Introduction
Propyl gallate (PG; 3,4,5-trihydroxybenzoic acid
propyl ester), a synthetic antioxidant, has been utilized for
decades as an additive for processed food, cosmetics, food packing,
and the pharmaceutical industry since it can be used to avoid
spoilage and decomposition (1).
Although PG is reported to have low toxicity, it has various
beneficial properties for tissue and cell functions. For example,
PG is an efficient protector of liver cells from lipid peroxidation
by oxygen radicals (2). In
addition, numerus studies have revealed that PG functions as an
antioxidant and a chemopreventive agent in vivo and in
vitro (3–5). In contrast, PG also exerts some
prooxidant effects (6,7). In addition, PG mediates its
cytotoxicity through the impediment of mitochondrial function in
hepatocytes, endothelial cells and testicular cells (8–10). PG
decreases the growth of microorganisms by constraining respiration
and nucleic acid synthesis (11).
Therefore, in order to clarify the discrepancies between the
different effects of PG as an antioxidant or a prooxidant, further
studies are required to re-evaluate its function and effects on
cells and tissues.
The cell cycle is the series of events that takes
place in a cell. The eukaryotic cell cycle consists of four
distinct phases: The G1 phase, S phase, G2 phase and M phase
(12,13). Proper progression throughout the
cell cycle depends on the expression level of a family of cyclins,
and the subsequent activation of cyclin-dependent kinases (CDKs)
(12). Regulation of the cell cycle
involves procedures crucial to cell survival, including the
recognition and repair of genetic damage as well as the prevention
of uncontrolled cell division (12,13).
Apoptosis is a cellular response to cytotoxicological drugs
(14,15). The signaling mechanism of apoptosis
commonly consists of two pathways: The mitochondrial pathway and
cell death receptor pathway (14–16).
The commencement of apoptosis in the mitochondrial pathway is
induced or accompanied by increasing BAX protein levels and
decreasing Bcl-2 protein levels, causing the loss of mitochondrial
membrane potential (MMP; ∆Ψm) (14). The focal point of this pathway is
the efflux of cytochrome c from mitochondria to the cytosol.
In the cytosol, cytochrome c forms an apoptosome complex
with apoptotic protease-activating factor 1 and caspase-9, inducing
the activation of a major executioner caspase, caspase-3 (15,17).
The cell death receptor pathway is distinguished by the connection
of cell death ligands to their death receptors with subsequent
stimulation of caspase-8 and caspase-3 activities (16). Caspase-3 activation can
systematically disassemble cells by severing key proteins,
particularly poly(ADP-ribose) polymerase (PARP). Therefore,
targeted inhibition of cell cycle progression and anti-apoptotic
pathways is an attractive concept for improving cancer treatment
strategies.
Lung cancer is the chief cause of cancer-related
death worldwide (18). Lung cancer
is classified into two main types: Small cell lung cancer (SCLC)
which accounts for 10–14% of all lung cancer cases, and non-SCLC
(NSCLC) which accounts for 85–90% (18,19).
NSCLC is additionally sorted into three subtypes in accordance with
histology: Adenocarcinoma, squamous-cell carcinoma, and large cell
carcinoma (19). The anti-growth
effects of PG have been confirmed in numerous cell types such as
pulmonary artery and umbilical vein endothelial cells (10,20),
testis cells (9), leukemia cells
(21), and hepatocellular carcinoma
(22). We also reported that PG
inhibits the growth of HeLa cells via apoptosis and glutathione
depletion (23,24). Although numerous studies have
suggested that PG plays a crucial role in cell death, very little
is known about the cytotoxic and anti-growth effects of PG in lung
cancer cells. In the present study, human SCLC Calu-6 and NSCLC
adenocarcinoma A549 cells were used to investigate the molecular
mechanism involved in the anti-growth effect of PG concerning
apoptosis as well as cell cycle arrest. PG inhibited the growth of
these lung cancer cells via apoptosis and G1 phase arrest of the
cell cycle.
Materials and methods
Cell culture
Human SCLC Calu-6 cells and NSCLC adenocarcinoma
A549 cells were obtained from the American Type Culture Collection
(ATCC). These cell lines were maintained in a standard humidified
incubator containing 5% CO2 at 37°C. The lung cancer
cells were cultured in RPMI-1640 medium containing 10% fetal bovine
serum (FBS) (Sigma-Aldrich; Merck KGaA) and 1%
penicillin-streptomycin (Gibco BRL; Thermo Fisher Scientific,
Inc.). Cells were grown in BD Falcon 100-mm plastic cell culture
dishes (BD Biosciences) and harvested with trypsin-EDTA (Gibco BRL;
Thermo Fisher Scientific, Inc.). Exponentially growing cells were
used for the experiments.
Reagents
PG was acquired from Sigma-Aldrich Co (Merck KGaA).
PG was dissolved in ethanol at 200 mM as a stock solution. Ethanol
(0.2%) was used as a control vehicle and did not influence cell
growth or cell death. Stock solution was wrapped in foil and kept
at 4°C or −20°C.
Cell growth inhibition assay
The influence of PG on the growth of lung cancer
cells was determined using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
Sigma-Aldrich; Merck KGaA) assays. Briefly, 5×104 cells
were seeded into each well of 96-well microtiter plates (Nunc).
After incubation with the designated doses of PG for 24 or 72 h, 20
µl of MTT solution [2 mg/ml in phosphate-buffered saline (PBS);
Gibco BRL; Thermo Fisher Scientific, Inc.] was added to each well.
The plates were incubated for 4 h at 37°C. The medium in plates was
removed via pipetting, and 100–200 µl of DMSO was added to each
well to solubilize formazan crystals. Optical density was measured
at 570 nm with a microplate reader (Synergy™ 2, BioTekR Instruments
Inc.). Each plate contained multiple wells at a given experimental
condition and multiple control wells. This procedure was replicated
for 2 to 4 plates per condition.
Cell cycle and sub-G1 cell
analysis
Cell cycle and sub-G1 distributions in cells were
determined using propidium iodide (PI, Sigma-Aldrich; Merck KGaA;
Ex/Em = 488 nm/617 nm) staining. Briefly, 1×106 cells in
BD Falcon 60-mm culture dishes (BD Biosciences) were incubated with
the designated concentrations of PG for 24 or 72 h. Cells were
washed twice with PBS and fixed in cold 70% ethanol. Cells were
again washed with PBS, and then incubated with 10 µg/ml PI
concurrently with RNase (Sigma-Aldrich; Merck KGaA) at a
concentration of 5×105 cells/ml in PBL at 37°C for 30
min. The proportions of cells in different phases of the cell cycle
or having sub-G1 DNA content were measured and analyzed with a
FACStar flow cytometer (BD Sciences).
