Introduction
Tumor metastasis typically forms secondary tumors in
other organs or tissues that originate from the primary tumor, and
is responsible for approximately 90% of cancer-related deaths
(1). Among epithelial tumors,
breast cancer is highly malignant and has a substantial probability
of metastasis (2). Degradation of
the extracellular matrix (ECM) by cancerous cells is mediated
through a variety of proteolytic enzymes, including the matrix
metalloproteinases (MMPs). The activity of MMPs in tumor cells
contributes to invasion and metastasis (3). MMP-9 is highly expressed in breast
cancer cells, and its abundant expression is associated with tumor
malignancy (4). MMP-9 secreted from
the tumor facilitates intravasation by destroying ECM components in
surrounding tissues and the resulting tumor cells in the
circulation can spread to distant organs through extravasation
(5). Furthermore, in human breast
cancer, increased MMP-9 expression is correlated with increased
lymph node metastasis and tumor size (6); thus, MMP regulation is considered a
therapeutic target for the prevention of metastasis.
Epidermal growth factor receptor (EGFR) is a
receptor tyrosine kinase (RTK), and it is involved in both
physiological and pathological epithelial cell processes (7). Regulating EGFR function is also
considered to be the main target for protection against cancer
metastasis (8). Ligand binding to
EGFRs leads to receptor dimerization and endocytosis (9). Subsequent phosphorylation of tyrosine
residues at the carboxyl-terminus of EGFR provides docking sites
for proteins with Src homology 2 and phosphotyrosine-binding
domains, and triggers signal transduction through
Ras-Raf-mitogen-activated protein kinase/extracellular
signal-regulated kinase 1/2, phosphoinositide 3 kinase, Akt, signal
transducer and transcriptions (STATs), phospholipase C γ 1, and
other pathways for cell growth, survival, proliferation, and
metastasis in mammalian cells (10).
Activated EGFRs are desensitized by promoting
receptor endocytosis (11). EGFR
endocytosis is directly linked to the decay of intracellular
signaling, and to the degradation of the receptor (12). After endocytosis, EGFR complexes can
return to the plasma membrane, but they can also be retained in
endosomes. Those retained in endosomes are eventually sorted to
early/late endosomes and lysosomes for degradation (13), and this degradation leads to signal
attenuation (14). Therefore,
regulating EGFR endocytosis is a potential therapeutic target for
signal termination (15).
α-arrestin is an identified tumor suppressor in
metastatic breast cancer (16), and
it is known to facilitate direct interactions between modulators of
plasma membrane RTKs, such as Grb2, SHP2, and E3 ubiquitin ligase
(17,18). Thioredoxin-interacting protein
(TXNIP), another α-arrestin family member, is associated with the
RTK-Rab5 complex and translocates together with this complex to
endosomes after ligand stimulation. These findings suggest that
TXNIP modulates RTK internalization and signaling (19).
The lipid
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) is naturally
found in deer antler, but its artificially synthesized version has
been used to explore its biological functions in neutropenia, oral
mucositis, and as an anti-inflammatory agent (20–22).
Specifically, PLAG has been shown to help resolve inflammation
originating from chemotherapy treatments (21,23),
where two common patient complications are neutropenia and oral
mucositis. Chemotherapy-induced metastasis remains a serious
problem (24), and as described
earlier, EGFR modulation is a therapeutic target as activation of
these receptors can contribute to tumor metastasis via
transcriptional activity of inversion-related genes (25).
In the present study, we investigated the
anti-metastatic activity of PLAG in EGF-stimulated cancer cells
after successful EGFR activation. The enhanced speed of
intracellular EGFR trafficking and its enhanced degradation were
examined in PLAG-treated MDA-MB-231 breast cancer cells. Our
results suggest that PLAG may be an anti-metastatic agent for
attenuating malignancy-related EGFR activation.
Materials and methods
Cell culture and reagents
MDA-MB-231 breast cancer cells were purchased from
the American Type Culture Collection (ATCC). Cells were grown in
Dulbecco modified Eagle's medium (DMEM; Welgene) containing 10%
fetal bovine serum (FBS; Tissue Culture Biologicals), 100 U/ml
penicillin, and 100 µg/ml streptomycin (antibiotic-antimycotic
solution; Welgene) at 37°C in a 5% CO2 atmosphere. All
cells tested mycoplasma-free by polymerase chain reaction (PCR) and
were used for experiments within 12 passages after thawing.
1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) and
1-palmitoyl 2-linoleoyl 3-hydroxylglycerol (PLH) were produced by
and obtained from Enzychem Lifescience Corporation (Jecheon,
Korea). Cells were pretreated with PLAH and PLH for 1 h, at doses
10–50 µg/ml.
Transwell cellular assays for invasion
and migration
The invasion and migration assays for MDA-MB-231
cells were performed in 24-well Transwell units (8-µm pore size,
Corning, Inc.). Transwells were coated with 1 mg/ml Matrigel
(Corning). Briefly, MDA-MB-231 cells (2×104/100 µl) were
either placed in the Matrigel-coated Transwells for the invasion
assay or in only the upper part of the Transwells for the migration
assay. The lower chamber was filled with medium containing 10, 25,
or 50 µg/ml PLAG for 1 h and 100 ng/ml EGF (Peprotech). After
incubation at 37°C for 24 h, non-invasive cells that remained on
top of the upper chamber were removed using cotton swabs. Cells
that invaded to the lower side of the membrane inserts were fixed
with formalin at 4°C for 10 min and then stained with cresyl violet
at RT for 5 min. The number of cells that migrated across the
membrane to the lower chamber were photographed under a light
microscope (Carl Zeiss).
Western blot analysis
MDA-MB-231 cells were seeded into 6 wells with a
density of 2×105 cells/ml and cultured without serum
overnight. For western blot analysis, PLAG- and EGF-stimulated
cells were lysed with radioimmunoprecipitation assay buffer (LPS
solution, Daejeon, Korea) containing protease inhibitors, and
debris was centrifuged at 4°C for 30 min at 16,609 × g). The
supernatant was diluted with 5X sample buffer. Equal protein
amounts were separated by sodium dodecyl sulfate-polyacrylamide 10%
gel electrophoresis and transferred to polyvinylidine fluoride
(PVDF) membranes (Millipore Corp.). The membranes were blocked with
2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS)
for 1 h. Membranes were incubated with antibodies to EGFR (cat. no.
4267; Cell Signaling Technology; dilution 1:1,000), phospho-EGFR
(Tyr 1068) (cat. no. 3777; Cell Signaling Technology, dilution
1:1,000), SHC (cat. no. 2432; Cell Signaling Technology; dilution
1:500), phospho-SHC (cat. no. 2434; Cell Signaling Technology;
1:1,000), ERK (cat. no. 4693; Cell Signaling Technology; dilution
1:2,000), phospho-ERK (cat. no. 9101; Cell Signaling Technology;
dilution 1:3,000), JNK (cat. no. 9252; Cell Signaling Technology;
dilution 1:1,000), phospho-JNK (cat. no. 4671, Cell Signaling
Technology; dilution 1:2,000), β-actin (cat. no. 3700; Cell
Signaling Technology; dilution 1:5,000), TXNIP (cat. no. 14715;
Cell Signaling Technology; dilution 1:1,000) and MMP-9 (cat. no.
AB19016; Millipore Corp; dilution 1:2,000) for overnight at 4°C.
After three washes in PBST, membranes were stained with
peroxidase-conjugated goat anti-rabbit IgG (cat. no. sc-2005; Santa
Cruz Biotechnology; dilution 1:5,000) or peroxidase-conjugated goat
anti-mouse IgG (cat. no. sc-2004; Santa Cruz Biotechnology;
dilution 1:5,000) for 1 h at room temperature. Immobilon Western
Chemiluminescent HRP Substrate was used for signal detection
(Millipore Corp). Densitometric analysis was performed using ImageJ
software (version 1.48; National Institutes of Health).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cells with Trizol
(Favorgen) according to the manufacturer's protocol. Approximately
500 ng of total RNA was used to prepare cDNA using M-MLV reverse
transcriptase (Promega). The following primers (Macrogen) were
used: 5′-CGAGAGAGACTCTACACCCAGGAC-3′ and
5′-CACTTCTTGTCGCTGTCAAAGT-3′ for MMP-9; 5′-CCATCACCATCTTCCAGGAG-3′
and 5′-ACAGTCTTCTGGGTGGCAGT-3′ for GAPDH;
5′-GCCACACTTACCTTGCCAAT-3′ and 5′-GGAGGAGCTTCTGGGGTATC-3′ for
TXNIP. PCR was performed under following conditions: 96°C for 30
sec, 60°C (MMP-9, TXNIP) or 58°C (GAPDH) for 30 sec, and 72°C for
30 sec, followed by 72°C for 5 min. PCR products were
electrophoresed using 2% agarose gels and stained with ethidium
bromide.
