Introduction
Axon-directing factor semaphorin 4D (Sema4D; also
called CD100), which was first discovered in the immune system in
1992 (1), is an important member of
the IV subfamily of the semaphorin superfamily. It exists in
membrane-bound and soluble forms. Soluble Sema4D is produced by
proteolytic cleaving of the Sema4D exodomain and is released into
the circulation, where it can bind and activate various receptors,
such as CD40, CD72 and Plexin-B1 (2).
Membrane Sema4D interacts with calmodulin via its C-terminal
domain, and the dissociation of this interaction induces its
cleavage and release of soluble Sema4D (3), which can be promoted by the stimulator
of interferon genes protein (4).
Sema4D has been indicated to be involved in the regulation of the
immune response in resting T cells and participate in the
activation of B lymphocytes and the activation and maturation of
antigen-presenting cells via the low affinity receptor CD72
(5). It has also been reported to be
associated with the activation of neutrophils and dendritic cells
(6,7),
and promote eosinophil migration (8).
Sema4D is highly expressed in prostate, colon, oral,
lung, pancreatic, breast and ovarian cancer, head and neck squamous
cell carcinoma and soft tissue sarcoma compared with healthy
tissues, and is involved in angiogenesis and invasion and migration
of tumor cells (9–20). Tumors overexpressing Sema4D have been
indicated to be highly invasive with a poor prognosis and
therapeutic response (10,12–16,21,22).
In chronic lymphocytic leukemia (CLL) cells, Sema4D has been
indicated to sustain viability and enhance proliferation (23). The interaction of Sema4D with
Plexin-B1 has been revealed to promote survival and growth and
inhibit apoptosis in B-CLL cells (24). Soluble Sema4D has been demonstrated to
enhance the metastasis of head and neck squamous cell carcinoma by
interacting with its receptor Plexin-B1, resulting in
epithelial-mesenchymal transition (25). A previous study utilizing a murine
carcinoma model has indicated that antibodies against Sema4D
induced an immune response in tumors via the activation of CD8 T
lymphocytes (26). Although
antibodies against Sema4D decrease proliferation, they have also
been reported to enhance invasion and metastasis in a pancreatic
neuroendocrine cancer mouse model and patients with pancreatic
neuroendocrine cancer (27).
Acute lymphoblastic leukemia (ALL), which affects
80–90 children per million annually in Italy (28), accounts for ~25% of childhood cancer
deaths, representing the most common malignancy in children
(29). The expression and function of
Sema4D is still unclear in ALL, and the aim of the present study
was to investigate the expression level of Sema4D in pediatric ALL
and its potential association with ALL development.
Materials and methods
Sample collection
Leukemia, including ALL and acute myeloid leukemia
(AML), was diagnosed according to standard clinical and laboratory
criteria (30). The present study
included newly diagnosed patients with pediatric leukemia and
healthy pediatric donors who presented no history of leukemia. The
samples of the healthy group and the patient group in the
experiment were collected from January 2018 to December 2018 in
Kunming Children's Hospital (Kunming, China). The age range of the
healthy group was 1–12 years old, including 15 males and 13
females; the age range of the patient group was 10 months to 13
years old, including 34 males and 27 females. The details of each
patient group are presented in Table
SI. Peripheral blood was collected from 18 patients with
pediatric leukemia and 6 healthy children, and peripheral blood
mononuclear cells (PBMCs) were isolated by density centrifugation
at 2,000 × g for 10 min at 4°C using Ficoll solution, followed by
washing with PBS. Plasma was collected from 55 patients with
pediatric leukemia and 22 healthy children for ELISA analysis. Of
the 18 patients with pediatric leukemia whose PBMCs were isolated,
the plasma samples of only 12 patients were included in the ELISA
analysis, as the amount of plasma obtained from the remaining 6
patients was insufficient.
Determination of soluble Sema4D in
plasma
Plasma was collected and stored at −80°C after total
blood was centrifuged at 2,000 × g for 10 min at 4°C. Soluble
Sema4D plasma levels were measured using Sema4D ELISA kit (cat. no.
MBS705483; MyBioSource, Inc.) according to the manufacturer's
instructions. In brief, a microtitration plate coated with
anti-Sema4D antibodies was incubated with plasma samples or Sema4D
protein standard for 2 h at 37°C. It was then incubated with
biotin-conjugated anti-Sema4D antibodies for 2 h at 37°C. After
being washed with the washing solution provided in the kit, it was
incubated with HRP-coupled streptavidin for 20 min at 37°C. and
then incubated with HRP substrate for 15 min at 37°C. Following
termination of the reaction, the absorbance was measured at 450
nm.
Cell culture and lentiviral
infection
Jurkat and BALL-1 cell lines (Shanghai Yihe Applied
Biotechnology Co., Ltd. China) were both cultured in RPMI-1640
supplemented with 10% FBS and 1% penicillin and streptomycin (all
from Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. The cell lines had been confirmed to be mycoplasma
free and authenticated by STR profiling. Sema4D cDNA (Sema4D) and
short hairpin (sh)RNA were subcloned into the lentiviral vector
PWPI-GFP (kindly provided by Dr John Basile, University of
Maryland) to obtain lentiviral Sema4D overexpression (Sema4D) and
shRNA (S4DshRNA) constructs, and the empty lentiviral vector
PWPI-GFP was used as negative control in further analysis. 293T
cells (Institute of Medical Biology, Chinese Academy of Medical
Sciences and Peking Union Medical College) were cultured in high
glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS
and 1% penicillin and streptomycin at 37°C with 5% CO2.
Lentiviruses were produced by transfecting 293T cells
(5×105 cells in six-wells plates incubated overnight),
with 1.5 µg lentiviral plasmid, 0.5 µg PVSVG plasmid and 1 µg
PASPAX plasmid (kindly provided by Dr John Basile, University of
Maryland) with Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.). Viral supernatants were collected 72 h after
transfection. Jurkat and BALL-1cells were infected with
lentiviruses at a MOI of 30 with 4 µg/ml polybrene (Beijing
Solarbio Science & Technology Co., Ltd.) for 72 h before
collection for subsequent analysis.
Western blot analysis
Jurkat and BALL-1 cells were lysed with RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.) containing
protease inhibitors. Protein concentration was determined using the
BCA method, and 50 µg protein/sample were separated by 10% SDS-PAGE
and transferred to PVDF membranes, which were blocked at room
temperature for 40 min using 5% BSA solution (Beijing Solarbio
Science & Technology Co., Ltd.). The membranes were incubated
at 4°C overnight with the following primary antibodies: Mouse
anti-Sema4D (1:1,000; cat. no. 610671; BD Biosciences), rabbit
anti-ERK (1:2,000; cat. no. 9126S), rabbit anti-phosphorylated
(p)-ERK (1:2,000; cat. no. 4376S), rabbit anti-AKT (1:2,000; cat.
no. 9272S), rabbit anti-p-AKT (1:2,000; cat. no. 9611S), rabbit
anti-PI3K (1:2,000; cat. no. 4257S), rabbit anti-p-PI3K (1:2,000;
cat. no. 4228S; all from Cell Signaling Technology, Inc.) and
rabbit anti-β-actin (1:100,000; cat. no. AC026; ABclonal Biotech
Co., Ltd.). Following primary antibody incubation, the membranes
were incubated at room temperature for 1 h with goat anti-mouse lgG
(H + L) (1:5,000; cat. no. 074-1806) and goat anti-rabbit lgG (H +
L) antibodies (1:5,000; cat. no. 074-1506; both from KPL). The
protein signal was detected using SuperSignal reagent
(MilliporeSigma) and quantified using ImageJ software v1.8.0
(National Institutes of Health).
Cell counting kit-8 (CCK-8) assay
Cell viability was analyzed by CCK-8 assay (Tongren
Institute of Chemistry). Jurkat and BALL-1 cells (5×106)
were lentivirally transduced for 72 h, and 5×104
cells/ml were seeded in 96-well plates. At 1, 2, 3, 4 and 5 days,
cells were incubated with 10 µl CCK-8 solution for 2 h at 37°C with
5% CO2 and the absorbance at 450 nm was measured.
Cell migration and invasion assay
Cell migratory capacity was detected by Transwell
assay. The lentivirally transduced Jurkat and BALL-1 cells were
cultured to logarithmic growth phase, adjusted to 1×104
cells/ml with RPMI-1640 basal medium containing 0.1% BSA (Beijing
Solarbio Science & Technology Co., Ltd.), and placed on top of
each compartment of 0.8 µm, 24-well Transwell chamber (Corning, New
York, USA), and RPMI-1640 medium containing 10% FBS was added to
lower chamber of the plates. After the cells were cultured for 24 h
at 37°C, the cell number in the lower chamber was counted using a
hemocytometer. Cell invasive capacity was also determined by
Transwell cell assay. A total of 100 µl 300 µg/ml Matrigel (BD
Biosciences) were thawed at 4°C overnight before being placed into
the upper chamber and incubated at 37°C for 1 h. The lentivirally
transduced Jurkat and BALL-1 cells were cultured to logarithmic
growth phase, adjusted to 1×104 cells/ml with RPMI-1640
basal medium containing 0.1% BSA and placed on top of each
compartment. RPMI-1640 medium with 10% FBS was then added to the
lower chambers. After the cells were cultured for 24 h at 37°C, the
cell number in the lower chamber was counted using a
hemocytometer.
