Introduction
The majority of patients with ovarian cancer may
suffer a relapse, and the development of platinum resistance
results in great challenges to clinical treatment; thus, much
effort has been placed into developing new drugs. Due to
polyphyllin VII having pharmacological effects, such as
anti-inflammatory (1), hemostatic
and analgesic (2), immune
regulation (3), and minor side
effects, it has been widely used in clinical applications. In
recent years, findings have shown that polyphyllin VII has obvious
anti-cancer activity against ovarian cancer cells (4) prostate (5), gastric (6), nasopharyngeal (7), and colon cancer (8). Yang et al found that
polyphyllin VII can regulate the expression of Bcl-2 and Bax, cause
a decrease in mitochondrial membrane potential (MMP), and induce
apoptosis in human erythroleukemia cell line K562 (9). Liu et al found in the study of
the hepatocellular carcinoma HepaRG that polyphyllin VII can
increase intracellular ROS production, decrease MMP, and accompany
activation of the caspase pathway and the release of cytochrome
c from the cytoplasm to induce mitochondrial apoptosis
(10). It is suggested that
polyphyllin VII may cause mitochondrial dysfunction of tumor cells
and induce apoptosis, although the specific mechanism of regulating
mitochondrial function is not clear. Therefore, investigating the
specific regulation mechanism of polyphyllin VII on mitochondrial
function may provide some targeted evidence for the clinical
treatment of ovarian cancer.
Mitochondria are not only the key organelles for
intracellular energy production, but also the basic platform for
cell signal transduction, playing a crucial role in cell signal
transduction, cell proliferation, differentiation, autophagy and
cellular immunity (11–13). Mitochondria are dynamic organelles,
whose imbalance in division and fusion often leads to changes in
mitochondrial structure and dysfunction (14). Although mitochondrial division often
occurs in apoptosis, some scholars believe that division is an
essential step in the process of apoptosis, although the specific
regulatory mechanism of mitochondrial division and apoptosis is
unclear. Mitochondrial fission is a multi-step process in which the
recruitment of GTPase DRP1 on mitochondria plays a key role
(15). During the process of cell
apoptosis, BAX activation co-locates BAX and DRP1 at mitochondrial
sites where division occurs (16),
and DRP1 stably binds to the mitochondrial outer membrane, leading
to mitochondrial fragmentation (17–19).
Hasnat et al found that triptolide can mediate Drp1
translocation to the outer mitochondrial membrane, leading to
increased mitochondrial division, accompanied by the release of
cytochrome c and activation of caspase-3, inducing L02 cell
death in human liver cells (20).
Li et al found that erucin could dephosphorylate DRP1
(Ser637) to mediate DRP1 mitochondrial translocation and induce
mitochondrial division and apoptosis in human MDA-MB-231 and MCF-7
breast cancer cells (21).
Therefore, the localization of DRP1 on mitochondria may mediate
mitochondrial dynamics and mitochondrial pathway apoptosis, and
this process is regulated by its phosphorylation level.
Protein phosphatase 2A (PP2A) is an important and
ubiquitous serine threonine phosphatase and a tumor suppressor
(22). PP2A is a critical negative
regulator of tumorigenesis, and affects protein synthesis, cell
proliferation, cell survival, cell migration, and invasiveness
(23). PP2A is inactivated in a
variety of malignancies, including breast (24), ovarian (25), cervical (26), and lung (27) cancer. Reactivating PP2A is an
effective way to fight cancer (28). Zhang et al found that
polyphyllin VII can inhibit the proliferation and invasion of
cisplatin-resistant SGC7901/DDP cells by regulating the PP2A/AKT
signaling axis (29). Kim et
al found that Drp1 may be a direct substrate of AKT, whose
activity is regulated by AKT, and their interaction induces
phosphorylation of Drp1, leading to mitochondrial division
(30). Therefore, we hypothesized
that polyphyllin VII could regulate the phosphorylation of DRP1 and
its mitochondrial localization by PP2A/AKT signaling axis.
In the present study, different concentrations of
polyphyllin VII were used to treat human ovarian cancer cells to
explore the association between changes in the mitochondrial
localization of DRP1 and apoptosis through changes in DRP1
phosphorylation levels. The results showed that polyphyllin VII can
significantly promote the translocation of DRP1 from the cytoplasm
to mitochondria and increase the binding of pro-apoptotic proteins
of the BCL-2 family to mitochondria, promoting the release of
cytochrome c into the cytoplasm. In addition, polyphyllin
VII-induced changes in DRP1 localization are regulated by the
dephosphorylation of DRP1 by PP2A/AKT. In summary, polyphyllin VII
regulates mitochondrial division through the PP2A/AKT/DRP1 axis,
and then promotes apoptosis, which provides a new idea for the
clinical treatment of ovarian cancer with polyphyllin VII.
Materials and methods
Cell culture and reagents
Ovarian cancer cell lines A2780 and SKOV3 were
purchased from the Chinese Academy of Medical Sciences and Peking
Union Medical College (Peking, China) and cultured under the
recommended conditions. Polyphyllin VII with a purity of 98% or
more was purchased from Sichuan Weikeqi Biotechnology Co., Ltd. The
drug was dissolved in methyl sulfoxide (DMSO) (Sigma-Aldrich; Merck
KGaA) and stored at −20°C. The negative control group was the low
concentration DMSO group. The final concentration of DMSO for all
treatments was consistently less than 0.1%. LB-100 was obtained
from Apexbio.
MTT cell viability assay
Cells were seeded in 96-well plates and treated with
different concentrations (0, 1, 2, 3 µM) of polyphyllin VII for 24
h. The original medium was then removed and 0.5 mg/ml MTT was added
for 4 h at 37°C. Formazan crystals were dissolved in 150 µl DMSO
and absorbance was measured at a wavelength of 490 nm.
Apoptosis analysis by flow
cytometry
According to the manufacturer's instructions, A2780
and SKOV3 cells were stained with an Annexin V-FITC apoptosis kit
(BD Bioscience) and analyzed using an Accuri C6 flow cytometer (BD
Biosciences).
Assessment of mitochondrial
depolarization
According to the manufacturer's instructions,
pre-treated A2780 and SKOV3 cells were suspended in 1 ml of
complete medium containing 10 µg/ml of JC-1 (Beyotime) at 37°C for
30 min. The cells were analyzed on an Accuri C6 flow cytometer (BD
Biosciences).
