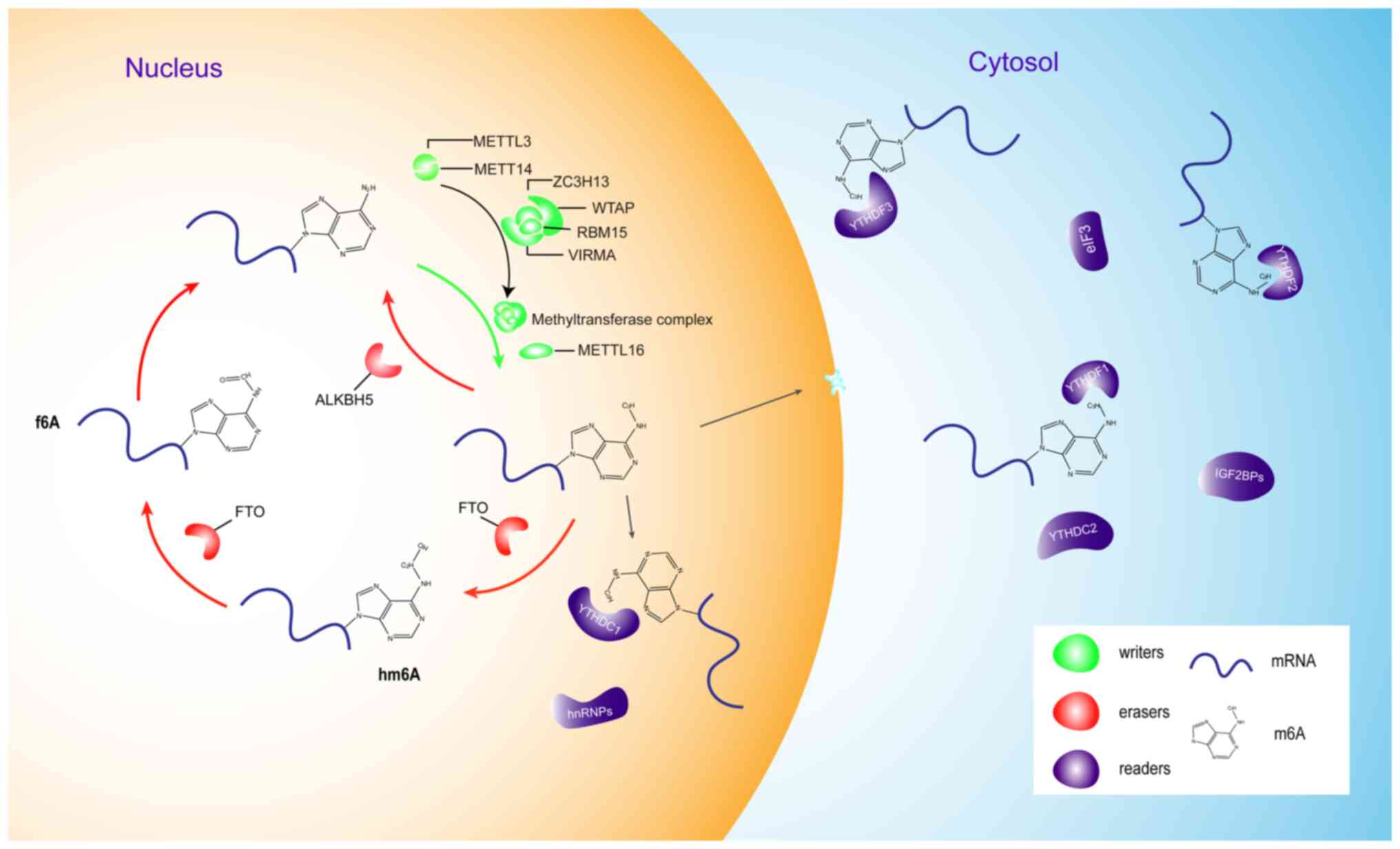
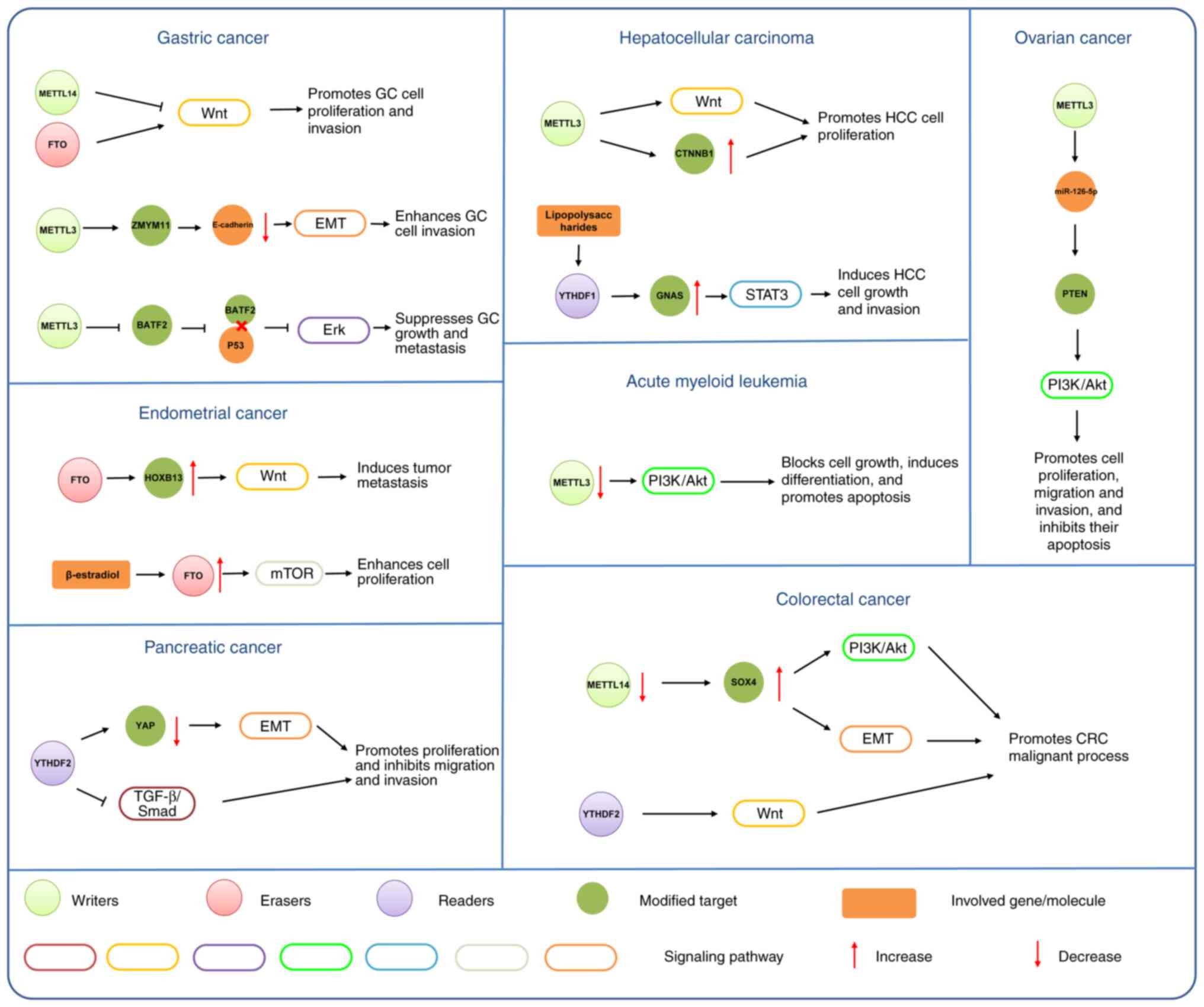
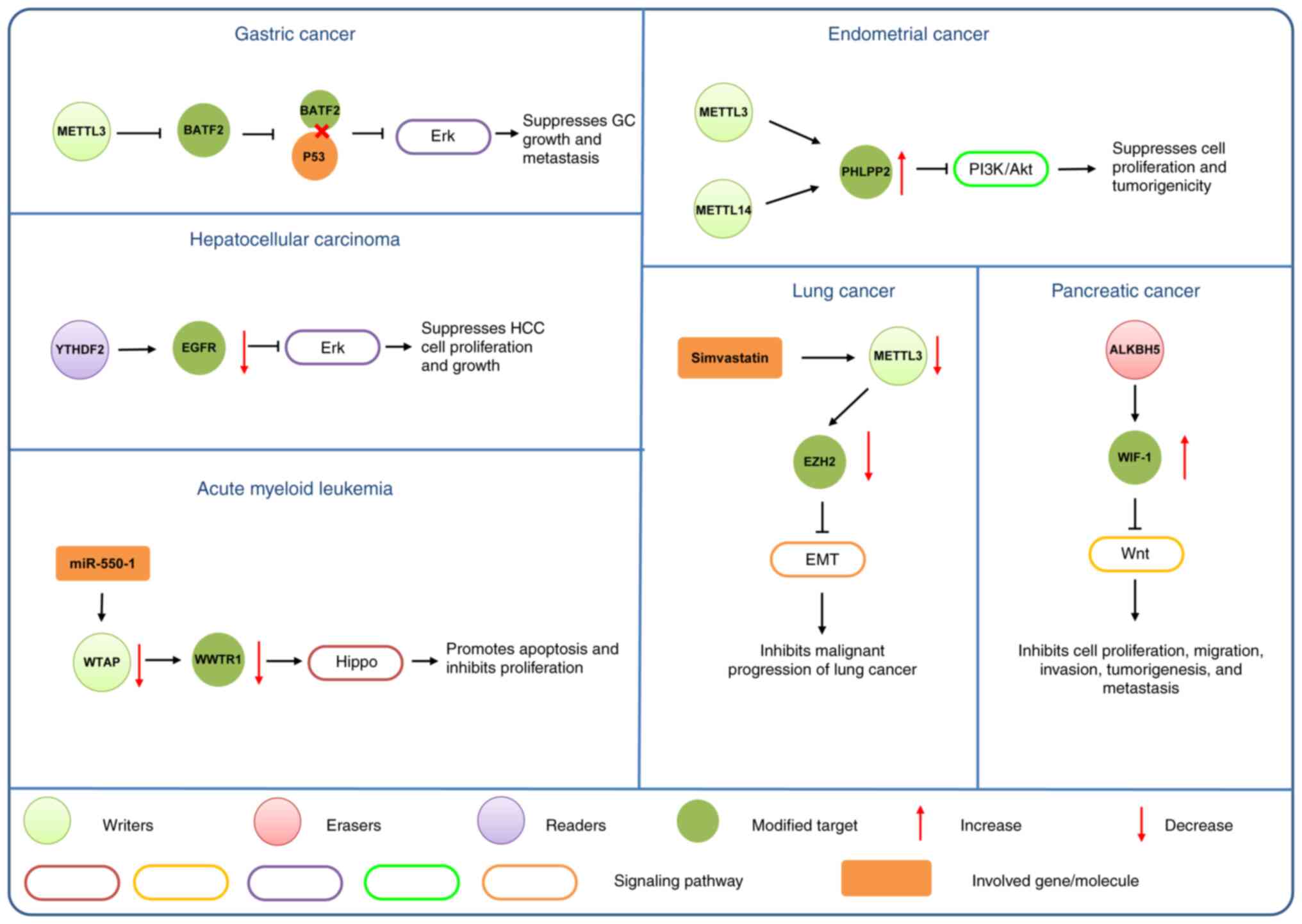
|
1
|
Holley RW, Everett GA, Madison JT and
Zamir A: Nucleotide sequences in the yeast alanine transfer
ribonucleic acid. J Biol Chem. 240:2122–2128. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desrosiers R, Friderici K and Rottman F:
Identification of methylated nucleosides in messenger RNA from
Novikoff hepatoma cells. Proc Natl Acad Sci USA. 71:3971–3975.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dominissini D, Moshitch-Moshkovitz S,
Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K,
Jacob-Hirsch J, Amariglio N, Kupiec M, et al: Topology of the human
and mouse m6A RNA methylomes revealed by m6A-seq. Nature.
485:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Hsu PJ, Chen YS and Yang YG:
Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers
and functions in RNA metabolism. Cell Res. 28:616–624. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer KD and Jaffrey SR: Rethinking
m6A readers, writers, and erasers. Annu Rev Cell Dev
Biol. 33:319–342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schumann U, Shafik A and Preiss T: METTL3
gains R/W access to the epitranscriptome. Mol Cell. 62:323–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ping XL, Sun BF, Wang L, Xiao W, Yang X,
Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al: Mammalian WTAP is
a regulatory subunit of the RNA N6-methyladenosine
methyltransferase. Cell Res. 24:177–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwartz S, Mumbach MR, Jovanovic M, Wang
T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N,
Cacchiarelli D, et al: Perturbation of m6A writers reveals two
distinct classes of mRNA methylation at internal and 5′ sites. Cell
Rep. 8:284–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haussmann IU, Bodi Z, Sanchez-Moran E,
Mongan NP, Archer N, Fray RG and Soller M: m6A
potentiates Sxl alternative pre-mRNA splicing for robust
Drosophila sex determination. Nature. 540:301–304. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muller S, Glaß M, Singh AK, Haase J, Bley
N, Fuchs T, Lederer M, Dahl A, Huang H, Chen J, et al: IGF2BP1
promotes SRF-dependent transcription in cancer in a m6A- and
miRNA-dependent manner. Nucleic Acids Res. 47:375–390. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang
Y, Yi C, Lindahl T, Pan T, Yang YG and He C: N6-methyladenosine in
nuclear RNA is a major substrate of the obesity-associated FTO. Nat
Chem Biol. 7:885–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng G, Dahl JA, Niu Y, Fedorcsak P,
Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al: ALKBH5
is a mammalian RNA demethylase that impacts RNA metabolism and
mouse fertility. Mol Cell. 49:18–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Narayan P and Rottman FM: An in vitro
system for accurate methylation of internal adenosine residues in
messenger RNA. Science. 242:1159–1162. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bodi Z, Button JD, Grierson D and Fray RG:
Yeast targets for mRNA methylation. Nucleic Acids Res.
38:5327–5335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hongay CF and Orr-Weaver TL:
Drosophila inducer of MEiosis 4 (IME4) is required for Notch
signaling during oogenesis. Proc Natl Acad Sci USA.
108:14855–14860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon KJ, Ringeling FR, Vissers C, Jacob F,
Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al:
Temporal control of Mammalian Cortical Neurogenesis by
m6A Methylation. Cell. 171:877–889 e817. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McIntyre ABR, Alexander N, Grigorev K,
Bezdan D, Sichtig H, Chiu CY and Mason CE: Single-molecule
sequencing detection of N6-methyladenine in microbial reference
materials. Nat Commun. 10:5792019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong T, Yuan Y, Chen Z, Xi K, Wang T, Xie
Y, He Z, Su H, Zhou Y, Tan ZJ, et al: Precise Antibody-Independent
m6A Identification via 4SedTTP-Involved and FTO-Assisted Strategy
at Single-Nucleotide Resolution. J Am Chem Soc. 140:5886–5889.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meyer KD, Saletore Y, Zumbo P, Elemento O,
Mason CE and Jaffrey SR: Comprehensive analysis of mRNA methylation
reveals enrichment in 3′ UTRs and near stop codons. Cell.
149:1635–1646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi H, Wei J and He C: Where, when, and
how: Context-Dependent functions of RNA methylation writers,
readers, and erasers. Mol Cell. 74:640–650. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaccara S, Ries RJ and Jaffrey SR:
Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell
Biol. 20:608–624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao BS, Roundtree IA and He C:
Post-transcriptional gene regulation by mRNA modifications. Nat Rev
Mol Cell Biol. 18:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu Y, Dominissini D, Rechavi G and He C:
Gene expression regulation mediated through reversible m6A RNA
methylation. Nat Rev Genet. 15:293–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warda AS, Kretschmer J, Hackert P, Lenz C,
Urlaub H, Höbartner C, Sloan KE and Bohnsack MT: Human METTL16 is a
N6-methyladenosine (m6A) methyltransferase
that targets pre-mRNAs and various non-coding RNAs. EMBO Rep.
18:2004–2014. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doxtader KA, Wang P, Scarborough AM, Seo
D, Conrad NK and Nam Y: Structural basis for regulation of METTL16,
an S-adenosylmethionine homeostasis factor. Mol Cell.
