Introduction
Gallbladder cancer (GBC) is a rare but fatal cancer
of the biliary tract, which is often encountered in developing
countries. The variation in the incidence of GBC across different
regions and races suggests that genetic and environmental factors
may play an important role in this type of cancer (1,2). Previous
studies have demonstrated that a number of factors may induce GBC
formation, including chronic gallbladder infections, specific
chemicals, exposure to heavy metals, even multiple dietary factors
(3,4).
Familial gallstones, long-term tobacco exposure and excessive
intake of fried foods may increase the risk of GBC (5). Treatment of GBC is often challenging,
mainly because it is difficult to diagnose at its early stages.
Furthermore, a propensity for liver infiltration and metastasis is
an important reason for the poor prognosis (6). Surgical treatment is currently the
mainstay of treatment for patients with GBC (7). Similar to other tumors, GBC is a disease
involving a variety of genetic factors. However, our knowledge of
the genetic and molecular changes associated with GBC is currently
limited (8,9) and there is an urgent need to elucidate
the specific processes and mechanisms involved in its early
occurrence and development.
MicroRNAs (miRNAs) are a group of endogenous, small
non-coding RNAs, 18–25 nucleotides in length, that are highly
conserved across species (10–12).
miRNAs bind to the 3′-untranslated region (UTR) of their target
mRNAs to cause their degradation or inhibit their transcription.
Accumulating evidence indicates that miRNAs play key roles in
regulating various biological processes, including cell
proliferation, differentiation and apoptosis (13,14). In
addition, miRNAs have become a key focus in cancer research in
recent years, and some miRNAs have been shown to inhibit or promote
tumorigenesis (15,16). Previous studies have demonstrated
that, during the development and progression of GBC, the expression
patterns of some miRNAs may change. For example, Kono et al
demonstrated that aberrant expression of miR-155 markedly increased
the proliferation and invasion ability of GBC cells, and the
survival prognosis of GBC patients with high levels of miR-155 was
worse compared with that of patients with lower levels of miR-155
(17). miR-1, miR-24 and miR-135 were
also reported to play important roles in GBC as tumor suppressors
(18–20). In most cases of primary GBC,
downregulation of miR-335 may be associated with tumor invasiveness
(21). miR-146b-5p has been shown to
be abnormally expressed in a variety of malignancies in humans,
including gastric cancer, thyroid cancer, osteosarcoma and glioma
(22–25). Although abnormal miR-146b-5p
expression levels have been reported in GBC, the specific role and
mechanism of action of miR-146b-5p in the development of GBC
requires further investigation (26).
Activation of the Toll-like receptor 4 (TLR4)
pathway leads to activation of IRE3, NF-κB and mitogen-activated
protein kinase via MyD88-dependent and non-dependent pathways, and
then induces the expression of type II IFN (IFN-γ) and
pro-inflammatory cytokines (27–30).
Activation of TLR4 was previously considered to be primarily
involved in the innate immune response to bacterial infection
(31). However, recent research has
demonstrated that activation of TLR4 participates in the
physiological processes of various cancer cells, and is associated
with the metastasis of certain cancers (32–36). In
some cancer cells (e.g., breast cancer cells), TLR4 functions as a
regulator through a dual TP53-dependent role. In the case of TP53
expression, activated TLR4 increases INF-γ secretion and inhibition
of breast cancer cell proliferation, thereby exerting an anticancer
effect. However, when TP53 is mutated, activated TLR4 induces the
secretion of growth factors to promote breast cancer cell
proliferation and exerts a cancer-promoting effect (37).
In the present study, the level of miR-146b-5p was
detected in 60 groups of human GBC and normal tissues. In addition,
the expression levels of miR-146b-5p were compared between human
GBC cells cultured in vitro and gallbladder epithelial
cells. Furthermore, the effects of miR-146b-5p overexpression on
the proliferation, migration, invasion and apoptosis of GBC cells
were investigated. Bioinformatics analysis identified TLR4 as the
possible target gene of miR-146b-5p. Therefore, the mRNA and
protein levels of TLR4 were investigated in GBC tissues and cells.
By overexpressing or inhibiting the expression of miR-146b-5p, it
was investigated whether there is a correlation between the
expression level of TLR4 and that of miR-146b-5p, and how the
overexpression of miR-146b-5p and TLR4 affects the proliferation,
migration and invasion ability of GBC cells. It was also
investigated whether these effects are mediated via regulation of
the NF-κB signaling pathway. In vivo experiments were also
performed to examine the effects of miR-146b-5p overexpression on
GBC cell proliferation and apoptosis.
Materials and methods
Patients and clinical samples
GBC samples and adjacent normal gallbladder tissue
samples were acquired from 60 surgical patients, and all specimens
had been clinically diagnosed and histologically confirmed at the
Jinling Hospital between July 2017 and June 2018. Patient informed
consent for participation was obtained at the time of the study.
The study protocol was approved by the Ethics Committee of Southern
Medical University (no. 2018-SR-052). Immediately after surgery,
clinical samples were divided into two groups: One group of samples
was immediately fixed in 4% paraformaldehyde and then embedded in
paraffin for later experiments, whereas the remaining samples were
placed in liquid nitrogen and preserved at −80°C to be further used
for mRNA and protein extraction.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from tissues
and cells, according to the manufacturer's protocols. For each
sample, 1 µg RNA was reversely transcribed into complementary DNA
(cDNA) using the Reverse Transcription System Kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). qPCR assay was used to
examine the expression of miR-146b-5p and TLR4. U6 was used as an
internal reference gene. The thermocycling conditions were as
follows: 95°C for 5 min, followed by 40 cycles at 95°C for 10 sec
and at 60°C for 30 sec. The primers used were as follows:
miR-146b-5p: 5′-TGAACTGAATTCATGGGTT-3′ (sense) and
5′-ATCTTGAGCTCCTCCGAAG-3′ (antisense); TLR4:
5′-AGCACTTCATCCAGAGCCGC-3′ (sense) and 5′-CGGTACAGCTCCACCTGCTG-3′
(antisense); U6: 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (sense) and
5′-CGCTTCAGAATTTGCGTGTCAT-3′ (antisense). The gene expression
levels were calculated using the 2−ΔΔCq method (16).
Cell cultures
The human GBC cell lines NOZ and GBC-SD were
purchased from Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). The normal human gallbladder
epithelium cell line HGBEC was obtained by cell isolation and
culture. GBC-SD cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.), NOZ cells were maintained in
William's E Medium (Gibco; Thermo Fisher Scientific, Inc.) and
HGBEC cells were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.),
penicillin and streptomycin (100 µg/ml) in an incubator with 5%
CO2 at 37°C.
Cell transfection
For upregulation or suppression of miR-146b-5p, all
the plasmids were procured from GenePharma. The plasmids were
marked with green fluorescent protein (GFP) and the miR-146b-5p
mimics, inhibitors and the negative controls (NC mimics and NC
inhibitors) were synthesized by GenePharma. The sequences were as
follows: miR-146b-5p mimics, sense 5′-UGAGAACUGAAUUCCAUGGGUU-3′ and
antisense 5′-CCCAUGGAAUUCAGUUCUCAUU-3′; and miR-146b-5p mimics NC,
sense 5′-UUCUCCGAACGUGUCACGUTT−3′ and antisense
5′-ACGUGACACGUUCGGAGAATT−3′. miR-146b-5p inhibitors:
5′-AACCCAUGGAAUUCAGUUCUCA-3′; miR-146b-5p inhibitors NC:
5′-CAGUACUUUUGUGUAGUACAA−3′. Cell transfection was performed with
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as
per the manufacturer's recommendations. RT-PCR analysis and green
fluorescence microscopy were performed to assess the transfection
efficiency.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Beyotime Institute of
Biotechnology) was used to detect the proliferation and activity of
GBC cells according to the manufacturer's protocol. Cells were
inoculated into a 96-well plate at 1×105 cells/well and
cultured for 24, 48 and 72 h. Subsequently, the cells were
incubated with CCK-8 solution with 5% CO2 at 37°C for
2–3 h. A microplate reader (Bio-Rad Laboratories, Inc.) was used to
measure the absorbance at 450 nm.
Cell colony formation assay
Cells in the logarithmic growth phase were digested
by 0.25% trypsin and counted. A cell suspension was prepared with
DMEM and the cell density reached 1×106 cells per liter.
Low melting point agarose (1.2%) mixed with equal amount of DMEM
was added to the Petri dish to cool and solidify as the bottom
agar. The cell suspension (0.2 ml) was added to the mixture of 0.7%
agarose and DMEM, and then coagulated in the Petri dish as the
upper agar. The cells were cultured for 2 weeks with 5%
CO2 at 37°C. The number of cell clones was observed
under an inverted microscope at a magnification of ×40 (Olympus
Corporation) and the colony formation rate was calculated.
Western blot assay
Lysis buffer (Thermo Fisher Scientific, Inc.) was
used to lyse cells/tissues, and the protein concentration in the
lysates was determined using a BCA Protein Assay Kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Equal amounts of protein (10 mg) were resolved by 12%
SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The
membranes were blocked with 5% BSA mixed with Tris-buffered
saline/0.1% Tween-20 (Beyotime Institute of Biotechnology), and
then incubated with primary antibodies overnight at 4°C. The
primary antibodies used in the present study were obtained from
Abcam and were as follows: Anti-GAPDH (cat. no. ab181602; 1:2,000),
anti-PCNA (cat. no. ab18197; 1:1,000), anti-cleaved-caspase-3 (cat.
no. ab2302; 1:1,000), anti-caspase-3 (cat. no. ab13847; 1:1,000),
anti-cleaved-caspase-9 (ab2304; 1:1,000), anti-caspase-9 (cat. no.
ab52298; 1:1,000), anti-Bax (cat. no. ab32503; 1:1,000), anti-Bcl-2
(cat. no. ab32124; 1:1,000), anti-cyclooxygenase-2 (anti-COX-2;
cat. no. ab23672; 1:1,000), anti-matrix metallopeptidase (MMP)-2
(cat. no. ab86607; 1:1,000), anti-MMP-9 (cat. no. ab76003;
1:1,000), anti-TLR4 (cat. no. ab13556; 1:1,000), anti-inhibitor of
nuclear factor (NF)-κB (anti-IκBα; cat. no. ab32518; 1:1,000),
anti-phosphorylated (p)-IκBα (cat. no. ab133462; 1:1,000),
anti-p-NF-κB (cat. no. ab222494; 1:1,000) and anti-Histone H3 (cat.
no. ab1791; 1:1,000). The membranes were then incubated with
corresponding secondary antibodies for 2 h at room temperature. The
protein bands were derived from the same membrane and exposed using
a Super Signal ECL kit (EMD Millipore) in a western blot detection
instrument and quantified by gray level analysis relative to GAPDH
or H3.
