Introduction
Endometrial cancer (EC), cervical cancer and ovarian
cancer (OC) are the three most common gynecological malignancies.
EC is the most common gynecological tumor in developed countries,
and its incidence and mortality are increasing (1). By contrast, the incidence of cervical
cancer is decreasing (1) due to the
extensive application of the human papillomavirus (HPV) vaccine and
the standard screening strategy. However, the incidence and
mortality of cervical cancer in developing countries are very
different from those in developed countries (2,3). A study
conducted in China revealed that cervical cancer had the highest
incidence and mortality among gynecological tumors in 2015
(4). OC is the most lethal cancer
(1), possibly due to the lack of
efficient early diagnostic methods, its high recurrence rate, and
its extensive chemoresistance.
Persistent HPV infection is the main cause of
cervical cancer (5), but the causes
of the other two gynecological cancers have not yet been clearly
determined, despite years of research. Multiple factors are
involved in EC and OC, among which family history, advanced age,
low fertility and low levels of female hormones are considered as
high-risk factors (6,7). Standard therapy includes surgery and
adjuvant therapy, such as chemotherapy and radiotherapy;
furthermore, an increasing number of active agents, such as
bevacizumab and poly(ADP-ribose) polymerase (PARP) inhibitors
(PARPi), have emerged in recent decades (5–7). However,
identifying characteristic markers is the key to improving the
diagnosis, treatment and prognosis of these tumors.
Sirtuin 1 (SIRT1), a member of the sirtuin protein
family, belongs to the family of type III histone deacetylases and
is mainly localized to the nucleus (8,9). SIRT1 can
deacetylate histone (H1, H3 and H4) and non-histone proteins [p53
and nuclear factor (NF)-κB] by consuming nicotinamide adenine
dinucleotide (NAD+), causing ADP-ribose to attach to the
acetyl moiety of the substrate, and releasing nicotinamide (NAM)
and 2′-O-acetyl-adenosine diphosphate-ribose (O-AcADPR) (10,11). SIRT1
regulates metabolism, aging, transcription, DNA damage and repair,
apoptosis, the cell cycle, inflammation, and tumor-related
processes, and it is the most extensively investigated sirtuin in
mammals (10,12). SIRT1 has been studied in the context
of breast cancer, colorectal cancer, liver cancer, melanoma and
several other cancers (13), but few
studies have reported the involvement of SIRT1 in gynecological
malignancies. The aim of the present review was to summarize the
pathogenic roles of SIRT1 in gynecological malignancies and reveal
the etiological association and therapeutic potential of SIRT1 in
EC, cervical cancer and OC.
Pathogenic function of SIRT1 in
malignancies
Genetic damage and repair
Recent research has shown that SIRT1 exerts
protective effects on normal cells by preventing DNA damage and
promoting DNA repair; however, SIRT1 may exert the same effects on
cancer cells, thereby promoting their survival, proliferation and
metastasis (13). In addition, SIRT1
exerts carcinogenic effects by deacetylating specific
molecules.
After deacetylating histones, SIRT1 affects the
chromatin topology and densely binds chromatin into
heterochromatin, thereby protecting DNA from the effects of
ultraviolet rays and oxidation and further inhibiting the onset of
malignancies (14,15). When damage occurs, SIRT1 binds to
damaged DNA fragments and changes their transcriptional activity by
histone deacetylase and adjacent promoter methylation mediated by
the recruitment of DNA methyltransferases (DNMTs) (16–18).
Histone methylation is coactivated by complexes consisting of E2H2,
DNMTs and γH2AX, and is involved in transcriptional silencing and
protection. Additionally, a previous review concluded that SIRT1
facilitates DNA damage repair (DDR), including homologous
recombination (HR) repair, non-homologous end-joining (NHEJ)
repair, mismatch repair and base excision repair, and regulates the
cell cycle through key molecules such as ataxia-telangiectasia
mutated (ATM), p53-binding protein 1, KU70, the FOXO family, MSH2,
MSH6 and apurinic/apyrimidinic endonuclease APEX1 (Fig. 1) (13,19).
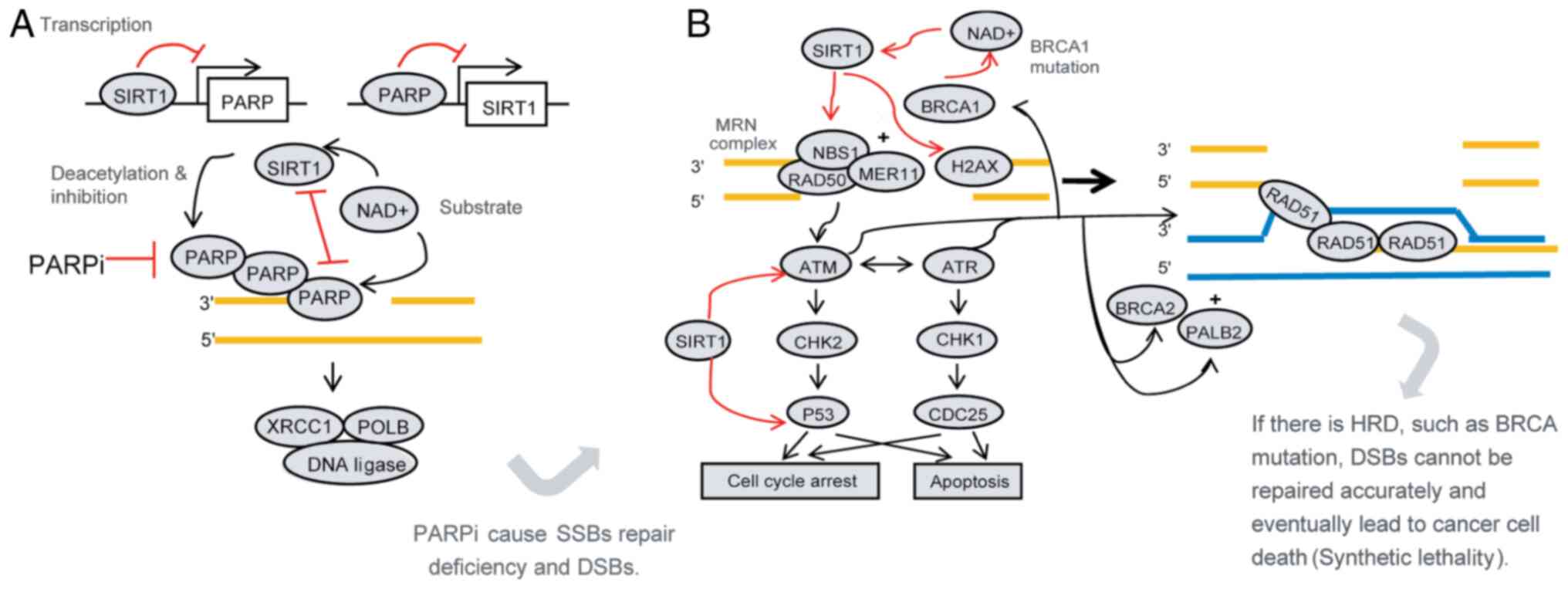 | Figure 1.Mechanism of PARPi in the treatment
of ovarian cancer and the possible role of SIRT1. Synthetic
lethality is the principle of PARPi; i.e., PARPi cause SSB repair
defects leading to DSBs. When HRD coexists, a large number of DSBs
cannot be repaired or are erroneously repaired, which eventually
leads to cancer cell death. (A) SIRT1 interacts with PARP. SIRT1
reduces the level and function of PARP protein through
transcription, post-translational modification (deacetylation) and
competition for substrates, which may affect the therapeutic effect
of PARPi. (B) SIRT1 promotes homologous recombination repair. SIRT1
increases the activity of NBS1, ATM and H2AX through deacetylation
and promotes HR. Furthermore, it promotes cell cycle arrest and
inhibits apoptosis, which promotes cell survival. When BRCA1 is
mutated, HR repair defects may be compensated to some extent by
increasing the expression of SIRT1. These findings indicate that
SIRT1 may affect the therapeutic efficacy of PARPi. PARPi,
poly(ADP-ribose) polymerase inhibitors; SSBs, single-strand breaks;
DSBs, double-strand breaks; HRD, homologous recombination
deficiency; NAD+, nicotinamide adenine dinucleotide;
XRCC1, X-ray repair complementing defective repair in Chinese
hamster cells 1; H2AX, histone H2AX; POLB, DNA polymerase-β; ATR,
ATR serine/threonine kinase; ATM, ATM serine/threonine kinase;
CHK1, checkpoint kinase 1; CHK2, checkpoint kinase 2; PALB2,
partner and localizer of BRCA2. |
The dissociation of SIRT1 from a previous binding
site and its subsequent specific binding to a damaged site to
assist in DDR may cause problems. One problem is that separation
may alter the chromatin conformation from heterochromatin to
euchromatin, lifting the transcriptional suppression of genes that
should be transcriptionally silent, which is likely to cause cancer
(18). Furthermore, the binding of
SIRT1 to damaged fragments should be transient, persisting just
long enough to permit repair; otherwise, genes that are supposed to
be silent may be expressed and serve as driving factors of
tumors.