71:1001–1011.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo J, Tang HW, Li J, Perrimon N and Yan
D: Xio is a component of the Drosophila sex determination
pathway and RNA N6-methyladenosine methyltransferase
complex. Proc Natl Acad Sci USA. 115:3674–3679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patil DP, Chen CK, Pickering BF, Chow A,
Jackson C, Guttman M and Jaffrey SR: m6A RNA methylation
promotes XIST-mediated transcriptional repression. Nature.
537:369–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu Y, Jia G, Pang X, Wang RN, Wang X, Li
CJ, Smemo S, Dai Q, Bailey KA, Nobrega MA, et al: FTO-mediated
formation of N6-hydroxymethyladenosine and N6-formyladenosine in
mammalian RNA. Nat Commun. 4:17982013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Zhao BS, Roundtree IA, Lu Z, Han
D, Ma H, Weng X, Chen K, Shi H and He C: N(6)-methyladenosine
modulates messenger RNA translation efficiency. Cell.
161:1388–1399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han
D, Fu Y, Parisien M, Dai Q, Jia G, et al:
N6-methyladenosine-dependent regulation of messenger RNA stability.
Nature. 505:117–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu
PJ, Liu C and He C: YTHDF3 facilitates translation and decay of
N6-methyladenosine-modified RNA. Cell Res. 27:315–328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Yang Y, Sun BF, Shi Y, Yang X,
Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al: FTO-dependent
demethylation of N6-methyladenosine regulates mRNA splicing and is
required for adipogenesis. Cell Res. 24:1403–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng S, Xiao W, Ju D, Sun B, Hou N, Liu Q,
Wang Y, Zhao H, Gao C, Zhang S, et al: Identification of entacapone
as a chemical inhibitor of FTO mediating metabolic regulation
through FOXO1. Sci Transl Med. 11:eaau71162019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben-Haim MS, Moshitch-Moshkovitz S and
Rechavi G: FTO: Linking m6A demethylation to adipogenesis. Cell
Res. 25:3–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Fu J and Zhou Y: A review in
research progress concerning m6A methylation and immunoregulation.
Front Immunol. 10:9222019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Z, Gao X, Shuai Y, Xing X and Ji J: The
m6A epitranscriptome opens a new charter in immune system logic.
Epigenetics. 1–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shulman Z and Stern-Ginossar N: The RNA
modification N(6)-methyladenosine as a novel regulator of the
immune system. Nat Immunol. 21:501–512. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geula S, Moshitch-Moshkovitz S,
Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V,
Peer E, Mor N, Manor YS, et al: Stem cells. m6A mRNA methylation
facilitates resolution of naive pluripotency toward
differentiation. Science. 347:1002–1006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roundtree IA, Evans ME, Pan T and He C:
Dynamic RNA modifications in gene expression regulation. Cell.
169:1187–1200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao S, Zeng X, Fan Y, Su Y, Ma Q, Zhu J
and Yao H: Gene polymorphism association with type 2 diabetes and
related gene-gene and gene-environment interactions in a uyghur
population. Med Sci Monit. 22:474–487. 2016.PubMed/NCBI
|
|
41
|
Kennedy EM, Bogerd HP, Kornepati AV, Kang
D, Ghoshal D, Marshall JB, Poling BC, Tsai K, Gokhale NS, Horner SM
and Cullen BR: Posttranscriptional m(6)A editing of HIV-1 mRNAs
enhances viral gene expression. Cell host microbe. 19:675–685.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tirumuru N, Zhao BS, Lu W, Lu Z, He C and
Wu L: N(6)-methyladenosine of HIV-1 RNA regulates viral infection
and HIV-1 Gag protein expression. Elife. 5:e155282016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gokhale NS, McIntyre ABR, McFadden MJ,
Roder AE, Kennedy EM, Gandara JA, Hopcraft SE, Quicke KM, Vazquez
C, Willer J, et al: N6-Methyladenosine in flaviviridae viral RNA
genomes regulates infection. Cell host microbe. 20:654–665. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y,
He C and Rana TM: Dynamics of human and viral RNA methylation
during Zika virus infection. Cell Host Microbe. 20:666–673. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu R, Li Q, Feng Z, Cai L and Xu Q: M6A
Reader YTHDF2 Regulates LPS-Induced inflammatory response. Int J
Mol Sci. 20:13232019. View Article : Google Scholar
|
|
46
|
Nichols J and Smith A: Naive and primed
pluripotent states. Cell Stem Cell. 4:487–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Batista PJ, Molinie B, Wang J, Qu K, Zhang
J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al:
m6A RNA modification controls cell fate transition in
mammalian embryonic stem cells. Cell stem cell. 15:707–719. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Buecker C, Srinivasan R, Wu Z, Calo E,
Acampora D, Faial T, Simeone A, Tan M, Swigut T and Wysocka J:
Reorganization of enhancer patterns in transition from naive to
primed pluripotency. Cell stem cell. 14:838–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu C, Wang X, Liu K, Roundtree IA, Tempel
W, Li Y, Lu Z, He C and Min J: Structural basis for selective
binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol.
10:927–929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu
Y, Gregory BD, Schultz RM and Wang PJ: Nuclear m6A reader YTHDC1
regulates alternative polyadenylation and splicing during mouse
oocyte development. PLoS Genet. 14:e10074122018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Z, Qian P, Shao W, Shi H, He XC, Gogol
M, Yu Z, Wang Y, Qi M, Zhu Y, et al: Suppression of m(6)A reader
Ythdf2 promotes hematopoietic stem cell expansion. Cell Res.
