Gastric cancer is the third leading cause of
cancer-related mortality worldwide (1). The incidence of gastric cancer is lowest
in Northern Europe and Northern America, and remains highest in
Eastern and Central Asia and Latin America (1–3). In all
confirmed cases of gastric cancer, >1/3 of cases occur in China.
Moreover, the incidence and mortality rates of gastric cancer in
China rank second amongst all cancer types and are second only to
lung cancer (4). The age-standardised
5-year survival rate of gastric cancer in the Chinese population
was only 35.9% in 2010–2014 (5).
Although immune checkpoint blocking therapy can improve the
survival rate of some patients with gastric cancer (6), not all patients can benefit from this
immunotherapy. Numerous patients with gastric cancer have problems
that should be addressed, such as hyperprogressive diseases
(7–12), low efficiency of a single drug
(13–15) and treatment-related adverse events
(TRAEs) (16–26).
For example, immune checkpoint inhibitors cause
imbalances in immunological tolerance, resulting in inflammatory
side effects which are called immune-related adverse events
(irAEs). Masuda et al reported that the development of irAEs
was closely associated with clinical responses of patients with
advanced gastric cancer in nivolumab monotherapy (27). Park et al revealed that irAEs
may predict overall survival (OS) as well as progression-free
survival (PFS) and represent meaningful biomarkers across different
types of cancer including gastric cancer (28).
All of these issues aforementioned may be associated
with the complex regulation of the tumour immune microenvironment,
as the immune contexture can convey important information
associated with prognosis and therapeutic responsiveness (29–35). The
tumour immune microenvironment is composed of innate immune cells,
adaptive immune cells and cytokines (CKs), amongst others. These
immune components form a complex regulatory network. Neutrophils
secrete tumour-promoting factors (36), while T cells and NK cells secrete
antitumour factors (37,38). Moreover, regulatory T cells (Tregs),
regulatory B cells and myeloid-derived suppressor cells (MDSCs)
secrete immunosuppressive cytokines (CKs) (37). It has been revealed that macrophages
secrete antitumour factors and tumour-promoting factors, depending
on their state of differentiation (39,40).
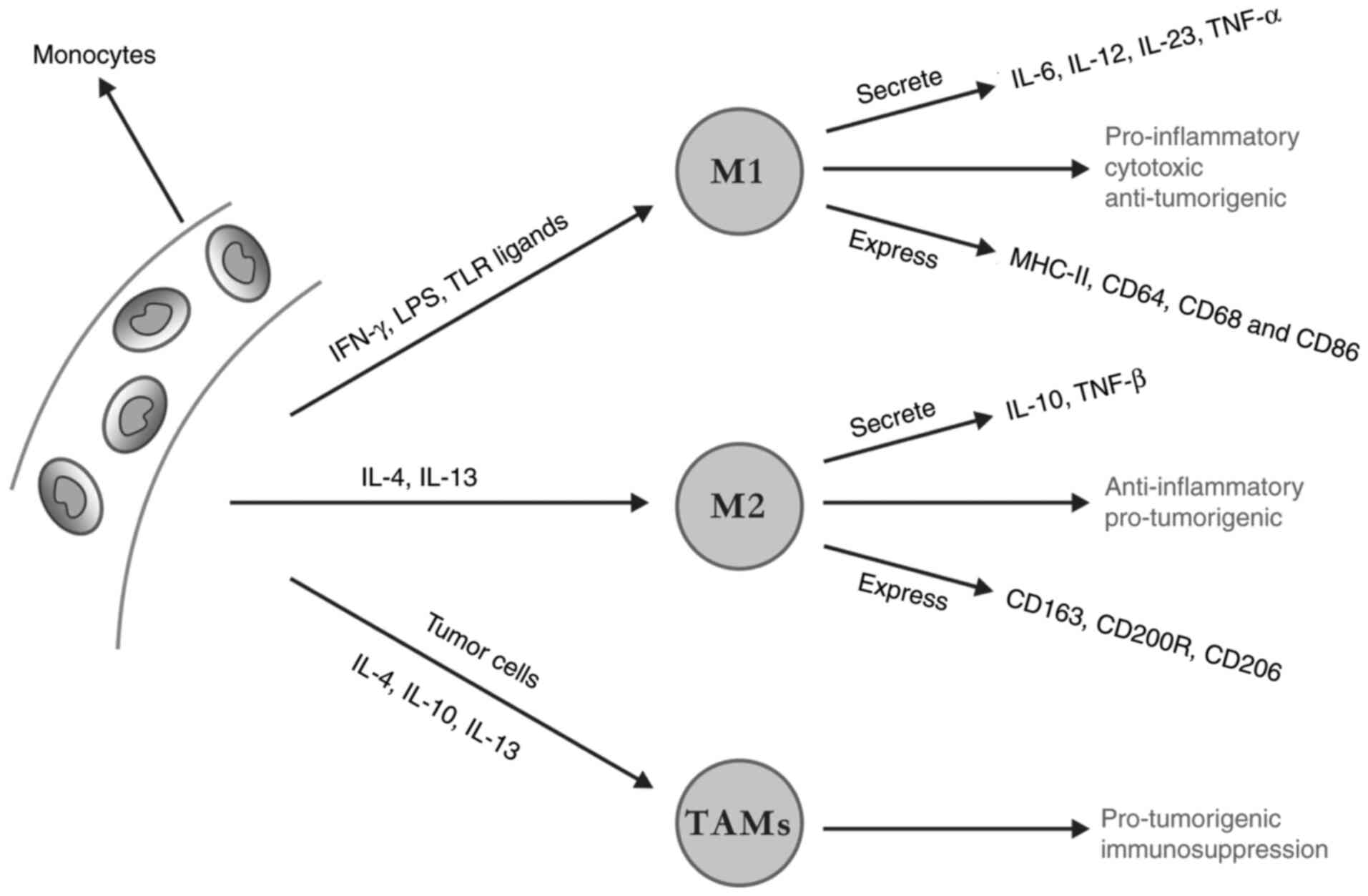
M1-type macrophages are activated by interferon-γ
(IFN-γ), lipopolysaccharide (LPS) and Toll-like receptor (TLR)
ligands. These macrophages can secrete CKs, such as IL-6, IL-12,
IL-23 and TNF-α, and these CKs exert pro-inflammatory, cytotoxic
and antitumour effects (46,47). On the other hand, M2-type macrophages
are activated by IL-4 and IL-13. These macrophages secrete CKs,
such as IL-10 and transforming growth factor-β (TGF-β), which
possess anti-inflammatory and tumour-promoting effects (47,48). With
regard to phenotype, M1-type macrophages highly express CD64, CD68,
CD86 and major histocompatibility complex (MHC) 2 (46,49), while
M2-type macrophages lowly express MHC2 and feature the expression
of CD163, CD200 receptor (CD200R) and CD206 (46,49).
Under the action of IL-4, IL-10 and IL-13,
macrophages can be recruited around tumour cells and eventually
differentiated into TAMs (Fig. 1).
Macrophages co-cultured with gastric cancer cells likely
differentiate into M2-type TAMs (54). M2-type TAMs have evident
immunosuppressive effects on diffuse-type and genomically
stable-type gastric cancer (55).
Furthermore, M2-type TAMs can promote peritoneal metastasis of
gastric cancer via the epidermal growth factor receptor signalling
pathway in the abdominal cavity of gastric cancer patients with
peritoneal metastasis (56).