Apoptosis analysis
Apoptosis was detected using PE Annexin V Apoptosis
Detection Kit I (BD Biosciences). After lentiviral transduction for
72 h, Jurkat and BALL-1 cells were washed with pre-chilled PBS and
were adjusted to 1×106 cells/ml with 1× Binding Buffer.
The cells were incubated at room temperature with 5 µl PE Annexin V
and 5 µl 7-AAD for 15 min before being analyzed by Attune NxT flow
cytometry (Thermo Fisher Scientific, Inc.), and the data were
analyzed using Treestar FlowJo version 10 software (Becton,
Dickinson and Company).
Cell cycle analysis
After lentiviral transduction for 72 h, Jurkat and
BALL-1 cells were washed with pre-chilled PBS, before being
suspended in pre-chilled PBS and fixed with ice-cold absolute
ethanol at −20°C overnight. After being washed with ice-cold PBS,
the cells were incubated with 50 µg/ml RNase A and 65 µg/ml
propidium iodide (BD Biosciences) at 4°C for 30 min before analysis
by Attune NxT flow cytometry (Thermo Fisher Scientific, Inc.), and
the data were analyzed using Treestar FlowJo version 10 software
(Becton, Dickinson and Company).
Overall survival analysis
The analysis was performed on the GEPIA website
(http://gepia.cancer-pku.cn) using the
median expression of Sema4D as cut-off to differentiate low and
high Sema4D expression groups and 95% confidence interval.
Statistical analysis
The statistical analysis was performed by SPSS
software version 21.0 (IBM Corp.). Experimental values are
presented as the mean ± SD. Statistical analysis in Fig. 1 and Figs.
3–6 was performed using one-way
ANOVA followed by Bonferroni's post hoc test. Statistical analysis
in Fig. 2 was performed using two-way
ANOVA followed by Bonferroni's post hoc test. Statistical analysis
in Fig. 7B was performed using
unpaired two-tailed Student's t-test. The correlation between the
level of p-PI3K, p-ERK or p-AKT and Sema4D in Fig. 7C was evaluated using Pearson's
correlation analysis (two-sided). P<0.05 was considered to
indicate a statistically significant difference.
Results
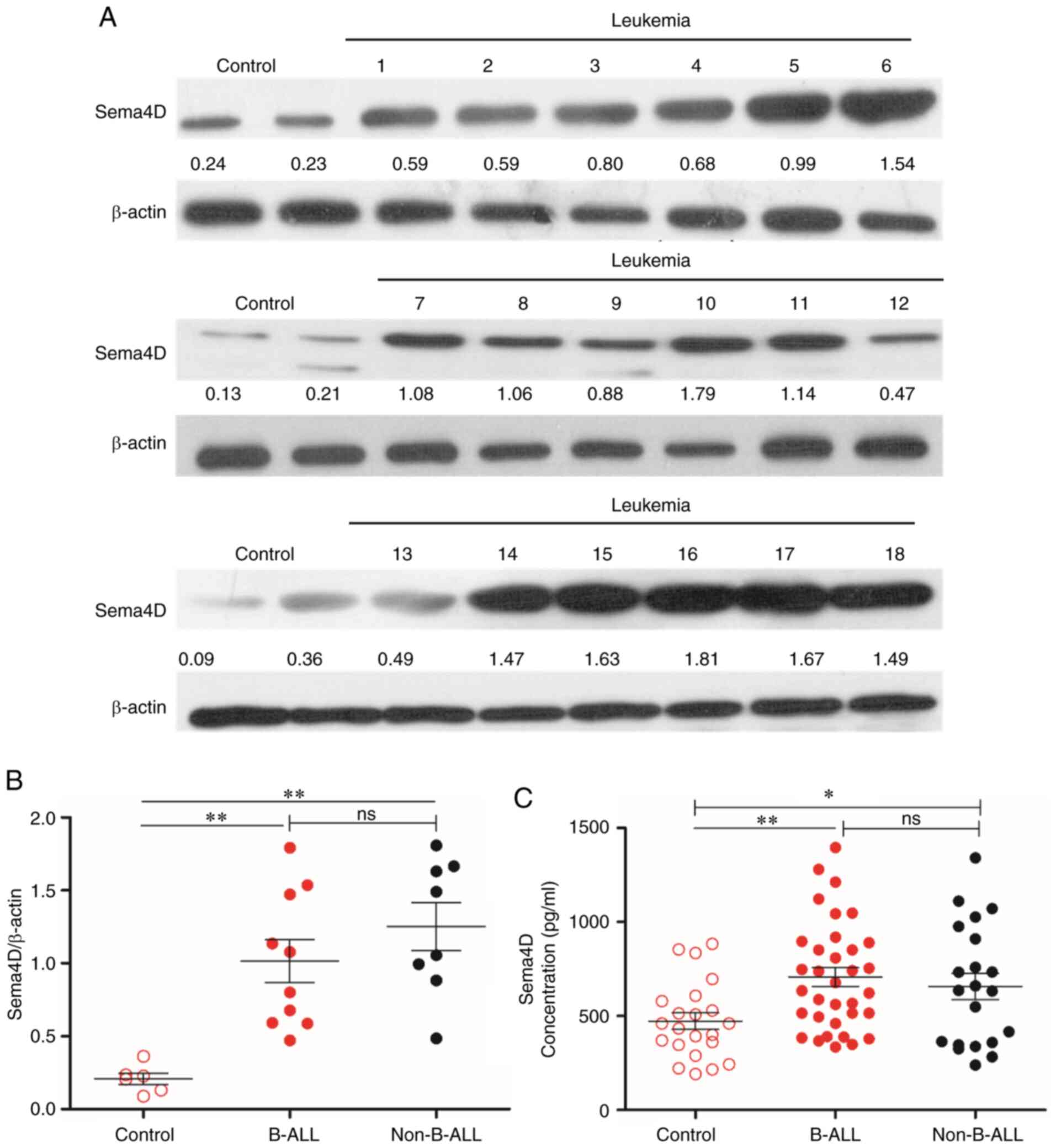
Sema4D is highly expressed in
pediatric leukemia
To investigate the expression level of Sema4D in
pediatric leukemia cells, the mononuclear cell lysates of 18
patients with pediatric leukemia and 6 healthy children were
subjected to western blot analysis. The clinical details of the 18
patients with pediatric leukemia are presented in Table SII. The results revealed that Sema4D
was highly expressed in patients with pediatric leukemia compared
with healthy participants (Fig. 1A).
As the majority of patients were B cell-ALL patients, it was
subsequently determined whether there were any differences in
Sema4D expression level between B cell-ALL and other types of
leukemia. Therefore, patients were divided into either B cell-ALL
(B-ALL) or non-B cell-ALL groups (Non-B-ALL). The analysis
indicated that Sema4D expression level in both groups was
significantly higher compared with that in the control group
(P<0.01), and there was no difference in Sema4D expression level
between the B-ALL and Non-B-ALL groups (Fig. 1B).
As Sema4D can be cleaved to functional soluble
Sema4D, which is secreted from cells (23), the level of soluble Sema4D in the
plasma of patients with leukemia was examined. The plasma of 55
pediatric patients with leukemia and 22 healthy children was
collected and analyzed by ELISA. The clinical details of the 55
pediatric patients with leukemia are listed in Table SII. The results revealed that the
level of soluble Sema4D in the B-ALL and Non-B-ALL groups was
higher compared with that in healthy children (P<0.01 for B-ALL;
P<0.05 for Non-B-ALL), and there was no difference in Sema4D
level between the B-ALL and Non-B-ALL groups (Fig. 1C).
Taken together, the results indicated that Sema4D
was highly expressed in patients with pediatric leukemia, and
soluble Sema4D was released into the circulation in these patients.
However, there was no difference in Sema4D levels between B-ALL and
other types of leukemia in both leukemia cells and plasma.
