According to statistics, peritoneal metastasis of
common malignancies is considered unresectable and poses a great
challenge in cancer treatment (1).
Previously, traditional intravenous chemotherapy was the
recommended option for patients with peritoneal metastasis, the
efficacy of which is largely limited by myelotoxicity (2,3). The
peritoneal plasma barrier and high interstitial pressure of tumor
tissues limit the accumulation of traditional intravenous agents in
the peritoneal cavity (4). Several
clinical trials have confirmed that intraperitoneal chemotherapy
can overcome these limitations and extend patient survival time
(5). Intraperitoneal drug delivery
under hyperthermia conditions, a procedure known as hyperthermic
intraperitoneal chemotherapy (HIPEC), has proven to be a more
effective interventional therapy for metastatic tumors of the
abdominal cavity (6,7). Since the standard nomenclature for HIPEC
was devised at the Fourth International Workshop on Peritoneal
Surface Malignancy in 2004 (8), its
clinical application has drawn great interest. However, as
demonstrated in the PRODIGE7 (9) and
PROPHYLOCHIP (10) trials, adjuvant
HIPEC does not appear to significantly improve patient survival. As
an adjuvant therapeutic method, the underlying molecular mechanisms
of HIPEC remain largely unknown, which may affect its clinical
application. The present review focuses on the direct and indirect
effects of HIPEC, particularly at the immunological level.
HIPEC is an important adjuvant treatment for
malignant tumors, malignant ascites and intraperitoneal metastases,
the aim of which is to perfuse chemotherapeutic agents into the
abdominal cavity at a constant temperature and for a specific
period (7,11). Giovanella et al (12) revealed that exposure to temperatures
at 42.5–43.0°C has a significantly greater lethal effect on
neoplastic cells compared with non-neoplastic human cells. There
are two clinical methods of HIPEC application, namely the
open-abdomen and closed-abdomen techniques (13). The open method is usually performed in
the operating room directly after surgery and has exhibited
significant heat loss in porcine models (14). A meta-analysis revealed that closed
HIPEC is used more frequently than the open method, and that the
choice of HIPEC method has no impact on the overall recurrence-free
survival rate of patients, though this finding requires further
verification (15).
CO2-HIPEC is a recently developed technology that
promises improved heat delivery into the ‘closed’ abdomen (14). The closed CO2-HIPEC system
(PRS-1.0 Combat) uses a CO2 recirculation system to
distribute the perfusate and increase pressure within the
peritoneal cavity, ensuring intra-abdominal thermal homogeneity, in
addition to optimal solution distribution and drug penetration
(16). Pressurized Intraperitoneal
Aerosol Chemotherapy (PIPAC) employs the CO2
recirculation method, using a PIPAC micropump to nebulize the
liquid cytotoxic drug into droplets of ~25-µm, generating a
polydisperse aerosol in the abdominal cavity that is more
effectively absorbed (17,18).
The clinical effects of HIPEC remain a
contradiction. Although HIPEC has been demonstrated to increase
intraperitoneal hydrostatic pressure to enhance drug delivery to
the peritoneal surface, and theoretically, to eliminate residual
microscopic peritoneal disease, the practice remains controversial
(26,27). Thus, investigations on the basic
molecular mechanisms of HIPEC may prove beneficial. HIPEC is also
known to exert direct and indirect antitumor effects by promoting
albuminous degeneration, inducing apoptosis and inhibiting
angiogenesis, which may indicate a synergistic effect between
hyperthermia and chemotherapy (28,29).
During hyperthermia, supranormal temperatures can
induce a distinct decrease in membrane and cytoplasmatic proteins,
resulting in tumor cell death (30,31). For
example, multidrug resistance-associated protein 1 and 3 are
responsible for the failure of several oncological treatments, such
as chemotherapy of taxanes, vinca alkaloids and anthracyclines,
while the expression of these proteins can be reduced by
hyperthermia (32). The expression
levels of membrane-bound cytoskeletal proteins, such as actin, are
also reduced by hyperthermia, which results in the subsequent loss
of integrin CD11a (also known as leukocyte function-associated
antigen-1) from the cell surface (33,34). CD11a
deficiency or blockade subsequently abrogates the aggregation of
Treg cells, enhancing the efficacy of anticancer treatments
(35). Furthermore, defects in the
tumor cell membrane result in enhanced membrane fluidity (36). As a result, hyperthermia-induced
membrane remodeling and the release of lipid signals can upregulate
cellular thermosensitivity (37). In
addition, activation of the purinergic receptor, P2X7, can be
potentiated by the associated changes in membrane fluidity, which
ultimately promotes tumor cell death (38). Apoptosis is one of the mechanisms
underlying hyperthermia-associated cell death, which occurs
alongside downregulation of p53 and Bcl-2 expression, as well as
upregulation of Bax expression and mitotic catastrophe (39,40).
Early trials have reported that hyperthermia can
result in nuclear DNA double-strand breaks (DSBs), single strand
breaks and chromosomal aberrations in tumor cells (41,42).
Through the enhancement of ataxia-telangiectasia mutated protein
(ATM) kinase activity, and an increase in cellular ATM
autophosphorylation, foci formation of phosphorylated H2AX (at
serine 139; γ-H2AX) can be induced by hyperthermia, which acts as a
specific indicator for the occurrence of DNA DSBs (43,44). While
DSBs are primarily repaired via non-homologous end joining,
hyperthermia increases the probability of incorrect DSB
reconnections (45). However,
hyperthermia appears to delay the repair of DNA DSBs caused by
cisplatin, doxorubicin or radiotherapy, rather than by direct
proteasomal DNA damage (46,47). Mild hyperthermia (41.0–42.5°C) has
also been reported to induce BRCA2 degradation and inhibit
homologous recombination, thus impeding DNA damage repair (41,48).
Cisplatin-induced adduct formation is also increased in
vivo, which is an important determinant of toxicity (49).
In 1994, researchers determined that hyperthermia
enhances the effects of intraperitoneal chemotherapy by increasing
the uptake of chemotherapeutic agents (50). As a result, the clinically effective
dose could be reduced to decrease the incidence of severe side
effects (51). Hyperthermia has been
demonstrated to enhance the sensitivity of tumors to chemotherapy
by the impairing DNA repair (39),
and to decrease the number of proliferating tumor cells when
combined with chemotherapeutic drugs (52). However, Cesna et al (28) suggested that without cisplatin,
hyperthermia does not synergistically effect cancer cell viability,
indicating that the interaction between chemotherapy and
hyperthermia requires further investigation.
