Cancer is the second leading cause of death
globally, responsible for ~9.6 million deaths in 2018 (1). The main forms of cancer treatments are
surgery, radiotherapy, chemotherapy and hormone therapy. Adjuvant
chemotherapy following surgery has been proven to decrease
recurrence and improve patient survival time (2,3). Natural
products have been major sources for the active ingredients in
numerous medicines. Compared with synthetic products, natural
products generally have fewer toxic effects and are less expensive.
Ginseng, derived from the rhizome and root of Panax ginseng
Meyer (P. ginseng), is a popular herbal medicine that has
been used in Asian countries (e.g., China, Japan and Korea) for
thousands of years. It is well known for its disease-preventing and
therapeutic effects.
The ginsenosides are classified as
20(S)-protopanaxadiol compounds (including ginsenosides Rb1, Rb2,
Rc, Rh2 and Rg3, among others) or 20(S)-protopanaxatriol compounds
(including ginsenosides Re, Rg1 and Rg2, among others). G-Rh2 has
been found to inhibit the growth of various human cancer cell
types, such as lung cancer cells (22–25), liver
cancer cells (27,28) and colorectal cancer cells (17,30,31). The
chemical formula for G-Rh2 is
C36H62O8, and the molecular weight
is 622.87; it is a white crystal with strong biological activity.
The antitumor activity of ginsenosides is related to factors such
as the type of ginsenoside, the substituents, and the number and
configuration of sugars. Due to the different spatial structures at
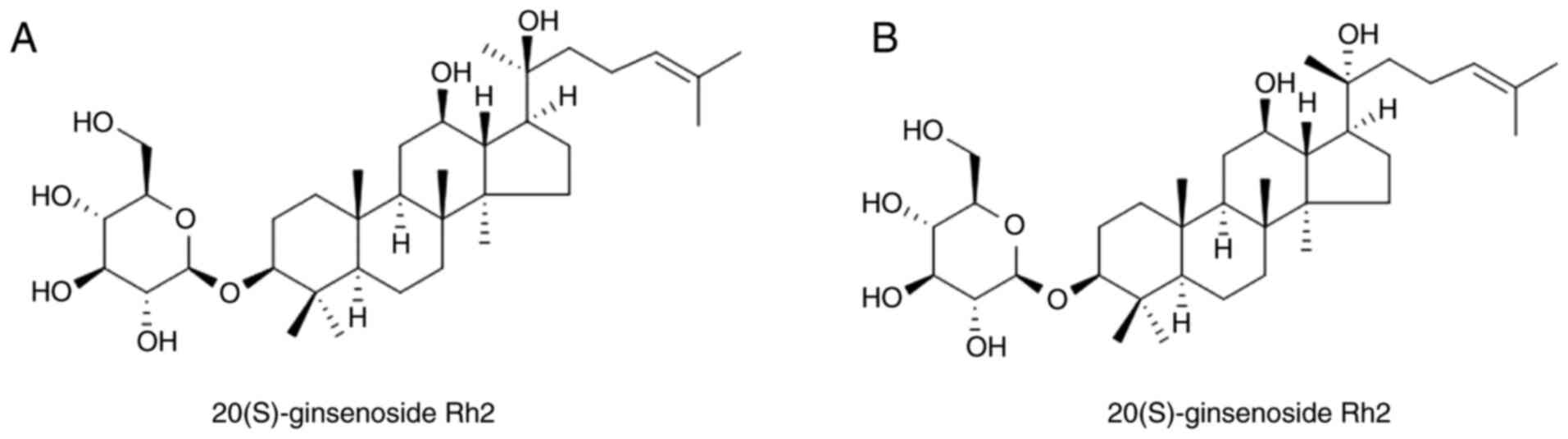
the C20 position, there are two stereoisomeric forms of G-Rh2:
20(S)-G-Rh2 and 20(R)-G-Rh2 (Fig. 1).
In comparison with 20(R)-G-Rh2, 20(S)-G-Rh2 shows more potent
anticancer activity among different cancer cells (35,46). One
study reported that the half maximal inhibitory concentration
values of 20(S)-G-Rh2 and 20(R)-G-Rh2 in A549 cells were 45.7 and
53.6 µM, respectively (46). Another
study compared the effect of 20(S)-G-Rh2 and 20(R)-G-Rh2 on LNCaP,
PC3 and DU145 cells. The results showed that 25 µM 20(S)-G-Rh2
inhibited LNCaP proliferation by 70%, PC3 cell proliferation by 40%
and DU145 cell proliferation by 20%, while 25 µM 20(R)-G-Rh2 did
not affect the proliferation of these cells (35). In addition, cytotoxic potency is
generally in the descending order of protopanaxadiol, 20(S)-G-Rh2
and then 20(R)-G-Rh2, indicating structure-related activities in
which the compound with less polar chemical structures possesses
higher cytotoxic activity towards cancer cells (47). As much of the literature does not
indicate whether 20(S)-G-Rh2 or 20(R)-G-Rh2 is used, the term G-Rh2
in this review includes both of these configurations.