A major feature of the tumour microenvironment of
gastric cancer is the chronic inflammation caused by
Helicobacter pylori (Hp) infection. This feature is the
classic determinant of gastric cancer (57). It has been revealed that Hp can damage
the immune response of M1-type macrophages and lead to the
differentiation of M2-like macrophages, thereby promoting reactive
oxygen species-induced macrophage apoptosis (58). Another major feature of the tumour
microenvironment of gastric cancer is hypoxia. For one thing,
macrophages and hypoxia serve an important role in regulating the
invasive ability of gastric cancer cells in vitro (59). For another thing, hypoxia decreases
the percentage of M1-type macrophages by targeting microRNA
(miR)-30c and mTOR in human gastric cancer (60). Furthermore, the upregulation of
endothelin-2 and vascular endothelial growth factor (VEGF) can
mediate the accumulation of TAMs in gastric cancer in hypoxic
areas, ultimately promoting the differentiation of M1-type
macrophages into M2-type macrophages (50).
When gastric cancer cells are stimulated by a
hypoxic environment, macrophages can be recruited in the tumour
microenvironment of gastric cancer and are differentiated into TAMs
(61). On the one hand, TAMs can
promote the activation of the hypoxia-related signalling pathways
and increase the activity of matrix metalloproteinases (50,61).
Moreover, TAMs facilitate the formation of microvessels in gastric
cancer (50,61). On the other hand, the expression of
vasohibin-1 tissue is significantly and positively correlated with
the expression of VEGF-A in gastric cancer. TAMs can upregulate
vasohibin-1 to promote angiogenesis in gastric cancer (62). In addition, thymidine phosphorylase
expressed by TAMs can promote angiogenesis in gastric cancer
(63).
M2-type TAMs serve an important role in the
angiogenesis of gastric cancer. M2-type macrophage culture medium
treated with high-mobility group protein B1 (HMGB1) can promote the
angiogenesis of human gastric cancer MKN-45 cell line in
vitro. It has also been revealed that CD163+ TAMs in
gastric cancer are associated with the increased density of
microvessels in cancer nests, tumour stroma and tumour invasive
margins, indicating that M2-type TAMs can promote angiogenesis in
gastric cancer (52).
Invasion and metastasis are important causes of poor
prognosis of patients with gastric cancer (64–73). These
processes represent a multistep biological cascade that leads to
widespread dissemination of gastric cancer cells in various tissues
(74). TAMs can induce the expression
of transcription factor forkhead box Q1 (FOXQ1) (75) and TGF-β1 (76) to promote the epithelial-mesenchymal
transition (EMT), invasion and metastasis of gastric cancer cells.
The coexistence of TAMs and TGF-β is associated with tumour
aggressiveness, which can be an independent prognostic factor for
gastric cancer (50). Moreover, the
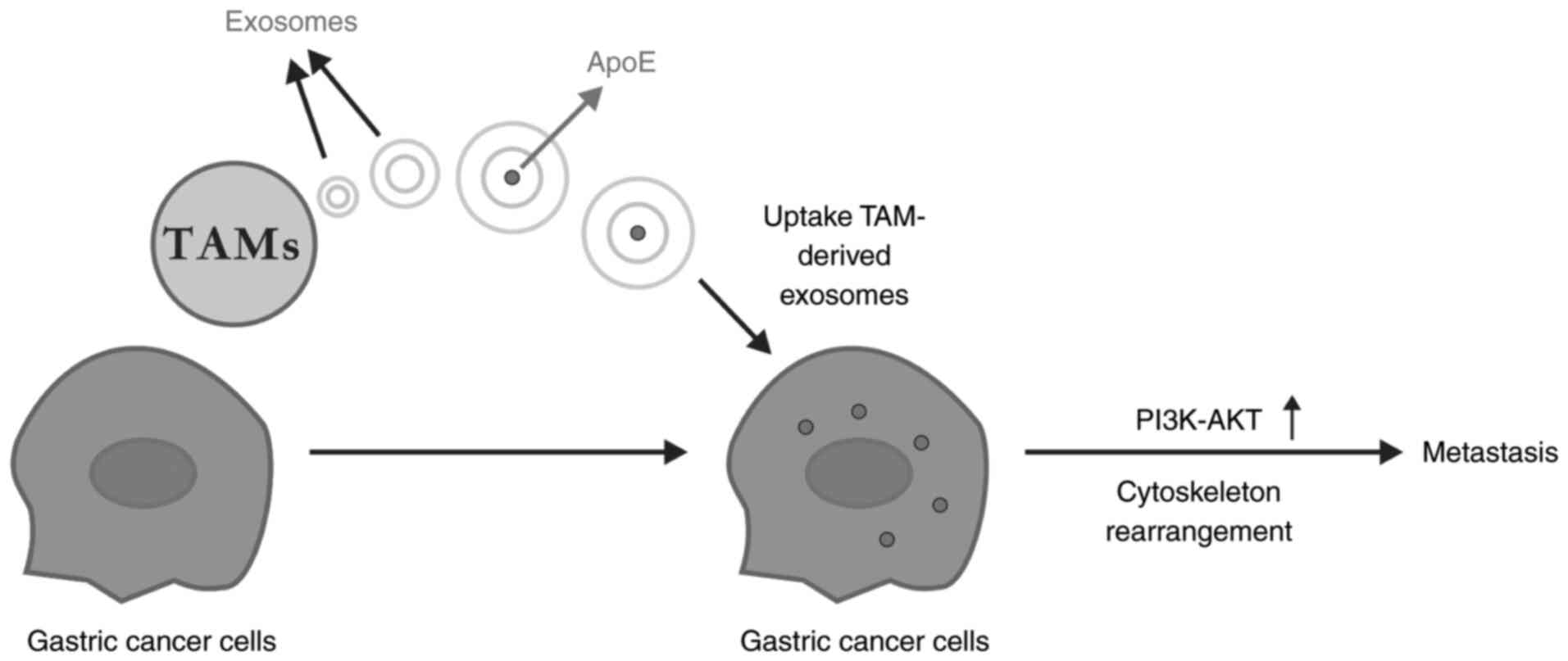
cytoskeleton rearrangement during EMT is an important mechanism of
tumour invasion and metastasis. TAM-derived exosomes can activate
the PI3K/AKT signalling pathway, thereby mediating the transfer of
apolipoprotein E from TAMs to gastric cancer cells, and ultimately
induce the cytoskeletal rearrangement and metastasis of gastric
cancer (77,78) (Fig. 2).
In addition, the expression of chemokine CXCL12 is closely
associated with the recruitment of M2-type TAMs in tumour invasive
margins. It has been suggested that CXCL12 may be involved in the
invasion of gastric cancer (52).
C-X-C Motif Chemokine Ligand 8 (CXCL8), ADAM metallopeptidase
domain (ADAM) 8, ADAM9, C-C motif chemokine ligand (CCL) 5,
secreted phosphoprotein 1, semaphorin 4D, TIMP metallopeptidase
inhibitor 3, T-cell immunoglobulin mucin family member 3 (Tim-3)
and Vinculin-2 are also indicated to be involved in the invasion
and metastasis of gastric cancer cells caused by TAMs (79–82).
TAMs can spread through the lymphatic vessels of
patients with gastric cancer, thus promoting the invasion and
metastasis of gastric cancer cells (83). The interaction between lymph
node-derived lymphatic endothelial cells and TAMs in gastric cancer
may be an important initial step in the progression of
lymphangiogenesis to lymph node metastasis (54). HMGB1 is also associated with the lymph
node metastasis of gastric cancer. HMGB1 can activate the receptor
for advanced glycosylation end products to increase the
tumour-promoting activity of M2-type macrophages and enhance the
invasive ability of the co-cultured human gastric cancer MKN-45
cells (84).
Cisplatin is a commonly used drug for the treatment
of advanced gastric cancer. However, long-term medication can
result in resistance. In addition to increased drug efflux and
enhanced anti-apoptotic effects caused by genetic changes in tumour
cells, the protection of the tumour microenvironment on tumour
cells can lead to drug resistance. Considering that the
overexpression of miR-21 has no effect on the ATP-binding cassette
transporter gene of gastric cancer cells in the tumour
microenvironment, TAM-derived exosomes can transport miR-21 from
M2-type TAMs to gastric cancer cells. This extracellular transport
can downregulate PTEN and enhance the activity of AKT, thereby
increasing the survival rate of gastric cancer cells (85). Thus, targeted therapy of miR-21
extracellular transport caused by TAM-derived exosomes may improve
the resistance of patients with gastric cancer to cisplatin.
