Hepatocellular carcinoma (HCC) is becoming a
critical malignancy affecting human health worldwide (1,2). The
5-year survival rate of patients with HCC is <12% (3,4).
Although surgical resection is the common therapeutic approach for
patients with HCC at an early stage, recurrence is observed in
>70% of patients within 5 years (5). Local metastasis and chemoresistance
remain the key causes of the poor prognosis of HCC (6).
It has been established that the coagulation and
fibrinolytic systems serve important roles in the human body by
maintaining the balance of the physiological coagulation process,
which is closely associated with liver functions, including
generation of clotting factors, and synthesis of fibrinolytic and
antifibrinolytic substances (7).
However, recent studies have indicated that both systems can also
influence the development and prognosis of HCC (8,9). It
has been demonstrated that elevated levels of serum urokinase
plasminogen activator (uPA) are associated with higher mortality of
patients with HCC after resection (8). The clinical course of certain tumor
types can be slowed down by protease inhibitors, which inhibit uPA
expression (9). Similarly, the
majority of patients with cancer exhibit some changes in the
coagulation and fibrinolytic systems (10,11).
However, it is unclear whether these changes have any effects on
the biological behavior of cancers and how they interact with each
other.
The blood coagulation and fibrinolytic systems are
mainly involved in maintaining a balance between thrombogenesis and
bleeding, and in keeping blood vessels unobstructed. However,
systemic activation of blood coagulation is also a prominent
characteristic of cancer, and various coagulation components have
been identified to be associated with malignant phenotypes
(10). For example, high fibrinogen
expression has been suggested to contribute to the poor prognosis
of patients with advanced cancer (11).
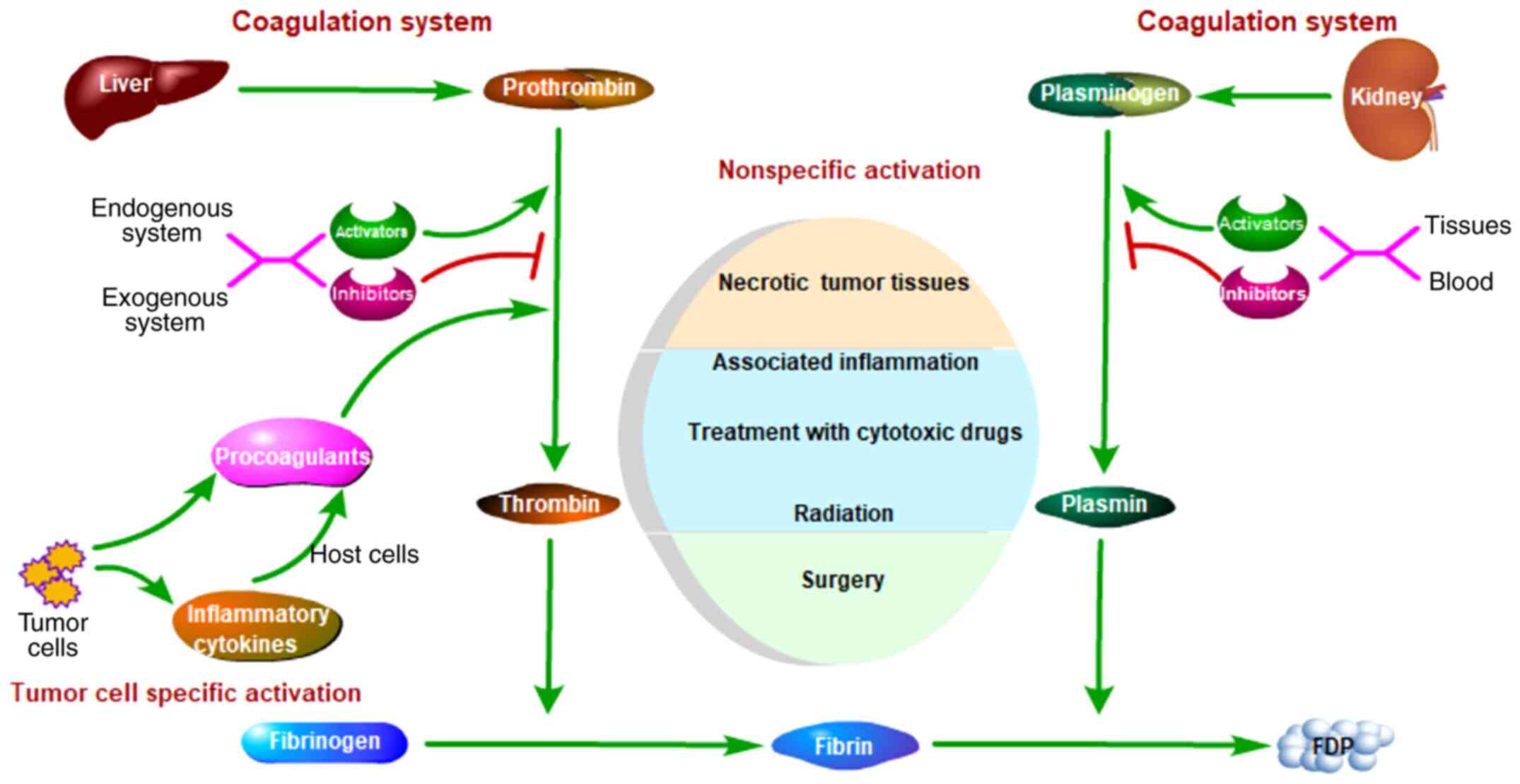
Coagulation activation in cancer can be classified
into two types: Non-specific and tumor cell-specific (Fig. 1). The causes of non-specific
activation include damaged tissues, inflammation, surgery,
chemotherapy drugs and radiation. The causes of tumor cell-specific
activation can be ‘direct’ or ‘indirect’. For example, tissue
factor (TF), which is expressed by tumor cells, can trigger
coagulation directly. Furthermore, certain cytokines, which are
expressed by tumor cells, may activate procoagulant expression of
endothelial cells, which can trigger coagulation indirectly.
