Introduction
Myelodysplastic syndromes (MDS) are malignant clonal
proliferative disorders of hematopoietic stem cells, which are
characterized by uni- or multi-lineage cytopenias, dysplasia of
bone marrow hematopoietic cells, and eventual progression to
leukemia in approximately one-third of patients (1). Residual normal hematopoietic cells and
malignant clonal hematopoietic cells often co-exist and compete
with each other for a considerable period of time in the bone
marrow of patients with MDS. When the malignant proliferation of
bone marrow clonal cells becomes dominant, the disease progresses
to acute myelocytic leukemia (AML) (2,3).
Patients with MDS with a bone marrow blast percentage >5%
present an increased risk of progressing to AML (1). At present, studies on the pathogenesis
of malignant clonal proliferation in MDS are primarily focused on
the involvement of gene mutations, downregulation of tumor
suppressor genes, epigenetic abnormalities (including
hypermethylation of genes and histone modification), abnormal
regulations of multiple signaling pathways associated with
proliferation, and abnormalities in bone marrow cell apoptosis
(4–8). However, bone marrow clonal cells in
MDS are also highly heterogeneous in terms of their morphology and
differentiation stages, which renders studies on the mechanisms
underlying malignant clonal proliferation in MDS difficult and
hampers progress in the development of therapeutics.
Type 1 insulin-like growth factor receptor (IGF-IR)
belongs to the tyrosine receptor family of proteins (9). IGF-IR serves an important role in
regulating cell growth, proliferation, transformation,
differentiation and apoptosis (9,10). It
has been demonstrated that IGF-IR expression is upregulated in bone
marrow cells of patients with multiple myeloma, AML, chronic
myeloid leukemia, acute lymphocytic leukemia as well as other
hematological malignancies, or other associated tumor cell lines
(11–15). Studies have also demonstrated that
IGF-IR serves an important role in regulating the biological
activity of cancer stem cells (16–18).
In our previous studies, it was demonstrated that overexpression of
IGF-IR was negatively correlated with apoptosis of bone marrow
mononuclear cells (BMNCs) in patients with MDS and IGF-IR was
primarily expressed on the surface of MDS clonal cells (19,20).
Since IGF-IR is a membrane receptor, our laboratory was able to
effectively purify MDS clonal cells by labeling IGF-IR using flow
cytometry. In addition, our data further revealed that the
proliferation of clonal cells was significantly inhibited by the
IGF-IR inhibitor picropodophyllin (PPP) (21). Our previous results indicated that
overexpression of IGF-IR was associated with proliferation of
clonal cells. The aim of the present study was to further
investigate the underlying mechanisms governing IGF-IR-mediated
clonal cell proliferation, and explore the potential of IGF-IR as a
therapeutic target of MDS.
Materials and methods
Antibodies and reagents
The following antibodies were used for western
blotting: p38 MAPK (product no. 9212), phosphorylated (p-)p38 MAPK
(product no. 4511), p44/42 MAPK (product no. 9102), p-p44/42 MAPK
(product no. 5726), GSK (product no. 5676), p-GSK (product no.
8566), Akt (product no. 9272), p-Akt (product no. 4060) and β-actin
(product no. 4970) were purchased from Cell Signaling Technology,
Inc.. PPP was purchased from Santa Cruz Biotechnology, Inc.. PPP
was dissolved in DMSO to a concentration of 0.5 mM. In a series of
experiments, CD34+ cells were incubated with 1 µM PPP in
the maintenance medium (StemSpan™ SFEM 09650; StemCell
Technologies).
Patients and isolation of
CD34+ cells
MDS was diagnosed in accordance with the minimum
diagnostic criteria (22). The
classification and prognostic risk scoring of MDS were performed
according to the World Health Organization (WHO) classification
system, the French-American-British classification (FAB) and the
International Prognostic Scoring System (IPSS) (23–25).
Chromosomal abnormalities of patients were described according to
International System for Human Cytogenetics Nomenclature (ISCN
2005) (26). From August 2014 to
March 2015, 8 MDS patients (5 males and 3 females) were included,
and all samples were obtained from patients at the time of the
initial diagnosis. Detailed clinicopathological characteristics of
patients recruited for the present study are presented in Table I. According to the Declaration of
Helsinki, all subjects signed informed consent, and the present
study was approved by the Ethics Committee of the Sixth Hospital
Affiliated with Shanghai Jiao Tong University. CD34+
cells were isolated using magnetic-activated cell sorting (MACS)
from BMNCs according to the manufacturer's protocol (Miltenyi
Biotec). The yield and purity of the positive CD34 cells were
evaluated using flow cytometry (FACS Calibur; BD Biosciences).
Typically, approximately 1–5×106 CD34+ cells
were obtained from patients with MDS and used for subsequent
biological experiments apart from western blotting.
 | Table I.Clinical characteristics of MDS
patients. |
Table I.
Clinical characteristics of MDS
patients.
| No. | Sex/age | WHO/FAB | IPSS | Karyotype by
G-banding |
|---|
| 1 | M/62 | RAEB2 | 2.0 |
46,XY,del(5)(q13q31)[18]/46,XY[2] |
| 2 | M/47 | RAEB1 | 1.0 | 46,XY[15] |
| 3 | M/73 | RAEB1 | 1.5 |
46,XY,del(5)(q15q31),inv(9)(p12q12)[10] |
| 4 | F/55 | RA | 0.5 | 47,XX,+8[15] |
| 5 | M/17 | RCMD | 1.0 | 47,XY,+8[25] |
| 6 | M/77 | RAEB1 | 1.0 | 46,XY[14] |
| 7 | F/34 | RAEBt | 2.5 | 47,XX,+8[12] |
| 8 | F/62 | RAEB2 | 3.0 |
48,X,-X,der(7)t(7;11)(q11q11),+3mar,inc[2]/46,XX[8] |
Cell lines and culture
SKM-1 cells were kindly gifted from Professor
Nakagawa (27). K562 cells were
obtained from the American Type Culture Collection. Cell lines were
maintained in complete medium (RPMI-1640 supplemented with 10% FBS,
1% glutamine, and 1% sodium pyruvate were purchased from Thermo
Fischer Scientific, Inc.).
Lentivirus-mediated cell
transfection
Three IGF-IR-short hairpin (sh)RNAs were inserted
into the LV1 vector purchased from Shanghai Genechem Co., Ltd.
(www.genechem.com.cn). The construction
of the IGF-IR-knockdown vector was confirmed using restriction
digest analysis and DNA sequencing. Lentiviral packaging was
performed using a four-plasmid system (pGLV1/U6/GFP Vector,
pRsv-REV, pMDlg-pRRE and pMD2G). After titre determination
(1-3×108 TU/ml), the shIGF-IR lentivirus was transfected
into SKM-1 cells. Briefly, 5×105 cells/well in a 6-well
plate were incubated with the virus and polybrene (10 µg/ml) in a
1-ml volume. The stably expressing cells were propagated in
complete RPMI-1640 medium at 37°C for 3–5 days prior to subsequent
experiments. There was no noticeable loss of GFP expression in the
established cultures observed throughout the experiments based on
fluorescence microscopy or flow cytometric analysis. Silencing
efficiency of the IGF-IR vector was evaluated using reverse
transcription-quantitative (RT-q)PCR. Cells with a decrease >70%
in IGF-IR mRNA expression post-transfection were used in subsequent
experiments (Fig. S1).
RNA extraction and RT-qPCR
Total RNA was extracted from 1×105 cells
from either SKM-1 or K562 cells using an RNeasy system (Qiagen,
Inc.) according to the manufacturer's instructions, and the RNA was
reverse transcribed into cDNA. cDNA was synthesized using
PrimeScript RT reagent kit (Takara Bio, Inc.). PCR amplification of
IGF-IR, p21 and MYC mRNA was performed on an ABI Prism 7500 System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with SYBR
Green Master mix (Takara Bio, Inc.). PCR was carried out under the
following cycling conditions: Initial denaturation at 9°C for 60
sec, followed by 40 cycles of denaturation at 95°C for 20 sec,
annealing at 60°C for 30 sec and extension at 72°C for 30 sec.