28:904–917. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lobo J, Costa AL, Cantante M, Guimarães R,
Lopes P, Antunes L, Braga I, Oliveira J, Pelizzola M, Henrique R
and Jerónimo C: m6A RNA modification and its
writer/reader VIRMA/YTHDF3 in testicular germ cell tumors: A role
in seminoma phenotype maintenance. J Transl Med. 17:792019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou J, Wan J, Shu XE, Mao Y, Liu XM, Yuan
X, Zhang X, Hess ME, Brüning JC and Qian SB:
N6-Methyladenosine guides mRNA alternative translation
during integrated stress response. Mol Cell. 69:636–647.e7. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu R, Liu Y, Yao Y, Zhao Y, Bi Z, Jiang Q,
Liu Q, Cai M, Wang F, Wang Y and Wang X: FTO regulates adipogenesis
by controlling cell cycle progression via m6A-YTHDF2
dependent mechanism. Biochim Biophys Acta Mol Cell Biol Lipids.
1863:1323–1330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu J, Zhang X, Chen K, Cheng Y, Liu S,
Xia M, Chen Y, Zhu H, Li Z and Cao X: CCR7 chemokine
receptor-inducible lnc-Dpf3 restrains dendritic cell migration by
inhibiting HIF-1α-mediated glycolysis. Immunity. 50:600–615.e15.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Han D, Liu J, Chen C, Dong L, Liu Y, Chang
R, Huang X, Liu Y, Wang J, Dougherty U, et al: Anti-tumour immunity
controlled through mRNA m(6)A methylation and YTHDF1 in dendritic
cells. Nature. 566:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu
H, Ni T, Zhang ZS, Zhang T, Li C, et al: Small-molecule targeting
of oncogenic FTO demethylase in acute myeloid leukemia. Cancer
Cell. 35:677–691.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li HB, Tong J, Zhu S, Batista PJ, Duffy
EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, et al:
m6A mRNA methylation controls T cell homeostasis by
targeting the IL-7/STAT5/SOCS pathways. Nature. 548:338–342. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kim GW, Imam H, Khan M and Siddiqui A:
N6-Methyladenosine modification of hepatitis B and C
viral RNAs attenuates host innate immunity via RIG-I signaling. J
Biol Chem. 295:13123–13133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mapperley C, van de Lagemaat LN, Lawson H,
Tavosanis A, Paris J, Campos J, Wotherspoon D, Durko J, Sarapuu A,
Choe J, et al: The mRNA m6A reader YTHDF2 suppresses
proinflammatory pathways and sustains hematopoietic stem cell
function. J Exp Med. 218:e202008292021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu
Z, Hu B, Zhou J, Zhao Z, Feng M, et al: YTHDF2 reduction fuels
inflammation and vascular abnormalization in hepatocellular
carcinoma. Mol Cancer. 18:1632019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin Z, Hsu PJ, Xing X, Fang J, Lu Z, Zou
Q, Zhang KJ, Zhang X, Zhou Y, Zhang T, et al:
Mettl3-/Mettl14-mediated mRNA N6-methyladenosine
modulates murine spermatogenesis. Cell Res. 27:1216–1230. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao BS and He C: ‘Gamete On’ for
m6A: YTHDF2 exerts essential functions in female
fertility. Mol Cell. 67:903–905. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Livneh I, Moshitch-Moshkovitz S, Amariglio
N, Rechavi G and Dominissini D: The m6A
epitranscriptome: Transcriptome plasticity in brain development and
function. Nat Rev Neurosci. 21:36–51. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lence T, Akhtar J, Bayer M, Schmid K,
Spindler L, Ho CH, Kreim N, Andrade-Navarro MA, Poeck B, Helm M and
Roignant JY: m6A modulates neuronal functions and sex
determination in Drosophila. Nature. 540:242–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin S, Choe J, Du P, Triboulet R and
Gregory RI: The m(6)A methyltransferase METTL3 promotes translation
in human cancer cells. Mol Cell. 62:335–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH,
Wang F, Wang TT, Xu QG, Zhou WP and Sun SH: METTL14 suppresses the
metastatic potential of hepatocellular carcinoma by modulating
N6 -methyladenosine-dependent primary MicroRNA
processing. Hepatology. 65:529–543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vu LP, Pickering BF, Cheng Y, Zaccara S,
Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al:
The N6-methyladenosine (m6A)-forming enzyme
METTL3 controls myeloid differentiation of normal hematopoietic and
leukemia cells. Nat Med. 23:1369–1376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang Q, Geng W, Guo H, Wang Z, Xu K, Chen
C and Wang S: Emerging role of RNA methyltransferase METTL3 in
gastrointestinal cancer. J Hematol Oncol. 13:572020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yue B, Song C, Yang L, Cui R, Cheng X,
Zhang Z and Zhao G: METTL3-mediated N6-methyladenosine modification
is critical for epithelial-mesenchymal transition and metastasis of
gastric cancer. Mol Cancer. 18:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang DD, Chen ZH, Yu K, Lu JH, Wu QN, Wang
Y, Ju HQ, Xu RH, Liu ZX and Zeng ZL: METTL3 promotes the
progression of gastric cancer via targeting the MYC pathway. Front
Oncol. 10:1152020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X,
Zhang X, Cao Y, Ma D, Zhu X, et al: m6A-dependent
glycolysis enhances colorectal cancer progression. Mol Cancer.