Apoptosis detection by Annexin V-FITC
staining
The cultured cells under different treatments were
washed three times with cold PBS. The cells were then suspended in
50 µl binding buffer and stained with Annexin V-FITC and propidium
iodide for 15 min at room temperature in the dark. Then, the cell
suspension was gently blended after adding 100 µl of binding
buffer, and the percentage of apoptotic cells was detected by flow
cytometry (LSR2; BD Biosciences) using FlowJo 10.06 software
(FlowJo LLC).
Wound healing assay
Cells (1×106) were inoculated on a 6-well
plate. After the cells had reached a confluence of ~100%, a linear
scratch was created in the cell monolayer with the tip of a 10-µl
micropipette. The detached cells were gently washed off with 1X PBS
and the remaining cells were cultured in serum-free medium with 5%
CO2 at 37°C. Finally, images were captured at 0 and 48 h
under a light microscope at a magnification of ×200 (Olympus
Corporation).
Transwell migration and invasion
assays
Transwell migration and invasion experiments were
performed using Boyden chambers (BD Biosciences). Cells
(1×106) were cultured in serum-free medium for 24 h and
digested with trypsin. Then, BSA-containing serum-free medium was
used to re-suspend the cells to a cell density of
5×105/ml. A total of 100 µl of cell suspension was added
to the upper chamber without Matrigel (for the migration assay) or
pre-coated with Matrigel (for the invasion assay) at 37°C for 30
min (BD Biosciences); 600 µl DMEM supplemented with 20% FBS was
added to the lower chamber. After 24 h of conventional culture at
37°C, the chamber was removed, washed twice with PBS, then fixed
with 4% formaldehyde at 37°C for 30 min. After 20 min of staining
with 0.1% crystal violet solution at 37°C, the cells in the upper
chamber were wiped off and the chamber was washed three times with
PBS. The cells were observed and counted under a microscope
(Olympus Corporation) at a magnification of ×200 in five random
fields of view.
Luciferase reporter assay
3′-UTR fragments of TLR4 containing wild-type or
mutant miR-146b-5p-binding sites were inserted into the psiCHECK-2
plasmids (Promega Corporation) to produce TLR4-WT and TLR4-Mut
constructs. Cells were co-transfected with indicated constructs and
miR-146b-5p mimic or NC mimic by using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. At 48 h post-transfection, luciferase
activity was determined with the Dual-Luciferase Reporter Assay
System (Promega Corporation) according to the manufacturer's
instructions and normalized to Renilla luciferase activity.
Immunohistochemical analysis
The tissue sections were maintained at room
temperature for 1 h, then immersed in xylene for 10 min to
deparaffinize. The sections were hydrated with different
concentration gradients of ethanol. Subsequently, the slides were
immersed in sodium citrate buffer (pH 6.0) and boiled for 30 min,
followed by incubation with anti-TLR4 (ab13867, 1:1,000, Abcam) and
anti-Ki-67 antibodies (ab15580, 1:1,000, Abcam) at 37°C according
to the immunohistochemistry protocol. The sections were observed
under a light microscope (CX23; Olympus Corporation) and images
were captured at a magnification of ×200.
Immunofluorescence analysis
The cells were washed with PBS and fixed in 1%
formalin in PBS at room temperature for 10 min. After fixing, the
cells were washed in PBS three times (5 min per wash), and
incubated for 3 min at 37°C in a solution of 0.01% Triton X-100 in
PBS, followed by washing three times in PBS for 5 min per wash and
blocking in 10% goat serum (Gibco; Thermo Fisher Scientific, Inc.)
for 1 h at room temperature. The cells were then incubated
overnight at 4°C with primary antibody against TLR4 (cat. no.
ab13556; 1:1,000, Abcam). On the following day, the cells were
washed in PBS three times (5 min per wash) and incubated with
secondary antibody (ab150081, 1:1,000, Abcam) for 2 h at room
temperature. After incubation, the cells were rinsed in PBS three
times (5 min per wash) and mounted in 50% triglyceride.
In vivo xenograft model
Female SPF BALB/c nude mice (age, 6 weeks; weight,
18–22 g) were provided by Nanjing Medical University Animal
Laboratory (Nanjing, China). The animal experimental protocols were
approved by the Institutional Animal Care and Use Committee of
Southern Medical University (no. GZZL-2018-0235). The animals were
housed at room temperature (18-25°C) with a 12-h light/dark cycle
and were given free access to a standard diet and tap water. The
BALB/c nude mice were randomly divided into blank and experimental
groups and weighed at the same time. GBC-SD cells transfected with
miR-146b-5p mimic or miR-146b-5p mimic NC in the logarithmic growth
phase were digested, centrifuged at 12,000 × g for 5 min at room
temperature, the old culture medium was discarded and the cells
were suspended in PBS. The cells were washed twice with PBS and
mixed with Matrigel at a ratio of 1:1 (on ice) to adjust the cell
density to 1×107/ml. The single-cell suspension
(1×106) was injected slowly into the axilla of nude
mice. According to the humane endpoints, the mice were continuously
observed for obvious decrease in activity, abnormal diet,
emaciation and progressive weight loss, at which point they would
have to be immediately anesthetized and sacrificed. None of the
animals reached the humane endpoints before the conclusion of the
study. During the experiments, animal health and behavior were
monitored every 2 days and the size of tumors was measured every 3
days. At 30 days after injection, the mice were sacrificed by
cervical dislocation after an intraperitoneal injection of sodium
pentobarbital (100 mg/kg). Death was confirmed by observing lack of
respiration and cardiac activity for 5 min.
Statistical analysis
The results are presented as mean and standard
error. Differences among multiple groups were analyzed by using
one-way ANOVA followed by Tukey's post hoc test. Student's t-test
was performed to evaluate statistical comparisons between two
independent groups. Statistical analyses were performed using SPSS
v19.0 (IBM Corp.). P<0.05 was considered to indicate
statistically significant differences.
Results
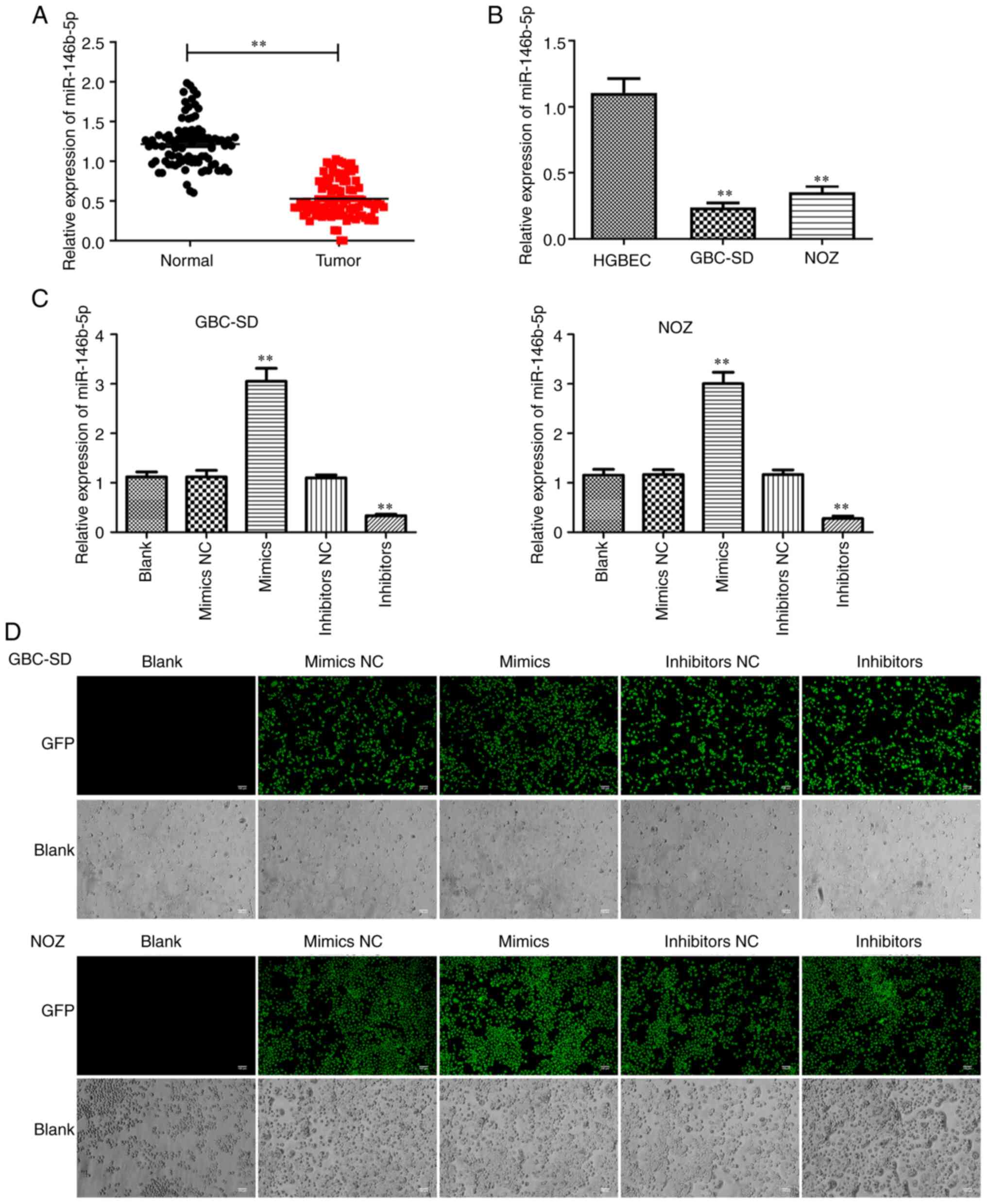
The expression of miR-146b-5p is
decreased in GBC tissues and cells
Examination of 60 pairs of human GBC and normal
tissues revealed a prominent decrease in miR-146b-5p expression in
GBC tissues compared with that in normal tissues (Fig. 1A). Furthermore, when compared with
gallbladder epithelial cells, the expression of miR-146b-5p in GBC
cell lines was significantly lower (Fig.