Programmed death protein 1 (PD-1) and its two
ligands programmed death-ligand 1 (PD-L1) as well as programmed
death-ligand 2 (PD-L2) serve as an immune checkpoint axis which can
suppress T-cell proliferation in carcinoma (86,87). While
the prognosis of gastric cancer remains poor, PD-1 and PD-L1/PD-L2
are promising prognostic biomarkers (88).
PD-1/PD-L1 signalling pathway has become the hot
spot of current immunotherapies for gastric cancer. D'Ignazio et
al observed a higher number of CD68+ macrophages
with a lower number of CD163+ macrophages and the
inhibition of the PD-1/PD-L1 in gastric and colorectal patients
treated with enteral immunonutrition (89). Consequently, there is an intricate
relationship between macrophages and the PD-1/PD-L1 signalling
pathway during the progression and treatment of gastric cancer.
PD-1 is one of the best-studied and most clinically
successful immune checkpoint drug targets. Kono et al
performed double immunohistochemical staining of PD-1 and CD68 in
gastric cancer tissue and found numerous
PD-1+CD68− tumour infiltrating cells
(90). They also determined the
frequency of PD-1+ macrophages in gastric cancer tissue
by flow cytometry. Flow cytometric analysis revealed that
PD-1+ macrophages in gastric cancer express more CD206,
indicating that these PD-1+ macrophages exhibited an
M2-type profile. Similarly, Wang et al revealed that
PD-1+ TAMs express an M2-type surface molecule, such as
a significant increase in the expression of CD206, and a clear
decrease in the expression of an M1-type surface molecule including
CD64 (91).
PD-L1 is a key protein upregulated by tumour cells
to suppress the immune response. PD-L1+ TAMs were
revealed to account for approximately 50% of all PD-L1+
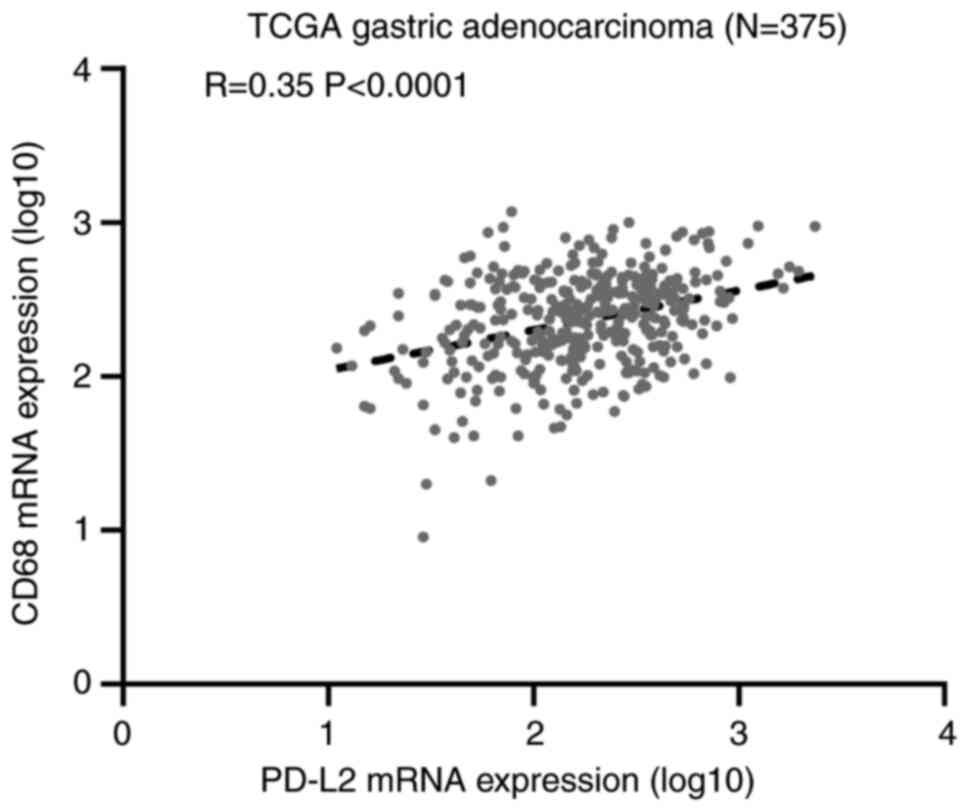
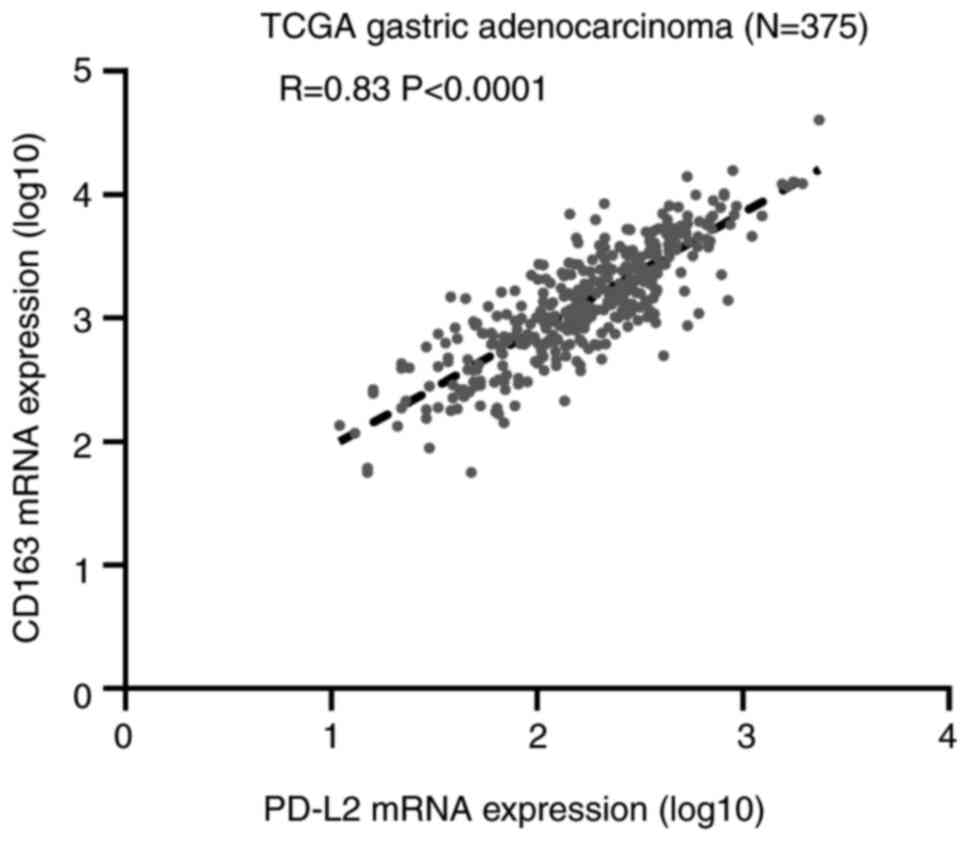
cells in gastric cancer (92). Harada
et al performed immunohistochemical staining of PD-L1, CD68
and CD163 in 217 gastric adenocarcinoma tissue specimens from the
tissue microarrays (93). These
authors observed that M2-type TAMs could promote the expression of
PD-L1 in gastric cancer cells. Moreover, the expression of PD-L1 in
gastric adenocarcinoma cells was examined, and a high density of
CD68+ cells and CD163+ cells was identified
(CD68, P=0.0002; CD163, P<0.0001; the P-value indicated that the
correlation between the expression of PD-L1 and CD163 was closer).
In addition, Huang et al also identified CD206+
macrophages to be most relevant to high PD-L1 expression (92).
