Introduction
Ovarian cancer is the most lethal gynecological
malignancy in developed countries (1). In 2018, approximately 295,400 new
cases of ovarian cancer were diagnosed and 184,800 patients with
ovarian cancer died worldwide (2).
Clear cell carcinoma (CCC) is one of the common histological types
of epithelial ovarian cancer (EOC) (3). The frequency of ovarian CCC (OCCC)
varies depending on ethnicity; CCC accounts for 11.7–26.9% of
Japanese EOC cases in comparison with 4.6–8.4% of EOC in North
America (4,5). About half of the OCCC cases are
diagnosed at stage I and have a good prognosis (6). However, advanced stage or recurrent
OCCC cases have worse prognosis than the other EOC subtypes due to
the resistance to standard platinum-based chemotherapy (7). Therefore, early detection and complete
resection are crucial in OCCC treatment. Cancer antigen 125 (CA125)
is currently the most frequently used serum biomarker for EOC.
However, CA125 is also elevated in benign conditions such as
endometrial cyst and peritonitis, menstruation and other
intra-abdominal malignancies (8).
Thus, CA125 does not necessarily distinguish malignancy.
Additionally, CA125 often fails to detect OCCC even at advanced
stages (9).
Tissue factor pathway inhibitor-2 (TFPI-2) protein,
a homologue of tissue factor pathway inhibitor (TFPI), is a
secreted protease inhibitor containing an N-terminal signal peptide
and Kunitz-type serine protease inhibitory domains (10). Despite its structural similarity to
TFPI, TFPI-2 has weak inhibitory activity against the tissue factor
blood coagulation pathway, which is initiated by the serine
protease tissue factor-coagulation factor VIIa complex, and instead
inhibits a wide variety of serine proteases, such as plasmin,
plasma kallikrein, trypsin and chymotrypsin (10). TFPI-2 is predominantly and highly
expressed in placenta (11,12). Although several studies have
examined the association between TFPI-2 and preeclampsia (13,14),
the biological function of TFPI-2 is not fully understood.
Many reports have shown that TFPI-2 is genetically
silenced in aggressive cancers, such as glioma (15), non-small cell lung cancer (16), pancreatic cancer (17), breast cancer (18), malignant melanoma (19) and hepatocellular carcinoma (20), indicating its tumor-suppressor
character. The anticancer functions of TFPI-2 are generally thought
to be mediated by its protease inhibitory activities, which lead to
inhibition of cell proliferation, invasion or angiogenesis and
augmentation of apoptosis (21,22).
Recent studies also suggest another tumor-suppressor aspect of
TFPI-2, demonstrating that exogenously applied TFPI-2 localized in
the nucleus of fibrosarcoma cells (23) and overexpressed TFPI-2 in breast
cancer cells negatively regulate matrix metalloproteinase-2 (MMP-2)
expression (24).
In contrast to the results showing epigenetic
silencing of TFPI-2 in several tumor types, we recently reported
that cultivated OCCC cells produce and secrete TFPI-2 into medium
and we initiated studies to develop TFPI-2 as a specific serum
biomarker for preoperative clinical diagnosis for OCCC (25,26).
Serum TFPI-2 level discriminated CCC from other histological types
of EOC and endometrial cyst (26),
which is a risk factor for CCC (27). Although we are considering that
serum TFPI-2 is derived from OCCC tumor cells, TFPI-2 expression
was also reported in endothelial cells, which are distributed
throughout the body (23).
Furthermore, non-secreted fractions of TFPI-2 were reported in
in vitro studies in other tumor types. Therefore, in the
present study, we examined TFPI-2 expression and localization of
TFPI-2 in multiple OCCC cell lines and in surgically removed OCCC
tissues including tissues of other EOC histologic types. We also
investigated the association between TFPI-2 expression and clinical
characteristics of OCCC patients to clarify the role of TFPI-2 in
OCCC.
Materials and methods
Cell lines and cell culture
The OCCC cell lines ES-2 (ATCC CRL-1978) and TOV-21G
(ATCC CRL-11730) were purchased from the American Type Culture
Collection. OVISE (JCRB1043), OVMANA (JCRB1045), OVTOKO (JCRB1048),
RMG-1 (JCRB0172) and HAC-2 (JCRB1359) cells were obtained from JCRB
Cell Bank. JHOC-5 (RCB1520), JHOC-7 (RCB1688), JHOC-8 (RCB1723) and
JHOC-9 (RCB2226) cell lines were from RIKEN Bioresource Center Cell
Bank. These OCCC cell lines were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) and
penicillin-streptomycin at 37°C in a humidified atmosphere of 5%
CO2.
Preparation of subcellular
fractions
Cells were cultured for 2 days in 100-mm plates
until they reached semi-confluency. Cells were washed with
phosphate-buffered saline (PBS) and then dissociated using Accutase
reagent (Nacalai Tesque) according to the manufacturer's
instruction. Dissociated cells were collected to prepare the whole
cell fraction (WCF). Plates were rinsed twice with PBS, and the
fraction that remained attached to the plate was collected by
scraping the plates with lysis buffer and was considered the
extracellular fraction (ECF). (NuPAGE NP0007, Thermo Fisher
Scientific, Inc.). The Nuclear Extract Kit (Active Motif Inc.) was
used for preparation of cytoplasmic and nuclear fractions from WCFs
according to the manufacturer's instructions. Cells were cultured
with 10 ml of RPMI-1640 medium supplemented with 10% FBS and
penicillin-streptomycin for 2 days in 100-mm plates. Culture medium
of semi-confluent cells was collected and centrifuged at 180 × g
for 3 min. The supernatant was obtained as conditioned medium
(CM).
Western blotting
Western blotting was performed using the NuPAGE
4–12% gradient Bis-Tris Protein Gel system (Thermo Fisher
Scientific, Inc.) with MOPS running buffer (Thermo Fisher
Scientific, Inc.). To detect TFPI-2, we used mouse monoclonal
anti-TFPI-2 antibody (clone 28Aa, 1 µg/ml, diluted 1:2,000) raised
against a synthetic peptide antigen corresponding to the N-terminal
of mature TFPI-2 protein after cleavage of the putative signal
peptide (13). Anti-vinculin
(V9131, diluted 1:10,000, Sigma-Aldrich; Merck KGaA), anti-Lamin A
(sc-20680, diluted 1:500, Santa Cruz Biotechnology, Inc.) and
anti-α-tubulin antibodies (T-9026, diluted 1:3,000, Sigma-Aldrich;
Merck KGaA) were used for protein loading controls. Secondary
antibody reaction was performed with peroxidase-conjugated
anti-mouse IgG (NA931, 1:100,000, Cytiva) or anti-rabbit IgG
(NA934, 1:100,000, Cytiva). Detection was performed using the
ImmunoStar LD enhanced chemiluminescence detection reagent
(FUJIFILM Wako Chemicals).
TFPI-2 concentration in CM
The TFPI-2 concentration in CM was measured on an
automated immunoassay analyzer (AIA) system (TOSOH, Japan) as
described previously (26).
Briefly, measurement of TFPI-2 using the AIA system was completed
as a sandwich-type, one-step immune fluorometric assay using two
different anti-TFPI-2 monoclonal antibodies, one of which was
coated on magnetic beads and the other was labeled with alkaline
phosphatase. As the calibration standard of the assay, recombinant
TFPI-2 protein was prepared from the CM of SP2/0 cells transfected
with the TFPI-2 expression vector and spiked into sample dilution
buffer.
