An adenovirus is a non-enveloped double-stranded DNA
virus with a symmetrical icosahedral structure (9). The genome is ~36 kb in length and can
encode >40 gene products (10).
These gene products are divided into three subtypes based on their
transcription start time, including early, middle and late stages
(11). Early gene products are
predominantly responsible for coding gene regulation, including the
E region, while late gene products are predominantly responsible
for coding structural proteins, including the L region (12,13). Among
adenovirus subtypes, adenovirus serotype 3 (Ad.3) and adenovirus
serotype 5 (Ad.5) are the most commonly studied subtypes, and Ad.5
is the most commonly used subtype (14). Following infection, the oncolytic
adenovirus initially recognizes specific receptors on the surface
of tumor cells and triggers their internalization (15). Subsequently, it enters tumor cells,
viral genomes migrate to the nucleus through microtubules, and
early viral proteins in the E1 region immediately begin to be
transcribed (16). The protein binds
to Rb to release the transcription factor, E2F, which also
activates the cell cycle, allowing oncolytic adenovirus-infected
cells to enter the S phase (6,17).
Concurrently, the E1A protein maintains p53 stability and inhibits
tumor growth by relying on the p53 pathway (18). The release of E2F also triggers the
coordinated activation of viral genes, which results in the
production of new virions, the lysis of infected cells and the
spread of viral offspring (19). The
oncolytic adenovirus continuously replicates in tumor cells,
eventually lysing tumor cells and infecting other tumor cells via
the same mechanism of action (20–23). Due
to the large loading capacity of the oncolytic adenovirus vector,
therapeutic genes are commonly inserted into the adenovirus vector
(24,25). Due to the continuous replication and
accumulation of adenoviruses in tumor cells, therapeutic genes are
expressed and thus spread, playing a synergistic antitumor role
(26).
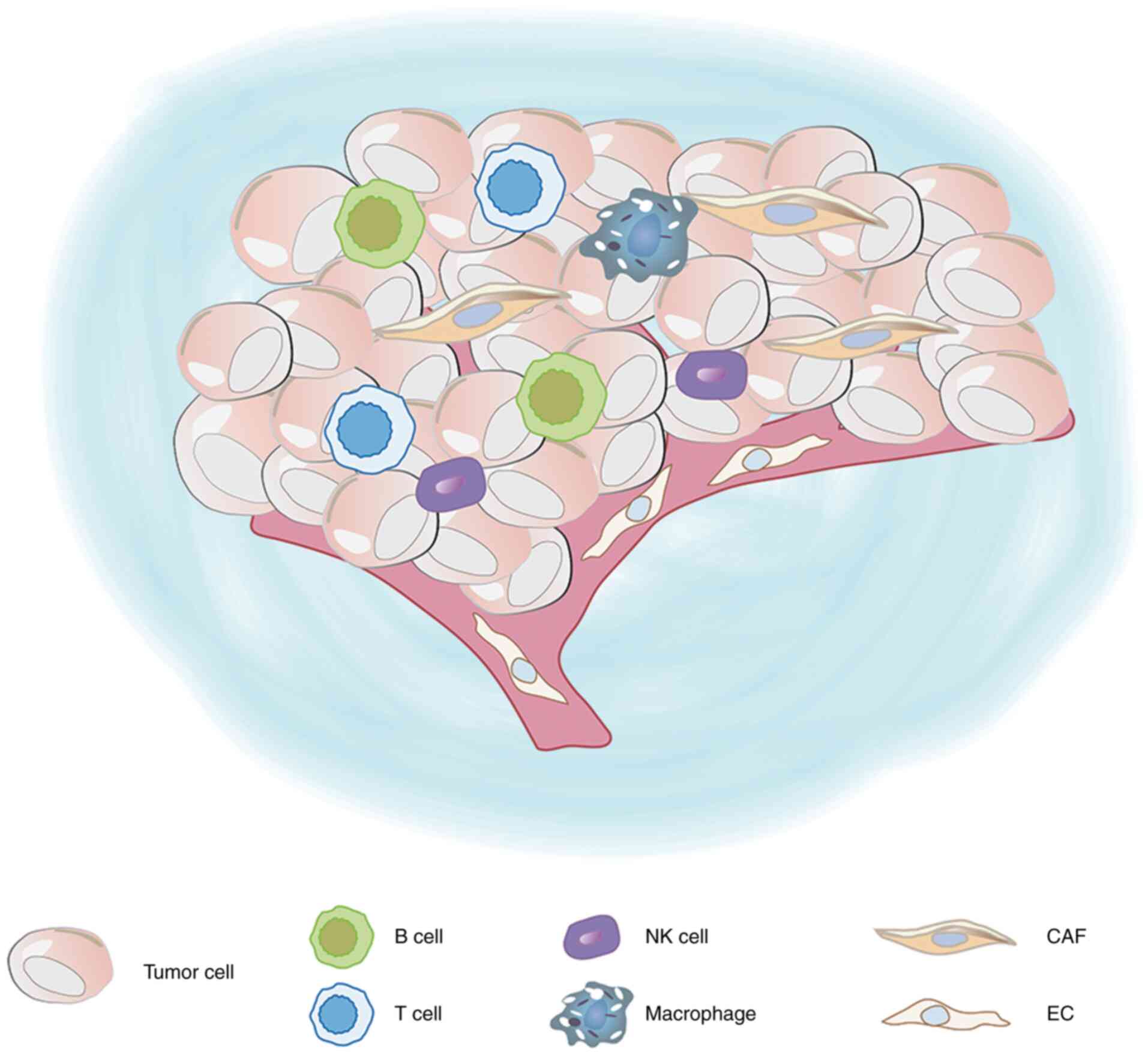
The TME is the internal environment for the growth
of tumors, which includes tumor cells (27,28),
stromal cells (tumor-associated vascular endothelial cells and
tumor-associated fibroblasts), immune cells [T lymphocytes and B
lymphocytes, tumor-associated macrophages (TAMs), dendritic cells
(DCs) and natural killer (NK) cells], the extracellular matrix