Intracellular ROS measurements
To detect intracellular hydrogen peroxide levels,
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma-Aldrich;
Merck KGaA) was used to measure intracellular ROS levels. Cell
fluorescence was measured using an Accuri C6 flow cytometer (BD
Biosciences).
Mitochondrial isolation
A Mitochondria Isolation Kit (Invent
Biotechnologies) was used to extract mitochondria as directed by
the manufacturer's protocol.
Western blot analysis
The A2780 and SKOV3 cells were lysed with 120 µl of
RIPA buffer (Beyotime). Cell lysates were sonicated for 30 sec
under ice and lysed at 4°C for 45 min. The cells were lysed by
centrifugation at 3,000 × g for 15 min, and the supernatant was
used to determine the protein concentration using Bio-Rad kit
(Pierce Biotechnology Inc.) and the samples were boiled for 5 min.
The cell lysates (10-20 µg) were resolved on 12% SDS-polyacrylamide
gels and transferred to polyvinylidene fluoride (PVDF) membranes.
The membranes were blocked with 5% skim milk for 1 h, and incubated
with primary antibodies overnight at 4°C. The following day,
membranes were washed with PBST and incubated with horseradish
peroxidase-conjugated secondary antibodies at 1:2,000 dilution for
1 h at room temperature. After washing the membranes with PBST,
immunodetection was performed using ECL reagent (Thermo Fisher
Scientific) and visualized using a Syngene Bio Imaging (Synoptics).
The primary antibodies used were anti-β-actin (1:1,000 dilution),
anti-cleaved caspase-3 (1:1,000 dilution), anti-Bax (1:1,000
dilution), anti-Bcl-2 (1:1,000 dilution), anti-Tom20 (1:1,000
dilution), anti-Drp1 (1:1,000 dilution), anti-cytochrome c
(1:1,000 dilution) (Cell Signaling Technology), anti-phospho-DRP1
(Ser637) (1:1,000 dilution), anti-Phospho-AKT (Ser473) (1:2,000
dilution) (Proteintech Group, Inc.).
Mitochondrial fragmentation
Cells were cultured on slides and exposed to
different treatments. The original complete medium was replaced
with serum-free medium containing MitoTracker Red, and the cells
were incubated at 37°C for 30 min and washed three times with PBS.
Cells were then examined under an Echo-lab Revolve microscope.
PP2A phosphatase activity assays
PP2A phosphatase activity was measured with a PP2A
Immunoprecipitation Phosphatase Assay Kit (Millipore). Treated
cells were collected and added to RIPA lysis buffer, and malachite
green was used to detect the free phosphate released by PP2A.
Immunofluorescence
Cells were cultured on slides and exposed to
different treatments. Immunofluorescence analysis was performed
following the addition of MitoTracker Red and DRP1 antibodies.
Cells were then examined under an Echo-lab Revolve microscope.
Xenograft models
Twelve female BALB/c nude mice aged 6-8 weeks
(Beijing Vital River Laboratory Animal Technology, Beijing, China)
were placed in a standard microisolator in non-pathogenic
conditions. Raised under the conditions of controlled temperature
(20-25°C) and light (12-h light/dark cycle), mice had free access
to food and tap water, cages were ventilated, and wood chips were