To summarize, both PD-1 and PD-L1 are markedly more
closely associated with M2-type TAMs in gastric cancer. Targeting
M2-type TAMs may represent an effective approach to modulate the
activity of anti-PD-1/PD-L1 agents and combined M2-type
TAM-centered strategies should be developed to maximize the
efficacy of anti-PD-1/PD-L1 agents in gastric cancer.
TAMs express PD-1 at a significantly higher level
compared with that in the surrounding healthy tissues. Wang et
al provided a new insight into possible manipulation of
PD-1+ TAM-mediated immunosuppression in gastric cancer
(91). These authors reported that
TAMs from patients with gastric cancer shared markedly increased
PD-1 levels, which promoted tumour progression by impairing the
antitumour functions of CD8+ T cells. Moreover,
PD-1+ TAMs possessed stronger immunosuppressive activity
of CD8+ T-cell function compared with PD-1−
TAMs. When PD-1+ TAMs interacted with PD-L1+
cells, IL-10 was produced in large quantities to induce the
dysfunction of CD8+ T cells and impaired the antitumour
immune response. These results indicated that PD-1 signal
immunotherapies may function through a direct effect on
PD-1+ TAMs.
Previous studies have addressed the important role
of lipids in immune cells, including myeloid-derived suppressor
cells and dendritic cells (96–98). Luo
et al provided evidence that lipid accumulation also
presents in TAMs (99). They
demonstrated that the effect of lipid accumulation conferred the
M2-type polarization of TAMs in gastric cancer. On the one hand,
lipid-accumulated TAMs in gastric cancer reduced phagocytic potency
against tumour cells. On the other hand, lipid-accumulated TAM
upregulated PD-L1 expression, which blocks antitumour T-cell
responses to support their immunosuppressive functions. There is an
abundance of lipids in the tumour microenvironment of gastric
cancer that can be acquired by TAMs. Increased serum lipid levels
are present in patients with gastric cancer and favour tumour
progression. Thus, exploring the mechanisms of lipid-laid TAMs
holds potential for the development of therapeutic interventions in
gastric cancer. Moreover, these authors also revealed that the
PI3-kinase-γ (PI3K-γ) signalling pathway may contribute to the
intrinsic lipid generation in TAMs in the murine gastric cancer
cell line MFC, and the reduced lipid accumulation in TAMs may be
due to the dominant M1-type TAMs after PI3K-γ inhibitor treatment.
To sum up, targeting of PI3K-γ signalling pathways in TAMs may
provide a novel potential approach to improve the long-term
survival of patients with gastric cancer.
DC-SIGN is one of the most widely researched C-type
lectin receptors, and these are mainly expressed on certain
macrophages and dendritic cells. Liu et al identified that
DC-SIGN+ TAMs were highly infiltrated in patients with
gastric cancer and this high infiltration of DC-SIGN+
TAMs was closely associated with a higher ratio of
Foxp3+ Tregs/CD8+ T cells (100). These CD8+ T cells in the
high DC-SIGN+ TAMs subgroup failed to exert antitumour
immunity. There were decreased expression levels of IFN-γ, granzyme
B and perforin, as well as increased expression levels of PD-1 and
CTLA-4 in the tumour microenvironment of gastric cancer, suggesting
that DC-SIGN+ TAMs in gastric cancer could promote an
immunoevasive tumour microenvironment. Conclusively,
DC-SIGN+ TAMs may be independent prognosticators for
gastric cancer and could improve the therapeutic strategy of
fluorouracil-based adjuvant chemotherapy and immune checkpoint
inhibitors.
The percentage of NK cells in tumour tissue is
significantly decreased in advanced gastric cancer, and this low
percentage of NK cells positively correlates with poor OS of
patients with gastric cancer. Peng et al investigated the
relationship between macrophages and NK cells in tumour tissue from
patients with gastric cancer, and their results demonstrated a role
for TAMs in NK-cell functional impairment (101). On the one hand, TAMs in gastric
cancer suppressed the expression of Ki-67, IFN-γ and TNF-α in NK
cells. On the other hand, TAMs in gastric cancer isolated from
tumour tissue produced higher TGFβ1 (a known inhibitor of NK cell
function) compared with those from non-tumour tissues, and flow
cytometric analysis revealed that TGFβ1 was absent on the surface
of TAMs in gastric cancer, suggesting that TAMs in gastric cancer
may secrete TGFβ1 to mediate NK-cell functional impairment. To
further confirm this hypothesis, an antibody against TGFβ1 was
added to the coculture system of TAMs in gastric cancer and NK
cells. Eventually, these authors demonstrated that TGFβ1 blockade
subsequently attenuated TAM-mediated suppression of Ki-67, IFN-γ
and TNF-α expression in NK cells. In conclusion, blockade of TGFβ1
could restore the function of NK cells and could be a useful
therapeutic strategy for patients with gastric cancer.
The treatment of gastric cancer involves surgical
resection, chemotherapy, radiation therapy and immunotherapy
(102). The current overall
treatment strategy for gastric cancer is a comprehensive treatment
based on surgery. Furthermore, radical gastrectomy is the only
radical treatment for gastric cancer. Although various therapies
have developed in recent years, the mortality rate of gastric
cancer remains high as the early stage of this cancer type is
asymptomatic (103). Thus,
traditional treatments must be improved, and novel treatment
regimens should be developed.
The most significant signalling pathway associated
with TAM recruitment and proliferation is CSF-1/CSF-1 receptor
(CSF-1R), vital to the transition from M1-type TAM into M2-type TAM
(50). The anti-CSF-1/CSF-1R
signalling pathway can reduce the infiltration of M2-type
macrophages into tumour tissues (50). Emactuzumab is a monoclonal antibody
directed against CSF-1R expressed by macrophages (113). Selicrelumab is a selective agonistic
cluster of differentiation 40 (CD40) monoclonal antibody (114), which has been tested clinically
along with tremelimumab (115).
Paclitaxel is one of the most effective
antineoplastic agents for the treatment of numerous forms of cancer
(117). Nab-paclitaxel was developed
to improve paclitaxel solubility and does not need premedication to
avoid infusion-related reactions associated with solvent-based
paclitaxel (118). Ramucirumab is
the first targeted drug approved by the U.S. Food and Drug
Administration for the treatment of advanced gastric cancer, after
failure of previous chemotherapy (119). The inhibition of ramucirumab on the
VEGF receptor 2 can reduce the immune infiltration of TAMs and the
release of CKs and chemokines, as well as inhibit the proliferation
and reproduction of gastric cancer cells and improve the clinical
prognosis of patients with gastric cancer (50).
It has been reported that the response rates with
pembrolizumab (a PD-1 inhibitor) treatment were limited to ~15% in
patients with advanced gastric cancer who had a PD-L1 combined
positive score of ≥1 (14). The
development of novel combination therapies is required to improve
the treatment response rates. Lenvatinib, a multi-kinase inhibitor,
increased the infiltration of CD8+ T cells and decreased
TAMs levels, as well as enhanced the activation of the IFN
signalling pathway and the antitumour function of PD-1 inhibitors
(121).
One reason for the controversy between TAMs and
gastric cancer prognosis is the absence of histological sites. Park
et al reported that CD163+ TAMs in the tumour
stroma and tumour invasive margins were associated with not only
size, depth of invasion, TNM staging, lymph node metastasis and
lymphatic invasion of gastric cancer, but also with poor OS and DFS
of patients with gastric cancer (52). Moreover, TAMs in cancer nests are
associated with histological types and poor DFS, but not with OS.
M2-type TAMs in the tumour stroma and tumour invasive margins have
a stronger influence on the progression and poor prognosis of
gastric cancer compared with the M2-type TAMs in the cancer nest.