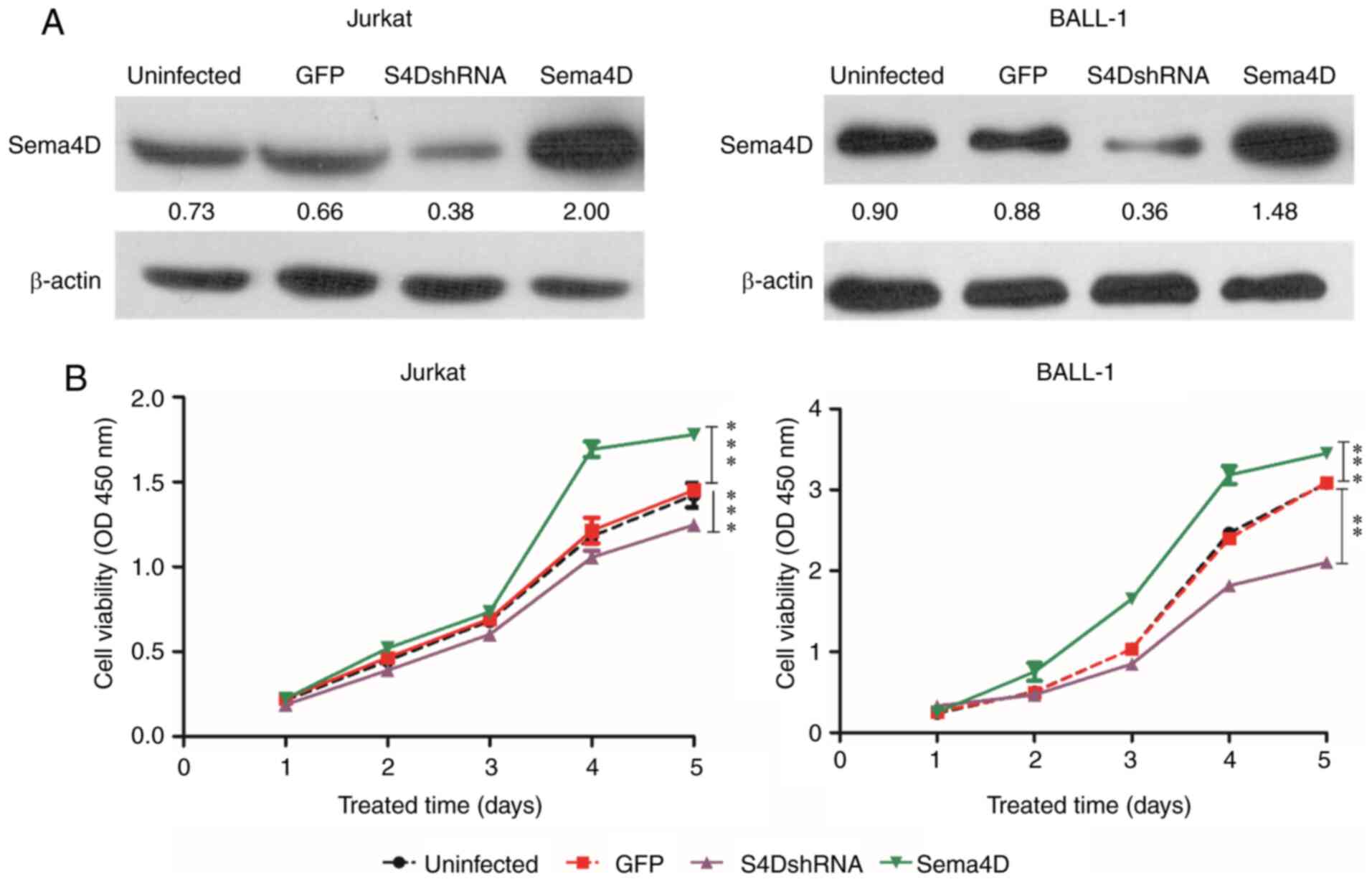
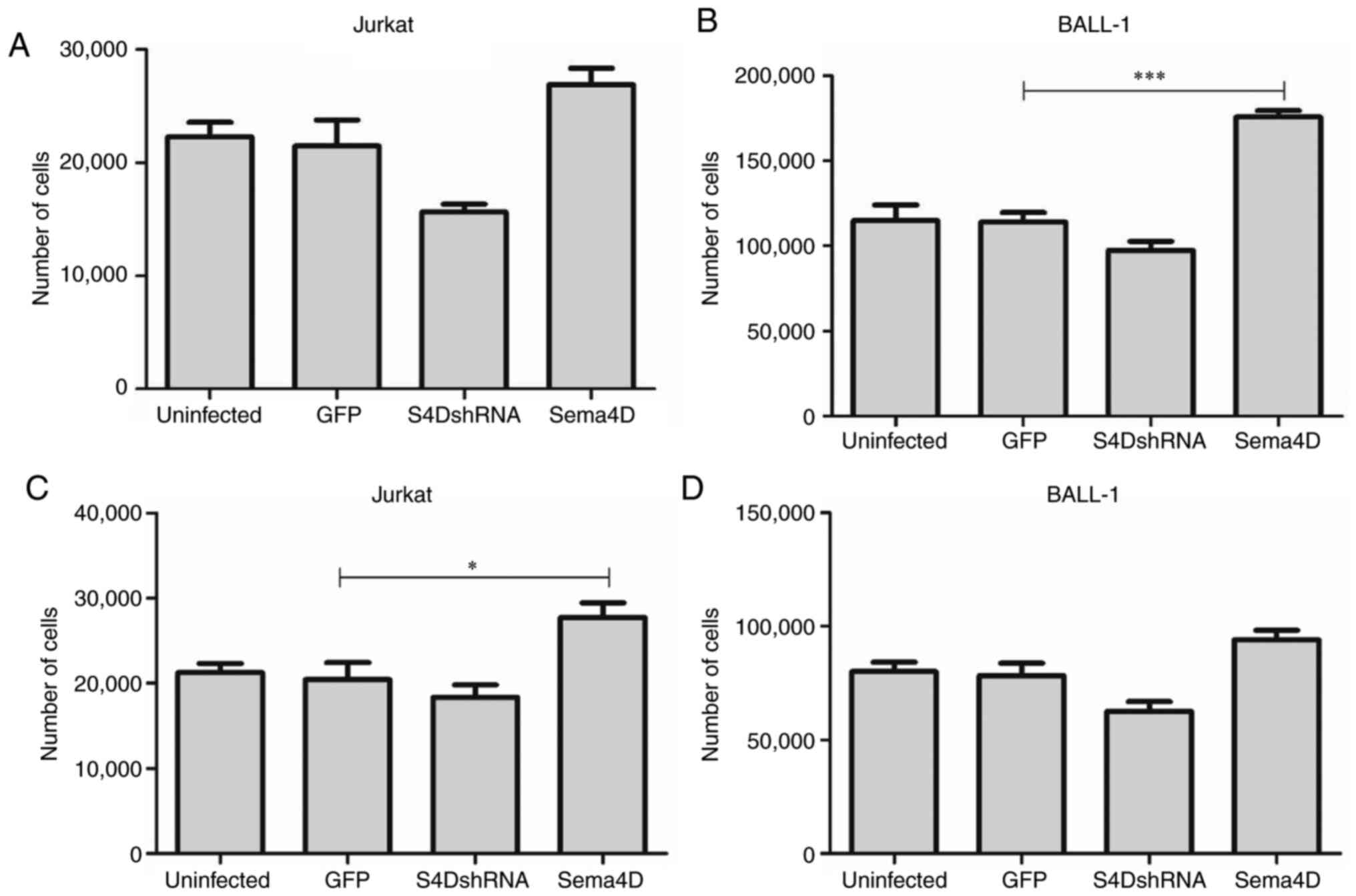
Sema4D promotes proliferation
As the results indicated that Sema4D was highly
expressed in pediatric leukemia, the potential role of Sema4D in
leukemia development was subsequently investigated. To examine the
function of Sema4D, the Jurkat cell line, which originates from T
cell ALL, and the BALL-1 cell line, which originates from B cell
ALL, were selected for the study. Sema4D overexpressing and
S4DshRNA lentiviruses were transduced into Jurkat and BALL-1 cells.
Sema4D expression level in Jurkat and BALL-1 cells was detected by
western blotting after transduction for 72 h. The results indicated
that Sema4D expression in both cell lines was reduced after
transduction with S4DshRNA, and increased after transduction with
the Sema4D overexpressing lentivirus compared with uninfected cells
or cells infected with the empty vector (GFP) (Fig. 2A). The viability of Jurkat and BALL-1
cells was assessed by CCK-8 assay after transduction for 72 h. The
results demonstrated that the viability of both Jurkat and BALL-1
cells was significantly decreased after transduction with S4DshRNA
(P<0.001 in Jurkat and P<0.01 in BALL-1), while it was
significantly increased after transduction with Sema4D
overexpression construct (both P<0.001) (Fig. 2B). The results suggested that Sema4D
promoted the proliferation of Jurkat and BALL-1 cells.