Due to the clinical similarity between exogenously
induced hyperthermia and natural fever, it has been speculated that
immune cells, including antigen presenting cells (APCs) and natural
killer (NK) cells, can be stimulated by hyperthermia to enhance the
antitumor effects of chemotherapeutic agents in the abdominal
cavity (53). The premise of these
immunological influences is that peptides released from dead tumor
cells can be internalized by APCs, including dendritic cells (DCs)
and macrophages, which subsequently activate cytotoxic T
lymphocytes (CTLs) (54,55). In this regard, hyperthermia can
substantially enhance the phagocytic potential of DCs and increase
DC infiltration by regulating the (C-C) receptor (CCR)7-(C-X-C
motif) ligand 21 axis (56). The
viability of NK cells is substantially reduced between 41–42°C,
while thermal stress activates NK cell cytotoxicity by activating
receptor NKG2D and its ligand, MHC class I-related chain A
(57).
The peritoneum is a visceral tissue with unique
immune functions, in which the largest cell fraction are the
peritoneum mesothelial cells (HPMCs) (58). The HPMCs comprise fibroblasts,
endothelial cells, macrophages and lymphocytes (59). Tumor-associated macrophages are the
primary immune cell population in the tumor peritoneal cavity and
can be stimulated to differentiate into two distinct subtypes:
Anti-tumorigenic M1 macrophages and pro-tumorigenic M2 macrophages,
of which M2 macrophages represent the majority (60,61). M2
macrophages inactivate CD8+ T cells through increased
expression of programmed cell death 1 ligand 1 and cytotoxic T
lymphocyte antigen 4 (62). In
addition, M2 macrophages accelerate tumor cell proliferation
through signal transducer and activator of transcription 3 (STAT3)
activation via transforming growth factor β (TGF-β), interleukin
(IL)-6 and IL-10 (63,65).
A study suggested that fever-like mild heat (~43°C)
can synergistically induce macrophage polarization from the M2 to
M1 phenotype during low-temperature photothermal therapy with a
lipid nanocomposite (66). However,
to the best of our knowledge, no studies have reported changes in
the immunological competency of the peritoneum in patients
undergoing HIPEC, which may indicate a determining factor for HIPEC
resistance, and thus requires further investigation. In addition,
hyperthermia may impair the functions and decrease the number of
immune cells (67,68), thus future studies should also focus
on the dual effects of hyperthermia.
Fever is commonly considered to be a protective
response following inflammation (69). Artificial HIPEC-induced fever appears
to evoke an inflammatory response with the release of cytokines,
such as IL-1, IL-6 and TGF-β (70).
IL-1 is expressed by malignant and infiltrating cell, and promotes
tumor progression and invasiveness (71). By activating STAT3, classical IL-6
signaling blocks DC maturation, inhibits T-cell activation and
enhances tumor cell proliferation (72). In addition, IL-6 and TGF-β are
considered to promote the differentiation of Th17 cells, which
support tumor progression by secreting immunosuppressive IL-17,
thus facilitating immune escape (73). Conflictingly, hyperthermia
phospho-regulates gp130, which is anchored to the endothelial cell
membrane of tumor microvessels (74).
Soluble IL-6 receptor α binds IL-6 and gp130 to activate IL-6
trans-signaling, which regulates high endothelial venule adhesion
and promotes the trafficking and recruitment of CD8+ T
cell into the tumor site (75).
The expression of molecular chaperone heat-shock
proteins (HSPs) in the abdominal cavity (including HSP-70, HSP-72
and HSP-92) can be upregulated by hyperthermic antineoplastic
agents, which protect the organs from heat-induced stress (76). Similar to other multidomain proteins,
HSPs recognize and bind unfolded/disordered sequences to facilitate
the folding/refolding of these sequences, or simply to present
themselves to the proteasome for destruction, protecting
intracellular proteins from stress-induced cellular damage
(77,78). The upregulation of HSP machinery in
cancerous cells may prevent the misfolding and degradation of
mutated and overexpressed oncoproteins (79). The highly conserved molecular
mechanisms of HSPs hinder the antiproliferative and apoptotic
effects of HIPEC on tumor cells (80). For example, upregulation of HSP27
resulting from HIPEC/CRS combination treatment may promote
thermotolerance and chemoresistance (81). However, chemotherapy can also suppress
HSP27 expression, rendering cancer cells sensitive to mild
hyperthermia (43°C) (82).
Professional APCs of the innate immune response can
be stimulated by extracellular HSPs, followed by cytokine release
and the expression of cell surface molecules (85). In addition, acting as a tumor vaccine,
the cross-presentation of HSP-bound peptide antigens to MHC class I
molecules can stimulate adaptive immunity via DCs (86,87),
leading to the efficient induction of antigen-specific CTLs
(88,89). There is also evidence that macrophages
can be activated by heat shock factor-1 through upregulation of the
inducible nitric oxide synthase gene (90).
The peritoneal cavity is a closed space that
consists of the peritoneum, abdominal organs and 50–70 ml
peritoneal fluid (91). The
peritoneum is an extensive serosal exchange membrane comprised of a
single layer of squamous HPMCs (~25 µm in diameter), collagen,
adipose tissue, lymphocytes, blood vessels and lymphatics (4). The so-called peritoneal-plasma barrier
comprises HPMCs, the subserosal interstitium and the capillary
walls (92). As the primary
absorption barrier, large molecular drugs in the peritoneal cavity
are absorbed slowly into the systemic blood circulation (93). The area under the concentration-time
curve (AUC) of drugs from the peritoneal cavity to the plasma
demonstrated the pharmacological advantage of intraperitoneal
administration (94).
As for the selection of intraperitoneal
chemotherapeutics for solid tumors, several factors must be
considered in addition to the selection of effective proliferation
inhibitors, such as the active form and half-life of agents
(112). The effects of peritoneal
delivery are based on direct contact between tumor cells and the
chemotherapeutic agent (113). As
these agents must be delivered in an active form, chemotherapeutics
that require activation by hepatic metabolization are considered
unsuitable (113).
Increasing evidence suggest that several
immunological factors can be induced by conventional
chemotherapeutic agents, including the composition, phenotype and
function of immune cells, as well as alterations in several
immune-related parameters (114,115).
For example, Latchman et al (116) revealed that the expression of T cell
inhibitory molecule programmed death receptor-ligand 2 (PD-L2) on
both human DCs and tumor cells is markedly decreased following
exposure to platinum-based chemotherapeutics. As a second ligand
for PD-1, PD-L2 inhibits T cell activation (117). In addition, Treg cells and
circulating myeloid-derived suppressor cells have been demonstrated
to be depleted by paclitaxel, gemcitabine and vinorelbine,
therapeutically enabling relevant tumor-targeting immune responses
(118–120). As for the immunological effects of
chemotherapeutics, immune effector cells may be stimulated, and
Treg cells may be depleted following the release of cytokines and
chemokines, while tumor-specific antigens containing tumor cell
peptides may be internalized by APCs following chemotherapeutic
exposure (121).