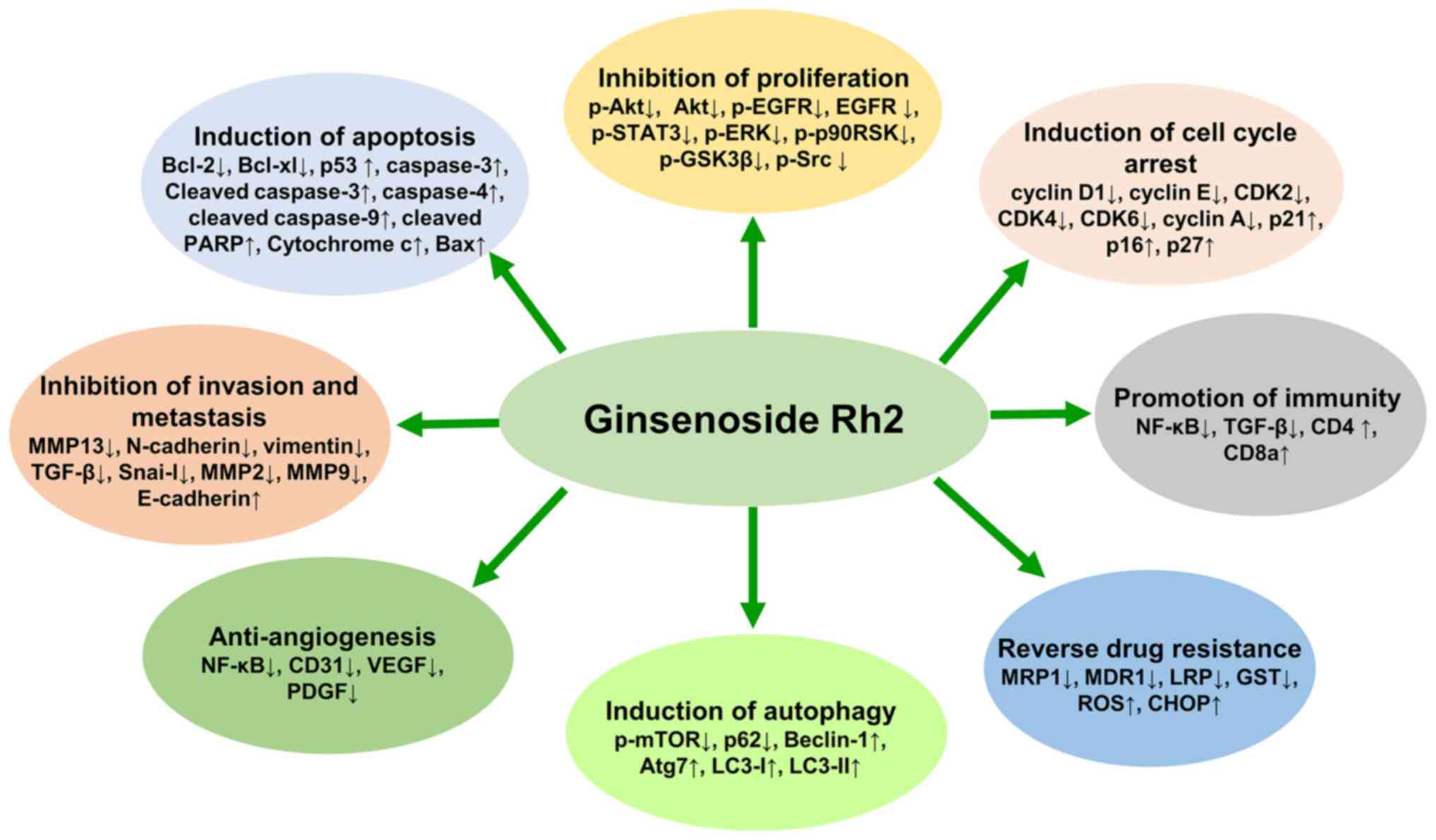
Previous studies have demonstrated that G-Rh2 exerts
significant anticancer activities through multiple molecular
mechanisms. The mechanisms are mainly related to cell cycle arrest,
apoptosis, proliferation, invasion, metastasis, angiogenesis,
autophagy and immunity, which are summarized in Table I and Fig.
2. Although the anticancer activity of G-Rh2 has been widely
investigated, the exact molecular mechanisms remain unclear. Based
on existing research, the possible mechanisms of action of G-Rh2
are described in this review.
The cell cycle is a controlled process involved in
the growth, differentiation and proliferation of eukaryotic cells
(48). Cells that undergo cell cycle
arrest lose their ability to replicate and divide. Cyclin-dependent
kinase (CDK) inhibitors are crucial for controlling the cell cycle
and cell proliferation (49). The CDK
inhibitor p21 plays a key role in the G1 phase cell
cycle checkpoint (50). G-Rh2 was
reported to induce cell cycle G1 phase arrest in MCF-7
cells by increasing p21 levels and decreasing CDK2 and cyclin
E-dependent kinase activities (32).
In human glioma A172 cells, G-Rh2 induced cell cycle G1
phase arrest by downregulating CDK4 and cyclin E (21). In human lung cancer A549 cells, G-Rh2
induced cell cycle G1 phase arrest by significantly
reducing the expression of CDK4 and cyclin D1 (51). Furthermore, G-Rh2 induced cell cycle
G1 phase arrest in HL-60 and U937 cells by
downregulating the expression of CDK4, CDK6, cyclin D1, cyclin D2,
cyclin D3 and cyclin E. G-Rh2-mediated G1 phase arrest
and differentiation are closely linked to the regulation of TGF-β
production in human leukemia cells (52).
Apoptosis (programmed cell death) plays an important
role in animal development and adult life by eliminating damaged
cells (53). The induction of
apoptosis in cancer cells can inhibit tumor growth (54). Treatment of colorectal cancer cells
with G-Rh2 has been proven to activate the p53 pathway, increasing
the expression of the proapoptotic regulator Bax and decreasing the
expression of the antiapoptotic regulator Bcl-2 (30). The extrinsic pathway and the intrinsic
pathway are two core apoptotic pathways in mammalian cells
(55). As shown in Table I, G-Rh2 can induce apoptosis via these
two pathways. It was previously shown that G-Rh2 induced apoptosis
by downregulating Bcl-2 and survivin and upregulating Bax, cleaved
caspase-3 and cleaved caspase-9 in human pancreatic cancer Bxpc-3
cells (20). G-Rh2-triggered
intrinsic apoptosis was related to the induced translocation of
cytosolic Bak and Bax to the mitochondria, cytochrome c
release and caspase-9 activation in HeLa and SW480 cells (56). G-Rh2 may induce apoptosis of Kasumi-1
and U937 leukemia cells via microRNA-21-modulated suppression of
Bcl-2 (57). In addition, cotreatment
with G-Rh2 and betulinic acid could induce apoptosis of HeLa, A549
and HepG2 cancer cells by enhancing caspase-8 expression,
cytochrome c release and Bax translocation (58).
