Introduction
Over 1 million individuals are diagnosed with colon
cancer (CC) annually worldwide, resulting in a high rate of
morbidity and mortality throughout China and certain western
countries (1,2). Despite major advances in diagnostic
and therapeutic strategies in recent decades, the prognosis of
patients with CC remains poor, which is mainly due to the
development of distant metastasis leading to low survival rates
(3,4). Moreover, the mechanisms underlying CC
tumorigenesis and metastasis remain elusive. Thus, it is crucial to
elucidate the molecular mechanism of CC development and progression
in order to develop novel effective therapeutic strategies for
patients with CC.
Long non-coding RNAs (lncRNAs) and microRNAs
(miRNAs) are two common types of non-coding RNAs with different
lengths of nucleotide chains, which play key regulatory roles in
human diseases, including cancer (5,6).
Mounting evidence has demonstrated that lncRNAs are involved in
cancer development, progression and metastasis, and may act as
tumor suppressor genes or oncogenes (7,8).
miRNAs may suppress or promote tumorigenesis through specifically
binding to the 3′-untranslated region (3-UTR) of the target mRNA,
leading to mRNA degradation (9).
Corroborating evidence revealed that lncRNAs and microRNAs may
serve as predictive or therapeutic biomarkers in human cancers
(10,11). Interestingly, various lncRNAs share
the same targeting sequences with certain miRNAs, while functioning
as competing endogenous RNAs (ceRNAs) or miRNA sponges (12–14).
Thus, it is crucial to clearly determine the lncRNA-miRNA
interaction and their regulatory network in cancer. Multiple ceRNA
networks have been investigated in CC (15–17).
For example, lncRNA ZNFX1-AS1 was demonstrated to promote
colorectal cancer progression and metastasis via interacting with
miR-144 to regulate EZH2 expression (18). Yu et al reported that lncRNA
CCAT2 regulated miR-145 in CC cells (19). However, the complicated
lncRNA-miRNA-mRNA regulatory network in CC requires additional
extensive investigation.
In the present study, the altered expression of
lncRNAs in CC tissues was screened in comparison with that in
normal tissues in order to identify potential lncRNA/miRNA
predictive biomarkers in CC. While multiple lncRNAs functioned as
potential tumor suppressors or promoters in CC, this investigation
was focused on the function of lncRNA RP11-619L19.2, which is
highly expressed in CC tissues and cell lines. The effects of
RP11-619L19.2 knockdown on CC cell proliferation, migration,
invasion and epithelial-to-mesenchymal transition (EMT) were
investigated, and the association between lncRNA PR11-619L19.2 and
miRNA-1271-5p was examined. Additionally, it was investigated
whether CD164 is the downstream target of miR-1271-5p, and whether
overexpression of CD164 would be able to reverse the effects of
RP11-619L19.2 knockdown. In summary, the aim of the present study
was to elucidate the detailed function of the
RP11-619L19.2/miR-1271-5p/CD164 axis in CC, in the hope of
identifying effective and predictive biomarkers as well as
therapeutic targets for patients with CC.
Materials and methods
Human samples
A total of 30 pairs of primary CC tissues with
matching adjacent normal tissues and 20 CC tissues with distant
metastasis were obtained from the Second Affiliated Hospital of
Xi'an Jiaotong University. The present study was approved by the
Ethics Committee of the Second Affiliated Hospital of Xi'an
Jiaotong University, and all patients enrolled provided their
signed informed consent. All tissues were frozen in liquid nitrogen
and stored at −80°C until total RNA and protein were extracted.
Cell lines
Three human CC cell lines (HCT-116, SW620 and DLD-1)
and the normal colonic epithelial cell line FHC were obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
All cell lines were cultured in DMEM supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 U/ml streptomycin, and incubated at 37°C in a 5%
CO2 atmosphere.
Cell transfection
The lentiviral vectors containing short hairpin RNAs
(shRNAs) targeting RP11-619L19.2 were designed and constructed by
GenePharma. The expression vector for CD164 overexpression was
obtained from GeneChem, Inc. The miR-1271-5p mimic and negative
control (NC) oligonucleotides were synthesized by Guangzhou RiboBio
Co., Ltd. CC cells were transfected with the abovementioned vectors
or oligonucleotides by using the Lipofectamine® 2000
Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and the transfection efficiency was verified by RT-qPCR (Fig. S1).
Luciferase reporter assay
Luciferase reporter plasmids containing
RP11-619L19.2 or CD164 with the wild-type (Wt RP11-619L19.2 or Wt
CD164) or mutant (Mut RP11-619L19.2 or Mut CD164)
miR-1271-5p-binding sites were obtained from GenePharma. HCT-116 or
SW620 cells were transfected with the miR-1271-5p mimic or miR-NC,
together with the reporter plasmids. Subsequently, 48 h after
transfection the luciferase activity was measured by a Dual
Luciferase Reporter Gene Assay Kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNAs were purified from cultured cells, CC
tissues or adjacent normal tissues by using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA using the PrimeScript™ kit (Takara
Biotechnology Co., Ltd.) at 42°C for 1 h followed by 95°C for 5
min. qPCR was conducted on a 7500 Real-Time PCR System™ (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with SYBR™ Green Master
Mix (Takara Biotechnology Co., Ltd.). The thermocycling conditions
used were as follows: Initial denaturation at 95°C for 3 min,
followed by 40 cycles of denaturation at 95°C for 10 sec and
annealing/elongation at 60°C for 30 sec. The primers used were as
follows: For RP11-619L19.2, 5′-ACTGGGAATGGAGGAAGA-3′ (forward) and
5′-TGAGAAAGGATTGAGGGAAAAG-3′ (reverse); for E-cadherin,
5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and 5′-GAGTCCTTCCACGATACCAA-3′
(reverse); for N-cadherin, 5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and
5′-GAGTCCTTCCACGATACCAA-3′ (reverse); for vimentin,
5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and 5′-GAGTCCTTCCACGATACCAA-3′
(reverse); for β-catenin, 5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and
5′-GAGTCCTTCCACGATACCAA-3′ (reverse); for Snail,
5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and 5′-GAGTCCTTCCACGATACCAA-3′
(reverse); for miR-1271-5p, 5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and
5′-GAGTCCTTCCACGATACCAA-3′ (reverse); and for GAPDH,
5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and 5′-GAGTCCTTCCACGATACCAA-3′
(reverse).