The deacetylation of targeted proteins may also be
involved in carcinogenesis. p53 is a critical tumor suppressor, and
deficiency in its expression exists in a variety of malignancies,
whereas its activity is significantly reduced when p53 is
deacylated (20). The functions of
the FOXO family members, which regulate the cell cycle, may be
inhibited by SIRT1, thereby enabling damage repair but arresting
apoptosis (21). As a result, many
tumor cells with defects in transcription have sufficient time to
correct these errors, survive and proliferate.
Metabolism
Tumor cells grow more rapidly compared with normal
cells and, thus, they require more energy and materials for
proliferation. We found reports of several metabolic changes in the
literature, among which glycolipid metabolism is the most
fundamental metabolic change (22–24). The
majority of tumor cells abide by the particular glucose metabolism
principle known as the ‘Warburg effect’, which refers to the
reliance of tumor cells on aerobic glycolysis instead of the
tricarboxylic acid cycle to obtain energy (25,26). A
likely explanation for this effect is that the process of
glycolysis will assemble the necessary materials for cancer cell
proliferation, since not all glucose is converted into
CO2. Furthermore, the accumulation of lactic acid (the
product of glycolysis) promotes cell proliferation and stimulates
neovascularization (27). Lipid
metabolism also contributes to the proliferation and development of
cancer cells. Lipid synthesis provides indispensable materials for
cell proliferation, whereas lipolysis offers energy apart from that
of glycolysis; both processes involve some essential
transcriptional factors, such as peroxisome proliferator-activated
receptor (PPAR)γ and sterol regulatory element-binding proteins
(SREBPs) (22,24,28). SIRT1
has broad and opposite functions in the glucose and lipid
metabolism of malignant cells (29–31), as
shown in Tables I–III. SIRT1 directly regulates the
expression, activity and localization of enzymes involved in
glycolysis, and it can also indirectly adjust glucose metabolism by
affecting glycolysis-related transcriptional factors (Table I) (32,33).
Similarly, the diverse effects of SIRT1 may also be observed in
lipid synthesis and lipolysis (Tables
II and III) (32,33).
 | Table I.Effects of SIRT1 on glycolysis in
various tissues. |
Table I.
Effects of SIRT1 on glycolysis in
various tissues.
| Regulation | Promoting | Inhibiting |
|---|
| Direct | In pancreatic
cancer, SIRT1 upregulates glucose transporters and other critical
enzymes; combination with GAPDH keeps it in the cytoplasm and
prevents it from entering the nucleus. | In the liver,
SIRT1, Forkhead box O3 and nuclear respiratory factor 1 form
complexes, increasing the expression of SIRT6, which would silence
glycolysis-associated genes. |
| Indirect | Increases HIF-1α,
c-Myc and liver kinase B1-AMP-activated protein kinase-dependent
glycolysis | SIRT1 can
deacetylate and inhibit the activity of P300, and further decrease
the transcription of HIF-1α; inhibition of glycolysis by activating
peroxisome proliferator-activated receptor γ coactivator 1α and
phosphoglycerate mutase 1 |
 | Table III.Effects of SIRT1 on lipolysis. |
Table III.
Effects of SIRT1 on lipolysis.
| Regulation | Promoting | Inhibiting |
|---|
| Direct | – | The complexes of
SIRT1 and SIRT6 increase lipolysis, providing more energy, and
decrease the use of glycolysis. |
| Indirect | Activates
peroxisome proliferator-activated receptor γ coactivator 1α;
upregulates the level of Forkhead box O1 and further increases the
activity of adipose triglyceride lipase. | – |
 | Table II.Effects of SIRT1 on lipid
synthesis. |
Table II.
Effects of SIRT1 on lipid
synthesis.
| Regulation | Promoting | Inhibiting |
|---|
| Direct | Acetic acid is
transferred into acetyl-CoA, a key enzyme of lipid synthesis, under
the action of ACSS1 and ACSS2. Deacetylation by SIRT1 increases the
activity of ACSS1 | The complexes of
SIRT1 and SIRT6 inhibit lipid synthesis |
| Indirect | – | Inhibits the
expression of sterol regulatory element-binding proteins, increases
the expression of AMP-activated protein kinase. |
Role of SIRT1 in non-gynecological
malignancies
SIRT1 is associated with tumorigenesis, but its role
varies among different organs and tissues; SIRT1 is upregulated in
patients with primary colon cancer, prostatic carcinoma, acute
myelogenous leukemia, cutaneous melanoma and non-melanoma skin
cancer, but it is downregulated in breast cancer and hepatocellular
carcinoma (13). However, Moore et
al (34) revealed that SIRT1 is
overexpressed in estrogen receptor (ER)-positive breast carcinoma,
suggesting suppressive and promoting actions in disparate molecular
types of malignancies. Moreover, Zhong et al (35) demonstrated that SIRT1 has a negative
impact on colorectal cancer by downregulating the expression of key
proteins that participate in double-strand break repair, such as
BRCA2, RAD50 and FANCA, by impacting the recruitment of hMOF, which
belongs to the histone acetyltransferase family. In chronic myeloid
leukemia, SIRT1 has been shown to induce BCR-ABL mutations and drug
resistance to tyrosine kinase inhibitors (36,37),
although Jeong et al (38)
reported that SIRT1 enhances the NHEJ pathway by deacetylating and
activating KU70.
SIRT1 may also promote tumor invasion and metastasis
(39,40). Studies on breast cancer have revealed
that SIRT1 can increase epithelial-to-mesenchymal transition (EMT)
by overexpressing related transcriptional factors, such as SNAIL
and Twist, to encourage local infiltration and distant metastasis
(41–43). In addition, SIRT1 decreases the level
of matrix metalloproteinases (MMPs) through the TGFβ pathway,
thereby reducing adhesion and connection among epithelial cells and
further promoting migration (43,44).
Chemotherapy and radiotherapy are widely used as
adjuvant postoperative treatments and they may prognosis. SIRT1 may
be involved in resistance to treatment; however, a putative
mechanism has not been proposed. The cytotoxic effects of platinum
involve the formation of cross-links between double strands of DNA
or within a single strand of DNA; the repair of these
misconnections require activation of the nucleotide excision repair
(NER) pathway (45,46). SIRT1 promotes the phosphorylation of
the ATR serine/threonine kinase and further increases the level of
cAMP, which can enhance NER (47).
Therefore, SIRT1 may be associated with platinum chemoresistance.
Additionally, SIRT1 promotes glycolysis to block the cell apoptosis
induced by radiotherapy and promotes cancer cell survival (48).
With the emergence of immunosuppressants, such as
programmed cell death-1 inhibitors and cytotoxic
T-lymphocyte-associated protein-4 inhibitors, immunotherapy has an
increasingly important role in the treatment of malignancies
(49). The human immune system
recognizes and kills tumor cells by a costimulatory signal, but a
coinhibitory signal may help these cells escape immune
surveillance. Immunosuppressants break inhibitory signals and
reactivate the immune system, enabling cytotoxic T lymphocytes
(CTLs) to recognize and kill tumor cells (50,51). In
addition, cancer cells can expose specific antigens after
chemotherapy and other treatments, triggering the immune response,
such as recruiting dendritic cells (DCs) and promoting their
maturation to present these antigens to CTLs (52–54).
Moreover, CTLs can produce a number of inflammatory factors, such
as interferon-γ, and mediate the long-term effects of chemotherapy
(54,55). SIRT1 may undermine the immune system
and indirectly change the effect of antitumor therapy. Research
focused on aging-related diseases has revealed that SIRT1 can limit
NF-κB pathway-mediated inflammation and reduce the number of
monocytes, which are the precursors of DCs and other cells of the
mononuclear phagocytic system (56–58). Other
studies have shown that stress increases SIRT1 expression and then
changes the glucocorticoid level, which inhibits the body's immune
system (34), whereas exogenous
administration of glucocorticoids counteracts the antitumor effect
of SIRT1 (59).
Role of SIRT1 in gynecological
malignancies
Pathogenic effect
EC
Traditionally, EC is divided into two subtypes based
on its histological characteristics (60). Type I EC is endometrioid EC (EEC),
accounting for ~80% of all EC cases, whereas the remaining
histological patterns, such as serous and clear cell EC, are
classified as type II EC.