used as bedding, which was replaced every 3 days. The protocol was
approved by the ethics committee of Jilin University National
Health Research Institute in accordance with the National
Institutes of Health Guidelines for the Care and Use of Laboratory
Animals. The mice were injected subcutaneously into the left flank
with 5×107 SKOV3 cells. When the average tumor volume of
the animals was 30 mm3, they were randomly divided into
four groups (n=3 per group) as follows: Control group, 1, 2 and 3
mg/kg groups. Tumor volume was measured every two days and
calculated using the formula: 0.5 × length × width2.
After 29 days of treatment, the mice were euthanized by cervical
dislocation and tumors were excised for western blot analysis.
Statistical analysis
Data are expressed as mean ± SD. Comparisons between
groups were performed using one-way analysis of variance and
Tukey's post hoc test. All the experiments were repeated three
times. The data were analyzed by Chi-square and Spearman's rank
correlation. All statistical analyses were performed using SPSS19.0
statistical software (SPSS Inc.). P<0.05 was considered
statistically significant.
Results
Polyphyllin VII affects cell viability
and induces apoptosis in A2780 and SKOV3 cells
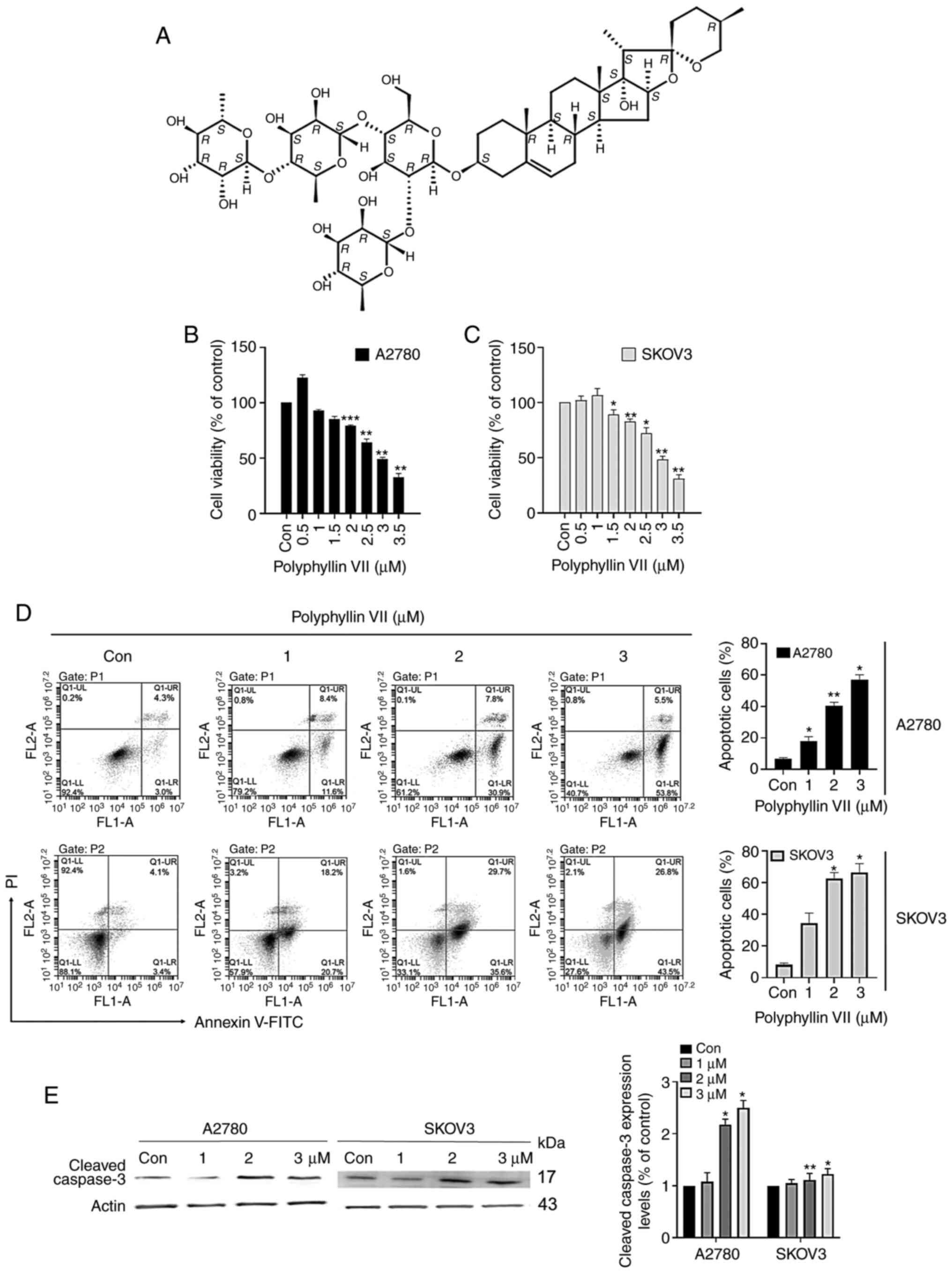
Fig. 1A shows the
chemical structure of polyphyllin VII. To investigate the effect of
polyphyllin VII on cell viability, we cultured cells with different
concentrations (0, 1, 2, 3 µM) for 24 h and tested cell
proliferation capacity with MTT. As shown in Fig. 1B and C, polyphyllin VII inhibited
cell activity in a dose-dependent manner. Polyphyllin VII (3 µM)
significantly decreased the viability of A2780 and SKOV3 cells.
These results suggested that polyphyllin VII can suppress the
viability of different human ovarian cell lines. To further
investigate the effect of polyphyllin VII on cell survival, we
assessed apoptosis in ovarian cancer cell lines. A2780 and SKOV3
cells were cultured for 24 h with 0, 1, 2 or 3 µM Polyphyllin VII
and flow cytometry analysis was performed with Annexin V-FITC/PI
double staining. We found that Polyphyllin VII promoted the early
and late apoptosis of A2780 and SKOV3 cells in a dose-dependent
manner (Fig. 1D). Moreover, the
expression of cleaved caspase-3 was significantly increased in the
two cells (Fig. 1E). These results
suggest that polyphyllin VII can induce apoptosis of human ovarian
cancer cells.
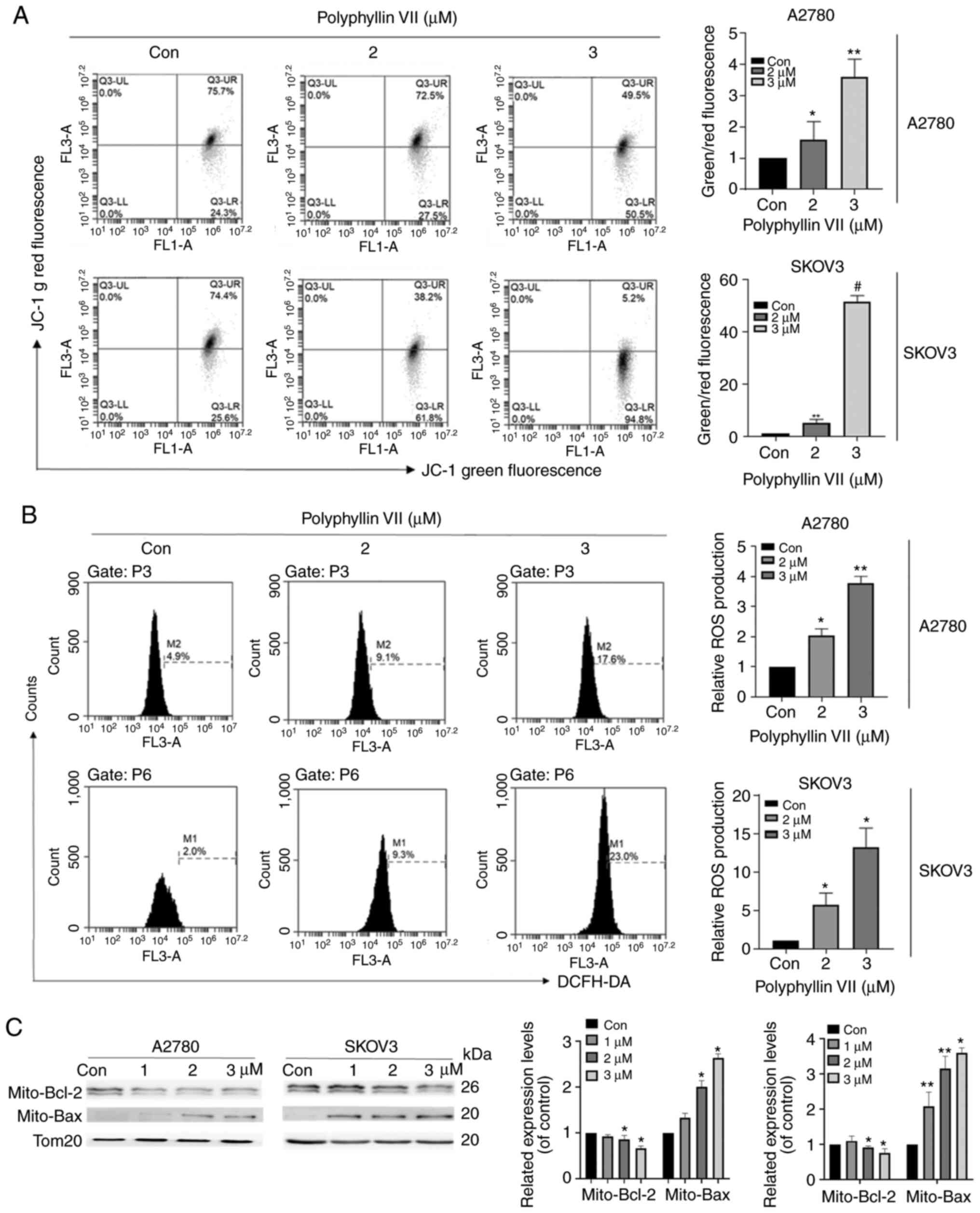
Polyphyllin VII promotes mitochondrial
dysfunction in A2780 and SKOV3 cells
A2780 and SKOV3 cells were stained with the ROS
indicator DCFH-DA to evaluate the impact of polyphyllin VII on ROS
production. The results showed that the ROS production in the two
cell lines was increased in a dose-dependent manner after treatment
with polyphyllin VII (Fig. 2A). In
addition, polyphyllin VII decreased the mitochondrial membrane
potential (Δψm), which was confirmed by JC-1 probe application
(Fig. 2B). With the increase in
polyphyllin VII concentration, the intensity of green fluorescence
increased gradually. As shown in Fig.