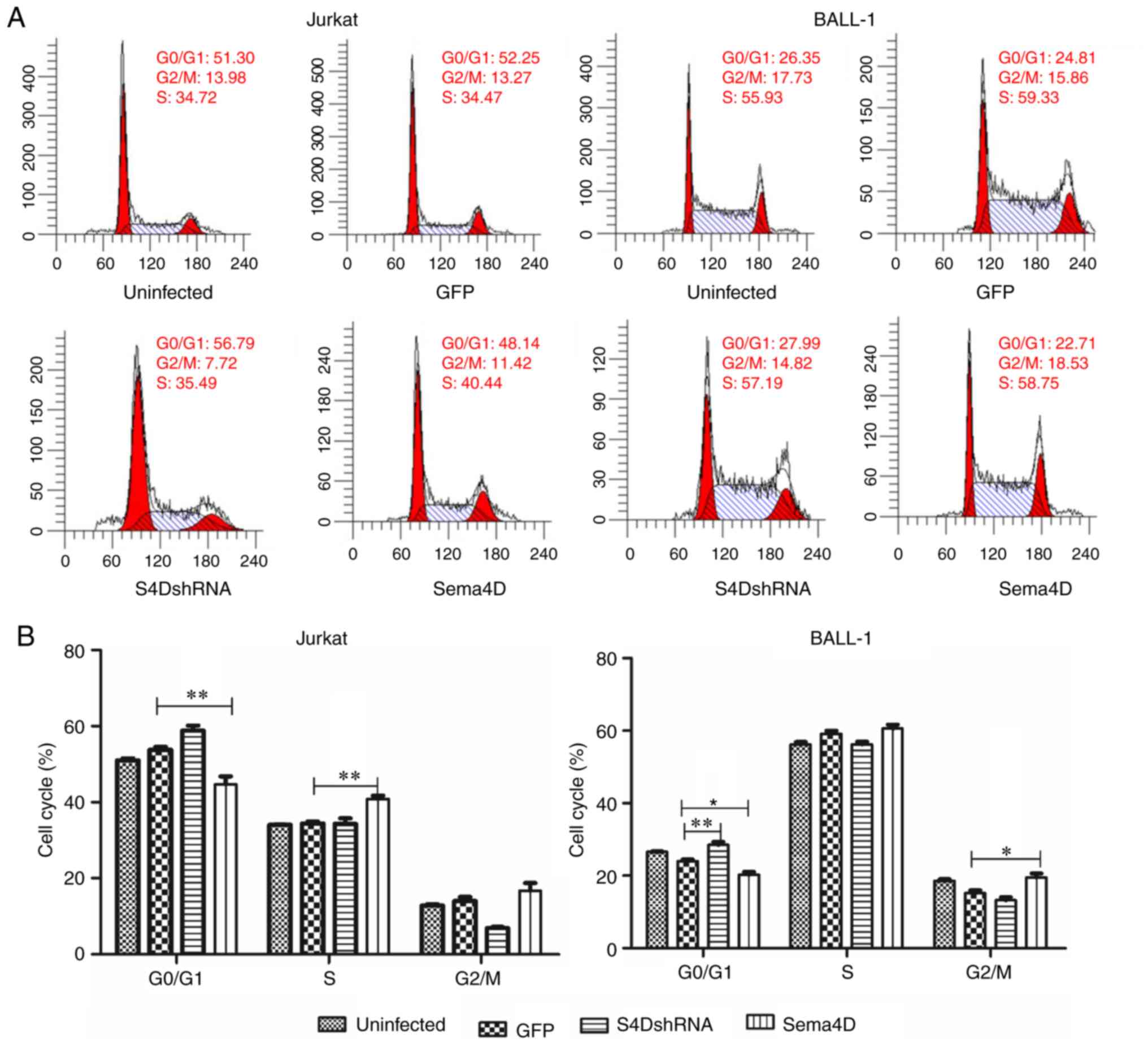
Sema4D modulates the
G0/G1 phase of the cell cycle
Subsequently, the effect of Sema4D on cell cycle was
examined in Jurkat and BALL-1 cells. The results revealed that the
percentage of cells in the G0/G1 phase
significantly increased after transduction with S4DshRNA
(P<0.01), and significantly decreased after transduction with
the Sema4D overexpression construct (P<0.05) in BALL-1 cells
(Fig. 3A and B). The cell percentage
in the G0/G1 phase also significantly
decreased after transduction with the Sema4D overexpression
construct in Jurkat cells (P<0.01), but was not altered after
transduction with S4DshRNA, as the percentage of Jurkat cells in
the G0/G1 phase was already high (Fig. 3A and B). As a consequence of the
decreased abundance of cells in the G0/G1
phase caused by Sema4D overexpression, the percentage of Jurkat
cells in the S phase or BALL-1 cells in the G2/M phase
increased significantly compared with the control (P<0.01 for
Jurkat; P<0.05 for BALL-1 cells). The results suggested that
downregulation of Sema4D induced cell cycle arrest at the
G0/G1 phase, and Sema4D modulated the
G0/G1 phase of the cell cycle.
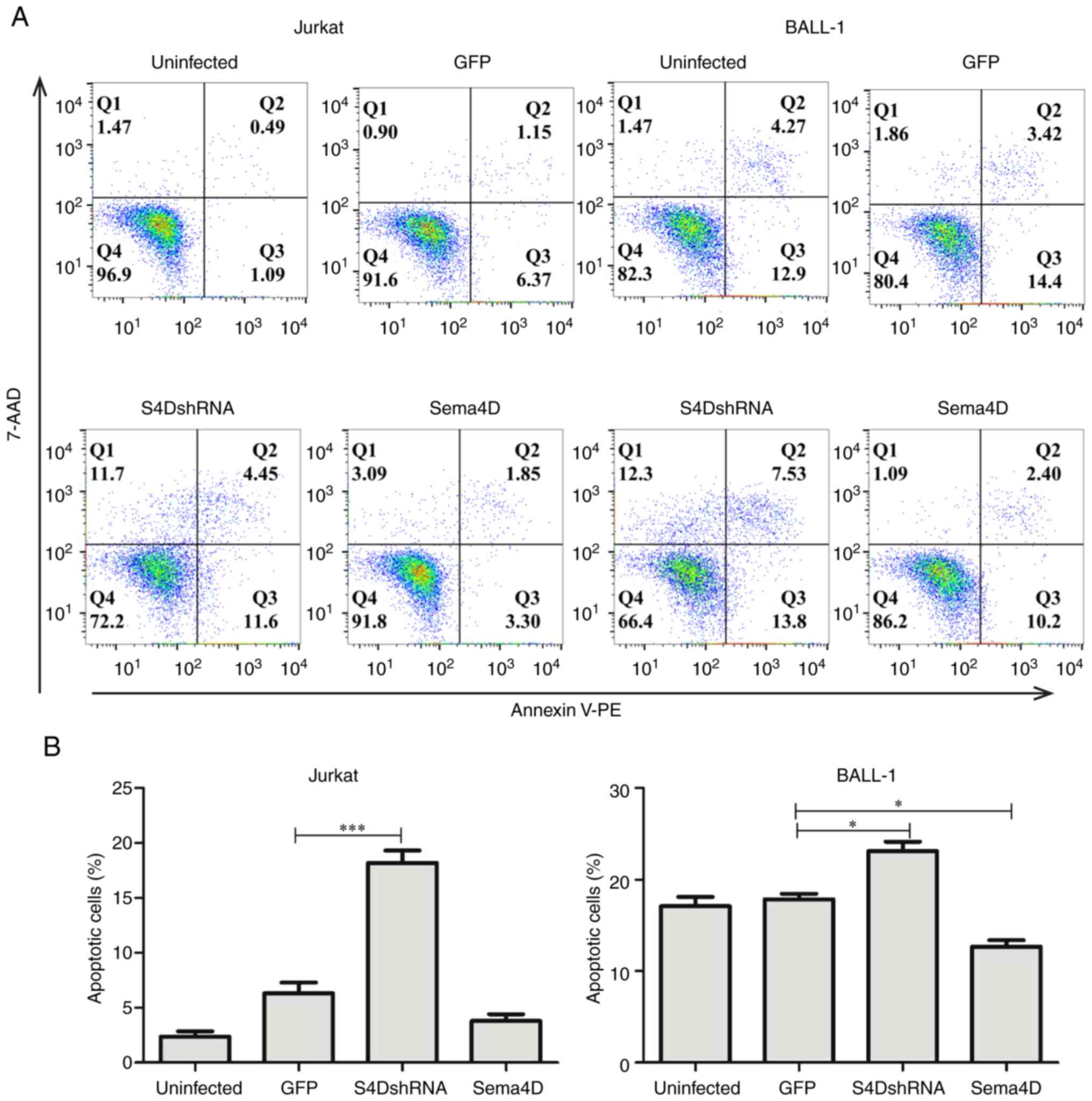
Sema4D inhibits apoptosis
The effect of Sema4D on apoptosis was also
investigated. In Jurkat cells, the number of apoptotic cells was
significantly higher in the S4DshRNA group (P<0.001; Fig. 4A and B). Similarly, in BALL-1 cells,
the number of apoptotic cells was significantly higher in the
S4DshRNA group (P<0.05) and was significantly lower in Sema4D
overexpression group compared with the GFP control group
(P<0.05) (Fig. 4A and B). These
results indicated that Sema4D protected leukemia cells from
apoptosis.