In another study, Wang et al revealed that, while
macrophages in healthy tissues and adjacent tissues had no effect
on the prognosis of patients with gastric cancer, the greater the
number of the combination of macrophages and Tregs in the tumour
tissue, the higher the survival rate of patients with gastric
cancer. Therefore, TAMs at different histological sites may have
different effects on the progression and prognosis of patients with
gastric cancer. Thus, future in-depth investigations of TAMs in
gastric cancer must consider the differences caused by various
histological sites (53).
The prognostic effects of different histological
types of TAMs on gastric cancer are significant. For example,
Kawahara et al observed that high-density TAMs were
significantly associated with the poor prognosis of patients with
intestinal-type gastric cancer but not with the survival of
patients with diffuse-type gastric cancer (63). In another study, Liu et al
conducted a multivariate survival analysis of 598 patients with
gastric cancer (123). These authors
reported that CD163+ M2-type TAMs were independent
prognostic factors. Moreover, it was revealed that expression
levels of CD163+ M2-type TAMs was low in signet-ring
cell carcinoma and mucinous adenocarcinoma, and was high in poorly
differentiated adenocarcinoma. However, the high-density M2-type
TAM infiltration in signet-ring cell carcinoma and mucinous
adenocarcinoma indicated a favourable prognosis. Therefore, the
prognostic significance of M2-type TAMs in gastric cancer in
different histological types should be further clarified.
TAMs serve a significant role in the development of
gastric cancer. The tumour-promoting mechanism of TAMs in gastric
cancer involves angiogenesis, invasion, metastasis, chemotherapy
resistance and immune tolerance. TAMs also demonstrated a
favourable application potential in the prognostic evaluation and
treatment of patients with gastric cancer. With the continuous
optimisation of technology and progression of research, the
findings of TAMs will gradually enter the clinical field and
provide references for the individualised treatment of patients
with gastric cancer.
Not applicable.
This work was supported by the National Natural
Science Foundation of China (grant nos. 81502088 and 81502621), the
Nanjing Medical Science and Technology Development Project (grant
no. YKK19136), the Medical Clinical Science and Technology
Development Fund of Jiangsu University (grant no. JLY20180033).
Not applicable.
ZZ searched the literature and drafted the
manuscript. ZY and HZ assisted with the critical revision of the
manuscript. QW, XJ and JW were involved in the conception of the
study. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI
|
|
3
|
Balakrishnan M, George R, Sharma A and
Graham DY: Changing trends in stomach cancer throughout the world.
Curr Gastroenterol Rep. 19:362017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kono K, Nakajima S and Mimura K: Current
status of immune checkpoint inhibitors for gastric cancer. Gastric
Cancer. 23:565–578. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki A, Nakamura Y, Mishima S, Kawazoe
A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, et al:
Predictive factors for hyperprogressive disease during nivolumab as
anti-PD1 treatment in patients with advanced gastric cancer.
Gastric Cancer. 22:793–802. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aoki M, Shoji H, Nagashima K, Imazeki H,
Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, et
al: Hyperprogressive disease during nivolumab or irinotecan
treatment in patients with advanced gastric cancer. ESMO Open.
4:e0004882019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogata T, Satake H, Ogata M, Hatachi Y and
Yasui H: Hyperprogressive disease in the irradiation field after a
single dose of nivolumab for gastric cancer: A case report. Case
Rep Oncol. 11:143–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamada T, Togashi Y, Tay C, Ha D, Sasaki
A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, et al:
PD-1+ regulatory T cells amplified by PD-1 blockade
promote hyperprogression of cancer. Proc Natl Acad Sci USA.
116:9999–10008. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takeoka T, Okada K, Matsuno H, Konishi K,
Ota H, Yokoyama S, Fukunaga M and Kobayashi K: Hyperprogressive
disease during treatment with nivolumab for recurrence of gastric
cancer. Gan To Kagaku Ryoho. 47:165–167. 2020.(In Japanese).
PubMed/NCBI
|
|
12
|
Togasaki K, Sukawa Y, Kanai T and Takaishi
H: Clinical efficacy of immune checkpoint inhibitors in the
treatment of unresectable advanced or recurrent gastric cancer: An
evidence-based review of therapies. Onco Targets Ther.
11:8239–8250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bang YJ, Ruiz EY, Van Cutsem E, Lee KW,
Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, et
al: Phase III, randomised trial of avelumab versus physician's
choice of chemotherapy as third-line treatment of patients with
advanced gastric or gastro-oesophageal junction cancer: Primary
analysis of JAVELIN Gastric 300. Ann Oncol. 29:2052–2060. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shitara K, Ozguroglu M, Bang YJ, Di
Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial.
Lancet. 392:123–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song X, Qi W, Guo J, Sun L, Ding A, Zhao
G, Li H, Qiu W and Lv J: Immune checkpoint inhibitor combination
therapy for gastric cancer: Research progress. Oncol Lett.
20:462020.PubMed/NCBI
|
|
16
|
Wang BC, Zhang ZJ, Fu C and Wang C:
Efficacy and safety of anti-PD-1/PD-L1 agents vs chemotherapy in
patients with gastric or gastroesophageal junction cancer: A
systematic review and meta-analysis. Medicine (Baltimore).
98:e180542019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung HC, Arkenau HT, Lee J, Rha SY, Oh
DY, Wyrwicz L, Kang YK, Lee KW, Infante JR, Lee SS, et al: Avelumab
(anti-PD-L1) as first-line switch-maintenance or second-line
therapy in patients with advanced gastric or gastroesophageal
junction cancer: phase 1b results from the JAVELIN Solid Tumor
trial. J Immunother Cancer. 7:302019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Zhang F, Zhou N, Gu YM, Zhang YT,
He YD, Wang L, Yang LX, Zhao Y and Li YM: Efficacy and safety of
immune checkpoint inhibitors in advanced gastric or
gastroesophageal junction cancer: A systematic review and
meta-analysis. Oncoimmunology. 8:e15815472019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Mo H, Zhang W, Chen X, Qu D, Wang
X, Wu D, Wang X, Lan B, Yang B, et al: Promising efficacy of
SHR-1210, a novel anti-programmed cell death 1 antibody, in
patients with advanced gastric and gastroesophageal junction cancer
in China. Cancer. 125:742–749. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doi T, Iwasa S, Muro K, Satoh T, Hironaka
S, Esaki T, Nishina T, Hara H, Machida N, Komatsu Y, et al: Phase 1
trial of avelumab (anti-PD-L1) in Japanese patients with advanced
solid tumors, including dose expansion in patients with gastric or
gastroesophageal junction cancer: The JAVELIN Solid Tumor JPN
trial. Gastric Cancer. 22:817–827. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Z, Guo Y and Zou CP: Oncological
outcomes of addition of anti-PD1/PD-L1 to chemotherapy in the
therapy of patients with advanced gastric or gastro-oesophageal
junction cancer: A meta-analysis. Medicine (Baltimore).
99:e183322020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients with first-line,
advanced gastric cancer: The KEYNOTE-062 phase 3 randomized
clinical trial. JAMA Oncol. 6:1571–1580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang YK, Bang YJ, Kondo S, Chung HC, Muro
K, Dussault I, Helwig C, Osada M and Doi T: Safety and tolerability
of bintrafusp alfa, a bifunctional fusion protein targeting TGFbeta
and PD-L1, in asian patients with pretreated recurrent or
refractory gastric cancer. Clin Cancer Res. 26:3202–3210. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taieb J, Moehler M, Boku N, Ajani JA,
Yañez Ruiz E, Ryu MH, Guenther S, Chand V and Bang YJ: Evolution of
checkpoint inhibitors for the treatment of metastatic gastric
cancers: Current status and future perspectives. Cancer Treat Rev.
66:104–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Mello RA, Lordick F, Muro K and
Janjigian YY: Current and future aspects of immunotherapy for
esophageal and gastric malignancies. Am Soc Clin Oncol Educ Book.
39:237–247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masuda K, Shoji H, Nagashima K, Yamamoto
S, Ishikawa M, Imazeki H, Aoki M, Miyamoto T, Hirano H, Honma Y, et
al: Correlation between immune-related adverse events and prognosis
in patients with gastric cancer treated with nivolumab. BMC Cancer.