Researchers have detected significantly higher
expression of SIRT1 in EC cells (ECCs) compared with that in normal
endometrial cells, indicating that SIRT1 may contribute to the
onset of EC (61,62). However, Bartosch et al
(63) investigated SIRT1 mRNA and
protein expression by quantitative PCR and immunohistochemistry,
respectively, in 76 patients with EC and 30 non-EC subjects, and
found that SIRT1 mRNA was underexpressed and SIRT1 protein was
overexpressed in ECs. Additionally, significantly lower levels of
SIRT1 mRNA were found in type II ECs compared with type I ECs, but
no significant associations were found between the SIRT1 protein
level and the histological subtype, grade, stage, or lymphovascular
invasion. These results suggest that the effects of SIRT1 on the
pathogenesis of EC are complex. Although the mechanism through
which SIRT1 promotes EC remains unclear, there are significant
correlations between SIRT1 expression and risk factors for EC.
EECs are estrogen-dependent, as the exposure to a
continuous estrogen overload unopposed by progesterone may lead to
endometrial hyperplasia, which may evolve into complex atypical
hyperplasia and, eventually, progress into cancer (6,64). The ER
pathway is the traditional pathway for 17β-estradiol (E2) (65), which is activated by the binding of E2
to ERα; the E2-ER complexes bind to the promoters of target genes
in the nucleus to regulate transcription with the help of
coactivators, such as P300, PPARγ and PPARγ coactivator 1α, leading
to cancer onset and progression (66–68). The
ER pathway is also linked to other signaling pathways, such as the
EGFR, PI3K and ERK pathways (69,70). In
addition to participating in the ER pathway, E2 can also bind to
G-protein coupled receptors (GPCRs) to exert similar effects
quickly through a non-classical pathway when ER is not expressed
(71).
SIRT1 affects the expression of ERα and the
secretion of E2, resulting in the development and maintenance of
estrogen-related cancer: E2-ERα complexes promote the expression of
SIRT1, which is consistent with the observation that SIRT1 is
overexpressed in most ERα-positive breast cancer samples (72). In turn, SIRT1 increases the ERα level
to enhance the carcinogenic effect of estrogen, forming a positive
feedback loop. Similarly, the SIRT1 inhibitor reduces the levels of
ERα and interferes with cellular proliferation (73), and E2-GPCR complexes have a parallel
function (74). Most importantly,
SIRT1 directly facilitates the secretion of E2 by proliferating the
expression and function of aromatase (75). However, several studies have indicated
that SIRT1 may suppress the function of ERα (34,76,77).
Other risk factors for ECCs are obesity, diabetes
and hypertension (6), and the roles
of SIRT1 in these diseases have been described in the literature.
SIRT1 has been shown to induce mitochondrial-related gene
expression and help maintain a healthy weight in mice (78). In animals fed a high-fat, high-sugar
diet, SIRT1 improves insulin sensitivity, maintains a normal
glucose level in the plasma, and reduces the complications of
diabetes (79,80). Insulin secretion is an ATP-dependent
process (81), while SIRT1 can
increase the level of ATP by inhibiting UCP2 to promote insulin
secretion and activate NeuroD and MafA, two transcription factors
that promote insulin synthesis (82,83). In
addition, SIRT1 can reduce the levels of low-density lipoprotein
and cholesterol, increase high-density lipoprotein, obstruct lipid
oxidation, platelet aggregation and vascular smooth muscle cell
proliferation, and protect cardiovascular cells (84).
Molecular studies have identified PTEN mutation as
the most common gene mutation driving EEC, and the PI3K/AKT pathway
is known to be affected by PTEN (6).
SIRT1 has been shown to inhibit the function of the PTEN protein by
deacetylation, thereby activating the PI3K/AKT pathway (85), indicating that SIRT1 may directly
promote the development of precancerous lesions.
Type II ECs are usually estrogen-independent, and
age is the main risk factor (6).
SIRT1 increases the lifespan and resists aging in disparate
species, as it is involved in the morphology and function of
telomerase (86). p53 acts as a
cancer-driving gene in type II ECs (6), while SIRT1 can limit the expression and
function of p53.
Cervical cancer
Close to 100% of cervical cancer cases are caused by
high-risk HPV, with HPV16 and HPV18 being responsible for 70% of
the cases (5). HPV is a small
double-stranded DNA virus encoding eight proteins (early stage: E1,
E2 and E4-E7; late-stage: L1 and L2), among which E6 and E7 are
responsible for progression from cervical intraepithelial neoplasia
to cancer (87,88).
SIRT1 is overexpressed in squamous intraepithelial
neoplasia and squamous cell carcinoma (SCC), and its expression is
increased in advanced illness (89,90).
Extensive variation in SIRT1 expression in SCC from low to high
suggests that SIRT1 may be an indicator of early lesions (90). Possible causes for the facilitating
effect of SIRT1 on cervical cancer are shown in Fig. 2.
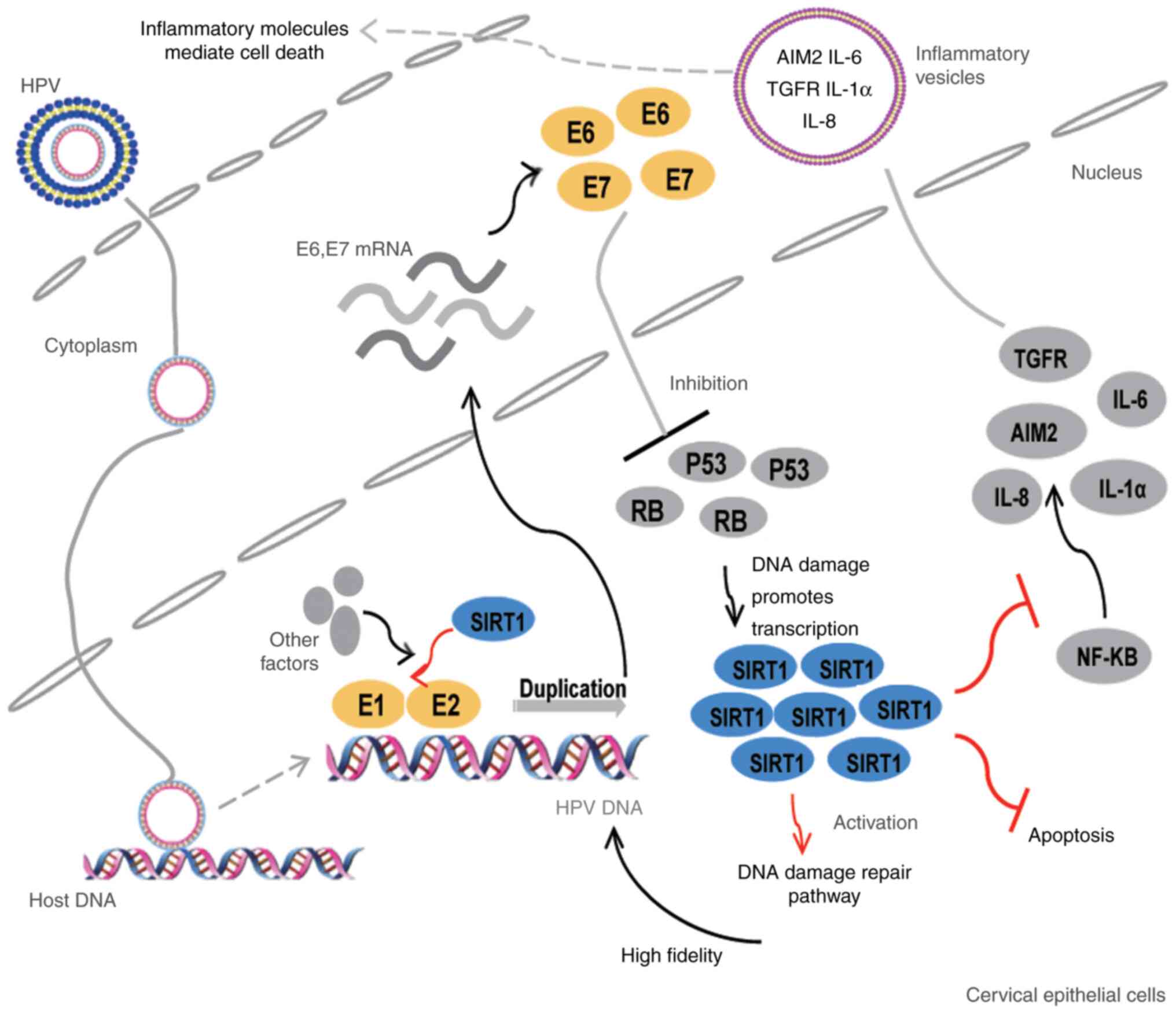 | Figure 2.SIRT1 promotes persistent HPV
infection and malignant transformation of cervical epithelial
cells. SIRT1 promotes the onset of cervical cancer as follows: i)
As a component of the E1-E2-replicating complex, it participates in
the DNA duplication of HPV; ii) HPV infection causes host DNA
damage and increases SIRT1 expression, which activates the DNA
damage repair process (particularly HR). As a result, high fidelity
of HPV DNA replication is ensured; iii) SIRT1 inhibits the
apoptosis of infected epithelial cells; iv) SIRT1 decreases the
transcription of inflammation-related factors, such as AIM2 and
ILs, thereby helping infected cells escape the innate antiviral
immunity. HPV, human papillomavirus; AIM2, absent in melanoma 2;
IL, interleukin; TGFR, transforming growth factor receptor; NF-κB,
nuclear factor κB; RB, retinoblastoma transcriptional
corepressor. |
SIRT1 cooperates with the E1 and E2 proteins to
ensure the quantity and high fidelity of HPV genome replication
(91,92). When infected with HPV, cervical
epithelial cells undergo DNA damage, leading to the recruitment of
DDR-associated proteins at the E1-E2 binding position in the
nucleus and the initiation of HR-dependent replication (5,87,88). SIRT1 is a component of the E1-E2
replication complex and a necessary molecule for initiating
replication (91). DDR also
stimulates the transcription of SIRT1, which deacetylates Nbs1 and
then activates ATM; these processes are followed by the recruitment
of the MRN complex and the enhancement of replication. Furthermore,
deacetylated Werner helicase displays a greater ability to work
with E1 and E2, guaranteeing the fidelity of viral replication
(92).