Sema4D promotes the migratory and
invasive abilities of leukemia cells
To evaluate the effects of Sema4D on cell invasion
and migration, Jurkat and BALL-1 cells were transduced with Sema4D
overexpression construct or S4DshRNA, and their migratory and
invasive abilities were examined by Transwell assay. In Jurkat
cells, no difference was observed in the invasive ability among the
GFP control, S4DshRNA and Sema4D overexpression groups (Fig. 5A), while the migratory ability
increased significantly in the Sema4D overexpression group compared
with the GFP control (P<0.05; Fig.
5C). In BALL-1 cells, compared with the GFP group, the invasive
ability increased significantly in the Sema4D overexpression group
(P<0.001; Fig. 5B), while no
difference was observed in the migratory ability among the GFP
control, S4DshRNA and Sema4D overexpression groups (Fig. 5D). As Jurkat and BALL-1 cell lines
originate from T-ALL and B-ALL, respectively, their different
migratory and invasive capabilities may be due to their original
characteristics. Overall, these data suggested that Sema4D
moderately promoted the invasive and migratory ability of ALL
cells.
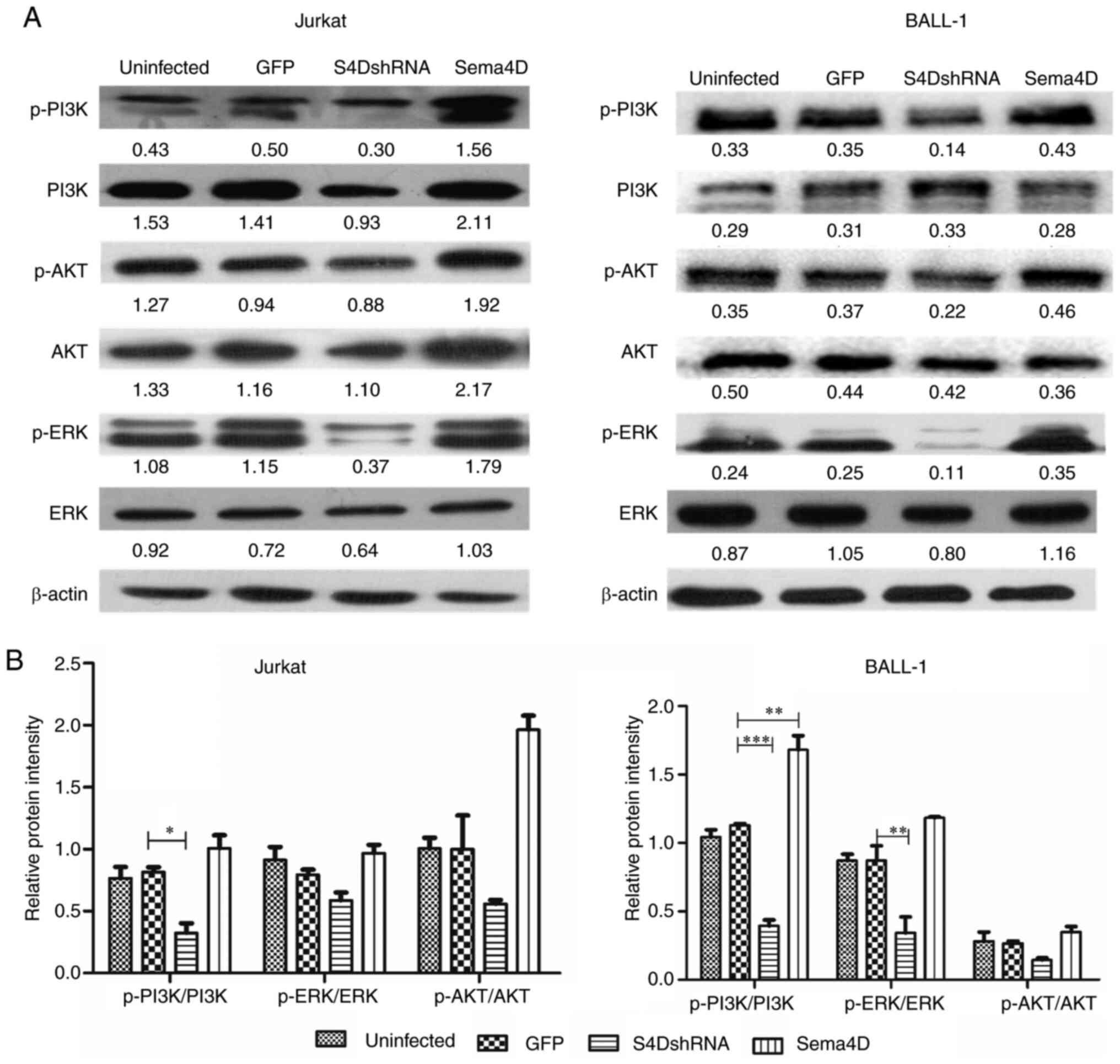
Sema4D activates the PI3K, ERK and AKT
proteins
As the results had indicated that Sema4D promoted
proliferation and inhibited apoptosis, it was subsequently
investigated how these effects were mediated. PI3K, AKT and ERK
proteins, which are widely expressed in a variety of tumor tissues
(31–35), promote cancer development by mediating
activation of downstream effectors (31,36–39). The
role of Sema4D in activating PI3K, AKT and ERK was examined in
Jurkat and BALL-1 cells. In Sema4D-overexpressing BALL-1 cells, the
phosphorylation level of PI3K was significantly increased compared
with the GFP control cells (P<0.01; Fig. 6). However, potentially owing to the
high endogenous expression of Sema4D in both Jurkat and BALL-1
cells, Sema4D overexpression did not consistently elevate the
phosphorylation level of PI3K, ERK and AKT.
In Sema4D knocked-down BALL-1 cells, the
phosphorylation level of PI3K and ERK significantly decreased
compared with the GFP control cells (P<0.001 for PI3K and
P<0.01 for ERK; Fig. 6A and B). In
Sema4D knocked-down Jurkat cells, the phosphorylation level of PI3K
was also significantly decreased (P<0.05; Fig. 6A and B). The alteration in the AKT
phosphorylation level was not statistically significant in both
Jurkat and BALL-1 cell lines. The Sema4D knockdown results
indicated that Sema4D mediated the activation of PI3K and ERK in
ALL cells.