Cell migration assay
In vitro wound healing assay was conducted to
assess the mobility of CC cells. After different treatments,
HCT-116 or SW620 cells were seeded into 6-well plates and cultured
until ~100% confluent. Then, each well was scratched with a 200-µl
sterile pipette tip, washed with PBS, and incubated with serum-free
DMEM at 37°C. The scratch area was photographed at 0 and 48 h to
evaluate wound closure rate.
Cell invasion assay
HCT-116 or SW620 cells were suspended in serum-free
medium at a density of 4×105 cells/ml at 48 h after
transfection. A 100-µl cell suspension was added to the upper
Matrigel-coated chambers (8.0 µm pore size, Corning, Inc.) and 600
µl of complete medium was added to the bottom chamber. After a 24 h
incubation, the invading cells were fixed in 4% paraformaldehyde
for 10 min, and stained with 0.1% crystal violet solution for 30
min at room temperature. The number of cells was calculated and
images were captured under a light microscope (Olympus Corporation)
at a magnification of ×200.
Cell proliferation assay
HCT-116 or SW620 cells transfected with negative
control, sh-RP11-619L19.2, miR-NC, miR-1271-5p mimics, or
sh-RP11-619L19.2 + pcDNA3.1-CD164 were seeded into 96-well plates
at 20,000 cells/well. After culture for the indicated time, 10 µl
CCK-8 reagent (Dojindo Molecular Technologies, Inc.) was added to
the cell culture medium and cell viability was assessed following
the manufacturer's instructions.
Western blotting
Once different treatments were completed, HCT-116 or
SW620 cell lysates were collected by using RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA) and protein concentration was measured
using a BCA assay kit (Pierce; Thermo Fisher Scientific, Inc.). All
samples were subjected to 10% SDS-PAGE electrophoresis and
transferred to a PVDF membrane (Roche Diagnostics). After blocking
in 5% non-fat milk in TBST (containing 0.05% Tween-20) for 1 h at
room temperature, the membranes were incubated with the following
primary antibodies (all from Abcam): Snail (1:1,000, ab216347),
E-cadherin (1:10,000, ab40772), N-cadherin (1:500, ab98952),
vimentin (1:1,000, ab20346), β-catenin (1:5,000, ab32572), or
β-actin (1:2,000, ab8226) at 4°C overnight. After washing three
times with TBST (5 min per wash), the membranes were then incubated
with HRP-conjugated goat anti-rabbit IgG (1:20,000, ab205718,
Abcam) at room temperature for 1 h. Band detection was performed
using an enhanced chemiluminescence kit (Pierce; Thermo Fisher
Scientific, Inc.).
Immunohistochemical staining
The immunohistochemical staining for CD164 was
performed as previously described (20). Briefly, tissue sections were
deparaffinized and incubated in an antigen retrieval solution
(Target Retrieval; Dako; Agilent Technologies, Inc.) at 95°C for 15
min. The sections were then incubated overnight at 4°C with a
monoclonal antibody against CD164 (1:100; HPA010636; Sigma-Aldrich;