Luciferase assay
AP-1 transcriptional activity was measured
indirectly using a pGL4-AP-1-luc plasmid-based luciferase reporter
assay (Promega) and Attractene transfection reagent (Qiagen)
according to the manufacturers' instructions. Cells were seeded
into 24-well plates at a density of 5×104/ml and the
luciferase-reporter plasmid (1 µg/well) was added for 24 h. Cells
were then starved and treated with different concentrations of PLAG
for 1 h, followed by stimulation with EGF (100 ng/ml) for 6 h.
Transient expression of the reporter gene was quantified using the
DualGlo1 luciferase assay system (Promega) in a TD-20/20 Turner
luminometer (Promega).
Immunoprecipitation assay
Protein G agarose beads (Roche) were used for
immunoprecipitation. Cells (2×105/ml) were seeded in
6-well plates and starved overnight. After EGF and PLAG treatment,
the cells were lysed with lysis buffer (25 mM Tris-HCl, pH 7.4; 150
mM NaCl, 1% NP-40, 1 mM EDTA, 5% glycerol). Cell lysates were
incubated with anti-EGFR (cat. no. sc-373746, Santa Cruz
Biotechnology, dilution 1:50) antibodies by gentle agitation for 6
h at 4°C. The beads were then added and incubated overnight. After
the reaction, the beads were washed using lysis buffer. Target
proteins were eluted in 1X sample buffer and analyzed by western
blotting using antibodies against EGFR (cat. no. 4267; Cell
Signaling Technology; dilution 1:1,000), ubiquitin (cat. no. 3933;
Cell Signaling Technology; dilution 1:1,000), c-Cbl (cat. no. 2747;
Cell Signaling Technology; dilution 1:1,000), EGFR pathway
substrate 15 (EPS15) (cat. no. sc-390259; Santa Cruz Biotechnology;
dilution 1:1,000), and TXNIP (cat. no. 14715; Cell Signaling
Technology; dilution 1:1,000).
Immunofluorescence staining
Cells (1×105/ml) were seeded onto cover
glasses in 24-well plates and cultured without serum overnight. For
visualization of surface EGFRs, cells were fixed with 3.7%
formaldehyde for 20 min. For visualization of EGFR-Rab5/Rab7
colocalizations, cells were fixed and then permeabilized with 0.2%
Triton X-100 for 20 min. After being washed with PBS twice and
blocked with 2% BSA, cells were incubated with anti-EGFR (cat. no.
352904; BioLegend, dilution 1:1,000), anti-Rab5 (cat. no. sc-47792;
Santa Cruz Biotechnology; dilution 1:1,000), and anti-Rab7 (cat.
no. sc-376362; Santa Cruz Biotechnology; dilution 1:1,000)
antibodies overnight at 4°C. Cells were then washed with PBS twice
and incubated with Alexa Fluor 488-conjugated secondary antibodies
(cat. no. A32723; Invitrogen; Thermo Fisher Scientific, Inc.;
dilution 1:1,000) for 1 h at room temperature. Finally, cells were
stained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen;
Thermo Fisher Scientific, Inc.). Fluorescence confocal microscopy
(Carl Zeiss, Thornwood, NY, USA) was used to assess labeling and
its distribution.
Flow cytometry analysis
After PLAG and EGF treatment, cells were collected
and placed in a solution of trypsin-EDTA. Collected cells were
washed with PBS and then blocked with FACS buffer (0.5% BSA in PBS)
for 30 min at 4°C. Blocked cells were incubated with PE-conjugated
anti-human EGFR antibody (cat. no. 352904, BioLegend) for 1 h at
4°C. Analyses were performed using a BD FACS Verse flow cytometer
(BD Biosciences), and the data were processed using FlowJo software
(version 10.6; Tree Star, USA).
Transient transfection with small
interfering RNA
TXNIP siRNA (cat. no. sc-270490) was purchased from
Santa Cruz Biotechnology, Inc. Scrambled siRNA (cat. no. sc-37007)
was used as the control. MDA-MB-231 breast cancer cells were
transfected with these siRNA duplex targeting constructs (40 nM)
and HiPerFect transfection reagent (Qiagen). After 60 h
incubations, cells were treated with PLAG (50 µg/ml) for 1 h and
EGF (100 ng/ml), and downregulation of target-gene expression was
evaluated by RT-PCR.
Statistical analysis
The data are presented as the mean ± SD of at least
three independent experiments. For statistical analysis, one-way
ANOVA followed by Turkey-Kramer post hoc test was performed using
GraphPad Prism version 5.0 (GraphPad Software, Inc.). P-value less
than 0.05 is considered to indicate statistical significance.
Results
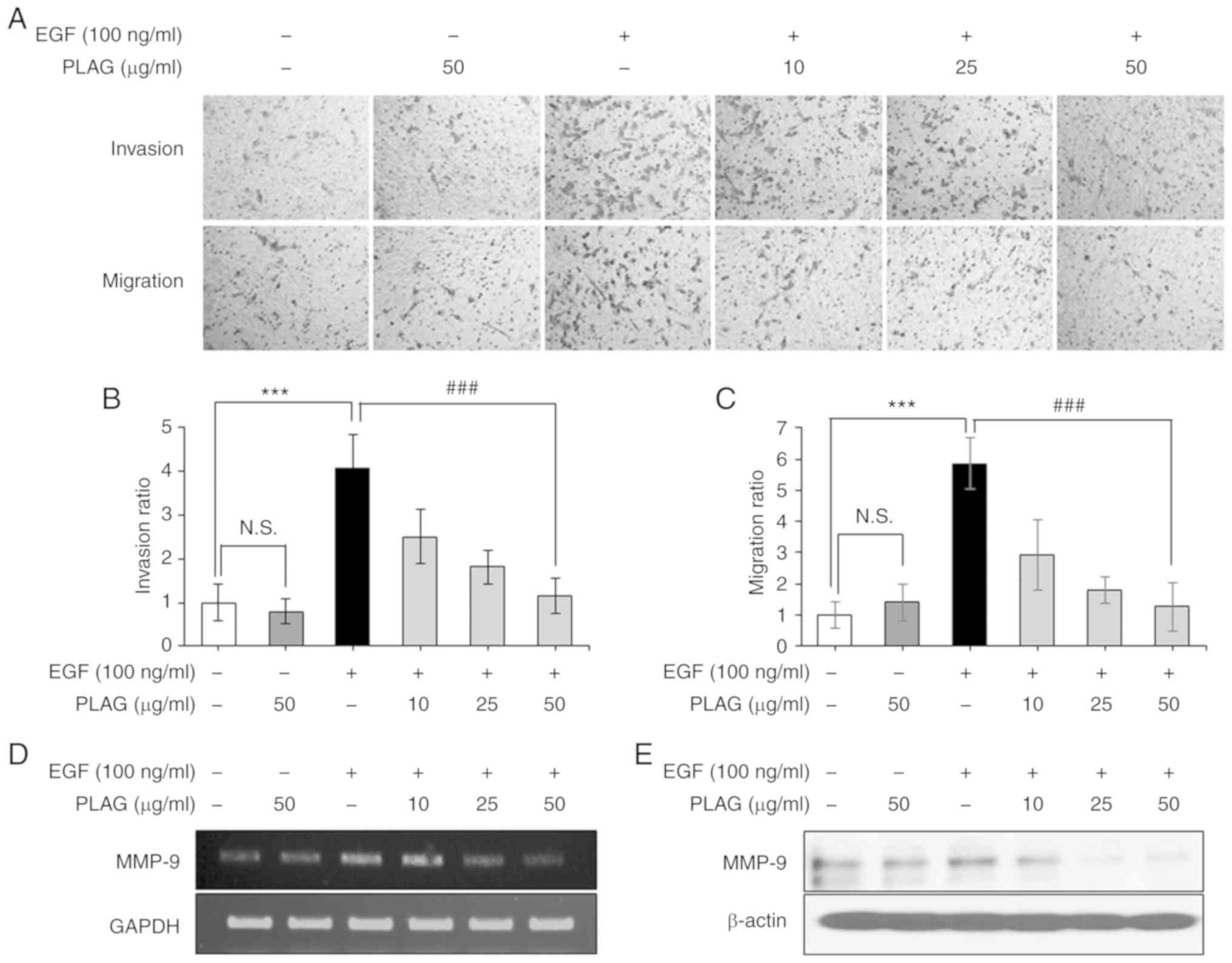
PLAG attenuates EGF-induced invasion
and migration of MDA-MB-231 cells and effectively downregulates
high expression of MMP-9
To evaluate the anti-metastatic effect of PLAG, we
investigated the inhibitory activity of PLAG on EGF-induced cell
invasiveness and mobility using Transwell assays. EGF-treated
MDA-MB-231 cells (100 ng/ml) showed high invasiveness and mobility,
whereas PLAG-treated cells (10, 25, or 50 µg/ml) exhibited
significantly reduced invasiveness and mobility in a dose-dependent
manner (Fig. 1A-C). As expected,
MMP-9 suppression alone by siMMP-9 reduced the invasiveness and
migration of EGF-treated cells (data not shown). PLAG treatment was
also investigated for modulation of invasion-associated MMP-9
expression. MMP-9 expression was examined in EGF-treated cells
using RT-PCR and western blotting analysis. Similar to the mobility
results, MMP-9 expression was high after EGF treatment alone, but
PLAG-treated cells showed significantly lower MMP-9 mRNA and
protein expressions (Fig. 1D and
E). These data indicate that PLAG affected EGF-induced tumor
cell motility by modulating the EGFR signaling pathway and its
downstream influences on gene expression (e.g., on MMPs).