1B). These results indicate that miR-146b-5p may play an
important role in GBC.
miR-146b-5p affects proliferation and
apoptosis of GBC cells
To explore the role of miR-146b-5p in GBC, GBC cell
lines were treated in vitro with miR-146b-5p mimics and
miR-146b-5p inhibitors. RT-qPCR analysis and the GFP fluorescence
reporter system were employed to confirm the efficiency of cell
transfection (Fig. 1C and D). Then,
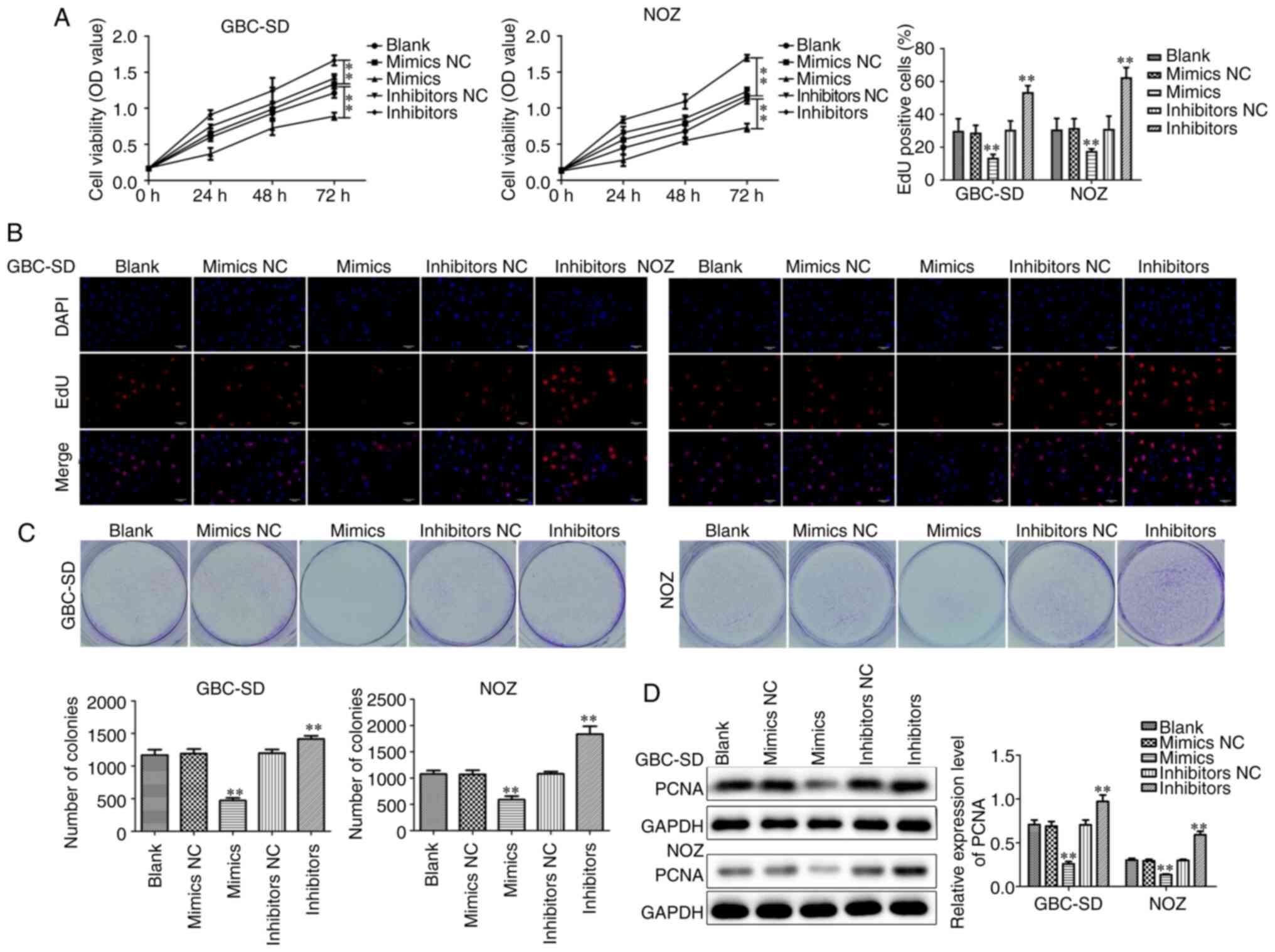
the CCK-8 assay was used to detect cell viability, and it was
observed that treatment with miR-146b-5p inhibitors can effectively
increase cell viability; conversely, treatment with miR-146b-5p
mimics resulted in decreased cell viability (Fig. 2A). These changes were the same in both
GBC cell lines and lasted for at least 72 h, suggesting a negative
correlation between the expression level of miR-146b-5p and the
viability of the GBC cells. Labeling proliferating cells with EdU
demonstrated that overexpression of miR-146b-5p notably reduced,
whereas inhibition of miR-146b-5p expression increased the number
of EdU+ cells (Fig. 2B).
By detecting the colony-forming ability of cells, it was observed
that overexpression of miR-146b-5p reduced the number of colonies,
whereas inhibiting the expression of miR-146b-5p increased colony
formation (Fig. 2C). Proliferating
cell nuclear antigen (PCNA), a marker of tumor cell deregulation,
may be used to objectively evaluate tumor cell proliferation. It
was observed that the expression of PCNA decreased following
treatment with miR-146b-5p mimics, while the expression of PCNA
increased moderately following treatment with miR-146b-5p
inhibitors (Fig. 2D). These data
indicate that overexpression of miR-146b-5p inhibits the
proliferation of GBC cells, while inhibiting the expression of
miR-146b-5p increases GBC cell proliferation ability.
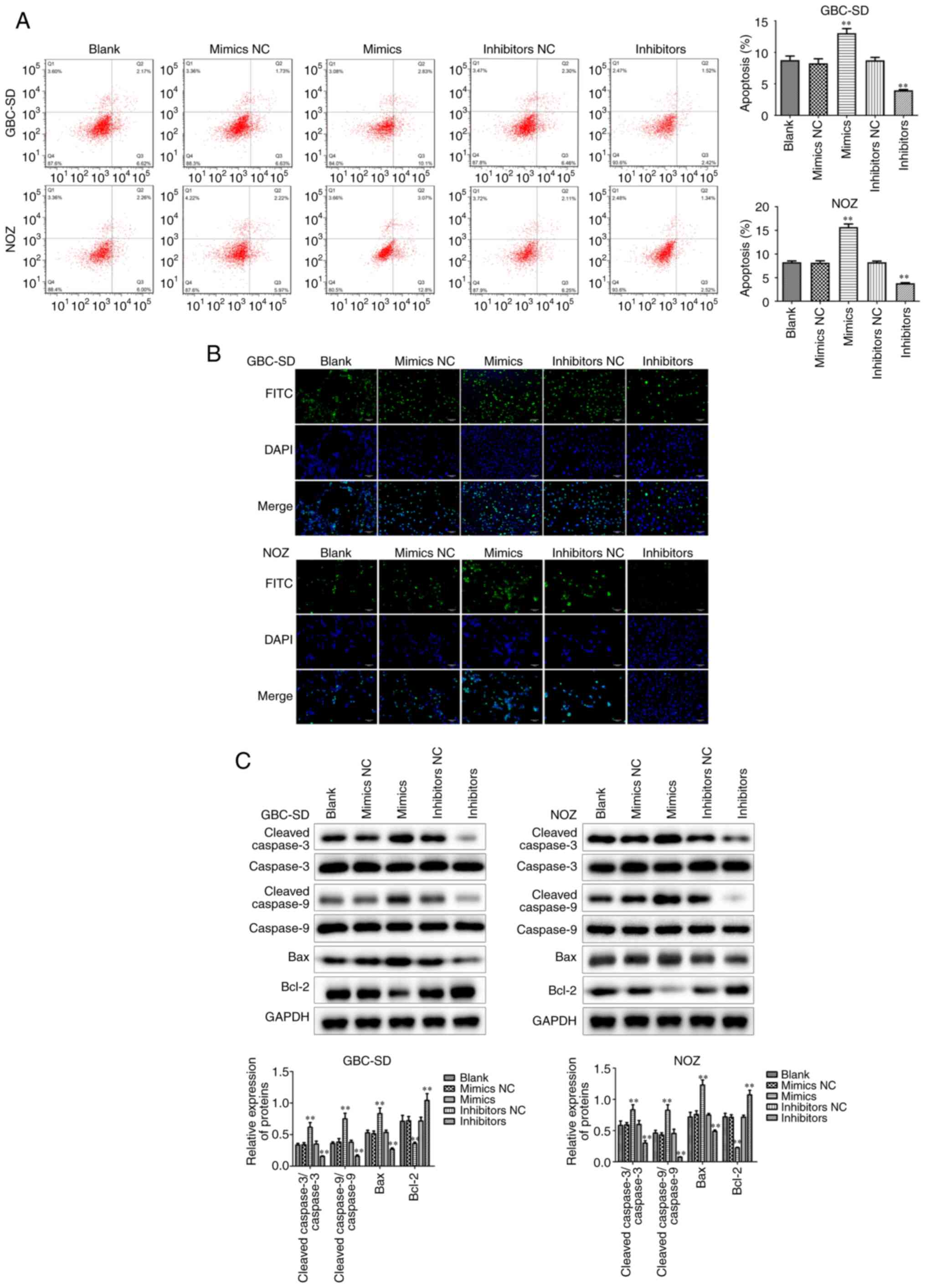
In addition to the abnormal proliferative capacity,
apoptosis escape is also one of the reasons why tumor cells are
difficult to eradicate. Flow cytometry displayed that miR-146b-5p
overexpression markedly increased apoptosis of GBC cells (Fig. 3A), which was confirmed by TUNEL
staining (Fig. 3B). Furthermore, it
was observed that the expression of the pro-apoptotic factors Bax,
cleaved-caspase-3 and cleaved-caspase-9 increased, whereas the
expression of the anti-apoptotic factor Bcl-2 decreased in the
group treated with miR-146b-5p mimics (Fig. 3C). By contrast, when the expression of
miR-146b-5p was inhibited, apoptosis of GBC cells also decreased.