Patients and sample collection
A total of 142 patients with a confirmed
histopathological diagnosis of EOC at Kanagawa Cancer Center
Hospital (KCCH), Japan were included in this study. Patients who
underwent treatment before primary debulking surgery or exploratory
laparotomy were excluded. Patients with other cancers were also
excluded. We examined all 71 EOC patients who matched the criteria
from 2014 to 2017 to evaluate the expression of TFPI-2 along with
the histological subtypes. Due to the small number of the included
cases, 8 patients with endometrioid carcinoma and 14 patients with
mucinous carcinoma were selected from the period before 2014 and
additionally examined. Formalin-fixed and paraffin-embedded (FFPE)
tissue sectioned to 4 µm-thickness were prepared from archives of
the Department of Pathology, KCCH. Whole tissue sections of tumors
of all enrolled patients were analyzed. Representative
non-neoplastic regions of the surgical specimens of EOC cases were
also examined in 18 cases, including endometrium and fallopian
tubal epithelium (CCC: 9, serous: 3, endometrioid: 3, mucinous: 3).
Written informed consent for research using specimens derived from
routine clinical procedures was obtained from all patients. The
experimental protocol of the present study was reviewed and
approved by the Institutional Review Board of KCCH (approval no.
Ethics-2018-10).
Immunohistochemical analysis of TFPI-2
expression
FFPE tissue specimens on glass slides were routinely
stained with hematoxylin and eosin. Deparaffinized and rehydrated
slides were immersed in 0.01 M citrate, pH 6.0 (Sigma-Aldrich;
Merck KGaA), and heat-induced antigen retrieval was performed in an
autoclave at 110°C for 15 min. Slides were cooled to room
temperature, washed in PBS and immersed in 3%
H2O2 diluted in methanol. For primary
antibody, 28Aa antibody was diluted to 5 µg/ml. Histofine
Simplestain Max PO (M) (Nichirei) and Histofine DAB Substrate kit
(Nichirei) were used to detect the labeled antigens. Placental
tissue was used as positive control for TFPI-2 staining (13). Non-specific mouse IgG was used as a
negative control. We conducted an absorption test to evaluate the
specificity of the staining. Antibodies were incubated with a
20-fold excess molar concentration of the antigen for 24 h prior to
the primary antibody reaction (28). The antigen for the 28Aa antibody is
the 14 amino acid residues corresponding to the N-terminus of
mature TFPI-2 protein, NH2-DAAQEPTGNNAEIC-COOH (13), linked to keyhole limpet hemocyanin.
We used another anti-TFPI-2 antibody B-7 (sc-48380, diluted 1:200,
Santa Cruz Biotechnology, Inc.) for detection of nuclear TFPI-2.
The B-7 antibody is a mouse monoclonal antibody that was raised
against peptides corresponding to amino acid residues 71–190 of
human TFPI-2. We also conducted an absorption test using placental
tissue with recombinant full-length TFPI-2 protein (OriGene) as
antigen. TFPI-2 protein staining (cytoplasmic and nuclear staining)
was scored by the H-score method (29). Briefly, the H-score was calculated
as the sum of the products of multiplying the staining intensity
(0, 1+, 2+, 3+) by percentage stained area. For example, in a case
with the intensity and percentage staining of 0+: 70%, 1+: 20%, 2+:
10% and 3+: 0%, the H-score is calculated as 40 (40=0×70 + 1×20 +
10×2 + 0×3). Under a pathologist supervision, automated scoring on
tumor regions was performed using Aperio's annotation software
‘Aperio Cytoplasm Algorithm’ (Leica Biosystem). We defined the
cut-off value for TFPI-2 positivity as an H-score of 1 to reduce
false negatives. We evaluated TFPI-2 expression within
extracellular matrix (ECM) as ‘positive’ or ‘negative.’ We analyzed
TFPI-2 expression and clinical characteristics of the OCCC
patients.
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistics 19 software (IBM Corp.). Clinicopathological parameters
were evaluated using Kruskal-Wallis test or Mann-Whitney U test for
continuous variables and Fisher's exact test for non-continuous
variables. Relationships between TFPI-2 expression and 5-year
overall survival were estimated by Kaplan-Meier method and compared
by log rank test. Cox regression analysis was used for multivariate
analysis of 5-year overall survival. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression, subcellular localization
and secretion of TFPI-2 in OCCC cell lines
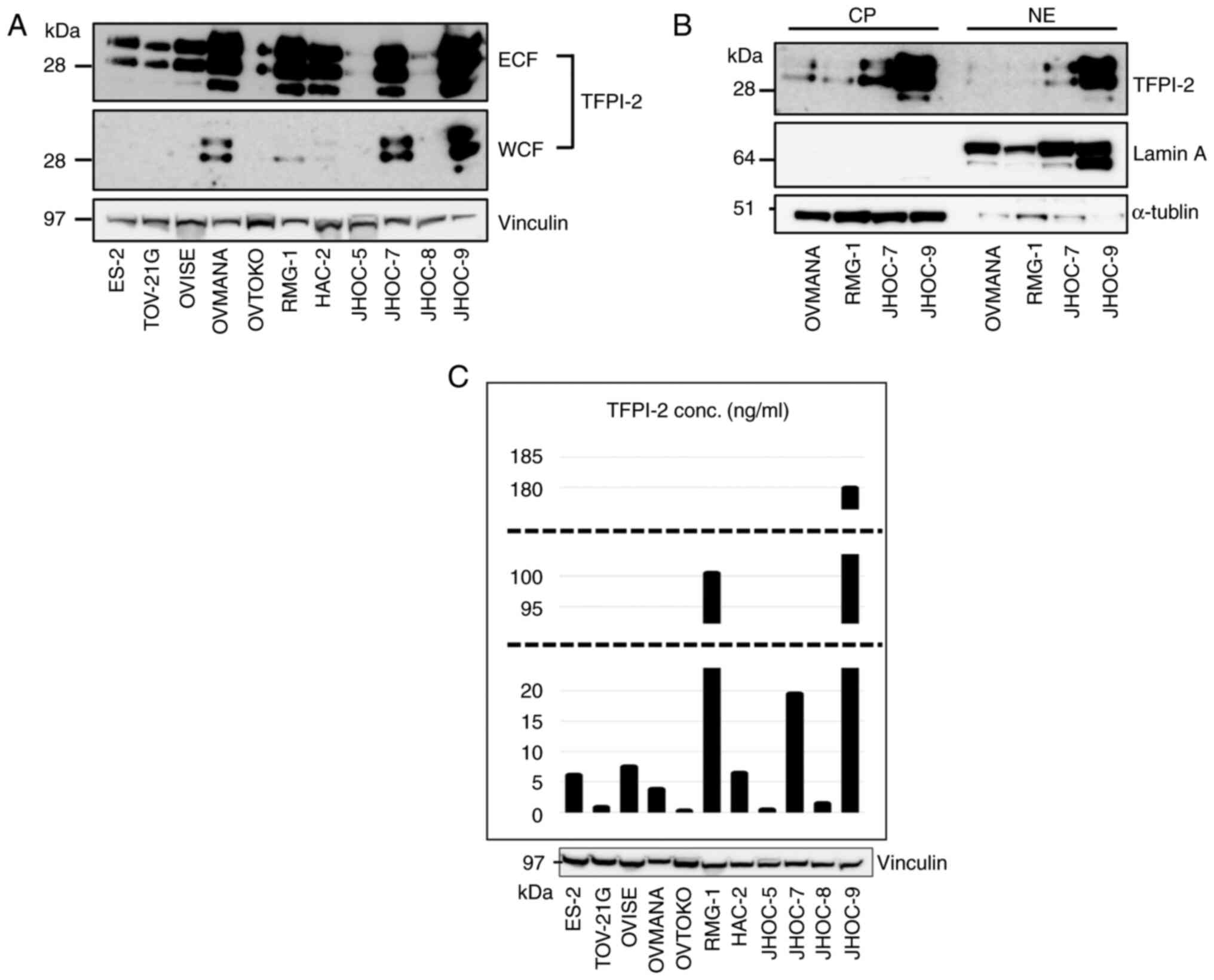
Western blotting using the monoclonal anti-TFPI-2
28Aa antibody (13) revealed that
TFPI-2 was expressed in 8 out of the 11 CCC cell lines examined
(Fig. 1A). All eight cell lines
showed TFPI-2 expression in ECF and four cell lines also expressed
TFPI-2 in the WCF. In all cell lines, TFPI-2 was much more abundant
in ECF than in WCF. We next fractionated TFPI-2 containing WCFs of
the four cell lines into nuclear and cytoplasmic fractions. TFPI-2
was detected in both cytoplasmic (CP) and nuclear fractions (NE)
(Fig. 1B). TFPI-2 polypeptides of
three molecular weights (27, 31, 33 kDa) (12) were observed in all 3 fractions, but
the larger two molecules were predominant (Fig. 1B). Three cell lines did not express
TFPI-2 in any fraction. We also examined TFPI-2 concentration in CM
(Fig. 1C). The amount of secreted
TFPI-2 in the CM was generally correlated to the levels in ECF.