Detection of apoptosis
Apoptosis was identified via Annexin V-fluorescein
isothiocyanate staining (FITC, Thermo Fisher Scientific, Inc.;
Ex/Em = 488/519 nm). Annexin V-FITC is used to detect
phosphatidylserine exposing cells thereby marking apoptotic cells.
Briefly, 1×106 cells in BD Falcon 60-mm culture dishes
(BD Biosciences) were incubated with the designated concentrations
of PG for 24 or 72 h. Cells were washed twice with cold PBS and
then suspended in 200 µl of binding buffer (10 mM HEPES/NaOH pH
7.4, 140 mM NaCl, 2.5 mM CaCl2) at a concentration of
5×105 cells/ml at 37°C for 30 min. Annexin V-FITC (2 µl)
was added to the solution, and cells were analyzed with a FACStar
flow cytometer (BD Sciences).
Measurement of mitochondrial membrane
potential (MMP; ΔΨm)
The MMP (ΔΨm) was monitored using a Rhodamine 123
cationic fluorescent dye (Sigma-Aldrich; Merck KGaA; Ex/Em =
485/535 nm), which preferentially enters into mitochondria with
high MMP (∆Ψm). Depolarization of MMP (∆Ψm) results in the loss of
Rhodamine 123 from the mitochondria and reduces the intracellular
fluorescence intensity of this dye. In brief, 1×106
cells in 60-mm culture dishes (Nunc) were incubated with the
indicated doses of PG for 24 h. Cells were washed twice with PBS
and incubated with Rhodamine 123 (0.1 mg/ml) at a concentration of
5×105 cells/ml in PBL at 37°C for 30 min. Rhodamine 123
staining intensities were determined using a FACStar flow cytometer
(BD Sciences). Rhodamine 123 negative (−) cells indicated the loss
of MMP (∆Ψm) in the lung cancer cells. MMP (∆Ψm) levels
in cells, except MMP (∆Ψm) loss cells, were expressed as
proportions compared with the control cells.
Western blot analysis
Protein expression levels were evaluated via western
blotting. Briefly, 5×106 cells in BD Falcon 100-mm
culture dishes (BD Biosciences) were incubated with the indicated
concentrations of PG for 24 h. Cells were washed with PBS and 4
volumes of lysis buffer (Intron Biotechnology) was added. Total
proteins (30 µg) were resolved via 8–15% SDS-PAGE gels and then
transferred to Immobilon-P PVDF membranes (Millipore) by
electroblotting. Membranes were probed with anti-Bcl-2 (cat. no.
2872, 1:1,000 dilution), anti-caspase-3 (cat. no. 9662, 1:1,000
dilution), anti-PARP (cat. no. 9542, 1:1,000 dilution),
anti-cleaved PARP (cat. no. 9541, 1:1,000 dilution) antibodies
(Cell Signaling Technology, Inc.), and anti-β-actin antibody
(sc-81178, 1:1,000 dilution, Santa Cruz Biotechnology, Inc.).
Membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (sc-2004 or sc-2005, 1:1,000 dilution, Santa
Cruz Biotechnology, Inc.). Western blots were developed using an
EZ-Western Lumi Pico ECL solution kit (DoGen, Korea).
Quantification of caspase-3 and
caspase-8 activities
The activities of caspase-3 and caspase-8 were
evaluated using Caspase-3 and Caspase-8 Colorimetric Assay Kits
(R&D Systems, Inc.). In brief, 1×106 cells were
incubated with the specified concentrations of PG for 24 h. Cells
were washed with PBS and 4 volumes of lysis buffer (Intron
Biotechnology) was added. Samples containing 50 µg of total protein
were added to 2X Reaction buffer containing DEVD-pNA for caspase-3
activity or IETD-pNA for caspase-8 activity in 96-well microtiter
plates (Nunc) and incubated at 37°C for 1 h. Optical density was
measured at 405 nm using a microplate reader (Synergy™ 2). Each
plate contained multiple wells of a given experimental condition as
well as multiple control wells. Caspase activity is expressed in
arbitrary absorbance units (absorbance at a wavelength of 405
nm).
Statistical analysis
The results represent the mean of at least three
independent experiments (mean ± SD). Data were analyzed using
Instat software (GraphPad Prism 5.0; GraphPad Software, Inc.).
One-way analysis of variance with post hoc analysis using Tukey's
multiple comparison test was applied to judge statistical
significance which was defined at P<0.05.
Results
Effects of PG on the growth of Calu-6
and A549 lung cancer cells
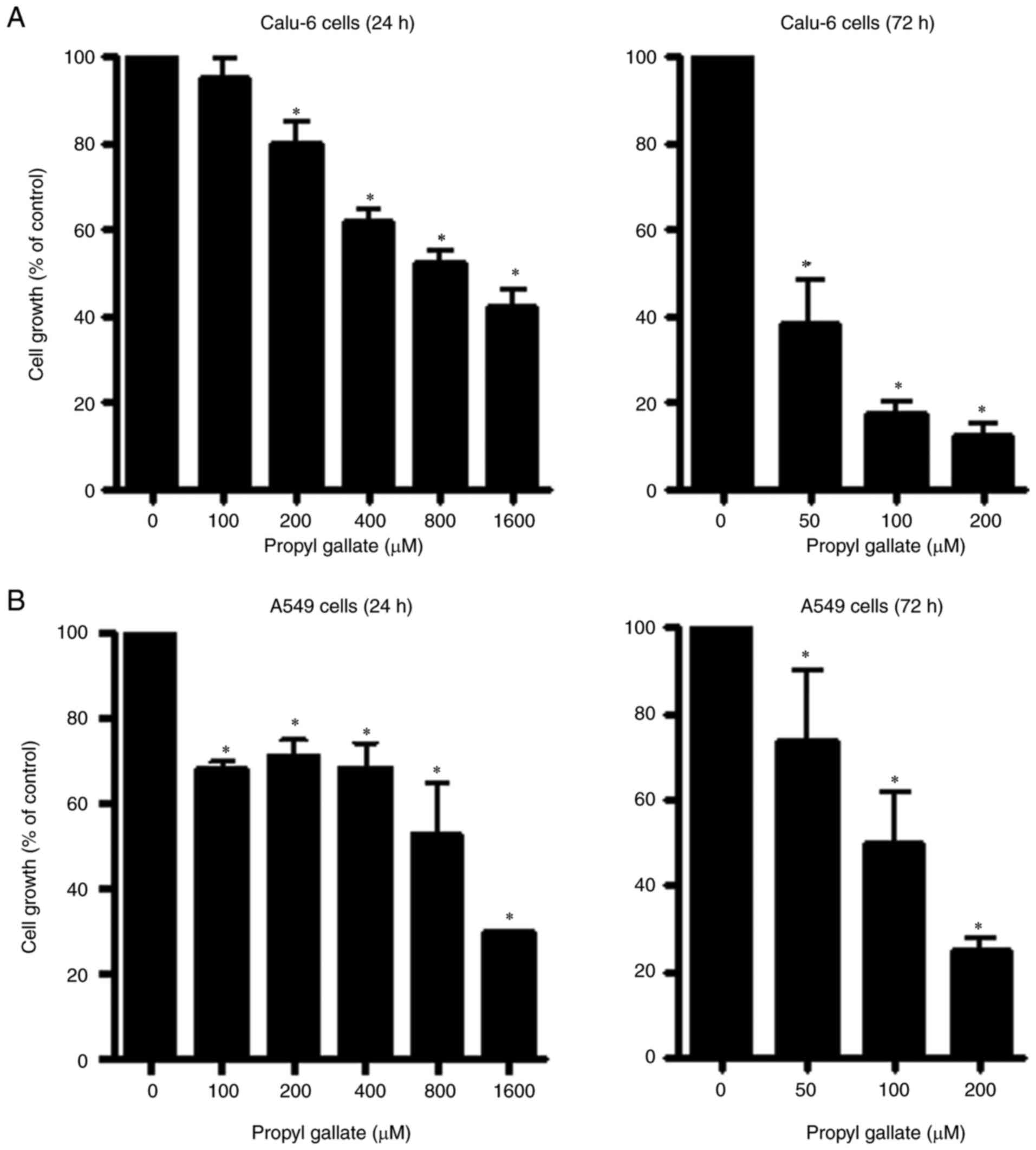
The effect of PG on the growth of Calu-6 and A549
lung cancer cell types was observed using MTT assays. A
dose-dependent reduction in cell growth was observed in Calu-6
cells with a half maximal inhibitory concentration
(IC50) of approximately 800 µM following treatment with
PG for 24 h (Fig. 1A).