PLAG promotes endocytosis and
ubiquitination of ligand-bound EGFR
EGF treatment induces MMPs through its cognate
receptor, EGFR. A PLAG-induced decrease in MMP expression results
from possible modifications to the EGFR signaling pathway,
including receptor internalization. We assessed EGFR
internalization by examining plasma membrane-localized EGFRs using
flow cytometry and confocal microscopy. EGF treatment reduced the
number of cell-surface EGFRs on MDA-MB-231 cells. These EGFRs were
internalized 5 min after EGF stimulation, and most EGFRs were also
internalized within 5 min in the PLAG-treated cells. EGFR
internalization induced by PLAG treatment was assessed by flow
cytometry at different time intervals (Fig. 2A-C) and by confocal microscope
analysis (Fig. 2D). Internalized
surface EGFRs did not return to the plasma membrane (data not
shown). Degradation of these internalized EGFRs was also examined
using an assay for ubiquitination. After EGF stimulation,
ubiquitinated EGFRs were observed at 30 min, and were sustained for
60 min in the immunoprecipitation assay. In PLAG-treated cells,
ubiquitinated EGFRs were observed 5 min after EGF treatment and
sustained for 30 min. Complexes with ubiquitin ligase, c-Cbl, and
EPS15 were also detected. These dissociated at earlier time points
in the PLAG-treated cells than in EGF-only treated cells,
suggesting that PLAG accelerates ligand-stimulated EGFR
internalization and degradation (Fig.
2E). These activities eventually led to EGFR desensitization
and contributed to preventing the mobility and invasiveness
mediated by EGFR activation.
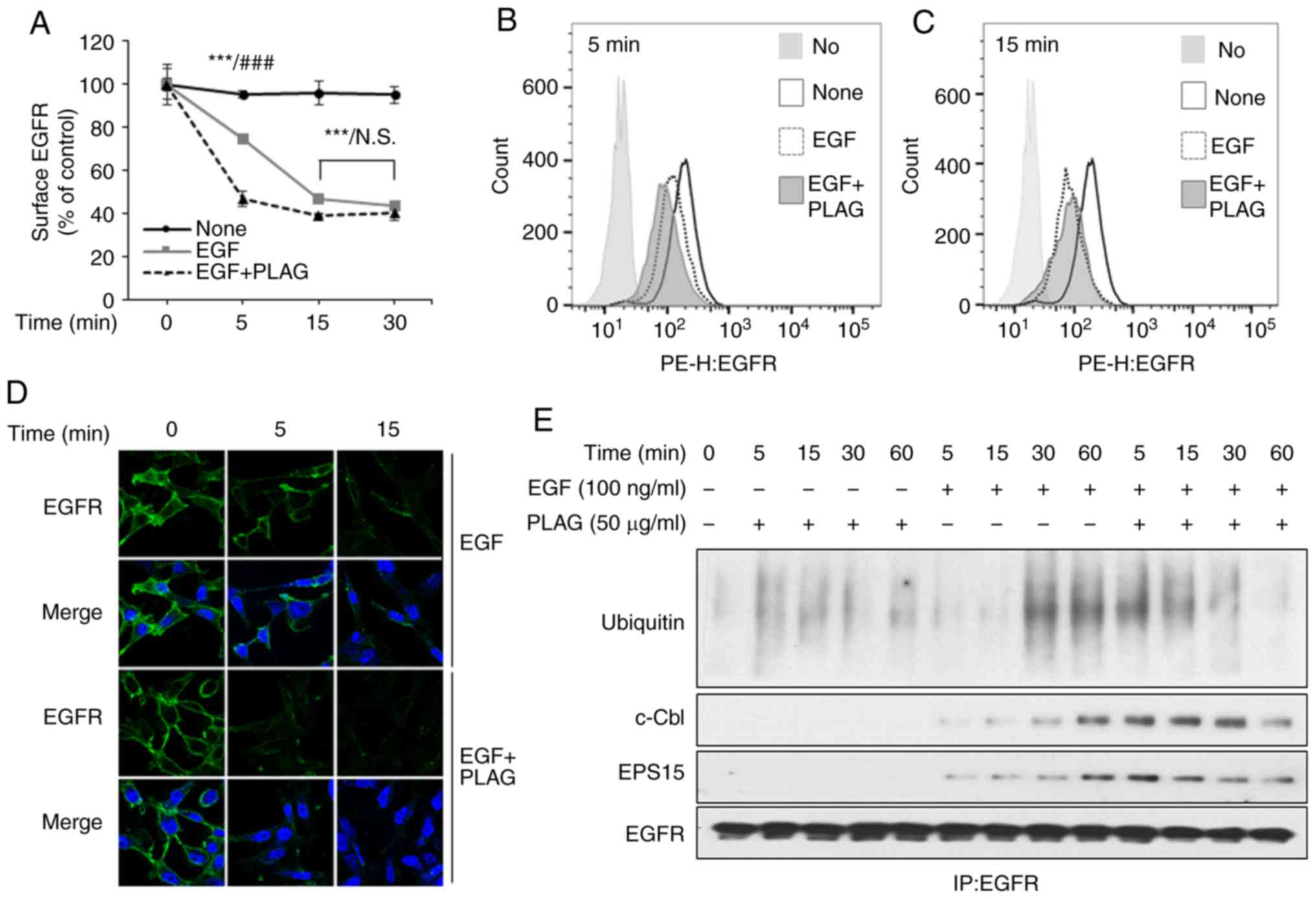 | Figure 2.Accelerated EGFR endocytosis and
ubiquitination in PLAG-treated cells. (A-D) MDA-MB-231 cells were
pretreated with PLAG (10, 25, or 50 µg/ml) for 1 h and then treated
with EGF (100 ng/ml). (A-C) Surface EGFRs were analyzed by flow
cytometry, and the data are represented by means ± SD. (D) Surface
EGFRs were detected by fluorescence confocal microscopy at ×1,000.