Relative expression of IGF-IR, p21, MYC and GAPDH genes was
calculated using the 2−ΔΔCq method (28). The primer sequences are presented in
Table S1.
Proliferation analyses
For the colony formation assays, cells were plated
in 6-well plates with methylcellulose medium containing SCF,
GM-CSF, IL-3 and erythropoietin (StemCell Technologies, Inc.) at
2×103 cells/well with two wells per condition. After 14
days of incubation in a humidified incubator at 37°C, the colonies
containing ≥30 cells were counted. For the cell proliferation
assay, SKM-1 and K562 cells were seeded in 96-well plates at a
density of 1×103 cells/well in triplicate. Cell Counting
Kit-8 (CCK-8; 10 µl; Dojindo Molecular Technologies, Inc.) was
added to each well after 24, 48, 72 or 96 h. The absorbance value
was read at 450 nm using an enzyme-labeled instrument. The
inhibition rate of cell proliferation was calculated as follows:
Percent of inhibition rate=[1-(ODknockdown
well-ODblank well)/(ODcontrol
well-ODblank well)]x100%. For the Trypan blue
staining assay, 4×105 CD34+ cells purified
from patients with MDS or cell lines were treated with 1 µM PPP for
24 h, and an equivalent volume of DMSO was used as a negative
control. The cells were stained using 0.4% Trypan Blue (Thermo
Fisher Scientific, Inc.) for 3 min at room temperature. The
unstained (viable) and stained (non-viable) cells were counted
separately in the hemacytometer and the survival of cells was
calculated.
Apoptosis detection
A total of 1×105 SKM-1 or K562 cells were
stained with anti-Annexin V-APC (Lianke Biotech Co., Ltd.) for 15
min at room temperature, and subsequently analyzed using flow
cytometry (FACSCalibur; BD Biosciences). Additionally, a total of
1×105 cells CD34+ from patients with MDS or
cell lines were treated with 1 µM PPP for 24 h. Apoptosis was
evaluated using flow cytometry (FACSCalibur; BD Biosciences) after
staining cells with anti-Annexin V-FITC and PI (BD Pharmingen; BD
Biosciences). The sum of the upper right and lower right quadrants
was used for calculating total apoptosis rates and subjected to
statistical analysis (BD CellQuest software 6.0; BD
Biosciences).
Cell cycle analysis
A total of 5×105 cells were washed with
cold PBS, re-suspended in 1 ml of DNA staining reagent (50 µg/ml
PI) and 10 µl RNase A was added (Lianke Biotech Co., Ltd.). Samples
were incubated in the dark for 30 min, and then analyzed using flow
cytometry (FACSCalibur; BD Biosciences). The percentage of cells in
the G0/G1, S and G2/M phases were calculated using BD CellQuest
software 6.0 (BD Biosciences).
Gene expression microarray (GEM)
A Genechip Primeview Human Gene Expression Array
(cat. no. 901837; Affymetrix; Thermo Fisher Scientific, Inc.) was
used for GEM analysis. The signal intensity was acquired using a
GeneChip Scanner 3000 (Affymetrix; Thermo Fisher Scientific, Inc.)
to generate cell intensity files. The statistical analysis was
performed using Partek Genomics Suite software 7.0 (Partek, Inc.).
Changes in expression >2-fold were considered relevant.
GO, pathway and pathway-net analyses
of differentially expressed genes
Pathway and GO (Gene Oncology) enrichment analysis
was used to determine significant pathways or ontology based on
differential gene expression using Kyoto Encyclopedia of Genes and
Genomes (KEGG) (www.kegg.jp) and GO resources
(genontology.org). A Fisher's exact test was used to determine
whether a pathway was significant, and the threshold of
significance was defined by the P-value and False Discovery Rate.
The Pathway-net (www.gminix.com) is the net interaction of the
significant pathways of the differentially expressed genes, and was
built according to the interaction amongst the pathways in the KEGG
analysis. This approach summarizes and identifies the pathway
interactions of genes differentially expressed in diseases.
Western blotting
SKM-1 and K562 cells (~1×107) were lysed
using cell lysis buffer (Cell Signaling Technology, Inc.). Protein
concentrations were determined using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology). Equal quantities of total
protein (30 µg/lane) were loaded on a 10% SDS-gel, resolved using
SDS-PAGE, and transferred to PVDF membranes (EMD Millipore). The
membranes were blocked with 5% non-fat dry milk in diluted
Tris-buffered saline with 0.1% Tween-20 (cat. no. 9997; Cell
Signaling Technology) for 1 h at room temperature and then were
incubated overnight at 4°C in 5% non-fat dry milk with 0.1%
Tween-20 that contained one of the following primary antibodies:
p38 MAPK, p-p38 MAPK, p44/42 MAPK, p-p44/42 MAPK, GSK, p-GSK, Akt,
p-Akt or β-actin (dilution 1:1,000; Cell Signaling Technology).
Horseradish peroxidase-conjugated antibodies to rabbit (cat. no.
7074; Cell Signaling Technology) or mouse (cat. no. 7076; Cell
Signaling Technology) were used as the secondary antibody. The
membranes were subsequently incubated with the corresponding
secondary antibody for 1 h at room temperature. Signals were
visualized using enhanced chemiluminescence method (product code
34577; Thermo Fisher Scientific, Inc.). An Epson Perfection 4490
Scanner was used to scan the films (EpsonEurope B.V.).
Statistical analysis
All statistical analysis was performed using SPSS
version 11 (IBM, Corp.). Differences among groups were compared
using an unpaired t-test or ANOVA test followed by Tukey's multiple
comparisons test. Differences among percentages were compared using
Chi-square test. All trials were repeated at least 3 times.
P<0.05 was considered to indicate a statistically
significant difference.
Results
IGF-IR is required for the maintenance
of clonal proliferation of MDS/leukemia cells
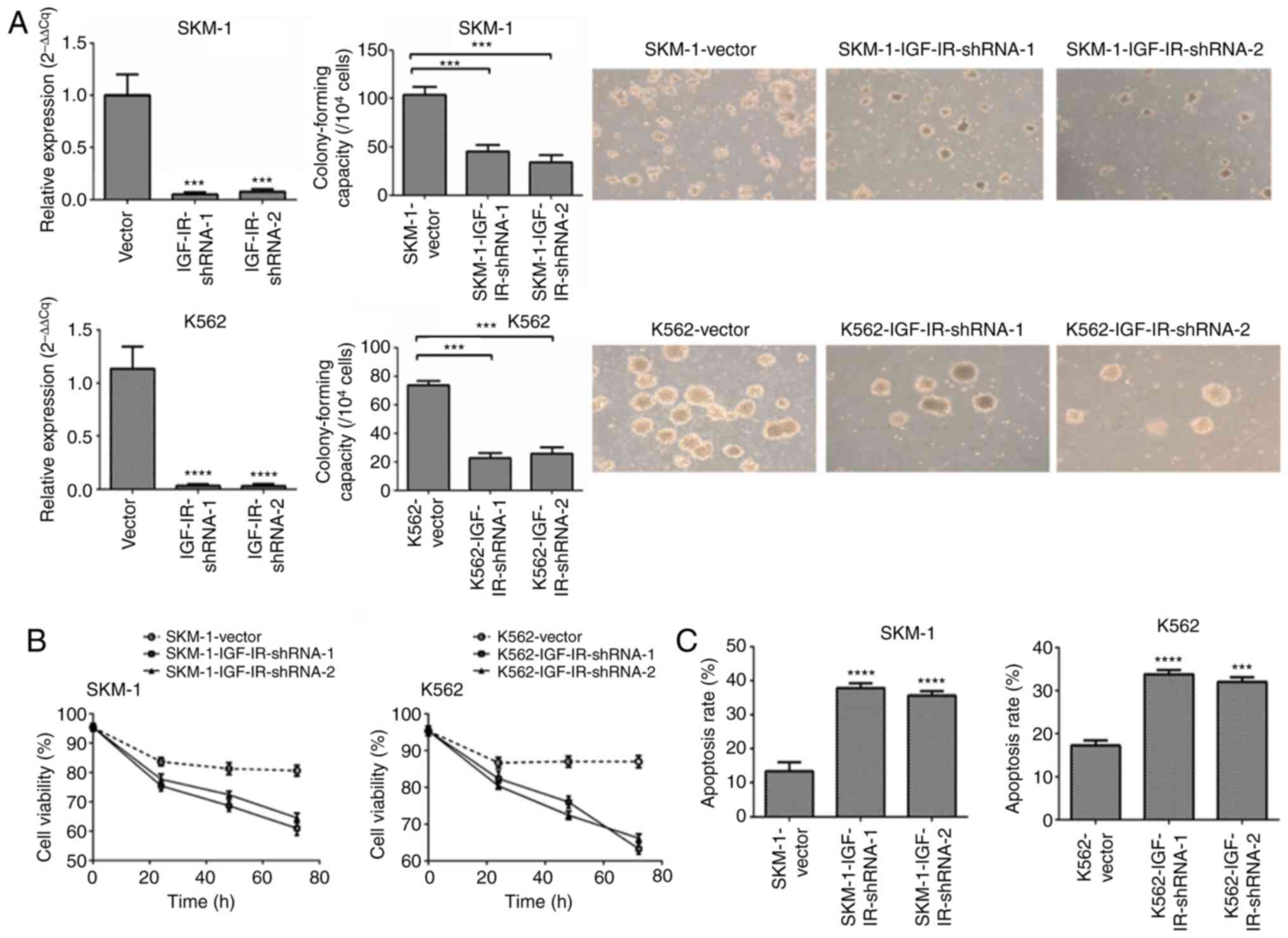
Transfection of MDS cells with IGF-IR shRNA
lentivirus resulted in a decrease in IGF-IR expression in the SKM-1
(MDS-derived leukemia cell line) and K562 (acute erythroid leukemia
cell line) cells (Figs. 1A and
S1). To evaluate the proliferation
dependence of tumor cells on IGF-IR, colony formation and CCK-8
proliferation assays were performed. The colony formation assays
revealed that SKM-1 and K562 cells with IGF-IR knockdown
significantly reduced colony formation compared with the control
cells (Fig. 1A). CCK-8 assays
revealed that knockdown of IGF-IR inhibited cell growth compared
with the control cells (Fig. 1B).