Merck KGaA). On the following day, the sections were incubated with
an HRP-conjugated secondary antibody (1:2,000; 7074, Cell Signaling
Technology, Inc.) at room temperature for 30 min; they were then
developed with diaminobenzidine at room temperature for 10 min and
counterstained with hematoxylin at room temperature for 1 min.
Statistical analysis
All data analyses were conducted using GraphPad
Prism 5.0 software (GraphPad Software, Inc.). The data are shown as
the mean ± SE. Student's t-test was used when two groups were
compared. When more than two groups were compared, one-way ANOVA
followed by Tukey's post hoc test was performed. P<0.05 was
considered to indicate statistically significant differences.
Results
RP11-619L19.2 is highly expressed in
CC tissues and cell lines
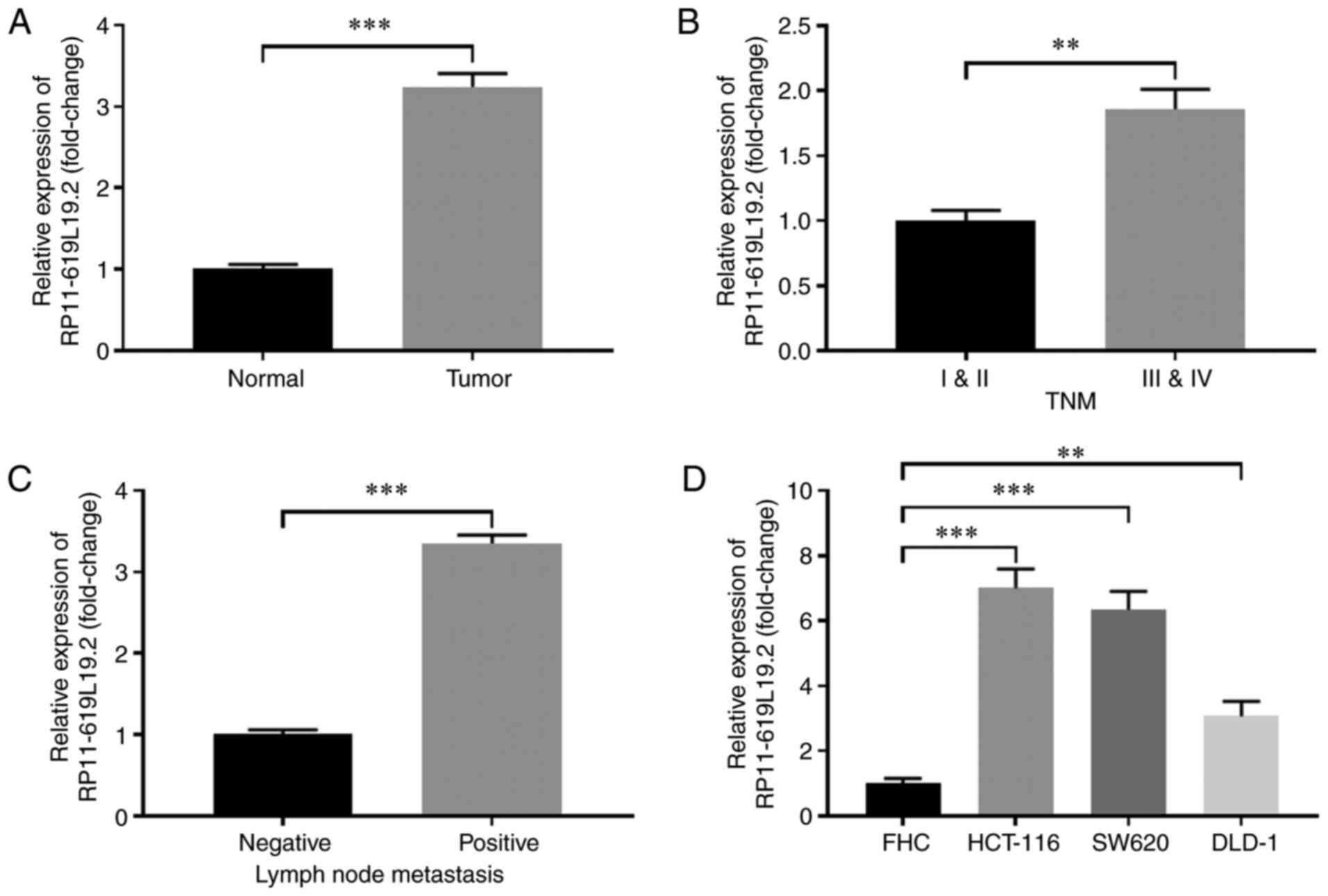
The expression profile of lncRNA RP11-619L19.2 in CC
tissues and paired normal tissues was first examined. As shown in
Fig. 1A, the expression of
RP11-619L19.2 was significantly higher in CC tissues compared with
that in normal tissues. Furthermore, CC tissues with advanced TNM
stage (III and V) exhibited markedly higher RP11-619L19.2 levels
compared with those with TNM stage I and II (Fig. 1B). In addition, CCs with lymph node
metastasis exhibited higher expression of RP11-619L19.2 compared
with tumors without lymph node metastasis (Fig. 1C). The expression of RP11-619L19.2
in CC cell lines was also analyzed. Compared with the control cell
line FHC, the CC cell lines HCT-116, SW620 and DLD-1 had notably
higher expression of RP11-619L19.2 (Fig. 1D). Thus, lncRNA RP11-619L19.2 was
highly expressed in CC tissues and cell lines.
Knockdown of RP11-619L19.2 inhibits CC
cell proliferation, migration, invasion and EMT
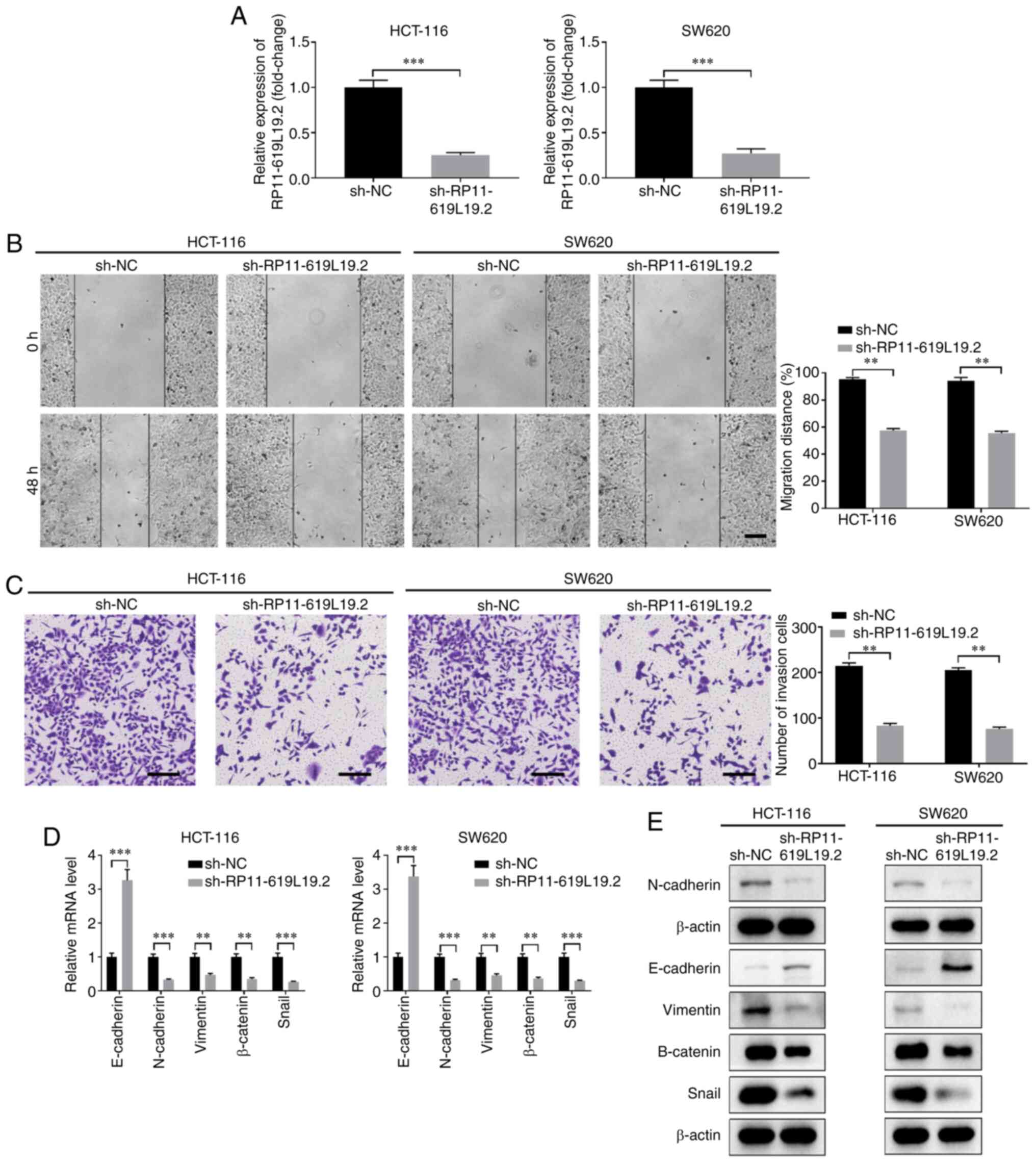
To investigate the function of RP11-619L19.2 in CC,
multiple shRNAs were screened to knock down the expression of
RP11-619L19.2 (data not shown). The knockdown efficiency of shRNA
targeting RP11-619L19.2 was confirmed in HCT-116 and SW620 cells
(Fig. 2A). Functionally, knockdown
of RP11-619L19.2 suppressed the proliferation of HCT-116 and SW620
cells (Fig. S2A and B). In
addition, knockdown of RP11-619L19.2 markedly suppressed the
migration and invasion of HCT-116 or SW620 cells, as demonstrated
by wound healing and Transwell assays (Fig. 2B and C). EMT is a critical process
for tumor invasion and metastasis (21). The expression of EMT-related genes
after RP11-619L19.2 knockdown was further analyzed. As shown in
Fig. 2D and E, knockdown of
RP11-619L19.2 significantly enhanced the expression of E-cadherin,
while decreasing the expression of N-cadherin, vimentin, β-catenin
and Snail at both the mRNA and protein levels. In conclusion,
RP11-619L19.2 regulated EMT-related gene expression and promoted
tumor cell proliferation, migration, invasion and metastasis in
CC.