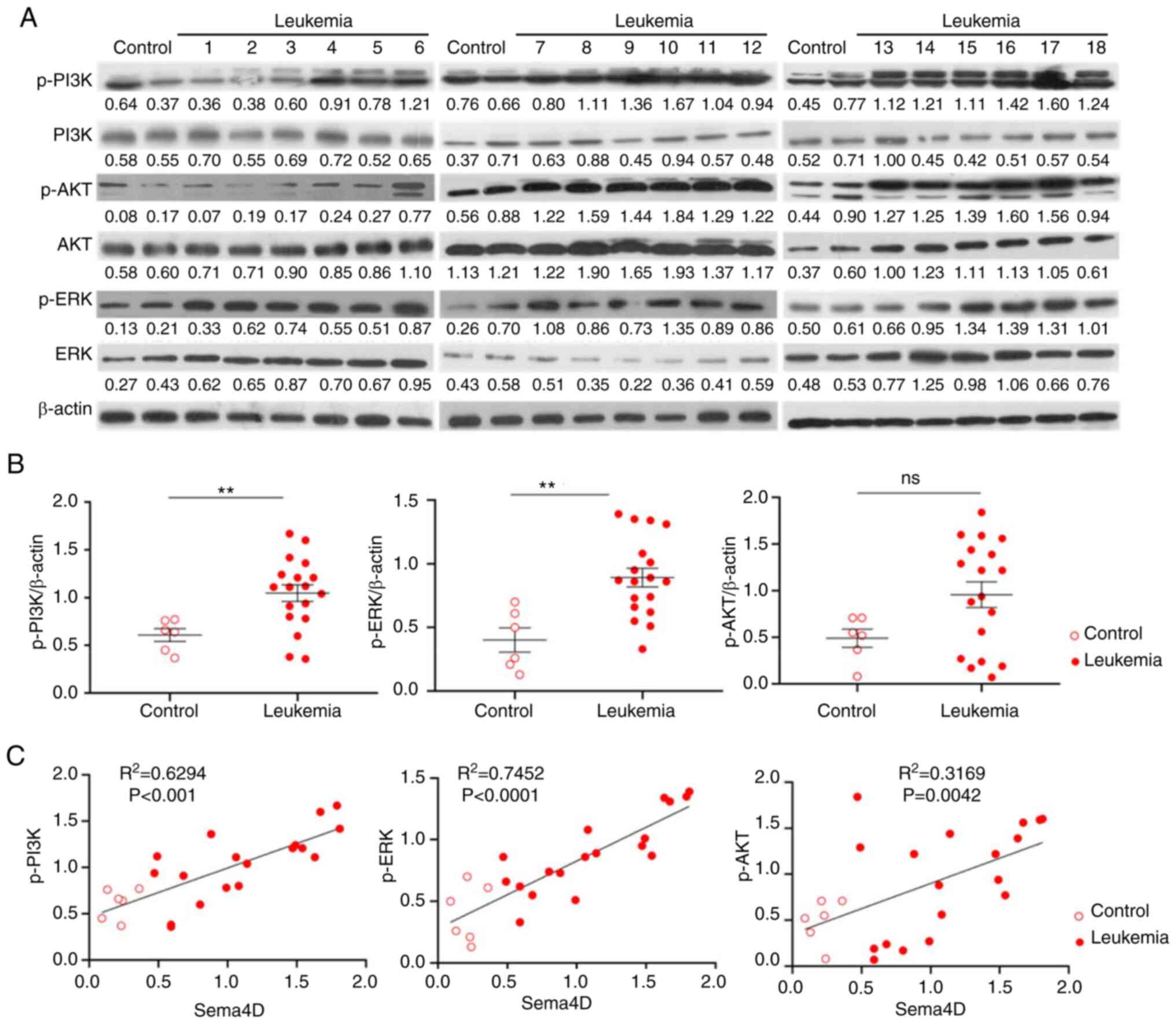
Phosphorylation of PI3K, ERK and AKT
is correlated with the expression of Sema4D in pediatric
leukemia
In order to ascertain the clinical relevance of
PI3K, ERK and AKT pathways, the expression and phosphorylation
levels of PI3K, ERK and AKT were analyzed by western blotting in 18
pediatric leukemia samples, in which the expression of Sema4D had
been examined (Fig. 1A and B). Since
no difference was observed in the expression level of Sema4D
between the B-ALL and Non-B-ALL groups, both groups were analyzed
together regarding the expression and phosphorylation of PI3K, ERK
and AKT. There was no significant difference in the expression
level of PI3K, ERK and AKT between patients with leukemia and
healthy control subjects (data not shown). The total
phosphorylation level of PI3K and ERK in the leukemia group was
significantly higher compared with the healthy control group
(P<0.01), and the total phosphorylation level of AKT in the
leukemia group was higher compared with the healthy control group
(all normalized to β-actin), but with no statistical significance
(Fig. 7A and B). The relative
phosphorylation level normalized to total PI3K, ERK and AKT was not
significantly different between the groups (data not shown).
Therefore, the results indicated that the total phosphorylation
level of PI3K, ERK and AKT was enhanced in pediatric leukemia. The
correlation of Sema4D expression with the phosphorylation level of
PI3K, ERK and AKT was further analyzed, and the results indicated
that Sema4D expression was significantly correlated with p-PI3K and
p-ERK (P<0.001 for p-PI3K; P<0.0001 for p-ERK) and moderately
correlated with p-AKT (P=0.0042), and the expression level of
Sema4D and the phosphorylation level of PI3K, ERK and AKT were
higher in patients with leukemia compared with healthy subjects
(Fig. 7C). The expression of Sema4D
was not correlated with the expression of PI3K, ERK and AKT
proteins (data not shown). The results depicted in Figs. 6 and 7
suggested that Sema4D activated the PI3K, ERK and AKT pathways in
leukemia cells.
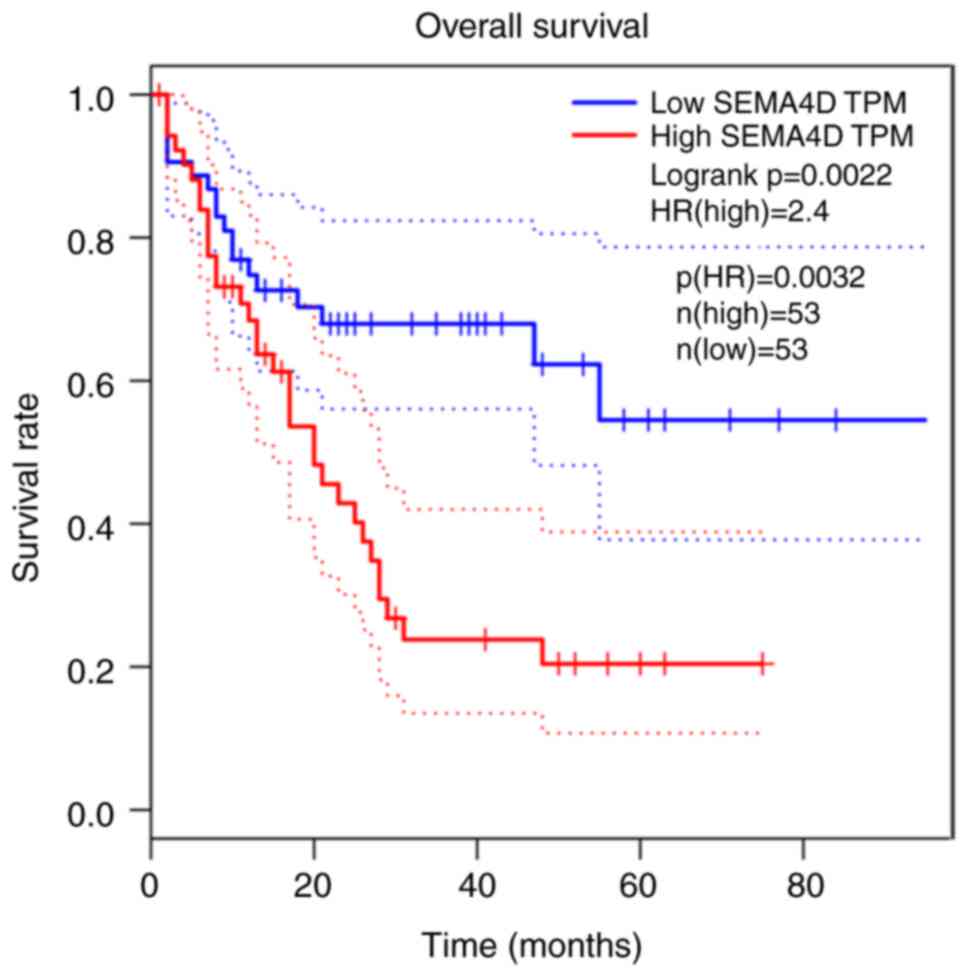
Sema4D overexpression is correlated
with poor prognosis in AML
As there were no ALL data in the GEPIA website,
overall survival was only analyzed in patients with AML, and it was
revealed that high expression of Sema4D was associated with shorter
overall survival in patients with AML (Fig. 8). Therefore, Sema4D may be a poor
prognostic biomarker in leukemia.
Discussion
Sema4D is highly expressed in several tumor tissues,
including breast, lung, colorectal, prostate, oral and pancreatic
cancer, head and neck squamous cell carcinoma and soft tissue
sarcoma (10,12,13,16,17).
Although Sema4D is expressed in B-CLL (40), its expression in other leukemia types
has not been reported. Therefore, the expression of Sema4D in
pediatric leukemia was examined in the present study. The results
demonstrated that there was high expression of Sema4D in PBMCs and
a high level of soluble Sema4D in the plasma of patients with
pediatric leukemia, with no difference between B-ALL and Non-B-ALL
groups. As Non-B-ALL mainly included AML and T cell-ALL, the data
suggested that the expression of Sema4D was upregulated in B-ALL
and other types of leukemia as well. Therefore, Sema4D may serve an
important role in the development of leukemia.
To understand the role of Sema4D in the development
of leukemia, its effects on proliferation, apoptosis, cell cycle,
invasion and migration were examined in the present study. The
results demonstrated that in BALL-1 cells, Sema4D knockdown induced
cell cycle arrest in the G0/G1 phase,
increased apoptosis and inhibited proliferation; on the contrary,
overexpression of Sema4D promoted cell division and proliferation
and inhibited apoptosis. In Jurkat cells, Sema4D knockdown
inhibited proliferation and promoted apoptosis, while Sema4D
overexpression resulted in a decreased abundance of cells in the
G0/G1 phase and promoted proliferation. Taken
together, these data suggested that Sema4D promoted proliferation
and inhibited apoptosis in leukemia cells. Sema4D overexpression
promoted the migratory ability of Jurkat cells and the invasive
ability of BALL-1 cells. Sema4D enhanced the invasive and migratory
abilities of leukemia cells, although the effect was dependent on
the cell origin. These results on the effect of Sema4D on invasion
and migration are consistent with a previous study in breast cancer
(20).