As the downstream target of E7, SIRT1 protects
HPV-infected cells from aging and apoptosis (93,94). E6
lowers the level of p53 and further inhibits cell apoptosis, while
the expression of retinoblastoma protein (e.g., pRb) is affected by
E7; these proteins contribute to cell cycle regulation. Both p53
and pRb are essential factors for the survival and proliferation of
cervical cancer cells (87,88). E7 upregulates SIRT1, and the latter
reduces apoptosis by deacetylating target proteins, such as FOXO3
and p53. By contrast, SIRT1 has been shown to be significantly
downregulated after E7 knockout, and specific knockout of SIRT1 has
been shown to induce tumor cell death (93,94).
SIRT1 may also help infected cells escape innate
antiviral immune responses in mammals (95). The overexpression of SIRT1 in tumor
cells suppresses the transcription of absent in melanoma 2 (AIM2),
a key factor of antiviral immunity, by NF-κB. When SIRT1 is
suppressed, the levels of AIM2 and associated inflammasome genes
increase and lead to infected cell death through the immune
response. Of note, SITR1-knockout cells release vesicles containing
AIM2 and inflammasomes, which can be transferred to adjacent cells
and cause cell death, demonstrating that the role of SIRT1 is not
confined to a single cell.
OC
The function of SIRT1 in epithelial OC displays
significant heterogeneity. Differentially expressed genes and
differential expression network analyses of the ArrayExpress
database have revealed that SIRT1 is a hub gene and pathogenic gene
for OC (96). Studies based on the
Hendrix, GEPIA and Bonome databases show the same expression
pattern of SIRT1 (97). However,
Kanda et al (98) reported
that SIRT1 is upregulated in epithelial OC, and peptide analysis of
the plasma verified that the level of SIRT1 precursor is higher in
patients with OC compared with that in healthy participants
(99).
This heterogeneity may be attributed to tumor
components. SIRT1 expression is significantly lower in cancer cells
compared with that in stromal cells (100). In tumor cells, SIRT1 deacetylates
and inhibits high mobility group box 1, a multifunctional
chromosome-related protein that promotes the occurrence and
development of OC, which results in the inhibition of metastasis
and angiogenesis (101).
In addition, different pathological types of OC may
also express varying levels of SIRT1. A study demonstrated that
SIRT1 was significantly increased in serous OC (SOC), but its level
was not associated with disease stage or grade, suggesting that
SIRT1 may facilitate tumorigenesis rather than progression
(98). However, another study
demonstrated that SOC exhibited the same level of SIRT1 as normal
tissues, and that this pattern was due to the high mutation
frequency of p53 in SOC, which limits the effect of p53
deacetylation on tumor promotion (102). In addition, SIRT1 may be associated
with malignant transformation in endometriosis, given its high
expression in endometriosis, endometrioid OC and clear cell OC
(102). Ovarian sex cord-stromal
tumors may also be associated with SIRT1. In granulosa cell tumors,
SIRT1 deacylates and inhibits FOXL2, causing cell cycle arrest and
DDR, which promotes tumorigenesis, whereas nicotinamide, a SIRT
suppressor, inhibits tumor cell proliferation (103). Furthermore, SIRT1 has been shown to
be overexpressed in yolk sac cell tumors, which promotes tumor cell
proliferation and differentiation and maintains the germ cell
state, which may cause recurrence, whereas SIRT1 inhibitors have
been shown to inhibit differentiation and invasiveness (104).
Tumor development
EC
SIRT1 overexpression promotes EC progression, which
is inhibited by specific SIRT1 inhibitors (61). As a synthetic inhibitor of histone
deacetylase, MHY2256 can significantly decrease SIRT1 levels,
thereby increasing the levels and activities of p53, p21 and other
cell cycle proteins, which induce autophagy and apoptosis (105). The cancer-promoting effect of SIRT1
may also stem from metabolic changes. SIRT1 can increase the
expression of SREBP-1, a nuclear lipogenic transcription factor,
and promote the biosynthesis of fatty acids and triglycerides
(62). However, miR-29c can bind to
the 3′ untranslated region of SIRT1 and inhibit its expression,
causing cell death (106); by
contrast, OGFRP1, a long non-coding (lnc)RNA, has the capacity to
reduce the production of miR-124-3p and further increase the level
of SIRT1 in cancer cells, thereby promoting invasion and metastasis
of ECs (107).
Cervical cancer
SIRT1 promotes the metastasis of cervical cancer. A
decrease in miR-29 in malignant cells can reduce SIRT1
transcriptional suppression; the resulting increased levels of
SIRT1 can promote EMT by increasing the expression and activity of
MMP9 and enhancing cell migration and invasion (108,109).
The lncRNA TUG1, which is upregulated in cancer cells and may act
as a molecular sponge, can bind to miR-138-5p and amplify SIRT1,
leading to cell proliferation via the Wnt/β-catenin pathway
(110), whereas the overexpression
of miR-138-5p can inhibit the transcription of SIRT1 and further
arrest cells in the G0/G1 phase, thereby inducing apoptosis
(111). However, it has been shown
that, when pyruvate augments the binding between the histone gene
promoter and SIRT1, heterochromatin density and genome imbalance
suppress cell proliferation (112).
OC
SIRT1 plays a dual role in OC metastasis. SIRT1 may
promote tumor metastasis. The deacetylation of cortactin mediated
by SIRT1 increases the expression of vimentin, a mesenchymal cell
marker, and reduces that of E-cadherin, an epithelial cell marker,
thereby promoting EMT and tumor cell migration (113,114).
SIRT1 expression has been shown to be correlated with ER expression
and to impact the effect of E2-ER complexes on EMT (115). Amyotrophic lateral sclerosis,
hypoxia and 15d-PGJ2 induce apoptosis and reduce the migration of
cells due to the reduced levels of SIRT1 (116–118).
However, other research has indicated that higher levels of SIRT1
in OC suppress invasion and metastasis by increasing the epithelial
phenotype (119). SIRT1 deacetylates
and activates KLF4, which binds to the CLDN5 promoter to maintain
the characteristics of epithelial cells (120). SIRT1 can also lower the EMT-inducing
zinc finger E-box binding homeobox 1 by deacetylating
hypoxia-inducible factor (HIF)1α (121). Rikkunshito, a Japanese herbal
medicine, can decrease EMT by stimulating the expression of SIRT1
(98).
SIRT1 also has opposing roles in OC cell
proliferation. SIRT1 reduces the levels of reactive oxygen species,
promoting tumor cell proliferation and reducing apoptosis (122). miR-494 and artesunate accelerate
cell death by downregulating SIRT1 (123,124).
Additionally, 15-deoxy-Δ12,14-prostaglandin J2, a SIRT1 inhibitor,
and its derivatives exert anticancer effects by blocking the cell
cycle and triggering apoptosis and autophagy (125). Calorie restriction and metformin
have been shown to increase the expression of SIRT1 in a mouse
model and cause significant decreases in the tumor load in the
peritoneum, liver, kidney, spleen and intestine; furthermore,
growth factors, such as insulin-like growth factor-1, insulin,
interleukin-1, monocyte chemoattractant protein-1 and vascular
endothelial growth factor, in the plasma and ascitic fluid have
been found to decrease the expression of SIRT1 (126,127).
Treatment
EC
Chemotherapy is a crucial method for the
postoperative adjuvant therapy of EC, and SIRT1 is closely
associated with platinum and paclitaxel resistance (61). Progesterone therapy is another common
adjuvant treatment; it is used mainly as conservative treatment of
patients who wish to preserve their fertility and as adjuvant
treatment for patients with advanced disease (6). Researchers have found that SIRT1 is
overexpressed in medroxyprogesterone acetate (MPA)-resistant tumors
and that the level of progesterone receptor (PR) expression is
decreased in those tumors (128). It
has been found that, after SIRT1 is knocked out, the levels of PR
are restored and tumor sensitivity to MPA is recovered, indicating
that SIRT1 participates in progesterone resistance.