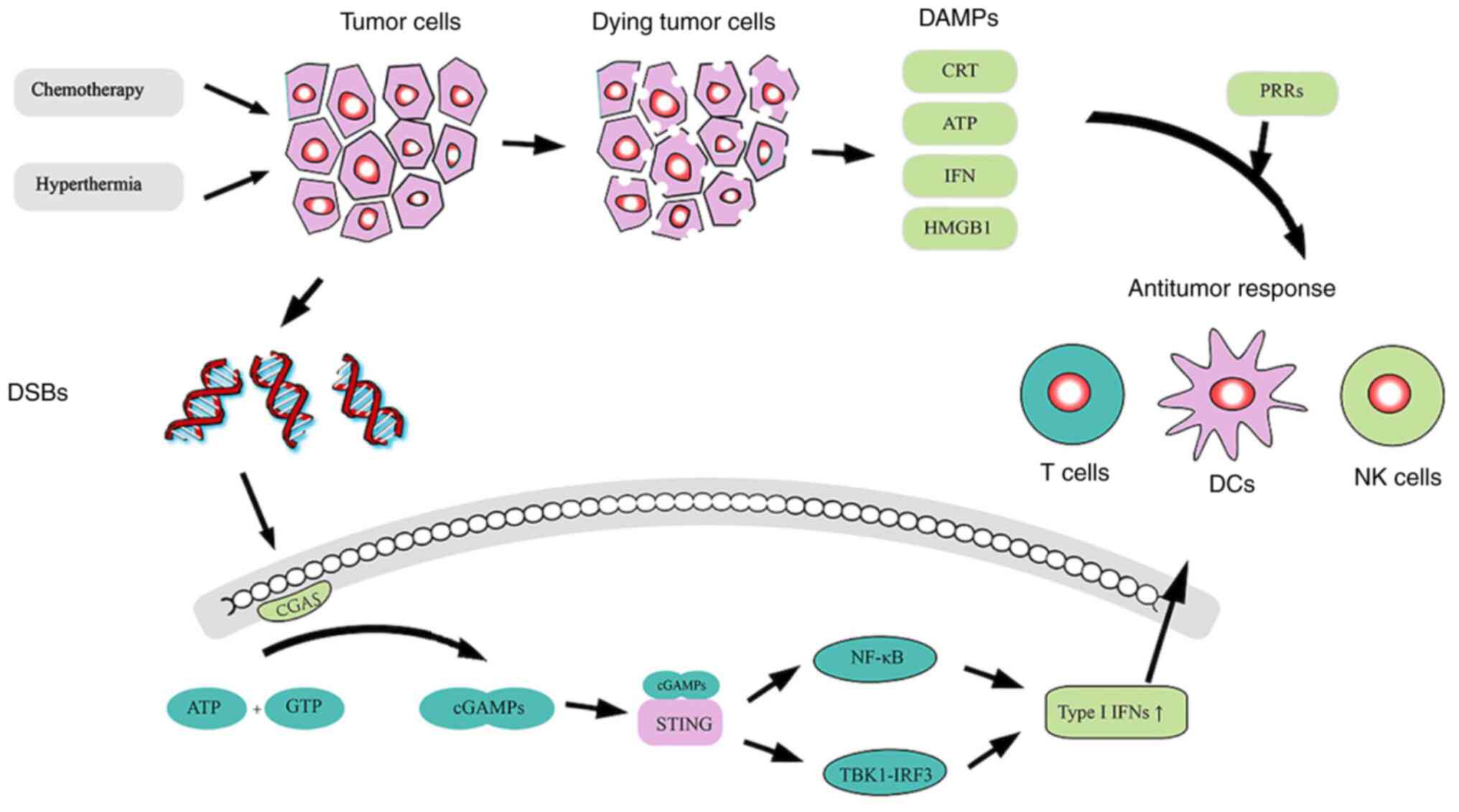
During chemotherapy-induced tumor cell death, the
release of DNA, a tumor-specific antigen, can enhance antitumor
immune responses via the cGAS-cGAMP-STING pathway (Fig. 1). The production of cGAMP can be
catalyzed by the interaction between dsDNA and cGAS, after which
cGAMP activates the adaptor protein STING, a second messenger in
APCs (128,129). The downstream NF-κB and TBK1-IRF3
pathways are then activated to induce the expression of type I
IFNs, which induce innate and adaptive immune responses, including
various immune cell subsets, such as NK cells, DCs, B cells and
effector T cells (130,131). As hyperthermia can promote tumor
cell DNA damage (43,44) and delay the repair of DSBs caused by
chemotherapy (48,49), it may be a sensitizer for such a
process. Thus, HIPEC may exert unexpected antitumor effects
following activation of the CGAS-cGAMP-STING pathway.
Following improvements by medical personnel, HIPEC
appears to increase the OS rate of patients more favorably with
peritoneal metastatic carcinoma compared with systemic
chemotherapy. However, the therapeutic effects of HIPEC have been
debated since the publication of the PRODIGE7 and PROPHYLOCHIP
trial results. Thus, additional clinical trials are required to
definitively establish the role of HIPEC in abdominal metastatic
adenocarcinoma.
HIPEC exhibits several noteworthy side effects, such
as visceral hemorrhage, fatigue and gastrointestinal or neurotoxic
effects (132,133). Intra-abdominal infection, peritoneal
recurrence and small bowel obstruction are also frequently observed
(134). Antibiotic-induced
intestinal dysbiosis can result in the failure of cancer
immunotherapy (135). Thus,
detrimental changes in the gut microbiome of patients undergoing
HIPEC may also occur (136,137). It was hypothesized that temperate
chemotherapy delivered into the abdominal cavity may also lead to
intestinal dysbiosis; however, further clarification is
required.
The present review focuses on the dual actions of
chemotherapeutic drugs and hyperthermia using HIPEC to clarify the
molecular mechanisms underlying the enhanced efficacy of HIPEC, and
to identify other therapies for its combinatory use. However, the
precise molecular indexes, not just the retrospective indexes,
require further investigation to predict patient prognosis.
Not applicable.
The present review was financially supported by the
National Natural Science Foundation of China (grant nos. 31700792
and 81801568) and the Maternal and Child Health Association
Foundation of Jiangsu (grant no. FYX202017).
Not applicable.
YZ and YW performed the literature review and
drafted the initial manuscript. JW and CW revised the manuscript
for important intellectual content and confirmed the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polyzos A, Tsavaris N, Kosmas C, Giannikos
L, Katsikas M, Kalahanis N, Karatzas G, Christodoulou K,
Giannakopoulos K, Stamatiadis D and Katsilambros N: A comparative
study of intraperitoneal carboplatin versus intravenous carboplatin
with intravenous cyclophosphamide in both arms as initial
chemotherapy for stage III ovarian cancer. Oncology. 56:291–296.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arshad U, Ploylearmsaeng SA, Karlsson MO,
Doroshyenko O, Langer D, Schömig E, Kunze S, Güner SA,
Skripnichenko R, Ullah S, et al: Prediction of exposure-driven
myelotoxicity of continuous infusion 5-fluorouracil by a
semi-physiological pharmacokinetic-pharmacodynamic model in
gastrointestinal cancer patients. Cancer Chemother Pharmacol.