Additionally, a 50 µM concentration of PG appeared to significantly
reduce the growth of Calu-6 cells by approximately 60% at 72 h
(Fig. 1A). Thus, the
IC50 of PG in Calu-6 cells at 72 h was less than 50 µM.
The growth of A549 cells was also reduced with an IC50
of ~800 µM after a 24-h incubation with PG (Fig. 1B). In addition, the IC50
of PG in A549 cells at 48 h was approximately 150 µM (data not
shown). Treatment with 100 µM PG decreased the growth of A549 cells
by approximately 50% at 72 h (Fig.
1B).
Effects of PG on the cell cycle
distribution of lung cancer cells
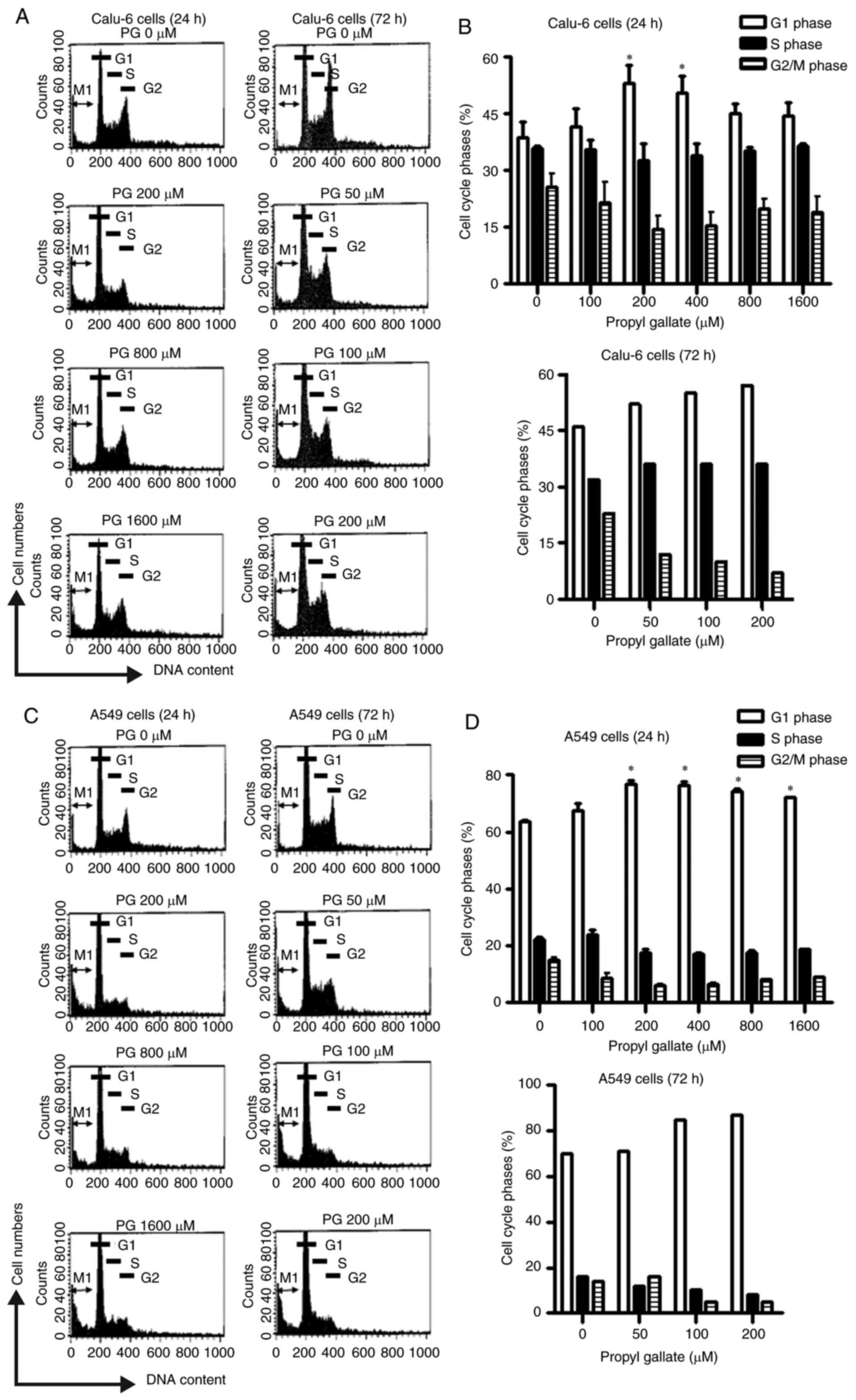
Because the growth inhibition of Calu-6 and A549
cells by PG could be explained by an arrest during cell cycle
progression, allocations of cells in different stages of the cell
cycle were observed after a 24- or 72-h incubation period with PG.
DNA flow cytometric analysis indicated that tested doses of PG
induced a G1 phase arrest of the cell cycle in Calu-6 cells at 24
and 72 h (Fig. 2A and B).