These results indicate that miR-146b-5p can inhibit cell
proliferation and promote apoptosis in GBC cells in
vitro.
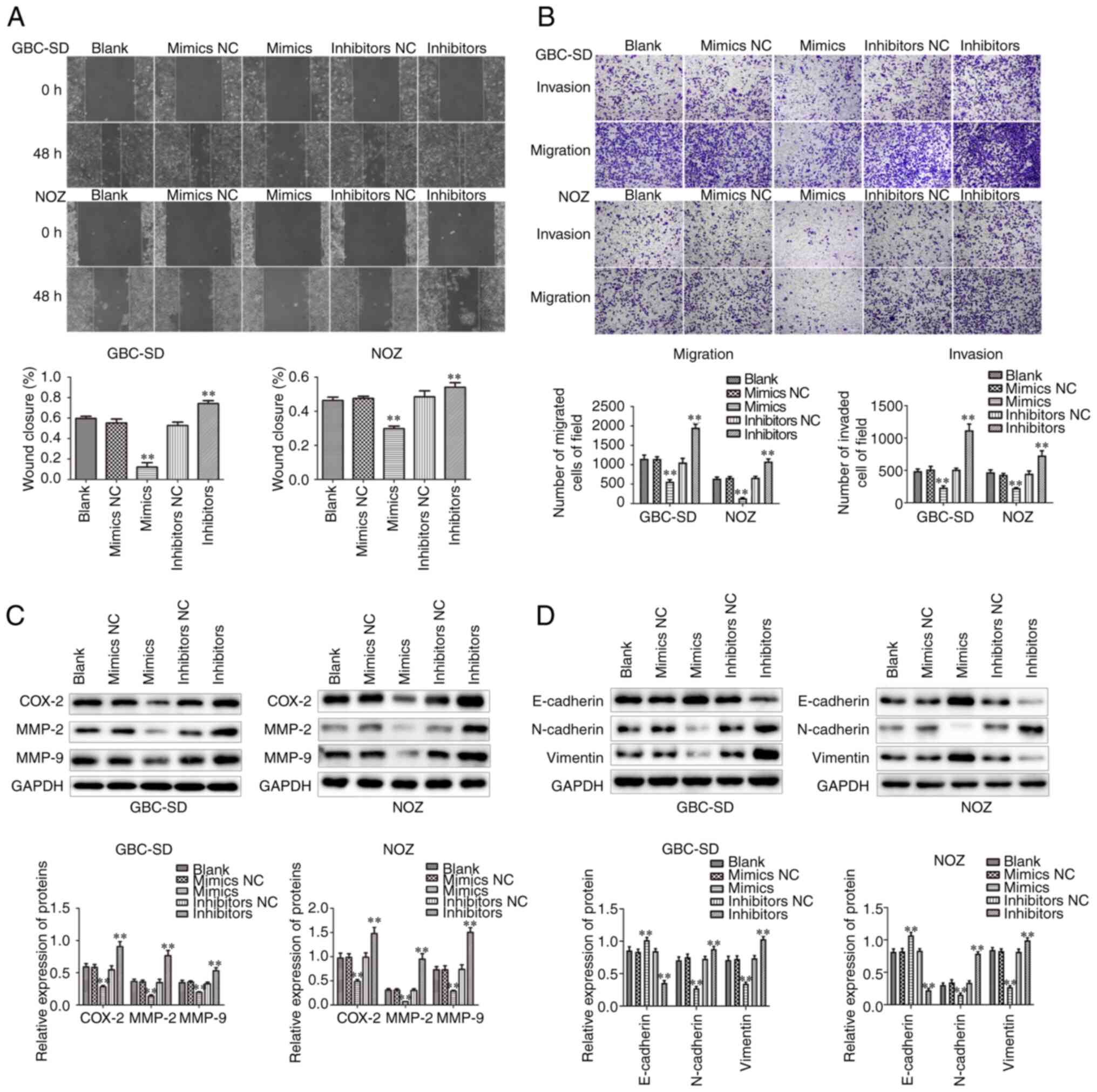
miR-146b-5p affects migration and
invasion of GBC cells
The migration and invasion abilities of cells under
different treatments was next evaluated by Transwell and wound
healing assays. It was observed that, when miR-146b-5p was
overexpressed, the migration and invasion ability of GBC cells
decreased. Conversely, inhibition of miR-146b-5p expression
significantly increased cell migration and invasion ability
(Fig. 4A and B). In addition, we
observed that overexpression of miR-146b-5p signally reduced the
expression of cell migration-associated proteins, such as MMP-2,
MMP-9 and COX-2, but inhibiting the expression of miR-146b-5p
significantly increased these protein expression levels of MMP-2,
MMP-9 and COX-2 (Fig. 4C).
Furthermore, miR-146b-5p overexpression reduced the protein
expression of N-cadherin and vimentin and increased the expression
of E-cadherin (Fig. 4D).
Collectively, these results indicate that miR-146b-5p
overexpression inhibited the proliferation, migration and invasion
of GBC cells and increased their apoptosis, suggesting that
miR-146b-5p may be involved in occurrence and development of
GBC.
miR-146b-5p targets TLR4 in GBC
tissues and cells
Although miR-146b-5p was found to play a crucial
role regulatory role in GBC, the underlying mechanism remains
elusive. Bioinformatics analysis revealed that TLR4 was target of
miR-146b-5p and the mutated binding sites between TLR4 and
miR-146b-5p were established (Fig.
5A). It was then observed by using luciferase reporter assay
that TLR4 is a direct target gene of miR-146b-5p, whereas the
mutated TLR4 was not degraded by miR-146b-5p (Fig. 5B). The Pearson curve distribution
revealed a negative correlation between the expressions of
miR-146b-5p and TLR4 (Fig. 5C). The
mRNA and protein levels of TLR4 were found to be increased in human
GBC tissues compared with those in normal tissues (Fig. 5D and E). Similarly, the mRNA and
protein levels of TLR4 in GBC cell lines were higher compared with
those in gallbladder epithelial cells (Fig. 5F and G). The miR-146b-5p inhibitor
treatment group exhibited a significantly increased expression of
TLR4 at both te mRNA and protein levels (Fig. 5H). The results mentioned above
demonstrated that miR-146b-5p directly targets and negatively
regulates TLR4, suggesting that miR-146b-5p may regulate GBC
through TLR4.