Sema4D has been indicated to phosphorylate tyrosine
kinase receptors [protein-tyrosine kinase 2-beta (Pyk2) or Src] and
ERK1/2, and phosphorylated Pyk2 and Src further activate the
PI3K/AKT signaling pathway and mediate cell invasion and migration
(41). AKT is widely expressed in
different types of tumors, such as colorectal, pancreatic, gastric
and non-small cell lung cancer cell (31,32,34,35).
It has been indicated to associate cancer-promoting molecules and
downstream signaling molecules in tumor development (42). It has also been demonstrated to
promote cell proliferation and inhibit apoptosis (31), and promote metastasis by regulating
Bcl2 and focal adhesion kinase expression (43–45). The
results of the present study revealed that knockdown of Sema4D
inhibited the phosphorylation PI3K and AKT in both Jurkat and
BALL-1 cell lines, suggesting that Sema4D activated the PI3K/AKT
signaling pathway, which was also supported by the fact that the
phosphorylation of PI3K and AKT was correlated with Sema4D
expression in pediatric leukemia samples.
ERK, which is a downstream protein of a variety of
growth factors (37–39), has been indicated to be activated in
oral, colorectal and gastric cancer (46–49). It
transfers extracellular signals to the nucleus to regulate cell
proliferation, differentiation and survival. The results of the
current study revealed that the phosphorylation level of ERK was
significantly decreased in Sema4D knocked-down BALL-1 cells. The
phosphorylation level of ERK was also increased in patients with
pediatric leukemia and was correlated with the expression level of
Sema4D. This indicated that Sema4D activated ERK signaling in
leukemia cells.
As the activation of PI3K, AKT and ERK is important
in regulating cell proliferation, invasion, migration and apoptosis
(31,42–45), we
speculate that Sema4D promoted leukemia development via activating
the PI3K/AKT and ERK pathways.
Soluble Sema4D, which is released by proteolytic
cleavage of the extracellular domain of transmembrane Sema4D, has
been indicated to be involved in infectious and inflammatory
diseases (2) and heart failure
(50). The finding of high levels of
soluble Sema4D in the plasma of patients with pediatric leukemia is
consistent with results in human head and neck cancer (25,51),
suggesting that Sema4D may be a potential biomarker for cancer
diagnosis. Soluble Sema4D has also been reported to participate in
cancer development by inducing metastasis (25), inhibiting differentiation (52) and suppressing the immune response
(53). However, the role of soluble
Sema4D in leukemia remains unknown, and further studies are
required.
In conclusion, the results of the present study
demonstrated that Sema4D was upregulated in the PBMCs of patients
with pediatric leukemia and soluble Sema4D was similarly increased
in the plasma of these patients. Sema4D was revealed to modulate
the cell cycle, promote cell proliferation and invasion and inhibit
apoptosis in ALL cells. It was indicated to serve an important role
in leukemia development via regulating the PI3K/AKT and ERK
signaling pathways. Sema4D may serve as a novel target for leukemia
diagnosis and treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81960033 to HJ,
31970868 to QS and 81460028 to MY), the Association Foundation
Program of Yunnan Science and Technology Department and Kunming
Medical University (grant no. 2019FE001-103 to HJ), the Foundation
of the CAMS Initiative for Innovative Medicine (CAMS-I2M) (grant
no. 2017-I2M-2-006 to QS), the Foundation of Yunnan Medical Science
and Technology (grant no. 2016NS124 to HJ), Yunnan Health Training
Project of High Level Talents (grant no. D-2017053 to HJ), Top
Young Experts Training Project for the Academy and Technology in
Kunming and Yunnan Province to HJ (grant no. 202005AC160066),
Postdoctoral Training Program of Yunnan Province (grant no.
Ynbh19035 to HJ) and Natural Science Foundation of Yunnan Province
(grant no. 2019-1-C-25318000002240 to HJ).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HJ and JT confirm the authenticity of the raw data.
HJ designed and performed the experiments. JT performed the
experiments and prepared the manuscript. SS, LX, LK, TH, WN, BZ and
CZ obtained and arranged the clinical specimens and performed
clinical analysis. LQ performed clinical analysis and western blot
of clinical samples. MY, QS and ZZ proposed the idea, designed and
supervised the project, analyzed and interpreted the data and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Children's Hospital of Kunming Medical
University (Kunming, China), and written informed consent was
obtained from each donor's guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Sema4D
|
semaphorin 4D
|
|
ALL
|
acute lymphoblastic leukemia
|
|
AML
|
acute myeloid leukemia
|
References
|
1
|
Hall KT, Boumsell L, Schultze JL,
Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM and
Freeman GJ: Human CD100, a novel leukocyte semaphorin that promotes
B-cell aggregation and differentiation. Proc Natl Acad Sci USA.
93:11780–11785. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maleki KT, Cornillet M and Björkström NK:
Soluble SEMA4D/CD100: A novel immunoregulator in infectious and
inflammatory diseases. Clin Immunol. 163:52–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mou P, Zeng Z, Li Q, Liu X, Xin X,
Wannemacher KM, Ruan C, Li R, Brass LF and Zhu L: Identification of
a calmodulin-binding domain in Sema4D that regulates its exodomain
shedding in platelets. Blood. 121:4221–4230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motani K and Kosako H: Activation of
stimulator of interferon genes (STING) induces ADAM17-mediated
shedding of the immune semaphorin SEMA4D. J Biol Chem.
293:7717–7726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ch'ng ES and Kumanogoh A: Roles of Sema4D
and Plexin-B1 in tumor progression. Mol Cancer. 9:2512010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishide M, Nojima S, Ito D, Takamatsu H,
Koyama S, Kang S, Kimura T, Morimoto K, Hosokawa T, Hayama Y, et
al: Semaphorin 4D inhibits neutrophil activation and is involved in
the pathogenesis of neutrophil-mediated autoimmune vasculitis. Ann
Rheum Dis. 76:1440–1448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao C, Luo Y, Zhang C, Zhu Z, Yang L,
Qiao H, Fu M, Wang G, Yao X and Li W: Negative regulation of
dendritic cell activation in psoriasis mediated via
CD100-plexin-B2. J Pathol. 250:409–419. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuda T, Nishide M, Maeda Y, Hayama Y,
Koyama S, Nojima S, Takamatsu H, Okuzaki D, Morita T, Nakatani T,
et al: Pathological and therapeutic implications of
eosinophil-derived semaphorin 4D in eosinophilic chronic
rhinosinusitis. J Allergy Clin Immunol. 145:843–854.e4. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu L, Bergmeier W, Wu J, Jiang H, Stalker
TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, et al:
Regulated surface expression and shedding support a dual role for
semaphorin 4D in platelet responses to vascular injury. Proc Natl
Acad Sci USA. 104:1621–1626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou H, Yang YH, Binmadi NO, Proia P and
Basile JR: The hypoxia-inducible factor-responsive proteins
semaphorin 4D and vascular endothelial growth factor promote tumor
growth and angiogenesis in oral squamous cell carcinoma. Exp Cell
Res. 318:1685–1698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sierra JR, Corso S, Caione L, Cepero V,
Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H,
Comoglio PM, et al: Tumor angiogenesis and progression are enhanced
by Sema4D produced by tumor-associated macrophages. J Exp Med.
205:1673–1685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou H, Binmadi NO, Yang YH, Proia P and
Basile JR: Semaphorin 4D cooperates with VEGF to promote
angiogenesis and tumor progression. Angiogenesis. 15:391–407. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruan SS, Li RC, Han Q, Liu J, Li GL, Song
YQ and Wu G: Expression and clinical significance of Semaphorin4D
in non-small cell lung cancer and its impact on malignant behaviors
of A549 lung cancer cells. J Huazhong Univ Sci Technolog Med Sci.
34:491–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Yang Y, Xiao J, Yang S, Liu Y, Kang
W, Li X and Zhang F: Semaphorin 4D expression is associated with a
poor clinical outcome in cervical cancer patients. Microvasc Res.
93:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Zhang L, Lv R and Zhang WQ:
Overexpression of Semaphorin4D indicates poor prognosis and prompts
monocyte differentiation toward M2 macrophages in epithelial
ovarian cancer. Asian Pac J Cancer Prev. 14:5883–5890. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simon JM, Mokhtari K, Genestie C, Bissery
A, Mazeron JJ and Jaillon P: Hypoxia-inducible factor lalpha
(HIF-1α) and carbonic anhydrase IX (CA9) expressions in
glioblastoma multiform to predict response to radiation therapy. J
Clin Oncol. 23 (Suppl 16):S15122005. View Article : Google Scholar
|
|
17
|
Ch'ng E, Tomita Y, Zhang B, He J, Hoshida
Y, Qiu Y, Morii E, Nakamichi I, Hamada K, Ueda T and Aozasa K:
Prognostic significance of CD100 expression in soft tissue sarcoma.