Particularly, concentrations of 200 and 400 µM PG showed a
significant increase in the G1 phase at 24 h (Fig. 2A and B). In addition, the tested
doses of PG induced a G1 phase arrest of the cell cycle in A549
cells at 24 h (Fig. 2C and D).
Treatment with 100 and 200 µM PG also increased the proportion of
cells at the G1 phase at 72 h (Fig. 2C
and D).
Effects of PG on cell death in lung
cancer cells
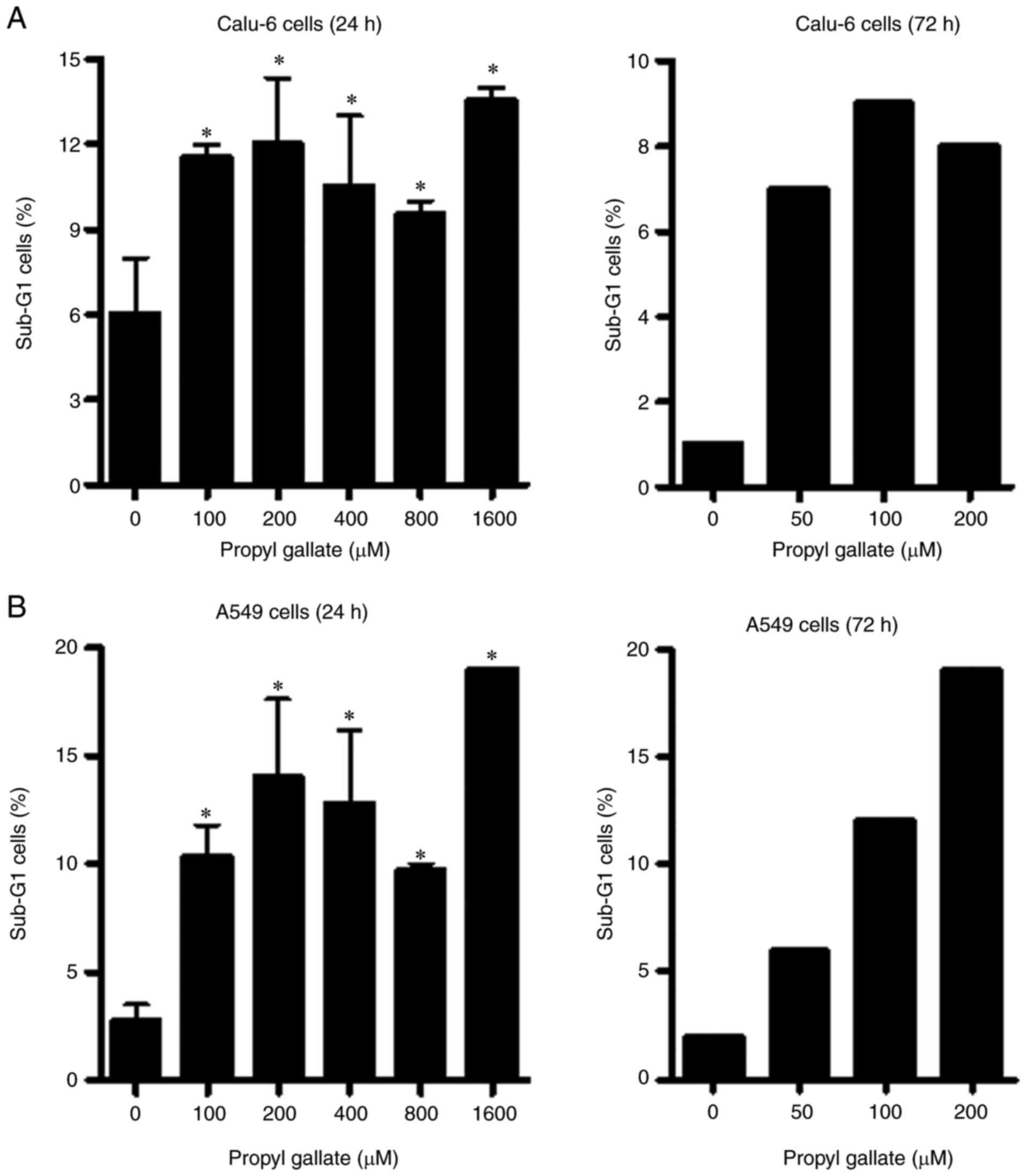
Whether PG induces cell death was evaluated using
sub-G1 cells and Annexin V-staining cells. As shown in Fig. 3A, the tested doses of PG increased
the quantity of sub-G1 cells in Calu-6 cells at 24 or 72 h, but the
effects were not dose-dependent. PG also increased the quantity of
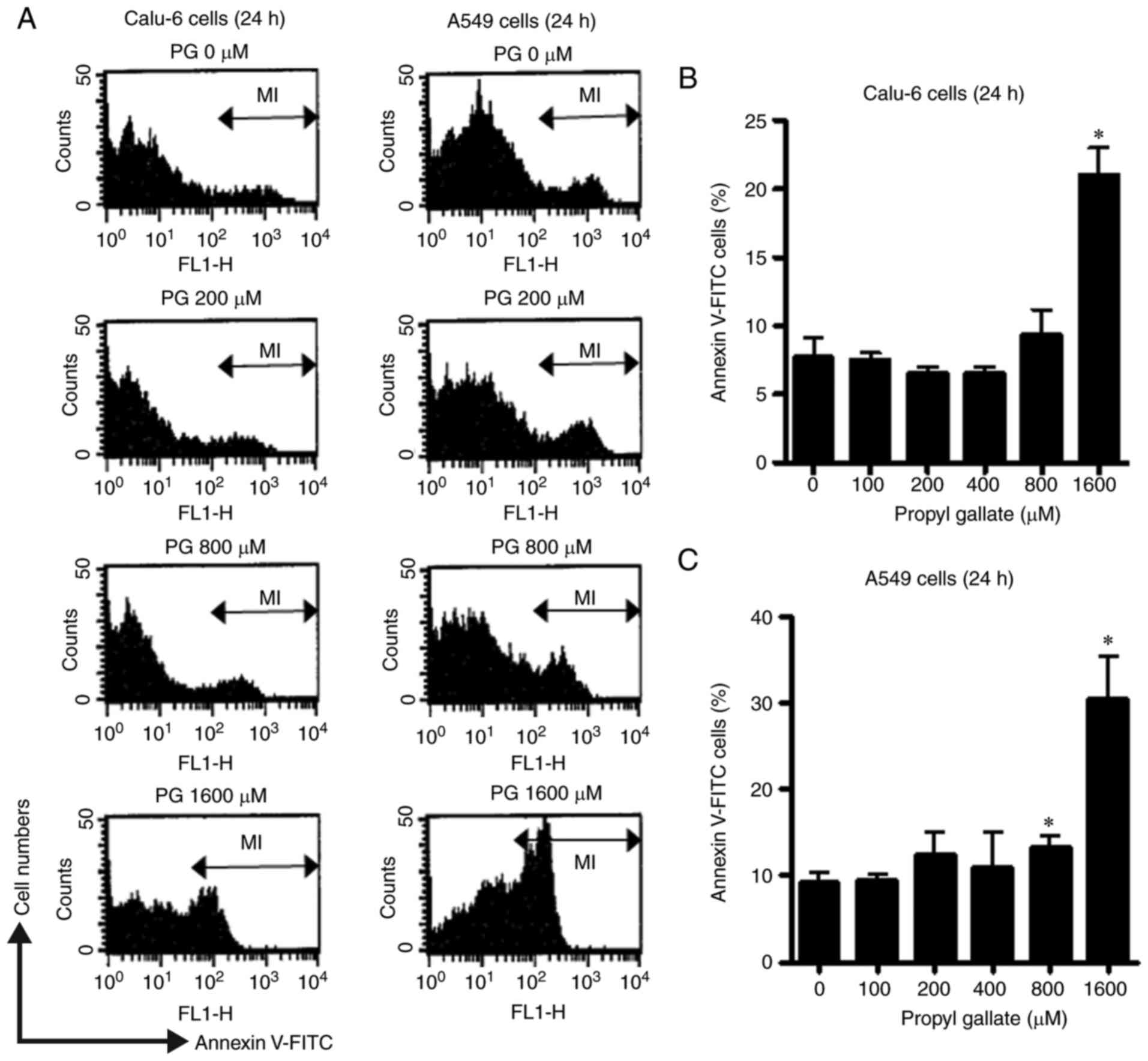
sub-G1 cells in A549 cells at 24 or 72 h (Fig. 3B). Furthermore, 100–400 µM PG did