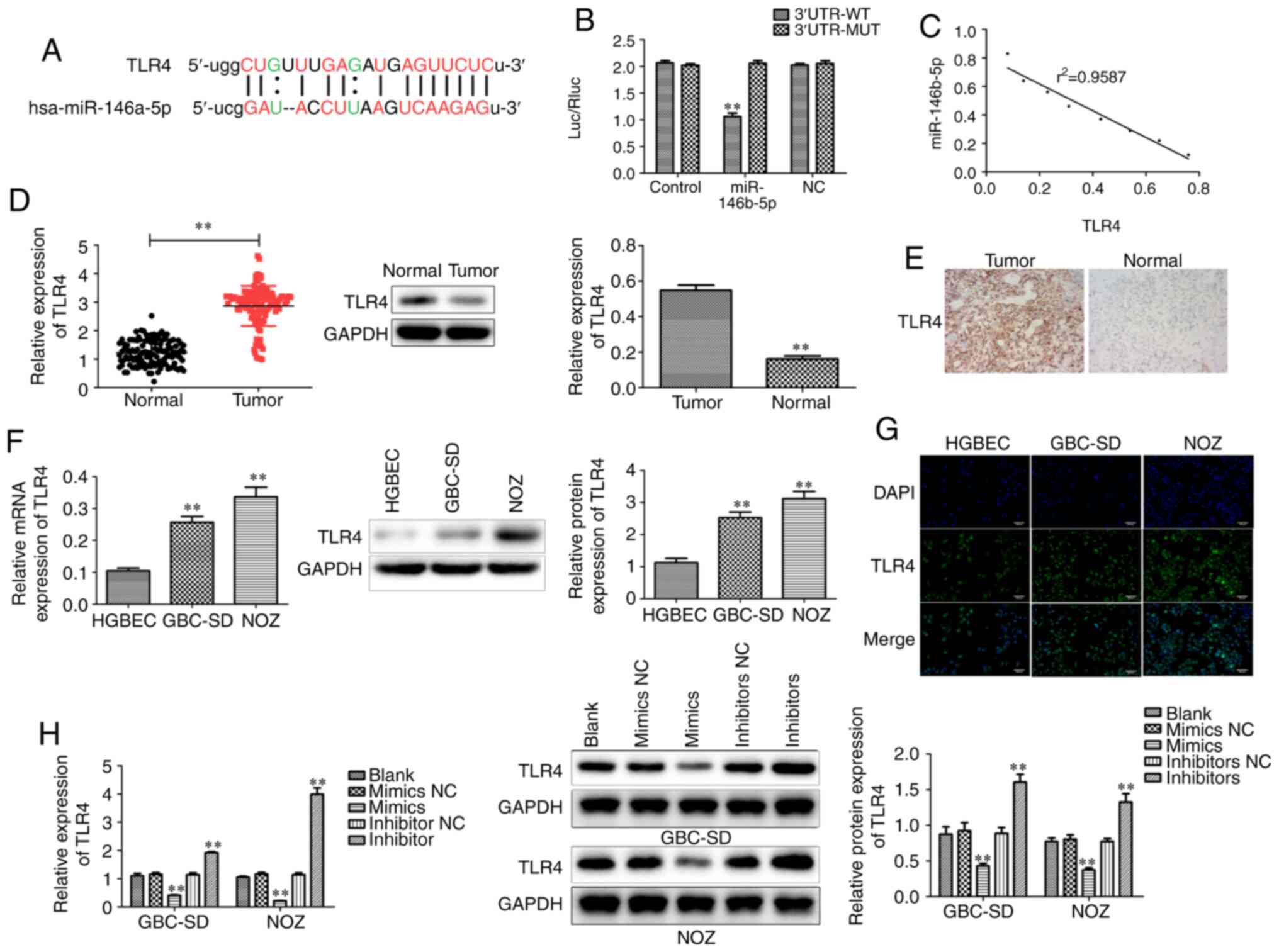 | Figure 5.miR-146b-5p directly targets TLR4 in
GBC tissues and cells. (A) Sequence alignment between miR-146b-5p
and its target gene TLR4. (B) Luciferase activities of reporters
containing the WT and MUT sequence of TLR4 in miR-146b-5p
mimic NC cells and miR-146b-5p mimic cells. (C) There was a
negative correlation between the expression of miR-146b-5p and
TLR4. (D) RT-qPCR analysis and western blotting indicated an
increase in the mRNA and protein level, respectively, of TLR4 in
GBC tissues compared to normal tissues. (E) Immunohistochemical
staining revealed differential expression of TLR4 between GBC and
normal tissues. (F) RT-qPCR analysis and western blotting indicated
an increase in mRNA and protein level, respectively, of TLR4 in GBC
cells compared to gallbladder epithelial cells. (G)
Immunofluorescence staining revealed differential expression of
TLR4 between GBC cells and gallbladder epithelial cells
(magnification, ×200). (H) Overexpression of miR-146b-5p
significantly reduced the mRNA and protein levels of TLR4, whereas
inhibition of miR-146b-5p expression significantly increased the
expression of TLR4. All experimental data are expressed as mean ±
SD and each assay was performed in triplicate. **P<0.01 vs.
blank group. GBC, gallbladder cancer; TLR4, Toll-like receptor 4;
RT-qPCR, reverse transcription-quantitative PCR; WT, wild-type;
MUT, mutant. |
Increased expression of TLR4
attenuates changes in GBC cells caused by overexpression of
miR-146b-5p via the NF-κB signaling pathway
To verify whether changes in TLR4 expression levels
are responsible for miR-146b-5p involvement in GBC, miR-146b-5p was
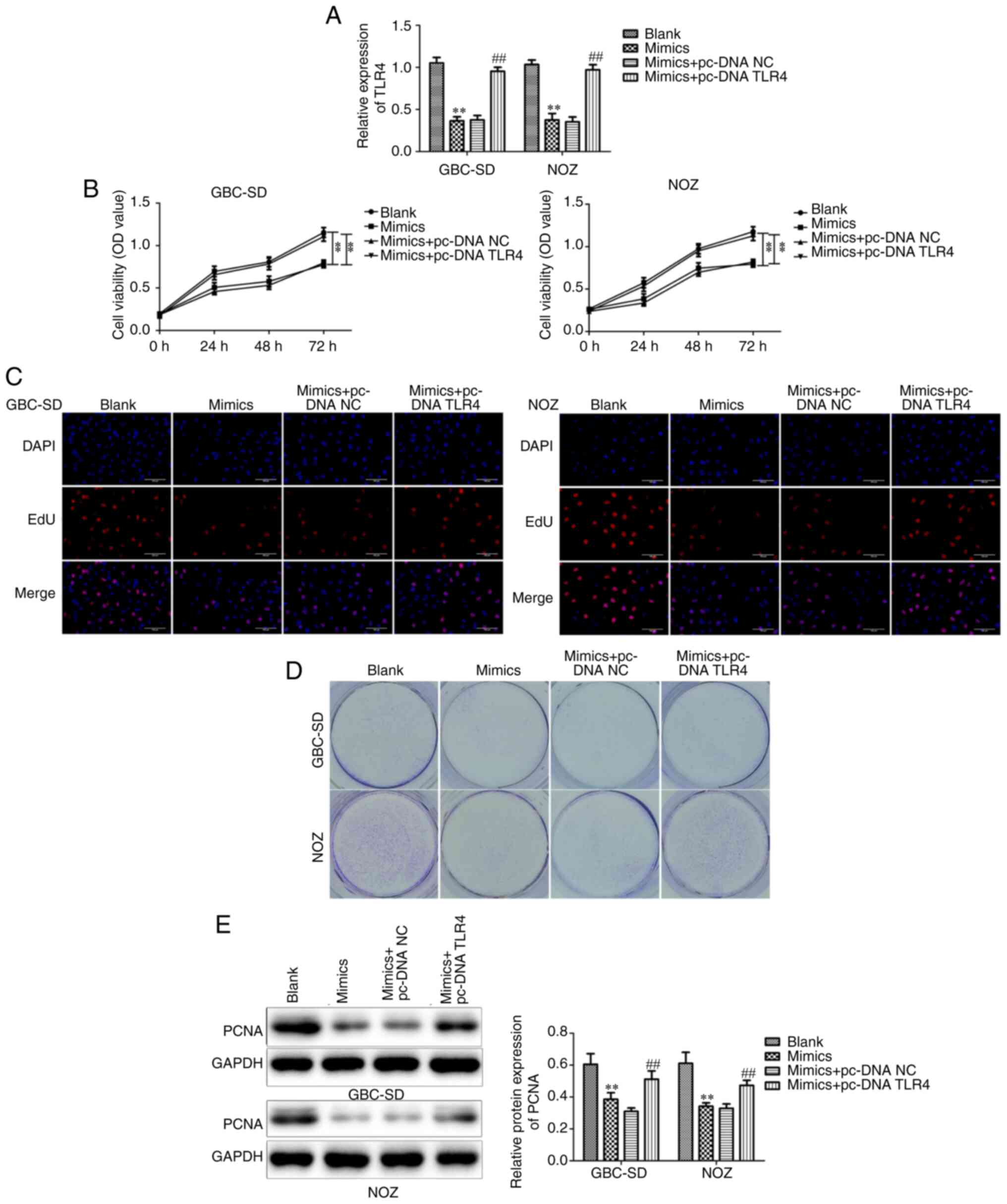
overexpressed while increasing TLR4 expression levels (Fig. 6A). The results demonstrated that the
decreased cell viability and proliferative capacity in the
miR-146b-5p mimics-treated group of GBC cells recovered after
overexpression of TLR4 (Fig. 6B-E).
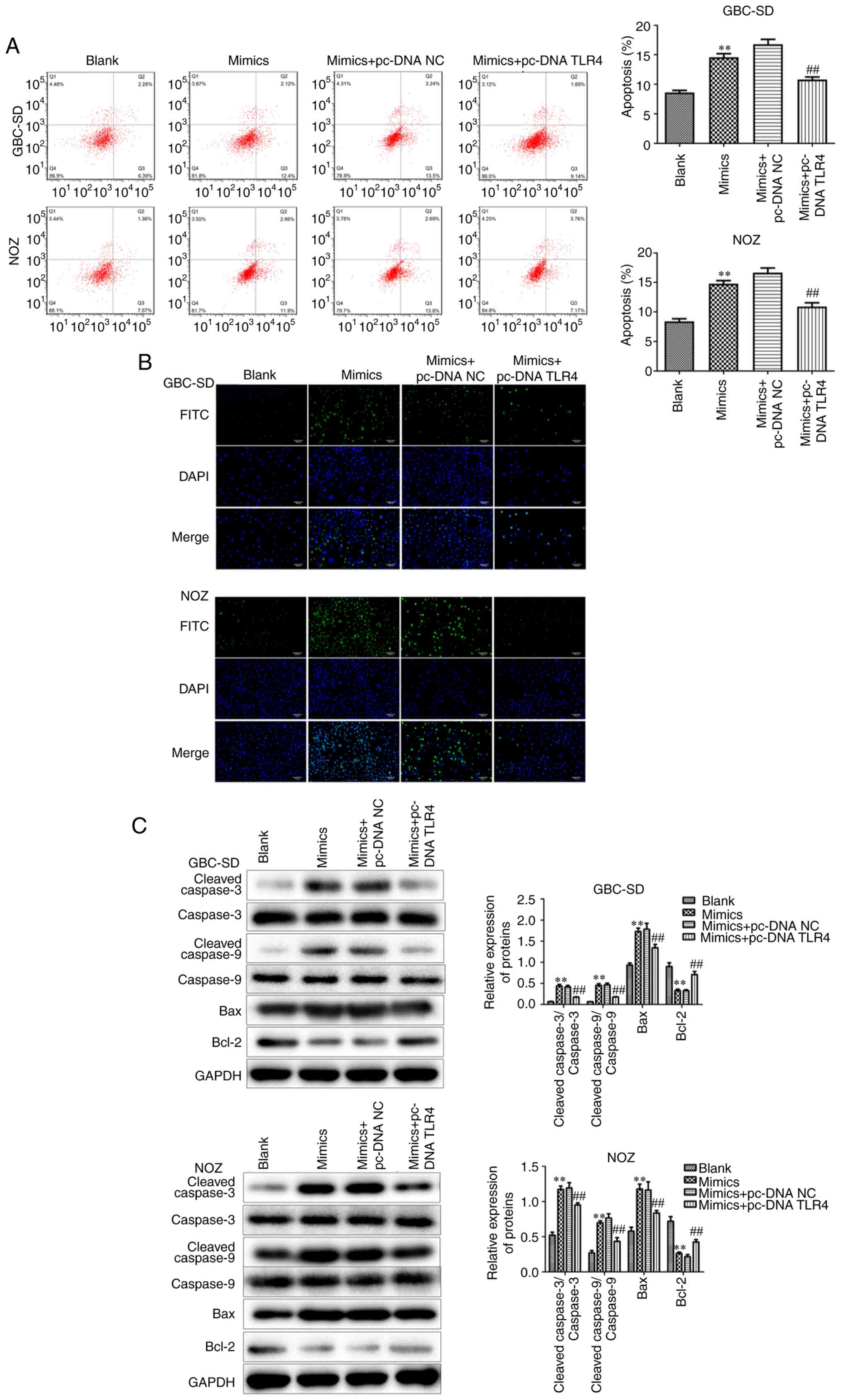
Conversely, the increased apoptosis in GBC cells in the miR-146b-5p
mimics-treated group was significantly reduced after overexpression
of TLR4 (Fig. 7A-C).