19:722020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen M, Wei L, Law CT, Tsang FH, Shen J,
Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al: RNA
N6-methyladenosine methyltransferase-like 3 promotes liver cancer
progression through YTHDF2-dependent posttranscriptional silencing
of SOCS2. Hepatology. 67:2254–2270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C,
Li S, Tan L, Mai D, Li G, et al: Excessive miR-25-3p maturation via
N6-methyladenosine stimulated by cigarette smoke
promotes pancreatic cancer progression. Nat Commun. 10:18582019.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Taketo K, Konno M, Asai A, Koseki J,
Toratani M, Satoh T, Doki Y, Mori M, Ishii H and Ogawa K: The
epitranscriptome m6A writer METTL3 promotes chemo- and
radioresistance in pancreatic cancer cells. Int J Oncol.
52:621–629. 2018.PubMed/NCBI
|
|
76
|
Liu T, Yang S, Sui J, Xu SY, Cheng YP,
Shen B, Zhang Y, Zhang XM, Yin LH, Pu YP and Liang GY: Dysregulated
N6-methyladenosine methylation writer METTL3 contributes to the
proliferation and migration of gastric cancer. J Cell Physiol.
235:548–562. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang P, Wang X, Zheng L and Zhuang C: Gene
signatures and prognostic values of m6A regulators in
hepatocellular carcinoma. Front Genet. 11:5401862020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhu D, Zhou J, Zhao J, Jiang G, Zhang X,
Zhang Y and Dong M: ZC3H13 suppresses colorectal cancer
proliferation and invasion via inactivating Ras-ERK signaling. J
Cell Physiol. 234:8899–8907. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Barros-Silva D, Lobo J, Guimaraes-Teixeira
C, Carneiro I, Oliveira J, Martens-Uzunova ES, Henrique R and
Jerónimo C: VIRMA-Dependent N6-Methyladenosine modifications
regulate the expression of long non-coding RNAs CCAT1 and CCAT2 in
prostate cancer. Cancers (Basel). 12:7712020. View Article : Google Scholar
|
|
80
|
Qian JY, Gao J, Sun X, Cao MD, Shi L, Xia
TS, Zhou WB, Wang S, Ding Q and Wei JF: KIAA1429 acts as an
oncogenic factor in breast cancer by regulating CDK1 in an
N6-methyladenosine-independent manner. Oncogene. 38:6123–6141.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C,
Huang H, Nachtergaele S, Dong L, Hu C, et al: FTO plays an
oncogenic role in acute myeloid leukemia as a
N6-Methyladenosine RNA demethylase. Cancer Cell.
31:127–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Van Der Werf I and Jamieson C: The yin and
yang of RNA methylation: An imbalance of erasers enhances
sensitivity to FTO demethylase small-molecule targeting in leukemia
stem cells. Cancer Cell. 35:540–541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhu T, Yong XLH, Xia D, Widagdo J and
Anggono V: Ubiquitination regulates the proteasomal degradation and
nuclear translocation of the fat mass and obesity-associated (FTO)
protein. J Mol Biol. 430:363–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun
G, Lu Z, Huang Y, Yang CG, et al: m6A RNA methylation
regulates the self-renewal and tumorigenesis of glioblastoma stem
cells. Cell Rep. 18:2622–2634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kwok CT, Marshall AD, Rasko JE and Wong
JJ: Genetic alterations of m(6)A regulators predict poorer survival
in acute myeloid leukemia. J Hematol Oncol. 10:392017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S,
Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, et al: m6A
demethylase ALKBH5 maintains tumorigenicity of glioblastoma
stem-like cells by sustaining FOXM1 expression and cell
proliferation program. Cancer Cell. 31:591–606 e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bai Y, Yang C, Wu R, Huang L, Song S, Li
W, Yan P, Lin C, Li D and Zhang Y: YTHDF1 regulates tumorigenicity
and cancer stem cell-like activity in human colorectal carcinoma.
Front Oncol. 9:3322019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nishizawa Y, Konno M, Asai A, Koseki J,
Kawamoto K, Miyoshi N, Takahashi H, Nishida N, Haraguchi N, Sakai
D, et al: Oncogene c-Myc promotes epitranscriptome m6A
reader YTHDF1 expression in colorectal cancer. Oncotarget.
9:7476–7486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tanabe A, Tanikawa K, Tsunetomi M, Takai
K, Ikeda H, Konno J, Torigoe T, Maeda H, Kutomi G, Okita K, et al:
RNA helicase YTHDC2 promotes cancer metastasis via the enhancement
of the efficiency by which HIF-1α mRNA is translated. Cancer Lett.
376:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Huang H, Weng H, Sun W, Qin X, Shi H, Wu
H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al: Recognition of RNA
N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and
translation. Nat Cell Biol. 20:285–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang C, Zhang M, Ge S, Huang W, Lin X,
Gao J, Gong J and Shen L: Reduced m6A modification predicts
malignant phenotypes and augmented Wnt/PI3K-Akt signaling in
gastric cancer. Cancer Med. 8:4766–4781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Korshunov A, Sahm F, Zheludkova O, Golanov
A, Stichel D, Schrimpf D, Ryzhova M, Potapov A, Habel A, Meyer J,
et al: DNA methylation profiling is a method of choice for
molecular verification of pediatric WNT-activated medulloblastomas.