not increase the percentage of Annexin V-stained cells in both
Calu-6 (Fig. 4A and B) and A549
cell lines (Fig. 4A and C) and 800
µM PG slightly increased the percentage of Annexin V-staining cells
in these cells (Fig. 4). However,
the percentage of Annexin V-stained cells in Calu-6 and A549 cells
was significantly increased after treatment with 1,600 µM PG at 24
h (Fig. 4).
Effects of PG on mitochondrial
membrane potential (MMP; ∆Ψm) in lung cancer cells
As apoptosis is closely related to the collapse of
MMP (∆Ψm), loss of MMP (∆Ψm) in PG-treated cells was evaluated
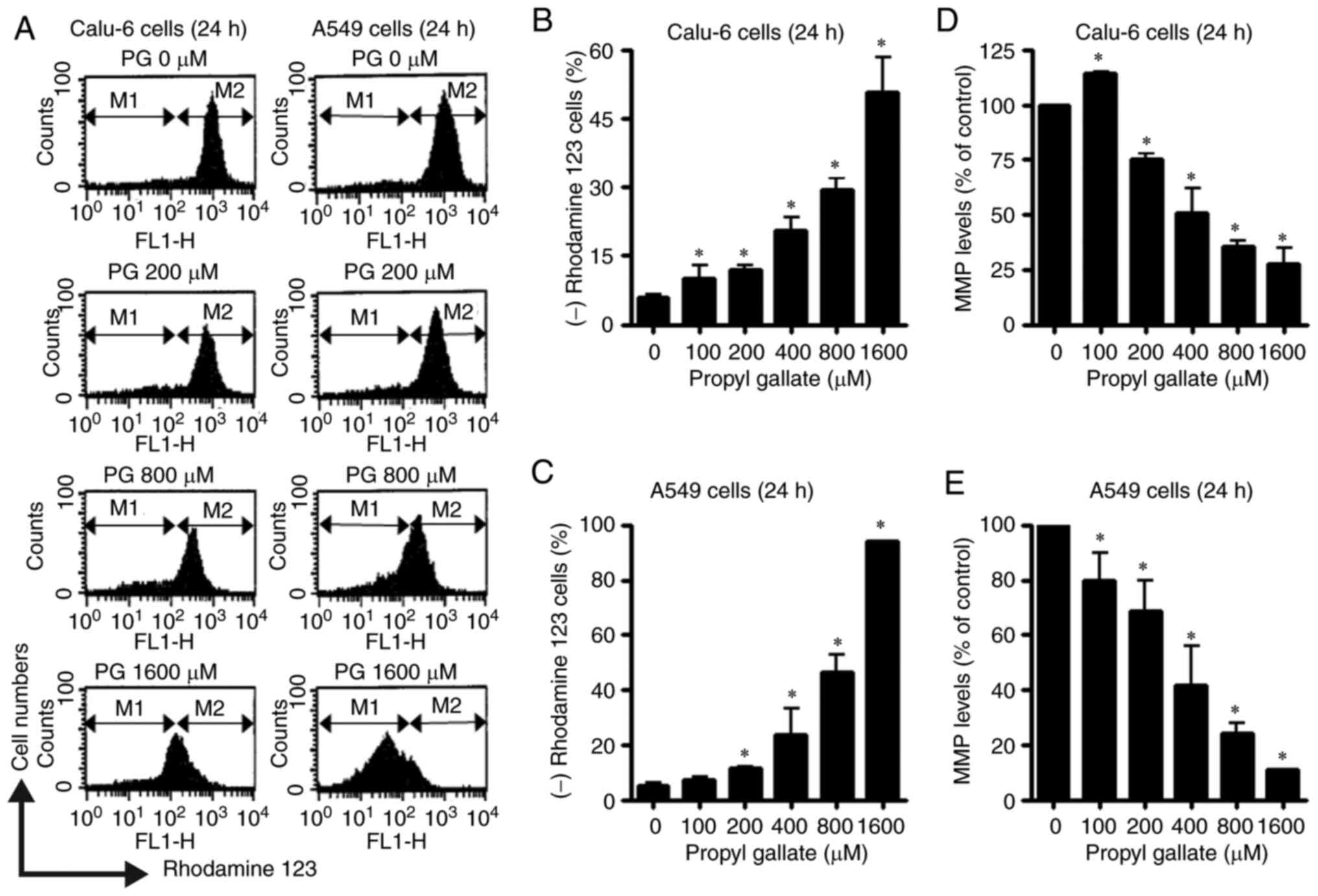
using Rhodamine 123 dye. Loss of MMP (∆Ψm) in both lung cancer cell
types was dose-dependently induced by PG at concentrations of
100–1,600 µM at 24 h (Fig. 5A-C).