85:711–722. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flessner MF: The transport barrier in
intraperitoneal therapy. Am J Physiol Renal Physiol. 288:F433–F442.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barlin JN, Dao F, Bou Zgheib N, Ferguson
SE, Sabbatini PJ, Hensley ML, Bell-McGuinn KM, Konner J, Tew WP,
Aghajanian C and Chi DS: Progression-free and overall survival of a
modified outpatient regimen of primary intravenous/intraperitoneal
paclitaxel and intraperitoneal cisplatin in ovarian, fallopian
tube, and primary peritoneal cancer. Gynecol Oncol. 125:621–624.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Driel WJ, Koole SN, Sikorska K,
Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT,
van der Velden J, Arts HJ, Massuger LFAG, et al: Hyperthermic
intraperitoneal chemotherapy in ovarian cancer. N Engl J Med.
378:230–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gonzalez-Moreno S: Peritoneal surface
oncology: A PROGRESS REPort. Eur J Surg Oncol. 32:593–596. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quénet F, Elias D, Roca L, Goéré D, Ghouti
L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, et al:
Cytoreductive surgery plus hyperthermic intraperitoneal
chemotherapy versus cytoreductive surgery alone for colorectal
peritoneal metastases (PRODIGE 7): a multicentre, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:256–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goéré D, Glehen O, Quenet F, Guilloit JM,
Bereder JM, Lorimier G, Thibaudeau E, Ghouti L, Pinto A, Tuech JJ,
et al: Second-look surgery plus hyperthermic intraperitoneal
chemotherapy versus surveillance in patients at high risk of
developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE
15): A randomised, phase 3 study. Lancet Oncol. 21:1147–1154. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
González-Moreno S, González-Bayón LA and
Ortega-Pérez G: Hyperthermic intraperitoneal chemotherapy:
Rationale and technique. World J Gastrointest Oncol. 2:68–75. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giovanella BC, Stehlin JS and Morgan AC:
Selective lethal effect of supranormal temperatures on human
neoplastic cells. Cancer Res. 36:3944–3950. 1976.PubMed/NCBI
|
|
13
|
Glehen O, Cotte E, Kusamura S, Deraco M,
Baratti D, Passot G, Beaujard AC and Noel GF: Hyperthermic
intraperitoneal chemotherapy: Nomenclature and modalities of
perfusion. J Surg Oncol. 98:242–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sánchez-García S, Padilla-Valverde D,
Villarejo-Campos P, Martín-Fernández J, García-Rojo M and
Rodríguez-Martínez M: Experimental development of an
intra-abdominal chemohyperthermia model using a closed abdomen
technique and a PRS-1.0 Combat CO2 recirculation system.
Surgery. 155:719–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leiting JL, Cloyd JM, Ahmed A, Fournier K,
Lee AJ, Dessureault S, Felder S, Veerapong J, Baumgartner JM,
Clarke C, et al: Comparison of open and closed hyperthermic
intraperitoneal chemotherapy: Results from the United States
hyperthermic intraperitoneal chemotherapy collaborative. World J
Gastrointest Oncol. 12:756–767. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sánchez-García S, Villarejo-Campos P,
Padilla-Valverde D, Amo-Salas M and Martín-Fernández J:
Intraperitoneal chemotherapy hyperthermia (HIPEC) for peritoneal
carcinomatosis of ovarian cancer origin by fluid and CO2
recirculation using the closed abdomen technique (PRS-1.0 Combat):
A clinical pilot study. Int J Hyperthermia. 32:488–495. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khosrawipour V, Khosrawipour T,
Diaz-Carballo D, Förster E, Zieren J and Giger-Pabst U: Exploring
the spatial drug distribution pattern of pressurized
intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol.
23:1220–1224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nadiradze G, Horvath P, Sautkin Y, Archid
R, Weinreich FJ, Königsrainer A and Reymond MA: Overcoming drug
resistance by taking advantage of physical principles: Pressurized
intraperitoneal aerosol chemotherapy (PIPAC). Cancers (Basel).
12:342019. View Article : Google Scholar
|
|
19
|
Verwaal VJ, van Ruth S, de Bree E, van
Sloothen GW, van Tinteren H, Boot H and Zoetmulder FA: Randomized
trial of cytoreduction and hyperthermic intraperitoneal
chemotherapy versus systemic chemotherapy and palliative surgery in
patients with peritoneal carcinomatosis of colorectal cancer. J
Clin Oncol. 21:3737–3743. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klaver CE, Musters GD, Bemelman WA, Punt
CJ, Verwaal VJ, Dijkgraaf MG, Aalbers AG, van der Bilt JD, Boerma
D, Bremers AJ, et al: Adjuvant hyperthermic intraperitoneal
chemotherapy (HIPEC) in patients with colon cancer at high risk of
peritoneal carcinomatosis; the COLOPEC randomized multicentre
trial. BMC Cancer. 15:4282015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reutovich MY, Krasko OV and Sukonko OG:
Hyperthermic intraperitoneal chemotherapy in serosa-invasive
gastric cancer patients. Eur J Surg Oncol. 45:2405–2411. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL,
Cheng FL, Zhou YF, Xiong B, Yonemura Y and Li Y: Cytoreductive
surgery and hyperthermic intraperitoneal chemotherapy improves
survival of patients with peritoneal carcinomatosis from gastric
cancer: Final results of a phase III randomized clinical trial. Ann
Surg Oncol. 18:1575–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spiliotis J, Halkia E, Lianos E, Kalantzi
N, Grivas A, Efstathiou E and Giassas S: Cytoreductive surgery and
HIPEC in recurrent epithelial ovarian cancer: A prospective
randomized phase III study. Ann Surg Oncol. 22:1570–1575. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glockzin G, Rochon J, Arnold D, Lang SA,
Klebl F, Zeman F, Koller M, Schlitt HJ and Piso P: A prospective
multicenter phase II study evaluating multimodality treatment of
patients with peritoneal carcinomatosis arising from appendiceal
and colorectal cancer: The COMBATAC trial. BMC Cancer. 13:672013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Leeuwen BL, Graf W, Pahlman L and
Mahteme H: Swedish experience with peritonectomy and HIPEC. HIPEC
in peritoneal carcinomatosis. Ann Surg Oncol. 15:745–753. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zivanovic O, Chi DS, Filippova O, Randall
LM, Bristow RE and O'Cearbhaill RE: It's time to warm up to
hyperthermic intraperitoneal chemotherapy for patients with ovarian
cancer. Gynecol Oncol. 151:555–561. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kusamura S, Azmi N, Fumagalli L, Baratti
D, Guaglio M, Cavalleri A, Garrone G, Battaglia L, Barretta F and
Deraco M: Phase II randomized study on tissue distribution and
pharmacokinetics of cisplatin according to different levels of
intra-abdominal pressure (IAP) during HIPEC (NCT02949791). Eur J
Surg Oncol. 47:82–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cesna V, Sukovas A, Jasukaitiene A,
Naginiene R, Barauskas G, Dambrauskas Z, Paskauskas S and Gulbinas
A: Narrow line between benefit and harm: Additivity of hyperthermia
to cisplatin cytotoxicity in different gastrointestinal cancer
cells. World J Gastroenterol. 24:1072–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ceelen W, Braet H, van Ramshorst G,
Willaert W and Remaut K: Intraperitoneal chemotherapy for
peritoneal metastases: An expert opinion. Expert Opin Drug Deliv.