Abnormal regulation of cell proliferation is the key
cause of cancer development and progression (59). Both activation of oncogenes and the
inactivation of tumor suppressor genes can promote the
proliferation of cancer cells. Protein kinase B (Akt) is one of the
well-known proto-oncogenes, and activated Akt promotes cancer cell
proliferation and survival (60).
G-Rh2 has been shown to inhibit HeLa and A172 cell proliferation by
suppressing the Akt pathway (15,21).
Another study revealed that G-Rh2 inhibited the proliferation of
HCT116 colon cancer cells by targeting PDZ-binding kinase/T-LAK
cell-originated protein kinase and downregulating the expression of
p-ERK1/2 and p-histone H3 (17).
G-Rh2 inhibits the proliferation of K562 and KG1-α cells by
suppressing the expression and activity of HDAC1, HDAC2, and HDCA6,
increasing histone H3 acetylation and regulating MAPK/JNK signaling
pathways (61). Additionally, G-Rh2
could inhibit cancer cell proliferation by suppressing endoplasmic
reticulum (ER) stress (22), by
inhibiting the Src/ERK signaling pathway (62) and by targeting microRNA-128 (63).
Metastasis is the main cause of cancer treatment
failure and recurrence. During metastasis, the cells become highly
plastic or adaptive, similar to stem cells. This change is called
epithelial-mesenchymal transition (EMT) (64). G-Rh2 could effectively inhibit tumor
metastasis by suppressing EMT. G-Rh2 has been found to inhibit
migration and invasion by increasing E-cadherin and suppressing
vimentin expression in endometrial cancer cells (65) and oral cancer cells (62). In addition, matrix metalloproteinases
(MMPs) play an important role in tumor metastasis, where they can
degrade the extracellular matrix and basement membrane (66). G-Rh2 was previously found to
effectively inhibit Bxpc-3 cell migration and invasion by
downregulating MMP-2 and MMP-9 (20).
Moreover, G-Rh2 was reported to significantly reduce the protein
levels of VEGF, MMP-2 and MMP-9 in co-cultured lung cancer cells
(23) and oral cancer cells (67). Therefore, G-Rh2 may play an inhibitory
role in the process of cancer metastasis.
Angiogenesis plays an important role in tumor growth
and metastasis by providing the necessary nutrients and oxygen
(68). TGF-β is a potent angiogenesis
inducer in vivo (69).
Anti-angiogenic treatment for tumors is considered a promising
therapeutic strategy (70). G-Rh2 was
reported to inhibit prostate cancer growth by impeding angiogenesis
via decreasing the expression of CD31, VEGF, platelet-derived
growth factor and CNNM1 in cancer cells (13). Furthermore, G-Rh2 could affect tumor
angiogenesis by downregulating JAM expression in tumors (71).
Autophagy is a catabolic process that degrades
cytoplasmic constituents and organelles in lysosomes (72). There is increasing evidence that
autophagy signaling is closely related to oncogenic signaling.
Autophagy may be one of the effective ways to prevent the formation
and progression of tumors (72).
Selective targeting of autophagy for cancer treatment has attracted
considerable attention (73). The
autophagy-related genes ATG5, ATG7, LC3B and beclin-1 were
upregulated after treatment with G-Rh2 in U937 and K562 cells
(74). The formation of
autophagosomes involves the conversion from cytosolic LC3-I to the
autophagosome-associating form of LC3-II (75). Treatment of U937 and K562 cells with
G-Rh2 was found to induce the conversion from LC3-I to LC3-II and
downregulate the protein level of p62 (74). G-Rh2 treatment increased autophagy
through upregulating autophagy-related proteins Beclin, Atg7 and
the ratio of LC3-II to LC3-I in A431 cells (76). Another study reported that G-Rh2 could
promote cell autophagy in human retinoblastoma cell lines Y79 and
RBL-13 by inactivating the phosphatidylinositol 3-kinase
(PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway (77).
Drug resistance is the leading cause of failure of
cancer chemotherapy. Several studies have suggested that G-Rh2 has
a role in reversing drug resistance and improving treatment
efficacy. G-Rh2 has been found to reverse oxaliplatin resistance in
colon cancer cells through decreasing the expression of
P-glycoprotein (78), and to reverse
drug resistance in Adriamycin-resistant human breast cancer MCF-7
cells (79). Another study reported
that G-Rh2 effectively reversed 5-flurouracil resistance in
colorectal cancer cells by regulating MDR1, MRP1, LRP and GST gene
expression (80). In addition, G-Rh2
could enhance the antitumor effects of SMI-4a by increasing
autophagy in melanoma cells (81) and
could enhance the anticancer effects of cisplatin and prolong the
survival time of nude mice (43).
These reports suggest that G-Rh2 has an adjunctive effect in cancer
chemotherapy and can delay the occurrence of drug resistance.
It is well known that certain cancer treatments can
temporarily weaken the immune system, and that enhancing the immune
response could play an important role in cancer treatment. Ginseng
has a long history of improving the immunity of patients in Asia. A
growing number of studies indicate that G-Rh2 can improve immunity.
One study reported that G-Rh2 enhanced the antitumor immunological
response by triggering CD4+ and CD8a+
T-lymphocyte infiltration in B16-F10 melanoma cells derived from
xenograft tumor tissues, as well as enhancing the cytotoxicity in
spleen lymphocytes (82). Another
study found that G-Rh2 could downregulate CASP1, INSL5 and OR52A1,
and upregulate CLINT1, ST3GAL4 and C1orf198 expression in MCF-7
cells, indicating that G-Rh2 induces epigenetic methylation changes
in genes involved in the immune response and tumorigenesis, thereby
contributing to enhanced immunogenicity and inhibiting the growth
of cancer cells (33). G-Rh2
suppressed T-cell acute lymphoblastic leukemia (T-ALL) by blocking
the PI3K/Akt/mTOR signaling pathway and enhanced immunity in the
spleen by downregulating IL-4, IL-6, IL-10, CD3 and CD45, and
upregulating IL-2 and INF-γ, and increased the number of natural
killer cells (83). These studies
indicate that G-Rh2 has the ability to enhance the immune response,
which may play a role in the prevention and treatment of
cancer.