After exposure to 800 µM PG, the proportions of cells with MMP
(∆Ψm) loss in Calu-6 and A549 cell lines were
approximately 30 and 45%, respectively (Fig. 5A-C). In relation to the levels of
MMP (∆Ψm) in both lung cancer cell lines at 24 h, 100 µM
PG increased the MMP (∆Ψm) level in Calu-6 cells whereas
PG at 200–1,600 µM significantly decreased the MMP (∆Ψm)
level in these cells (Fig. 5A and
D). Treatment with 100–1,600 µM PG also reduced MMP
(∆Ψm) levels in A549 cells (Fig. 5A and E). The levels of MMP
(∆Ψm) were approximately 30 and 20% in Calu-6 and A549
cell lines treated with 800 µM PG, respectively (Figs. 5A, D and E).
Effects of PG on apoptosis-related
protein levels and activities of caspases in lung cancer cells
Since PG induced cell death in both lung cancer cell
types, the levels of apoptosis-related proteins were assessed by
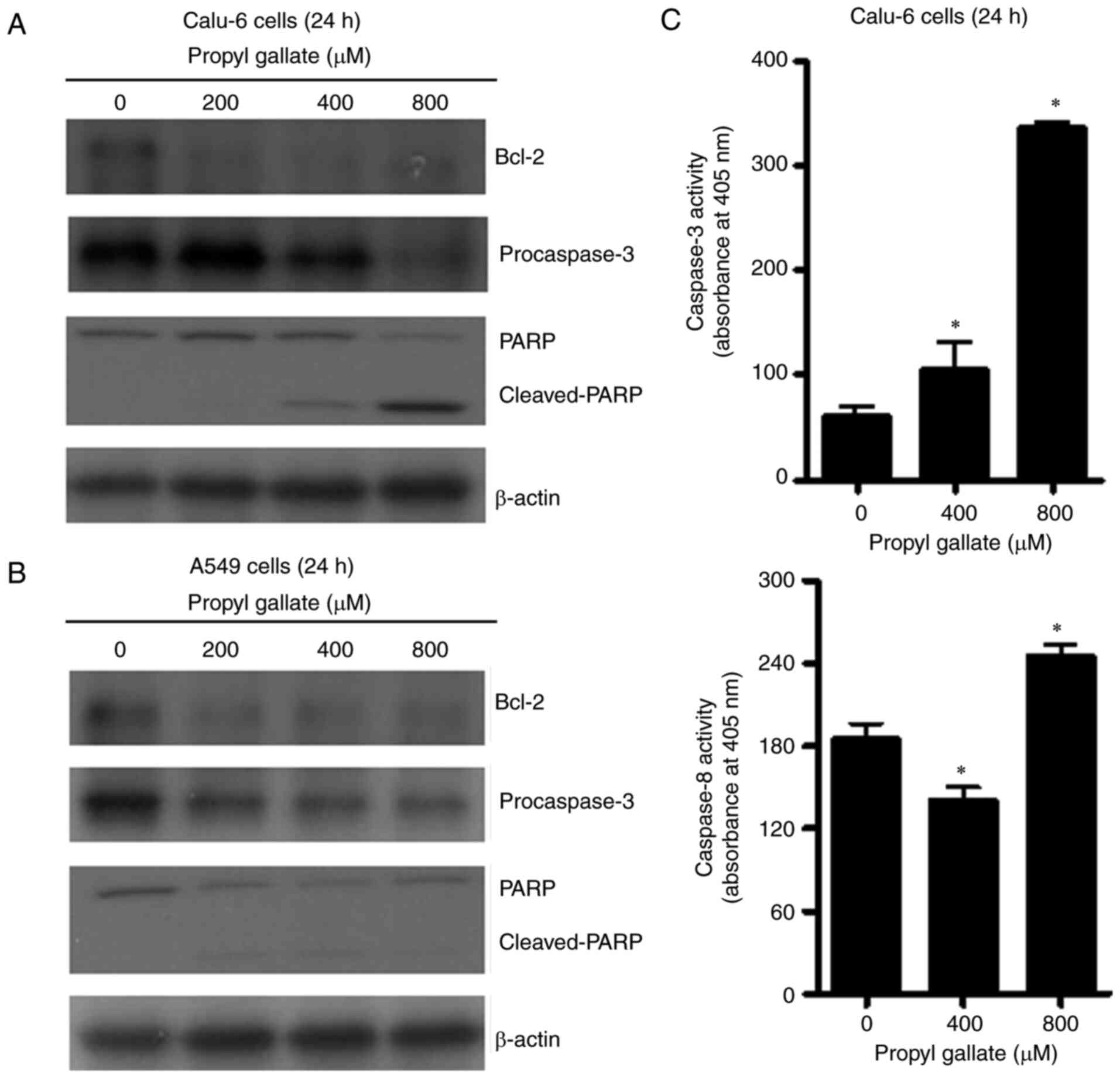
western blot analysis. Examination of Bcl-2 regulation in
PG-treated cells revealed that Bcl-2 protein levels were reduced
following treatment with 200–800 µM PG in both lung cancer cell
types (Fig. 6A and B). Caspase-3
plays an essential role as an executioner in apoptosis (25). Whether PG activates caspase-3 in
PG-treated lung cancer cells was also examined. Levels of
procaspase-3 (32 kDa precursor) were visibly reduced following
treatment with 400–800 µM PG in the Calu-6 cells (Fig. 6A) and following treatment with
200–800 µM PG in the A549 cells (Fig.
6B). Cleavage of PARP provides one of the most recognizable
markers of apoptosis (26). While
the intact 116 kDa moiety of PARP was reduced in the 400–800 µM
PG-treated Calu6 cells, the cleaved form of PARP increased in these
cells (Fig. 6A). PG at 200–800 µM
also reduced the intact form of PARP in the A549 cells (Fig. 6B). Furthermore, 400–800 µM PG
treatment significantly increased caspase-3 activities in the
Calu-6 cells (Fig. 6C). Caspase-8,
which is correlated with the cell death receptor pathway (25), was also significantly activated by
treatment with 800 µM PG in Calu-6 cells (Fig. 6C). However, 400 µM PG downregulated
the activity of caspase-8, compared with basal activity of the
control cells (Fig. 6C).
Discussion
Although propyl gallate (PG) is used to preserve and
stabilize medicinal provisions included on the US Food and Drug
Administration list (27), the
latent toxicity of PG has been inspected to assess various in
vivo or in vitro toxicological properties (2,28–30).
In the present study, the anti-growth effects of PG were examined
in Calu-6 and A549 lung cancer cell lines in relation to apoptosis
as well as cell cycle arrest. Treatment with PG decreased the
growth of both lung cancer cell types with an IC50 of
approximately 800 µM at 24 h. Similarly, 800 µM of PG was found to
reduce the growth of HeLa cells by approximately 50% at 24 h
(23). However, 800 µM PG was found
to strongly reduce the growth of umbilical vein and pulmonary
artery endothelial cells by more than 50% at 24 h and the
susceptibility of pulmonary artery endothelial cells to PG was
higher than that of umbilical vein endothelial cells (20). In addition, 400 µM PG did not
significantly inhibit the growth of primary human pulmonary
fibroblasts at 24 h (31). Because
PG can damage mitochondria which leads to ATP depletion (9,20,23,32),
the varying susceptibility to PG is perhaps owing to the different
basal activities of mitochondria and antioxidant enzymes that each
cell type has. Thus, the toxicity of PG should be carefully studied
and interpreteted in vivo and in vitro depending on
PG concentration, incubation time, and experimental target
cells.