RMG-1 and OVMANA cells strongly expressed TFPI-2 in ECF by western
blotting. In contrast, TFPI-2 concentration was high in CM in RMG-1
cells but low in OVMANA cells.
Immunohistochemical analysis of TFPI-2
expression in surgically removed EOC tissues
FFPE samples prepared from 142 patients including 77
OCCC and 65 non-CCC EOC cases were subjected to
immunohistochemistry (IHC). The patient clinical information is
shown in Table I. The mean age of
patients at surgery was 57 years (range 36–84 years).
 | Table I.Clinicopathological characteristics
of the 142 epithelial ovarian cancer patients. |
Table I.
Clinicopathological characteristics
of the 142 epithelial ovarian cancer patients.
|
Characteristics | OCCC (n=77) | SC (n=20) | EMC (n=19) | MOC (n=17) | Others (n=9) | P-value |
|---|
| Period (year) | 2005 to 2017 | 2014 to 2017 | 2011 to 2017 | 2005 to 2017 | 2014 to 2017 |
|
| Age in years,
median (range) | 58 (36–75) | 67.5 (37–80) | 54 (38–83) | 55 (38–84) | 60 (47–83) | P=0.0875 |
| Parity (%) |
|
|
|
|
| P=0.024 |
| No
(0) | 36 (46.8) | 4 (20.0) | 7 (36.8) | 3 (17.6) | 6 (66.7) |
|
| Yes
(≥1) | 41 (53.2) | 16 (80.0) | 12 (63.1) | 14 (82.4) | 3 (33.3) |
|
| Menopausal status
(%) |
|
|
|
|
| P=0.149 |
|
Premenopause | 18 (23.4) | 2 (10.0) | 7 (36.8) | 7 (41.2) | 3 (33.3) |
|
|
Postmenopause | 59 (76.6) | 18 (90.0) | 12 (63.1) | 10 (58.8) | 6 (66.7) |
|
| CA125 (%) |
|
|
|
|
| P=0.321 |
|
<35 | 24 (31.2) | 2 (10.0) | 7 (36.8) | 5 (29.4) | 2 (22.2) |
|
|
≥35 | 53 (68.8) | 18 (90.0) | 12 (63.1) | 12 (70.6) | 7 (77.8) |
|
| FIGO (%) |