Cervical cancer
SIRT1 has been shown to increase multidrug
resistance-associated protein (MRP) expression on the cell
membrane, inducing chemotherapy resistance in paclitaxel-resistant
tumor cells (129). In addition,
SIRT1 is partly responsible for the transformation of
chemoresistance, as it transfers deacetylated FOXO1 and ATP-binding
cassette transporter p-glycoprotein and MRP to neighboring cells
(130). Moreover, SIRT1 induces
p53-dependent chemoresistance by p53 deacetylation; for example,
the response to doxorubicin was shown to be restored in
SIRT1-knockout cells (131).
Surgery after two to three cycles of neoadjuvant
chemotherapy (NACT) is an alternative treatment for patients with
locally advanced cervical cancer whose tumors are too large to
operate or are at an inoperable stage (3). Currently, clinicians lack predictive
indicators for evaluating NACT efficiency; therefore, disease
progression during NACT may occur in some patients. SIRT1 may be
used as such a predictor. Teramae et al (132) divided patients with locally advanced
cervical cancer who had received NACT into two groups based on the
level of SIRT1; the weighted score of the low group was ≤4, whereas
that of the high group was ≥6. They found that the patients in the
low-SIRT1 group significantly benefited from NACT, indicating that
an elevated SIRT1 level is a negative indicator of NACT.
OC
SIRT1 is highly expressed in SOC patients with
chemoresistance. Its high expression in these patients is due to
its stimulating effect on MRP (133,134).
Cancer stem cells (CSCs) are a possible source of drug resistance
and a cause of relapse in malignant tumors. Researchers have found
that the percentage of EpCAM+CD45+ (CSC
potential surface marker) cells in ascites is increased in patients
with high-SIRT1 expression, and that hypoxia increases SIRT1 levels
by affecting HIF1α and NF-κB, thereby maintaining the stemness
characteristics of CSCs (135).
Following exposure to cytotoxic drugs, intracellular SIRT1 levels
have been shown to be upregulated and to further improve the
transcription of stemness-associated genes, leading to acquired
chemotherapy resistance (114).
Recently, PARPi, which cause defects in
single-strand break repair, have become the most promising targeted
drugs for OC and were recommended by the National Comprehensive
Cancer Network guidelines as first-line maintenance therapy
(136). PARPi kill tumor cells when
HR defects exist, of which the BRCA mutation is the most common,
also known as ‘synthetic lethality’ (Fig.
1) (137). Moreover, the damage
caused by the continuous binding of PARP-PARPi to DNA is far more
lethal than the reduction in PARP protein; therefore, the amount
and function of PARP proteins are crucial for PARPi efficacy
(138). SIRT1 may suppress the
synthesis and function of PARP (139) and impair the therapeutic effect of
PARPi (Fig. 1). As both PARP and
SIRT1 require NAD+, they have a competitive
relationship. SIRT1 can post-translationally modify PARP and affect
its function; when overexpressed, SIRT1 can deacetylate PARP-1 and
inhibit its function; SIRT1 can also specifically bind to the
promoter of PARP and inhibit its transcription. In addition, SIRT1
promotes HR repair and impedes PARPi (Fig. 1). Notably, the loss of the BRCA1
function increases NAD+ synthesis and SIRT1 activity,
suggesting that SIRT1 may partially compensate for the HR repair
effect when BRCA1 is defective (140).
Prognosis
There is abundant evidence suggesting that SIRT1
overexpression is responsible for poor prognosis in EC (61), cervical cancer (95,108,110)
and OC (97,98,102).
After HPV infection of cervical epithelial cells, increased SIRT1
expression is closely associated with poor prognosis. Similarly,
decreases in miR-29a and miR-138-5p levels lead to an increase in
SIRT1 levels and poor prognosis. High levels of SIRT1 in OC,
particularly SOC, are associated with reductions in the
progression-free and overall survival of patients with OC.
Conclusions
The present review summarized the possible
pathogenic role of SIRT1 in malignant tumors, which involves DDR
and metabolism. The articles included in this review were focused
on different pathways and factors. As a result, SIRT1 was shown to
serve a dual role in cancer promotion as well as cancer
suppression. In addition, the functions of SIRT1 in
non-gynecological malignancies are discussed in the present review,
and exhibit tissue specificity. Furthermore, in this review, the
possible roles of SIRT1 in the occurrence, development and
treatment of gynecological malignancies were discussed. Although
the role of SIRT1 in the pathogenesis of cervical cancer has been
elucidated, further research is needed to understand the mechanisms
underlying the involvement of SIRT1 in EC and OC. SIRT1 is
associated with multiple risk factors for EC, such as high estrogen
levels, hypertension, metabolic syndrome, driving mutations, and
disruptions in certain signaling pathways; we herein aimed to
summarize gaps in research and identify topics for further
research. Additionally, SIRT1 is associated with the invasiveness,
metastasis and treatment resistance of tumors. Therefore, SIRT1 may
serve as an indicator of poor prognosis and as a target for cancer
treatment. Most importantly, we herein highlighted the ability of
SIRT1 to interact with PARPi. Since SIRT1 counteracts the
therapeutic effect of PARPi, it was hypothesized that SIRT1
inhibitors may act synergistically with PARPi; however, the
application of PARPi and SIRT1 inhibitors in OC requires further
investigation. Finally, although SIRT1 is involved in the stemness
regulation of CSCs, no articles on the niche of CSCs and SIRT1 were
found. However, we observed that the role of SIRT1 in the CSC niche
had become a research hotspot for other types of cancer, such as
breast, lung and gastrointestinal cancer; therefore, it may
represent a promising topic for future research in the field of
gynecological oncology.
Acknowledgements
Not applicable.
Funding
This project was supported by CAMS Innovation Fund
for Medical Sciences (grant no. CIFMS-2017-I2M-1-002) and by the
Fund of The National Key R&D Program of China 2016YFC1303700
(affiliated project 2016YFC1303701).
Availability of data and materials
The data that support the findings of the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JC, LP and HC equally contributed to the study
conception and design and material preparation. The first draft of
the manuscript was written by JC, and all authors have critically
revised the manuscript for important intellectual content. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaccarella S, Lortet-Tieulent J, Plummer
M, Franceschi S and Bray F: Worldwide trends in cervical cancer
incidence: Impact of screening against changes in disease risk
factors. Eur J Cancer. 49:3262–3273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
WHO. Cervical cancer, . World Health
Organization; Geneva: 2018, http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/enJanuary
10–2020
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nogueiras R, Habegger KM, Chaudhary N,
Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT
and Tschöp MH: Sirtuin 1 and sirtuin 3: Physiological modulators of
metabolism. Physiol Rev. 92:1479–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morris BJ: Seven sirtuins for seven deadly
diseases of aging. Free Radic Biol Med. 56:133–171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bitterman KJ, Anderson RM, Cohen HY,
Latorre-Esteves M and Sinclair DA: Inhibition of silencing and
accelerated aging by nicotinamide, a putative negative regulator of
yeast sir2 and human SIRT1. J Biol Chem. 277:45099–45107. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guarente L and Franklin H: Epstein
lecture: Sirtuins, aging, and medicine. N Engl J Med.
364:2235–2244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alves-Fernandes DK and Jasiulionis MG: The
role of SIRT1 on DNA damage response and epigenetic alterations in
cancer. Int J Mol Sci. 20:31532019. View Article : Google Scholar
|
|
14
|
O'Hagan HM: Chromatin modifications during
repair of environmental exposure-induced DNA damage: A potential
mechanism for stable epigenetic alterations. Environ Mol Mutagen.
55:278–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ura K, Araki M, Saeki H, Masutani C, Ito
T, Iwai S, Mizukoshi T, Kaneda Y and Hanaoka F: ATP-dependent
chromatin remodeling facilitates nucleotide excision repair of
UV-induced DNA lesions in synthetic dinucleosomes. EMBO J.
20:2004–2014. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kala R, Shah HN, Martin SL and Tollefsbol
TO: Epigenetic-based combinatorial resveratrol and pterostilbene
alters DNA damage response by affecting SIRT1 and DNMT enzyme
expression, including SIRT1-dependent γ-H2AX and telomerase
regulation in triple-negative breast cancer. BMC Cancer.
15:6722015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding N, Bonham EM, Hannon BE, Amick TR,
Baylin SB and O'Hagan HM: Mismatch repair proteins recruit DNA
methyltransferase 1 to sites of oxidative DNA damage. J Mol Cell
Biol. 8:244–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng CX: SIRT1, is it a tumor promoter or
tumor suppressor? Int J Biol Sci. 5:147–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dobbin MM, Madabhushi R, Pan L, Chen Y,
Kim D, Gao J, Ahanonu B, Pao PC, Qiu Y, Zhao Y and Tsai LH: SIRT1
collaborates with ATM and HDAC1 to maintain genomic stability in
neurons. Nat Neurosci. 16:1008–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ong ALC and Ramasamy TS: Role of
Sirtuin1-p53 regulatory axis in aging, cancer and cellular
reprogramming. Ageing Res Rev. 43:64–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guarente L: Sirtuins in aging and disease.