17:511–522. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y and Calderwood SK: Autophagy,
protein aggregation and hyperthermia: A mini-review. Int J
Hyperthermia. 27:409–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed K, Zaidi SF, Mati-Ur-Rehman, Rehman
R and Kondo T: Hyperthermia and protein homeostasis: Cytoprotection
and cell death. J Therm Biol. 91:1026152020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franke K, Kettering M, Lange K, Kaiser WA
and Hilger I: The exposure of cancer cells to hyperthermia, iron
oxide nanoparticles, and mitomycin C influences membrane multidrug
resistance protein expression levels. Int J Nanomedicine.
8:351–363. 2013.PubMed/NCBI
|
|
33
|
Luchetti F, Mannello F, Canonico B,
Battistelli M, Burattini S and Falcieri E: Integrin and
cytoskeleton behaviour in human neuroblastoma cells during
hyperthermia-related apoptosis. Apoptosis. 9:635–648. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luchetti F, Canonico B, Della Felice M,
Burattini S, Battistelli M, Papa S and Falcieri E: Hyperthermia
triggers apoptosis and affects cell adhesiveness in human
neuroblastoma cells. Histol Histopathol. 18:1041–1052.
2003.PubMed/NCBI
|
|
35
|
Onishi Y, Fehervari Z, Yamaguchi T and
Sakaguchi S: Foxp3+ natural regulatory T cells
preferentially form aggregates on dendritic cells in vitro and
actively inhibit their maturation. Proc Natl Acad Sci USA.
105:10113–10118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alvarez-Berríos MP, Castillo A, Mendéz J,
Soto O, Rinaldi C and Torres-Lugo M: Hyperthermic potentiation of
cisplatin by magnetic nanoparticle heaters is correlated with an
increase in cell membrane fluidity. Int J Nanomedicine.
8:1003–1013. 2013.PubMed/NCBI
|
|
37
|
Csoboz B, Balogh GE, Kusz E, Gombos I,
Peter M, Crul T, Gungor B, Haracska L, Bogdanovics G, Torok Z, et
al: Membrane fluidity matters: Hyperthermia from the aspects of
lipids and membranes. Int J Hyperthermia. 29:491–499. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Andrade Mello P, Bian S, Savio LEB,
Zhang H, Zhang J, Junger W, Wink MR, Lenz G, Buffon A, Wu Y and
Robson SC: Hyperthermia and associated changes in membrane fluidity
potentiate P2X7 activation to promote tumor cell death. Oncotarget.
8:67254–67268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Oorschot B, Granata G, Di Franco S,
Ten Cate R, Rodermond HM, Todaro M, Medema JP and Franken NA:
Targeting DNA double strand break repair with hyperthermia and
DNA-PKcs inhibition to enhance the effect of radiation treatment.
Oncotarget. 7:65504–65513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mehta IS, Kulashreshtha M, Chakraborty S,
Kolthur-Seetharam U and Rao BJ: Chromosome territories reposition
during DNA damage-repair response. Genome Biol. 14:1352013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Warters RL and Henle KJ: DNA degradation
in chinese hamster ovary cells after exposure to hyperthermia.
Cancer Res. 42:4427–4432. 1982.PubMed/NCBI
|
|
42
|
Takahashi A, Matsumoto H, Nagayama K,
Kitano M, Hirose S, Tanaka H, Mori E, Yamakawa N, Yasumoto J, Yuki
K, et al: Evidence for the involvement of double-strand breaks in
heat-induced cell killing. Cancer Res. 64:8839–8845. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takahashi A, Mori E, Somakos GI, Ohnishi K
and Ohnishi T: Heat induces gammaH2AX foci formation in mammalian
cells. Mutat Res. 656:88–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hunt CR, Pandita RK, Laszlo A, Higashikubo
R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R,
et al: Hyperthermia activates a subset of ataxia-telangiectasia
mutated effectors independent of DNA strand breaks and heat shock
protein 70 status. Cancer Res. 67:3010–3017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
El-Awady RA, Dikomey E and Dahm-Daphi J:
Heat effects on DNA repair after ionising radiation: Hyperthermia
commonly increases the number of non-repaired double-strand breaks
and structural rearrangements. Nucleic Acids Res. 29:1960–1966.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Muenyi CS, States VA, Masters JH, Fan TW,
Helm CW and States JC: Sodium arsenite and hyperthermia modulate
cisplatin-DNA damage responses and enhance platinum accumulation in
murine metastatic ovarian cancer xenograft after hyperthermic
intraperitoneal chemotherapy (HIPEC). J Ovarian Res. 4:92011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Oei AL, Vriend LE, Crezee J, Franken NA
and Krawczyk PM: Effects of hyperthermia on DNA repair pathways:
One treatment to inhibit them all. Radiat Oncol. 10:1652015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Krawczyk PM, Eppink B, Essers J, Stap J,
Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist
MR, et al: Mild hyperthermia inhibits homologous recombination,
induces BRCA2 degradation, and sensitizes cancer cells to poly
(ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA.