The apoptosis rate was determined using flow cytometry, and the
mean apoptotic rate was significantly higher in SKM-1 and K562
cells with IGF-IR knockdown compared with the control cells
(Figs. 1C and S2). Collectively, these data indicated
that IGF-IR was required for the maintenance of malignant
proliferation of MDS/leukemia cells.
Identification of IGF-IR-associated
signaling pathways based on GEM and bioinformatic analyses
To determine the mechanism by which IGF-IR regulated
the proliferation of MDS clonal cells, gene expression profiling on
SKM-1 cells with IGF-IR-knockdown (n=3) and control cells (n=3) was
performed. Gene expression profiling identified 1,654
differentially expressed genes (954 downregulated genes and 700
upregulated genes). Gene Ontology (GO) analysis of these genes was
also performed. The results of pathway analysis revealed that the
pathways significantly affected by upregulated genes, included MAPK
signaling, apoptosis, transcriptional dysregulation in cancer, p53
signaling, and Notch signaling (all P<0.001), and the pathways
significantly affected by downregulated genes included metabolism,
insulin signaling, PI3K-Akt signaling and Toll-like receptor
signaling (P<0.01) (Fig. 2A).
The results of GO analysis revealed that the ontologies
significantly affected by upregulated genes included DNA-dependent
transcription, negative regulation of cell proliferation, apoptosis
signaling pathway, positive regulation of NF-κB transcriptional
factor activity and cell cycle arrest (all P<0.001), and the
ontologies significantly affected by downregulated genes included
metabolic processes, innate immunity, cell adhesion, protein
phosphorylation and inflammatory response (P<0.001) (Fig. 2B). Further pathway-net analysis
revealed that MAPK was a key node among the downregulated genes as
a result of IGF-IR knockdown, and the primary upregulated genes
were apoptosis-associated pathway genes (Fig. 2C). Collectively, the results
indicated that knockdown of IGF-IR resulted in abnormal MAPK
signaling, which may underlie the dysregulated cell proliferation
and apoptosis.
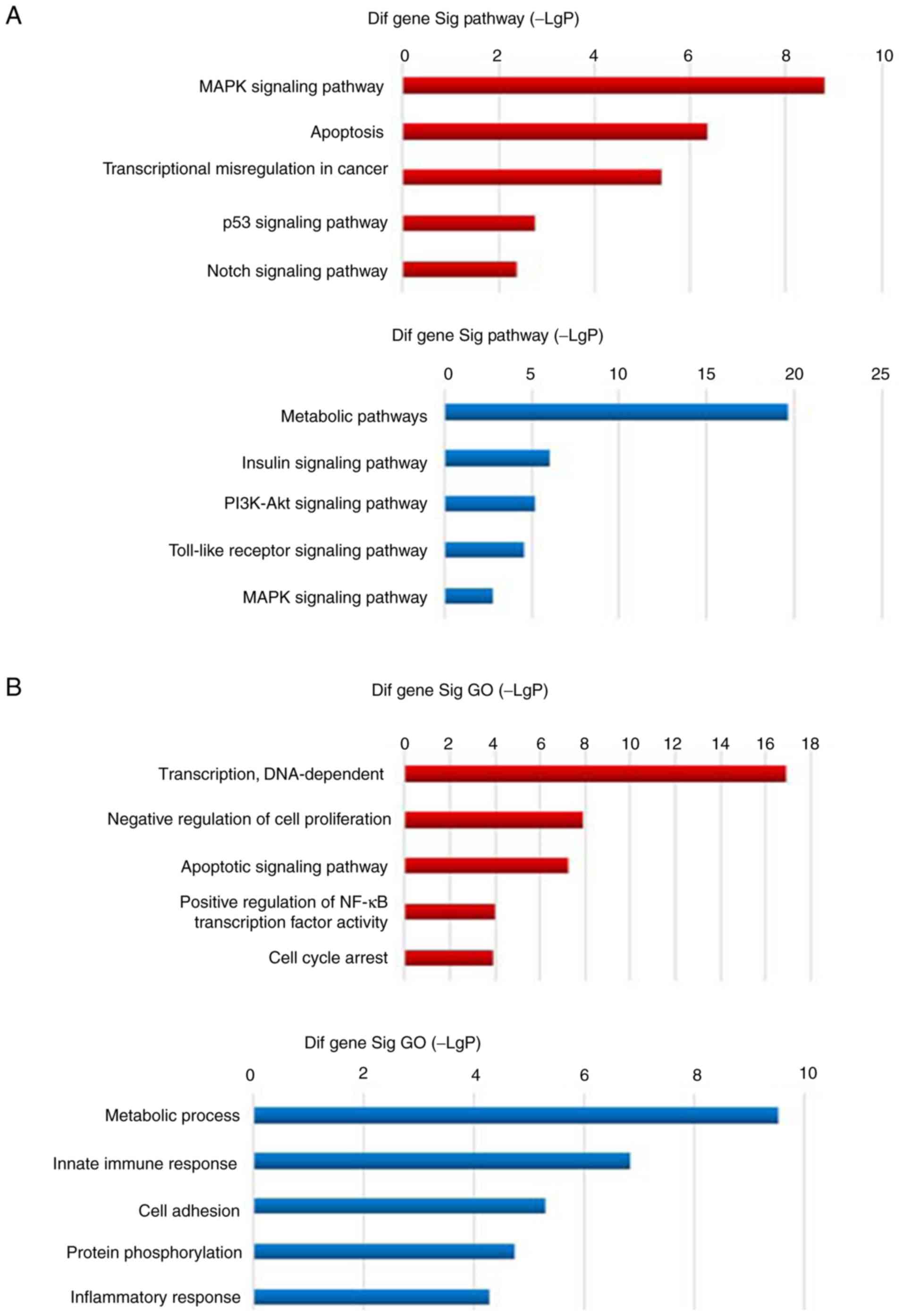 | Figure 2.Gene expression profile analysis of
IGF-IR knockdown in SKM-1 cells. (A) Pathway analysis revealed that
the pathways significantly affected by genes upregulated in the
IGF-IR knockdown cells, included MAPK signaling, apoptosis,
transcriptional dysregulation in cancer, p53 signaling and Notch
signaling, and the pathways significantly affected by downregulated
genes in the knockdown cells included metabolism, insulin
signaling, PI3K-Akt signaling and Toll-like receptor signaling. (B)
GO analysis revealed that the ontologies significantly affected by
upregulated genes in the knockdown cells included DNA-dependent
transcription, negative regulation of cell proliferation, apoptosis