Reciprocal repression is observed
between RP11-619L19.2 and miR-1271-5p
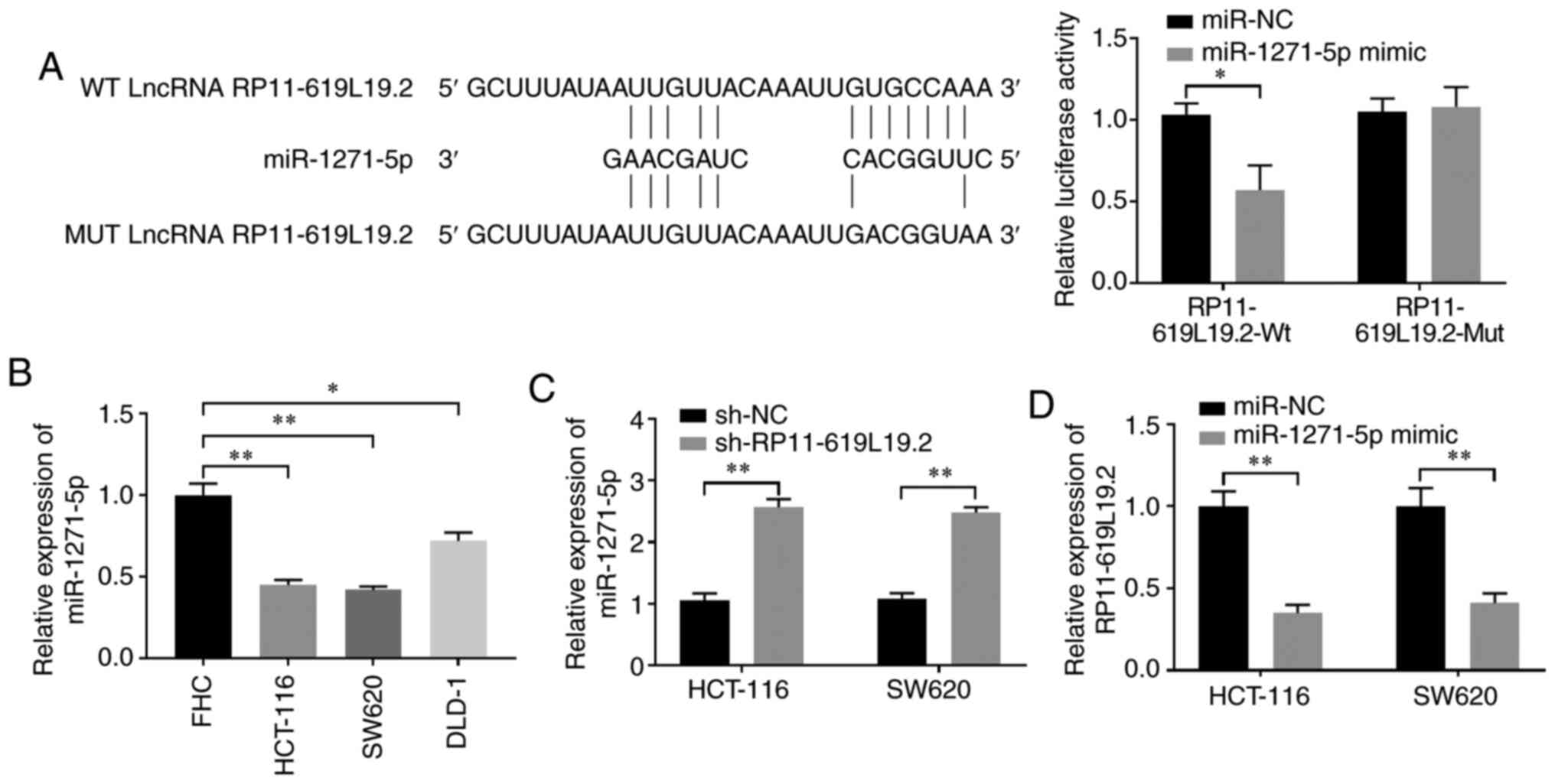
Given the crucial interaction between lncRNA and
miRNA, it was further investigated whether miRNA was involved in
the regulation of RP11-619L19.2 expression. DIANA tools were
employed to search for miRNAs that may interact with RP11-619L19.2
(22). miR-1271-5p was identified
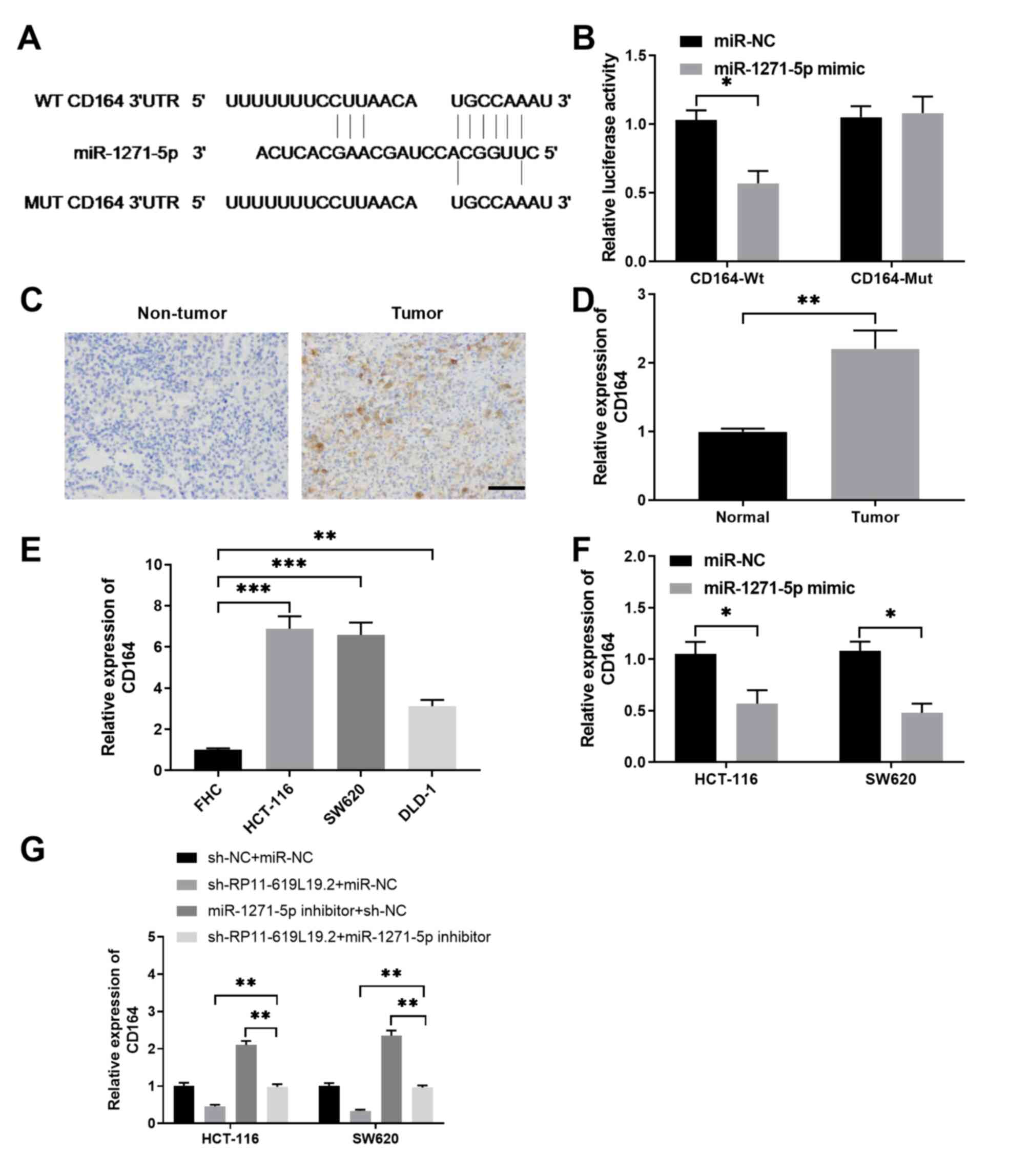
to have putative binding sites for RP11-619L19.2 (Fig. 3A). Dual luciferase reporter assay
demonstrated that miR-1271-5p mimics inhibited luciferase activity
in HCT-116 cells transfected with the reporter vector containing Wt
lncRNA RP11-619L19.2 sequences, while no significant suppression
was found in HCT-116 cells transfected with the reporter vector
containing mutated RP11-619L19.2 sequences (Fig. 3A). In addition, the expression of
miR-1271-5p was significantly lower in CC cell lines (HCT-116,
SW620 and DLD-1) compared with that in control FHC cells (Fig. 3B). Moreover, it was demonstrated
that RP11-619L19.2 knockdown could enhance the expression of
miR-1271-5p in HCT-116 and SW620 cells, which further confirmed the
reciprocal repression between RP11-619L19.2 and miR-1271-5p
(Fig. 3C). By contrast, HCT-116 or
SW620 cells transfected with miR-1271-5p mimics significantly
downregulated the expression of RP11-619L19.2 compared with cells
transfected with the miR-NC control (Fig. 3D).