|
|
|
|
| P<0.001 |
|
I/II | 61 (79.2) | 3 (15.0) | 17 (89.4) | 16 (94.1) | 5 (55.6) |
|
|
III/IV | 16 (20.8) | 17 (85.0) | 2 (10.5) | 1 (5.9) | 4 (44.4) |
|
| Site of specimen
(%) |
|
|
|
|
| P<0.001 |
| Primary
site | 77 (100) | 17 (85.0) | 19 (100) | 17 (100) | 7 (77.8) |
|
|
Omentum | 0 (0) | 3 (15.0) | 0 (0) | 0 (0) | 2 (22.2) |
|
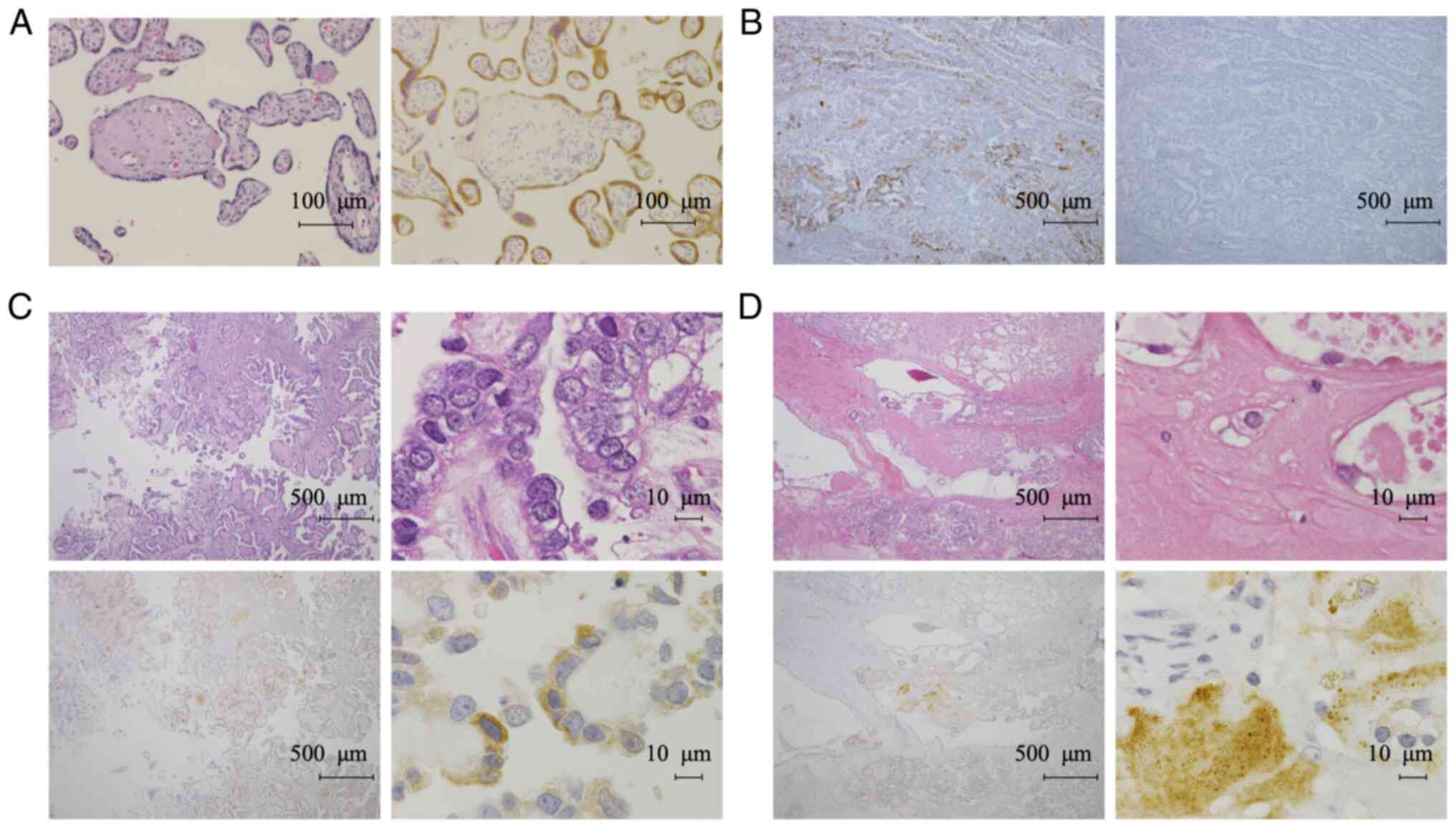
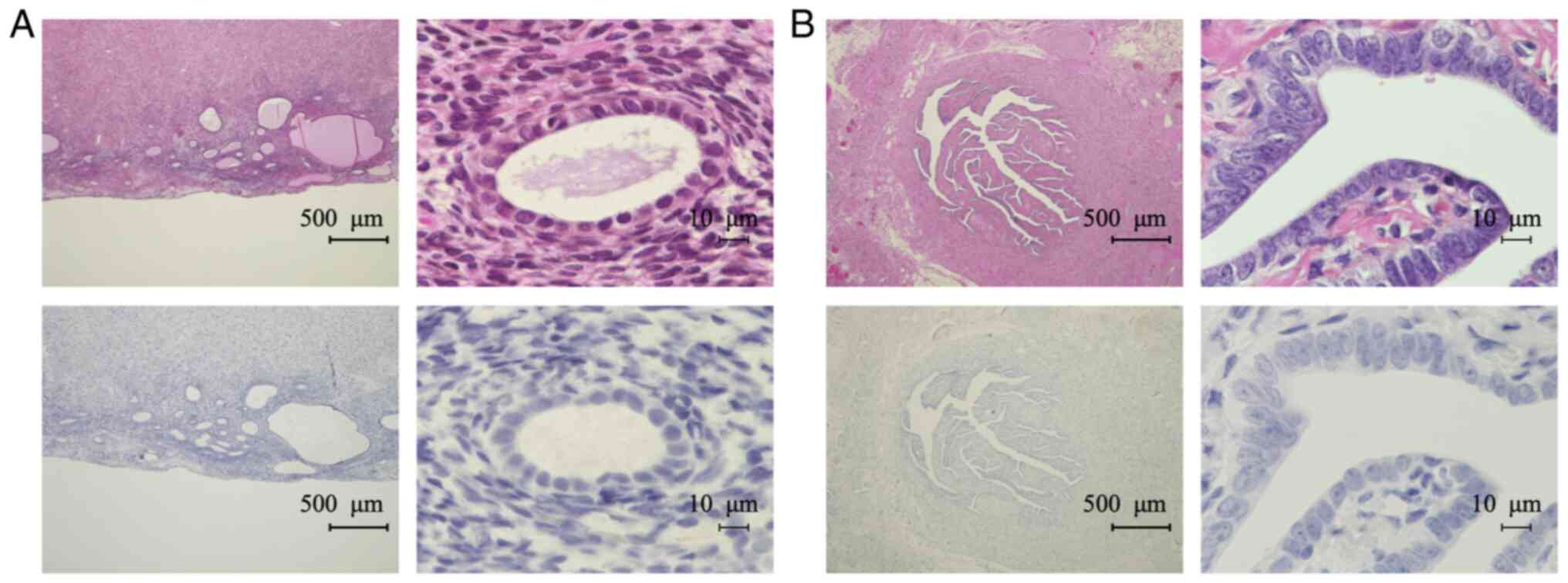
Experiments with placental tissue confirmed that the
antibody stained the cytoplasm of syncytiotrophoblasts, as reported
previously (13) (Fig. 2A). We confirmed the specificity of
the antibody by an absorption test using the immunized antigen for
the 28Aa antibody (Fig. 2B). IHC
revealed TFPI-2 in the cytoplasm of tumor cells and in the ECM of
OCCC tissues (Fig. 2C and D). We
did not detect any nuclear TFPI-2 staining using the 28Aa antibody.
Therefore, we next assessed the localization of TFPI-2 using
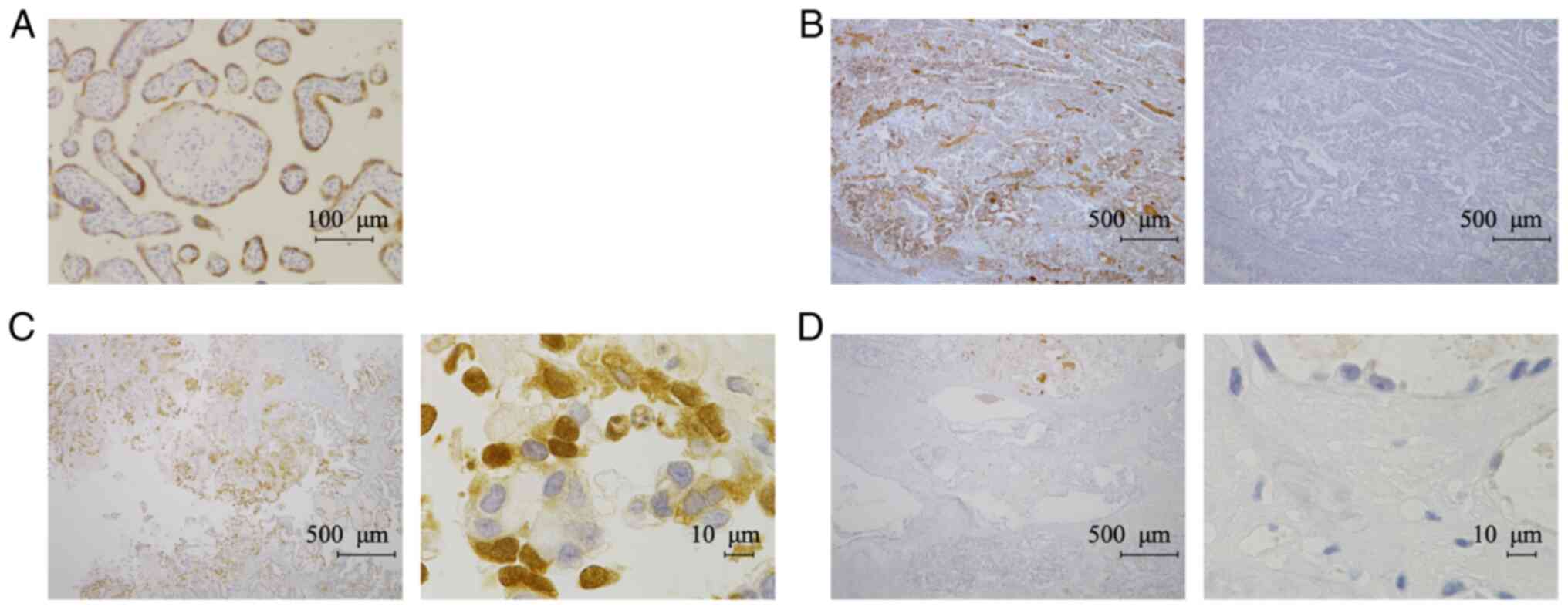
another TFPI-2 antibody (B-7). We confirmed that the B-7 antibody
also stained the cytoplasm of syncytiotrophoblasts in placental
tissue (Fig. 3A). The specificity
of the B-7 antibody was confirmed by absorption test (Fig. 3B). We detected TFPI-2 both in the
nucleus and cytoplasm with the B-7 antibody (Fig. 3C); however, signals in ECM were
weaker than in staining with the 28Aa antibody (Figs. 2D and 3D). Therefore, we decided to use the B-7
antibody to evaluate nuclear and cytoplasmic expression of TFPI-2,
while the 28Aa antibody was used to evaluate TFPI-2 expression in
ECM.