signaling pathway, positive regulation of NF-κB transcriptional
factor activity and cell cycle arrest, and the ontologies
significantly affected by downregulated genes included metabolic
processes, innate immunity, cell adhesion, protein phosphorylation
and inflammatory response. Gene expression profile analysis of
IGF-IR knockdown in SKM-1 cells. (C) Further pathway-net analysis
revealed that MAPK was a key node among genes downregulated as a
result of IGF-IR knockdown, and the main upregulated genes were
apoptosis-related pathway genes. IGF-IR, type 1 insulin-like growth
factor receptor; GO, gene ontology. |
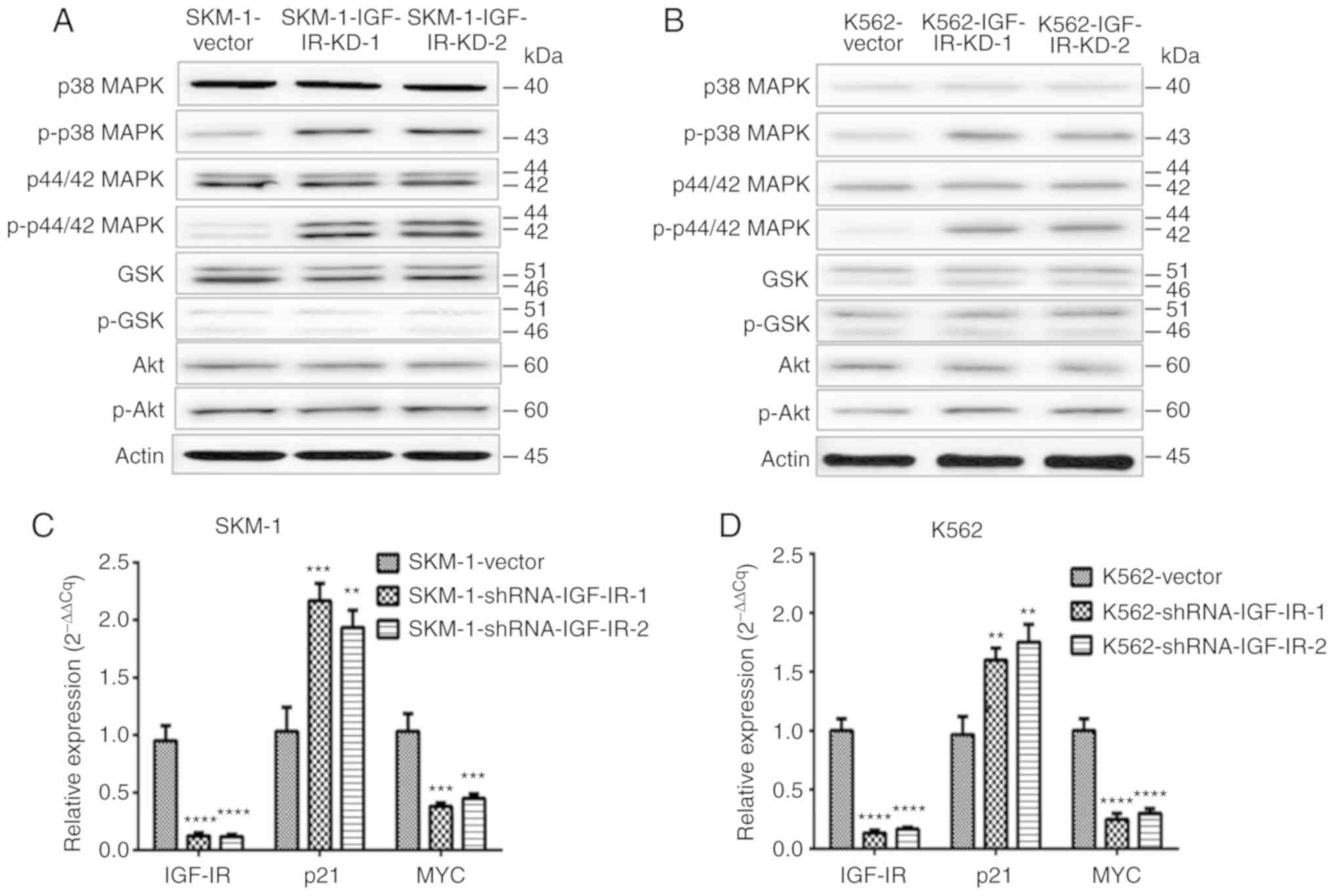
Knockdown of IGF-IR activates the MAPK
signaling pathway in MDS/leukemia cells
In order to validate the results of gene expression
microarray and bioinformatics analysis, expression of specific key
signaling pathway proteins associated with hematological diseases
was determined using western blotting and RT-qPCR. The results
determined the expression levels of relevant proteins. It was
revealed that MAPK signaling characterized by p-p38 MAPK and
p-p44/42 MAPK was activated by knockdown of IGF-IR in SKM-1 and
K562 cells (Fig. 3A and B). p21 and
MYC are considered critical target genes of MAPK signaling. The
knockdown of IGF-IR increased the expression of p21 whereas the
expression of MYC was decreased (Fig.
3C and D). Knockdown of IGF-IR activated the MAPK signaling
pathway, which may lead to an increase in the expression of the
pro-apoptotic gene p21, and a decrease in the pro-proliferative
gene MYC.
Effect of IGF-IR inhibitor PPP on the
properties of MDS/leukemia cells in vitro
In order to examine whether IGF-IR may serve as a
therapeutic target for treatment of MDS, PPP, an IGF-IR specific
inhibitor was used. PPP was added to the two cell lines SKM-1 and
K562, whereas an equivalent volume of DMSO was added to the control
group. Cells were harvested after treatment with PPP for 0, 24, 48,
72, 96 and 168 h, and Trypan blue staining was performed. The
number of viable cells was counted, and cell viability was
calculated. The cell viability of the two cell lines was reduced in
a time-dependent manner. The mean cell viability of SKM-1 cells was
reduced from 97.2 to 16.6% (P<0.001), and the mean cell
viability of K562 cells was reduced from 97.1 to 70.3% (P=0.007)
(Fig. 4A). The apoptotic rate was
determined using flow cytometry and it was revealed that PPP
induced cell apoptosis in vitro. The mean apoptotic rate of
SKM-1 cells was increased from 1.7 to 6.2% (P=0.002), and
the mean apoptotic rate of K562 cells increased from 1.0 to 7.2%
(P=0.024) (Fig. 4B). In
addition, it was also determined that PPP significantly induced
arrest of the cell cycle at the G2/M phase and decreased the
percentage of cells in the S phase in SKM-1 and K562 cells
(Fig. 4C).