19:9742019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park R, Lopes L and Saeed A:
Anti-PD-1/L1-associated immune-related adverse events as harbinger
of favorable clinical outcome: Systematic review and meta-analysis.
Clin Transl Oncol. 23:100–109. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fridman WH, Zitvogel L, Sautes-Fridman C
and Kroemer G: The immune contexture in cancer prognosis and
treatment. Nat Rev Clin Oncol. 14:717–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lazar DC, Avram MF, Romosan I, Cornianu M,
Taban S and Goldis A: Prognostic significance of tumor immune
microenvironment and immunotherapy: Novel insights and future
perspectives in gastric cancer. World J Gastroenterol.
24:3583–3616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang WH, Wang WQ, Gao HL, Yu XJ and Liu
L: The tumor immune microenvironment in gastroenteropancreatic
neuroendocrine neoplasms. Biochim Biophys Acta Rev Cancer.
1872:1883112019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan X, Jin J, Yan L, Liu L, Li Q and Xu Y:
The impaired anti-tumoral effect of immune surveillance cells in
the immune microenvironment of gastric cancer. Clin Immunol.
219:1085512020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rojas A, Araya P, Gonzalez I and Morales
E: Gastric tumor microenvironment. Adv Exp Med Biol. 1226:23–35.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kamath SD, Kalyan A and Benson AB III:
Pembrolizumab for the treatment of gastric cancer. Expert Rev
Anticancer Ther. 18:1177–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oya Y, Hayakawa Y and Koike K: Tumor
microenvironment in gastric cancers. Cancer Sci. 111:2696–2707.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ocana A, Nieto-Jimenez C, Pandiella A and
Templeton AJ: Neutrophils in cancer: Prognostic role and
therapeutic strategies. Mol Cancer. 16:1372017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aktas ON, Ozturk AB, Erman B, Erus S,
Tanju S and Dilege S: Role of natural killer cells in lung cancer.
J Cancer Res Clin Oncol. 144:997–1003. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jackaman C, Tomay F, Duong L, Abdol Razak
NB, Pixley FJ, Metharom P and Nelson DJ: Aging and cancer: The role
of macrophages and neutrophils. Ageing Res Rev. 36:105–116. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tevis KM, Cecchi RJ, Colson YL and
Grinstaff MW: Mimicking the tumor microenvironment to regulate
macrophage phenotype and assessing chemotherapeutic efficacy in
embedded cancer cell/macrophage spheroid models. Acta Biomater.
50:271–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Yan Y, Yang Y, Wang L, Li M and
Wang J, Liu X, Duan X and Wang J: High infiltration of
tumor-associated macrophages influences poor prognosis in human
gastric cancer patients, associates with the phenomenon of EMT.
Medicine (Baltimore). 95:e26362016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang XL, Jiang JT and Wu CP: Prognostic
significance of tumor-associated macrophage infiltration in gastric
cancer: A meta-analysis. Genet Mol Res. 152016.doi:
10.4238/gmr15049040.
|
|
44
|
Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y,
Zheng L and Li L: Association of intra-tumoral infiltrating
macrophages and regulatory T cells is an independent prognostic
factor in gastric cancer after radical resection. Ann Surg Oncol.
18:2585–2593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou K, Cheng T, Zhan J, Peng X, Zhang Y,
Wen J, Chen X and Ying M: Targeting tumor-associated macrophages in
the tumor microenvironment. Oncol Lett. 20:2342020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aras S and Zaidi MR: TAMeless traitors:
Macrophages in cancer progression and metastasis. Br J Cancer.
117:1583–1591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Petty AJ and Yang Y: Tumor-associated
macrophages: Implications in cancer immunotherapy. Immunotherapy.
9:289–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fan X, Zhang H, Cheng Y, Jiang X, Zhu J
and Jin T: Double roles of macrophages in human neuroimmune
diseases and their animal models. Mediators Inflamm.
2016:84892512016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Jiao X, Meng Y, Chen H, Griffin N,
Gao X and Shan F: Methionine enkephalin (MENK) inhibits human
gastric cancer through regulating tumor associated macrophages
(TAMs) and PI3K/AKT/mTOR signaling pathway inside cancer cells. Int
Immunopharmacol. 65:312–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gambardella V, Castillo J, Tarazona N,
Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, Roselló
S, Roda D, Huerta M, Cervantes A and Fleitas T: The role of
tumor-associated macrophages in gastric cancer development and
their potential as a therapeutic target. Cancer Treat Rev.
86:1020152020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim KJ, Wen XY, Yang HK, Kim WH and Kang
GH: Prognostic implication of M2 macrophages are determined by the
proportional balance of tumor associated macrophages and tumor
infiltrating lymphocytes in microsatellite-unstable gastric
carcinoma. PLoS One. 10:e01441922015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Park JY, Sung JY, Lee J, Park YK, Kim YW,
Kim GY, Won KY and Lim SJ: Polarized CD163+ tumor-associated
macrophages are associated with increased angiogenesis and CXCL12
expression in gastric cancer. Clin Res Hepatol Gastroenterol.
40:357–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu JY, Peng CW, Yang GF, Hu WQ, Yang XJ,
Huang CQ, Xiong B and Li Y: Distribution pattern of tumor
associated macrophages predicts the prognosis of gastric cancer.
Oncotarget. 8:92757–92769. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tauchi Y, Tanaka H, Kumamoto K, Tokumoto
M, Sakimura C, Sakurai K, Kimura K, Toyokawa T, Amano R, Kubo N, et
al: Tumor-associated macrophages induce capillary morphogenesis of
lymphatic endothelial cells derived from human gastric cancer.
Cancer Sci. 107:1101–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ge S, Xia X, Ding C, Zhen B, Zhou Q, Feng
J, Yuan J, Chen R, Li Y, Ge Z, et al: A proteomic landscape of
diffuse-type gastric cancer. Nat Commun. 9:10122018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yamaguchi T, Fushida S, Yamamoto Y,
Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I,
Munesue S, et al: Tumor-associated macrophages of the M2 phenotype
contribute to progression in gastric cancer with peritoneal
dissemination. Gastric Cancer. 19:1052–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Plummer M, de Martel C, Vignat J, Ferlay
J, Bray F and Franceschi S: Global burden of cancers attributable
to infections in 2012: A synthetic analysis. Lancet Glob Health.
4:e609–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hardbower DM, Asim M, Murray-Stewart T,
Casero RA Jr, Verriere T, Lewis ND, Chaturvedi R, Piazuelo MB and
Wilson KT: Arginase 2 deletion leads to enhanced M1 macrophage
activation and upregulated polyamine metabolism in response to
Helicobacter pylori infection. Amino Acids. 48:2375–2388. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shen Z, Kauttu T, Seppanen H, Vainionpää
S, Ye Y, Wang S, Mustonen H and Puolakkainen P: Both macrophages
and hypoxia play critical role in regulating invasion of gastric
cancer in vitro. Acta Oncol. 52:852–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhihua Y, Yulin T, Yibo W, Wei D, Yin C,
Jiahao X, Runqiu J and Xuezhong X: Hypoxia decreases macrophage
glycolysis and M1 percentage by targeting microRNA-30c and mTOR in
human gastric cancer. Cancer Sci. 110:2368–2377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Osinsky S, Bubnovskaya L, Ganusevich I,
Kovelskaya A, Gumenyuk L, Olijnichenko G and Merentsev S: Hypoxia,
tumour-associated macrophages, microvessel density, VEGF and matrix
metalloproteinases in human gastric cancer: Interaction and impact
on survival. Clin Transl Oncol. 13:133–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shen Z, Yan Y, Ye C, Wang B, Jiang K, Ye
Y, Mustonen H, Puolakkainen P and Wang S: The effect of Vasohibin-1
expression and tumor-associated macrophages on the angiogenesis in
vitro and in vivo. Tumour Biol. 37:7267–7276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kawahara A, Hattori S, Akiba J, Nakashima
K, Taira T, Watari K, Hosoi F, Uba M, Basaki Y, Koufuji K, et al:
Infiltration of thymidine phosphorylase-positive macrophages is
closely associated with tumor angiogenesis and survival in
intestinal type gastric cancer. Oncol Rep. 24:405–415. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao B, Mei D, Zhang J, Luo R, Lu H, Xu H
and Huang B: Impact of skip lymph node metastasis on the prognosis
of gastric cancer patients who underwent curative gastrectomy. J
BUON. 24:693–700. 2019.PubMed/NCBI
|
|
66
|
Liu XJ, Li SL, Li JS, Lu H, Yin LL, Zheng
WF and Wang WC: Long non-coding RNA ZEB1-AS1 is associated with
poor prognosis in gastric cancer and promotes cancer cell
metastasis. Eur Rev Med Pharmacol Sci. 22:2624–2630.