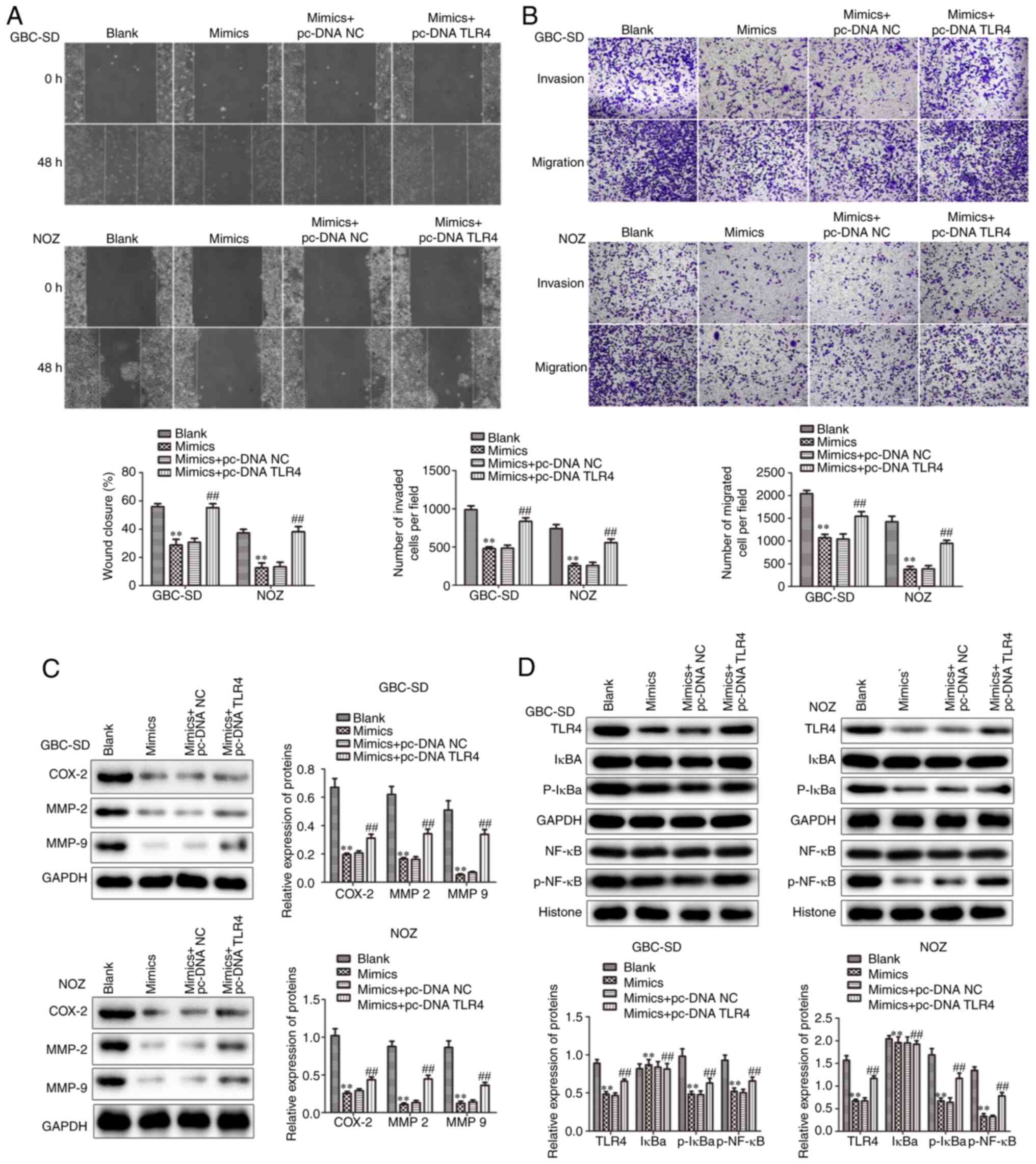
Furthermore, it was investigated whether TLR4 could
reverse the decrease in the migration and invasion of GBC cells
caused by overexpression of miR-146b-5p. Increased expression of
TLR4 was found to attenuate the inhibition of migration and
invasion of GBC cells caused by miR-146b-5p overexpression
(Fig. 8A-C). These results suggest
that increased TLR4 expression may reverse the changes in GBC cells
caused by overexpression of miR-146b-5p.
It is well known that NF-κB is primarily involved in
immune responses and inflammation, however, increasing evidence
supports its pivotal role in tumorigenesis (38–41). The
present study demonstrated that, after overexpressing miR-146b-5p,
there was no significant change in cytoplasmic IκBα levels, but the
p-IκBα levels decreased, indicating a decrease in NF-κB nuclear
import. However, this change was restored after simultaneous
overexpression of TLR4 (Fig. 8D).
These results indicate that TLR4 may be involved in the regulation
of GBC by increasing the phosphorylation of NF-κB and its transport
into the nucleus to activate downstream target genes.
Overexpression of miR-146b-5p inhibits
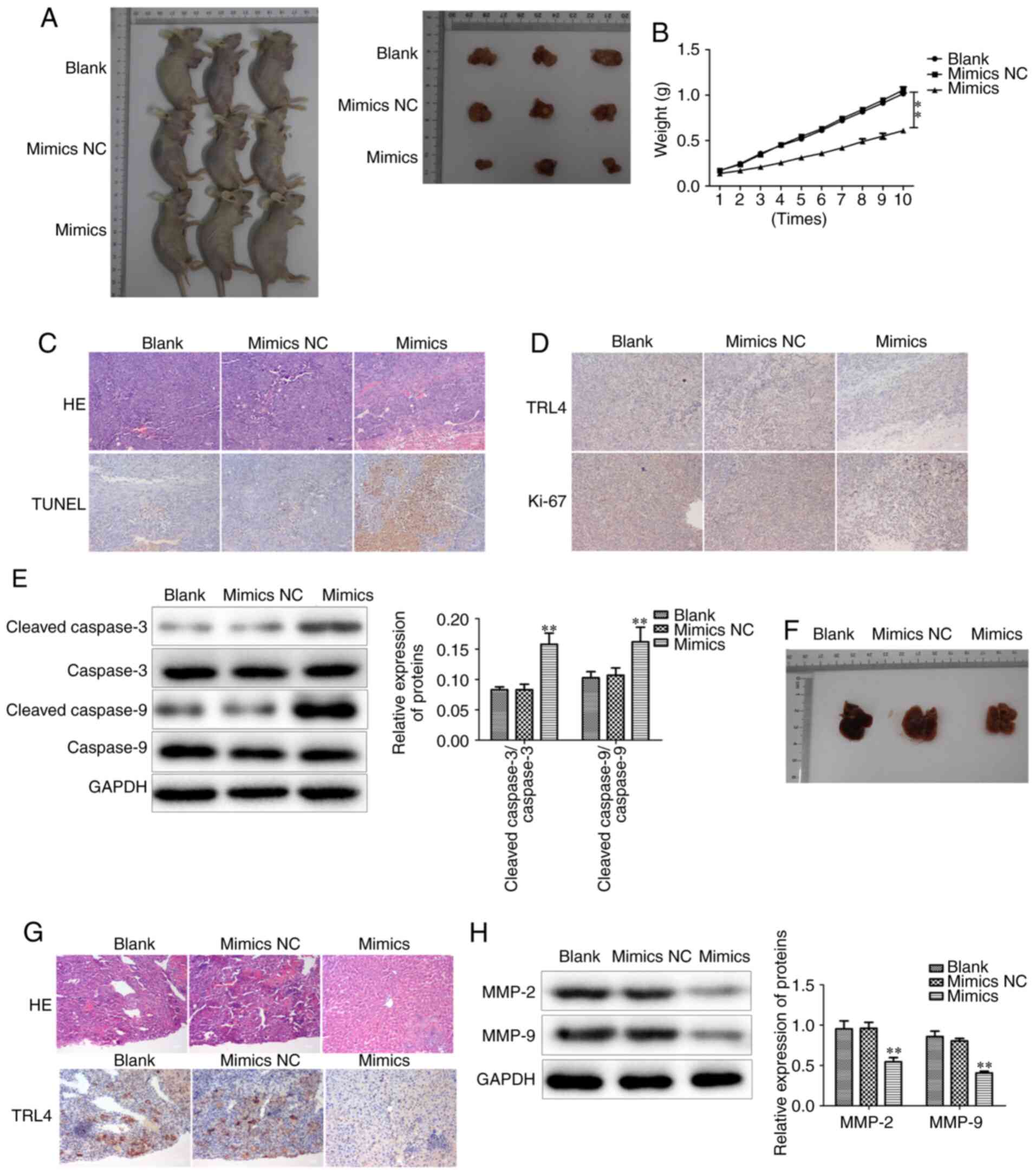
tumor growth and metastasis in vivo
To further investigate whether miR-146b-5p has the
same effect in vivo, GBC-SD cells with different treatments
were transplanted into BALB/c nude mice to observe tumor growth. It
was observed that the tumors of the miR-146b-5p mimics-treated
group had smaller volume and weight compared with those in the
control group (Fig. 9A and B).