Neuro Oncol. 21:214–221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang L, Wan Y, Zhang Z, Jiang Y, Lang J,
Cheng W and Zhu L: FTO demethylates m6A modifications in HOXB13
mRNA and promotes endometrial cancer metastasis by activating the
WNT signalling pathway. RNA Biol. Nov 5–2020.(Epub ahead of print).
doi: 10.1080/15476286.2020.1841458. View Article : Google Scholar
|
|
94
|
Liu L, Wang J, Sun G, Wu Q, Ma J, Zhang X,
Huang N, Bian Z, Gu S, Xu M, et al: m6A mRNA methylation
regulates CTNNB1 to promote the proliferation of hepatoblastoma.
Mol Cancer. 18:1882019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Le PN, McDermott JD and Jimeno A:
Targeting the Wnt pathway in human cancers: Therapeutic targeting
with a focus on OMP-54F28. Pharmacol Ther. 146:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li Z, Chen Y, An T, Liu P, Zhu J, Yang H,
Zhang W, Dong T, Jiang J, Zhang Y, et al: Nuciferine inhibits the
progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug
signaling pathway. J Exp Clin Cancer Res. 38:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li M, Bu X, Cai B, Liang P, Li K, Qu X and
Shen L: Biological role of metabolic reprogramming of cancer cells
during epithelialmesenchymal transition (Review). Oncol Rep.
41:727–741. 2019.PubMed/NCBI
|
|
98
|
Chen J, Sun Y, Xu X, Wang D, He J, Zhou H,
Lu Y, Zeng J, Du F, Gong A and Xu M: YTH domain family 2
orchestrates epithelial-mesenchymal transition/proliferation
dichotomy in pancreatic cancer cells. Cell Cycle. 16:2259–2271.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen WW, Qi JW, Hang Y, Wu JX, Zhou XX,
Chen JZ, Wang J and Wang HH: Simvastatin is beneficial to lung
cancer progression by inducing METTL3-induced m6A modification on
EZH2 mRNA. Eur Rev Med Pharmacol Sci. 24:4263–4270. 2020.PubMed/NCBI
|
|
100
|
Aoki M and Fujishita T: Oncogenic roles of
the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 407:153–189.
2017.PubMed/NCBI
|
|
101
|
Li X, Tang J, Huang W, Wang F, Li P, Qin
C, Qin Z, Zou Q, Wei J, Hua L, et al: The M6A methyltransferase
METTL3: Acting as a tumor suppressor in renal cell carcinoma.
Oncotarget. 8:96103–96116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhao Q, Zhao Y, Hu W, Zhang Y, Wu X, Lu J,
Li M, Li W, Wu W, Wang J, et al: m6A RNA modification
modulates PI3K/Akt/mTOR signal pathway in gastrointestinal cancer.
Theranostics. 10:9528–9543. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B,
Li C, Sun L, Qin J, Xu T, et al: METTL14-mediated
N6-methyladenosine modification of SOX4 mRNA inhibits tumor
metastasis in colorectal cancer. Mol Cancer. 19:1062020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bi X, Lv X, Liu D, Guo H, Yao G, Wang L,
Liang X and Yang Y: METTL3-mediated maturation of miR-126-5p
promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR
pathway. Cancer Gene Ther. Sep 16–2020.(Epub ahead of print). doi:
10.1038/s41417-020-00222-3. View Article : Google Scholar
|
|
105
|
Liu J, Eckert MA, Harada BT, Liu SM, Lu Z,
Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, et al:
m6A mRNA methylation regulates AKT activity to promote
the proliferation and tumorigenicity of endometrial cancer. Nat
Cell Biol. 20:1074–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Salaroglio IC, Mungo E, Gazzano E, Kopecka
J and Riganti C: ERK is a pivotal player of chemo-immune-resistance
in cancer. Int J Mol Sci. 20:25052019. View Article : Google Scholar
|
|
107
|
Zhong L, Liao D, Zhang M, Zeng C, Li X,
Zhang R, Ma H and Kang T: YTHDF2 suppresses cell proliferation and
growth via destabilizing the EGFR mRNA in hepatocellular carcinoma.
Cancer Lett. 442:252–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sun HL, Zhu AC, Gao Y, Terajima H, Fei Q,
Liu S, Zhang L, Zhang Z, Harada BT, He YY, et al: Stabilization of
ERK-Phosphorylated METTL3 by USP5 Increases m6A
methylation. Mol Cell. 80:633–647.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Xie JW, Huang XB, Chen QY, Ma YB, Zhao YJ,
Liu LC, Wang JB, Lin JX, Lu J, Cao LL, et al: m6A
modification-mediated BATF2 acts as a tumor suppressor in gastric
cancer through inhibition of ERK signaling. Mol Cancer. 19:1142020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ghazi T, Nagiah S and Chuturgoon AA:
Fusaric acid decreases p53 expression by altering promoter
methylation and m6A RNA methylation in human hepatocellular
carcinoma (HepG2) cells. Epigenetics. 1–13. 2020.(Epub ahead of
print).
|
|
111
|
Ding H, Zhang X, Su Y, Jia C and Dai C:
GNAS promotes inflammation-related hepatocellular carcinoma
progression by promoting STAT3 activation. Cell Mol Biol Lett.