Patients with tumors can be divided into three groups according to
specific coagulation activation. Certain patients exhibit
generation of thrombin and fibrin by tumor cells but minor systemic
coagulation activation, and these patients belong to the direct
activation group (12). Other
patients exhibit marked and deadly coagulation activation but small
tumor tissues, and these patients belong to the indirect activation
group (13). The remaining patients
who do not belong to either of these groups may exhibit only
limited activation of coagulation (14).
Animal models suggest that numerous drugs that
regulate coagulation activities also have antitumor effects
(15,16). These drugs can attenuate tumor
growth, decrease metastasis and prolong survival time. Furthermore,
the majority of the components associated with coagulation and
fibrinolysis contribute to HCC in some way (Table I).
Coagulation factor VII (FVII) combines with TF to
initiate the exogenous coagulation signaling pathway, which
activates the coagulation cascade and platelets (17). Upregulation of TF has been
identified in numerous types of cancer, including HCC. Zhou et
al (18) demonstrated that both
plasma and tissue samples from patients with HCC exhibited high
levels of TF, and this promoted invasion and metastasis via
regulation of the AKT/ERK-EGFR signaling pathway (19). Mouse models and clinical follow-up
revealed that TF expression is associated with angiogenesis and
invasiveness of HCC (20,21). The plasma levels of TF in patients
with HCC are associated with the degree of tumor differentiation,
tumor size and occurrence of hepatic cirrhosis (22).
Hepatic metastasis of colorectal cancer (CRC) is
closely associated with TF expression, and TF knockdown inhibits
hepatic metastasis in vitro and in vivo via
activation of apoptosis and autophagy (23,24).
Furthermore, a TF-FVIIa inhibitor (FFRFVIIa) was found to be able
to attenuate hepatic metastasis of CRC (25). Neaud et al (26) revealed that TF pathway inhibitor-2
could induce invasion of HCC cells following binding to the
TF-FVIIa complex, and this could be inhibited in a dose-dependent
manner by an antibody against human factor VII.
FVII/proteinase-activated receptor 2 (PAR2) promotes
migration of HCC cells via activation of the MEK/ERK, TSC complex
subunit 2 and mTOR signaling pathways (27). Additionally, Chen et al
(28) demonstrated that TF, FVII
and PAR2 increased the invasion and migration of HCC cells via mTOR
signaling to inhibit autophagy. Furthermore, Lin et al
indicated that the four common polymorphisms in the promoter region
of the FVII gene (−122T/C, −323ins10-bp, −401G/T and −402G/A) had
no effects on the incidence, survival and recurrence in patients
with HCC (29).
Coagulation factors VIII (FVIII) and XII (FXII) may
also contribute to HCC to some extent. Dihydrodiosgenin was found
to inhibit the lung metastasis of mouse HCC cells by decreasing
FVIII secretion via the PI3K, MAPK and NF-κB signaling pathways,
and this led to anoikis of HCC cells (30,31).
Astrocyte elevated gene-1 induced marked upregulation of FXII
levels, and increased angiogenesis and progression of HCC (32,33).
uPA, uPA receptor (uPAR), and plasminogen activator
inhibitor (PAI)-1 and PAI-2, are four components of the uPA system.
All of them may contribute to the metastasis of HCC.
Plasminogen can be converted to plasmin by uPA, and
promotes angiogenesis and tumor growth, degrading the extracellular
matrix (ECM) and activating pro-MMPs (34). Matrix metalloproteinases (MMPs),
particularly MMP-2 and MMP-9, are able to degrade type IV collagen
(an ECM component) to promote invasion and metastasis of cancer
(35). Previous studies have
demonstrated that uPA could activate MMP-2/MMP-9 to enhance the
invasion and migration of HCC (36,37).
Higher expression levels of uPA have been observed
in aggressive HCC cells compared with levels in HCC cells with low
levels of invasiveness (9).
Patients with HCC, particularly those with a portal tumor embolus
and metastasis, also exhibit high levels of uPA and uPAR. The
positive rates of uPA and uPAR in patients with HCC were found to
be 72 and 86%, respectively, and a positive rate of uPAR of 100%
was observed in patients who died within 2 years after surgery
(8). Phosphorylation of ERK1/2 and
PI3K/Akt, and activation of the protein tyrosine kinase 2 (FAK),
NF-κB and STAT3 signaling pathways may contribute to these
processes (38,39). Hepatocyte growth factor (HGF) was
demonstrated to promote the invasion of HCC cells by enhancing the
levels of uPA and uPAR. Monoclonal antibodies against uPAR were
found to inhibit tumor cell invasion mediated by HGF in a
dose-dependent manner (40).
Bicyclol inhibited the invasiveness of HCC cells by decreasing the
expression levels of uPAR (41). A
novel inhibitor of uPA, serine peptidase inhibitor Kazal type 13,
also inhibited intrahepatic metastasis of HCC in vivo
(9).