Hematoxylin and eosin (HE) staining demonstrated that
overexpression of miR-146b-5p reduced the aggressive phenotype of
the tumor tissues (Fig. 9C).
Furthermore, the proliferation and apoptosis of tumor cells were
examined, and it was observed that the mouse tumors from cells
treated with miR-146b-5p mimics exhibited higher cell apoptosis and
lower proliferation rates compared with the control group (Fig. 9C-E).
Subsequently, tumor metastasis was evaluated by
analyzing metastatic nodes in liver tissues at the end of the
experiments. The results revealed that restoring miR-146b-5p
expression significantly decreased liver metastasis in vivo
(Fig. 9F), and HE staining confirmed
a reduced number of metastatic tumor cells in liver tissue in
miR-146b-5p mimics transfection group (Fig. 9G). In addition, the expression of
MMP-2 and MMP-9 was found to be decreased in the miR-146b-5p mimics
group (Fig. 9H). Collectively, these
results indicate that miR-146b-5p inhibits the growth and
aggressiveness of xenografted tumors in BALB/c nude mice, and
upregulating miR-146b-5p expression inhibits GBC liver metastasis
in vivo, suggesting that it may represent an effective
target for the treatment of GBC.
Discussion
The present study demonstrated that human GBC
tissues exhibited lower expression level of miR-146b-5p compared
with normal tissue, and its expression level was correlated with
clinicopathological characteristics. Inhibiting the expression
level of miR-146b-5p in GBC cells cultured in vitro
significantly increased cell viability, proliferation, migration
and invasion, and reduced cell apoptosis. Conversely,
overexpression of miR-146b-5p inhibited GBC cell viability,
proliferative and invasive ability, and increased apoptosis.
Bioinformatics analysis and prediction identified TLR4 as a
miR-146b-5p target, which was confirmed by our results. In
addition, a significant increase in the expression level of TLR4 in
human GBC tissues was detected. Overexpression of TLR4 alleviated
inhibition of GBC cell characteristics caused by overexpression of
miR-146b-5p. Furthermore, it was observed that, after
overexpression of miR-146b-5p, the level of p-IκBα in the cytoplasm
decreased, while the expression level of p-NF-κB in the nucleus
increased, and these changes were inhibited by simultaneously
overexpressing TLR4. This may uncover the mechanism through which
miR-146b-5p is involved in the regulation of the development of
GBC. The BALB/c nude mouse xenograft experiments also demonstrated
that miR-146b-5p reduces tumor growth in vivo by inhibiting
cell proliferation and promoting cell apoptosis. Taken together,
these results indicate that upregulating the expression of
miR-146b-5p may be a new and valuable clinical approach to the
treatment of GBC.
As the fifth most usual gastrointestinal cancer
globally, GBC has a clear regional bias (42). In addition, in some high-prevalence
areas of GBC, the incidence of women is 2.3 times that of men,
suggesting the effect of sex on GBC susceptibility (43,44). It
was previously demonstrated that estrogen increases the risk of
developing cholesterol gallstones to some extent, and the
supersaturation of cholesterol in the bile participates in the
development of GBC through gallstone-mediated mechanisms (45); however, this is questioned by other
research groups (46). It was also
reported that some cancers differ at the molecular level between
sexes, including small RNA levels (47). Differences in expression of
sex-dependent small RNAs are prevalent in mammals and birds
(48–52). We herein demonstrated that human GBC
tissues express lower levels of miR-146b-5p compared with normal
tissues. It is not clear whether the expression of microRNA-146b-5p
is also affected by sex, and this association requires further
investigation of a larger number of samples.
The majority of patients with GBC are often
diagnosed in the late stages of the disease, due in part to the
lack of reliable tumor markers. Thus far, only two markers, namely
carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9, have
been detected in late-stage GBC, but their specificity is poor
(53). Therefore, it is necessary to
identify biomarkers that can quickly and accurately diagnose GBC.
Although other groups have also investigated the possibility of
different markers of other types of tumors as markers of GBC, the
results reported are highly inconsistent (54–56). Some
research groups have tried to analyze the GBC genome in an attempt
to find a way to diagnose and treat GBC, but with little success
(57). There is increasing evidence
that circulating miRNAs secreted by pathological tissues into the
humoral circulation system may serve as markers for early diagnosis
of diseases, such as myocardial infarction, endocrine cancer and
coronary artery disease (58–61). By examining blood samples, it may be
possible to analyze the difference in the expression level of
miR-146b-5p, which may prove to be an effective method for clinical
diagnosis of early GBC.
A variety of inflammatory mediators released during
chronic inflammation have been shown to induce DNA methylation and
post-translational modification of proto-oncogenes and/or tumor
suppressor genes (62). The effects
of DNA methylation modifications on GBC have been extensively
investigated. It has been demonstrated that the expression level of
TLR4 is observably enhanced in human GBC tissues, and that
activation of TLR4 signaling leads to an excessive inflammatory
response (63). The present study
confirmed that TLR4 is a direct target gene of miR-146b-5p
(Fig. 5). The long-term
overactivation of TLR4 involved in inflammation, which is
negatively regulated by miR-146b-5p, may be among the main causes
of GBC occurrence and development. This finding constitutes
powerful evidence that miR-146b-5p may be a useful biomarker for
the clinical diagnosis of early GBC.
In summary, the present study demonstrated that
miR-146b-5p is involved in the regulation of GBC, and that
overexpression of miR-146b-5p may reduce the expression of its
target gene, TLR4, thereby inhibiting the sustained activation of
NF-κB. Furthermore, overexpression of miR-146b-5p in vivo
may partially reduce GBC volume and suppress the aggressive
phenotypical characteristics. However, the development of GBC may
be a multi-factorial process, and further research is required to
elucidate whether it involves more complicated regulatory
mechanisms.
Acknowledgements
Not applicable.
Funding
The present study was supported by Nanjing Medical
Science and technique Development Foundation (grant no.
QRX17105).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WJ designed the experiments. BO was the major
contributor to writing the manuscript. NP, HZ and CX performed the
experiments. All the authors have read and approved the final
version of the manuscript for publication.
Ethics approval and consent to
participate
Informed patient consent for participation was
obtained at the time of the study. The present study was approved
by the Ethics Committee of Southern Medical University (no.
2018-SR-052). The animal experimental protocols were approved by
the Institutional Animal Care and Use Committee of Southern Medical
University (no. GZZL-2018-0235).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andia ME, Hsing AW, Andreotti G and
Ferreccio C: Geographic variation of gallbladder cancer mortality
and risk factors in Chile: A population-based ecologic study. Int J
Cancer. 123:1411–1416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pilgrim CH, Groeschl RT, Christians KK and
Gamblin TC: Modern perspectives on factors predisposing to the
development of gallbladder cancer. HPB (Oxford). 15:839–844. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iyer P, Barreto SG, Sahoo B, Chandrani P,
Ramadwar MR, Shrikhande SV and Dutt A: Non-typhoidal Salmonella DNA
traces in gallbladder cancer. Infect Agent Cancer. 11:122016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain K, Sreenivas V, Velpandian T, Kapil U
and Garg PK: Risk factors for gallbladder cancer: A case-control
study. Int J Cancer. 132:1660–1666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
7
|
Yang XW, Yang J, Li L, Man XB, Zhang BH,
Shen F and Wu MC: Analysis of the relationships between
clinicopathologic factors and survival in gallbladder cancer
following surgical resection with curative intent. PLoS One.
7:e515132012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasatomi E, Tokunaga O and Miyazaki K:
Precancerous conditions of gallbladder carcinoma: Overview of
histopathologic characteristics and molecular genetic findings. J
Hepatobiliary Pancreat Surg. 7:556–567. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rashid A: Cellular and molecular biology
of biliary tract cancers. Surg Oncol Clin N Am. 11:995–1009. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mo YY: MicroRNA regulatory networks and
human disease. Cell Mol Life Sci. 69:3529–3531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson CD, Esquela-Kerscher A, Stefani G,
Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J,
Shingara J, et al: The let-7 microRNA represses cell proliferation
pathways in human cells. Cancer Res. 67:7713–7722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okayama H, Schetter AJ and Harris CC:
MicroRNAs and inflammation in the pathogenesis and progression of
colon cancer. Dig Dis. 30 (Suppl 2):S9–S15. 2012. View Article : Google Scholar
|
|
16
|
Schetter AJ, Heegaard NH and Harris CC:
Inflammation and cancer: Interweaving microRNA, free radical,
cytokine and p53 pathways. Carcinogenesis. 31:37–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kono H, Nakamura M, Ohtsuka T, Nagayoshi
Y, Mori Y, Takahata S, Aishima S and Tanaka M: High expression of
microRNA-155 is associated with the aggressive malignant behavior
of gallbladder carcinoma. Oncol Rep. 30:17–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Letelier P, García P, Leal P, Álvarez H,
Ili C, López J, Castillo J, Brebi P and Roa JC: miR-1 and miR-145
act as tumor suppressor microRNAs in gallbladder cancer. Int J Clin
Exp Pathol. 7:1849–1867. 2014.PubMed/NCBI
|
|
19
|
Zhou H, Wang Y, Zha R, Ding J, Liang L, Hu
J, Shen H, Chen Z, Guo W, Zhao Y, et al: MicroRNA-26a acts as a
tumor suppressor inhibiting gallbladder cancer cell proliferation
by directly targeting HMGA2. Int J Oncol. 44:2050–2058. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou H, Guo W, Zhao Y, Wang Y, Zha R, Ding
J, Liang L, Yang G, Chen Z, Ma B and Yin B: MicroRNA-135a acts as a
putative tumor suppressor by directly targeting very low density
lipoprotein receptor in human gallbladder cancer. Cancer Sci.