Cold Spring Harb Symp Quant Biol. 72:483–488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamanaka RB and Chandel NS: Targeting
glucose metabolism for cancer therapy. J Exp Med. 209:211–215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maan M, Peters JM, Dutta M and Patterson
AD: Lipid metabolism and lipophagy in cancer. Biochem Biophys Res
Commun. 504:582–589. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kleszcz R, Paluszczak J and Baer-Dubowska
W: Targeting aberrant cancer metabolism - The role of sirtuins.
Pharmacol Rep. 67:1068–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taylor DM, Maxwell MM, Luthi-Carter R and
Kazantsev AG: Biological and potential therapeutic roles of sirtuin
deacetylases. Cell Mol Life Sci. 65:4000–4018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haigis MC and Guarente LP: Mammalian
sirtuins-emerging roles in physiology, aging, and calorie
restriction. Genes Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chalkiadaki A and Guarente L: The
multifaceted functions of sirtuins in cancer. Nat Rev Cancer.
15:608–624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye X, Li M, Hou T, Gao T, Zhu WG and Yang
Y: Sirtuins in glucose and lipid metabolism. Oncotarget.
8:1845–1859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu S, Dong Z, Ke X, Hou J, Zhao E, Zhang
K, Wang F, Yang L, Xiang Z and Cui H: The roles of sirtuins family
in cell metabolism during tumor development. Semin Cancer Biol.
57:59–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moore RL, Dai Y and Faller DV: Sirtuin 1
(SIRT1) and steroid hormone receptor activity in cancer. J
Endocrinol. 213:37–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong J, Ji L, Chen H, Li X, Zhang J, Wang
X, Wu W, Xu Y, Huang F, Cai W and Sun ZS: Acetylation of hMOF
modulates H4K16ac to regulate DNA repair genes in response to
oxidative stress. Int J Biol Sci. 13:923–934. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shah NP, Nicoll JM, Nagar B, Gorre ME,
Paquette RL, Kuriyan J and Sawyers CL: Multiple BCR-ABL kinase
domain mutations confer polyclonal resistance to the tyrosine
kinase inhibitor imatinib (STI571) in chronic phase and blast
crisis chronic myeloid leukemia. Cancer Cell. 2:117–125. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roth M, Wang Z and Chen WY: SIRT1 and LSD1
competitively regulate KU70 functions in DNA repair and mutation
acquisition in cancer cells. Oncotarget. 7:50195–50214. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeong J, Juhn K, Lee H, Kim SH, Min BH,
Lee KM, Cho MH, Park GH and Lee KH: SIRT1 promotes DNA repair
activity and deacetylation of Ku70. Exp Mol Med. 39:8–13. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Byles V, Zhu L, Lovaas JD, Chmilewski LK,
Wang J, Faller DV and Dai Y: SIRT1 induces EMT by cooperating with
EMT transcription factors and enhances prostate cancer cell
migration and metastasis. Oncogene. 31:4619–4629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Simic P, Williams EO, Bell EL, Gong JJ,
Bonkowski M and Guarente L: SIRT1 suppresses the
epithelial-to-mesenchymal transition in cancer metastasis and organ
fibrosis. Cell Rep. 3:1175–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakopoulou L, Tsirmpa I, Alexandrou P,
Louvrou A, Ampela C, Markaki S and Davaris PS: MMP-2 protein in
invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on
overall survival. Breast Cancer Res Treat. 77:145–155. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdelmawgoud H and El Awady RR: Effect of
Sirtuin 1 inhibition on matrix metalloproteinase 2 and Forkhead box
O3a expression in breast cancer cells. Genes Dis. 4:240–246. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi L, Tang X, Qian M, Liu Z, Meng F, Fu
L, Wang Z, Zhu WG, Huang JD, Zhou Z and Liu B: A SIRT1-centered
circuitry regulates breast cancer stemness and metastasis.
Oncogene. 37:6299–6315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
García-Vizcaíno EM, Liarte S,
Alonso-Romero JL and Nicolás FJ: Sirt1 interaction with active
Smad2 modulates transforming growth factor-β regulated
transcription. Cell Commun Signal. 15:502017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ceccaldi R, O'Connor KW, Mouw KW, Li AY,
Matulonis UA, D'Andrea AD and Konstantinopoulos PA: A unique subset
of epithelial ovarian cancers with platinum sensitivity and PARP
inhibitor resistance. Cancer Res. 75:628–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jarrett SG, Carter KM, Bautista RM, He D,
Wang C and D'Orazio JA: Sirtuin 1-mediated deacetylation of XPA DNA
repair protein enhances its interaction with ATR protein and
promotes cAMP-induced DNA repair of UV damage. J Biol Chem.
293:19025–19037. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Joo HY, Woo SR, Shen YN, Yun MY, Shin HJ,
Park ER, Kim SH, Park JE, Ju YJ, Hong SH, et al: SIRT1 interacts
with and protects glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
from nuclear translocation: Implications for cell survival after
irradiation. Biochem Biophys Res Commun. 424:681–686. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Esfahani K, Roudaia L, Buhlaiga N, Del
Rincon SV, Papneja N and Miller WH Jr: A review of cancer
immunotherapy: From the past, to the present, to the future. Curr
Oncol. 27 (Suppl 2):S87–S97. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Van Coillie S, Wiernicki B and Xu J:
Molecular and cellular functions of CTLA-4. Adv Exp Med Biol.
1248:7–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
52
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunological effects of conventional chemotherapy
and targeted anticancer agents. Cancer Cell. 28:690–714. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma Y, Adjemian S, Mattarollo SR, Yamazaki
T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh
K, et al: Anticancer chemotherapy-induced intratumoral recruitment
and differentiation of antigen-presenting cells. Immunity.
38:729–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma Y, Pitt JM, Li Q and Yang H: The
renaissance of anti-neoplastic immunity from tumor cell demise.
Immunol Rev. 280:194–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vacchelli E, Ma Y, Baracco EE, Sistigu A,
Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et
al: Chemotherapy-induced antitumor immunity requires formyl peptide
receptor 1. Science. 350:972–978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Morris BJ: Seven sirtuins for seven deadly
diseases of aging. Free Radic Biol Med. 56:133–171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qureshi AA, Guan XQ, Reis JC, Papasian CJ,
Jabre S, Morrison DC and Qureshi N: Inhibition of nitric oxide and
inflammatory cytokines in LPS-stimulated murine macrophages by
resveratrol, a potent proteasome inhibitor. Lipids Health Dis.
11:762012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lanzilli G, Cottarelli A, Nicotera G,
Guida S, Ravagnan G and Fuggetta MP: Anti-inflammatory effect of
resveratrol and polydatin by in vitro IL-17 modulation.
Inflammation. 35:240–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang H, Xia L, Chen J, Zhang S, Martin V,
Li Q, Lin S, Chen J, Calmette J, Lu M, et al:
Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced
antitumor immunity. Nat Med. 25:1428–1441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Asaka R, Miyamoto T, Yamada Y, Ando H,
Mvunta DH, Kobara H and Shiozawa T: Sirtuin 1 promotes the growth
and cisplatin resistance of endometrial carcinoma cells: A novel
therapeutic target. Lab Invest. 95:1363–1373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin L, Zheng X, Qiu C, Dongol S, Lv Q,
Jiang J, Kong B and Wang C: SIRT1 promotes endometrial tumor growth
by targeting SREBP1 and lipogenesis. Oncol Rep. 32:2831–2835. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bartosch C, Monteiro-Reis S, Almeida-Rios
D, Vieira R, Castro A, Moutinho M, Rodrigues M, Graça I, Lopes JM
and Jerónimo C: Assessing sirtuin expression in endometrial
carcinoma and non-neoplastic endometrium. Oncotarget. 7:1144–1154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang P, Chandra V and Rastinejad F:
Structural overview of the nuclear receptor superfamily: Insights
into physiology and therapeutics. Annu Rev Physiol. 72:247–272.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Burns KA and Korach KS: Estrogen receptors
and human disease: An update. Arch Toxicol. 86:1491–1504. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sanchez R, Nguyen D, Rocha W, White JH and
Mader S: Diversity in the mechanisms of gene regulation by estrogen
receptors. Bioessays. 24:244–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Weigel NL and Zhang Y: Ligand-independent
activation of steroid hormone receptors. J Mol Med (Berl).