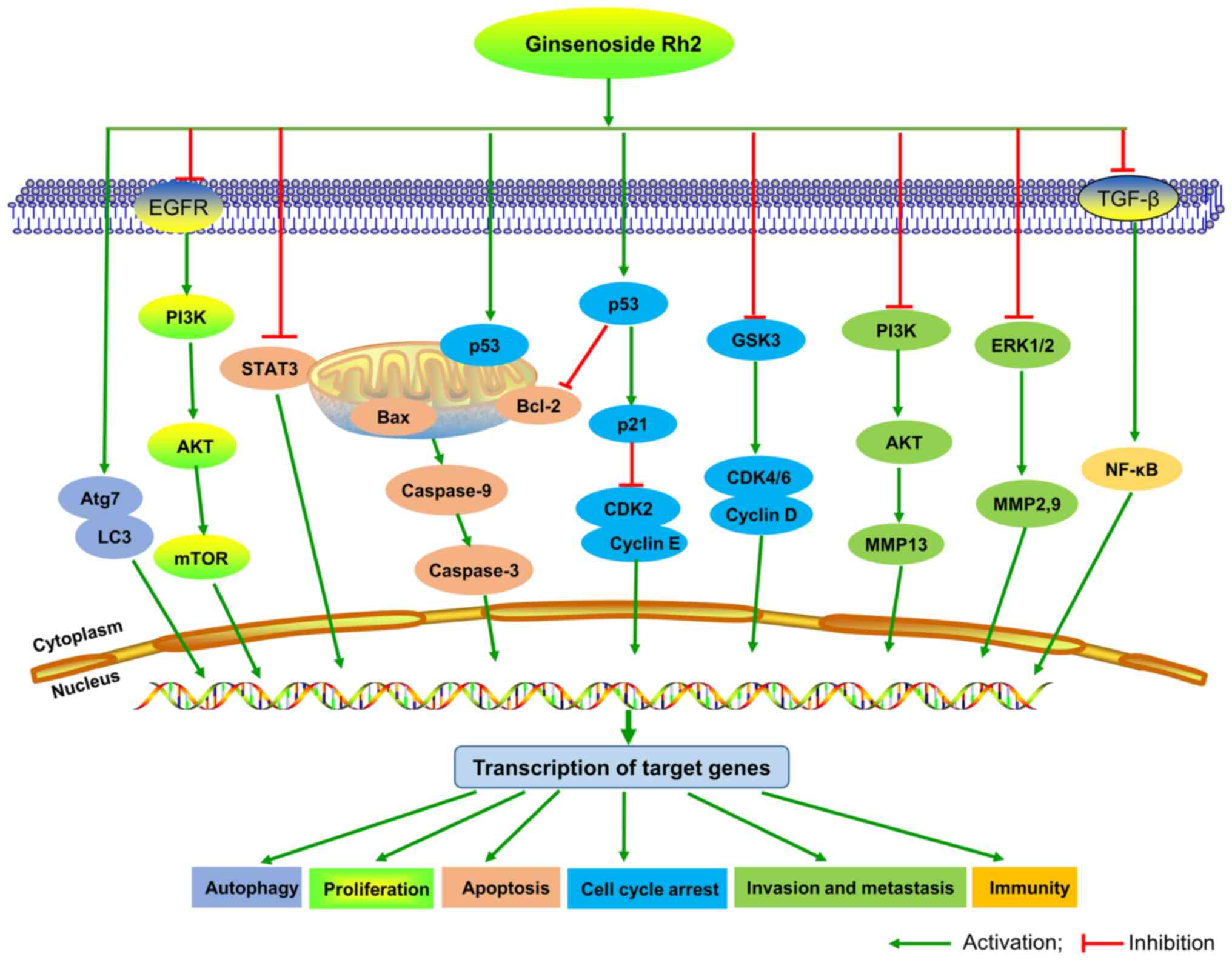
According to current studies, the anticancer
signaling pathway of G-Rh2 remains unclear. It was reported that
G-Rh2 suppresses growth of oral squamous cell carcinoma cells by
decreasing ROS, MMP-2 and VEGF (67).
G-Rh2 inhibits glioma cell growth by targeting microRNA-128
(63) and inhibiting epidermal growth
factor receptor (89). G-Rh2 induces
leukemia cell differentiation and cell cycle arrest by upregulating
TGF-β expression (52) and inducing
apoptosis by inducing the release of mitochondrial cytochrome
c and the activation of caspase-3 and −9 (90). In neuroblastoma cells, G-Rh2 induces
apoptosis via activation of caspase-1 and −3 and upregulation of
Bax (91). G-Rh2 suppresses cancer
cell migration and invasion by downregulating the expression levels
of MMP3 (92) and MMP13 (93), and by regulating CDKN2A-2B gene
cluster transcription (84). G-Rh2
exerts anticancer activity in T-cell acute lymphoblastic leukemia
cells (94), glioblastoma multiforme
cells (95) and osteosarcoma cells
(96) by suppressing the
PI3K/Akt/mTOR signaling pathway. In addition, G-Rh2-induced DNA
damage and autophagy in vestibular schwannoma is dependent on LAMP2
transcriptional suppression (97),
and it improves the cisplatin effect in lung adenocarcinoma A549
cells by repressing superoxide generation and PD-L1 expression
(98). Based on these reports, we
hypothesize that PI3K/Akt/mTOR could be an important signaling
pathway for G-Rh2 to exert its activity, which provides some
context for further research on G-Rh2. The potential signaling
pathways of G-Rh2 in cancer are demonstrated in Fig. 3.