108:9851–9856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang J, Zhao B, Chen S, Wang Y, Zhang Y,
Wang Y, Wei D, Zhang L, Rong G and Weng Y: Near-infrared light
irradiation induced Mild hyperthermia enhances glutathione
depletion and DNA interstrand cross-link formation for efficient
chemotherapy. ACS Nano. 14:14831–14845. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ohno S, Siddik ZH, Kido Y, Zwelling LA and
Bull JM: Thermal enhancement of drug uptake and DNA adducts as a
possible mechanism for the effect of sequencing hyperthermia on
cisplatin-induced cytotoxicity in L1210 cells. Cancer Chemother
Pharmacol. 34:302–306. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sato I, Umemura M, Mitsudo K, Kioi M,
Nakashima H, Iwai T, Feng X, Oda K, Miyajima A, Makino A, et al:
Hyperthermia generated with ferucarbotran (Resovist®) in
an alternating magnetic field enhances cisplatin-induced apoptosis
of cultured human oral cancer cells. J Physiol Sci. 64:177–183.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Clavel CM, Nowak-Sliwinska P, Păunescu E,
Griffioen AW and Dyson PJ: In vivo evaluation of
small-molecule thermoresponsive anticancer drugs potentiated by
hyperthermia. Chem Sci. 6:2795–2801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Peer AJ, Grimm MJ, Zynda ER and Repasky
EA: Diverse immune mechanisms may contribute to the survival
benefit seen in cancer patients receiving hyperthermia. Immunol
Res. 46:137–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Borst J, Ahrends T, Bąbała N, Melief CJM
and Kastenmüller W: CD4+T cell help in cancer immunology
and immunotherapy. Nat Rev Immunol. 18:635–647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schuijs MJ, Hammad H and Lambrecht BN:
Professional and ‘Amateur’ Antigen-Presenting Cells In Type 2
Immunity. Trends Immunol. 40:22–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Evans SS, Repasky EA and Fisher DT: Fever
and the thermal regulation of immunity: The immune system feels the
heat. Nat Rev Immunol. 15:335–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E
and Repasky EA: Enhancement of natural killer (NK) cell
cytotoxicity by fever-range thermal stress is dependent on NKG2D
function and is associated with plasma membrane NKG2D clustering
and increased expression of MICA on target cells. J Leukoc Biol.
82:1322–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang L, Liu F, Peng Y, Sun L and Chen G:
Changes in expression of four molecular marker proteins and one
microRNA in mesothelial cells of the peritoneal dialysate effluent
fluid of peritoneal dialysis patients. Exp Ther Med. 6:1189–1193.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rynne-Vidal A, Au-Yeung CL,
Jiménez-Heffernan JA, Pérez-Lozano ML, Cremades-Jimeno L, Bárcena
C, Cristóbal-García I, Fernández-Chacón C, Yeung TL, Mok SC, et al:
Mesothelial-to-mesenchymal transition as a possible therapeutic
target in peritoneal metastasis of ovarian cancer. J Pathol.
242:140–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pathria P, Louis TL and Varner JA:
Targeting Tumor-associated macrophages in cancer. Trends Immunol.
40:310–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li X, Liu R, Su X, Pan Y, Han X, Shao C
and Shi Y: Harnessing tumor-associated macrophages as aids for
cancer immunotherapy. Mol Cancer. 18:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Farhood B, Najafi M and Mortezaee K:
CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J
Cell Physiol. 234:8509–8521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S
and Jian Z: IL-6/STAT3 pathway intermediates M1/M2 macrophage
polarization during the development of hepatocellular carcinoma. J
Cell Biochem. 119:9419–9432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Qu D, Qin Y, Liu Y, Liu T, Liu C, Han T,
Chen Y, Ma C and Li X: Fever-inducible lipid nanocomposite for
boosting cancer therapy through synergistic engineering of a tumor
microenvironment. ACS Appl Mater Interfaces. 12:32301–32311. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Frey B, Weiss EM, Rubner Y, Wunderlich R,
Ott OJ, Sauer R, Fietkau R and Gaipl US: Old and new facts about
hyperthermia-induced modulations of the immune system. Int J
Hyperthermia. 28:528–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Agarwal SS, Katz EJ and Loeb LA: Effect of
hyperthermia on the survival of normal human peripheral blood
mononuclear cells. Cancer Res. 43:3124–3126. 1983.PubMed/NCBI
|
|
68
|
Harden LM, Kent S, Pittman QJ and Roth J:
Fever and sickness behavior: Friend or foe? Brain Behav Immun.
50:322–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang L, Zhang Y, Xue Y, Wu Y, Wang Q, Xue
L, Su Z and Zhang C: Transforming weakness into strength:
Photothermal-therapy-induced inflammation enhanced
cytopharmaceutical chemotherapy as a combination anticancer
treatment. Adv Mater. 31:e18059362019.PubMed/NCBI
|
|
70
|
Mantovani A, Barajon I and Garlanda C:
IL-1 and IL-1 regulatory pathways in cancer progression and
therapy. Immunol Rev. 281:57–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kitamura H, Ohno Y, Toyoshima Y, Ohtake J,
Homma S, Kawamura H, Takahashi N and Taketomi A:
Interleukin-6/STAT3 signaling as a promising target to improve the
efficacy of cancer immunotherapy. Cancer Sci. 108:1947–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Appenheimer MM, Chen Q, Girard RA, Wang WC
and Evans SS: Impact of fever-range thermal stress on
lymphocyte-endothelial adhesion and lymphocyte trafficking. Immunol
Invest. 34:295–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chonov DC, Ignatova MMK, Ananiev JR and
Gulubova MV: IL-6 Activities in the Tumour Microenvironment. Part
1. Open Access Maced J Med Sci. 7:2391–2398. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wagner AC, Weber H, Jonas L, Nizze H,
Strowski M, Fiedler F, Printz H, Steffen H and Göke B: Hyperthermia
induces heat shock protein expression and protection against
cerulein-induced pancreatitis in rats. Gastroenterology.
111:1333–1342. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Burd R, Dziedzic TS, Xu Y, Caligiuri MA,
Subjeck JR and Repasky EA: Tumor cell apoptosis, lymphocyte
recruitment and tumor vascular changes are induced by low
temperature, long duration (fever-like) whole body hyperthermia. J
Cell Physiol. 177:137–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hartl FU and Hayer-Hartl M: Molecular
chaperones in the cytosol: From nascent chain to folded protein.