(ECM) and signaling molecules such as IL-4 and IL-10 (29–31). The
ECM includes various proteins, glycoproteins, proteoglycans and
other biochemical substances, which regulate vascular endothelial
cells and fibroblasts, and promote tumor growth and cell migration
(32) (Fig.
1). In the TME, tumor blood vessels are constantly supplying
oxygen and nutrients to support tumor growth (28,32–34). When
the tumor is exposed to hypoxic conditions locally, tumor blood
vessels receive signal stimulation and generate branches from
existing blood vessels (35,36). However, the structure of these tumor
vessels differs from that of normal vessels, with absence of
basement membranes, uneven diameter and size of the tubes, and
short circuit of arteries and veins, resulting in tumor
interstitial hypertension (37,38). Under
hypoxic conditions, tumor cells undergo glycolysis and produce more
lactic acid, which lowers the pH of the TME (39). Proton transport channels exist in all
parts of tumor tissues, which transfer the metabolized
H+ out of tumor tissues and maintain the pH in the TME
(40–42). However, pH reduction in normal tissues
results in necrosis, which is more conducive to tumor metastasis
and growth (43). Among the myeloid
progenitors cells located in the TME, myeloid-derived suppressor
cells (MDSCs), mast cells and most TAMs play key roles in promoting
tumor development (44). MDSCs are
immunosuppressive precursors of DCs, macrophages and granulocytes
(35,45). MDSCs maintain a normal tissue dynamic
balance in response to a variety of systemic infections and
injuries (33). Several animal models
have demonstrated that MDSCs can promote tumor angiogenesis and
disrupt the main mechanisms of immune surveillance by interfering
with antigen presentation, T-cell activation and NK cell killing of
DCs (46,47). It has also been reported that mast
cells are recruited into the tumor, where they release factors that
promote endothelial cell proliferation to promote tumor
angiogenesis (48–50). Increasing evidence suggests that
microenvironment-mediated external stimulation plays a key role in
tumor cell survival and drug resistance (28,51). The
complexity of the TME makes it difficult for the traditional
oncolytic virus to reverse the conditions set by the TME while
targeting tumor cells (27,52). The traditional oncolytic virus can
only inhibit the growth of tumor cells to a certain extent.