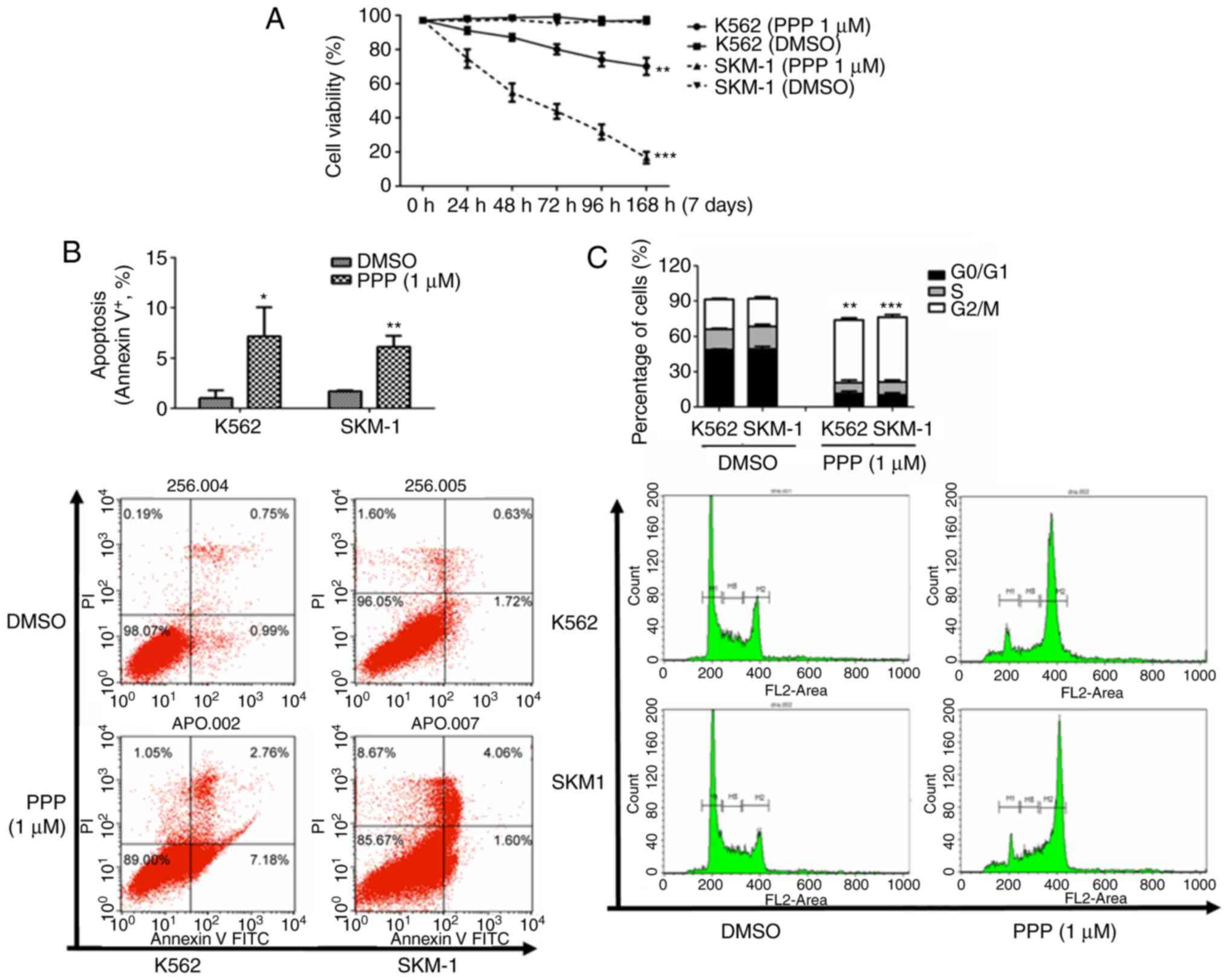 | Figure 4.PPP inhibits proliferation, promotes
apoptosis and induces cell cycle arrest at the G2/M phase in
MDS/leukemia cell lines. (A) PPP inhibited proliferation of both
SKM-1 and K562 cell lines. The mean cell viability of SKM-1 cells
was reduced from 97.2 to 16.6%, and the mean cell viability of K562
cell reduced from 97.1 to 70.3%. (B) PPP promoted apoptosis. The
mean apoptotic rate of the SKM-1 cell line increased from 1.7 to
6.2%, and the mean apoptotic rate of the K562 cell line increased
from 1.0 to 7.2%. *P<0.05, **P<0.01 (unpaired t-test). (C)
PPP significantly induced G2/M phase block and decreased the
percentage of cells in the S phase in SKM-1 and K562 cells. M1
represents G0/G1 phase cells, M2 represents S phase cells, M3
represents G2/M phase cells, and M1-M3 was flanked by fragments and
clumps of cells, thus, the sum of the three was <100%.
**P<0.01, ***P<0.001 (Chi-square test). PPP,
picropodophyllin; MDS, myelodysplastic syndrome. |
Effect of PPP on the properties of
CD34+ cells obtained from patients with MDS
The purity of positive CD34 cells sorted by MACS was
~90%. Treatment with PPP significantly reduced the cell viability
of CD34+ cells of the 8 patients with MDS. Averaging the
viability across all 8 groups of cells, the mean cell viability was
reduced from 84.2 to 67.1%. Additionally, proliferation was also
significantly reduced following treatment with PPP to 60.1% after
48 h of drug treatment (P=0.003). When cells were treated for time
periods longer than 48 h, proliferation appeared to increase.
Individually, inhibition of cell proliferation was most significant
in the 8 patients after either 48 or 72 h of drug treatment.
Thereafter, proliferation exhibited varying degrees of recovery,
and the proliferation in the cells obtained from certain patients
recovered to pre-treatment levels. Cell viability before and after
drug treatment of each patient was compared with the lowest level
of proliferation, respectively, and the differences were found to
be statistically significant (Fig.
5A; Tables I and SII). PPP increased the rate of apoptosis
in the CD34+ cells of 6 MDS patients, of which, the
differences of 2 patients were statistically significant (Fig. 5B). The cell cycles of the
CD34+ cells of 7 MDS patients were arrested in the G2/M
phase, among which the differences of 6 patients were statistically
significant. The percentage of cells in the S-phase of 5 patients
was reduced, among which the reduction in the S-phase in nos. 7 and
8 were the most significant, and the differences were statistically
significant (Fig. 5C).
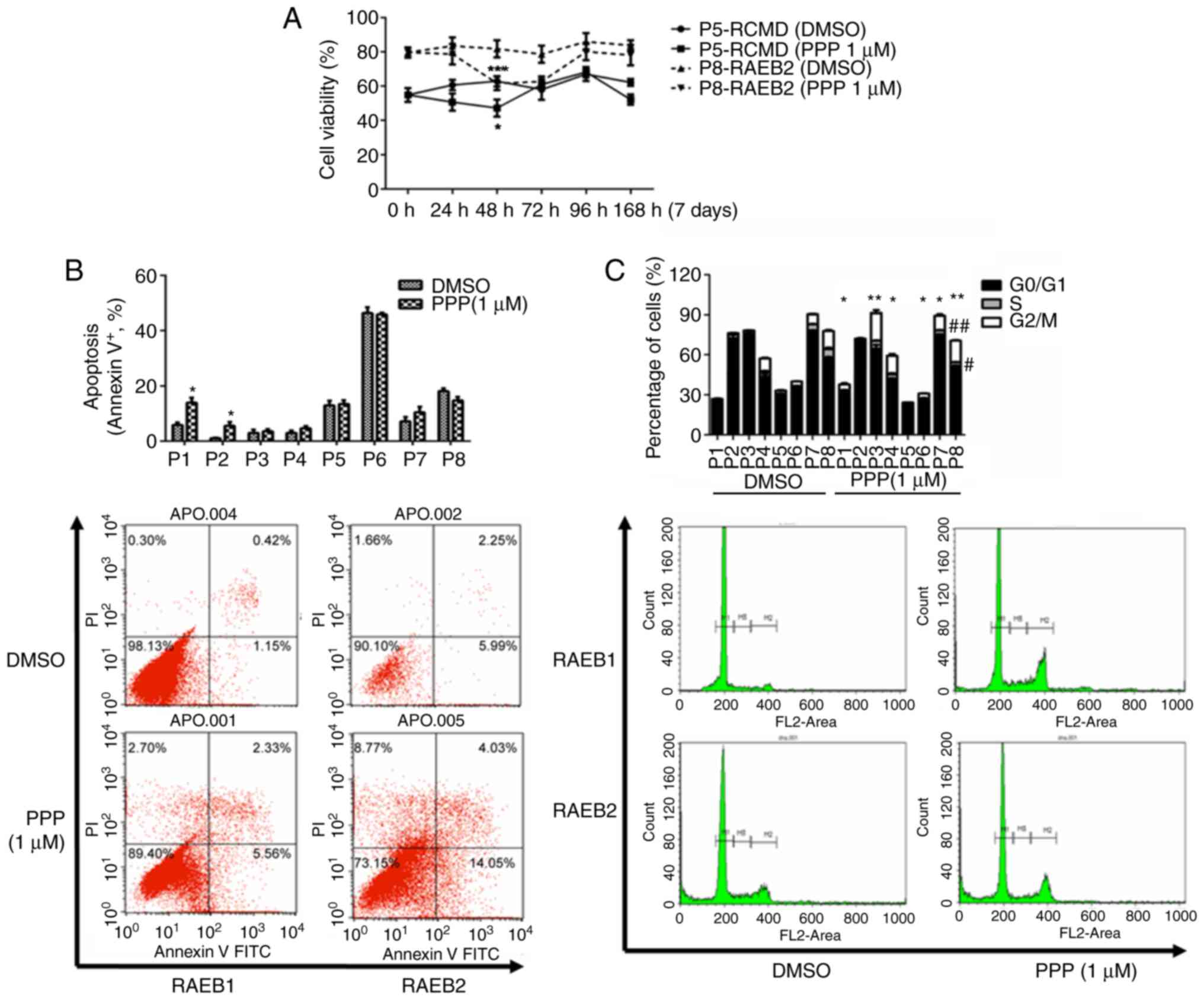 | Figure 5.PPP inhibits proliferation, promotes
apoptosis and induces cell cycle arrest in G2/M in CD34+
cells of patients with MDS. (A) Following PPP treatment, cell
viability of CD34+ cells of 8 patients with MDS was also
significantly reduced. Collectively, the mean cell viability was
reduced from 84.2 to 67.1%. The decrease in proliferation was the
most significant, decreasing to 60.1% after 48 h of treatment,
after which proliferation increased to varying degrees in the
different groups of cells obtained from patients. Representative
graphs for two patients (RCMD and RAEB2) are presented. (B) PPP
increased apoptosis in the CD34+ cells of 6 MDS
patients, among which the differences of 2 patients were
statistically significant. *P<0.05 (unpaired t-test). (C) The
cell cycles of the CD34+ cells of 7 MDS patients were
arrested in the G2/M phase when treated with PPP. The percentage of
cells in the S-phase in 5 patients was reduced, among which the
reduction in patients no. 7 and 8 were the most significant. M1
represents G0/G1 phase cells, M2 represents S phase cells, M3
represents G2/M phase cells, and M1-M3 was flanked by fragments and
clumps of cells, thus, the sum of the three was <100%.
