5a-Ror2 signaling in aggressiveness of
tongue squamous cell carcinoma and production of matrix
metalloproteinase-2 via ΔNp63β-mediated epithelial-mesenchymal
transition. Oral Oncol. 69:15–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Jiao Y, Liu Y, Zhang M, Wang Z,
Li Y, Li T, Zhao X and Wang D: Sinomenine hydrochloride inhibits
the metastasis of human glioblastoma cells by suppressing the
expression of matrix Metalloproteinase-2/-9 and reversing the
endogenous and exogenous Epithelial-mesenchymal transition. Int J
Mol Sci. 9(pii): E8442018. View Article : Google Scholar
|
|
22
|
Lee H, Ko JH, Baek SH, Nam D, Lee SG, Lee
J, Yang WM, Um JY, Kim SH, Shim BS and Ahn KS: Embelin inhibits
invasion and migration of MDA-MB-231 breast cancer cells by
suppression of CXC chemokine receptor 4, matrix
Metalloproteinases-9/2, and Epithelial-mesenchymal transition.
Phytother Res. 30:1021–1032. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chatterjee K, Jana S, DasMahapatra P and
Swarnakar S: EGFR-mediated matrix metalloproteinase-7 up-regulation
promotes epithelial-mesenchymal transition via ERK1-AP1 axis during
ovarian endometriosis progression. FASEB J. 32:4560–4572. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong
XY, Gu Q, Yao Z and Sun BC: Promotion of hepatocellular carcinoma
metastasis through matrix metalloproteinase activation by
epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med.
15:691–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen GH, Huang CH, Luo HY and Jiang ST:
Effect of small interfering RNAs on matrix metalloproteinase 1
expression. Biotechnol Rep (Amst). 4:5–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu M, Hu Y, Zhang MF, Luo KJ, Xie XY, Wen
J, Fu JH and Yang H: MMP1 promotes tumor growth and metastasis in
esophageal squamous cell carcinoma. Cancer Lett. 377:97–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langenskiöld M, Ivarsson ML, Holmdahl L,
Falk P, Kåbjörn-Gustafsson C and Angenete E: Intestinal mucosal
MMP-1-a prognostic factor in colon cancer. Scand J Gastroenterol.
48:563–569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bianco BC, Scotti FM, Vieira DS, Biz MT,
Castro RG and Modolo F: Immunohistochemical expression of matrix
metalloproteinase-1, matrix metalloproteinase-2 and matrix
metalloproteinase-9, myofibroblasts and Ki-67 in actinic cheilitis
and lip squamous cell carcinoma. Int J Exp Pathol. 96:311–318.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang QM, Lv L, Tang Y, Zhang L and Wang
LF: MMP-1 is overexpressed in triple-negative breast cancer tissues
and the knockdown of MMP-1 expression inhibits tumor cell malignant
behaviors in vitro. Oncol Lett. 17:1732–1740.
2019.PubMed/NCBI
|
|
30
|
Eiró N, González LO, Atienza S,
González-Quintana JM, Beridze N, Fernandez-Garcia B,
Pérez-Fernández R, García-Caballero T, Schneider J and Vizoso FJ:
Prediction of metastatic breast cancer in non-sentinel lymph nodes
based on metalloprotease-1 expression by the sentinel lymph node.
Eur J Cancer. 49:1009–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grimm M, Lazariotou M, Kircher S, Stuermer
L, Reiber C, Höfelmayr A, Gattenlöhner S, Otto C, Germer CT and von
Rahden BH: MMP-1 is a (pre-)invasive factor in Barrett-associated
esophageal adenocarcinomas and is associated with positive lymph
node status. J Transl Med. 8:992010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Pan J, Li L, Wang Z, Xiao W and
Li N: Survey of risk factors contributed to lymphatic metastasis in
patients with oral tongue cancer by immunohistochemistry. J Oral
Pathol Med. 40:127–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lassig AAD, Joseph AM, Lindgren BR and
Yueh B: Association of oral cavity and oropharyngeal cancer
biomarkers in surgical drain fluid with patient outcomes. JAMA
Otolaryngol Head Neck Surg. 143:670–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weiss MB, Abel EV, Mayberry MM, Basile KJ,
Berger AC and Aplin AE: TWIST1 is an ERK1/2 effector that promotes
invasion and regulates MMP-1 expression in human melanoma cells.
Cancer Res. 72:6382–6392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fuhrman-Luck RA, Stansfield SH, Stephens
CR, Loessner D and Clements JA: Prostate Cancer-associated
Kallikrein-related peptidase 4 activates matrix Metalloproteinase-1
and Thrombospondin-1. J Proteome Res. 15:2466–2478. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang C, Li Y, Guo Y, Zhang Z, Lian G,
Chen Y, Li J, Su Y, Li J, Yang K, et al: MMP1/PAR1/SP/NK1R
paracrine loop modulates early perineural invasion of pancreatic
cancer cells. Theranostics. 8:3074–3086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Juncker-Jensen A, Deryugina EI, Rimann I,
Zajac E, Kupriyanova TA, Engelholm LH and Quigley JP: Tumor MMP-1
activates endothelial PAR1 to facilitate vascular intravasation and
metastatic dissemination. Cancer Res. 73:4196–4211. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong F, Eibach M, Bartsch JW, Dolga AM,
Schlomann U, Conrad C, Schieber S, Schilling O, Biniossek ML,
Culmsee C, et al: The metalloprotease-disintegrin ADAM8 contributes
to temozolomide chemoresistance and enhanced invasiveness of human
glioblastoma cells. Neuro Oncol. 17:1474–1485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanrahan K, O'Neill A, Prencipe M, Bugler
J, Murphy L, Fabre A, Puhr M, Culig Z, Murphy K and Watson RW: The
role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in
mediating Docetaxel-resistant prostate cancer. Mol Oncol.
11:251–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pal I, Rajesh Y, Banik P, Dey G, Dey KK,
Bharti R, Naskar D, Chakraborty S, Ghosh SK, Das SK, et al:
Prevention of epithelial to mesenchymal transition in colorectal
carcinoma by regulation of the E-cadherin-β-catenin-vinculin axis.
Cancer Lett. 452:254–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Derycke LD and Bracke ME: N-cadherin in
the spotlight of cell-cell adhesion, differentiation,
embryogenesis, invasion and signalling. Int J Dev Biol. 48:463–476.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang D, Rai B, Qi F, Liu T, Wang J, Wang X
and Ma B: Influence of the Twist gene on the invasion and
metastasis of colon cancer. Oncol Rep. 39:31–44. 2018.PubMed/NCBI
|