2C, we observed that compared with the control group,
mitochondrial BAX expression in the polyphyllin VII-treated group
was significantly upregulated, while mitochondrial BCL-2 expression
was significantly reduced.
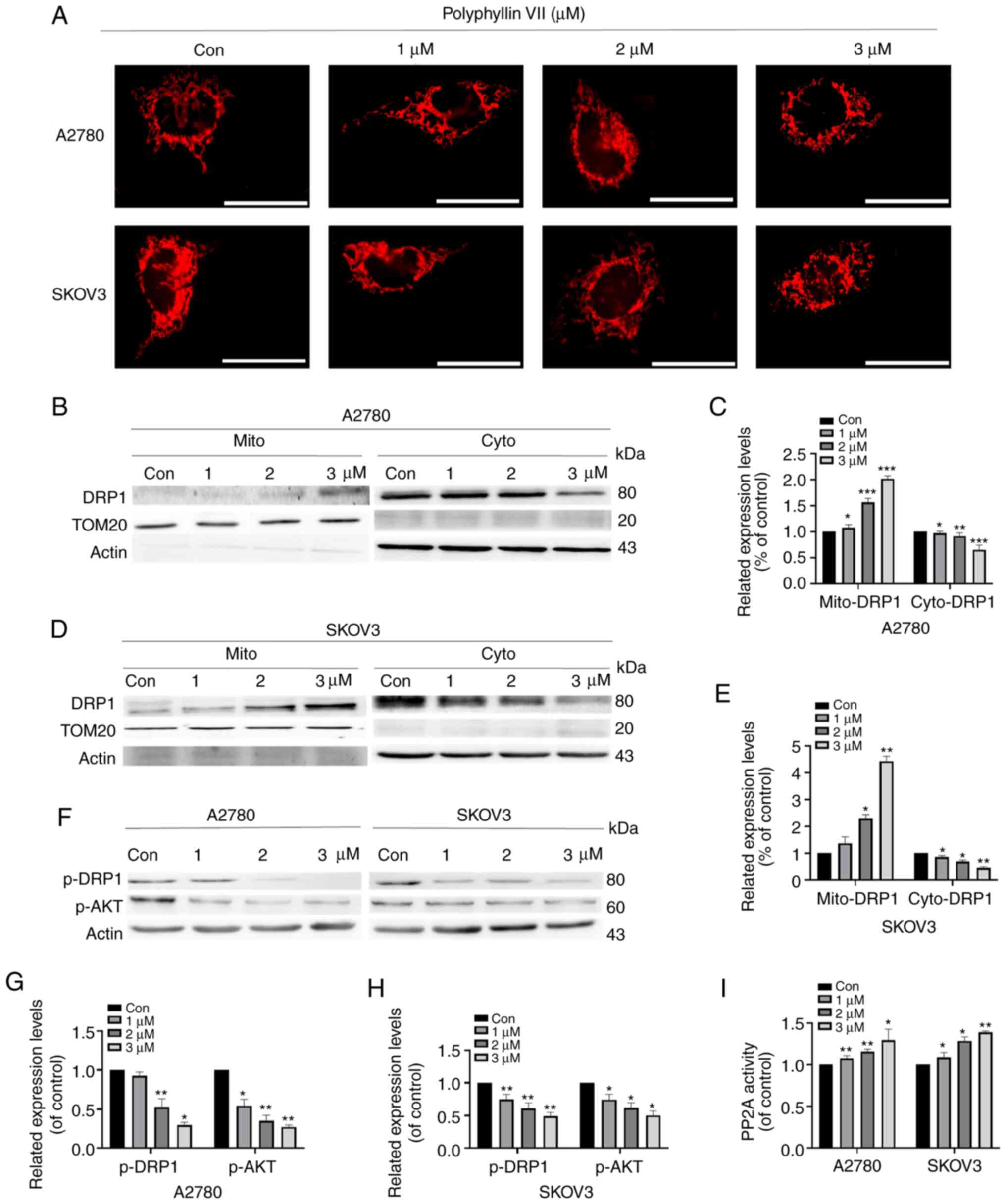
Polyphyllin VII intensifies
DRP1-dependent mitochondrial fission
We used fluorescence microscopy to observe
Mito-tracker red-stained mitochondrial morphology to evaluate
changes in mitochondrial dynamics in A2780 and SKOV3 cells. As
shown in Fig. 3A, after polyphyllin
VII treatment at concentrations of 1, 2, and 3 µM, the proportion
of mitochondrial fragmentation gradually increased compared with
the control group. After treatment with 3 µM polyphyllin VII,
approximately 50% of the A2780 and SKOV3 cells were fragmented from
the original grid-like mitochondrial structure. Additionally, we
extracted mitochondria to detect protein expression by western blot
analysis. DRP1 translocation to mitochondria was increased
following polyphyllin VII treatment, and this phenomenon was most
obvious at a concentration of 3 µM (Fig. 3B-E). Mitochondrial localization of
DRP1 is regulated by phosphorylation of DRP1 at Ser637. We found
that the expression of phosphorylated DRP1 decreased with the
increase in polyphyllin VII concentration. Studies have shown that
DRP1 is a direct downstream molecule of AKT, and AKT can
continuously regulate the activity of DRP1 (31,32).
AKT-DRP1 interactions induce phosphorylation of DRP1, leading to
mitochondrial division (Fig.
3F-H).
It has been shown that polyphyllin VII can inhibit
tumor cell vitality via the PP2A/AKT pathway (29). We found that polyphyllin VII can
downregulate phosphorylated AKT, and we hypothesized that
polyphyllin VII can further regulate DRP1 through the regulation of
AKT by PP2A. Therefore, to further examine the activation of PP2A
upstream of AKT, we applied the PP2A immunoprecipitation
phosphatase assay kit to detect the activity of PP2A, and found
that the activity of PP2A was increased with increased polyphyllin
VII concentration (Fig. 3I).