As one of the main active components of ginseng,
G-Rh2 has a wide range of pharmacological effects and plays a
therapeutic role in numerous diseases. A number of studies have
demonstrated that G-Rh2 exerts excellent anticancer activity in
vitro and in vivo. G-Rh2 exerts its anticancer activity
by inducing apoptosis, autophagy, cell cycle arrest and immunity,
as well as by inhibiting proliferation, invasion, metastasis and
angiogenesis. In addition, G-Rh2 in combination with specific
anticancer drugs can overcome drug resistance and enhance the
immune response. In summary, G-Rh2 exerts anticancer effects in
vitro and in vivo, and is a promising agent for cancer
prevention and treatment.
Not applicable.
No funding was received.
Not applicable.
HBZ, SP, HH, EK and JY collected the literature and
designed the study. HBZ drafted the manuscript. SKC, ZYR and MK
revised the manuscript. ZYR and MK confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
All authors declare hat they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shahab D, Gabriel E, Attwood K, Ma WW,
Francescutti V, Nurkin S and Boland PM: Adjuvant chemotherapy is
associated with improved overall survival in locally advanced
rectal cancer after achievement of a pathologic complete response
to chemoradiation. Clin Colorectal Cancer. 16:300–307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujita K, Taneishi K, Inamoto T, Ishizuya
Y, Takada S, Tsujihata M, Tanigawa G, Minato N, Nakazawa S, Takada
T, et al: Adjuvant chemotherapy improves survival of patients with
high-risk upper urinary tract urothelial carcinoma: A propensity
score-matched analysis. BMC Urol. 17:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu JM, Yao Q and Chen C: Ginseng
compounds: An update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou J, Xue J, Lee M, Liu L, Zhang D, Sun
M, Zheng Y and Sung C: Ginsenoside Rh2 improves learning and memory
in mice. J Med Food. 16:772–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian Y, Huang R, Li S, Xie R, Qian B,
Zhang Z, Li L, Wang B, Tian C, Yang J, et al: Ginsenoside Rh2
reverses cyclophosphamide-induced immune deficiency by regulating
fatty acid metabolism. J Leukoc Biol. 106:1089–1100. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi WY, Lim HW and Lim CJ:
Anti-inflammatory, antioxidative and matrix metalloproteinase
inhibitory properties of 20(R)-ginsenoside Rh2 in cultured
macrophages and keratinocytes. J Pharm Pharmacol. 65:310–316. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang XP, Li KR, Yu Q, Yao MD, Ge HM, Li
XM, Jiang Q, Yao J and Cao C: Ginsenoside Rh2 inhibits vascular
endothelial growth factor-induced corneal neovascularization. FASEB
J. 32:3782–3791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Gao Y, Yi X and Ding Y:
Ginsenoside Rh2 suppresses neovascularization in xenograft
psoriasis model. Cell Physiol Biochem. 36:980–987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi K, Kim M, Ryu J and Choi C:
Ginsenosides compound K and Rh(2) inhibit tumor necrosis
factor-alpha-induced activation of the NF-kappaB and JNK pathways
in human astroglial cells. Neurosci Lett. 421:37–41. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bae EA, Kim EJ, Park JS, Kim HS, Ryu JH
and Kim DH: Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1
and protein kinase A pathway in
lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial
cells. Planta Med. 72:627–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Wang H, Liu Y, Li C, Qi P and Bao
J: Antihyperglycemic effect of ginsenoside Rh2 by inducing islet
β-cell regeneration in mice. Horm Metab Res. 44:33–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang YQ, Huang HX, Han ZD, Li W, Mai ZP
and Yuan RQ: Ginsenoside Rh2 Inhibits Angiogenesis in Prostate
Cancer by Targeting CNNM1. J Nanosci Nanotechnol. 19:1942–1950.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tong-Lin Wu T, Tong YC, Chen IH, Niu HS,
Li Y and Cheng JT: Induction of apoptosis in prostate cancer by
ginsenoside Rh2. Oncotarget. 9:11109–11118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi X, Yang J and Wei G: Ginsenoside
20(S)-Rh2 exerts anti-cancer activity through the Akt/GSK3β
signaling pathway in human cervical cancer cells. Mol Med Rep.
17:4811–4816. 2018.PubMed/NCBI
|
|
16
|
Wang YS, Lin Y, Li H, Li Y, Song Z and Jin
YH: The identification of molecular target of (20S) ginsenoside Rh2
for its anti-cancer activity. Sci Rep. 7:124082017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Yuan D, Xing T, Su H, Zhang S, Wen
J, Bai Q and Dang D: Ginsenoside Rh2 inhibiting HCT116 colon cancer
cell proliferation through blocking PDZ-binding kinase/T-LAK
cell-originated protein kinase. J Ginseng Res. 40:400–408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han S, Jeong AJ, Yang H, Bin Kang K, Lee
H, Yi EH, Kim BH, Cho CH, Chung JW, Sung SH and Ye SK: Ginsenoside
20(S)-Rh2 exerts anti-cancer activity through targeting
IL-6-induced JAK2/STAT3 pathway in human colorectal cancer cells. J
Ethnopharmacol. 194:83–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MJ, Yun H, Kim DH, Kang I, Choe W, Kim
SS and Ha J: AMP-activated protein kinase determines apoptotic
sensitivity of cancer cells to ginsenoside-Rh2. J Ginseng Res.
38:16–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang XP, Tang GD, Fang CY, Liang ZH and
Zhang LY: Effects of ginsenoside Rh2 on growth and migration of
pancreatic cancer cells. World J Gastroenterol. 19:1582–1592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li KF, Kang CM, Yin XF, Li HX, Chen ZY, Li
Y, Zhang Q and Qiu YR: Ginsenoside Rh2 inhibits human A172 glioma
cell proliferation and induces cell cycle arrest status via
modulating Akt signaling pathway. Mol Med Rep. 17:3062–3068.
2018.PubMed/NCBI
|
|
22
|
Ge G, Yan Y and Cai H: Ginsenoside Rh2
inhibited proliferation by inducing ROS mediated ER stress
dependent apoptosis in lung cancer cells. Biol Pharma Bull.