Overexpression of miR-1271-5p
suppresses CC cell proliferation, migration, invasion and EMT
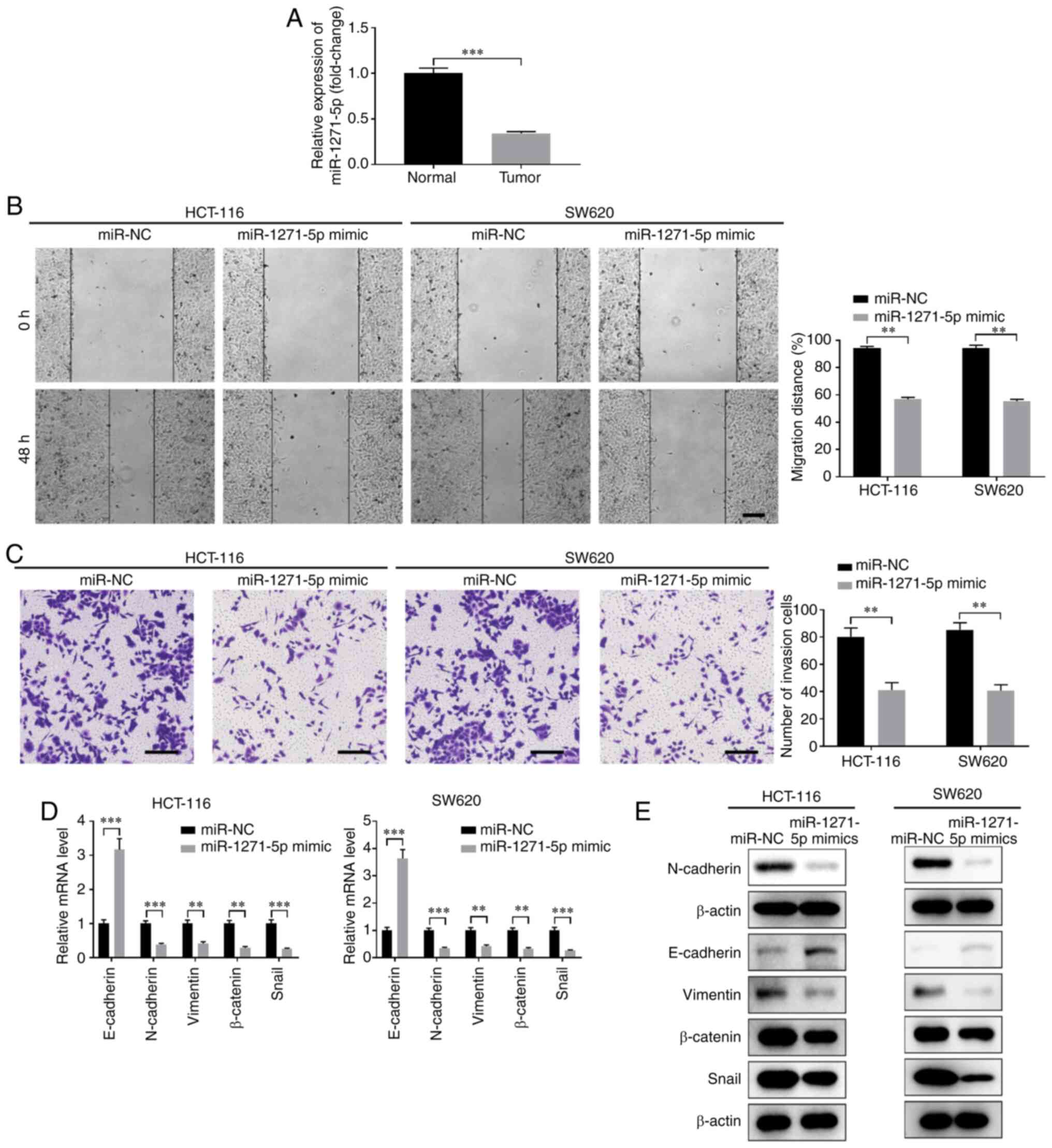
Next, the function of miR-1271-5p in CC development
was evaluated. The expression of miR-1271-5p was markedly lower in
CC tissues compared with that in normal tissues (Fig. 4A). Overexpression of miR-1271-5p
using miR-1271-5p mimics significantly inhibited the proliferation,
migration and invasion of HCT-116 and SW620 cells (Fig. 4B and C, Fig. S2C and D). Furthermore,
overexpression of miR-1271-5p suppressed EMT, with enhanced
expression of E-cadherin and decreased expression of N-cadherin,
vimentin, β-catenin and Snail at both the mRNA and protein levels
(Fig. 4D and E).
CD164 is a direct target of
miR-1271-5p
To explore how miR-1271-5p regulates CC metastasis,
bioinformatics analysis was performed and the targets of
miR-1271-5p were predicted by TargetScan. As shown in Fig. 5A, miR-1271-5p had the complementary
binding sequences targeting the 3′-UTR of CD164. Luciferase
reporter assay demonstrated that miR-1271-5p specifically inhibited
the luciferase activity in HCT-116 cells transfected with reporter
vector containing the Wt 3′-UTR of CD164, but not in HCT-116 cells
transfected with reporter vector containing the Mut 3′-UTR of CD164
(Fig. 5B). Immunohistochemical
staining of CD164 was performed, and the results revealed that