(E) EGFR-binding protein and ubiquitination were confirmed by
western blotting via co-immunoprecipitation. Statistical
significance was determined by ANOVA (Tukey's test). ***P<0.005,
compared with the None group; ###P<0.005, compared
with the EGF only treated group. N.S., not significant; PLAG,
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol; EGF, epidermal
growth factor; EGFR, epidermal growth factor receptor; EPS15, EGFR
pathway substrate 15. |
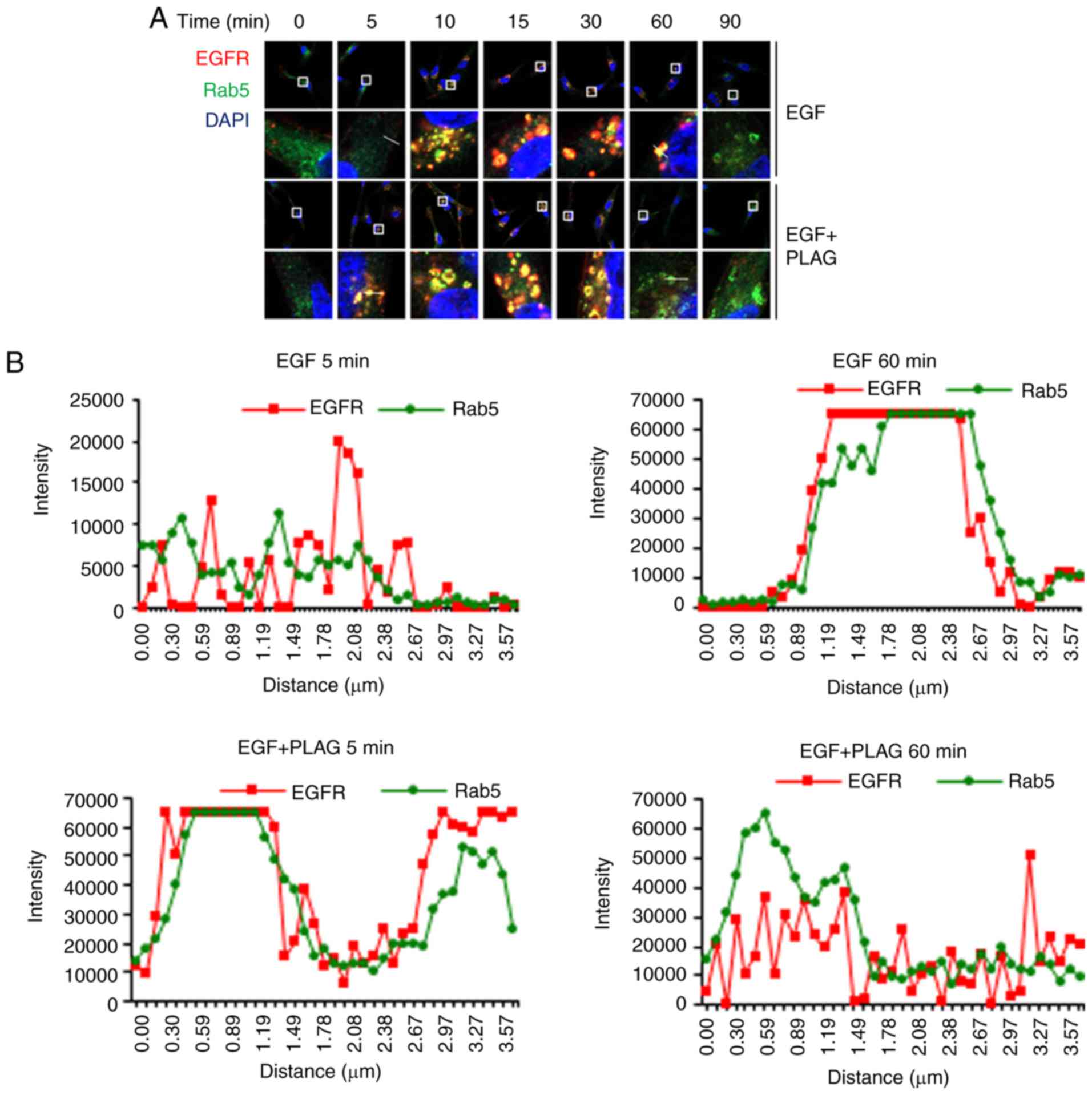
PLAG accelerates intracellular
trafficking and degradation of EGFRs
Degradation of the internalized EGFRs was further
investigated using a colocalization assay with Rab5 and Rab7. Rab5
is required for intracellular trafficking of EGFR to endosomal
compartments, leading to EGFR degradation (26). Rab7 regulates membrane trafficking
at the late endosome-lysosome stage (27).
In confocal microscope images, the overlap of red
PE-conjugated EGFRs with green Alexa 488-bound Rab5 appeared
yellow, indicating colocalization of EGFRs and Rab5. Yellow EGFR
and Rab5 complex assemblies appeared 10 min after EGF stimulation
and were sustained for 60 min. The same yellow EGFR and Rab5
complexes in PLAG-treated cells were detected within 5 min and
dissociated by 30 min (Fig. 3A).
Colocalization of EGFR and Rab5 was quantified by measuring
fluorescence intensity in the images (Fig. 3B). These data indicate that PLAG
accelerates not only the assembly, but also the degradation, of
EGFR complexes.
Using the same assay, the colocalization EGFR and
Rab7, a late endosome marker, was observed at 15 min and
disappeared at 120 min in EGF-only stimulated cells. In contrast,
PLAG-treated cells exhibited EGFR-Rab7 colocalization within 5 min
and dissociated by 90 min (Fig.
3C). This colocalization of EGFR and Rab7 was also verified by
measuring fluorescence intensity in the confocal images (Fig. 3D). These colocalization assay
results show that PLAG may accelerate ligand-bound EGFR
intracellular trafficking and EGFR degradation via lysosome
sorting.
Signals originating from EGFR
activation are attenuated in PLAG-treated cells
The data presented above demonstrated that EGFR
degradation occurs later (at 90 min) in EGF-treated cells than in
PLAG-treated cells (at 60 min). Western blot analysis was used to
assess whether this accelerated EGFR degradation influenced
EGFR-dependent signaling. EGFR degradation was observed at 120 min,
and the decay of phosphorylated EGFRs was detected at 60 min in
PLAG-treated cells; earlier than the degradation and decay times
for EGF-only treated cells. In addition, phosphorylated SHC, ERK,
and JNK induced by EGF stimulation was sustained for 120 min. In
contrast, for PLAG-treated cells, phosphorylated SHC, ERK, and JNK
were dephosphorylated at 60 min (Fig.
4A). The proportions of degraded EGFR (Fig. 4B) and phosphorylated EGFR (Fig. 4C), as well as those of
phosphorylated SHC, ERK, and JNK, were all monitored over time
(Fig. 4D-F). Similar studies have
reported that herbacetin accelerates the internalization and
degradation of EGFR, and subsequently suppressed the activation of
the downstream kinase, ERK (28).
In addition, the adaptor protein SHC has an essential role in the
integration of EGFR signaling (29). Similar to the EGFR degradation
findings, kinase-associated EGFR signaling for MMP-9 expression was
also terminated earlier in PLAG-treated cells. Based on PLAG's
unique mechanism for attenuating EGFR signaling, we further
characterized kinase-activated AP-1, a major transcription factor
regulating MMP-9 expression, using a luciferase assay. AP-1
activity induced by EGF treatment was reduced in PLAG-treated cells
in a dose-dependent manner (Fig.
4G). These results provide further evidence for the potential
role of PLAG in blocking metastasis-inducing EGFR activation.
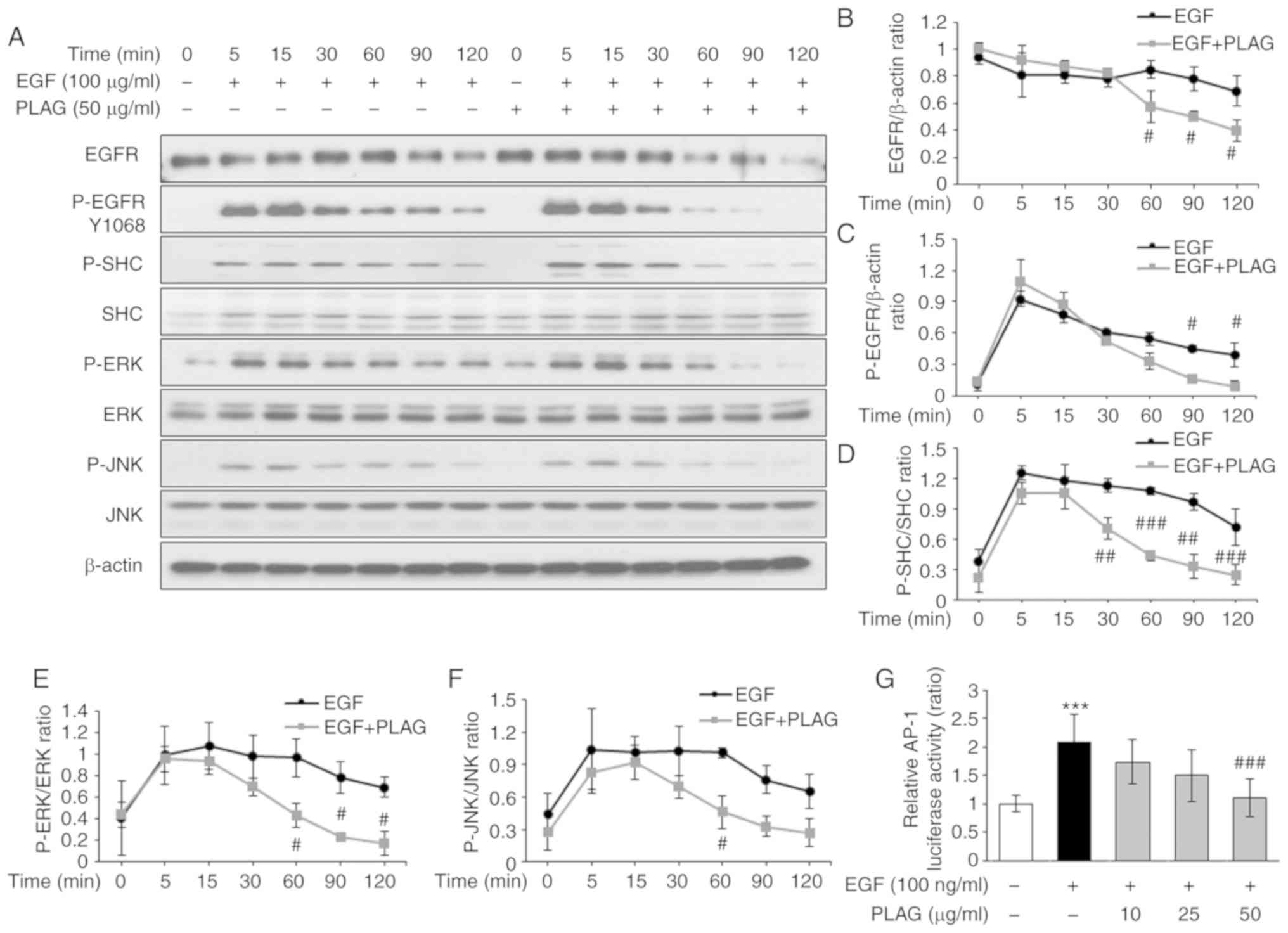 | Figure 4.Attenuation of EGF-signaling by PLAG.