PAI-1 and PAI-2 are regulators of uPA and uPAR
involved in activating plasminogen, initiating signal transduction
and inducting cell chemotaxis (42). High PAI-1 levels in tumors are
associated with poor prognosis; however, the PAI-2 level may be
associated with a beneficial prognosis (43,44).
In a Taiwanese population, PAI-1 genotypes were considered as a
critical factor which led to higher susceptibility and pathological
development in patients with HCC (45). Furthermore, patients with CRC with
liver metastasis were demonstrated to have higher plasma levels of
PAI-1, and the levels were associated with tumor differentiation,
tumor size, Duke's stage and lymphatic metastasis (46). Elevated plasma levels of PAI-1 have
been suggested to be a predictor of unfavorable prognosis in
patients with HCC with serpin family E member 1 4G/4G polymorphism
undergoing transarterial chemoembolization (47).
Other studies have made opposite observations
regarding the effects of PAI-1 on HCC. Wang et al (48) revealed that berberine could trigger
cell apoptosis by generating reactive oxygen species, and could
inhibit the migration and invasion of HCC cells. This may be
associated with the upregulation of PAI-1, which could decrease the
expression levels of cyclooxygenase-2 (Cox-2), NF-κB, uPA and uPAR
via inactivation of the p38 and ERK1/2 signaling pathways (48).
There is limited research on PAI-2 in HCC. PAI-2 was
found to inhibit invasion of HCC MHCC97-H cells via uPA- and
retinoblastoma/transcription factor E2F1-associated mechanisms
(49).
DCP is produced in HCC cells in the absence of
vitamin K or the presence of vitamin K antagonists, and may
stimulate growth, invasion and metastasis of HCC. Previous research
has indicated that 44–81% of patients with HCC exhibit elevated
serum levels of DCP (50). DCP is
widely used as a serologic tumor marker for the diagnosis and
follow-up of HCC (51). Although
its sensitivity is lower than that of α-fetoprotein (AFP), it has a
higher specificity than AFP. A number of studies have suggested
that higher levels of DCP may be associated with poor tumor
behavior and prognosis of patients with HCC (52,53).
Additionally, positive serum DCP expression is closely associated
with vascular invasion, intrahepatic metastasis, TNM stage and
recurrence in patients with HCC (54).
DCP induces the proliferation of HCC cells via
activation of the MET proto-oncogene receptor tyrosine kinase
(Met)-Jak family tyrosine kinases-STAT signaling pathway (55). Furthermore, binding of DCP to c-Met
can activate various downstream effectors, such as
autophosphorylation of EGFR, to increase the proliferation and
angiogenesis of HCC (56). Yue
et al (57) suggested that
DCP could increase the activity of MMP-2/MMP-29 via activation of
the ERK1/2-MAPK signaling pathway to promote the growth and
metastasis of HCC.
It is crucial for DCP generation to decrease vitamin
K uptake through cytoskeletal changes in the process of
epithelial-to-fibroblastoid conversion induction by chemical
methods (58). Hypoxia can induce
the production of DCP in HCC cells in this way (59). Vitamin K2 can inhibit the generation
of DCP, and DCP levels can also be decreased by vitamin K2
analogues in patients with HCC (60,61).
In addition to the aforementioned components of the
coagulation and fibrinolytic systems, there are other factors that
also contribute to HCC to a certain extent.
Thrombomodulin (TM) acts as a natural anticoagulant
factor and is present at high levels in endothelial cells to
maintain unobstructed circulation. TM has been demonstrated to
serve roles in inflammation, thrombosis and carcinogenesis
(62). A study of TM in 141
patients with HCC who underwent surgery suggested that TM may
inhibit tumor cell invasion to the portal vein and prevent
intrahepatic metastasis (63).
Furthermore, silencing of TM expression markedly increased the
migration of HCC cells by decreasing E-cadherin levels and
increasing zinc finger E-box binding homeobox 1 (ZEB1) levels
(64).
Thrombin is a serine protease and serves multiple
roles in coagulation. A previous study revealed that thrombin could
induce changes in osteopontin (OPN) activity (a potential
therapeutic target for inhibiting HCC metastasis) (65). Furthermore, another study
demonstrated that these changes served a key role in the aggressive
phenotype of HCC mediated by OPN via activation of integrin β1-FAK,
and thrombin was associated with poor prognosis in HCC (66). Several thrombin inhibitors available
for clinical treatment, including non-specific anticoagulants and
thrombin-specific inhibitors, have been demonstrated to inhibit
metastasis in experimental models (10).
Anoikis is critical to ensure that cells contact the
ECM appropriately and to limit the invasiveness of cancer. However,
numerous cancer cells can survive by suppressing anoikis to
stimulate tissue invasion and to confer metastatic abilities to
cancer cells (67). Resistance to
anoikis is a prerequisite for the metastasis of epithelial cancer
cells (68,69). As a typical malignant tumor derived
from epithelial cells, HCC obtains the ability of anoikis
resistance to survive in the bloodstream, and to subsequently
metastasize and become more resistant to anticancer agents
(70).
Various coagulation components may be involved in
the anoikis resistance of HCC. Cellular communication network
factor 3 downstream genes TF and thrombin, which are positively
associated with the malignant phenotype of HCC and vascular
thrombosis, prolong survival time and improve pulmonary metastasis
in vivo. Mechanistically, ERK and NF-κB signaling are
activated in order to maintain cell survival by inhibiting anoikis
(71).
Dihydrodiosgenin inhibits lung metastasis of mouse
HCC cells by attenuating the adhesion of cancer cells and platelets
to endothelial cells by decreasing FVIII production. The direct
antitumor activity may involve the PI3K, MAPK and NF-κB signaling
pathways, which lead to anoikis of HCC cells (30,31).