76:469–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cenni B and Picard D: Ligand-independent
activation of steroid receptors: New roles for old players. Trends
Endocrinol Metab. 10:41–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kinyamu HK and Archer TK: Modifying
chromatin to permit steroid hormone receptor-dependent
transcription. Biochim Biophys Acta. 1677:30–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Perissi V and Rosenfeld MG: Controlling
nuclear receptors: The circular logic of cofactor cycles. Nat Rev
Mol Cell Biol. 6:542–554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Filardo EJ, Quinn JA, Bland KI and
Frackelton AR Jr: Estrogen-induced activation of Erk-1 and Erk-2
requires the G protein-coupled receptor homolog, GPR30, and occurs
via trans-activation of the epidermal growth factor receptor
through release of HB-EGF. Mol Endocrinol. 14:1649–1660. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Elangovan S, Ramachandran S, Venkatesan N,
Ananth S, Gnana-Prakasam JP, Martin PM, Browning DD, Schoenlein PV,
Prasad PD, Ganapathy V and Thangaraju M: SIRT1 is essential for
oncogenic signaling by estrogen/estrogen receptor α in breast
cancer. Cancer Res. 71:6654–6664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yao Y, Li H, Gu Y, Davidson NE and Zhou Q:
Inhibition of SIRT1 deacetylase suppresses estrogen receptor
signaling. Carcinogenesis. 31:382–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Santolla MF, Avino S, Pellegrino M, De
Francesco EM, De Marco P, Lappano R, Vivacqua A, Cirillo F,
Rigiracciolo DC, Scarpelli A, et al: SIRT1 is involved in oncogenic
signaling mediated by GPER in breast cancer. Cell Death Dis.
6:e18342015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Holloway KR, Barbieri A, Malyarchuk S,
Saxena M, Nedeljkovic-Kurepa A, Cameron Mehl M, Wang A, Gu X and
Pruitt K: SIRT1 positively regulates breast cancer associated human
aromatase (CYP19A1) expression. Mol Endocrinol. 27:480–490. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim MY, Woo EM, Chong YT, Homenko DR and
Kraus WL: Acetylation of estrogen receptor alpha by p300 at lysines
266 and 268 enhances the deoxyribonucleic acid binding and
transactivation activities of the receptor. Mol Endocrinol.
20:1479–1493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu Z, Yang Y, Li B, Li Y, Xia K, Yang Y,
Li X, Wang M, Li S and Wu H: Checkpoint suppressor 1 suppresses
transcriptional activity of ERα and breast cancer cell
proliferation via deacetylase SIRT1. Cell Death Dis. 9:5592018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Moynihan KA, Grimm AA, Plueger MM,
Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt M and Imai S:
Increased dosage of mammalian Sir2 in pancreatic beta cells
enhances glucose-stimulated insulin secretion in mice. Cell Metab.
2:105–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bordone L, Motta MC, Picard F, Robinson A,
Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A,
et al: Sirt1 regulates insulin secretion by repressing UCP2 in
pancreatic beta cells. PLoS Biol. 4:e312006. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kitamura YI, Kitamura T, Kruse JP, Raum
JC, Stein R, Gu W and Accili D: FoxO1 protects against pancreatic
beta cell failure through NeuroD and MafA induction. Cell Metab.
2:153–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fontana L, Meyer TE, Klein S and Holloszy
JO: Long-term calorie restriction is highly effective in reducing
the risk for atherosclerosis in humans. Proc Natl Acad Sci USA.
101:6659–6663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ikenoue T, Inoki K, Zhao B and Guan KL:
PTEN acetylation modulates its interaction with PDZ domain. Cancer
Res. 68:6908–6912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hubbard BP and Sinclair DA: Small molecule
SIRT1 activators for the treatment of aging and age-related
diseases. Trends Pharmacol Sci. 35:146–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Vonsky M, Shabaeva M, Runov A, Lebedeva N,
Chowdhury S, Palefsky JM and Isaguliants M: Carcinogenesis
associated with human papillomavirus infection. Mechanisms and
potential for immunotherapy. Biochemistry (Mosc). 84:782–799. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Almeida AM, Queiroz JA, Sousa F and Sousa
Â: Cervical cancer and HPV infection: Ongoing therapeutic research
to counteract the action of E6 and E7 oncoproteins. Drug Discov
Today. 24:2044–2057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Velez-Perez A, Wang XI, Li M and Zhang S:
SIRT1 overexpression in cervical squamous intraepithelial lesions
and invasive squamous cell carcinoma. Hum Pathol. 59:102–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Singh S, Kumar PU, Thakur S, Kiran S, Sen
B, Sharma S, Rao VV, Poongothai AR and Ramakrishna G:
Expression/localization patterns of sirtuins (SIRT1, SIRT2, and
SIRT7) during progression of cervical cancer and effects of sirtuin
inhibitors on growth of cervical cancer cells. Tumour Biol.
36:6159–6171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Das D, Smith N, Wang X and Morgan IM: The
deacetylase SIRT1 regulates the replication properties of human
papillomavirus 16 E1 and E2. J Virol. 91:e00102–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Das D, Bristol ML, Smith NW, James CD,
Wang X, Pichierri P and Morgan IM: Werner helicase control of human
papillomavirus 16 E1-E2 DNA replication is regulated by SIRT1
deacetylation. mBio. 10:e00263–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Allison SJ, Jiang M and Milner J:
Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity
protein in human cervical cancer cells. Aging (Albany NY).
1:316–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Brooks CL and Gu W: Anti-aging protein
SIRT1: A role in cervical cancer? Aging (Albany NY). 1:278–280.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
So D, Shin HW, Kim J, Lee M, Myeong J,
Chun YS and Park JW: Cervical cancer is addicted to SIRT1 disarming
the AIM2 antiviral defense. Oncogene. 37:5191–5204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lu X, Wang J, Shan X and Li Y: Selecting
key genes associated with ovarian cancer based on differential
expression network. J BUON. 22:48–57. 2017.PubMed/NCBI
|
|
97
|
Sun X, Wang S and Li Q: Comprehensive
analysis of expression and prognostic value of sirtuins in ovarian
cancer. Front Genet. 10:8792019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kanda R, Miyagawa Y, Wada-Hiraike O,
Hiraike H, Fukui S, Nagasaka K, Ryo E, Fujii T, Osuga Y and Ayabe
T: Rikkunshito attenuates induction of epithelial-mesenchymal
switch via activation of Sirtuin1 in ovarian cancer cells. Endocr
J. 67:379–386. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Dufresne J, Bowden P, Thavarajah T,
Florentinus-Mefailoski A, Chen ZZ, Tucholska M, Norzin T, Ho MT,
Phan M, Mohamed N, et al: The plasma peptides of ovarian cancer.
Clin Proteomics. 15:412018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shin DH, Choi YJ, Jin P, Yoon H, Chun YS,
Shin HW, Kim JE and Park JW: Distinct effects of SIRT1 in cancer
and stromal cells on tumor promotion. Oncotarget. 7:23975–23987.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jiang W, Jiang P, Yang R and Liu DF:
Functional role of SIRT1-induced HMGB1 expression and acetylation
in migration, invasion and angiogenesis of ovarian cancer. Eur Rev
Med Pharmacol Sci. 22:4431–4439. 2018.PubMed/NCBI
|
|
102
|
Mvunta DH, Miyamoto T, Asaka R, Yamada Y,
Ando H, Higuchi S, Ida K, Kashima H and Shiozawa T: Overexpression
of SIRT1 is associated with poor outcomes in patients with ovarian
carcinoma. Appl Immunohistochem Mol Morphol. 25:415–421. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Benayoun BA, Georges AB, L'Hôte D,
Andersson N, Dipietromaria A, Todeschini AL, Caburet S, Bazin C,
Anttonen M and Veitia RA: Transcription factor FOXL2 protects
granulosa cells from stress and delays cell cycle: Role of its
regulation by the SIRT1 deacetylase. Hum Mol Genet. 20:1673–1686.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kojima YA, Assylbekova B, Zhao B, Nugent E
and Brown RE: Morphoproteomics identifies the EZH2 and SIRT1
pathways as potential blocks to differentiation in yolk sac tumor
of the ovary and provides therapeutic options: A case study. Ann
Clin Lab Sci. 47:88–91. 2017.PubMed/NCBI
|
|
105
|
De U, Son JY, Sachan R, Park YJ, Kang D,
Yoon K, Lee BM, Kim IS, Moon HR and Kim HS: A new synthetic histone
deacetylase inhibitor, MHY2256, induces apoptosis and autophagy
cell death in endometrial cancer cells via p53 acetylation. Int J
Mol Sci. 19:27432018. View Article : Google Scholar
|
|
106
|
Van Sinderen M, Griffiths M, Menkhorst E,
Niven K and Dimitriadis E: Restoration of microRNA-29c in type I
endometrioid cancer reduced endometrial cancer cell growth. Oncol
Lett. 18:2684–2693. 2019.PubMed/NCBI
|
|
107
|
Lv Y, Chen S, Wu J, Lin R, Zhou L, Chen G,
Chen H and Ke Y: Upregulation of long non-coding RNA OGFRP1
facilitates endometrial cancer by regulating miR-124-3p/SIRT1 axis
and by activating PI3K/AKT/GSK-3β pathway. Artif Cells Nanomed
Biotechnol. 47:2083–2090. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nan P, Niu Y, Wang X and Li Q: MiR-29a
function as tumor suppressor in cervical cancer by targeting SIRT1
and predict patient prognosis. Onco Targets Ther. 12:6917–6925.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Edatt L, Maurya AK, Raji G, Kunhiraman H
and Kumar SVB: MicroRNA106a regulates matrix metalloprotease 9 in a
sirtuin-1 dependent mechanism. J Cell Physiol. 233:238–248. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhu J, Shi H, Liu H, Wang X and Li F: Long
non-coding RNA TUG1 promotes cervical cancer progression by
regulating the miR-138-5p-SIRT1 axis. Oncotarget. 8:65253–65264.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ou L, Wang D, Zhang H, Yu Q and Hua F:
Decreased expression of miR-138-5p by lncRNA H19 in cervical cancer
promotes tumor proliferation. Oncol Res. 26:401–410. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ma R, Wu Y, Zhai Y, Hu B, Ma W, Yang W, Yu
Q, Chen Z, Workman JL, Yu X and Li S: Exogenous pyruvate represses
histone gene expression and inhibits cancer cell proliferation via
the NAMPT-NAD+-SIRT1 pathway. Nucleic Acids Res.