*P<0.05, **P<0.01 vs. the G2/M phase; #P<0.05,
##P<0.01 vs. the S phase (Chi-square test). PPP,
picropodophyllin; MDS, myelodysplastic syndrome. |
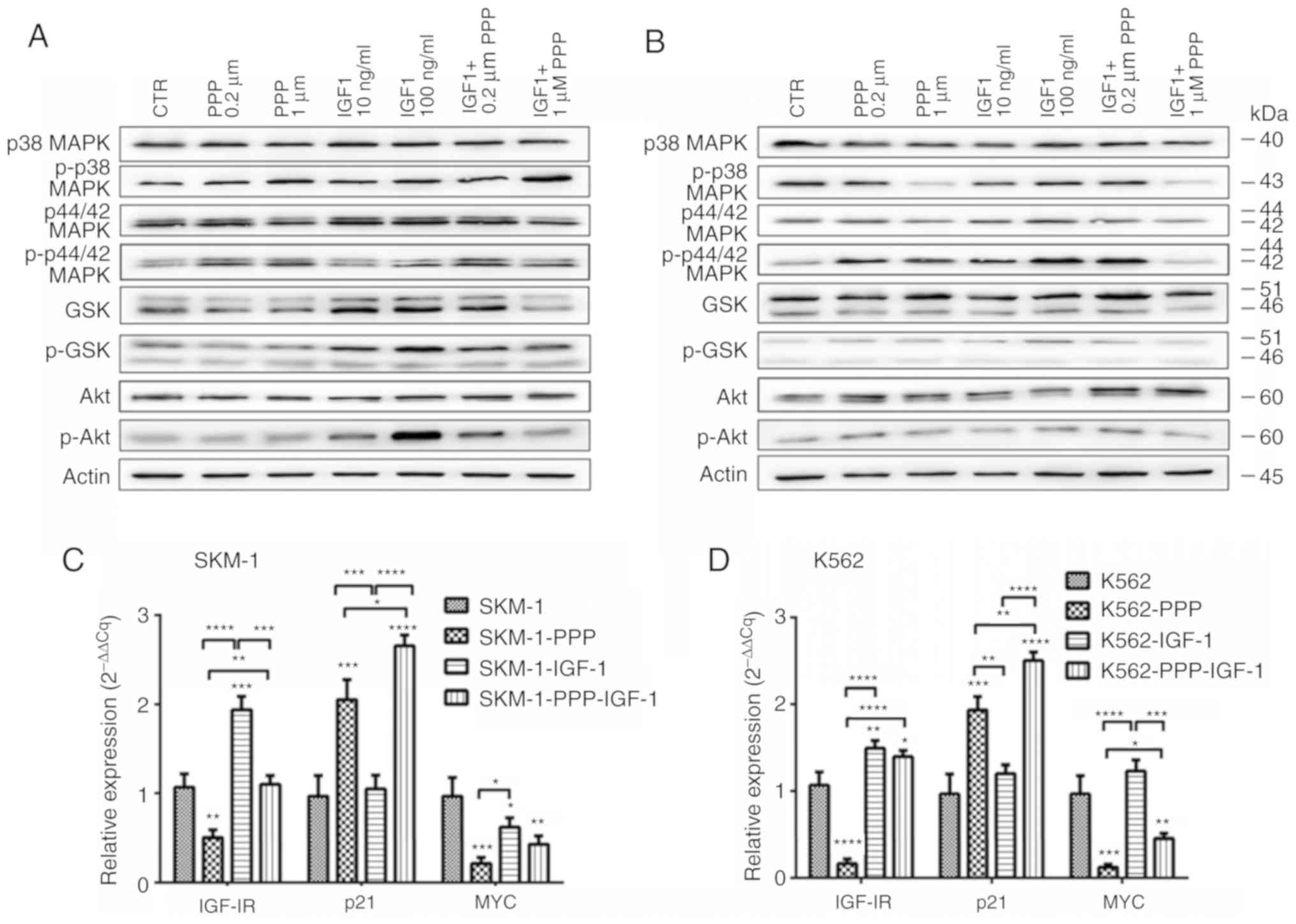
PPP activates the MAPK signaling
pathway in MDS/leukemia cells
As aforementioned, IGF-IR knockdown activated the
MAPK signaling pathway in SKM-1 and K562 cells. As an inhibitor of
IGF-IR, the effect of PPP on MAPK was investigated to validate the
data from IGF-IR knockdown. PPP upregulated the expression of MAPK
signaling-related proteins including p-p38 MAPK and p-p44/42 MAPK
in SKM-1 cells (Fig. 6A). PPP also
increased the expression of p-p44/42 MAPK, whereas the expression
of p-p38 MAPK in K562 cells was not altered, possibly due to
characteristics of the cell line and the specific effects of PPP on
this cell line (Fig. 6B). PPP
increased the expression of p21 and reduced the expression of MYC
(Fig. 6C and D). Collectively, PPP
may be considered a potential therapeutic agent for treatment of
MDS.
Discussion
Numerous studies have used genome sequencing
technologies to study MDS bone marrow cells, and IGF-IR mutations
have not been identified as of yet, to the best of our knowledge
(29–31). Similarly, IGF-IR mutations have not
been identified in solid tumor cells (32). However, our previous studies
revealed that the high expression of IGF-IR was mainly in MDS
clonal cells (19,20), suggesting that the changes in IGF-IR
gene expression levels may be associated with the pathogenesis of
MDS, thereby regulating the proliferation of MDS clonal cells.
In order to study the mechanism by which IGF-IR
regulates MDS clonal cell proliferation, RNA interference was used
to knockdown IGF-IR in SKM-1 and K562 cell lines in vitro,
and the changes in the biological activity of the cells was
assessed. The results demonstrated that cell proliferation rates of
SKM-1 and K562 cell lines following IGF-IR knockdown were
significantly reduced, and the apoptotic rates were significantly
increased. This suggests that the downregulation of IGF-IR may
inhibit the proliferation of MDS/leukemia cell lines and induce
apoptosis. These results are consistent with the results reported
on IGF-IR in other types of solid tumors (9,10,32).