105:956–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng HH, Zhang YD, Gong LS, Liu WD and
Zhang Y: Increased expression of microRNA-335 predicts a favorable
prognosis in primary gallbladder carcinoma. Onco Targets Ther.
6:1625–1630. 2013.PubMed/NCBI
|
|
22
|
Yoon SO, Kim EK, Lee M, Jung WY, Lee H,
Kang Y, Jang YJ, Hong SW, Choi SH and Yang WI: NOVA1 inhibition by
miR-146b-5p in the remnant tissue microenvironment defines occult
residual disease after gastric cancer removal. Oncotarget.
7:2475–2495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang
X and Fan Y: MiR-146b-5p promotes metastasis and induces
epithelial-mesenchymal transition in thyroid cancer by targeting
ZNRF3. Cell Physiol Biochem. 35:71–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu E, Zhao J, Ma J, Wang C, Zhang C, Jiang
H, Cheng J, Gao R and Zhou X: miR-146b-5p promotes invasion and
metastasis contributing to chemoresistance in osteosarcoma by
targeting zinc and ring finger 3. Oncol Rep. 35:275–283. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Xu J, Li H, Sun C, Yu L, Li Y, Shi
C, Zhou X, Bian X, Ping Y, et al: miR-146b-5p functions as a tumor
suppressor by targeting TRAF6 and predicts the prognosis of human
gliomas. Oncotarget. 6:29129–29142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai J, Xu L, Cai Z, Wang J, Zhou B and Hu
H: MicroRNA-146b-5p inhibits the growth of gallbladder carcinoma by
targeting epidermal growth factor receptor. Mol Med Rep.
12:1549–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Byrd-Leifer CA, Block EF, Takeda K, Akira
S and Ding A: The role of MyD88 and TLR4 in the LPS-mimetic
activity of Taxol. Eur J Immunol. 31:2448–2457. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang JM, Zhang GN, Shi Y, Zha X, Zhu Y,
Wang MM, Lin Q, Wang W, Lu HY, Ma SQ, et al: Atractylenolide-I
sensitizes human ovarian cancer cells to paclitaxel by blocking
activation of TLR4/MyD88-dependent pathway. Sci Rep. 4:38402014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawasaki T and Kawai T: Toll-like receptor
signaling pathways. Front Immunol. 5:4612014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang AC, Ma YB, Wu FX, Ma ZF, Liu NF, Gao
R, Gao YS and Sheng XG: TLR4 induces tumor growth and inhibits
paclitaxel activity in MyD88-positive human ovarian carcinoma in
vitro. Oncol Lett. 7:871–877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawasaki K, Akashi S, Shimazu R, Yoshida
T, Miyake K and Nishijima M: Mouse toll-like receptor 4.MD-2
complex mediates lipopolysaccharide-mimetic signal transduction by
Taxol. J Biol Chem. 275:2251–2254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rajput S, Volk-Draper LD and Ran S: TLR4
is a novel determinant of the response to paclitaxel in breast
cancer. Mol Cancer Ther. 12:1676–1687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tichomirowa MA, Theodoropoulou M, Daly AF,
Yassouridis A, Hansen S, Lu J, Lange M, Goldbrunner RH, Stalla GK
and Renner U: Toll-like receptor-4 is expressed in meningiomas and
mediates the antiproliferative action of paclitaxel. Int J Cancer.
123:1956–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ustinova EE, Shurin GV, Gutkin DW and
Shurin MR: The role of TLR4 in the paclitaxel effects on neuronal
growth in vitro. PLoS One. 8:e568862013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ran S: The role of TLR4 in
chemotherapy-driven metastasis. Cancer Res. 75:2405–2410. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Volk-Draper L, Hall K, Griggs C, Rajput S,
Kohio P, DeNardo D and Ran S: Paclitaxel therapy promotes breast
cancer metastasis in a TLR4-dependent manner. Cancer Res.
74:5421–5434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haricharan S and Brown P: TLR4 has a
TP53-dependent dual role in regulating breast cancer cell growth.
Proc Natl Acad Sci USA. 112:E3216–E3225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Greten FR and Karin M: The IKK/NF-kappaB
activation pathway-a target for prevention and treatment of cancer.
Cancer Lett. 206:193–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cogswell PC, Guttridge DC, Funkhouser WK
and Baldwin AS Jr: Selective activation of NF-kappa B subunits in
human breast cancer: Potential roles for NF-kappa B2/p52 and for
Bcl-3. Oncogene. 19:1123–1131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boutros C, Gary M, Baldwin K and
Somasundar P: Gallbladder cancer: Past, present and an uncertain
future. Surg Oncol. 21:e183–e191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Randi G, Franceschi S and La Vecchia C:
Gallbladder cancer worldwide: Geographical distribution and risk
factors. Int J Cancer. 118:1591–1602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Everson GT, McKinley C and Kern F Jr:
Mechanisms of gallstone formation in women. Effects of exogenous
estrogen (Premarin) and dietary cholesterol on hepatic lipid
metabolism. J Clin Invest. 87:237–246. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barreto SG, Haga H and Shukla PJ: Hormones
and gallbladder cancer in women. Indian J Gastroenterol.
28:126–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan Y, Liu L, Chen H, Wang Y, Xu Y, Mao
H, Li J, Mills GB, Shu Y, Li L and Liang H: Comprehensive
characterization of molecular differences in cancer between male
and female patients. Cancer Cell. 29:711–722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Warnefors M, Mössinger K, Halbert J,
Studer T, VandeBerg JL, Lindgren I, Fallahshahroudi A, Jensen P and
Kaessmann H: Sex-biased microRNA expression in mammals and birds
reveals underlying regulatory mechanisms and a role in dosage
compensation. Genome Res. 27:1961–1973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo L, Zhang Q, Ma X, Wang J and Liang T:
miRNA and mRNA expression analysis reveals potential sex-biased
miRNA expression. Sci Rep. 7:398122017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duttagupta R, Jiang R, Gollub J, Getts RC
and Jones KW: Impact of cellular miRNAs on circulating miRNA
biomarker signatures. PLoS One. 6:e207692011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang K, Yuan Y, Cho JH, McClarty S, Baxter
D and Galas DJ: Comparing the MicroRNA spectrum between serum and
plasma. PLoS One. 7:e415612012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Langevin SM, Stone RA, Bunker CH, Grandis
JR, Sobol RW and Taioli E: MicroRNA-137 promoter methylation in
oral rinses from patients with squamous cell carcinoma of the head
and neck is associated with gender and body mass index.
Carcinogenesis. 31:864–870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Srivastava K, Srivastava A and Mittal B:
Potential biomarkers in gallbladder cancer: Present status and
future directions. Biomarkers. 18:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He CZ, Zhang KH, Li Q, Liu XH, Hong Y and
Lv NH: Combined use of AFP, CEA, CA125 and CAl9-9 improves the
sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol.
13:872013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zur B, Holdenrieder S, Walgenbach-Brünagel
G, Albers E and Stoffel-Wagner B: Method comparison for
determination of the tumor markers AFP, CEA, PSA and free PSA
between Immulite 2000 XPI and Dimension Vista 1500. Clin Lab.
58:97–105. 2012.PubMed/NCBI
|
|
56
|
Zhang D, Yu M, Xu T and Xiong B:
Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of
colorectal liver metastasis in Chinese population.
Hepatogastroenterology. 60:1297–1301. 2013.PubMed/NCBI
|
|
57
|
Sicklick JK, Fanta PT, Shimabukuro K and
Kurzrock R: Genomics of gallbladder cancer: The case for
biomarker-driven clinical trial design. Cancer Metastasis Rev.
35:263–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Olivieri F, Antonicelli R, Capogrossi MC
and Procopio AD: Circulating microRNAs (miRs) for diagnosing acute
myocardial infarction: An exciting challenge. Int J Cardiol.
167:3028–3029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhao S, Yan L and Zhao Z: Up-regulation of
miR-203 inhibits the growth of cervical cancer cells by inducing
cell cycle arrest and apoptosis. Eur J Gynaecol Oncol. 40:791–795.
2019.
|
|
60
|
Fichtlscherer S, De Rosa S, Fox H,
Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T,
Müller-Ardogan M, et al: Circulating microRNAs in patients with
coronary artery disease. Circ Res. 107:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Keller A and Meese E: Can circulating
miRNAs live up to the promise of being minimal invasive biomarkers
in clinical settings? Wiley Interdiscip Rev RNA. 7:148–156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hussain SP and Harris CC: Inflammation and
cancer: An ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mara MA, Good M and Weitkamp JH: Innate
and adaptive immunity in necrotizing enterocolitis. Semin Fetal
Neonatal Med. 23:394–399. 2018. View Article : Google Scholar : PubMed/NCBI
|