The H-score method using automated scoring software
was applied to evaluate TFPI-2 staining (Fig. S1). The H-scores and staining
categorization of EOC tissues are shown in Table II. Among OCCC cases, 52/77 (67.5%)
specimens were positive for TFPI-2; among these samples, 35/77
(45.5%) showed cytoplasmic staining, 10/77 (13.0%) showed nuclear
staining and 35/77 (45.5%) showed staining in ECM (shown as a Venn
diagram in Fig. S2). All cases
with positive nuclear staining also showed positive staining in the
cytoplasm, and 7/77 (9.1%) cases showed positive staining in all
three fractions (Fig. S2). In
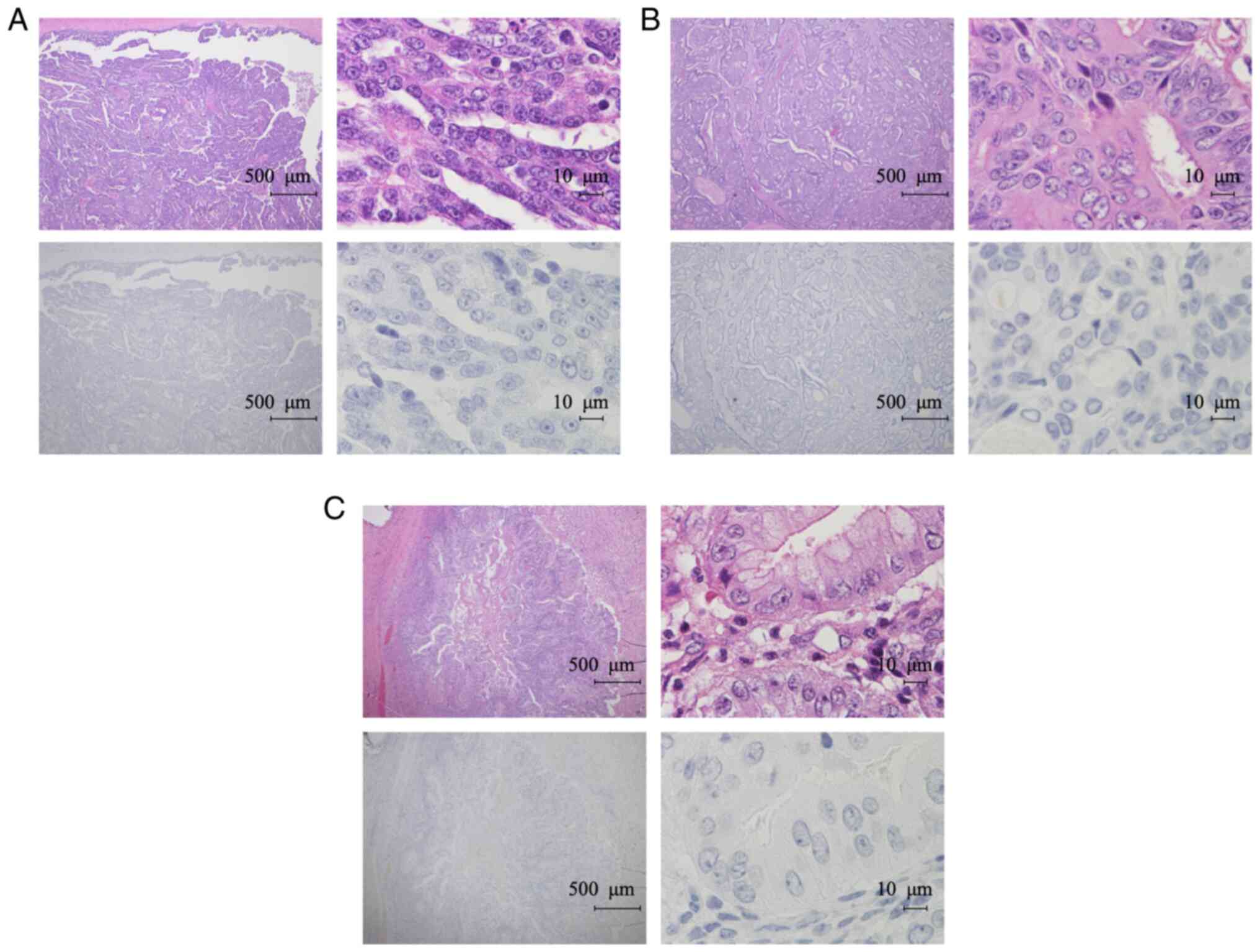
contrast, TFPI-2 was not detected in any of the non-CCC cases
(Fig. 4A-C). TFPI-2 expression
levels evaluated by IHC distinguished CCC from non-CCC with 67.5%
sensitivity and 100% specificity. Previous studies showed that
TFPI-2 is expressed in endometrium (30,31).
Therefore, we next performed IHC for the non-tumor samples using
B-7 antibody in the same manner. Out of 18 cases, 17 cases were
negative for TFPI-2 in endometrium cells (Fig. 5A). In one case (5.6%), endometrium
cells were focally positive for TFPI-2. Fallopian tube epithelial
cells were all negative for TFPI-2 expression (Fig. 5B).
 | Table II.TFPI-2 expression score according to
subcellular localization. |
Table II.
TFPI-2 expression score according to
subcellular localization.
| Subcellular
localization | H-score | CCC (n=77) n
(%) | Non-CCC (n=65) n
(%) |
|---|
| Nuclear |
|
|
|
|
Negative | 0 | 67 (87.0) | 65 (100) |
|
Positive | 1–9 | 1 (1.3) | 0 (0) |
|
| 10–29 | 4 (5.2) | 0 (0) |
|
| 30- | 5 (6.5) | 0 (0) |
| Cytoplasm |
|
|
|
|
Negative | 0 | 42 (54.5) | 65 (100) |
|
Positive | 1–9 | 20 (26.0) | 0 (0) |
|
| 10–29 | 9 (11.7) | 0 (0) |
|
| 30- | 6 (7.8) | 0 (0) |
| ECM |
|
|
|
|
Negative |
| 42 (54.5) | 65 (100) |
|
Positive |
| 35 (45.5) | 0 (0) |
We next statistically analyzed the correlations
between TFPI-2 cytoplasmic expression and clinicopathological
characteristics of the OCCC patients according to previous studies
(32,33). We examined patient age, parity,
menopausal status, rate of elevated serum CA125 level (>35 U/ml)
and distribution of cancer stage (FIGO: International Federation of
Gynecology and Obstetrics staging and TNM classification) in
univariate analysis according to the cytoplasmic expression status
for TFPI-2 (Table III). The
median patient age was significantly younger for patients positive
for TFPI-2 than for patients negative for TFPI-2 (56 vs. 60.5
years, respectively; P=0.019). Parity, menopausal status, rate of
elevated serum level of CA125, FIGO and TNM staging did not
significantly correlate with TFPI-2 expression. Kaplan-Meier
analysis showed that the 5-year overall survival was not
significantly affected by TFPI-2 expression (P=0.621, log-rank
test) (Fig. S3A). Multivariate
analysis revealed that TFPI-2 expression was not an independent
prognostic factor (Table SI).
Analyses with nuclear and ECM TFPI-2 expression showed similar
results (Fig. S3B-D, Tables SI–SIV).
 | Table III.Clinicopathological characteristic
and TFPI-2 cytoplasmic expression in 77 CCC samples. |
Table III.