40:2117–2124. 2017. View Article : Google Scholar
|
|
23
|
Li H, Huang N, Zhu W, Wu J, Yang X, Teng
W, Tian J, Fang Z, Luo Y, Chen M and Li Y: Modulation the crosstalk
between tumor-associated macrophages and non-small cell lung cancer
to inhibit tumor migration and invasion by ginsenoside Rh2. BMC
Cancer. 18:5792018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An IS, An S, Kwon KJ, Kim YJ and Bae S:
Ginsenoside Rh2 mediates changes in the microRNA expression profile
of human non-small cell lung cancer A549 cells. Oncol Rep.
29:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Zhang Y, Song W, Zhang Y, Dong X
and Tan M: Ginsenoside Rh2 inhibits migration of lung cancer cells
under hypoxia via mir-491. Anticancer Agents Med Chem.
19:1633–1641. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng CC, Yang SM, Huang CY, Chen JC,
Chang WM and Hsu SL: Molecular mechanisms of ginsenoside
Rh2-mediated G1 growth arrest and apoptosis in human lung
adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 55:531–540.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen W, Chu S, Li H and Qiu Y:
MicroRNA-146a-5p enhances ginsenoside Rh2-induced
anti-proliferation and the apoptosis of the human liver cancer cell
line HepG2. Oncol Lett. 16:5367–5374. 2018.PubMed/NCBI
|
|
28
|
Chen W and Qiu Y: Ginsenoside Rh2 Targets
EGFR by up-regulation of miR-491 to enhance anti-tumor activity in
hepatitis B virus-related hepatocellular carcinoma. Cell Biochem
Biophys. 72:325–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian J, Li J, Jia JG, Jin X, Yu DJ, Guo
CX, Xie B and Qian LY: Ginsenoside-Rh2 inhibits proliferation and
induces apoptosis of human gastric cancer SGC-7901 side population
cells. Asian Pac J Cancer Prev. 17:1817–1821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li B, Zhao J, Wang CZ, Searle J, He TC,
Yuan CS and Du W: Ginsenoside Rh2 induces apoptosis and
paraptosis-like cell death in colorectal cancer cells through
activation of p53. Cancer Lett. 301:185–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu C, Liu F, Qian W, Zhang T and Li F:
Combined effect of sodium selenite and Ginsenoside Rh2 on hct116
human colorectal carcinoma cells. Arch Iran Med. 19:23–29.
2016.PubMed/NCBI
|
|
32
|
Oh M, Choi YH, Choi S, Chung H, Kim K, Kim
SI, Kim DK and Kim ND: Anti-proliferating effects of ginsenoside
Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 14:869–875.
1999.PubMed/NCBI
|
|
33
|
Lee H, Lee S, Jeong D and Kim SJ:
Ginsenoside Rh2 epigenetically regulates cell-mediated immune
pathway to inhibit proliferation of MCF-7 breast cancer cells. J
Ginseng Res. 42:455–462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi S, Kim TW and Singh SV: Ginsenoside
Rh2-mediated G1 phase cell cycle arrest in human breast cancer
cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of
cyclin-dependent kinases. Pharm Res. 26:2280–2288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Shimizu K, Yu H, Zhang C, Jin F and
Kondo R: Stereospecificity of hydroxyl group at C-20 in
antiproliferative action of ginsenoside Rh2 on prostate cancer
cells. Fitoterapia. 81:902–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Q, Hong B, Wu S and Niu T:
Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour
Biol. 36:2377–2381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao Q and Zheng J: Ginsenoside Rh2
inhibits prostate cancer cell growth through suppression of
microRNA-4295 that activates CDKN1A. Cell Prolif. 51:e124382018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia T, Wang YN, Zhou CX, Wu LM, Liu Y,
Zeng QH, Zhang XL, Yao JH, Wang M and Fang JP: Ginsenoside Rh2 and
Rg3 inhibit cell proliferation and induce apoptosis by increasing
mitochondrial reactive oxygen species in human leukemia Jurkat
cells. Mol Med Rep. 15:3591–3598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia T, Wang J, Wang Y, Wang Y, Cai J, Wang
M, Chen Q, Song J, Yu Z, Huang W and Fang J: Inhibition of
autophagy potentiates anticancer property of 20(S)-ginsenoside Rh2
by promoting mitochondria-dependent apoptosis in human acute
lymphoblastic leukaemia cells. Oncotarget. 7:27336–27349. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang J, Peng K, Wang L, Wen B, Zhou L,
Luo T, Su M, Li J and Luo Z: Ginsenoside Rh2 inhibits proliferation
and induces apoptosis in human leukemia cells via TNF-alpha
signaling pathway. Acta Biochim Biophys Sin (Shanghai). 48:750–755.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Y, Liu ZH, Xia J, Li XP, Li KQ, Xiong
W, Li J and Chen DL: 20(S)-ginsenoside Rh2 inhibits the
proliferation and induces the apoptosis of KG-1a cells through the
Wnt/β-catenin signaling pathway. Oncol Rep. 36:137–146. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakata H, Kikuchi Y, Tode T, Hirata J,
Kita T, Ishii K, Kudoh K, Nagata I and Shinomiya N: Inhibitory
effects of ginsenoside Rh2 on tumor growth in nude mice bearing
human ovarian cancer cells. Jpn J Cancer Res. 89:733–740. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tode T, Kikuchi Y, Kita T, Hirata J,
Imaizumi E and Nagata I: Inhibitory effects by oral administration
of ginsenoside Rh2 on the growth of human ovarian cancer cells in
nude mice. J Cancer Res Clin Oncol. 120:24–26. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li N, Lin Z, Chen W, Zheng Y, Ming Y,
Zheng Z, Huang W, Chen L, Xiao J and Lin H: Corilagin from longan
seed: Identification, quantification, and synergistic cytotoxicity
on SKOv3ip and hey cells with ginsenoside Rh2 and 5-fluorouracil.