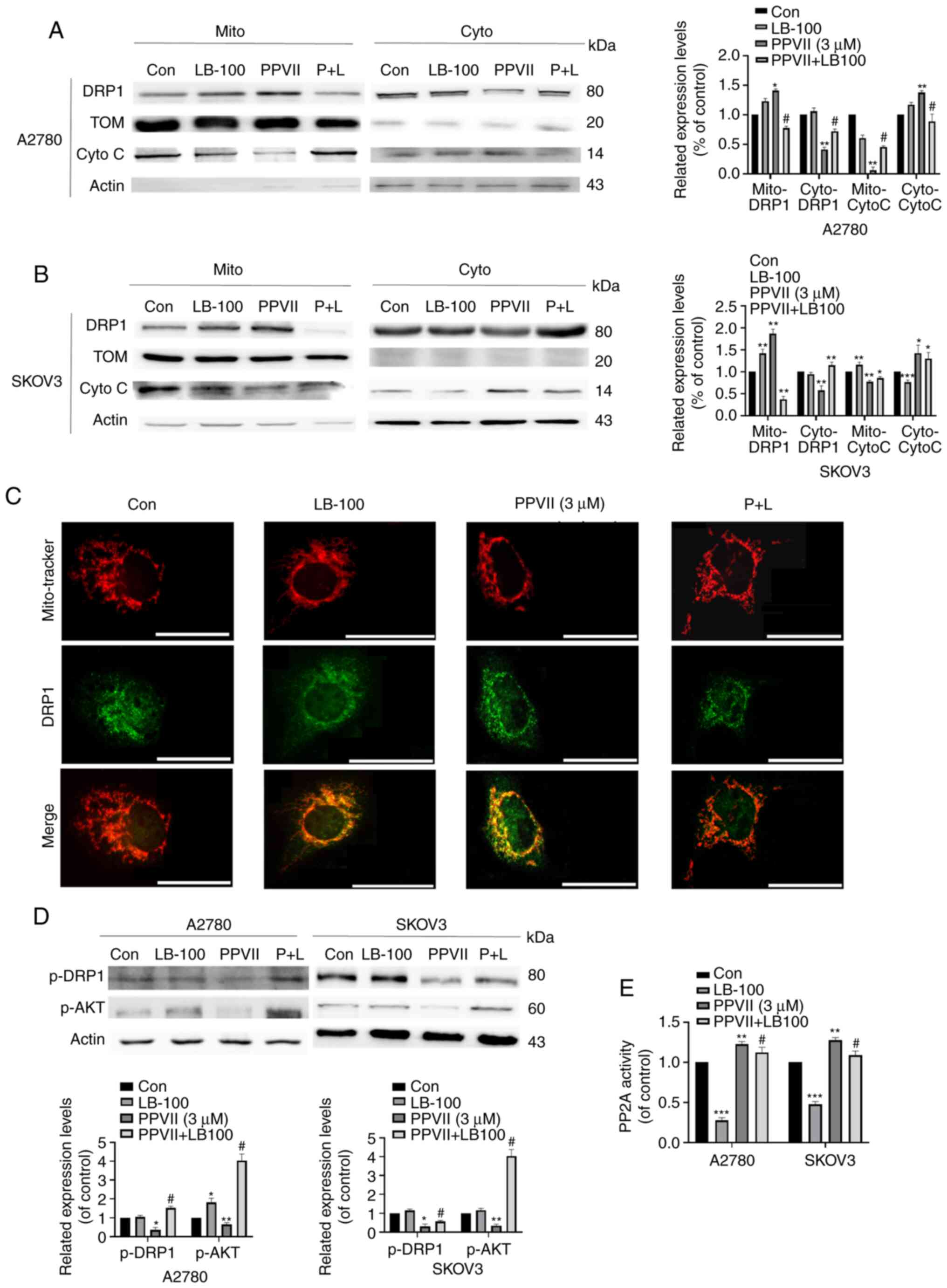
Polyphyllin VII regulates the
PP2A/AKT/DRP1 signaling axis in A2780 and SKOV3 cells
To further confirm the role of the PP2A pathway in
mediating the inhibition of polyphyllin VII on the proliferation of
ovarian cancer cells, we introduced LB-100, a specific inhibitor of
PP2A, to examine the changes of other molecules in the pathway. The
results showed that LB-100 could reverse the increasing effect of
DRP1 mitochondrial localization in the LB-100 group pretreated with
polyphyllin VII (Fig. 4A and B),
which was also confirmed by immunofluorescence (Fig. 4C). Cytochrome c was
significantly released in the cytoplasm after treatment with
polyphyllin VII, which was inhibited by LB-100 (Fig. 4A and B). In addition, p-AKT and
p-DRP1 expression decreased after polyphyllin VII treatment, which
was largely changed by LB-100 pretreatment (Fig. 4D). As shown in Fig. 4E, LB-100 can significantly inhibit
PP2A activity effectively, and it can reverse the increase of PP2A
activity induced by polyphyllin VII. Based on the above results, we
concluded that polyphyllin VII changes DRP1 localization in
mitochondrial by regulating the PP2A/AKT/DRP1 signaling axis,
thereby promoting mitochondrial division.