Clinicopathological characteristic
and TFPI-2 cytoplasmic expression in 77 CCC samples.
|
Characteristics | Negative
(n=42) | Positive
(n=35) | P-value |
|---|
| Age in years,
median (range) | 60.5 (36–74) | 56 (39–75) | P=0.019 |
| Parity, n (%) |
|
|
|
| No
(0) | 20 (47.6) | 16 (45.7) |
|
| Yes
(≥1) | 22 (52.4) | 19 (54.3) | P=0.990 |
| Menopausal status,
n (%) |
|
|
|
|
Premenopause | 7 (16.7) | 11 (31.4) |
|
|
Postmenopause | 35 (83.3) | 24 (68.6) | P=0.177 |
| CA125 (U/ml), n
(%) |
|
|
|
|
<35 | 13 (31.0) | 11 (31.4) |
|
|
≥35 | 29 (69.0) | 24 (68.6) | P=0.990 |
| FIGO, n (%) |
|
|
|
|
I/II | 32 (76.2) | 29 (82.9) |
|
|
III/IV | 10 (23.8) | 6 (17.1) | P=0.577 |
| pT |
|
|
|
|
pT1/2 | 33 (78.6) | 29 (82.9) |
|
|
pT3 | 9 (21.4) | 6 (17.1) | P= 0.775 |
| pN |
|
|
|
|
pN0 | 8 (19.0) | 4 (11.4) |
|
|
pN1 | 1 (2.4) | 0 (0) |
|
|
pNx | 33 (78.6) | 31 (88.6) | P=0.441 |
| M |
|
|
|
| M0 | 41 (97.6) | 33 (94.3) |
|
| M1 | 1 (2.4) | 2 (5.7) | P=0.588 |
Discussion
In the present study, we found that tissue factor
pathway inhibitor-2 (TFPI-2) is expressed in surgically removed
ovarian clear cell carcinoma (OCCC) tissues. We previously
identified TFPI-2 as a CCC biomarker using secretome-based analysis
of CM derived from OCCC cell lines (25,26)
and reported that TFPI-2 may be a useful serum biomarker for OCCC
patients. The confirmation of TFPI-2 expression in OCCC tumor cells
in surgical tissues using IHC strongly supports the development of
TFPI-2 as a serum tumor biomarker.
We demonstrated that TFPI-2 is localized in the
nucleus as well as the cytoplasm and extracellular fraction (ECF)
of cultivated OCCC cells. TFPI-2 has been characterized as a
secreted protein (23) that
contains a signal peptide at its N-terminus, and mature TFPI-2
protein is secreted into the ECF through the endoplasmic reticulum
and secretory pathway (11,34). A recent study, however, showed that
TFPI-2 was also localized in the nucleus and cytoplasm in
endothelial cell lines (23), and
TFPI-2 exogenously added to culture medium in vitro was
rapidly internalized and distributed in both nucleus and
cytoplasmic fractions. A nuclear localization signal was found in
the C-terminal tail of TFPI-2 (23). In the nucleus, TFPI-2 regulates
MMP-2 gene transcription through the interaction with AP-2a, a
transcription factor important for the expression of many genes
(24). In the cytoplasm, TFPI-2
regulates ERK signaling and interacts with a-actinin-4 and
myosin-9, resulting in increased cancer cell activities (35). Consistent with the in vitro
study, we confirmed the nuclear, cytoplasm, and extracellular
matrix (ECM) subcellular localization of TFPI-2 in surgically
resected OCCC tissues. We detected TFPI-2 mainly in the ECF in
vitro; however, the four cell lines with the highest expression
of TFPI-2 also expressed TFPI-2 in both the nucleus and cytoplasm.
Three different molecular sized TFPI-2 polypeptides, which are
speculated to be derived from differential glycosylation events
(12), were detected in all three
fractions. Taken together, these findings suggest that mature
TFPI-2, after cleavage of the signal peptide and posttranslational
modifications, might be retained in the cytoplasm or internalized
after secretion and distributed into the cytoplasm or nucleus when
large amounts of TFPI-2 are produced. In OCCC OVMANA cells, the
level of secreted TFPI-2 was not as high as its expression in ECF.
In contrast, the majority of secreted TFPI-2 in ES-2 cells seemed
to be retained in the medium. The mechanisms regulating TFPI-2
localization remain to be elucidated.
In this study, we demonstrated the specificity of
TFPI-2 for CCC in IHC. CCC is pathologically diagnosed based on
morphologic features such as hobnail cells with clear cytoplasm
(3). However, tumors containing
clear cells with heterogeneous features are not reproducibly
diagnosed (3). Currently,
hepatocyte nuclear factor-1β (HNF-1β) immunohistochemical
expression (sensitivity, 82.5–85.2%; specificity, 76.5–95.2%)
(36,37), Napsin A (38) and glypican-3 (39) are candidates for CCC IHC markers. In
this study, we showed that TFPI-2 was only identified in CCC
tissues and not in non-CCC EOC tissues. This result is well
consistent with The Human Protein Atlas data, which examined TFPI-2
expression in limited numbers of EOC surgical specimens by IHC but
did not detect any cases with positive TFPI-2 expression (serous
0/5, mucinous 0/4, endometrioid 0/2 cases; CCC cases were not
enrolled) (40). Our results showed
that TFPI-2 expression distinguished CCC from non-CCC with a
sensitivity of 67.5% and specificity of 100%. The high specificity
of TFPI-2 may support its use for diagnosis of OCCC in combination
with existing markers. We propose TFPI-2 as an IHC biomarker for
histopathological diagnostics as well as serum biomarker for OCCC
patients.
We found that all serous carcinoma cases in the
current study group were negative for TFPI-2 in IHC. We previously
showed that serum TFPI-2 levels greater than 345 pg/ml can
pre-operatively discriminate OCCC from other EOC subtypes and
borderline ovarian tumors with a sensitivity of 71.4% and
specificity of 85.7% (25,26). Additionally, we found that serum
TFPI-2 level was also increased in 29.4% of serous carcinoma
patients (26). In this study, all
serous carcinoma cases were negative for TFPI-2 despite setting the
H-score cut-off value very low. Considering our IHC results, we
speculate that the elevation of TFPI-2 in the serum of serous
carcinoma patients was derived from non-tumor cells such as
endothelial cells (23) or
platelets (41), although the
numbers of examined serous carcinoma cases were limited and the
putative mechanisms are currently unclear.
We then examined the clinical significance of TFPI-2
expression in OCCC tissues but did not identify any significant
association between TFPI-2 expression in the primary site and
aggressiveness of the OCCC cases. This is not consistent with
published data from other cancer types, which showed that low
expression of TFPI-2 in IHC is associated with poor survival in
breast and pancreatic cancer patients (32,33).
The tumor suppressor-like activity of TFPI-2 suggested by these
reports are consistent with in vitro and animal experiments
showing that secreted TFPI-2 reduces invasiveness, through
preventing ECM degeneration by inhibiting proteases, such as
plasmin or MMPs (42,43). In many cancer types, TFPI-2
expression is epigenetically silenced by aberrant methylation of
CpG islands in the TFPI-2 promoter (16,20).
In contrast, our study showed that TFPI-2 is elevated in the serum
of OCCC patients and is certainly expressed in OCCC tumor cells.
These findings suggest that the roles of TFPI-2 may vary depending
on the cancer type and that the function of TFPI-2 in ovarian CCC
is unique compared with its role in other cancers. In this study,
we excluded cases that received neoadjuvant therapies to precisely
evaluate the TFPI-2 expression dynamics in OCCC tissues, and
therefore the enrolled patients were predicted to have an inherent
good prognosis and likely to be in early stages. This bias could be
another possibility to explain the negative correlation of TFPI-2
expression and clinical aggressiveness in OCCC tissue. Further
studies are needed to elucidate the potential value of TFPI-2 as a
prognostic marker or monitoring marker for OCCC patients.