(A-F) Western blot analysis of EGFR degradation and downstream
phosphorylation. (G) To assess luciferase activity, MDA-MB-231
cells were transfected with constructs containing the AP-1
promoter. Cells treated with PLAG and EGF were cultured for 6 h,
and luciferase activity was determined. Statistical significance
was determined by ANOVA (Tukey's test). ***P<0.005, compared
with the untreated group; #P<0.05,
##P<0.01 and ###P<0.005, compared with
the EGF group. N.S., not significant; PLAG,
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol; EGF, epidermal
growth factor; EGFR, epidermal growth factor receptor; p-,
phosphorylated. |
PLAG accelerates EGFR internalization
via TXNIP production
Our results demonstrated that PLAG reduces MMP-9
expression in EGF-stimulated cells. The data suggest that
ligand-bound EGFRs affect intracellular trafficking and activate
signals for MMP-9 expression, and that PLAG accelerates EGFR
internalization and degradation, resulting in a reduced MMP-9
signal. Therefore, we focused on molecules modulated by PLAG
treatment that are involved in receptor trafficking. In
PLAG-treated cells, TXNIP mRNA and protein expression were elevated
(Fig. 5A). Immunoprecipitation
using the EGFR antibody revealed that elevated TXNIP was complexed
with EGFR in the PLAG-treated cells (Fig. 5B). These assembly results were
corroborated using RT-PCR in TXNIP-silenced cells, where TXNIP
knockdown was detected (Fig. 5C).
In addition, the accelerated internalization of surface EGFRs was
no longer observed in the TXNIP-silenced cells (Fig. 5D). In the Transwell invasion and
migration assays, TXNIP-silenced cells did not show PLAG-induced
reductions in invasiveness and mobility (Fig. 5E-G). Downregulation of MMP-9
expression in PLAG-treated cells was also not observed after
TXNIP-silencing (Fig. 5H). These
results indicate that PLAG promotes the internalization of
receptors by increasing the expression of TXNIP and that increased
EGFR degradation reduces MMP-9 expression.
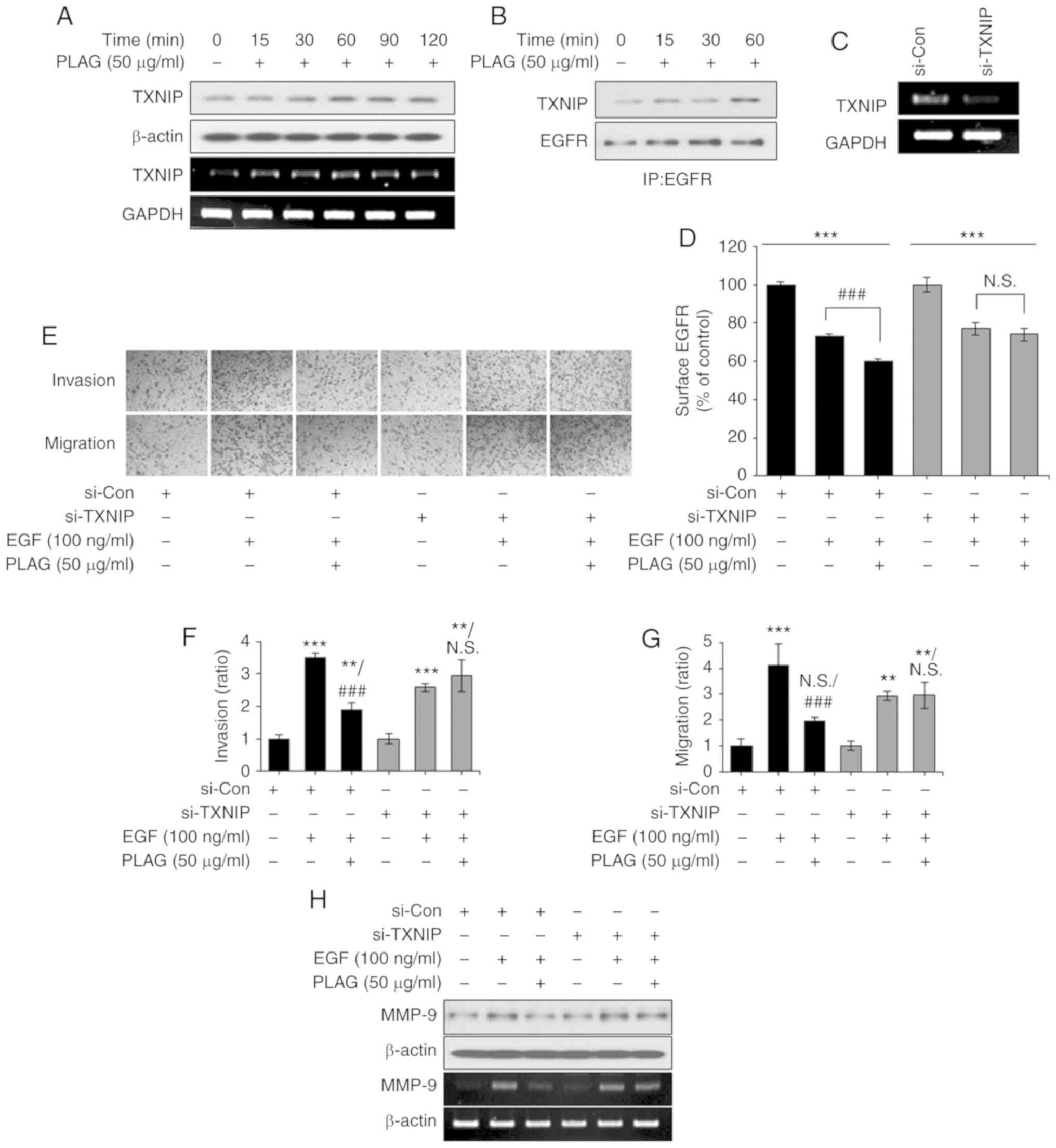 | Figure 5.TXNIP induction by PLAG treatment.
(A) TXNIP mRNA and protein expression was analyzed by RT-PCR and
western blotting, respectively. (B) EGFR-bound TXNIP was confirmed
by western blotting via co-immunoprecipitation in PLAG-only treated
cells. (C) TXNIP knockdown was confirmed by RT-PCR. (D) After
treatment with PLAG and EGF, EGFR internalization was analyzed by
flow cytometry. (E-G) PLAG did not reduce EGF-induced cell
migration and invasion in TXNIP-silenced cells. Invasive and
migrating cells were counted in the assay at ×200. (H) MMP-9
expression was not modified in the si-TXNIP treated cells. MMP-9
expression was analyzed by RT-PCR 6 h, and by western blotting 24
h, after stimulation. Statistical significance was determined by
ANOVA (Tukey's test). **P<0.01 and ***P<0.005, compared with
the untreated group; ###P<0.005, compared with the
EGF group. N.S., not significant; PLAG,
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol; EGF, epidermal
growth factor; EGFR, epidermal growth factor receptor; MMP-9,
matrix metalloproteinase 9; TXNIP, thioredoxin-interacting protein;
si-Con, scrambled siRNA transfected MDA-MB-231 breast cancer cells;
si-TXNIP, TXNIP siRNA transfected MDA-MB-231 breast cancer
cells. |
PLAG has specificity for blocking cell
migration and invasion
PLAG's specificity for inhibiting EGF-induced
invasiveness and high mobility was verified by direct comparison
with another diacylglycerol, 1-palmitoyl 2-linoleoyl
3-hydroxylglycerol (PLH) (23). In
the Transwell invasion and migration assays, EGF-only treated
cancer cells (100 ng/ml) showed high invasiveness and mobility,
whereas PLAG-treated cells (25 or 50 µg/ml) showed a significant
reduction in invasiveness and mobility in a dose-dependent manner;
this was not seen in the PLH-treated cells (25 or 50 µg/ml)
(Fig. 6A-C). PLAG and PLH
treatments were further compared for the modulation of MMP-9
expression. Whereas PLAG-treatment (50 µg/ml) of EGF-treated cells
reduced MMP-9 expression, PLH treatment (50 µg/ml) did not
(Fig. 6D). We also compared TXNIP
expression and complexing with EGFR using the two treatment
conditions. In PLAG-treated cells, TXNIP mRNA and protein
expression was enhanced, but in PLH-treated cells TXNIP mRNA and
protein expression was not (Fig.