The results of the gene expression profile and pathway-net analysis
of SKM-1 cells with IGF-IR knockdown indicated that the
downregulation of IGF-IR resulted in abnormalities in MAPK
signaling, apoptosis as well as other signaling pathways, thereby
leading to abnormal cell proliferation and apoptosis. Subsequently,
western blotting was used to confirm that IGF-IR knockdown resulted
in a significant increase in the expression of two proteins, p-p38
MAPK and p-p44/42 MAPK, indicating that IGF-IR primarily regulated
the proliferative and anti-apoptotic activities of MDS/leukemia
cell lines via inhibition of the MAPK signaling pathway. p21 and
MYC are considered critical target genes of MAPK signaling
(33,34). The results of the mRNA changes in
p21 and MYC confirmed that the MAPK pathway was activated. Although
the majority of studies have demonstrated that the p-p44/42 MAPK
pathway exerts an anti-apoptotic role, p-p44/42 MAPK signaling has
also been demonstrated to exhibit a pro-apoptotic effect, such as
in neurons (35), platelets
(36) and cardiomyocytes (37). Studies have demonstrated that
overactivation of MAPK and TGF-β signaling pathways in low-risk MDS
can promote excessive apoptosis of hematopoietic stem cells,
whereas the AKT/PI3K, PI3K/mTOR and EGF signaling pathways are
overactivated in high-risk MDS (38–41).
In the present study, the inhibitory effects of IGF-IR on the MAPK
signaling pathway may also serve an important role in high-risk
MDS.
The results of the aforementioned in vitro
experiments revealed that IGF-IR may serve as an oncogene in
regulating the proliferation of MDS clonal cells. To further
clarify whether IGF-IR could be used as a novel therapeutic target
for treatment of MDS, a specific inhibitor of IGF-IR was used to
perform intervention experiments in the primary MDS cells to
observe the changes in the biological activity of these cells. PPP
is an IGF-IR-specific tyrosine kinase inhibitor that can
specifically reduce the phosphorylation of tyrosine residue Y1136
of IGF-IR, and thus inhibit the activity of IGF-IR, without
affecting the activity of IR (9).
PPP (clinical drug name is AXL1717) (42,43) is
currently undergoing phase I/II clinical trials, and the existing
data demonstrated that PPP has multiple clinical efficacies with
only mild side effects. In the present study, PPP was used to treat
cells in vitro in two cell lines (SKM-1 and K562,) and
primary CD34+ cells isolated from 8 patients with MDS,
and it was revealed that cell proliferation was significantly
inhibited. However, the proliferation of CD34+ cells
from MDS patients gradually recovered after 48 or 72 h of PPP
treatment, which may be associated with the heterogeneity of
CD34+ cells (such as the co-existence of normal cells
and clonal cells in CD34+ cells from MDS patients).
After treatment with PPP, the apoptotic rates of the two cell lines
and CD34+ cells from 4 of the patients with MDS were
significantly increased, whereas the apoptotic rates of
CD34+ cells from the other 4 patients with MDS were not
significantly altered, although the number of dead cells
significantly increased. Furthermore, following treatment with PPP,
the cell cycles of the cell lines and CD34+ cells from 7
of the MDS patients were arrested in the G2/M phase, and the
majority of the cells also exhibited a significant decrease in the
percentage of cells in the S-phase. Collectively, this indicated
that inhibition of IGF-IR activity using PPP resulted in a
reduction in DNA synthesis and cell cycle arrest, thus
significantly reducing the number of cells entering cell division.
This result was consistent with that induced by knockdown of IGF-IR
using RNA interference. Recently, the effect of IGF-IR inhibitors
on acute lymphoblastic cell lines was studied (44), and the results suggested that
OSI-906 (IGF-IR/IR inhibitor) inhibited ERK activation, and NT157
(IGF-IR-IRS1/2 inhibitor) induced ERK activation. Although their
targets were different from PPP, they all affected the MAPK
signaling pathway. Different drugs have different effects on the
MAPK signaling pathway, and to complicate matters further the same
drug, such as PPP, may exhibit varying effects on the MAPK
signaling pathway in different cell lines based on the results of
the present study. Collectively, this highlights the complexity of
the mechanisms of inhibitors.
In conclusion, knockdown of IGF-IR activity using
RNA interference or with a specific inhibitor inhibited
proliferation and induced apoptosis in MDS cells, either in
established cell lines or primary cultured cells isolated from MDS
patients, thus resulting in arrest of the cell cycle. IGF-IR may
promote MDS cell proliferation, and inhibit apoptosis primarily
through inhibition of the MAPK signaling pathway. IGF-IR thus may
serve as a potential therapeutic target for treatment of MDS.
Supplementary Material
Supporting Data
Acknowledgements
We thank Shanghai Qiming, Inc. for providing
assistance in the bioinformatics analysis.
Funding
The present study was funded by the National Natural
Science Foundation of China (nos. 81100341, 81570108 and
81400090).
Availability of data and materials
The datasets supporting the conclusions of this
article are included within this article and its additional images.
Raw data are available from the corresponding author on reasonable
request.
Authors' contributions
QH, QZ, CC and FX performed all the experiments. QH
and QZ cultured the cells and performed the RT-qPCR and western
blotting. QH and FX wrote the manuscript. WS and JG performed the
flow cytometric analysis. ZZ and SZ collected the bone marrow
samples of patients. QH and QZ performed the statistical analysis.
XL conceived the study and participated in its design. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Sixth Hospital Affiliated with Shanghai Jiao Tong
University, and all patients provided informed consent for the
utilization of their tissue samples in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tiu R, Gondek L, O'Keefe C and Maciejewski
JP: Clonality of the stem cell compartment during evolution of
myelodysplastic syndromes and other bone marrow failure syndromes.
Leukemia. 21:1648–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steensma DP, Bejar R, Jaiswal S, Lindsley
RC, Sekeres MA, Hasserjian RP and Ebert BL: Clonal hematopoiesis of
indeterminate potential and its distinction from myelodysplastic
syndromes. Blood. 126:9–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi H, Qingxia Z, Xiao L, Lingyun W, Feng
X, Zheng Z and Chunkang C: Recurrent abnormal clones in
myelodysplastic syndrome marrow originate from cells at a
pluripotent stem level and maintain their early differentiation
potency. Cancer Invest. 33:369–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith MA, Choudhary GS, Pellagatti A, Choi
K, Bolanos LC, Bhagat TD, Gordon-Mitchell S, Von Ahrens D, Pradhan
K, Steeples V, et al: U2AF1 mutations induce oncogenic IRAK4
isoforms and activate innate immune pathways in myeloid
malignancies. Nat Cell Biol. 21:640–650. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kennedy AL and Shimamura A: Genetic
predisposition to MDS: Clinical features and clonal evolution.
Blood. 133:1071–1085. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nolte F and Hofmann WK: Molecular
mechanisms involved in the progression of myelodysplastic syndrome.
Future Oncol. 6:445–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Issa JP: Epigenetic changes in the
myelodysplastic syndrome. Hematol Oncol Clin North Am. 24:317–330.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Zhao L, Wei X, Guo Q, Zhu X, Wei
R, Yin X, Zhang Y, Wang B and Li X: Integrated bioinformatic
analysis of microarray data reveals shared gene signature between
MDS and AML. Oncol Lett. 16:5147–5159. 2018.PubMed/NCBI
|
|
9
|
Gao J, Chang YS, Jallal B and Viner J:
Targeting the insulin-like growth factor axis for the development
of novel therapeutics in oncology. Cancer Res. 72:3–12. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guerreiro AS, Boller D, Doepfner KT and
Arcaro A: IGF-IR: Potential role in antitumor agents. Drug News
Perspect. 19:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strömberg T, Ekman S, Girnita L, Dimberg
LY, Larsson O, Axelson M, Lennartsson J, Hellman U, Carlson K,
Osterborg A, et al: IGF-1 receptor tyrosine kinase inhibition by
the cyclolignan PPP induces G2/M-phase accumulation and apoptosis
in multiple myeloma cells. Blood. 107:669–678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tazzari PL, Tabellini G, Bortul R, Papa V,
Evangelisti C, Grafone T, Martinelli G, McCubrey JA and Martelli
AM: The insulin-like growth factor-I receptor kinase inhibitor
NVP-AEW541 induces apoptosis in acute myeloid leukemia cells
exhibiting autocrine insulin-like growth factor-I secretion.