In conclusion, we confirmed the expression of TFPI-2
in clinical OCCC tissues and confirmed the nuclear, cytoplasm, and
ECF/ECM subcellular localization of TFPI-2 in cultivated OCCC cells
and surgical tissues. We also demonstrated the high specificity of
TFPI-2 expression in OCCC tissues. TFPI-2 expression in IHC may
support its use for diagnosis of OCCC in combination with existing
markers.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to express our appreciation
to Masahiko Sakaguchi for his valuable and constructive suggestions
for statistical analysis. We would also like to thank the members
of the Department of Gynecology and the Department of Pathology of
Kanagawa Cancer Center Hospital for their cooperation with this
research.
Funding
This study was funded by Tosoh Corporation, Japan.
SM and NO are employees of the Tosoh Corporation. SM and NO
provided technical support for the experiments by analyzing TFPI-2
concentration in CM. The submission fee was provided by Tosoh
Corporation. EM obtained a grant from Tosoh Corporation, outside
the submitted work. YM obtained grants from Tosoh Corporation, both
for this work and outside the submitted work.
Availability of data and materials
The datasets generated and analyzed during the
current study are available from the corresponding author on
reasonable request. Aperio's annotation software is available at
https://www.leicabiosystems.com/digital-pathology/Accessed
13/07/2010.
Authors' contributions
YO contributed to the methodology, software, formal
analysis, investigation, and writing of the original draft. SK
contributed to the methodology, writing of the review and editing.
YN contributed to the investigation. MY contributed to the
investigation. TT contributed to the investigation. SS contributed
to the investigation. SM contributed to the investigation. NO
contributed to the investigation. HK contributed the resources and
conducted the data curation. TY conducted the validation and
contributed to the resources. EM was responsible for the
conceptualization and supervision. YM contributed to the
conceptualization, validation and writing of the review and editing
as well as the supervision. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experimental protocol of the present study was
reviewed and approved by the Institutional Review Board of Kanagawa
Cancer Center Hospital (approval no. ethics-2018-10). Written
informed consent was obtained from the patients for publication of
the study and accompanying images.
Patient consent for publication
Not applicable.
Competing interests
SM and NO are employees of the Tosoh Corporation,
which is now developing an in vitro diagnosis approach for
ovarian CCC patients by evaluating blood TFPI-2 concentration. EM
obtained a grant from Tosoh Corporation, outside the submitted
work. YM obtained grants from Tosoh Corporation, both for this work
and outside the submitted work. The other authors have no conflicts
of interest directly relevant to the content of this article.
Authors' information
ORCID: Yukihide Ota: 0000-0002-5167-1918; Shiro
Koizume: 0000-0002-9132-5286; Etsuko Miyagi:
0000-0002-5492-0844.
Glossary
Abbreviations
Abbreviations:
|
TFPI-2
|
tissue factor pathway inhibitor-2
|
|
OCCC
|
ovarian clear cell carcinoma
|
|
EOC
|
epithelial ovarian cancer
|
|
CA125
|
cancer antigen 125
|
|
PBS
|
phosphate-buffered saline
|
|
WCF
|
whole cell fraction
|
|
ECF
|
extracellular fraction
|
|
ECM
|
extracellular matrix
|
|
CM
|
conditioned medium
|
|
AIA
|
automated immunoassay analyzer
|
|
KCCH
|
Kanagawa Cancer Center Hospital
|
|
FFPE
|
formalin-fixed and
paraffin-embedded
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization, International
Agency for Research on Cancer, Cancer Fact Sheets. http://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf2018
4–September. 2020
|
|
3
|
Soslow RA: Histologic subtypes of ovarian
carcinoma: An overview. Int J Gynecol Pathol. 27:161–174.
2008.PubMed/NCBI
|
|
4
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee AW, Navajas EE and Liu L: Clear
differences in ovarian cancer incidence and trends by ethnicity
among Asian Americans. Cancer Epidemiol. 61:142–149. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shu CA, Zhou Q, Jotwani AR, Iasonos A,
Leitao MM Jr, Konner JA and Aghajanian CA: Ovarian clear cell
carcinoma, outcomes by stage: The MSK experience. Gynecol Oncol.
139:236–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anglesio MS, Carey MS, Köbel M, Mackay H
and Huntsman DG; Vancouver Ovarian Clear Cell Symposium Speakers, :
Clear cell carcinoma of the ovary: A report from the first ovarian
clear cell symposium, June 24th, 2010. Gynecol Oncol. 121:407–415.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer T and Rustin GJ: Role of tumour
markers in monitoring epithelial ovarian cancer. Br J Cancer.
82:1535–1538. 2000.PubMed/NCBI
|
|
9
|
Kudoh K, Kikuchi Y, Kita T, Tode T, Takano
M, Hirata J, Mano Y, Yamamoto K and Nagata I: Preoperative
determination of several serum tumor markers in patients with
primary epithelial ovarian carcinoma. Gynecol Obstet Invest.
47:52–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sierko E, Wojtukiewicz MZ and Kisiel W:
The role of tissue factor pathway inhibitor-2 in cancer biology.
Semin Thromb Hemost. 33:653–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyagi Y, Koshikawa N, Yasumitsu H, Miyagi
E, Hirahara F, Aoki I, Misugi K, Umeda M and Miyazaki K: cDNA
cloning and mRNA expression of a serine proteinase inhibitor
secreted by cancer cells: Identification as placental protein 5 and
tissue factor pathway inhibitor-2. J Biochem. 116:939–942. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao CN, Reddy P, Liu Y, O'Toole E, Reeder
D, Foster DC, Kisiel W and Woodley DT: Extracellular
matrix-associated serine protease inhibitors (Mr 33,000, 31,000,
and 27,000) are single-gene products with differential
glycosylation: cDNA cloning of the 33-kDa inhibitor reveals its
identity to tissue factor pathway inhibitor-2. Arch Biochem
Biophys. 335:82–92. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogawa M, Yanoma S, Nagashima Y, Okamoto N,
Ishikawa H, Haruki A, Miyagi E, Takahashi T, Hirahara F and Miyagi
Y: Paradoxical discrepancy between the serum level and the
placental intensity of PP5/TFPI-2 in preeclampsia and/or
intrauterine growth restriction: Possible interaction and
correlation with glypican-3 hold the key. Placenta. 28:224–232.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karaszi K, Szabo S, Juhasz K, Kiraly P,
Kocsis-Deak B, Hargitai B, Krenacs T, Hupuczi P, Erez O, Papp Z, et
al: Increased placental expression of placental protein 5
(PP5)/tissue factor pathway inhibitor-2 (TFPI-2) in women with
preeclampsia and HELLP syndrome: Relevance to impaired trophoblast
invasion? Placenta. 76:30–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao CN, Lakka SS, Kin Y, Konduri SD,
Fuller GN, Mohanam S and Rao JS: Expression of tissue factor
pathway inhibitor 2 inversely correlates during the progression of
human gliomas. Clin Cancer Res. 7:570–576. 2001.PubMed/NCBI
|
|
16
|
Rollin J, Iochmann S, Bléchet C, Hubé F,
Régina S, Guyétant S, Lemarié E, Reverdiau P and Gruelet Y:
Expression and methylation status of tissue factor pathway
inhibitor-2 gene in non-small-cell lung cancer. Br J Cancer.