6E). TXNIP and EGFR complexes were enhanced in PLAG-treated
cells, but in PLH-treated cells, they were not (Fig. 6F). These data indicate that PLAG
shows specificity for inhibiting EGF-induced tumor cell mobility
and suggest that the acetylated 3-position of the molecule is
important for recognition of its cognate receptor and for
activation of related signaling pathways.
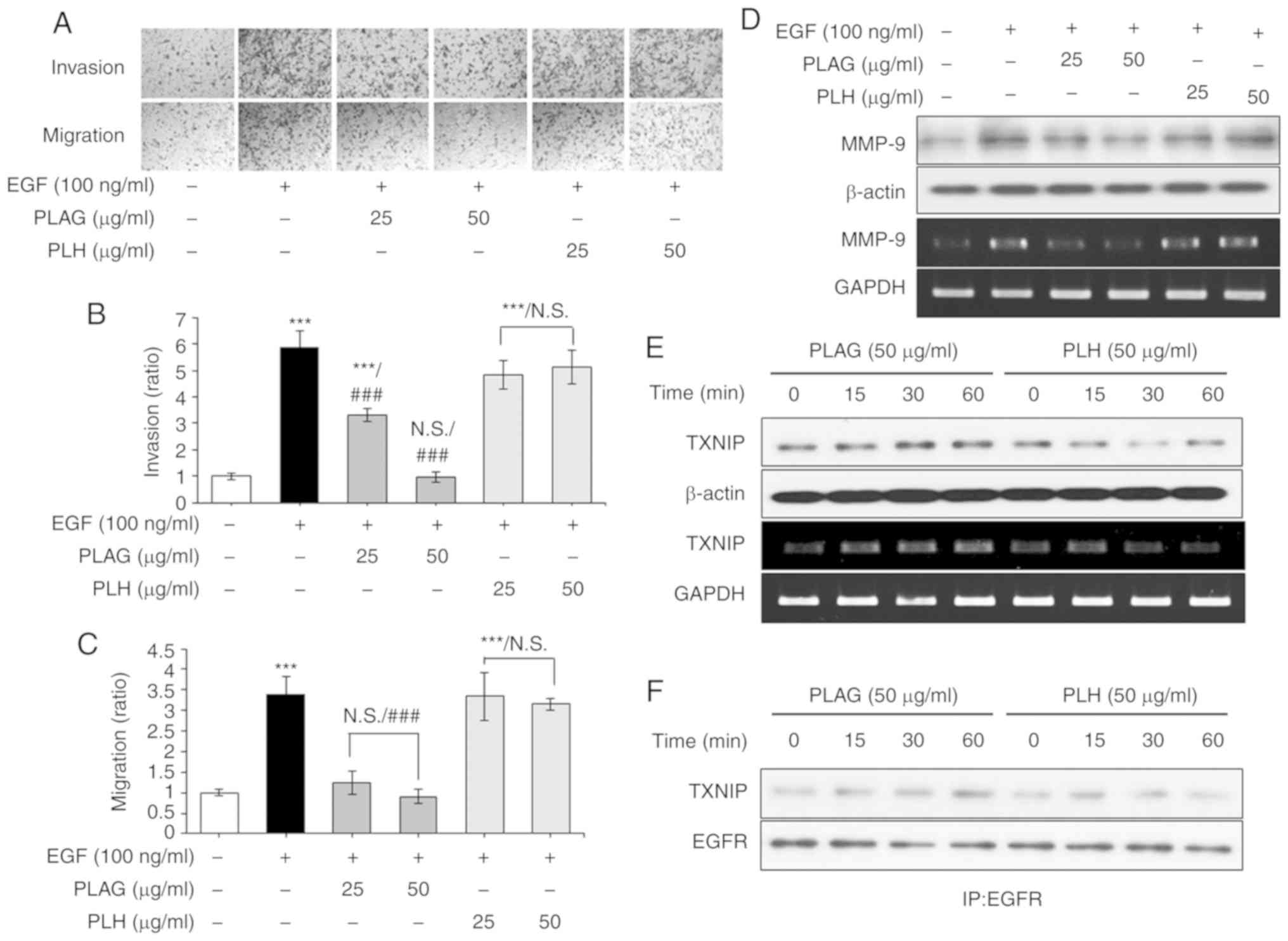 | Figure 6.PLAG-specific treatment induces
TXNIP. (A-C) PLH did not inhibit EGF-induced cell migration and
invasion. Cells were counted in the invasion and migration cell
assays. (D) MMP-9 expression was not modified in the PLH-treated
cells. MMP-9 expression was analyzed by RT-PCR at 6 h, and by
western blotting at 24 h, after stimulation. (E) TXNIP mRNA and
protein expression was analyzed by RT-PCR and western blotting,
respectively. (F) EGFR and TXNIP complexing was confirmed by
western blotting via co-immunoprecipitation in PLAG- and
PLH-treated cells. Statistical significance was determined by ANOVA
(Tukey's test). ***P<0.005, compared with the untreated group;
###P<0.005, compared with the EGF group. N.S., not
significant; PLAG, 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol;
EGF, epidermal growth factor; EGFR, epidermal growth factor
receptor; TXNIP, thioredoxin-interacting protein; PLH, 1-palmitoyl
2-linoleoyl 3-hydroxylglycerol. |
Discussion
EGFR ubiquitination induces receptor degradation and
consequently attenuates EGFR signaling (30). Upon EGF stimulation, several
endocytic accessory factors are also ubiquitinated and cotraffic
with EGFR along the endocytic pathway (15,31).
Recruitment of the E3 ubiquitin ligase, c-Cbl, to activated EGFRs
is a key event for receptor ubiquitination and is also associated
with EGFR degradation (32).
Trafficking ubiquitinated receptors into the endosome requires
multiple proteins, one of which is the scaffolding protein EPS15
(33). EPS15 has a
ubiquitin-interacting motif that mediates the early steps of EGFR
endocytosis (34,35). Rab5, an early endosome marker, is
also a checkpoint protein that plays a role in the endocytic
pathway, designating whether or not an endocytosed receptor is
sorted to the late endosome/lysosome compartment or recycled back
to the plasma membrane (36). For
early and late endosomes, Rab7 complexes are essential for
endocytic trafficking and lysosomal degradation (37).