2018.PubMed/NCBI
|
|
67
|
Wei Y, Zhang F, Zhang T, Zhang Y, Chen H,
Wang F and Li Y: LDLRAD2 overexpression predicts poor prognosis and
promotes metastasis by activating Wnt/β-catenin/EMT signaling
cascade in gastric cancer. Aging (Albany NY). 11:8951–8968. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xiao T and Jie Z: MiR-21 Promotes the
invasion and metastasis of gastric cancer cells by activating
epithelial-mesenchymal transition. Eur Surg Res. 60:208–218. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yao Z, Yuan T, Wang H, Yao S, Zhao Y, Liu
Y, Jin S, Chu J, Xu Y, Zhou W, et al: MMP-2 together with MMP-9
overexpression correlated with lymph node metastasis and poor
prognosis in early gastric carcinoma. Tumour Biol.
39:10104283177004112017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fan Y, Wang YF, Su HF, Fang N, Zou C, Li
WF and Fei ZH: Decreased expression of the long noncoding RNA
LINC00261 indicate poor prognosis in gastric cancer and suppress
gastric cancer metastasis by affecting the epithelial-mesenchymal
transition. J Hematol Oncol. 9:572016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang W, Song ZJ, Wang Y, Zhong WF, Kang P
and Yang Y: Elevated long non-coding RNA LINC00958 was associated
with metastasis and unfavorable prognosis in gastric cancer. Eur
Rev Med Pharmacol Sci. 23:598–603. 2019.PubMed/NCBI
|
|
72
|
Xie QP, Xiang C, Wang G, Lei KF and Wang
Y: MACC1 upregulation promotes gastric cancer tumor cell metastasis
and predicts a poor prognosis. J Zhejiang Univ Sci B. 17:361–366.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Han F, Zhang L, Qiu W and Yi X: TRAF6
promotes the invasion and metastasis and predicts a poor prognosis
in gastric cancer. Pathol Res Pract. 212:31–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Turajlic S and Swanton C: Metastasis as an
evolutionary process. Science. 352:169–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Guo J, Yan Y, Yan Y, Guo Q, Zhang M, Zhang
J and Goltzman D: Tumor-associated macrophages induce the
expression of FOXQ1 to promote epithelial-mesenchymal transition
and metastasis in gastric cancer cells. Oncol Rep. 38:2003–2010.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu
HY, Wang JS and Duan XY: High tumor-associated macrophages
infiltration is associated with poor prognosis and may contribute
to the phenomenon of epithelial-mesenchymal transition in gastric
cancer. Onco Targets Ther. 9:3975–3983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zheng P, Luo Q, Wang W, Li J, Wang T, Wang
P, Chen L, Zhang P, Chen H, Liu Y, et al: Tumor-associated
macrophages-derived exosomes promote the migration of gastric
cancer cells by transfer of functional Apolipoprotein E. Cell Death
Dis. 9:4342018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ying X, Wu Q, Wu X, Zhu Q and Wang X,
Jiang L, Chen X and Wang X: Epithelial ovarian cancer-secreted
exosomal miR-222-3p induces polarization of tumor-associated
macrophages. Oncotarget. 7:43076–43087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Su CY, Fu XL, Duan W, Yu PW and Zhao YL:
High density of CD68+ tumor-associated macrophages predicts a poor
prognosis in gastric cancer mediated by IL-6 expression. Oncol
Lett. 15:6217–6224. 2018.PubMed/NCBI
|
|
80
|
Wang Z, Yin N, Zhang Z, Zhang Y, Zhang G
and Chen W: Upregulation of T-cell immunoglobulin and mucin-domain
containing-3 (Tim-3) in monocytes/macrophages associates with
gastric cancer progression. Immunol Invest. 46:134–148. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ding H, Zhao L, Dai S, Li L, Wang F and
Shan B: CCL5 secreted by tumor associated macrophages may be a new
target in treatment of gastric cancer. Biomed Pharmacother.
77:142–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lin C, He H, Liu H, Li R, Chen Y, Qi Y,
Jiang Q, Chen L, Zhang P, Zhang H, et al: Tumour-associated
macrophages-derived CXCL8 determines immune evasion through
autonomous PD-L1 expression in gastric cancer. Gut. 68:1764–1773.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Go Y, Tanaka H, Tokumoto M, Sakurai K,
Toyokawa T, Kubo N, Muguruma K, Maeda K, Ohira M and Hirakawa K:
Tumor-associated macrophages extend along lymphatic flow in the
Pre-metastatic lymph nodes of human gastric cancer. Ann Surg Oncol.
23 (Suppl 2):S230–S235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rojas A, Delgado-Lopez F, Perez-Castro R,
Gonzalez I, Romero J, Rojas I, Araya P, Añazco C, Morales E and
Llanos J: HMGB1 enhances the protumoral activities of M2
macrophages by a RAGE-dependent mechanism. Tumour Biol.
37:3321–3329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dong Y, Sun Q and Zhang X: PD-1 and its
ligands are important immune checkpoints in cancer. Oncotarget.
8:2171–2186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T,
Wang Q and Jiang J: PD-1 and PD-L1 co-expression predicts favorable
prognosis in gastric cancer. Oncotarget. 8:64066–64082. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
D'Ignazio A, Kabata P, Ambrosio MR, Polom
K, Marano L, Spagnoli L, Ongaro A, Pieretti L, Marrelli D, Biviano
I and Roviello F: Preoperative oral immunonutrition in
gastrointestinal surgical patients: How the tumour microenvironment
can be modified. Clin Nutr ESPEN. 38:153–159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kono Y, Saito H, Miyauchi W, Shimizu S,
Murakami Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y,
Nakayama Y, et al: Increased PD-1-positive macrophages in the
tissue of gastric cancer are closely associated with poor prognosis
in gastric cancer patients. BMC Cancer. 20:1752020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang
P, Yang J, He W, Chen H, Jiao Z and Li Y: Tumor-derived exosomes
induce PD1+ macrophage population in human gastric
cancer that promotes disease progression. Oncogenesis. 7:412018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang YK, Wang M, Sun Y, Di Costanzo N,
Mitchell C, Achuthan A, Hamilton JA, Busuttil RA and Boussioutas A:
Macrophage spatial heterogeneity in gastric cancer defined by
multiplex immunohistochemistry. Nat Commun. 10:39282019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Harada K, Dong X, Estrella JS, Correa AM,
Xu Y, Hofstetter WL, Sudo K, Onodera H, Suzuki K, Suzuki A, et al:
Tumor-associated macrophage infiltration is highly associated with
PD-L1 expression in gastric adenocarcinoma. Gastric Cancer.