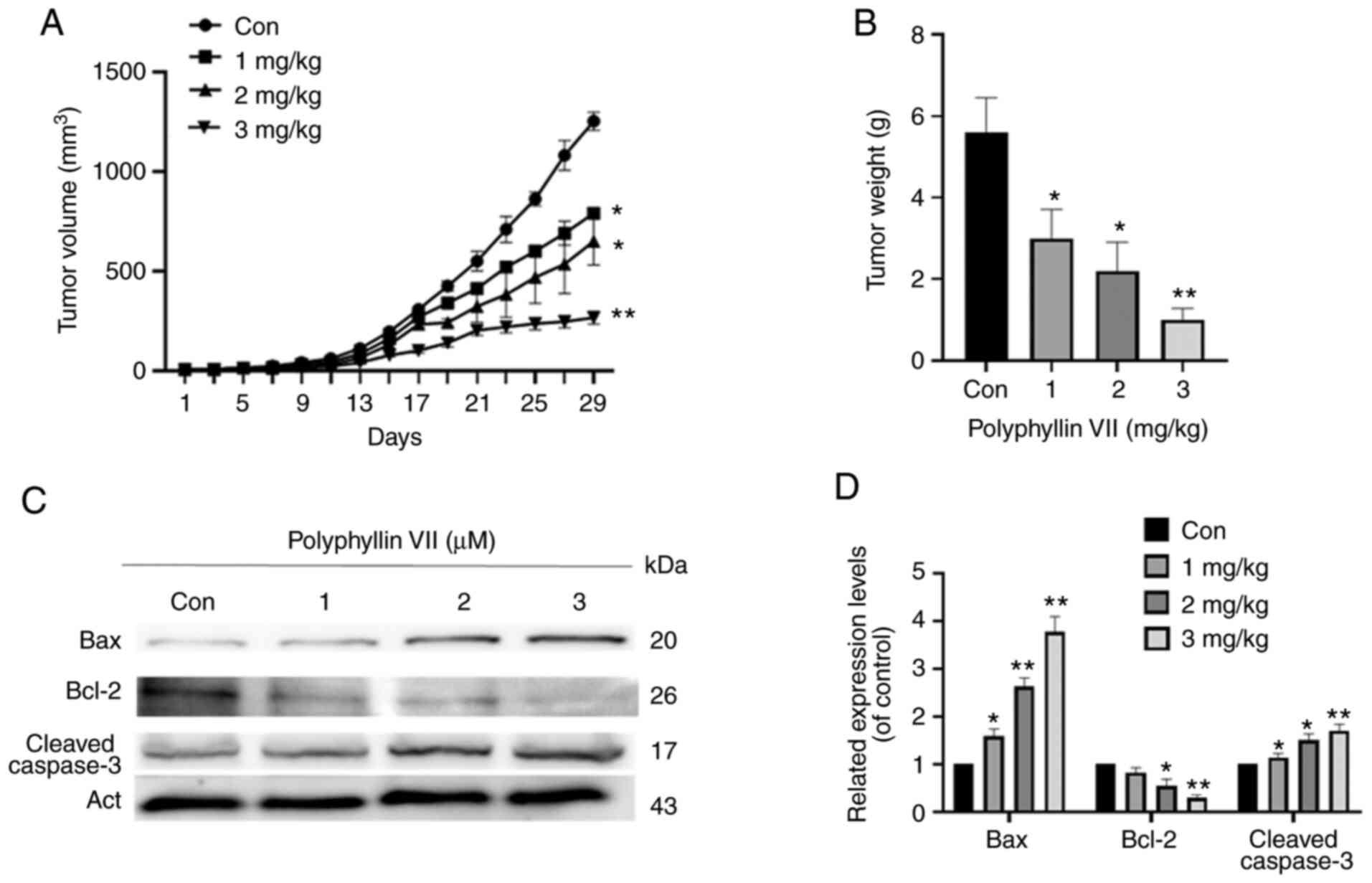
Polyphyllin VII inhibits tumor growth
in vivo
To study the effects of polyphyllin VII treatment
in vivo, we constructed a xenograft tumor model in nude mice
(Fig. 5A and B). Compared with the
control, the tumor growth was significantly inhibited, and the
weight of nude mice was significantly decreased (Fig. 5C and D). The ratio of BAX/BCL-2 was
upregulated and the expression of cleaved caspase-3 was
significantly increased (Fig. 5C).
These results further suggest that polyphyllin VII is a potential
treatment for ovarian cancer.
Discussion
Although the anti-tumor effect of polyphyllin VII on
various tumors including ovarian cancer has been widely studied
(5,6,29), the
changes in mitochondrial function and dynamics are still poorly
understood. To the best of our knowledge, this study is the first
to report that regulation of the PP2A/AKT/DRP1 axis promotes
mitochondrial division and inhibits the proliferation of human
ovarian cancer cells. We selected the most commonly used ovarian
cancer cell lines A2780 and SKOV3 to study the anticancer effects
of polyphyllin VII. Although A2780 and SKOV3 we used may not
represent the most common type of high-grade serous ovarian cancer
(HGSOC), our findings may have therapeutic value in patients with
ovarian cancer.
Mitochondria are the energy stations of the cell and
the main agents of cell death. They have multiple mechanisms that
control their homeostasis, among which constant fission and fusion
are important dynamics (33).
Imbalances in mitochondrial division and fusion often lead to
changes in mitochondrial structure and dysfunction. Our study found
that ovarian cells generated substantial amounts of ROS, which was
accompanied by Dym collapse after polyphyllin VII treatment.
Therefore, we aimed to determine whether this mitochondrial
dysfunction was related to increased mitochondrial division, and
whether it was a good point from which to start exploring new ways
to combat cancer. DRP1 mediates mitochondrial division, and can
translocate from the cytoplasm to the mitochondria and contract the
mitochondria so that mitochondrial division results in two
independent organelles. Dysfunctional mitochondria may lose their
ability to fuse by activating the mitochondrial division mechanism,
preventing the damaged mitochondria from re-entering the healthy
mitochondrial network (34). There
is increasing evidence that post-translational modification of DRP1
is an important mechanism for regulating its function (14). Under the stimulation of apoptosis,
the mitochondrial network collapses into a small spherical
structure, and the BCL-2 family has an important role in regulating
mitochondrial morphology (35).
During the process of cell apoptosis, BAX activation co-locates BAX
and DRP1 at mitochondrial sites where division occurs (16), and DRP1 stably binds to the
mitochondrial outer membrane, leading to mitochondrial
fragmentation (17–19). It has been shown that DRP1 activates
and oligomerizes BAX by promoting the formation of an intermediate,
creating a pore and leading to permeability of the mitochondrial
outer membrane (MOM) (36).
Mitochondrial division is accompanied by MOM permeability,
mitochondrial crest disorder, and the release of cytochrome
c (37,38). Our results showed that polyphyllin
VII treatment could increase DRP1 localization in mitochondria,
upregulate the mitochondrial BAX/BCL-2 ratio, and increase the
release of cytochrome c. Phosphorylated DRP1 (Ser637) can
regulate DRP1 localization in mitochondria and induce mitochondrial
division. Our study showed that polyphyllin VII can dephosphorylate
DRP1, thus recruiting DRP1 to mitochondria. The process of
mitochondrial fission runs through cell proliferation and is
affected by various kinases. AKT, which directly regulates DRP1, is
a serine/threonine kinase that has a significant role in cell
proliferation and survival (30,32).