Additionally, various coagulation-associated factors
contribute to anoikis or have anti-anoikis effects in other types
of cancer. Coagulation factor IXa was found to attenuate cell
adhesion to the matrix and induces anoikis of epidermal cancer
cells by increasing MAPK levels (72). Versteeg et al (73) observed that the TF-FVIIa complex
enhanced invasion and migration of baby hamster kidney cells via
inhibition of anoikis, which was mediated via the PI3K and MAPK
signaling pathways.
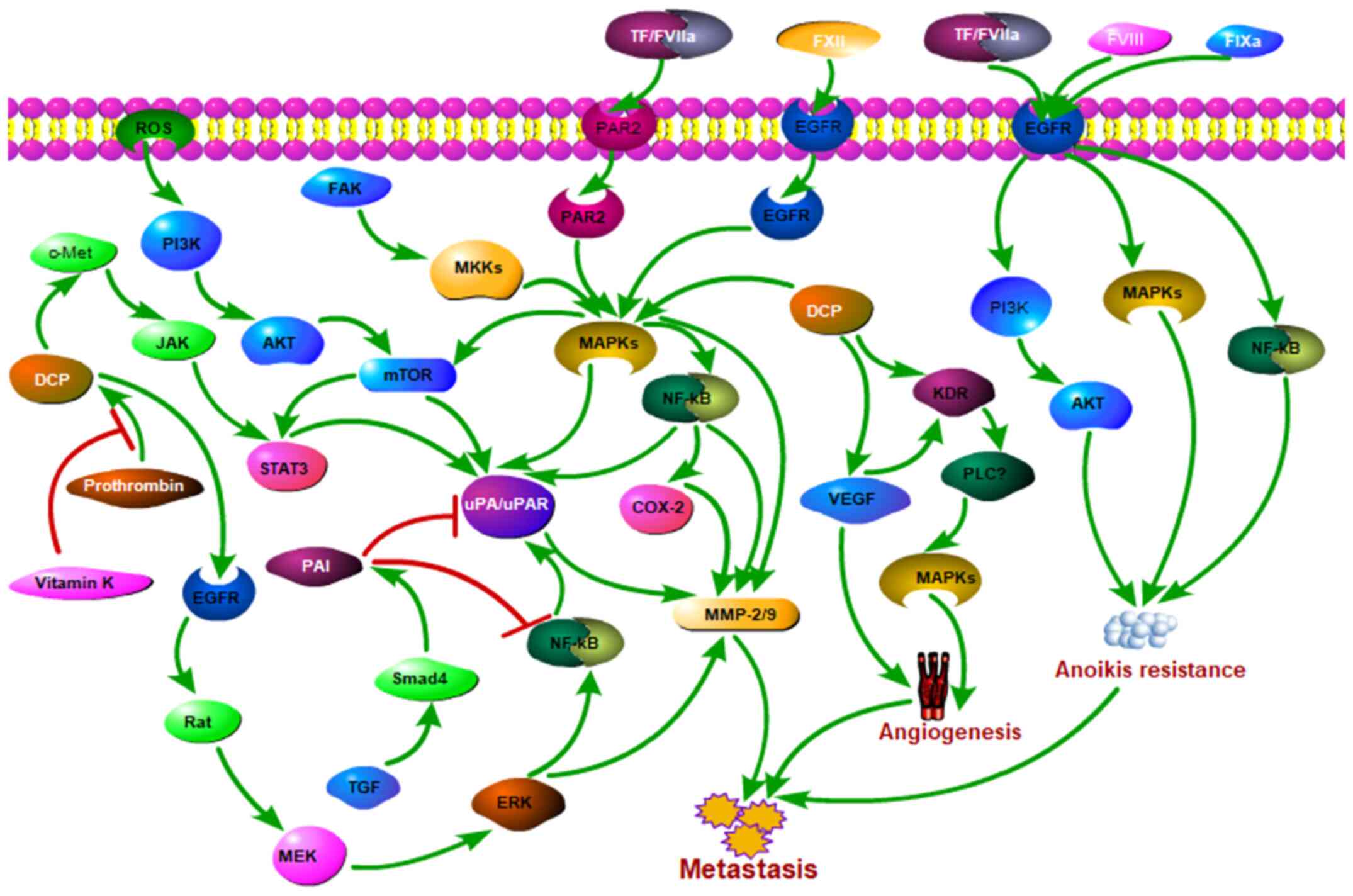
The liver, unlike other organs, is the main site of
coagulation and fibrinolytic factor production. Acquisition of
anoikis resistance is a prerequisite for cancer metastasis. In the
present review, it was speculated that coagulation- and
fibrinolysis-related factors may contribute to intrahepatic
metastasis of HCC via resistance to anoikis (Fig. 2).
Immunotherapy, particularly immune checkpoint
therapy targeting programmed death-ligand 1 (PD-L1), cytotoxic
T-lymphocyte associated protein 4 (CTLA-4), T-cell immunoglobulin
mucin family member 3 (TIM3) and lymphocyte activating 3 (LAG3),
serves an important role in tumor treatment. However, most solid
tumors only have a 15–30% effective response rate to immune
checkpoint inhibitors (74).