Food Chem Toxicol. 119:133–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim JH and Choi JS: Effect of ginsenoside
Rh-2 via activation of caspase-3 and Bcl-2-insensitive pathway in
ovarian cancer cells. Physiol Res. 65:1031–1037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang C, Yu H and Hou J: Effects of 20
(S)-ginsenoside Rh2 and 20 (R)-ginsenoside Rh2 on proliferation and
apoptosis of human lung adenocarcinoma A549 cells. Zhongguo Zhong
Yao Za Zhi. 36:1670–1674. 2011.(In Chinese). PubMed/NCBI
|
|
47
|
Dong H, Bai LP, Wong VK, Zhou H, Wang JR,
Liu Y, Jiang ZH and Liu L: The in vitro structure-related
anti-cancer activity of ginsenosides and their derivatives.
Molecules. 16:10619–10630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Harashima H, Dissmeyer N and Schnittger A:
Cell cycle control across the eukaryotic kingdom. Trends Cell Biol.
23:345–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Roskoski R Jr: Cyclin-dependent protein
serine/threonine kinase inhibitors as anticancer drugs. Pharmacol
Res. 139:471–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu X, Sun Y, Yue L, Li S, Qi X, Zhao H,
Yang Y, Zhang C and Yu H: JNK pathway and relative transcriptional
factor were involved in ginsenoside Rh2-mediated G1 growth arrest
and apoptosis in human lung adenocarcinoma A549 cells. Genet Mol
Res. 15:2016. View Article : Google Scholar
|
|
52
|
Chung KS, Cho SH, Shin JS, Kim DH, Choi
JH, Choi SY, Rhee YK, Hong HD and Lee KT: Ginsenoside Rh2 induces
cell cycle arrest and differentiation in human leukemia cells by
upregulating TGF-β expression. Carcinogenesis. 34:331–340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Plati J, Bucur O and Khosravi-Far R:
Dysregulation of apoptotic signaling in cancer: Molecular
mechanisms and therapeutic opportunities. J Cell Biochem.
104:1124–1149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guo XX, Li Y, Sun C, Jiang D, Lin YJ, Jin
FX, Lee SK and Jin YH: p53-dependent Fas expression is critical for
Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells.
Protein Cell. 5:224–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang X and Wang Y: Ginsenoside Rh2
mitigates pediatric leukemia through suppression of Bcl-2 in
leukemia cells. Cell Physiol Biochem. 37:641–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Q, Li Y, Wang X, Fang X, He K, Guo X,
Zhan Z, Sun C and Jin YH: Co-treatment with ginsenoside Rh2 and
betulinic acid synergistically induces apoptosis in human cancer
cells in association with enhanced capsase-8 activation, bax
translocation, and cytochrome c release. Mol Carcinog. 50:760–769.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu ZH, Li J, Xia J, Jiang R, Zuo GW, Li
XP, Chen Y, Xiong W and Chen DL: Ginsenoside 20(s)-Rh2 as potent
natural histone deacetylase inhibitors suppressing the growth of
human leukemia cells. Chem Biol Interact. 242:227–234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang H, Yi J, Kim E, Choo Y, Hai H, Kim
K, Kim EK, Ryoo Z and Kim M: 20(S)-Ginsenoside Rh2 suppresses oral
cancer cell growth by inhibiting the Src-Raf-ERK signaling pathway.
Anticancer Res. 41:227–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu N, Wu GC, Hu R, Li M and Feng H:
Ginsenoside Rh2 inhibits glioma cell proliferation by targeting
microRNA-128. Acta Pharmacol Sin. 32:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ishay-Ronen D, Diepenbruck M, Kalathur
RKR, Sugiyama N, Tiede S, Ivanek R, Bantug G, Morini MF, Wang J,
Hess C and Christofori G: Gain fat-lose metastasis: Converting
invasive breast cancer cells into adipocytes inhibits cancer
metastasis. Cancer Cell. 35:17–32.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim JH, Kim M, Yun SM, Lee S, No JH, Suh
DH, Kim K and Kim YB: Ginsenoside Rh2 induces apoptosis and
inhibits epithelial-mesenchymal transition in HEC1A and Ishikawa
endometrial cancer cells. Biomed Pharmacother. 96:871–876. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang BP, Li B, Cheng JY, Cao R, Gao ST,
Huang CJ, Li RP, Ning J, Liu B and Li ZG: Anti-cancer Effect of
20(S)-Ginsenoside-Rh2 on oral squamous cell carcinoma cells via the
decrease in ROS and downregulation of MMP-2 and VEGF. Biomed
Environ Sci. 33:713–717. 2020.PubMed/NCBI
|
|
68
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Goumans MJ, Liu Z and ten Dijke P:
TGF-beta signaling in vascular biology and dysfunction. Cell Res.
19:116–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Q, Wu MQ, Zhao LH, Yang HK and Lv XH:
Effect of ginsenoside Rh2 on transplanted-tumor and expression of
JAM in mice. Zhongguo Zhong Yao Za Zhi. 33:2116–2119. 2008.(In
Chinese). PubMed/NCBI
|
|
72
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ryan KM: p53 and autophagy in cancer:
Guardian of the genome meets guardian of the proteome. Eur J
Cancer. 47:44–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhuang J, Yin J, Xu C, Mu Y and Lv S:
20(S)-Ginsenoside Rh2 Induce the Apoptosis and Autophagy in U937
and K562 Cells. Nutrients. 10:3282018. View Article : Google Scholar
|
|
75
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu S, Chen M, Li P, Wu Y, Chang C, Qiu Y,
Cao L, Liu Z and Jia C: Ginsenoside rh2 inhibits cancer stem-like
cells in skin squamous cell carcinoma. Cell Physiol Biochem.
36:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li M, Zhang D, Cheng J, Liang J and Yu F:
Ginsenoside Rh2 inhibits proliferation but promotes apoptosis and
autophagy by down-regulating microRNA-638 in human retinoblastoma
cells. Exp Mol Pathol. 108:17–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ma J, Gao G, Lu H, Fang D, Li L, Wei G,
Chen A, Yang Y, Zhang H and Huo J: Reversal effect of ginsenoside
Rh2 on oxaliplatin-resistant colon cancer cells and its mechanism.
Exp Ther Med. 18:630–636. 2019.PubMed/NCBI
|
|
79
|
Zhou B, Xiao X, Xu L, Zhu L, Tan L, Tang
H, Zhang Y, Xie Q and Yao S: A dynamic study on reversal of
multidrug resistance by ginsenoside Rh(2) in adriamycin-resistant
human breast cancer MCF-7 cells. Talanta. 88:345–351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu GW, Liu YH, Jiang GS and Ren WD: The
reversal effect of Ginsenoside Rh2 on drug resistance in human
colorectal carcinoma cells and its mechanism. Hum Cell. 31:189–198.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lv DL, Chen L, Ding W, Zhang W, Wang HL,
Wang S and Liu WB: Ginsenoside G-Rh2 synergizes with SMI-4a in
anti-melanoma activity through autophagic cell death. Chin Med.
13:112018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang M, Yan SJ, Zhang HT, Li N, Liu T,
Zhang YL, Li XX, Ma Q, Qiu XC, Fan QY and Ma BA: Ginsenoside Rh2
enhances the antitumor immunological response of a melanoma mice
model. Oncol Lett. 13:681–685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xia T, Zhang B, Li Y, Fang B, Zhu XX, Xu
BC, Zhang J, Wang M and Fang JP: New insight into 20(S)-ginsenoside
Rh2 against T-cell acute lymphoblastic leukemia associated with the
gut microbiota and the immune system. Eur J Med Chem.
203:1125392020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li Q, Li B, Dong C, Wang Y and Li Q:
20(S)-Ginsenoside Rh2 suppresses proliferation and migration of
hepatocellular carcinoma cells by targeting EZH2 to regulate
CDKN2A-2B gene cluster transcription. Eur J Pharmacol. 815:173–180.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shi Q, Shi X, Zuo G, Xiong W, Li H, Guo P,
Wang F, Chen Y, Li J and Chen DL: Anticancer effect of
20(S)-ginsenoside Rh2 on HepG2 liver carcinoma cells: Activating
GSK-3β and degrading β-catenin. Oncol Rep. 36:2059–2070. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qian T, Cai Z, Wong RN and Jiang ZH:
Liquid chromatography/mass spectrometric analysis of rat samples
for in vivo metabolism and pharmacokinetic studies of ginsenoside
Rh2. Rapid Commun Mass Spectrom. 19:3549–3554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Qi Z, Chen L, Li Z, Shao Z, Qi Y, Gao K,
Liu S, Sun Y, Li P and Liu J: Immunomodulatory effects of
(24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on
cyclophosphamide-induced immunosuppression and their anti-tumor
effects study. Int J Mol Sci. 20:8362019. View Article : Google Scholar
|
|
88
|
Gu Y, Wang GJ, Sun JG, Jia YW, Wang W, Xu
MJ, Lv T, Zheng YT and Sai Y: Pharmacokinetic characterization of
ginsenoside Rh2, an anticancer nutrient from ginseng, in rats and
dogs. Food Chem Toxicol. 47:2257–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li S, Gao Y, Ma W, Guo W, Zhou G, Cheng T
and Liu Y: EGFR signaling-dependent inhibition of glioblastoma
growth by ginsenoside Rh2. Tumour Biol. 35:5593–5598. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xia T, Wang JC, Xu W, Xu LH, Lao CH, Ye QX
and Fang JP: 20S-Ginsenoside Rh2 induces apoptosis in human
Leukaemia Reh cells through mitochondrial signaling pathways. Biol
Pharm Bull. 37:248–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim YS and Jin SH: Ginsenoside Rh2 induces
apoptosis via activation of caspase-1 and −3 and up-regulation of
Bax in human neuroblastoma. Arch Pharm Res. 27:834–839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shi Q, Li J, Feng Z, Zhao L, Luo L, You Z,
Li D, Xia J, Zuo G and Chen D: Effect of ginsenoside Rh2 on the
migratory ability of HepG2 liver carcinoma cells: Recruiting
histone deacetylase and inhibiting activator protein 1
transcription factors. Mol Med Rep. 10:1779–1785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Guan N, Huo X, Zhang Z, Zhang S, Luo J and
Guo W: Ginsenoside Rh2 inhibits metastasis of glioblastoma
multiforme through Akt-regulated MMP13. Tumour Biol. 36:6789–6795.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xia T, Zhang J, Zhou C, Li Y, Duan W,
Zhang B, Wang M and Fang J: 20(S)-Ginsenoside Rh2 displays efficacy
against T-cell acute lymphoblastic leukemia through the
PI3K/Akt/mTOR signal pathway. J Ginseng Res. 44:725–737. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li S, Guo W, Gao Y and Liu Y: Ginsenoside
Rh2 inhibits growth of glioblastoma multiforme through mTor. Tumour
Biol. 36:2607–2612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li C, Gao H, Feng X, Bi C, Zhang J and Yin
J: Ginsenoside Rh2 impedes proliferation and migration and induces
apoptosis by regulating NF-κB, MAPK, and PI3K/Akt/mTOR signaling
pathways in osteosarcoma cells. J Biochem Mol Toxicol.
34:e225972020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang D, Li X and Zhang X: Ginsenoside Rh2
induces DNA damage and autophagy in vestibular schwannoma is
dependent of LAMP2 transcriptional suppression. Biochem Biophys Res
Commun. 522:300–307. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen Y, Zhang Y, Song W, Zhang Y, Dong X
and Tan M: Ginsenoside Rh2 improves the cisplatin anti-tumor effect
in lung adenocarcinoma A549 cells via superoxide and PD-L1.
Anticancer Agents Med Chem. 20:495–503. 2020. View Article : Google Scholar : PubMed/NCBI
|