CD164 was highly expressed in CC tissues compared with that in
non-tumor control tissues (Fig. 5C and
D). It was also confirmed that CD164 expression was
significantly higher in CC cell lines compared with that in FHC
control cells, indicating that the expression of CD164 was
negatively correlated with miR-1271-5p expression (Fig. 5E). Furthermore, overexpression of
miR-1271-5p was able to suppress the expression of CD164 in HCT-116
or SW620 cells (Fig. 5F),
validating that CD164 was a direct target of miR-1271-5p. Moreover,
while knockdown of RP11-619L19.2 inhibited CD164 expression and
inhibition of miR-1271-5p enhanced CD164 expression in HCT-116 or
SW620 cells, inhibition of miR-1271-5p antagonized the inhibitory
effect of RP11-619L19.2 knockdown (Fig.
5G).
Overexpression of CD164 reverses the
antimetastatic activity of RP11-619L19.2 knockdown in CC cells
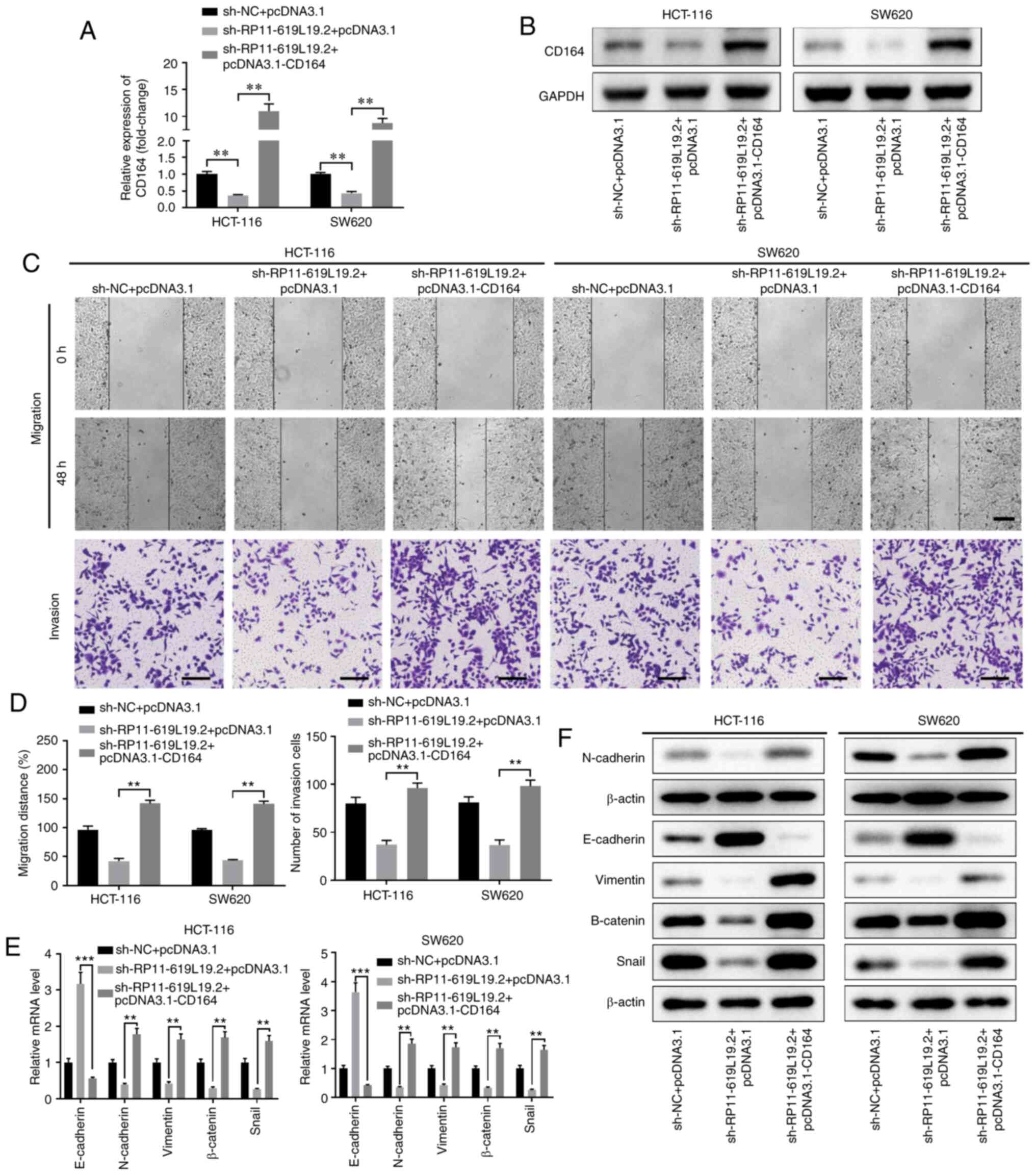
To determine whether RP11-619L19.2 exerted its
effects through the miR-1271-5p/CD164 axis in CC cells, rescue
experiments were performed. CD164 overexpression reversed the
inhibitory effect of RP11-619L19.2 knockdown on CD164 expression
(Fig. 6A and B). Functionally,
while sh-RP11-619L19.2 inhibited CC cell prolifer-ation, migration
and invasion, overexpression of CD164 together with RP11-619L19.2
antagonized the inhibition mediated by RP11-619L19.2 knockdown
(Fig. 6C and D, Fig. S2E and F).