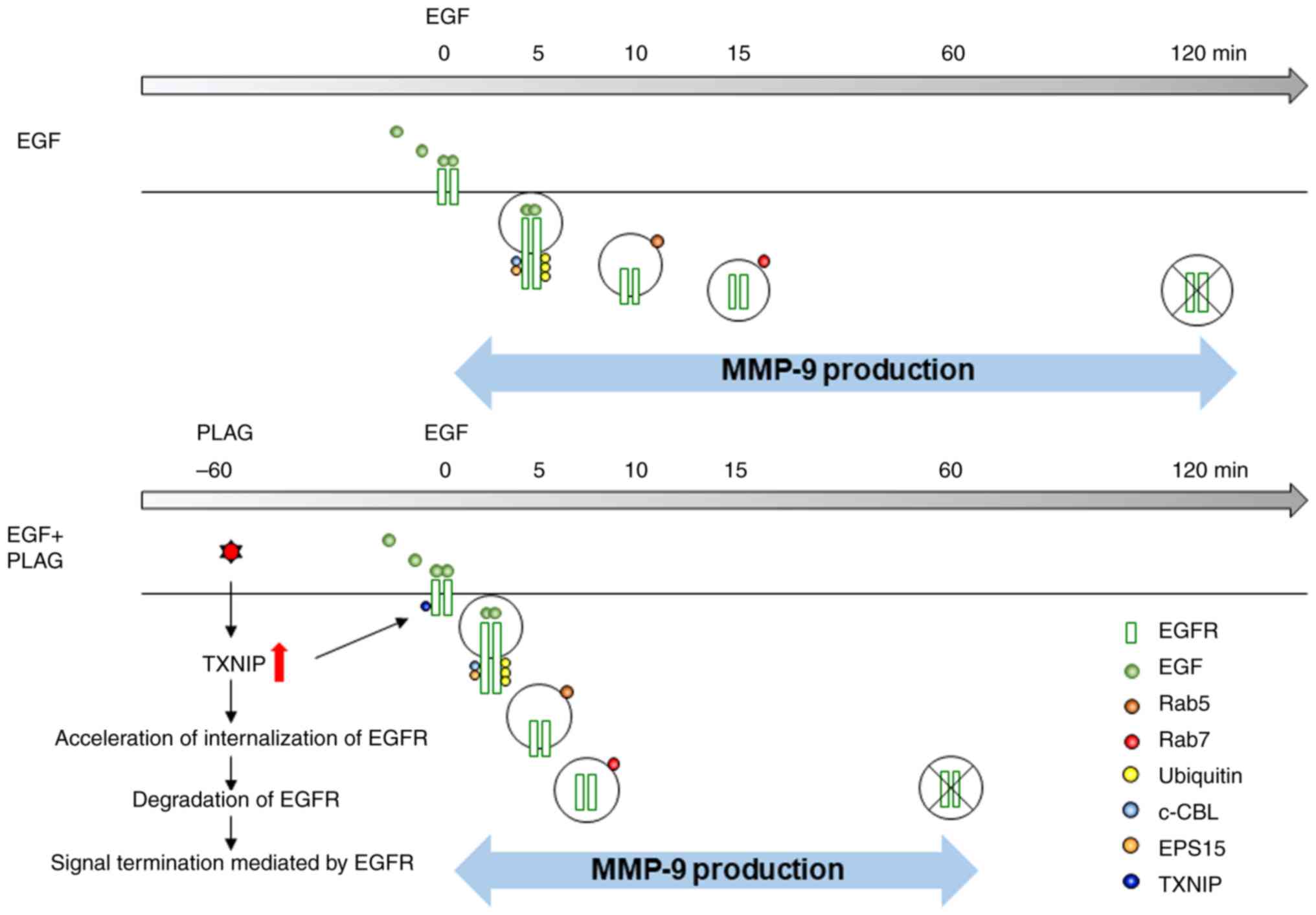
Our results have demonstrated that PLAG accelerates
endocytosis, ubiquitination, and lysosomal degradation of
ligand-bound EGFRs. The complexing of c-Cbl and EPS15 with EGFR was
verified by immunoprecipitation with anti-EGFR antibody, and EGFR
localization to early/late endosomes was assessed by examining
complexes using anti-Rab5 and anti-Rab7 antibodies. PLAG-only
treatment did not induce EGFR endocytosis, which means that PLAG
does not activate EGFR on its own. PLAG-cotreated cells showed
similar surface EGFR levels compared with EGF-only treated cells.
However, EGFR endocytosis was increased the most by PLAG
pretreatment when compared with other groups. These results
demonstrate that PLAG does not induce EGFR endocytosis by itself,
and it requires about 60 min to exhibit its effect in EGFR
endocytosis (Fig. S1). PLAG
induced weak ubiquitination, but c-Cbl and EPS15 were not complexed
with EGFR. EGFR ubiquitination can be induced by other factors such
as calcium and oxidative stress (12,38).
In the present study, PLAG increased calcium inflex (data not
shown). PLAG induced weak ubiquitination through other factors, not
through EGF-mediated ubiquitin ligase molecules. This result
indicates that PLAG has no effect itself in the EGF-mediated
process. Formation of these complexes and EGFR localization to
endosomes was accelerated in PLAG-treated cells, suggesting that
PLAG promoted EGFR degradation.
Modulation of EGFR signaling pathways was
investigated in PLAG-treated cells. The EGF-bound receptor is known
to activate multiple signaling pathways, including MAPK,
phosphatidylinositol 3-kinase (PIK3/Akt, and nuclear factor (NF)-κB
(39,40). Of interest here, the expression of
MMP-9 is associated with the metastasis of breast cancer cells
(41,42). MMP expression in breast cancer cells
is mediated by the MAPK signaling pathway (43). As intermediary signaling molecules
for the process of EGFR-mediated MMP-9 expression, the
phosphorylation states of SHC, ERK, and JNK were investigated.
EGFR, SHC, ERK, and JNK phosphorylation was detected at 5 min and
was sustained for 120 min in EGF-treated cells, but most of these
signals disappeared by 60 min in 50 µg/ml PLAG-treated cells,
suggesting that enhanced EGFR degradation in PLAG-treated cells
limited downstream MMP-9 expression. As part of this downstream
regulation, the transcription factor AP-1 (an MMP-9 promoter) was
activated in EGF-treated cells, and PLAG treatment attenuated its
enhanced activity in a dose-dependent manner.
As the results demonstrated, signaling molecules,
including SHC and c-Cbl, were complexed with EGFRs and
co-internalized into endosomes, resulting in degradation. This
process was accelerated by PLAG treatment. The EGFR-dependent
signaling for MMP-9 expression was reduced, and the invasiveness of
these breast cancer cells was consequently attenuated by PLAG
treatment. PLAG-only treatment exhibited no effect on invasion and
migration and MMP-9 expression. This decreased MMP-9 expression by
PLAG was initiated during EGFR internalization by the induction of
thioredoxin-interacting protein (TXNIP) expression, and enhanced
TXNIP expression is associated with trafficking to the
endosome/lysosome, supporting the interaction with ubiquitin
ligases (44). Therefore, in
PLAG-treated cells, increased TXNIP expression promoted EGFR
internalization and subsequent degradation.
PLAG specificity was verified by treating cells with
a closely related diacylglycerol, PLH, and then comparing the two
sets of results from the EGF-induced invasion and migration assays.
In these assays, PLAG significantly reduced the invasiveness and
migration of cells. But in PLH-treated cells, even though PLH has a
similar lipid structure, these reductions were not observed. These
PLAG results were regulated through the expression of TXNIP, and
PLH did not alter TXNIP expression.
PLAG therapy has been shown to mitigate the effects
of various diseases. Recent reports have documented that PLAG
controlled neutrophil recruitment by regulating the trafficking of
Toll-like receptors and enhanced efferocytosis through membrane
redistribution of G protein-coupled receptors (45,46).
We believe that these published studies have shown the ability of
PLAG to modulate the movement of receptors, thus we hypothesized
that PLAG may also modulate the trafficking of receptor tyrosine
kinases (RTKs). We demonstrated that PLAG accelerates EGFR
trafficking via TXNIP and that it reduces cancer cell invasiveness
and mobility (Fig. 7). These
results suggest that PLAG could be a specific agent for blocking
metastasis in breast cancer via TXNIP regulation.
Supplementary Material
Supporting Data
Acknowledgements
We thank Jimin Kim from Enzychem Lifesciences for
her review of the manuscript.
Funding
This study was supported by the Korea Research
Institute of Bioscience and Biotechnology (KRIBB) Research
Initiative Program (KGM5251911), and grant nos. IGM0201811 and
IGM0171911 from Enzychem Lifesciences.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author by reasonable
request.
Authors' contributions
JWK made substantial contributions to the concept
and design of the study. KHY acquired, analyzed and interpreted the
data. SC made substantial contributions to the manuscript and
critically revised it for important intellectual content. GTK
assisted with technical support. SYY analyzed the data and prepared
the figures. All authors approved the final version of the
manuscript and agreed to be accountable for all aspects of the
research to ensure that the accuracy or integrity of any part of
the work is appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DC and Radisky ES: Tumor cell-produced matrix
metalloproteinase 9 (MMP-9) drives malignant progression and
metastasis of basal-like triple negative breast cancer. Oncotarget.
5:2736–2749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shay G, Lynch CC and Fingleton B: Moving
targets: Emerging roles for MMPs in cancer progression and
metastasis. Matrix Biol 44–46. 200–206. 2015. View Article : Google Scholar
|
|
4
|
Radisky ES, Raeeszadeh-Sarmazdeh M and
Radisky DC: Therapeutic potential of matrix metalloproteinase
inhibition in breast cancer. J Cell Biochem. 118:3531–3548. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
6
|
Zhang M, Teng XD, Guo XX, Li ZG, Han JG
and Yao L: Expression of tissue levels of matrix metalloproteinases
and their inhibitors in breast cancer. Breast. 22:330–334. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers. 9:522017.
View Article : Google Scholar
|
|
8
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Chen X and Wang Z: Dimerization
drives EGFR endocytosis through two sets of compatible endocytic
codes. J Cell Sci. 128:935–950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kyriakopoulou K, Kefali E, Piperigkou Z,
Bassiony H and Karamanos NK: Advances in targeting epidermal growth
factor receptor signaling pathway in mammary cancer. Cell Signal.