Protein phosphatase A (PP2A) can regulate multiple
cellular processes by dephosphorylating many critical cellular
molecules such as PKC, AKT, β-catenin, and c-Myc, and plays an
important role as a tumor suppressor. PP2A has been shown to
regulate various biological processes in humans, such as cell DNA
replication, transcription, translation, cell cycle, cell
proliferation, apoptosis, and migration, and has also been shown to
regulate cell transformation and cancer (39–42).
PP2A is deactivated in various malignancies, including breast
(43), ovarian (44), cervical (45), and lung carcinoma (46). Partial loss of PP2A phosphatase
activity may lead to unrestricted carcinogenic kinase activity,
triggering carcinogenic signals. Reactivation of PP2A is an
effective way to antagonize cancer, and our study showed that
polyphyllin VII can activate PP2A and inactivate AKT and DRP1. The
application of PP2A inhibitors further confirmed that the mechanism
of action of polyphyllin VII is through the PP2A/AKT/DRP1 axis.
To summarize, the aim of this study was to explore
new drugs that promote cancer cell apoptosis by regulating
mitochondrial dynamics and to identify new therapies to prevent and
treat cancer progression. Our results showed that polyphyllin VII
can increase Drp1 localization in mitochondria, thus exacerbating
mitochondrial division and leading to apoptosis. We also found that
the above changes are due to the regulation of the PP2A/AKT/DRP1
signaling axis. However, further studies are required to verify
these results.
Acknowledgements
Not applicable.
Funding
The present study was funded by Natural Science
Foundation of Jilin Province (no. 20180101134C).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ contributed the experimental design and wrote the
draft. ZL and XD collected and sorted out relevant literature,
conducted experiments and collected data. JW and LS carried out
data analysis and revised the manuscript. LF and YZ made
substantial contributions to the interpretation and analysis of the
data, drafting the study and revising it critically for important
intellectual content. All authors have read and reviewed the final
manuscript.
Ethics approval and consent to
participate
The protocol was approved by the ethics committee of
Jilin University National Health Research Institute in accordance
with National Institutes of Health Guidelines for the Care and Use
of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang C, Li C, Jia X, Wang K, Tu Y, Wang
R, Liu K, Lu T and He C: In vitro and in vivo anti-inflammatory
effects of polyphyllin VII through downregulating MAPK and NF-ĸB
pathways. Molecules. 24:8752019. View Article : Google Scholar
|
|
2
|
Liu Z, Li N, Gao W, Man S, Yin S and Liu
C: Comparative study on hemostatic, cytotoxic and hemolytic
activities of different species of Paris L. J Ethnopharmacol.
142:789–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang XF, Cui Y, Huang JJ, Zhang YZ, Nie
Z, Wang LF, Yan BZ, Tang YL and Liu Y: Immuno-stimulating
properties of diosgenyl saponins isolated from Paris polyphylla.
Bioorg Med Chem Lett. 17:2408–2413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al Sawah E, Marchion DC, Xiong Y, Ramirez
IJ, Abbasi F, Boac BM, Bush SH, Bou Zgheib N, McClung EC,
Khulpateea BR, et al: The Chinese herb polyphyllin D sensitizes
ovarian cancer cells to cisplatin-induced growth arrest. J Cancer
Res Clin Oncol. 141:237–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Sun Z, Deng J, Liu J, Ma K, Si Y,
Zhang T, Feng T, Liu Y and Tan Y: Polyphyllin I inhibits invasion
and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling
in prostate cancer. Int J Oncol. 53:1279–1288. 2018.PubMed/NCBI
|
|
6
|
He J, Yu S, Guo C, Tan L, Song X, Wang M,
Wu J, Long Y, Gong D, Zhang R, et al: Polyphyllin I induces
autophagy and cell cycle arrest via inhibiting PDK1/Akt/mTOR signal
and downregulating cyclin B1 in human gastric carcinoma HGC-27
cells. Biomed Pharmacother. 117:1091892019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong F, Gu W, Jiang J, Liu X and Jiang H:
Anticancer activity of polyphyllin I in nasopharyngeal carcinoma by
modulation of lncRNA ROR and P53 signalling. J Drug Target.
27:806–811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin LT, Uen WC, Choong CY, Shi YC, Lee BH
and Tai CJ and Tai CJ: Paris polyphylla inhibits colorectal cancer
cells via inducing autophagy and enhancing the efficacy of
chemotherapeutic drug doxorubicin. Molecules. 24:21022019.
View Article : Google Scholar
|
|
9
|
Yang C, Cai H and Meng X: Polyphyllin D
induces apoptosis and differentiation in K562 human leukemia cells.
Int Immunopharmacol. 36:17–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Dong X, Wang W, You L, Yin X, Yang
C, Sai N, Leng X and Ni J: Molecular mechanisms of apoptosis in
HepaRG cell line induced by polyphyllin VI via the fas death
pathway and mitochondrial-dependent pathway. Toxins (Basel).
10:2012018. View Article : Google Scholar
|
|
11
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adam-Vizi V and Chinopoulos C:
Bioenergetics and the formation of mitochondrial reactive oxygen
species. Trends Pharmacol Sci. 27:639–645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choudhury AR and Singh KK: Mitochondrial
determinants of cancer health disparities. Semin Cancer Biol.
47:125–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tilokani L, Nagashima S, Paupe V and
Prudent J: Mitochondrial dynamics: Overview of molecular
mechanisms. Essays Biochem. 62:341–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cannino G, Ciscato F, Masgras I,
Sanchez-Martin C and Rasola A: Metabolic plasticity of tumor cell
mitochondria. Front Oncol. 8:3332018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karbowski M, Lee YJ, Gaume B, Jeong SY,
Frank S, Nechushtan A, Nechushtan A, Santel A, Fuller M, Smith CL
and Youle RJ: Spatial and temporal association of Bax with
mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J
Cell Biol. 159:931–938. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karbowski M, Arnoult D, Chen H, Chan DC,
Smith CL and Youle RJ: Quantitation of mitochondrial dynamics by
photolabeling of individual organelles shows that mitochondrial
fusion is blocked during the Bax activation phase of apoptosis. J
Cell Biol. 164:493–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brooks C, Wei Q, Feng L, Dong G, Tao Y,
Mei L, Xie ZJ and Dong Z: Bak regulates mitochondrial morphology
and pathology during apoptosis by interacting with mitofusins. Proc
Natl Acad Sci USA. 104:11649–11654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wasiak S, Zunino R and McBride HM: Bax/Bak
promote sumoylation of DRP1 and its stable association with
mitochondria during apoptotic cell death. J Cell Biol. 177:439–450.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hasnat M, Yuan Z, Ullah A, Naveed M, Raza
F, Baig MMFA, Khan A, Xu D, Su Y, Sun L, et al:
Mitochondria-dependent apoptosis in triptolide-induced
hepatotoxicity is associated with the Drp1 activation. Toxicol Mech
Methods. 30:124–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li G, Zhou J, Budhraja A, Hu X, Chen Y,
Cheng Q, Liu L, Zhou T, Li P, Liu E and Gao N: Mitochondrial
translocation and interaction of cofilin and Drp1 are required for
erucin-induced mitochondrial fission and apoptosis. Oncotarget.