In the present study, PG induced the apoptosis of
Calu-6 and A549 cells, as demonstrated by the proportions of sub-G1
and Annexin V-stained cells. In addition, PG treatment clearly
decreased the Bcl-2 levels, along with an increase in the cleavage
form of PARP. DNA flow cytometry indicated that PG induced arrest
at the G1 phase of the cell cycle in both lung cancer cell types at
24 and 72 h. Similarly, PG was previously found to induce G1 phase
arrest of the cell cycle along with an increase in p27, a
cyclin-dependent kinase (CDK) inhibitor (CDKI), in HeLa cells
(23). In the present study,
treatment with 800 µM PG inhibited the growth of lung cancer cells
by approximately 50% but this dose of PG induced cell death by
approximately 10% in view of the percentages of the sub-G1 cells
and Annexin V-stained cells. Thus, the significant G1 phase arrest
by PG is another conceivable underlying mechanism for the
inhibition of cell growth. It is worthwhile to study whether PG
affects the expression levels of cyclin, CDKs, and CDKIs to induce
cell cycle arrest, especially at the G1 phase in lung cancer cells.
Collectively, PG inhibits the growth of lung cancer cells via G1
phase arrest of the cell cycle as well as apoptosis.
Apoptosis is closely related to the failure of
mitochondrial membrane potential [MMP (∆Ψm)] (33), and PG can cause a breakdown in MMP
(∆Ψm) (9,20,23,32).
In the present study, likewise, PG dose-dependently induced the
loss of MMP (∆Ψm) and reduced the MMP (∆Ψm) level in lung cancer
cells. The degree of cells with MMP (∆Ψm) loss in the PG-treated
Calu-6 (SCLC; small cell lung cancer) cells was lower than that in
the PG-treated A549 (NSCLC; non-small cell lung cancer) cells. For
example, 800 µM of PG increased the percentage of cells with MMP
(∆Ψm) loss in the Calu-6 and A549 cell lines by
approximately 30 and 45%, respectively. While the level of MMP
(∆Ψm) was approximately 30% in the 800 µM PG-treated
Calu-6 cells, the MMP (∆Ψm) level in A549 cells was
approximately 20%. Interestingly, the degree of MMP (∆Ψm) loss in
the PG-treated lung cells was higher than that of the Annexin
V-stained cells. Furthermore, the proportions of Annexin V-stained
cells in the PG-treated Calu-6 cells were lower than those in the
A549 cells. These results imply that PG initially influences the
mitochondrial membrane, especially in adenocarcinoma A549 (NSCLC)
cells, which precedes the next step in apoptosis.
Apoptosis involves the mitochondrial (intrinsic) and
cell death receptor (extrinsic) pathways (15). Caspase-3 plays a critical role as an
executioner of apoptosis. The levels of procaspase-3 (32 kDa
precursor) were reduced in the PG-treated lung cancer cells, which
suggests that activation of caspase-3 occurred in these cells. In
fact, PG treatment upregulated the activity of caspase-3 in Calu-6
cells. In particular, the activation of caspase-8 was observed in
apoptosis of Calu-6 cells induced by PG. However, 400 µM PG that
showed a slightly decreased amount of Annexin V-stained cells
lessened caspase-8 activity, compared with the basal activity of
the control cells. Further research is required to elucidate the
exact mechanism that is involved. Caspase-8 activation is linked
with the cell death receptor pathway of apoptosis (25,34).
PG-induced apoptosis in lung cancer cells involved both extrinsic
and intrinsic pathways.
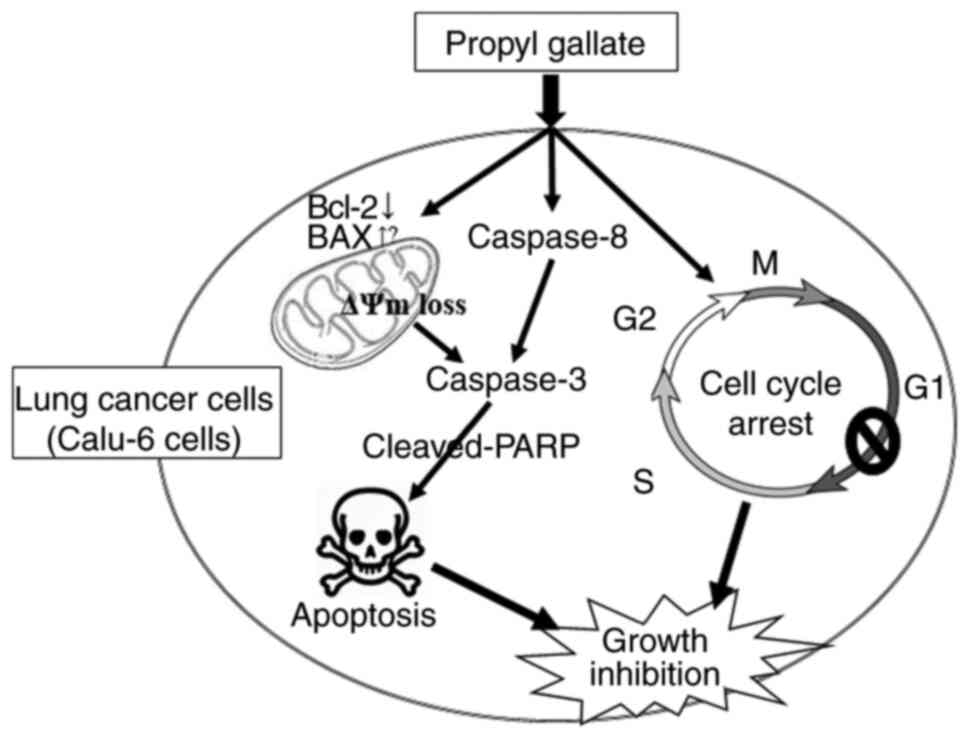
In conclusion, PG treatment inhibited the growth of
lung cancer cells, especially Calu-6 cells via caspase-dependent
apoptosis as well as G1 phase arrest of the cell cycle (Fig. 7). The presented data provides
valuable information for understanding the cytotoxicological
effects of PG in lung cancer cells in view of apoptosis and cell
cycle arrest. Since PG has been reported to exert prooxidant
properties (6,7), it is worth studying whether PG induces
apoptosis of Calu-6 and A549 lung cancer cells through oxidative
stress in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant
(2019R1I1A2A01041209) of the Basic Science Research Program through
the National Research Foundation (NRF) funded by the Ministry of
Education, Republic of Korea.