Moreover, overexpression of CD164 promoted EMT, with
decreased expression of E-cadherin and enhanced expression of
N-cadherin, vimentin, β-catenin and Snail at both the mRNA and
protein levels (Fig. 6E and F).
Taken together, these results indicated that the antimetastatic
activity of RP11-619L19.2 knockdown in CC cells was mediated by
CD164.
Discussion
Genomic and transcriptomic analysis by
next-generation sequencing has led to a well-characterized
molecular profiling of CC. Moreover, numerous lncRNAs have been
found to be dysregulated in CC (23,24).
We also screened for lncRNAs with altered expression in CC tissues
in the present study (data not shown). To the best of our
knowledge, this is the first study to report that lncRNA
RP11-619L19.2 is highly expressed in CC tissues and cell lines.
Furthermore, the expression level of RP11-619L19.2 was found to be
positively correlated with advanced TNM stage and lymph node
metastasis of CC. Functionally, knockdown of RP11-619L19.2
inhibited cell proliferation, migration, invasion and EMT, which
indicated that RP11-619L19.2 may act as an oncogene promoting CC
development.
As there are only few studies investigating the
function and mechanism of action of RP11-619L19.2 in CC, we
performed bioinformatics analysis using DIANA tools to identify the
potential miRNAs involved in the regulation of RP11-619L19.2
expression. RP11-619L19.2 was shown to act as a miR-1271-5p sponge,
and there was a reciprocal repression between the expression of
RP11-619L19.2 and that of miR-1271-5p. miR-1271-5p has been
reported as a tumor suppressor in several different types of
tumors. Chen et al reported that miR-1271-5p suppressed cell
proliferation and induced cell apoptosis via regulating ZIC2 in
acute myeloid leukemia (25). In
another study, miR-1271-5p negatively regulated FOXK2 and inhibited
cell growth and hepatocellular carcinoma development (26). lncRNA UCA was demonstrated to
regulate the miR-1271-5p/HGF axis in multiple myeloma by
controlling cell apoptosis and proliferation (27). Our findings revealed that
miR-1271-5p also acted as a tumor suppressor in CC, while
overexpression of miR-1271-5p suppressed cell proliferation,
migration, invasion and EMT.
To further address the mechanism underlying the role
of miR-1271-5p in CC, CD164 was identified as a direct target of
miR-1271-5p by bioinformatics analysis. CD164 belongs to the
sialomucin family, which regulates cell proliferation, adhesion and
migration of hematopoietic progenitor cells (28). In addition, CD164 has been reported
to regulate tumor development and progression (29). Tang et al reported that
inhibition of CD164 resulted in suppressed tumor cell
proliferation, mobility and metastasis in CC cell lines (30). Furthermore, CD164 has been
identified as a new biomarker in acute lymphoblastic leukemia
(31). The present study also
demonstrated that CD164 had a function similar to that of
RP11-619L19.2, promoting cell proliferation, migration, invasion,
and EMT in CC cell lines.
Although there was sufficient evidence to support
the presence of an interaction network involving RP11-619L19.2,
miR-1271-5p and CD164 in CC, whether other miRNAs may also interact
with RP11-619L19.2 and whether miR-1271-5p controls multiple
targets besides CD164 were not fully addressed. Furthermore, it was
demonstrated that RP11-169L192 and CD164 acted as oncogenes, while
miR-1271-5p acted as a tumor suppressor in regulating CC cell
migration, invasion and EMT; however, in vivo CC models must
be employed to further investigate the function of the
RP11-619L19.2/miR-1271-5p/CD164 axis.
In conclusion, the results of the present study
revealed that lncRNA RP11-619L19.2 functions as a ceRNA in
regulating miR-1271-5p/CD164 and controlling CC cell migration and
invasion. These findings provide new insight into the
lncRNA/miRNA/mRNA network in CC, which may represent a novel
diagnostic and predictive biomarker, as well as a target for the
treatment of patients with CC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
and Development Project of Shaanxi Province (grant no.
S2018YBSF0274).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
XZ, XS and HZ conceived and designed the
experiments; XZ, SL and DZ performed the experiments; XZ and DZ
analyzed and interpreted the data; XZ wrote the manuscript; XS and
HZ revised the manuscript. All the authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and the study protocol was approved by the Ethics
Committee of The Second Affiliated Hospital of Xi'an Jiaotong
University.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi RE and Corcione F: Worldwide burden of colorectal cancer: A
review. Updates Surg. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang ML, Peng P, Wu CX, Gong YM, Zhang
SW, Chen WQ and Bao PP: Report of breast cancer incidence and
mortality in China registry regions, 2008–2012. Zhonghua Zhong Liu
Za Zhi. 41:315–320. 2019.(In Chinese). PubMed/NCBI
|
|
4
|
Telang NT, Li G and Katdare M: Prevention
of early-onset familial/hereditary colon cancer: New models and
mechanistic biomarkers (R-eview). Int J Oncol. 28:1523–1529.