Additionally, anti-programmed cell death protein 1 (PD1) therapy
has been demonstrated to only have a 14.3% overall response rate
for the treatment of patients with advanced HCC who have been
previously treated with sorafenib (75). Studies have suggested that tumor
PD-L1 levels may be associated with the response to PD-L1/PD1
blockade (76,77). Previous studies have revealed that
low expression levels of PD-L1 in numerous types of HCC could be
induced by stimulation of interferon (INF)-γ, targeted therapies
and MYC activation, which decreased antitumor immunity and enhanced
HCC progression (78–81).
The majority of patients with tumors present with
hypercoagulability, and increasing evidence has demonstrated that
the coagulation and fibrinolytic systems are associated with tumor
progression. A common characteristic of HCC is intrahepatic
metastasis, which leads to a poor prognosis of patients with HCC.
Studies have demonstrated that coagulation and fibrinolytic factors
may regulate the metastasis of HCC in two ways. First, by
conferring anoikis resistance to HCC cells; second, by promoting
HCC cell escape from immune attacks. In conclusion, it not only
protects HCC cells themselves in terms of survival, but also
changes the tumor immune microenvironment, both of which eventually
lead to intrahepatic metastasis of HCC.
Various treatment methods currently exist for
advanced HCC. Molecular targeted therapy and immunotherapy have
become popular research topics. However, targeted therapy or
immunotherapy only show efficacy in certain patients with HCC,
while they have poor efficacy or are even ineffective in the
majority of patients. The authors of the present review are
currently registering a relevant clinical trial and establishing
related animal models, aiming to improve the efficacy of
immunotherapy by changing the coagulation and fibrinolysis status
of patients with HCC and exploring a specific molecular target, in
order to eventually improve the prognosis of patients with HCC.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (81670594, 81470791, 81376597);
the Basic Research Innovation Group Program of Gansu Province
(1606RJIA328); the Scientific Research of Health Services Program
of Gansu Province (GSWSKY2017-09); the Talent Staff Fund of the
Second Hospital of Lanzhou University (ynyjrckyzx2015-1-01);
Talents Innovation and Entrepreneurship Program of Lanzhou City
(2017-RC-62); the Cuiying Scientific and Technological Innovation
Program of Lanzhou University Second Hospital (CY2017-ZD01);
Fundamental Research Funds for the Central Universities
(lzujbky-2017-79); Key Project of Science and Technology in Gansu
Province (19ZD2WA001).
All information included in this Review is
documented by relevant and current references.
XL, BG and BW wrote the manuscript; XL, AL and HC
designed the paper; ZF, YM and HL revised the manuscript. All
authors read and approved the final manuscript and agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulik L and El-Serag HB: Epidemiology and
management of hepatocellular carcinoma. Gastroenterology.
56:477–491. 2019. View Article : Google Scholar
|
|
4
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roayaie S, Jibara G, Tabrizian P, Park JW,
Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M, et al: The role
of hepatic resection in the treatment of hepatocellular cancer.
Hepatology. 62:440–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabrizian P, Jibara G, Shrager B, Schwartz
M and Roayaie S: Recurrence of hepatocellular cancer after
resection: Patterns, treatments, and prognosis. Ann Surg.
261:947–955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanoh Y: Coagulation and fibrinolytic
system. Nihon Rinsho. 74 (Suppl 4):S301–S305. 2016.(In
Japanese).
|
|
8
|
Tsai MC, Yen YH, Chang KC, Hung CH, Chen
CH, Lin MT and Hu TH: Elevated levels of serum urokinase
plasminogen activator predicts poor prognosis in hepatocellular
carcinoma after resection. BMC Cancer. 19:11692019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei L, Lun Y, Zhou X, He S, Gao L, Liu Y,
He Z, Li B and Wang C: Novel urokinase-plasminogen activator
inhibitor SPINK13 inhibits growth and metastasis of hepatocellular
carcinoma in vivo. Pharmacol Res. 143:73–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zacharski LR: Anticoagulants in cancer
treatment: Malignancy as a solid phase coagulopathy. Cancer Lett.
186:1–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lip GY, Chin BS and Blann AD: Cancer and
the prothrombotic state. Lancet Oncol. 3:27–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zacharski LR, Wojtukiewicz MZ, Costantini
V, Ornstein DL and Memoli VA: Pathways of coagulation/fibrinolysis
activation in malignancy. Semin Thromb Hemost. 18:104–116. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Falanga A, Marchetti M and Vignoli A:
Coagulation and cancer: Biological and clinical aspects. J Thromb
Haemost. 11:223–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thodiyil PA and Kakkar AK: Variation in
relative risk of venous thromboembolism in different cancers.
Thromb Haemost. 87:1076–1077. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nadir Y: Decreasing tumor growth and
angiogenesis by inhibition of coagulation. Semin Thromb Hemost.
45:622–628. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H, Cao D, Liu Y, Wang H, Ke X and Ci
T: Low molecular weight heparin-based reduction-sensitive
nanoparticles for antitumor and anti-metastasis of orthotopic
breast cancer. Biomater Sci. 6:2172–2188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furie B and Furie BC: The molecular basis
of blood coagulation. Cell. 53:505–518. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Q, Huang T, Wang YF, Zhou XB, Liang
LJ and Peng BG: Role of tissue factor in hepatocellular carcinoma
genesis, invasion and metastasis. Chin Med J (Engl). 124:3746–3751.