47:11132–11150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang Y, Zhang M, Dong H, Yong S, Li X,
Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, et al: Deacetylation
of cortactin by SIRT1 promotes cell migration. Oncogene.
28:445–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mvunta DH, Miyamoto T, Asaka R, Yamada Y,
Ando H, Higuchi S, Ida K, Kashima H and Shiozawa T: SIRT1 regulates
the chemoresistance and invasiveness of ovarian carcinoma cells.
Transl Oncol. 10:621–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Pinton G, Nilsson S and Moro L: Targeting
estrogen receptor beta (ERβ) for treatment of ovarian cancer:
Importance of KDM6B and SIRT1 for ERβ expression and functionality.
Oncogenesis. 7:152018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ding YH, Zhou ZW, Ha CF, Zhang XY, Pan ST,
He ZX, Edelman JL, Wang D, Yang YX, Zhang X, et al: Alisertib, an
Aurora kinase A inhibitor, induces apoptosis and autophagy but
inhibits epithelial to mesenchymal transition in human epithelial
ovarian cancer cells. Drug Des Devel Ther. 9:425–464.
2015.PubMed/NCBI
|
|
117
|
Sun L, Li H, Chen J, Iwasaki Y, Kubota T,
Matsuoka M, Shen A, Chen Q and Xu Y: PIASy mediates hypoxia-induced
SIRT1 transcriptional repression and epithelial-to-mesenchymal
transition in ovarian cancer cells. J Cell Sci. 126:3939–3947.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
de Jong E, Winkel P, Poelstra K and
Prakash J: Anticancer effects of 15d-prostaglandin-J2 in wild-type
and doxorubicin-resistant ovarian cancer cells: Novel actions on
SIRT1 and HDAC. PLoS One. 6:e251922011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yang T, Zhou R, Yu S, Yu S, Cui Z, Hu P,
Liu J, Qiao Q and Zhang J: Cytoplasmic SIRT1 inhibits cell
migration and invasion by impeding epithelial-mesenchymal
transition in ovarian carcinoma. Mol Cell Biochem. 459:157–169.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang X, Chen J, Sun L and Xu Y: SIRT1
deacetylates KLF4 to activate Claudin-5 transcription in ovarian
cancer cells. J Cell Biochem. 119:2418–2426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ray U, Roy SS and Chowdhury SR:
Lysophosphatidic acid promotes epithelial to mesenchymal transition
in ovarian cancer cells by repressing SIRT1. Cell Physiol Biochem.
41:795–805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hou M, Zuo X, Li C, Zhang Y and Teng Y:
Mir-29b regulates oxidative stress by targeting SIRT1 in ovarian
cancer cells. Cell Physiol Biochem. 43:1767–1776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yang A, Wang X, Yu C, Jin Z, Wei L, Cao J,
Wang Q, Zhang M, Zhang L, Zhang L and Hao C: MicroRNA-494 is a
potential prognostic marker and inhibits cellular proliferation,
migration and invasion by targeting SIRT1 in epithelial ovarian
cancer. Oncol Lett. 14:3177–3184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chen X, Zhang XL, Zhang GH and Gao YF:
Artesunate promotes Th1 differentiation from CD4+ T
cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz
J Med Biol Res. 52:e79922019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Tae IH, Park EY, Dey P, Son JY, Lee SY,
Jung JH, Saloni S, Kim MH and Kim HS: Novel SIRT1 inhibitor
15-deoxy-delta12,14-prostaglandin J2 and its derivatives exhibit
anticancer activity through apoptotic or autophagic cell death
pathways in SKOV3 cells. Int J Oncol. 53:2518–2530. 2018.PubMed/NCBI
|
|
126
|
Al-Wahab Z, Mert I, Tebbe C, Chhina J,
Hijaz M, Morris RT, Ali-Fehmi R, Giri S, Munkarah AR and Rattan R:
Metformin prevents aggressive ovarian cancer growth driven by
high-energy diet: Similarity with calorie restriction. Oncotarget.
6:10908–10923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Al-Wahab Z, Tebbe C, Chhina J, Dar SA,
Morris RT, Ali-Fehmi R, Giri S, Munkarah AR and Rattan R: Dietary
energy balance modulates ovarian cancer progression and metastasis.
Oncotarget. 5:6063–6075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang Y, Zhang L, Che X, Li W, Liu Z and
Jiang J: Roles of SIRT1/FoxO1/SREBP-1 in the development of
progestin resistance in endometrial cancer. Arch Gynecol Obstet.
298:961–969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xia X and Zhou X: Knockdown of SIRT1
inhibits proliferation and promotes apoptosis of
paclitaxel-resistant human cervical cancer cells. Cell Mol Biol
(Noisy-le-grand). 64:36–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Raji GR, Sruthi TV, Edatt L, Haritha K,
Sharath Shankar S and Sameer Kumar VB: Horizontal transfer of
miR-106a/b from cisplatin resistant hepatocarcinoma cells can alter
the sensitivity of cervical cancer cells to cisplatin. Cell Signal.
38:146–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chen H, Zhang W, Cheng X, Guo L, Xie S, Ma
Y, Guo N and Shi M: β2-AR activation induces chemoresistance by
modulating p53 acetylation through upregulating Sirt1 in cervical
cancer cells. Cancer Sci. 108:1310–1317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Teramae M, Fukuda T, Wada T, Kawanishi M,
Imai K, Yamauchi M, Yasui T and Sumi T: Sirtuin1 expression
predicts the efficacy of neoadjuvant chemotherapy for locally
advanced uterine cervical cancer. Mol Clin Oncol. 3:73–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shuang T, Wang M, Zhou Y and Shi C:
Over-expression of Sirt1 contributes to chemoresistance and
indicates poor prognosis in serous epithelial ovarian cancer (EOC).
Med Oncol. 32:2602015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Akhter MZ, Sharawat SK, Kumar V, Kochat V,
Equbal Z, Ramakrishnan M, Kumar U, Mathur S, Kumar L and
Mukhopadhyay A: Aggressive serous epithelial ovarian cancer is
potentially propagated by EpCAM+CD45+
phenotype. Oncogene. 37:2089–2103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Björklund M, Roos J, Gogvadze V and
Shoshan M: Resveratrol induces SIRT1- and energy-stress-independent
inhibition of tumor cell regrowth after low-dose platinum
treatment. Cancer Chemother Pharmacol. 68:1459–1467. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
National Comprehensive Cancer Network, .
(NCCN) Clinical Practice Guidelines in Oncology. Ovarian Cancer.
Version 3. 2019.https://www.nccn.org/professionals/physician_gls/f_guidelines.aspNovember
26–2019
|
|
137
|
Lord CJ and Ashworth A: BRCAness
revisited. Nat Rev Cancer. 16:110–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Talens F, Jalving M, Gietema JA and Van
Vugt MA: Therapeutic targeting and patient selection for cancers
with homologous recombination defects. Expert Opin Drug Discov.
12:565–581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Chung HT and Joe Y: Antagonistic crosstalk
between SIRT1, PARP-1, and −2 in the regulation of chronic
inflammation associated with aging and metabolic diseases. Integr
Med Res. 3:198–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
El Ramy R, Magroun N, Messadecq N,
Gauthier LR, Boussin FD, Kolthur-Seetharam U, Schreiber V, McBurney
MW, Sassone-Corsi P and Dantzer F: Functional interplay between
Parp-1 and SirT1 in genome integrity and chromatin-based processes.
Cell Mol Life Sci. 66:3219–3234. 2009. View Article : Google Scholar : PubMed/NCBI
|
