Leukemia. 21:886–896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi P, Chandra J, Sun X, Gergely M, Cortes
JE, Garcia-Manero G, Arlinghaus RB, Lai R and Amin HM: Inhibition
of IGF-IR tyrosine kinase induces apoptosis and cell cycle arrest
in imatinib-resistant chronic myeloid leukaemia cells. J Cell Mol
Med. 14:1777–1792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whelan JT, Ludwig DL and Bertrand FE:
HoxA9 induces insulin-like growth factor-1 receptor expression in
B-lineage acute lymphoblastic leukemia. Leukemia. 22:1161–1169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schillaci R, Galeano A, Becu-Villalobos D,
Spinelli O, Sapia S and Bezares RF: Autocrine/paracrine involvement
of insulin-like growth factor-I and its receptor in chronic
lymphocytic leukaemia. Br J Haematol. 130:58–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malaguarnera R and Belfiore A: The
emerging role of insulin and insulin-like growth factor signaling
in cancer stem cells. Front Endocrinol (Lausanne). 5:102014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muraguchi T, Nanba D, Nishimura EK and
Tashiro T: IGF-1R deficiency in human keratinocytes disrupts
epidermal homeostasis and stem cell maintenance. J Dermatol Sci.
94:298–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teng CF, Jeng LB and Shyu WC: Role of
insulin-like growth factor 1 receptor signaling in stem cell
stemness and therapeutic efficacy. Cell Transplant. 27:1313–1319.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi H, Xiao L, Lingyun W, Ying T, Yi-Zhi L,
Shao-Xu Y and Quan P: Expression of type 1 insulin-like growth
factor receptor in marrow nucleated cells in malignant
hematological disorders: Correlation with apoptosis. Ann Hematol.
85:95–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Q, Li X, Zhang Z, Zhang Q, Xu F, Yang
L, Tao Y and Liu Y: Overexpression of IGF-IR in malignant clonal
cells in bone marrow of myelodysplastic syndromes. Cancer Invest.
28:983–988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Q, Chang CK, Xu F, Zhang QX, Shi WH and
Li X: Purification of bone marrow clonal cells from patients with
myelodysplastic syndrome via IGF-IR. PLoS One. 10:e01403722015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valent P, Horny HP, Bennett JM, Fonatsch
C, Germing U, Greenberg P, Haferlach T, Haase D, Kolb HJ, Krieger
O, et al: Definitions and standards in the diagnosis and treatment
of the myelodysplastic syndromes: Consensus statements and report
from a working conference. Leuk Res. 31:727–736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vardiman JW, Harris NL and Brunning RD:
The world health organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delacrétaz F, Schmidt PM, Piguet D,
Bachmann F and Costa J: Histopathology of myelodysplastic
syndromes. The FAB classification (proposals) applied to bone
marrow biopsy. Am J Clin Pathol. 87:180–186. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greenberg P, Cox C, LeBeau MM, Morel P,
Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, et
al: International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 89:2079–2088. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shaffer LG and Tommerup N: ISCN: An
international system for human cytogenetics nomenclature. S Karger;
Basel 2005: 2005
|
|
27
|
Nakagawa T, Matozaki S, Murayama T,
Nishimura R, Tsutsumi M, Kawaguchi R, Yokoyama Y, Hikiji K, Isobe T
and Chihara K: Establishment of a leukaemic cell line from a
patient with acquisition of chromosomal abnormalities during
disease progression in myelodysplastic syndrome. Br J Haematol.
85:469–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hosono N: Genetic abnormalities and
pathophysiology of MDS. Int J Clin Oncol. 24:885–892. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu F, Wu LY, Chang CK, He Q, Zhang Z, Liu
L, Shi WH, Guo J, Zhu Y, Zhao YS, et al: Whole-exome and targeted
sequencing identify ROBO1 and ROBO2 mutations as
progression-related drivers in myelodysplastic syndromes. Nat
Commun. 6:88062015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonçalves AC, Alves R, Baldeiras I,
Cortesão E, Carda JP, Branco CC, Oliveiros B, Loureiro L, Pereira
A, Nascimento Costa JM, et al: Genetic variants involved in
oxidative stress, base excision repair, DNA methylation, and folate
metabolism pathways influence myeloid neoplasias susceptibility and
prognosis. Mol Carcinog. 56:130–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuen JS and Macaulay VM: Targeting the
type 1 insulin-like growth factor receptor as a treatment for
cancer. Expert Opin Ther Targets. 12:589–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Narla G, Sangodkar J and Ryder CB: The
impact of phosphatases on proliferative and survival signaling in
cancer. Cell Mol Life Sci. 75:2695–2718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, Li N, Xiang R and Sun P: Emerging
roles of the p38 MAPK and PI3K/AKT/mTOR pathways in
oncogene-induced senescence. Trends Biochem Sci. 39:268–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Q, Chen M, Liu H, Yang L, Yang T and He
G: The dual role of ERK signaling in the apoptosis of neurons.
Front Biosci (Landmark Ed). 19:1411–1417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paul M, Manikanta K, Hemshekhar M,
Sundaram MS, Naveen S, Ramesh TN, Kemparaju K and Girish KS:
Bisdemethoxycurcumin promotes apoptosis in human platelets via
activation of ERK signaling pathway. Toxicol In Vitro.
63:1047432019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang DX, Ma DY, Yao ZQ, Fu CY, Shi YX,
Wang QL and Tang QQ: ERK1/2/p53 and NF-κB dependent-PUMA activation
involves in doxorubicin-induced cardiomyocyte apoptosis. Eur Rev
Med Pharmacol Sci. 20:2435–2442. 2016.PubMed/NCBI
|
|
38
|
Navas TA, Mohindru M, Estes M, Ma JY,
Sokol L, Pahanish P, Parmar S, Haghnazari E, Zhou L, Collins R, et
al: Inhibition of overactivated p38 MAPK can restore hematopoiesis
in myelodysplastic syndrome progenitors. Blood. 108:4170–4177.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhagat TD, Zhou L, Sokol L, Kessel R,
Caceres G, Gundabolu K, Tamari R, Gordon S, Mantzaris I, Jodlowski
T, et al: miR-21 mediates hematopoietic suppression in MDS by
activating TGF-β signaling. Blood. 121:2875–2881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Follo MY, Mongiorgi S, Bosi C, Cappellini
A, Finelli C, Chiarini F, Papa V, Libra M, Martinelli G, Cocco L
and Martelli AM: The Akt/mammalian target of rapamycin signal
transduction pathway is activated in high-risk myelodysplastic
syndromes and influences cell survival and proliferation. Cancer
Res. 67:4287–4294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boehrer S, Adès L, Braun T, Galluzzi L,
Grosjean J, Fabre C, Le Roux G, Gardin C, Martin A, de Botton S, et
al: Erlotinib exhibits antineoplastic off-target effects in AML and
MDS: A preclinical study. Blood. 111:2170–2180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ekman S, Frödin JE, Harmenberg J, Bergman
A, Hedlund A, Dahg P, Alvfors C, Ståhl B, Bergström S and Bergqvist
M: Clinical phase I study with an insulin-like growth factor-1
receptor inhibitor: Experiences in patients with squamous non-small
cell lung carcinoma. Acta Oncol. 50:441–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu X, Sooman L, Wickström M, Fryknäs M,
Dyrager C, Lennartsson J and Gullbo J: Alternative cytotoxic
effects of the postulated IGF-IR inhibitor picropodophyllin in
vitro. Mol Cancer Ther. 12:1526–1536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rodrigues Alves APN, Fernandes JC,
Fenerich BA, Coelho-Silva JL, Scheucher PS, Simões BP, Rego EM,
Ridley AJ, Machado-Neto JA and Traina F: IGF1R/IRS1 targeting has
cytotoxic activity and inhibits PI3K/AKT/mTOR and MAPK signaling in
acute lymphoblastic leukemia cells. Cancer Lett. 456:59–68. 2019.
View Article : Google Scholar : PubMed/NCBI
|