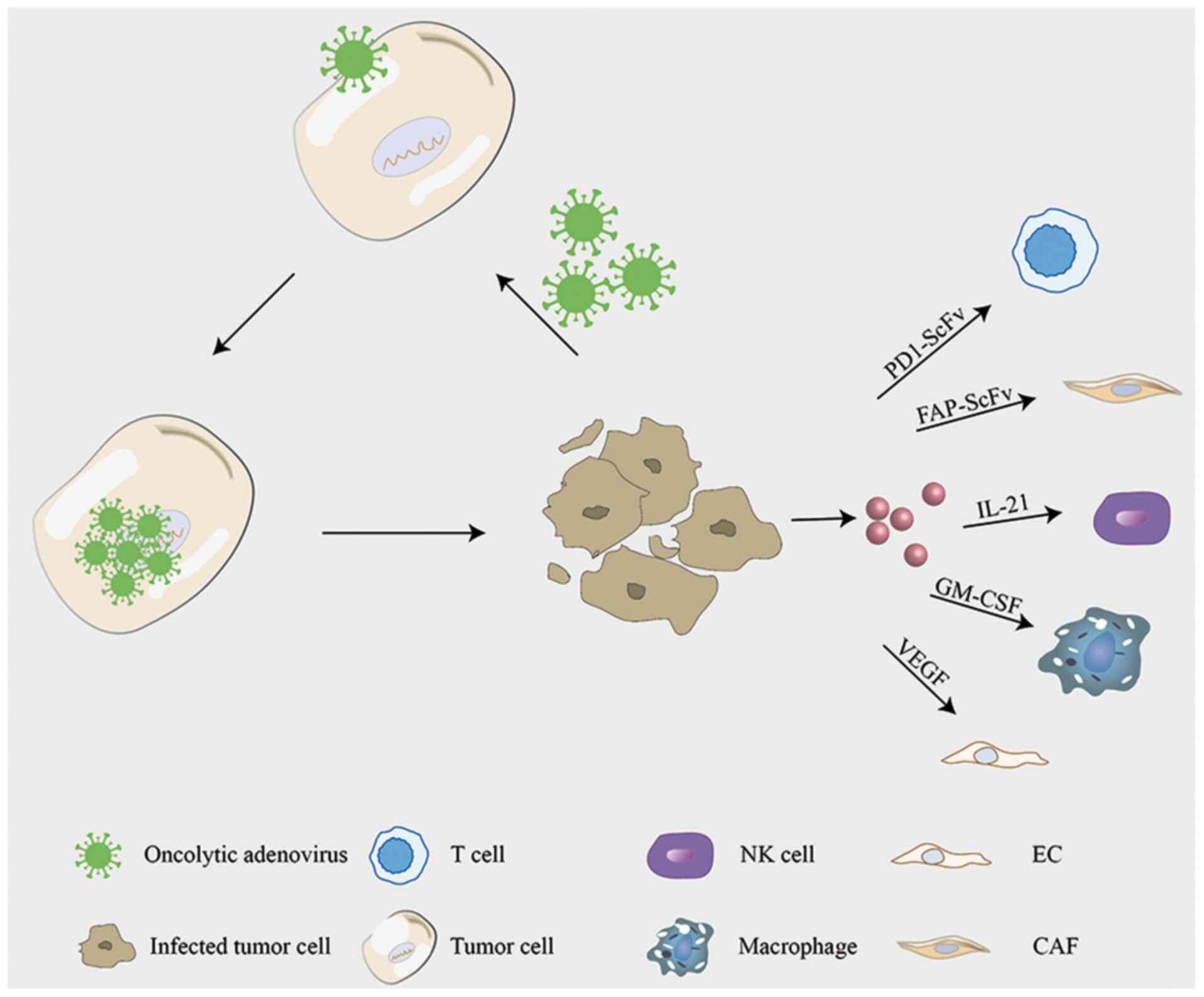
Owing to the constant improvement of genetic
engineering techniques, it is getting easier to develop oncolytic
adenovirus constructs with required properties. Preclinical trials
involve wild-type and recombinant oncolytic adenovirus (Table I), aimed to reverse the TME while
suppressing tumor cells (6,53) (Fig. 2).
Several oncolytic adenoviruses are currently undergoing clinical
trials as antitumor agents, and notably some progress has been made
in reversing the TME (Table II). An
ongoing clinical trial is testing RGD (Delta-24-rgd), a genetically
modified oncolytic adenovirus, as an agent against glioma. The
first results obtained in the phase I trials indicate that 20% of
patients showed durable responses and CD8+ T cells
infiltrated the tumor in large quantities (54). TILT-123 in preclinical studies altered
the cytokine balance in the TME towards Th1 and resulted in a
significant increase of the survival rate in severe combined
immunodeficiency (SCID) mice with human tumors (55,56). The
currently ongoing phase I trial is recruiting patients with solid
tumors to evaluate the safety.
DCs are derived from the bone marrow and play a key
role in inducing and maintaining antitumor immunity (57,58).
Infiltration of mature DCs into the tumor can enhance immune
activation and increase the recruitment of antitumor immune
effector cells and pathways (58,59).
However, in the TME, the antigen-presenting function of DCs may be
lost or inefficient (60). Tumor
cells can inhibit the function of DCs or change the TME by
recruiting immunosuppressive DCs (61). CD40 is a member of the tumor necrosis
factor receptor family and is expressed in DCs, which is a target
for infiltrating T cells (62). CD40L
is instantaneously expressed in T cells, which activates the
maturation of DCs and triggers the immune response. Adenovirus
delivery of CD40L induces DC activation, thereby inducing a Th1
immune response (63). The oncolytic
virus restricts CD40L expression in cancer cells, thus decreasing
systemic exposure and weakening the systemic immune response
(64,65). Currently, there are already two phase
I/II clinical trials involving LOAd703 that are recruiting
patients. One of the studies is recruiting patients with pancreatic
cancer to evaluate whether it supports the current treatment
standards for pancreatic cancer and whether it can improve the
survival rate of patients. Another study recruited patients with
malignant melanoma and monitored their tumor response, immune
response, virus shedding and survival rate. One of the major
virulence factors of bacteria was Helicobacter pylori
neutrophil-activating protein (HP-NAP), which is a TLR-2 agonist
capable of chemotaxis of neutrophils, and monocytes and stimulates
them to produce reactive oxygen species (66,67).