2011.PubMed/NCBI
|
|
19
|
Huang SZ, Wei MN, Huang JR, Zhang ZJ,
Zhang WJ, Jiang QW, Yang Y, Wang HY, Jin HL, Wang K, et al:
Targeting TF-AKT/ERK-EGFR pathway suppresses the growth of
hepatocellular carcinoma. Front Oncol. 9:1502019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dupuy E, Hainaud P, Villemain A,
Bodevin-Phèdre E, Brouland JP, Briand P and Tobelem G: Tumoral
angiogenesis and tissue factor expression during hepatocellular
carcinoma progression in a transgenic mouse model. J Hepatol.
38:793–802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poon RT, Lau CP, Ho JW, Yu WC, Fan ST and
Wong J: Tissue factor expression correlates with tumor angiogenesis
and invasiveness in human hepatocellular carcinoma. Clin Cancer
Res. 9:5339–5345. 2003.PubMed/NCBI
|
|
22
|
Panasiuk A, Zak J, Panasiuk B and
Prokopowicz D: Increase in expression of monocytic tissue factor
(CD142) with monocytes and blood platelet activation in liver
cirrhosis. Blood Coagul Fibrinolysis. 18:739–744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian M, Wan Y, Tang J, Li H, Yu G, Zhu J,
Ji S, Guo H, Zhang N, Li W, et al: Depletion of tissue factor
suppresses hepatic metastasis and tumor growth in colorectal cancer
via the downregulation of MMPs and the induction of autophagy and
apoptosis. Cancer Biol Ther. 12:896–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Tian ML, Yu G, Liu YC, Wang X, Zhang
J, Ji SQ, Zhu J, Wan YL and Tang JQ: Hyperthermia synergizes with
tissue factor knockdown to suppress the growth and hepatic
metastasis of colorectal cancer in orthotopic tumor model. J Surg
Oncol. 106:689–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zerbib P, Grimonprez A, Corseaux D,
Mouquet F, Nunes B, Petersen LC, Susen S, Ung A, Hebbar M, Pruvot
FR, et al: Inhibition of tissue factor-factor VIIa proteolytic
activity blunts hepatic metastasis in colorectal cancer. J Surg
Res. 153:239–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Neaud V, Hisaka T, Monvoisin A, Bedin C,
Balabaud C, Foster DC, Desmoulière A, Kisiel W and Rosenbaum J:
Paradoxical pro-invasive effect of the serine proteinase inhibitor
tissue factor pathway inhibitor-2 on human hepatocellular carcinoma
cells. J Biol Chem. 275:35565–35569. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai MC, Chen KD, Wang CC, Huang KT, Wu
CH, Kuo IY, Chen LY, Hu TH, Goto S, Nakano T, et al: Factor VII
promotes hepatocellular carcinoma progression through ERK-TSC
signaling. Cell Death Discov. 1:150512015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen KD, Wang CC, Tsai MC, Wu CH, Yang HJ,
Chen LY, Nakano T, Goto S, Huang KT, Hu TH, et al: Interconnections
between autophagy and the coagulation cascade in hepatocellular
carcinoma. Cell Death Dis. 5:e12442014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CC, Wu CH, Chen LY, Tsai MC, Elsarawy
AM and Huang KT: Coagulation factor VII gene polymorphisms are not
associated with the occurrence or the survival of hepatocellular
carcinoma: A report of 37 cases. Cancer Biol Med. 15:275–281. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Wang X, Cheng S, Du J, Deng Z, Zhang
Y, Liu Q, Gao J, Cheng B and Ling C: Diosgenin induces G2/M cell
cycle arrest and apoptosis in human hepatocellular carcinoma cells.
Oncol Rep. 33:693–698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuang M, Xin G, Wei Z, Li S, Xing Z, Ji
C, Du J, Niu H and Huang W: Dihydrodiosgenin inhibits endothelial
cell-derived factor VIII and platelet-mediated hepatocellular
carcinoma metastasis. Cancer Manag Res. 11:4871–4882. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J,
Shah K, Fisher PB and Sarkar D: Molecular mechanism of
chemoresistance by astrocyte elevated gene-1. Cancer Res.
70:3249–3258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Srivastava J, Siddiq A, Emdad L,
Santhekadur PK, Chen D, Gredler R, Shen XN, Robertson CL, Dumur CI,
Hylemon PB, et al: Astrocyte elevated gene-1 promotes
hepatocarcinogenesis: Novel insights from a mouse model.
Hepatology. 56:1782–1791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Danø K, Andreasen PA, Grøndahl-Hansen J,
Kristensen P, Nielsen LS and Skriver L: Plasminogen activators,
tissue degradation, and cancer. Adv Cancer Res. 44:139–266. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL,
Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ, et al: Lentiviral-mediated
miRNA against osteopontin suppresses tumor growth and metastasis of
human hepatocellular carcinoma. Hepatology. 48:1834–1842. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)-alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai JP, Hsiao PC, Yang SF, Hsieh SC, Bau
DT, Ling CL, Pai CL and Hsieh YH: Licochalcone a suppresses
migration and invasion of human hepatocellular carcinoma cells
through downregulation of MKK4/JNK via NF-κB mediated urokinase
plasminogen activator expression. PLoS One. 9:e865372014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weng CJ, Chou CP, Ho CT and Yen GC:
Molecular mechanism inhibiting human hepatocarcinoma cell invasion
by 6-shogaol and 6-gingerol. Mol Nutr Food Res. 56:1304–1314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN,
Lee HJ, Eun JY, Kim HG, Yoon SS, Lee DS, et al: Role of hepatocyte
growth factor/c-Met signaling in regulating urokinase plasminogen
activator on invasiveness in human hepatocellular carcinoma: A
potential therapeutic target. Clin Exp Metastasis. 25:89–96. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun H and Liu GT: Inhibitory effect of
anti-hepatitis drug bicyclol on invasion of human hepatocellular
carcinoma MHCC97-H cells with high metastasis potential and its
relative mechanisms. J Asian Nat Prod Res. 11:576–583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blasi F and Carmeliet P: uPAR: A versatile
signalling orchestrator. Nat Rev Mol Cell Biol. 3:932–943. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sakakibara T, Hibi K, Koike M, Fujiwara M,
Kodera Y, Ito K and Nakao A: Plasminogen activator inhibitor-1 as a
potential marker for the malignancy of colorectal cancer. Br J
Cancer. 93:799–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou L, Jin Y, Cui QC, Jin KM, Zhou WX and
Xing BC: Low expression of PAI-2 as a novel marker of portal vein
tumor thrombosis and poor prognosis in hepatocellular carcinoma.