Availability of data and materials
Data collected during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WHP conceived and designed the study, performed the
experiments, and wrote the manuscript. WHP agrees to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Woo Hyun Park: ORCID:
0000-0003-4341-5188.
Glossary
Abbreviations
Abbreviations:
|
PG
|
propyl gallate
|
|
NSCLC
|
non-small cell lung cancer
|
|
SCLC
|
small cell lung cancer
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
MMP (∆Ψm)
|
mitochondrial membrane potential
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
FITC
|
fluorescein isothiocyanate
|
References
|
1
|
Final report on the amended safety
assessment of propyl gallate. Int J Toxicol. 26 (Suppl 3):89–118.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu TW, Fung KP, Zeng LH, Wu J and Nakamura
H: Propyl gallate as a hepatoprotector in vitro and in vivo.
Biochem Pharmacol. 48:419–422. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reddan JR, Giblin FJ, Sevilla M,
Padgaonkar V, Dziedzic DC, Leverenz VR, Misra IC, Chang JS and Pena
JT: Propyl gallate is a superoxide dismutase mimic and protects
cultured lens epithelial cells from H2O2 insult. Exp Eye Res.
76:49–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CH, Liu TZ, Chen CH, Wong CH, Chen
CH, Lu FJ and Chen SC: The efficacy of protective effects of tannic
acid, gallic acid, ellagic acid, and propyl gallate against
hydrogen peroxide-induced oxidative stress and DNA damages in
IMR-90 cells. Mol Nutr Food Res. 51:962–968. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirose M, Yada H, Hakoi K, Takahashi S and
Ito N: Modification of carcinogenesis by alpha-tocopherol,
t-butylhydroquinone, propyl gallate and butylated hydroxytoluene in
a rat multi-organ carcinogenesis model. Carcinogenesis.
14:2359–2364. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi H, Oikawa S, Hirakawa K and
Kawanishi S: Metal-mediated oxidative damage to cellular and
isolated DNA by gallic acid, a metabolite of antioxidant propyl
gallate. Mutat Res. 558:111–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawanishi S, Oikawa S and Murata M:
Evaluation for safety of antioxidant chemopreventive agents.
Antioxid Redox Signal. 7:1728–1739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakagawa Y and Tayama S: Cytotoxicity of
propyl gallate and related compounds in rat hepatocytes. Arch
Toxicol. 69:204–208. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ham J, Lim W, Park S, Bae H, You S and
Song G: Synthetic phenolic antioxidant propyl gallate induces male
infertility through disruption of calcium homeostasis and
mitochondrial function. Environ Pollut. 248:845–856. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han YH, Moon HJ, You BR and Park WH:
Propyl gallate inhibits the growth of calf pulmonary arterial
endothelial cells via glutathione depletion. Toxicol In Vitro.
24:1183–1189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boyd I and Beveridge EG: Relationship
between the antibacterial activity towards escherichia coli NCTC
5933 and the physico-chemical properties of some esters of
3,4,5-trihydroxybenzoic acid (Gallic acid). Microbios. 24:173–184.
1979.PubMed/NCBI
|
|
12
|
Martinez-Alonso D and Malumbres M:
Mammalian cell cycle cyclins. Semin Cell Dev Biol. 2020.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dalton S: Linking the cell cycle to cell
fate decisions. Trends Cell Biol. 25:592–600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huska JD, Lamb HM and Hardwick JM:
Overview of BCL-2 family proteins and therapeutic potentials.
Methods Mol Biol. 1877:1–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung C: Restoring the switch for cancer
cell death: Targeting the apoptosis signaling pathway. Am J Health
Syst Pharm. 75:945–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Yue P, Zhou Z, Khuri FR and Sun SY:
Death receptor regulation and celecoxib-induced apoptosis in human
lung cancer cells. J Natl Cancer Inst. 96:1769–1780. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wurstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Z, Li M, Chen Z, Zhan C, Lin Z and Wang
Q: Advances in clinical trials of targeted therapy and
immunotherapy of lung cancer in 2018. Transl Lung Cancer Res.
8:1091–1106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carter BW, Lichtenberger JP III,
Benveniste MK, de Groot PM, Wu CC, Erasmus JJ and Truong MT:
Revisions to the TNM staging of lung cancer: Rationale,
significance, and clinical application. Radiographics. 38:374–391.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han YH, Moon HJ, You BR, Yang YM, Kim SZ,
Kim SH and Park WH: Propyl gallate inhibits the growth of
endothelial cells, especially calf pulmonary arterial endothelial
cells via caspase-independent apoptosis. Int J Mol Med. 25:937–944.
2010.PubMed/NCBI
|
|
21
|
Chen CH, Lin WC, Kuo CN and Lu FJ: Role of
redox signaling regulation in propyl gallate-induced apoptosis of
human leukemia cells. Food Chem Toxicol. 49:494–501. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei PL, Huang CY and Chang YJ: Propyl
gallate inhibits hepatocellular carcinoma cell growth through the
induction of ROS and the activation of autophagy. PLoS One.
14:e02105132019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han YH and Park WH: Propyl gallate
inhibits the growth of HeLa cells via regulating intracellular GSH
level. Food Chem Toxicol. 47:2531–2538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han YH, Moon HJ, You BR and Park WH: The
anti-apoptotic effects of caspase inhibitors on propyl
gallate-treated HeLa cells in relation to reactive oxygen species
and glutathione levels. Arch Toxicol. 83:825–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daniel JW: Metabolic aspects of
antioxidants and preservatives. Xenobiotica. 16:1073–1078. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dacre JC: Long-term toxicity study of
n-propyl gallate in mice. Food Cosmet Toxicol. 12:125–129. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosin MP and Stich HF: Enhancing and
inhibiting effects of propyl gallate on carcinogen-induced
mutagenesis. J Environ Pathol Toxicol. 4:159–167. 1980.PubMed/NCBI
|
|
30
|
Abdo KM, Huff JE, Haseman JK and Alden CJ:
No evidence of carcinogenicity of D-mannitol and propyl gallate in
F344 rats or B6C3F1 mice. Food Chem Toxicol. 24:1091–1097. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park WH: Effects of antioxidants and MAPK
inhibitors on cell death and reactive oxygen species levels in
H2O2-treated human pulmonary fibroblasts. Oncol Lett. 5:1633–1638.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakagawa Y, Nakajima K, Tayama S and
Moldeus P: Metabolism and cytotoxicity of propyl gallate in
isolated rat hepatocytes: effects of a thiol reductant and an
esterase inhibitor. Mol Pharmacol. 47:1021–1027. 1995.PubMed/NCBI
|
|
33
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|