Cancer. 110:164–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato S, Kubota K, Shimamura T, Shinohara
Y, Kobayashi N, Watanabe S, Yoneda M, Inamori M, Nakamura F,
Ishiguro H, et al: Semaphorin 4D, a lymphocyte semaphorin, enhances
tumor cell motility through binding its receptor, plexinB1, in
pancreatic cancer. Cancer Sci. 102:2029–2037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soone J, Chen Y, Shustef EM and Scott GA:
Sema4D, the ligand for Plexin B1, suppresses c-Met activation and
migration and promotes melanocyte survival and growth. J Invest
Dermatol. 132:1230–1238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang H, Chen C, Sun Q, Wu J, Qiu L, Gao
C, Liu W, Yang J, Jun N and Dong J: The role of semaphorin 4D in
tumor development and angiogenesis in human breast cancer. Onco
Targets Ther. 9:5737–5750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding X, Qiu L, Zhang L, Xi J, Li D, Huang
X, Zhao Y, Wang X and Sun Q: The role of semaphorin 4D as a
potential biomarker for antiangiogenic therapy in colorectal
cancer. Onco Targets Ther. 9:1189–1204. 2016.PubMed/NCBI
|
|
22
|
Basile JR, Holmbeck K, Bugge TH and
Gutkind JS: MT1-MMP controls tumor-induced angiogenesis through the
release of semaphorin 4D. J Biol Chem. 282:6899–6905. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Granziero L, Circosta P, Scielzo C,
Frisaldi E, Stella S, Geuna M, Giordano S, Ghia P and
Caligaris-Cappio F: CD100/Plexin-B1 interactions sustain
proliferation and survival of normal and leukemic CD5+ B
lymphocytes. Blood. 101:1962–1969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deaglio S, Vaisitti T, Bergui L, Bonello
L, Horenstein AL, Tamagnone L, Boumsell L and Malavasi F: CD38 and
CD100 lead a network of surface receptors relaying positive signals
for B-CLL growth and survival. Blood. 105:3042–3050. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Qiao H, Guo W, Liu Y, Yang L, Liu
Y, Jin B, Fu M, Wang G and Li W: CD100-plexin-B1 induces
epithelial-mesenchymal transition of head and neck squamous cell
carcinoma and promotes metastasis. Cancer Lett. 455:1–13. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clavijo PE, Friedman J, Robbins Y, Moore
EC, Smith E, Zauderer M, Evans EE and Allen CT: Semaphorin4D
inhibition improves response to immune-checkpoint blockade via
attenuation of MDSC recruitment and function. Cancer Immunol Res.
7:282–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zuazo-Gaztelu I, Pàez-Ribes M, Carrasco P,
Martín L, Soler A, Martínez-Lozano M, Pons R, Llena J, Palomero L,
Graupera M and Casanovas O: Antitumor effects of anti-Semaphorin 4D
antibody unravel a novel proinvasive mechanism of
vascular-targeting agents. Cancer Res. 79:5328–5341. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brillantino C, Rossi E, Bifano D, Minelli
R, Tamasi S, Mamone R, Bignardi E, Zeccolini R, Zeccolini M and
Vallone G: An unusual onset of pediatric acute lymphoblastic
leukemia. J Ultrasound. Apr 23–2020.(Epub ahead of print).
View Article : Google Scholar
|
|
29
|
Cheson BD, Cassileth PA, Head DR, Schiffer
CA, Bennett JM, Bloomfield CD, Brunning R, Gale RP, Grever MR,
Keating MJ, et al: Report of the national cancer
institute-sponsored workshop on definitions of diagnosis and
response in acute myeloid leukemia. J Clin Oncol. 8:813–819. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cortes JE and Kantarjian HM: Acute
lymphoblastic leukemia. A comprehensive review with emphasis on
biology and therapy. Cancer. 76:2393–2417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung DC: The genetic basis of colorectal
cancer: Insights into critical pathways of tumorigenesis.
Gastroenterology. 119:854–865. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou W, Fu XQ, Liu J and Yu HG: RNAi
knockdown of the Akt1 gene increases the chemosensitivity of
gastric cancer cells to cisplatin both in vitro and in vivo. Regul
Pept. 176:13–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, Danino M, Karlan BY and Slamon DJ:
Overexpression of AKT2/protein kinase Bbeta leads to up-regulation
of beta1 integrins, increased invasion, and metastasis of human
breast and ovarian cancer cells. Cancer Res. 63:196–206.
2003.PubMed/NCBI
|
|
34
|
Lee MW, Kim DS, Lee JH, Lee BS, Lee SH,
Jung HL, Sung KW, Kim HT, Yoo KH and Koo HH: Roles of AKT1 and AKT2
in non-small cell lung cancer cell survival, growth, and migration.
Cancer Sci. 102:1822–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schlieman MG, Fahy BN, Ramsamooj R,
Beckett L and Bold RJ: Incidence, mechanism and prognostic value of
activated AKT in pancreas cancer. Br J Cancer. 89:2110–2115. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan DP, Zhang YM, Hu XC, Li JJ and Zhang
W: Activation of AKT/ERK confers non-small cell lung cancer cells
resistance to vinorelbine. Int J Clin Exp Patho. 7:134–143.
2013.
|
|
37
|
Lin Z, Zhang C, Zhang M, Xu D, Fang Y,
Zhou Z, Chen X, Qin N and Zhang X: Targeting cadherin-17
inactivates Ras/Raf/MEK/ERK signaling and inhibits cell
proliferation in gastric cancer. PLoS One. 9:e852962014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park JI: Growth arrest signaling of the
Raf/MEK/ERK pathway in cancer. Front Biol (Beijing). 9:95–103.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu S, Fan L, Pan X, Sun Y and Zhao H:
Integrin αv promotes proliferation by activating ERK 1/2 in the
human lung cancer cell line A549. Mol Med Rep. 11:1266–1271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei L, Li H, Tamagnone L and You H:
Semaphorins and their receptors in hematological malignancies.
Front Oncol. 9:3822019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Basile JR, Gavard J and Gutkind JS:
Plexin-B1 utilizes RhoA and Rho kinase to promote the
integrin-dependent activation of Akt and ERK and endothelial cell
motility. J Biol Chem. 282:34888–34895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan F, Huang Y, Pei Q, Liu H, Pei H and
Zhu H: Matrix stiffness mediates stemness characteristics via
activating the Yes-associated protein in colorectal cancer cells. J
Cell Biochem. Sep 14–2018.(Epub ahead of print).
|
|
44
|
Jeong KY: Inhibiting focal adhesion
kinase: A potential target for enhancing therapeutic efficacy in
colorectal cancer therapy. World J Gastrointest Oncol. 10:290–292.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng Q, Wang B, Gao J, Xin N, Wang W,
Song X, Shao Y and Zhao C: CD155 knockdown promotes apoptosis via
AKT/Bcl-2/Bax in colon cancer cells. J Cell Mol Med. 22:131–140.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ai X, Wu Y, Zhang W, Zhang Z, Jin G, Zhao
J, Yu J, Lin Y, Zhang W, Liang H, et al: Targeting the ERK pathway
reduces liver metastasis of Smad4-inactivated colorectal cancer.
Cancer Biol Ther. 14:1059–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zinn RL, Gardner EE, Marchionni L, Murphy
SC, Dobromilskaya I, Hann CL and Rudin CM: ERK phosphorylation is
predictive of resistance to IGF-1R inhibition in small cell lung
cancer. Mol Cancer Ther. 12:1131–1139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yan L, Gu H, Li J, Xu M, Liu T, Shen Y,
Chen B and Zhang G: RKIP and 14-3-3ε exert an opposite effect on
human gastric cancer cells SGC7901 by regulating the ERK/MAPK
pathway differently. Dig Dis Sci. 58:389–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koyama T, Ogawara K, Kasamatsu A, Okamoto
A, Kasama H, Minakawa Y, Shimada K, Yokoe H, Shiiba M, Tanzawa H
and Uzawa K: ANGPTL3 is a novel biomarker as it activates ERK/MAPK
pathway in oral cancer. Cancer Med. 4:759–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu Q, Dong N, Wang Q, Yi W, Wang Y, Zhang
S, Gu H, Zhao X, Tang X, Jin B, et al: Increased levels of plasma
soluble Sema4D in patients with heart failure. PLoS One.
8:e642652013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Derakhshandeh R, Sanadhya S, Lee Han K,
Chen H, Goloubeva O, Webb TJ and Younis RH: Semaphorin 4D in human
head and neck cancer tissue and peripheral blood: A dense fibrotic
peri-tumoral stromal phenotype. Oncotarget. 9:111262018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang YH, Buhamrah A, Schneider A, Lin YL,
Zhou H, Bugshan A and Basile JR: Semaphorin 4D promotes skeletal
metastasis in breast cancer. PLoS One. 11:e01501512016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tamagnone L and Franzolin G: Targeting
semaphorin 4D in cancer: A look from different perspectives. Cancer
Res. 79:5146–5148. 2019. View Article : Google Scholar : PubMed/NCBI
|