World J Surg. 37:608–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weng CJ, Tsai CM, Chen YC, Hsieh YH, Lin
CW, Liu YF, Su SC, Chen MK and Yang SF: Evaluation of the
association of urokinase plasminogen activator system gene
polymorphisms with susceptibility and pathological development of
hepatocellular carcinoma. Ann Surg Oncol. 17:3394–3401. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen H, Peng H, Liu W, Sun Y, Su N, Tang
W, Zhang X, Wang J, Cui L, Hu P, et al: Silencing of plasminogen
activator inhibitor-1 suppresses colorectal cancer progression and
liver metastasis. Surgery. 158:1704–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Divella R, Daniele A, Abbate I, Savino E,
Casamassima P, Sciortino G, Simone G, Gadaleta-Caldarola G, Fazio
V, Gadaleta CD, et al: Circulating levels of PAI-1 and SERPINE1
4G/4G polymorphism are predictive of poor prognosis in HCC patients
undergoing TACE. Transl Oncol. 8:273–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Wang N, Li H, Liu M, Cao F, Yu X,
Zhang J, Tan Y, Xiang L and Feng Y: Up-Regulation of PAI-1 and
down-regulation of uPA are involved in suppression of invasiveness
and motility of hepatocellular carcinoma cells by a natural
compound berberine. Int J Mol Sci. 17:5772016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jin Y, Liang ZY, Zhou WX and Zhou L:
Plasminogen activator inhibitor 2 (PAI2) inhibits invasive
potential of hepatocellular carcinoma cells in vitro via uPA- and
RB/E2F1-related mechanisms. Hepatol Int. 13:180–189. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pang RW, Joh JW, Johnson PJ, Monden M,
Pawlik TM and Poon RT: Biology of hepatocellular carcinoma. Ann
Surg Oncol. 15:962–971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kudo A, Shinoda M, Ariizumi S, Kumamoto T,
Katayama M, Otsubo T, Endo I, Kitagawa Y, Tanabe Y, Yamamoto M, et
al: Des-gamma-carboxy prothrombin affects the survival of HCC
patients with marginal liver function and curative treatment:
ACRoS1402. J Cancer Res Clin Oncol. 2020.(Epub ahead of print).
View Article : Google Scholar
|
|
52
|
Li Y and Chen J: Serum des-gamma-carboxy
prothrombin for diagnosis of adult primary cancer in liver. J Coll
Physicians Surg Pak. 29:972–976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cai Z, Chen G, Zeng Y, Dong X, Li Z, Huang
Y, Xin F, Qiu L, Xu H, Zhang W, et al: Comprehensive liquid
profiling of circulating tumor DNA and protein biomarkers in
long-term follow-up patients with hepatocellular carcinoma. Clin
Cancer Res. 25:5284–5294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang W, Miki K, Kokudo N, Sugawara Y,
Imamura H, Minagawa M, Yuan LW, Ohnishi S and Makuuchi M:
Des-gamma-carboxy prothrombin in cancer and non-cancer liver tissue
of patients with hepatocellular carcinoma. Int J Oncol. 22:969–975.
2003.PubMed/NCBI
|
|
55
|
Suzuki M, Shiraha H, Fujikawa T, Takaoka
N, Ueda N, Nakanishi Y, Koike K, Takaki A and Shiratori Y:
Des-gamma-carboxy prothrombin is a potential autologous growth
factor for hepatocellular carcinoma. J Biol Chem. 280:6409–6415.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gao FJ, Cui SX, Chen MH, Cheng YN, Sun LR,
Ward SG, Kokudo N, Tang W and Qu XJ: Des-gamma-carboxy prothrombin
increases the expression of angiogenic factors in human
hepatocellular carcinoma cells. Life Sci. 83:815–820. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yue P, Gao ZH, Xue X, Cui SX, Zhao CR,
Yuan Y, Yin Z, Inagaki Y, Kokudo N, Tang W and Qu XJ:
Des-γ-carboxyl prothrombin induces matrix metalloproteinase
activity in hepatocellular carcinoma cells by involving the ERK1/2
MAPK signalling pathway. Eur J Cancer. 47:1115–1124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Murata K and Sakamoto A: Impairment of
clathrin-mediated endocytosis via cytoskeletal change by epithelial
to fibroblastoid conversion in HepG2 cells: A possible mechanism of
des-gamma-carboxy prothrombin production in hepatocellular
carcinoma. Int J Oncol. 33:1149–1155. 2008.PubMed/NCBI
|
|
59
|
Murata K, Suzuki H, Okano H, Oyamada T,
Yasuda Y and Sakamoto A: Hypoxia-induced des-gamma-carboxy
prothrombin production in hepatocellular carcinoma. Int J Oncol.