21:31–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nakayama Y, Mimura K, Kua LF, Okayama H,
Min AKT, Saito K, Hanayama H, Watanabe Y, Saito M, Momma T, et al:
Immune suppression caused by PD-L2 expression on tumor cells in
gastric cancer. Gastric Cancer. 23:961–973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Colaprico A, Silva TC, Olsen C, Garofano
L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM,
Castiglioni I, et al: TCGAbiolinks: An R/Bioconductor package for
integrative analysis of TCGA data. Nucleic Acids Res. 44:e712016.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Al-Khami AA, Zheng L, Del Valle L, Hossain
F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC
and Ochoa AC: Exogenous lipid uptake induces metabolic and
functional reprogramming of tumor-associated myeloid-derived
suppressor cells. Oncoimmunology. 6:e13448042017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hossain F, Al-Khami AA, Wyczechowska D,
Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T,
Zou W, et al: Inhibition of fatty acid oxidation modulates
immunosuppressive functions of myeloid-derived suppressor cells and
enhances cancer therapies. Cancer Immunol Res. 3:1236–1247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gardner JK, Mamotte CD, Patel P, Yeoh TL,
Jackaman C and Nelson DJ: Mesothelioma tumor cells modulate
dendritic cell lipid content, phenotype and function. PLoS One.
10:e01235632015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Luo Q, Zheng N, Jiang L, Wang T, Zhang P,
Liu Y, Zheng P, Wang W, Xie G, Chen L, et al: Lipid accumulation in
macrophages confers protumorigenic polarization and immunity in
gastric cancer. Cancer Sci. 111:4000–4011. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu X, Cao Y, Li R, Gu Y, Chen Y, Qi Y, Lv
K, Wang J, Yu K, Lin C, et al: Poor clinical outcomes of
intratumoral dendritic cell-specific intercellular adhesion
molecule 3-grabbing non-integrin-positive macrophages associated
with immune evasion in gastric cancer. Eur J Cancer. 128:27–37.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Peng LS, Zhang JY, Teng YS, Zhao YL, Wang
TT, Mao FY, Lv YP, Cheng P, Li WH, Chen N, et al: Tumor-associated
monocytes/macrophages impair NK-cell function via TGFβ1 in human
gastric cancer. Cancer Immunol Res. 5:248–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sitarz R, Skierucha M, Mielko J, Offerhaus
GJA, Maciejewski R and Polkowski WP: Gastric cancer: Epidemiology,
prevention, classification, and treatment. Cancer Manag Res.
10:239–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Salati M, Orsi G, Smyth E, Aprile G,
Beretta G, De Vita F, Di Bartolomeo M, Fanotto V, Lonardi S, Morano
F, et al: Gastric cancer: Translating novels concepts into clinical
practice. Cancer Treat Rev. 79:1018892019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhao D, Plotnikoff N, Griffin N, Song T
and Shan F: Methionine enkephalin, its role in immunoregulation and
cancer therapy. Int Immunopharmacol. 37:59–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tian J, Jiao X, Wang X, Geng J, Wang R,
Liu N, Gao X, Griffin N and Shan F: Novel effect of methionine
enkephalin against influenza A virus infection through inhibiting
TLR7-MyD88-TRAF6-NF-κB p65 signaling pathway. Int Immunopharmacol.
55:38–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Meng Y, Gao X, Chen W, Plotnikoff NP,
Griffin N, Zhang G and Shan F: Methionine enkephalin (MENK) mounts
antitumor effect via regulating dendritic cells (DCs). Int
Immunopharmacol. 44:61–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang DM, Wang GC, Yang J, Plotnikoff NP,
Griffin N, Han YM, Qi RQ, Gao XH and Shan FP: Inhibition of the
growth of human melanoma cells by methionine enkephalin. Mol Med
Rep. 14:5521–5527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yue Z, Si T, Pan Z, Cao W, Yan Z, Jiang Z
and Ouyang H: Sophoridine suppresses cell growth in human
medulloblastoma through FoxM1, NF-κB and AP-1. Oncol Lett.
14:7941–7946. 2017.PubMed/NCBI
|
|
109
|
Zhuang H, Dai X, Zhang X, Mao Z and Huang
H: Sophoridine suppresses macrophage-mediated immunosuppression
through TLR4/IRF3 pathway and subsequently upregulates CD8(+) T
cytotoxic function against gastric cancer. Biomed Pharmacother.
121:1096362020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Speiser DE, Ho PC and Verdeil G:
Regulatory circuits of T cell function in cancer. Nat Rev Immunol.
16:599–611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y,
Wang F, Duan X, Li J, Zhang W and Wang H: A Natural CCR2 antagonist
relieves tumor-associated macrophage-mediated immunosuppression to
produce a therapeutic effect for liver cancer. EBioMedicine.
22:58–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li X, Yao W, Yuan Y, Chen P, Li B, Li J,
Chu R, Song H, Xie D, Jiang X and Wang H: Targeting of
tumour-infiltrating macrophages via CCL2/CCR2 signalling as a
therapeutic strategy against hepatocellular carcinoma. Gut.
66:157–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gomez-Roca CA, Italiano A, Le Tourneau C,
Cassier PA, Toulmonde M, D'Angelo SP, Campone M, Weber KL, Loirat
D, Cannarile MA, et al: Phase I study of emactuzumab single agent
or in combination with paclitaxel in patients with
advanced/metastatic solid tumors reveals depletion of
immunosuppressive M2-like macrophages. Ann Oncol. 30:1381–1392.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Piechutta M and Berghoff AS: New emerging
targets in cancer immunotherapy: The role of cluster of
differentiation 40 (CD40/TNFR5). ESMO Open. 4:e0005102019.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bajor DL, Mick R, Riese MJ, Huang AC,
Sullivan B, Richman LP, Torigian DA, George SM, Stelekati E, Chen
F, et al: Long-term outcomes of a phase I study of agonist CD40
antibody and CTLA-4 blockade in patients with metastatic melanoma.
Oncoimmunology. 7:e14689562018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Machiels JP, Gomez-Roca C, Michot JM,
Zamarin D, Mitchell T, Catala G, Eberst L, Jacob W, Jegg AM,
Cannarile MA, et al: Phase Ib study of anti-CSF-1R antibody
emactuzumab in combination with CD40 agonist selicrelumab in
advanced solid tumor patients. J Immunother Cancer. 8:e0011532020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Alqahtani FY, Aleanizy FS, El Tahir E,
Alkahtani HM and AlQuadeib BT: Paclitaxel. Profiles Drug Subst
Excip Relat Methodol. 44:205–238. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Shitara K, Takashima A, Fujitani K, Koeda
K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K,
et al: Nab-paclitaxel versus solvent-based paclitaxel in patients
with previously treated advanced gastric cancer (ABSOLUTE): An
open-label, randomised, non-inferiority, phase 3 trial. Lancet
Gastroenterol Hepatol. 2:277–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Mehta R, Kommalapati A and Kim RD: The
impact of ramucirumab treatment on survival and quality of life in
patients with gastric cancer. Cancer Manag Res. 12:51–57. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Bando H, Shimodaira H, Fujitani K,
Takashima A, Yamaguchi K, Nakayama N, Takahashi T, Oki E, Azuma M,
Nishina T, et al: A phase II study of nab-paclitaxel in combination
with ramucirumab in patients with previously treated advanced
gastric cancer. Eur J Cancer. 91:86–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kawazoe A, Fukuoka S, Nakamura Y, Kuboki
Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N,
Higuchi T, et al: Lenvatinib plus pembrolizumab in patients with
advanced gastric cancer in the first-line or second-line setting
(EPOC1706): An open-label, single-arm, phase 2 trial. Lancet Oncol.
21:1057–1065. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Liu X, Xu D, Huang C, Guo Y, Wang S, Zhu
C, Xu J, Zhang Z, Shen Y, Zhao W and Zhao G: Regulatory T cells and
M2 macrophages present diverse prognostic value in gastric cancer
patients with different clinicopathologic characteristics and
chemotherapy strategies. J Transl Med. 17:1922019. View Article : Google Scholar : PubMed/NCBI
|