Science. 295:1852–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Calderwood SK and Gong J: Heat shock
proteins promote cancer: It's a protection racket. Trends Biochem
Sci. 41:311–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pelz JO, Vetterlein M, Grimmig T, Kerscher
AG, Moll E, Lazariotou M, Matthes N, Faber M, Germer CT,
Waaga-Gasser AM and Gasser M: Hyperthermic intraperitoneal
chemotherapy in patients with peritoneal carcinomatosis: Role of
heat shock proteins and dissecting effects of hyperthermia. Ann
Surg Oncol. 20:1105–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kepenekian V, Aloy MT, Magné N, Passot G,
Armandy E, Decullier E, Sayag-Beaujard A, Gilly FN, Glehen O and
Rodriguez-Lafrasse C: Impact of hyperthermic intraperitoneal
chemotherapy on Hsp27 protein expression in serum of patients with
peritoneal carcinomatosis. Cell Stress Chaperones. 18:623–630.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mu C, Wu X, Zhou X, Wolfram J, Shen J,
Zhang D, Mai J, Xia X, Holder AM, Ferrari M, et al: Chemotherapy
sensitizes therapy-resistant cells to Mild hyperthermia by
suppressing heat shock protein 27 expression in triple-negative
breast cancer. Clin Cancer Res. 24:4900–4912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zunino B, Rubio-Patiño C, Villa E, Meynet
O, Proics E, Cornille A, Pommier S, Mondragón L, Chiche J, Bereder
JM, et al: Hyperthermic intraperitoneal chemotherapy leads to an
anticancer immune response via exposure of cell surface heat shock
protein 90. Oncogene. 35:261–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Isambert N, Delord JP, Soria JC,
Hollebecque A, Gomez-Roca C, Purcea D, Rouits E, Belli R and
Fumoleau P: Debio0932, a second-generation oral heat shock protein
(HSP) inhibitor, in patients with advanced cancer-results of a
first-in-man dose-escalation study with a fixed-dose extension
phase. Ann Oncol. 26:1005–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Larson N, Gormley A, Frazier N and
Ghandehari H: Synergistic enhancement of cancer therapy using a
combination of heat shock protein targeted HPMA copolymer-drug
conjugates and gold nanorod induced hyperthermia. J Control
Release. 170:41–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Taha EA, Ono K and Eguchi T: Roles of
extracellular HSPs as biomarkers in immune surveillance and immune
evasion. Int J Mol Sci. 20:45882019. View Article : Google Scholar
|
|
86
|
Mukhopadhaya A, Mendecki J, Dong X, Liu L,
Kalnicki S, Garg M, Alfieri A and Guha C: Localized hyperthermia
combined with intratumoral dendritic cells induces systemic
antitumor immunity. Cancer Res. 67:7798–7806. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang HG, Mehta K, Cohen P and Guha C:
Hyperthermia on immune regulation: A temperature's story. Cancer
Lett. 271:191–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Torigoe T, Tamura Y and Sato N: Heat shock
proteins and immunity: Application of hyperthermia for
immunomodulation. Int J Hyperthermia. 25:610–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Calderwood SK, Theriault JR and Gong J:
How is the immune response affected by hyperthermia and heat shock
proteins? Int J Hyperthermia. 21:713–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lee S, Son B, Park G, Kim H, Kang H, Jeon
J, Youn H and Youn B: Immunogenic effect of hyperthermia on
enhancing radiotherapeutic efficacy. Int J Mol Sci. 19:27952018.
View Article : Google Scholar
|
|
91
|
van Baal JO, Van de Vijver KK, Nieuwland
R, van Noorden CJ, van Driel WJ, Sturk A, Kenter GG, Rikkert LG and
Lok CA: The histophysiology and pathophysiology of the peritoneum.
Tissue Cell. 49:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kastelein AW, Vos LMC, de Jong KH, van
Baal JOAM, Nieuwland R, van Noorden CJF, Roovers JWR and Lok CAR:
Embryology, anatomy, physiology and pathophysiology of the
peritoneum and the peritoneal vasculature. Semin Cell Dev Biol.
92:27–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
de Bree E, Michelakis D, Stamatiou D,
Romanos J and Zoras O: Pharmacological principles of
intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum.
2:47–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ceelen WP and Flessner MF: Intraperitoneal
therapy for peritoneal tumors: Biophysics and clinical evidence.
Nat Rev Clin Oncol. 7:108–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
van Ruth S, Mathôt RA, Sparidans RW,
Beijnen JH, Verwaal VJ and Zoetmulder FA: Population
pharmacokinetics and pharmacodynamics of mitomycin during
intraoperative hyperthermic intraperitoneal chemotherapy. Clinical
Pharmacokinet. 43:131–143. 2004. View Article : Google Scholar
|
|
96
|
Cashin PH, Ehrsson H, Wallin I, Nygren P
and Mahteme H: Pharmacokinetics of cisplatin during hyperthermic
intraperitoneal treatment of peritoneal carcinomatosis. Eur J Clin
Pharmacol. 69:533–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Leinwand JC, Bates GE, Allendorf JD,
Chabot JA, Lewin SN and Taub RN: Body surface area predicts plasma
oxaliplatin and pharmacokinetic advantage in hyperthermic
intraoperative intraperitoneal chemotherapy. Ann Surg Oncol.
20:1101–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
de Bree E, Rosing H, Filis D, Romanos J,
Melisssourgaki M, Daskalakis M, Pilatou M, Sanidas E, Taflampas P,
Kalbakis K, et al: Cytoreductive surgery and intraoperative
hyperthermic intraperitoneal chemotherapy with paclitaxel: A
clinical and pharmacokinetic study. Ann Surg Oncol. 15:1183–1192.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
de Bree E, Rosing H, Beijnen JH, Romanos
J, Michalakis J, Georgoulias V and Tsiftsis DD: Pharmacokinetic
study of docetaxel in intraoperative hyperthermic i.p. chemotherapy
for ovarian cancer. Anticancer Drugs. 14:103–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Nicoletto MO, Padrini R, Galeotti F,
Ferrazzi E, Cartei G, Riddi F, Palumbo M, De Paoli M and Corsini A:
Pharmacokinetics of intraperitoneal hyperthermic perfusion with
mitoxantrone in ovarian cancer. Cancer Chemother Pharmacol.
45:457–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rossi CR, Mocellin S, Pilati P, Foletto M,
Quintieri L, Palatini P and Lise M: Pharmacokinetics of
intraperitoneal cisplatin and doxorubicin. Surg Oncol Clin N Am.
12:781–794. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Choi YH: Interpretation of drug
interaction using systemic and local tissue exposure changes.
Pharmaceutics. 12:4172020. View Article : Google Scholar
|
|
103
|
Tentes AA, Kyziridis D, Kakolyris S,
Pallas N, Zorbas G, Korakianitis O, Mavroudis C, Courcoutsakis N
and Prasopoulos P: Preliminary results of hyperthermic
intraperitoneal intraoperative chemotherapy as an adjuvant in
resectable pancreatic cancer. Gastroenterol Res Pract.
2012:5065712012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lemoine L, Thijssen E, Carleer R, Cops J,
Lemmens V, Eyken PV, Sugarbaker P and der Speeten KV: Body surface
area-based versus concentration-based intraperitoneal perioperative
chemotherapy in a rat model of colorectal peritoneal surface
malignancy: Pharmacologic guidance towards standardization.
Oncotarget. 10:1407–1424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lemoine L, Thijssen E, Carleer R, Geboers
K, Sugarbaker P and van der Speeten K: Body surface area-based vs
concentration-based perioperative intraperitoneal chemotherapy
after optimal cytoreductive surgery in colorectal peritoneal
surface malignancy treatment: COBOX trial. J Surg Oncol.