25:82020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang Z, Zhou D, Lai Y, Liu Y, Tao X, Wang
Q, Zhao G, Gu H, Liao H, Zhu Y, et al: Estrogen induces endometrial
cancer cell proliferation and invasion by regulating the fat mass
and obesity-associated gene via PI3K/AKT and MAPK signaling
pathways. Cancer Lett. 319:89–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhu Y, Shen J, Gao L and Feng Y: Estrogen
promotes fat mass and obesity-associated protein nuclear
localization and enhances endometrial cancer cell proliferation via
the mTOR signaling pathway. Oncol Rep. 35:2391–2397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z,
Chen J, Jiang Z, Zhang Y, Xu G, Zhang J, et al: METTL3-mediated
m6A modification of HDGF mRNA promotes gastric cancer
progression and has prognostic significance. Gut. 69:1193–1205.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN,
Chen ZH, Zeng ZL, Wang F, Zheng J, et al: METTL3 facilitates tumor
progression via an m6A-IGF2BP2-dependent mechanism in
colorectal carcinoma. Mol Cancer. 18:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li Y, Zheng D, Wang F, Xu Y, Yu H and
Zhang H: Expression of demethylase genes, FTO and ALKBH1, is
associated with prognosis of gastric cancer. Dig Dis Sci.
64:1503–1513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang S, Chai P and Jia R and Jia R: Novel
insights on m6A RNA methylation in tumorigenesis: A
double-edged sword. Mol Cancer. 17:1012018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H,
Wu M, Liang Y, Zhu F, Zhang Y, Zhang X, et al: The m(6)A
methyltransferase METTL3 promotes bladder cancer progression via
AFF4/NF-kappaB/MYC signaling network. Oncogene. 38:3667–3680. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li F, Yi Y, Miao Y, Long W, Long T, Chen
S, Cheng W, Zou C, Zheng Y, Wu X, et al:
N6-Methyladenosine modulates nonsense-mediated mRNA
decay in human glioblastoma. Cancer Res. 79:5785–5798. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang K, Jiang L, Zhang Y and Chen C:
Progression of thyroid carcinoma is promoted by the m6A
methyltransferase METTL3 through regulating m6A
methylation on TCF1. Onco Targets Ther. 13:1605–1612. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Weng H, Huang H, Wu H, Qin X, Zhao BS,
Dong L, Shi H, Skibbe J, Shen C, Hu C, et al: METTL14 inhibits
hematopoietic stem/progenitor differentiation and promotes
leukemogenesis via mRNA m6A modification. Cell Stem
Cell. 22:191–205.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Bansal H, Yihua Q, Iyer SP, Ganapathy S,
Proia DA, Penalva LO, Uren PJ, Suresh U, Carew JS, Karnad AB, et
al: WTAP is a novel oncogenic protein in acute myeloid leukemia.
Leukemia. 28:1171–1174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chen Y, Peng C, Chen J, Chen D, Yang B, He
B, Hu W, Zhang Y, Liu H, Dai L, et al: WTAP facilitates progression
of hepatocellular carcinoma via m6A-HuR-dependent epigenetic
silencing of ETS1. Mol Cancer. 18:1272019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cheng X, Li M, Rao X, Zhang W, Li X, Wang
L and Huang G: KIAA1429 regulates the migration and invasion of
hepatocellular carcinoma by altering m6A modification of ID2 mRNA.
Onco Targets Ther. 12:3421–3428. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Xu D, Shao W, Jiang Y, Wang X, Liu Y and
Liu X: FTO expression is associated with the occurrence of gastric
cancer and prognosis. Oncol Rep. 38:2285–2292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li J, Han Y, Zhang H, Qian Z, Jia W, Gao
Y, Zheng H and Li B: The m6A demethylase FTO promotes the growth of
lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem
Biophys Res Commun. 512:479–485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li J, Zhu L, Shi Y, Liu J, Lin L and Chen
X: m6A demethylase FTO promotes hepatocellular carcinoma
tumorigenesis via mediating PKM2 demethylation. Am J Transl Res.
11:6084–6092. 2019.PubMed/NCBI
|
|
128
|
Chao Y, Shang J and Ji W:
ALKBH5-m6A-FOXM1 signaling axis promotes proliferation
and invasion of lung adenocarcinoma cells under intermittent
hypoxia. Biochem Biophys Res Commun. 521:499–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lin X, Chai G, Wu Y, Li J, Chen F, Liu J,
Luo G, Tauler J, Du J, Lin S, et al: RNA m(6)A methylation
regulates the epithelial mesenchymal transition of cancer cells and
translation of Snail. Nat Commun. 10:20652019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li J, Xie H, Ying Y, Chen H, Yan H, He L,
Xu M, Xu X, Liang Z, Liu B, et al: YTHDF2 mediates the mRNA
degradation of the tumor suppressors to induce AKT phosphorylation
in N6-methyladenosine-dependent way in prostate cancer. Mol Cancer.
19:1522020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Dixit D, Prager BC, Gimple RC, Poh HX,
Wang Y, Wu Q, Qiu Z, Kidwell RL, Kim LJ, Xie Q, et al: The RNA m6A
reader YTHDF2 maintains oncogene expression and is a targetable
dependency in glioblastoma stem cells. Cancer Discov. 11:480–499.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chang G, Shi L, Ye Y, Shi H, Zeng L,
Tiwary S, Huse JT, Huo L, Ma L, Ma Y, et al: YTHDF3 induces the
translation of m6A-enriched gene transcripts to promote
breast cancer brain metastasis. Cancer Cell. 38:857–871 e7. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ma L, Chen T, Zhang X, Miao Y, Tian X, Yu
K, Xu X, Niu Y, Guo S, Zhang C, et al: The m6A reader
YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing
SLC7A11-dependent antioxidant function. Redox Biol. 38:1018012021.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wu Y, Yang X, Chen Z, Tian L, Jiang G,
Chen F, Li J, An P, Lu L, Luo N, et al: m6A-induced
lncRNA RP11 triggers the dissemination of colorectal cancer cells
via upregulation of Zeb1. Mol Cancer. 18:872019. View Article : Google Scholar : PubMed/NCBI
|