92:775–783. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato N, Parker AR, Fukushima N, Miyagi Y,
Iacobuzio-Donahue CA, Eshleman JR and Gogginset M: Epigenetic
inactivation of TFPI-2 as a common mechanism associated with growth
and invasion of pancreatic ductal adenocarcinoma. Oncogene.
24:850–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo H, Lin Y, Zhang H, Liu J, Zhang N, Li
Y, Kong D, Tang Q and Ma D: Tissue factor pathway inhibitor-2 was
repressed by CpG hypermethylation through inhibition of KLF6
binding in highly invasive breast cancer cells. BMC Mol Biol.
8:1102007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nobeyama Y, Okochi-takada E, Furuta J,
Miyagi Y, Kikuchi K, Yamamoto A, Nakanishi Y, Nakagawa H and
Ushijima T: Silencing of tissue factor pathway inhibitor-2 gene in
malignant melanomas. Int J Cancer. 121:301–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong C, Ng Y, Lee JM, Wong CC, Cheung O,
Chan C, Tung EK, Ching Y and Ng IO: Tissue factor pathway
inhibitor-2 as a frequently silenced tumor suppressor gene in
hepatocellular carcinoma. Hepatology. 45:1129–1138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Qin X, Zhou J, Tu Z, Bi X, Li W, Fan
X and Zhang Y: Tissue factor pathway inhibitor-2 inhibits the
growth and invasion of hepatocellular carcinoma cells and is
inactivated in human hepatocellular carcinoma. Oncol Lett.
2:779–783. 2011.PubMed/NCBI
|
|
22
|
Lavergne M, Jourdan ML, Blechet C,
Guyetant S, Pape AL, Heuze-Vourc'h N, Courty Y, Lerondel S, Sobilo
J, Iochmann S and Reverdiau P: Beneficial role of overexpression of
TFPI-2 on tumour progression in human small cell lung cancer. FEBS
Open Bio. 3:291–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kempaiah P, Chand HS and Kisiel W: Human
tissue factor pathway inhibitor-2 is internalized by cells and
translocated to the nucleus by the importin system. Arch Biochem
Biophys. 482:58–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Zeng Y, Chen S, Li D, Li W, Zhou
Y, Singer RH and Gu W: Localization of TFPI-2 in the nucleus
modulates MMP-2 gene expression in breast cancer cells. Sci Rep.
7:135752017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arakawa N, Miyagi E, Nomura A, Morita E,
Ino Y, Ohtake N, Miyagi Y, Hirahara F and Hirano H: Secretome-Based
identification of TFPI2, A novel serum biomarker for detection of
ovarian clear cell adenocarcinoma. J Proteome Res. 12:4340–4350.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arakawa N, Kobayashi H, Yonemoto N,
Masuishi Y, Ino Y, Shigetomi H, Furukawa N, Ohtake N, Miyagi Y,
Hirahara F, et al: Clinical significance of tissue factor pathway
inhibitor 2, a serum biomarker candidate for ovarian clear cell
carcinoma. PLoS One. 11:e01656092016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pearce CL, Templeman C, Rossing MA, Lee A,
Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund
KG, et al: Association between endometriosis and risk of
histological subtypes of ovarian cancer: A pooled analysis of
case-control studies. Lancet Oncol. 13:385–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cinti S, Matteis RD, Picó C, Ceresi E,
Obrador A, Maffeis C, Oliver J and Palou A: Secretory granules of
endocrine and chief cells of human stomach mucosa contain leptin.
Int J Obes Relat Metab Disord. 24:789–793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pirker R, Pereira JR, von Pawel J,
Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE,
Paz-Ares L, Störkel S, et al: EGFR expression as a predictor of
survival for first-line chemotherapy plus cetuximab in patients
with advanced non-small-cell lung cancer: Analysis of data from the
phase 3 FLEX study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wojtukiewicz MZ, Sierko E, Zimnoch L,
Kozlowski L and Kisiel W: Immunohistochemical localization of
tissue factor pathway inhibitor-2 in human tumor tissue. Thromb
Haemost. 90:140–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Altmäe S, Salumets A, Bjuresten K, Kallak
TK, Wånggren K, Landgren BM, Hovatta O and Stavreus-Evers A: Tissue
factor and tissue factor pathway inhibitors TFPI and TFPI2 in human
secretory endometrium-possible link to female infertility. Reprod
Sci. 18:666–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhai LL, Cai CY, Wu Y and Tang ZG:
Correlation and prognostic significance of MMP-2 and TFPI-2
differential expression in pancreatic carcinoma. Int J Clin Exp
Pathol. 8:682–691. 2015.PubMed/NCBI
|
|
33
|
Xu C, Wang H, He H, Zheng F, Chen Y, Zhang
J, Lin X, Ma D and Zhang H: Low expression of TFPI-2 associated
with poor survival outcome in patients with breast cancer. BMC
Cancer. 13:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sprecher CA, Kisiel W, Mathewes S and
Foster DC: Molecular cloning, expression, and partial
characterization of a second human tissue-factor-pathway inhibitor.
Proc Natl Acad Sci USA. 91:3353–3357. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang G, Huang W, Li W, Chen S, Chen W,
Zhou Y, Peng P and Gu W: TFPI-2 suppresses breast cancer cell
proliferation and invasion through regulation of ERK signaling and
interaction with actinin-4 and myosin-9. Sci Rep. 8:144022018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Köbel M, Kalloger SE, Carrick J, Huntsman
D, Asad H, Oliva E, Ewanowich CA, Soslow RA and Gilks CB: A limited
panel of immunomarkers can reliably distinguish between clear cell
and high-grade serous carcinoma of the ovary. Am J Surg Pathol.
33:14–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang W, Cheng X, Ji J, Zhang J and Li Q:
The application value of HNF-1β transcription factor in the
diagnosis of ovarian clear cell carcinoma. Int J Gynecol Pathol.
35:66–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamashita Y, Nagasaka T, Naiki-Ito A, Sato
S, Suzuki S, Toyokuni S, Ito M and Takahashi S: Napsin A is a
specific marker for ovarian clear cell adenocarcinoma. Mod Pathol.
28:111–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maeda D, Ota S, Takazawa Y, Aburatani H,
Nakagawa S, Yano T, Taketani Y, Kodama T and Fukayama M: Glypican-3
expression in clear cell adenocarcinoma of the ovary. Mod Pathol.
22:824–832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
The Human Protein Atlas: TFPI2. https://www.proteinatlas.org/ENSG00000105825-TFPI2April
8–2020
|
|
41
|
Vadivel K, Ponnuraj SM, Kumar Y, Zaiss AK,
Bunce MW, Camire RM, Wu L, Evseenko D, Herschman HR, Bajaj MS and
Bajaj SP: Platelets contain tissue factor pathway inhibitor-2
derived from megakaryocytes and inhibits fibrinolysis. J Biol Chem.
289:31647–31661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rao CN, Cook B, Liu Y, Chilukuri K, Stack
MS, Foster DC, Kisiel W and Woodley DT: HT-1080 fibrosarcoma cell
matrix degradation and invasion are inhibited by the
matrix-associated serine protease inhibitor TFPI-2/33 kDa MSPI. Int
J Cancer. 76:749–756. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin M, Udagawa K, Miyagi E, Nakazawa T,
Hirahara F, Yasumitsu H, Miyazaki K, Nagashima Y, Aoki I and Miyagi
Y: Expression of serine proteinase inhibitor PP5/TFPI-2/MSPI
decreases the invasive potential of human choriocarcinoma cells in
vitro and in vivo. Gynecol Oncol. 83:325–333. 2001. View Article : Google Scholar : PubMed/NCBI
|