119:999–1010. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liesenfeld LF, Hillebrecht HC, Klose J,
Schmidt T and Schneider M: Impact of perfusate concentration on
hyperthermic intraperitoneal chemotherapy efficacy and toxicity in
a rodent model. J Surg Res. 253:262–271. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Shah DK, Shin BS, Veith J, Tóth K,
Bernacki RJ and Balthasar JP: Use of an anti-vascular endothelial
growth factor antibody in a pharmacokinetic strategy to increase
the efficacy of intraperitoneal chemotherapy. J Pharmacol Exp Ther.
329:580–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gremonprez F, Descamps B, Izmer A, Vanhove
C, Vanhaecke F, De Wever O and Ceelen W: Pretreatment with
VEGF(R)-inhibitors reduces interstitial fluid pressure, increases
intraperitoneal chemotherapy drug penetration, and impedes tumor
growth in a mouse colorectal carcinomatosis model. Oncotarget.
6:29889–29900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li H, Mao X, Liu K, Sun J, Li B, Malyar
RM, Liu D, Pan C, Gan F and Liu Y: A pilot study of combination
intraperitoneal recombinant human endostatin and chemotherapy for
refractory malignant ascites secondary to ovarian cancer. Med
Oncol. 31:9302014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cristea MC, Frankel P, Synold T, Rivkin S,
Lim D, Chung V, Chao J, Wakabayashi M, Paz B, Han E, et al: A phase
I trial of intraperitoneal nab-paclitaxel in the treatment of
advanced malignancies primarily confined to the peritoneal cavity.
Cancer Chemother Pharmaco. 83:589–598. 2019. View Article : Google Scholar
|
|
111
|
Shamsi M, Sedaghatkish A, Dejam M,
Saghafian M, Mohammadi M and Sanati-Nezhad A: Magnetically assisted
intraperitoneal drug delivery for cancer chemotherapy. Drug Deliv.
25:846–861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sugarbaker PH and Van der Speeten K:
Surgical technology and pharmacology of hyperthermic perioperative
chemotherapy. J Gastrointest Oncol. 7:29–44. 2016.PubMed/NCBI
|
|
113
|
Dakwar GR, Shariati M, Willaert W, Ceelen
W, De Smedt SC and Remaut K: Nanomedicine-based intraperitoneal
therapy for the treatment of peritoneal carcinomatosis-Mission
possible? Adv Drug Deliv Rev. 108:13–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunological effects of conventional chemotherapy
and targeted anticancer Agents. Cancer Cell. 28:690–714. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Coffelt SB and de Visser KE:
Immune-mediated mechanisms influencing the efficacy of anticancer
therapies. Trends Immunol. 36:198–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
117
|
Pfistershammer K, Klauser C, Pickl WF,
Stöckl J, Leitner J, Zlabinger G, Majdic O and Steinberger P: No
evidence for dualism in function and receptors: PD-L2/B7-DC is an
inhibitory regulator of human T cell activation. Eur J Immunol.
36:1104–1113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Schiavoni G, Sistigu A, Valentini M,
Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D'Urso MT,
Belardelli F, et al: Cyclophosphamide synergizes with type I
interferons through systemic dendritic cell reactivation and
induction of immunogenic tumor apoptosis. Cancer Res. 71:768–778.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wu J and Waxman DJ: Metronomic
cyclophosphamide eradicates large implanted GL261 gliomas by
activating antitumor Cd8 T-cell responses and immune memory.
Oncoimmunology. 4:e10055212015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen C, Chen Z, Chen D, Zhang B, Wang Z
and Le H: Suppressive effects of gemcitabine plus cisplatin
chemotherapy on regulatory T cells in nonsmall-cell lung cancer. J
Int Med Res. 43:180–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zitvogel L, Galluzzi L, Smyth MJ and
Kroemer G: Mechanism of action of conventional and targeted
anticancer therapies: Reinstating immunosurveillance. Immunity.
39:74–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kepp O, Senovilla L, Vitale I, Vacchelli
E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N,
et al: Consensus guidelines for the detection of immunogenic cell
death. Oncoimmunology. 3:e9556912014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Garg AD and Agostinis P: Cell death and
immunity in cancer: From danger signals to mimicry of pathogen
defense responses. Immunol Rev. 280:126–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Krysko DV, Garg AD, Kaczmarek A, Krysko O,
Agostinis P and Vandenabeele P: Immunogenic cell death and DAMPs in
cancer therapy. Nat Rev Cancer. 12:860–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Banchereau J, Briere F, Caux C, Davoust J,
Lebecque S, Liu YJ, Pulendran B and Palucka K: Immunobiology of
dendritic cells. Annu Rev Immunol. 18:767–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Buqué A and Galluzzi L: Modeling tumor
immunology and immunotherapy in Mice. Trends Cancer. 4:599–601.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Curiel TJ: Immunotherapy: A useful
strategy to help combat multidrug resistance. Drug Resist Updat.
15:106–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen Q, Sun L and Chen ZJ: Regulation and
function of the cGAS-STING pathway of cytosolic DNA sensing. Nat
Immunol. 17:1142–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Li A, Yi M, Qin S, Song Y, Chu Q and Wu K:
Activating cGAS-STING pathway for the optimal effect of cancer
immunotherapy. J Hematol Oncol. 12:352019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Le Bon A, Thompson C, Kamphuis E, Durand
V, Rossmann C, Kalinke U and Tough DF: Cutting edge: Enhancement of
antibody responses through direct stimulation of B and T cells by
type I IFN. J Immunol. 176:2074–2078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fuertes MB, Woo SR, Burnett B, Fu YX and
Gajewski TF: Type I interferon response and innate immune sensing
of cancer. Trends Immunol. 34:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Bhagwandin SB, Naffouje S and Salti G:
Delayed presentation of major complications in patients undergoing
cytoreductive surgery plus hyperthermic intraperitoneal
chemotherapy following hospital discharge. J Surg Oncol.
111:324–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Blaj S, Nedelcut S, Mayr M, Leebmann H,
Leucuta D, Glockzin G and Piso P: Re-operations for early
postoperative complications after CRS and HIPEC: Indication,
timing, procedure, and outcome. Langenbecks Arch Surg. 404:541–546.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Dreznik Y, Hoffman A, Hamburger T,
Ben-Yaacov A, Dux Y, Jacoby H, Berger Y, Nissan A and Gutman M:
Hospital readmission rates and risk factors for readmission
following cytoreductive surgery (CRS) and hyperthermic
intraperitoneal chemotherapy (HIPEC) for peritoneal surface
malignancies. Surgeon. 16:278–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Panebianco C, Andriulli A and Pazienza V:
Pharmacomicrobiomics: Exploiting the drug-microbiota interactions
in anticancer therapies. Microbiome. 6:922018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Routy B, Gopalakrishnan V, Daillère R,
Zitvogel L, Wargo JA and Kroemer G: The gut microbiota influences
anticancer immunosurveillance and general health. Nat Rev Clin
Oncol. 15:382–396. 2018. View Article : Google Scholar : PubMed/NCBI
|