6:1834–1849. 2005. View Article : Google Scholar
|
|
22
|
O'Connor CW, Perl A, Leonard D, Sangodkar
J and Narla G: Therapeutic targeting of PP2A. Int J Biochem Cell
Biol. 96:182–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raman D and Pervaiz S: Redox inhibition of
protein phosphatase PP2A: Potential implications in oncogenesis and
its progression. Redox Biol. 27:1011052019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rincon R, Cristobal I, Zazo S, Arpi O,
Menendez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J, et
al: PP2A inhibition determines poor outcome and doxorubicin
resistance in early breast cancer and its activation shows
promising therapeutic effects. Oncotarget. 6:4299–4314. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugiyama M, Imai A, Furui T and Tamaya T:
Independent action of serine/threonine protein phosphatase in
ovarian cancer plasma membrane and cytosol during
gonadotropin-releasing hormone stimulation. Oncol Rep.
10:1885–1889. 2003.PubMed/NCBI
|
|
26
|
Zheng HY, Shen FJ, Tong YQ and Li Y: PP2A
Inhibits cervical cancer cell migration by dephosphorylation of
p-JNK, p-p38 and the p-ERK/MAPK signaling pathway. Curr Med Sci.
38:115–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nader CP, Cidem A, Verrills NM and Ammit
AJ: Protein phosphatase 2A (PP2A): A key phosphatase in the
progression of chronic obstructive pulmonary disease (COPD) to lung
cancer. Respir Res. 20:2222019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perrotti D and Neviani P: Protein
phosphatase 2A: A target for anticancer therapy. Lancet Oncol.
14:e229–e238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Huang P, Liu X, Xiang Y, Zhang T,
Wu Y, Xu J, Sun Z, Zhen W, Zhang L, et al: Polyphyllin I inhibits
growth and invasion of cisplatin-resistant gastric cancer cells by
partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol
Sci. 137:305–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim DI, Lee KH, Gabr AA, Choi GE, Kim JS,
Ko SH and Han HJ: Aβ-induced Drp1 phosphorylation through Akt
activation promotes excessive mitochondrial fission leading to
neuronal apoptosis. Biochim Biophys Acta. 1863:2820–2834. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Wang HY, Wu B, Cheng CY, Xiao W,
Wang ZZ, Yang YY, Li P and Yang H: Ginkgolide K attenuates neuronal
injury after ischemic stroke by inhibiting mitochondrial fission
and GSK-3β-dependent increases in mitochondrial membrane
permeability. Oncotarget. 8:44682–44693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tao A, Xu X, Kvietys P, Kao R, Martin C
and Rui T: Experimental diabetes mellitus exacerbates
ischemia/reperfusion-induced myocardial injury by promoting
mitochondrial fission: Role of down-regulation of myocardial Sirt1
and subsequent Akt/Drp1 interaction. Int J Biochem Cell Biol.
105:94–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samanta K, Douglas S and Parekh AB:
Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+
oscillations, store-operated Ca2+ entry and
Ca2+-dependent gene expression in response to receptor
stimulation. PLoS One. 9:e1011882014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simula L, Nazio F and Campello S: The
mitochondrial dynamics in cancer and immune-surveillance. Semin
Cancer Biol. 47:29–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Montessuit S, Somasekharan SP, Terrones O,
Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ,
Bossy-Wetzel E, Basañez G, Meda P and Martinou JC: Membrane
remodeling induced by the dynamin-related protein Drp1 stimulates
bax oligomerization. Cell. 142:889–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YJ, Jeong SY, Karbowski M, Smith CL
and Youle RJ: Roles of the mammalian mitochondrial fission and
fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell.
15:5001–5011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frank S, Gaume B, Bergmann-Leitner ES,
Leitner WW, Robert EG, Catez F, Smith CL and Youle RJ: The role of
dynamin-related protein 1, a mediator of mitochondrial fission, in
apoptosis. Dev Cell. 1:515–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alberts AS, Thorburn AM, Shenolikar S,
Mumby MC and Feramisco JR: Regulation of cell cycle progression and
nuclear affinity of the retinoblastoma protein by protein
phosphatases. Proc Natl Acad Sci USA. 90:388–392. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Glenn GM and Eckhart W: Mutation of a
cysteine residue in polyomavirus middle T antigen abolishes
interactions with protein phosphatase 2A, pp60c-src, and
phosphatidylinositol-3 kinase, activation of c-fos expression, and
cellular transformation. J Virol. 67:1945–1952. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ronne H, Carlberg M, Hu GZ and Nehlin JO:
Protein phosphatase 2A in saccharomyces cerevisiae: Effects on cell
growth and bud morphogenesis. Mol Cell Biol. 11:4876–4884. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wlodarchak N and Xing Y: PP2A as a master
regulator of the cell cycle. Crit Rev Biochem Mol Biol. 51:162–184.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tseng LM, Liu CY, Chang KC, Chu PY, Shiau
CW and Chen KF: CIP2A is a target of bortezomib in human triple
negative breast cancer cells. Breast Cancer Res. 14:R682012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bockelman C, Lassus H, Hemmes A, Leminen
A, Westermarck J, Haglund C, Bützow R and Ristimäki A: Prognostic
role of CIP2A expression in serous ovarian cancer. Br J Cancer.
105:989–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shih IeM, Panuganti PK, Kuo KT, Mao TL,
Kuhn E, Jones S, Velculescu VE, Kurman RJ and Wang TL: Somatic
mutations of PPP2R1A in ovarian and uterine carcinomas. Am J
Pathol. 178:1442–1447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu P, Xu XL, Huang Q, Zhang ZH and Zhang
YB: CIP2A with survivin protein expressions in human non-small-cell
lung cancer correlates with prognosis. Med Oncol. 29:1643–1647.
2012. View Article : Google Scholar : PubMed/NCBI
|