2006.PubMed/NCBI
|
|
5
|
Yang S, Sun Z, Zhou Q, Wang W, Wang G,
Song J, Li Z, Zhang Z, Chang Y, Xia K, et al: MicroRNAs, long
noncoding RNAs, and circular RNAs: Potential tumor biomarkers and
targets for colorectal cancer. Cancer Manag Res. 10:2249–2257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue M, Zhuo Y and Shan B: MicroRNAs, long
noncoding RNAs, and their functions in human disease. Methods Mol
Biol. 1617:1–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renganathan A and Felley-Bosco E: Long
Noncoding RNAs in Cancer and Therapeutic Potential. Adv Exp Med
Biol. 1008:199–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang G, Pian C, Chen Z, Zhang J, Xu M,
Zhang L and Chen Y: Identification of cancer-related miRNA-lncRNA
biomarkers using a basic miRNA-lncRNA network. PLoS One.
13:e01966812018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hon KW, Abu N, Ab Mutalib NS and Jamal R:
miRNAs and lncRNAs as predictive biomarkers of response to FOLFOX
therapy in colorectal cancer. Front Pharmacol. 9:8462018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao M and Li W: Transcriptional factor
regulation network and competitive endogenous RNA (ceRNA) network
determining response of esophageal squamous cell carcinomas to
neoadjuvant chemoradiotherapy. PeerJ. 7:e66682019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Li H, Zheng B, Sun L, Yuan Y and
Xing C: Competitive endogenous RNA (ceRNA) regulation network of
lncRNA-miRNA-mRNA in colorectal carcinogenesis. Dig Dis Sci.
64:1868–1877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou RS, Zhang EX, Sun QF, Ye ZJ, Liu JW,
Zhou DH and Tang Y: Integrated analysis of lncRNA-miRNA-mRNA ceRNA
network in squamous cell carcinoma of tongue. BMC Cancer.
19:7792019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng Y, Geng L, Wang K, Sun J, Xu W, Gong
S and Zhu Y: Long noncoding RNA expression signatures of colon
cancer based on the ceRNA network and their prognostic value. Dis
Markers. 2019:76367572019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Z, Fu P, Yu Z, Zhen F and Gu Y:
Comprehensive Analysis of lncRNA-miRNA- mRNA network ascertains
prognostic factors in patients with colon cancer. Technol Cancer
Res Treat. 18:15330338198532372019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang XJ, Wang W and Hann SS: Interactions
among lncRNAs, miRNAs and mRNA in colorectal cancer. Biochimie.
163:58–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi L, Hong X, Ba L, He X, Xiong Y, Ding
Q, Yang S and Peng G: Long non-coding RNA ZNFX1-AS1 promotes the
tumor progression and metastasis of colorectal cancer by acting as
a competing endogenous RNA of miR-144 to regulate EZH2 expression.
Cell Death Dis. 10:1502019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Y, Nangia-Makker P, Farhana L and
Majumdar APN: A novel mechanism of lncRNA and miRNA interaction:
CCAT2 regulates miR-145 expression by suppressing its maturation
process in colon cancer cells. Mol Cancer. 16:1552017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang AF, Chen MW, Huang SM, Kao CL, Lai
HC and Chan JY: CD164 regulates the tumorigenesis of ovarian
surface epithelial cells through the SDF-1α/CXCR4 axis. Mol Cancer.
12:1152013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Reczko M, Maragkakis M, Dalamagas TM and Hatzigeorgiou
AG: DIANA-LncBase: Experimentally verified and computationally
predicted microRNA targets on long non-coding RNAs. Nucleic Acids
Res. 41:D239–D245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marisa L, de Reynies A, Duval A, Selves J,
Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D,
Ayadi M, et al: Gene expression classification of colon cancer into
molecular subtypes: Characterization, validation, and prognostic
value. PLoS Med. 10:e10014532013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Xu J, Hong J, Tang R, Zhang X and
Fang JY: Long noncoding RNA profiles identify five distinct
molecular subtypes of colorectal cancer with clinical relevance.
Mol Oncol. 8:1393–1403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Yang S, Zeng J and Chen M:
miR12715p inhibits cell proliferation and induces apoptosis in
acute myeloid leukemia by targeting ZIC2. Mol Med Rep. 19:508–514.
2019.PubMed/NCBI
|
|
26
|
Lin MF, Yang YF, Peng ZP, Zhang MF, Liang
JY, Chen W, Liu XH and Zheng YL: FOXK2, regulted by miR-1271-5p,
promotes cell growth and indicates unfavorable prognosis in
hepatocellular carcinoma. Int J Biochem Cell Biol. 88:155–161.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y and Chen L: Downregulation of
lncRNA UCA1 facilitates apoptosis and reduces proliferation in
multiple myeloma via regulation of the miR-1271-5p/HGF axis. J Chin
Med Assoc. 82:699–709. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doyonnas R, Yi-Hsin Chan J, Butler LH,
Rappold I, Lee-Prudhoe JE, Zannettino AC, Simmons PJ, Bühring HJ,
Levesque JP and Watt SM: CD164 monoclonal antibodies that block
hemopoietic progenitor cell adhesion and proliferation interact
with the first mucin domain of the CD164 receptor. J Immunol.
165:840–851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin J, Xu K, Wei J, Heimberger AB, Roth JA
and Ji L: MicroRNA-124 suppresses tumor cell proliferation and
invasion by targeting CD164 signaling pathway in non-small cell
lung cancer. J Gene Ther. 2:62016.PubMed/NCBI
|
|
30
|
Tang J, Zhang L, She X, Zhou G, Yu F,
Xiang J and Li G: Inhibiting CD164 expression in colon cancer cell
line HCT116 leads to reduced cancer cell proliferation, mobility,
and metastasis in vitro and in vivo. Cancer Invest. 30:380–389.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coustan-Smith E, Song G, Clark C, Key L,
Liu P, Mehrpooya M, Stow P, Su X, Shurtleff S, Pui CH, et al: New
markers for minimal residual disease detection in acute
lymphoblastic leukemia. Blood. 117:6267–6276. 2011. View Article : Google Scholar : PubMed/NCBI
|