51:99–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto H, Higa-Nakamine S, Noguchi N,
Maeda N, Kondo Y, Toku S, Kukita I and Sugahara K: Desensitization
by different strategies of epidermal growth factor receptor and
ErbB4. J Pharmacol Sci. 124:287–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conte A and Sigismund S: Chapter six-the
ubiquitin network in the control of EGFR endocytosis and signaling.
Prog Mol Biol Transl Sci. 141:225–276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salova AV, Belyaeva TN, Leontieva EA and
Kornilova ES: EGF receptor lysosomal degradation is delayed in the
cells stimulated with EGF-Quantum dot bioconjugate but earlier key
events of endocytic degradative pathway are similar to that of
native EGF. Oncotarget. 8:44335–44350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan X, Lambert PF, Rapraeger AC and
Anderson RA: Stress-induced EGFR trafficking: Mechanisms,
functions, and therapeutic implications. Trends Cell Biol.
26:352–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomas A, Futter CE and Eden ER: EGF
receptor trafficking: Consequences for signaling and cancer. Trends
Cell Biol. 24:26–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arakaki AKS, Pan WA, Lin H and Trejo J:
The α-arrestin ARRDC3 suppresses breast carcinoma invasion by
regulating G protein-coupled receptor lysosomal sorting and
signaling. J Biol Chem. 293:3350–3362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu S, Wu X, Zong M, Tempel W, Loppnau P
and Liu Y: Structural basis for a novel interaction between TXNIP
and Vav2. FEBS Lett. 590:857–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spindel ON, Yan C and Berk BC:
Thioredoxin-interacting protein mediates nuclear-to-plasma membrane
communication: Role in vascular endothelial growth factor 2
signaling. Arterioscler Thromb Vasc Biol. 32:1264–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SY, Shi X, Pang J, Yan C and Berk BC:
Thioredoxin-interacting protein mediates sustained VEGFR2 signaling
in endothelial cells required for angiogenesis. Arterioscler Thromb
Vasc Biol. 33:737–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin IS, Ahn KS, Shin NR, Lee HJ, Ryu HW,
Kim JW, Sohn KY, Kim HJ, Han YH and Oh SR: Protective effect of
EC-18, a synthetic monoacetyldiglyceride on lung inflammation in a
murine model induced by cigarette smoke and lipopolysaccharide. Int
Immunopharmacol. 30:62–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoo N, Lee HR, Shin SH, Sohn KY, Kim HJ,
Han YH, Chong S, Kim MH, Yoon SY and Kim JW: PLAG
(1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol) augments the
therapeutic effect of pegfilgrastim on gemcitabine-induced
neutropenia. Cancer Lett. 377:25–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HR, Yoo N, Kim JH, Sohn KY, Kim HJ,
Kim MH, Han MY, Yoon SY and Kim JW: The therapeutic effect of PLAG
against oral mucositis in hamster and mouse model. Front Oncol.
6:2092016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong J, Kim YJ, Lee DY, Moon BG, Sohn KY,
Yoon SY and Kim JW: 1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol
(PLAG) attenuates gemcitabine-induced neutrophil extravasation.
Cell Biosci. 9:42019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karagiannis GS, Condeelis JS and Oktay MH:
Chemotherapy- induced metastasis: Mechanisms and translational
opportunities. Clin Exp Metastasis. 35:269–284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng H, Yang Y, Hong YG, Wang MC, Yuan
SX, Wang ZG, Bi FR, Hao LQ, Yan HL and Zhou WP: Tropomodulin 3
modulates EGFR-PI3K-AKT signaling to drive hepatocellular carcinoma
metastasis. Mol Carcinog. 58:1897–1907. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou X, Xie S, Wu S, Qi Y, Wang Z, Zhang
H, Lu D, Wang X, Dong Y, Liu G, et al: Golgi phosphoprotein 3
promotes glioma progression via inhibiting Rab5-mediated
endocytosis and degradation of epidermal growth factor receptor.
Neuro-oncol. 19:1628–1639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ng EL, Gan BQ, Ng F and Tang BL: Rab
GTPases regulating receptor trafficking at the late
endosome-lysosome membranes. Cell Biochem Funct. 30:515–523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li L, Fan P, Chou H, Li J, Wang K and Li
H: Herbacetin suppressed MMP9 mediated angiogenesis of malignant
melanoma through blocking EGFR-ERK/AKT signaling pathway.
Biochimie. 162:198–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Begley MJ, Yun CH, Gewinner CA, Asara JM,
Johnson JL, Coyle AJ, Eck MJ, Apostolou I and Cantley LC:
EGF-receptor specificity for phosphotyrosine-primed substrates
provides signal integration with Src. Nat Struct Mol Biol.
22:983–990. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goh LK and Sorkin A: Endocytosis of
receptor tyrosine kinases. Cold Spring Harb Perspect Biol.
5:a0174592013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Merrifield CJ and Kaksonen M: Endocytic
accessory factors and regulation of clathrin-mediated endocytosis.
Cold Spring Harb Perspect Biol. 6:a0167332014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lyle CL, Belghasem M and Chitalia VC:
c-Cbl: An important regulator and a target in angiogenesis and
tumorigenesis. Cells. 8:4982019. View Article : Google Scholar
|
|
33
|
Gucwa AL and Brown DA: UIM
domain-dependent recruitment of the endocytic adaptor protein Eps15
to ubiquitin-enriched endosomes. BMC Cell Biol. 15:342014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garvalov BK, Foss F, Henze AT, Bethani I,
Gräf-Höchst S, Singh D, Filatova A, Dopeso H, Seidel S, Damm M, et
al: PHD3 regulates EGFR internalization and signalling in tumours.
Nat Commun. 5:55772014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tong D, Liang YN, Stepanova AA, Liu Y, Li
X, Wang L, Zhang F and Vasilyeva NV: Increased Eps15 homology
domain 1 and RAB11FIP3 expression regulate breast cancer
progression via promoting epithelial growth factor receptor
recycling. Tumour Biol. 39:10104283176910102017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan W, Liu B, Wang X, Li T, Xue H, Mo X,
Yang S, Ding S and Han W: CMTM3 decreases EGFR expression and
EGF-mediated tumorigenicity by promoting Rab5 activity in gastric
cancer. Cancer Lett. 386:77–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guerra F and Bucci C: Multiple roles of
the small GTPase Rab7. Cells. 5:342016. View Article : Google Scholar
|
|
38
|
Mukherjee R, Das A, Chakrabarti S and
Chakrabarti O: Calcium dependent regulation of protein
ubiquitination-Interplay between E3 ligases and calcium binding
proteins. Biochim Biophys Acta Mol Cell Res. 1864:1227–1235. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sasaki T, Hiroki K and Yamashita Y: The
role of epidermal growth factor receptor in cancer metastasis and
microenvironment. BioMed Res Int. 2013:5463182013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suen KM, Lin CC, Seiler C, George R,
Poncet-Montange G, Biter AB, Ahmed Z, Arold ST and Ladbury JE:
Phosphorylation of threonine residues on Shc promotes ligand
binding and mediates crosstalk between MAPK and Akt pathways in
breast cancer cells. Int J Biochem Cell Biol. 94:89–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Majumder A, Ray S and Banerji A: Epidermal
growth factor receptor-mediated regulation of matrix
metalloproteinase-2 and matrix metalloproteinase-9 in MCF-7 breast
cancer cells. Mol Cell Biochem. 452:111–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saby C, Collin G, Sinane M, Buache E, Van
Gulick L, Saltel F, Maquoi E and Morjani H: DDR1 and MT1-MMP
expression levels are determinant for triggering BIK-mediated
apoptosis by 3D type I collagen matrix in invasive basal-like
breast carcinoma cells. Front Pharmacol. 10:4622019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cepeda MA, Evered CL, Pelling JJH and
Damjanovski S: Inhibition of MT1-MMP proteolytic function and
ERK1/2 signalling influences cell migration and invasion through
changes in MMP-2 and MMP-9 levels. J Cell Commun Signal.
11:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Masutani H, Yoshihara E, Masaki S, Chen Z
and Yodoi J: Thioredoxin binding protein (TBP)-2/Txnip and
α-arrestin proteins in cancer and diabetes mellitus. J Clin Biochem
Nutr. 50:23–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee HR, Shin SH, Kim JH, Sohn KY, Yoon SY
and Kim JW: 1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG)
rapidly resolves LPS-induced acute lung injury through the
effective control of neutrophil recruitment. Front Immunol.
10:21772019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim GT, Hahn KW, Sohn KY, Yoon SY and Kim
JW: PLAG enhances macrophage mobility for efferocytosis of
apoptotic neutrophils via membrane redistribution of P2Y2. FEBS J.
286:5016–5029. 2019. View Article : Google Scholar : PubMed/NCBI
|