36:161–170. 2010.PubMed/NCBI
|
|
60
|
Mizuta T, Ozaki I, Eguchi Y, Yasutake T,
Kawazoe S, Fujimoto K and Yamamoto K: The effect of menatetrenone,
a vitamin K2 analog, on disease recurrence and survival in patients
with hepatocellular carcinoma after curative treatment: A pilot
study. Cancer. 106:867–872. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Otsuka M, Kato N, Shao RX, Hoshida Y,
Ijichi H, Koike Y, Taniguchi H, Moriyama M, Shiratori Y, Kawabe T
and Omata M: Vitamin K2 inhibits the growth and invasiveness of
hepatocellular carcinoma cells via protein kinase A activation.
Hepatology. 40:243–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi CS, Shi GY, Hsiao HM, Kao YC, Kuo KL,
Ma CY, Kuo CH, Chang BI, Chang CF, Lin CH, et al: Lectin-like
domain of thrombomodulin binds to its specific ligand Lewis Y
antigen and neutralizes lipopolysaccharide-induced inflammatory
response. Blood. 112:3661–3670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Suehiro T, Shimada M, Matsumata T,
Taketomi A, Yamamoto K and Sugimachi K: Thrombomodulin inhibits
intrahepatic spread in human hepatocellular carcinoma. Hepatology.
21:1285–1290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang MT, Wei PL, Liu JJ, Liu DZ,
Huey-Chun H, An J, Wu CC, Wu CH, Ho YS, Yang YY and Chang YJ:
Knockdown of thrombomodulin enhances HCC cell migration through
increase of ZEB1 and decrease of E-cadherin gene expression. Ann
Surg Oncol. 17:3379–3385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hasegawa M, Nakoshi Y, Iino T, Sudo A,
Segawa T, Maeda M, Yoshida T and Uchida A: Thrombin-cleaved
osteopontin in synovial fluid of subjects with rheumatoid
arthritis. J Rheumatol. 36:240–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xue YH, Zhang XF, Dong QZ, Sun J, Dai C,
Zhou HJ, Ren N, Jia HL, Ye QH and Qin LX: Thrombin is a therapeutic
target for metastatic osteopontin-positive hepatocellular
carcinoma. Hepatology. 52:2012–2022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liotta LA and Kohn E: Anoikis: Cancer and
the homeless cell. Nature. 430:973–974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kim YN, Koo KH, Sung JY, Yun UJ and Kim H:
Anoikis resistance: An essential prerequisite for tumor metastasis.
Int J Cell Biol. 2012:3068792012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang X, Cheng SL, Bian K, Wang L, Zhang
X, Yan B, Jia LT, Zhao J, Gammoh N, Yang AG and Zhang R:
MicroRNA-26a promotes anoikis in human hepatocellular carcinoma
cells by targeting alpha5 integrin. Oncotarget. 6:2277–2289. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jia Q, Xue T, Zhang Q, Cheng W, Zhang C,
Ma J, Bu Y, Yu S and Liu Q: CCN3 is a therapeutic target relating
enhanced stemness and coagulation in hepatocellular carcinoma. Sci
Rep. 7:138462017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ishikawa T, Kitano H, Mamiya A, Kokubun S
and Hidai C: The first EGF domain of coagulation factor IX
attenuates cell adhesion and induces apoptosis. Biosci Rep.
36:e003402016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Versteeg HH, Spek CA, Richel DJ and
Peppelenbosch MP: Coagulation factors VIIa and Xa inhibit apoptosis
and anoikis. Oncogene. 23:410–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7:3062019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang J, Bu X, Wang H, Zhu Y, Geng Y,
Nihira NT, Tan Y, Ci Y, Wu F, Dai X, et al: Cyclin D-CDK4 kinase
destabilizes PD-L1 via cullin 3-SPOP to control cancer immune
surveillance. Nature. 553:91–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Casey SC, Tong L, Li Y, Do R, Walz S,
Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M and Felsher
DW: MYC regulates the antitumor immune response through CD47 and
PD-L1. Science. 352:227–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li N, Wang J, Zhang N, Zhuang M, Zong Z,
Zou J, Li G, Wang X, Zhou H, Zhang L and Shi Y: Cross-talk between
TNF-α and IFN-γ signaling in induction of B7-H1 expression in
hepatocellular carcinoma cells. Cancer Immunol Immunother.
67:271–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiang J, Zhang N, Sun H, Su L, Zhang C, Xu
H, Feng J, Wang M, Chen J, Liu L, et al: Disruption of SIRT7
increases the efficacy of checkpoint inhibitor via MEF2D regulation
of programmed cell Death 1 Ligand 1 in hepatocellular carcinoma
cells. Gastroenterology. 158:664–678.e24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan
AWH, Tong JH, Wong J, Chong CCN, Lai PBS, et al: Hepatoma-intrinsic
CCRK inhibition diminishes myeloid-derived suppressor cell
immunosuppression and enhances immune-checkpoint blockade efficacy.
Gut. 67:931–944. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nakagawa H, Umemura A, Taniguchi K,
Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E,
Hidalgo J, et al: ER stress cooperates with hypernutrition to
trigger TNF-dependent spontaneous HCC development. Cancer Cell.
26:331–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Febbraio MA, Reibe S, Shalapour S, Ooi GJ,
Watt MJ and Karin M: Preclinical models for studying NASH-Driven
HCC: How useful are they? Cell Metab. 29:18–26. 2019. View Article : Google Scholar : PubMed/NCBI
|