HP-NAP also induced Th1-polarized immune responses by stimulating
the secretion of interleukin (IL)-12 and IL-23 and other
pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α
and IL-8 (68).
Ag-presenting-HP-NAP-activated DCs effectively amplified Ag
specific T cells, an important characteristic of mature DCs
(69). HP-NAP-activated DCs resulted
in Th1 cytokine secretion, with high IL-12 expression, relatively
low IL-10 secretion and migrated to CCL19 (69).
TAMs are divided into specific M1-like macrophage
subsets and specific M2-like macrophage subsets, and M1-like
macrophage subsets are activated by the classical pathway and exert
notable antitumor effects (70,71). In
the TME, specific M2-like macrophage subsets are the most common,
and their cytokines IL-6, TNF, IL-1 and IL-23 promote tumor growth
and metastasis and silence T-cell function (72,73).
Selective removal of specific M2-like macrophage subsets has become
a research hotspot. Granulocyte-macrophage colony-stimulating
factor (GM-CSF) can affect macrophages, promote their rapid
differentiation to mature macrophages, prolong the life of mature
macrophages, and enhance their cellular immune function (74). By inserting GM-CSF into the Ad5
vector, ONCS-102 induced notable antitumor immunity (75). ONCOS-102 is currently being assessed
in two phase I clinical trials in advanced peritoneal malignancies
and malignant pleural mesothelioma. Ad-CD-GMCSF is an adenovirus
carrying cytomegalovirus promoter cytosine deaminase (CD) and
GM-CSF (75). Adenovirus vectors
expressing CD and GM-CSF are well tolerated in refractory tumors
(5). CD47 is a cell surface
transmembrane protein present in normal tissues (76). It is highly expressed in malignant
tumor cells and binds to signaling regulatory protein-α (SIRPα)
expressed on macrophages to inhibit macrophage phagocytosis,
resulting in immune escape. SIRPα-FC fusion protein inserted into
the oncolytic adenovirus vector blocks the binding of CD47 to
macrophages, leading to a large increase in macrophage infiltration
in tumor tissues, thus enhancing the antitumor effect (77). MMAD-IL13 loaded with IL13 demonstrated
enhanced antitumor effects by inducing apoptosis in the TME in
vivo, and decreased the percentage of specific M2-like
macrophages (78). Scott et al
(79) constructed a set of bivalent
and trivalent T-cell adapters (BiTEs/TriTEs), which can
specifically recognize CD3ε on T cells and the folate receptor or
CD206 on specific M2-like macrophages. T-cell adapters were used to
specifically direct the cytotoxicity of endogenous T cells to
M2-like macrophages and deplete M2-like macrophages in tumor
tissues. There was a significant increase in specific M1-like
macrophage fraction among surviving macrophages, indicating a
reversal of macrophage type in the TME (79).
NK cells have a notable antitumor effect in the
initial stages of tumors, which can eliminate tumor cells (30). However, at the advanced tumor stage,
NK cells gradually lose their antitumor ability and become
dysfunctional (80). IL-21 is
involved in NK cell differentiation (81), and the oncolytic adenovirus equipped
with IL-21 exerts an obvious inhibitory effect on the proliferation
of tumor cells (82). Similarly, NK
cells can be activated by IL-15 (83), and oncolytic adenovirus (Ad-E2F/IL15),
which expresses IL-15, can lyse tumor cells and coordinate with
immune cells to enhance the antitumor response (84).
CAFs are a major component of the tumor stroma,
which regulate the TME and influence the behavior of tumor cells,
and play crucial roles in the occurrence, development, invasion and
metastasis of tumors (85,86). Fibroblasts in the TME secrete growth
factors such as hepatocyte growth factor, fibroblast growth factor
and CXCL12 (87), which promote the
growth and survival of malignant cells, and act as chemokines to
induce the migration of other cells into the TME (88,89).
Concurrently, CAFs form a barrier in tumors and prevent the
effective penetration and transmission of oncolytic virus, thus
limiting its efficacy (90,91). By modifying oncolytic adenovirus, the
effects of tumor cells and CAFs will be inhibited at the same time
(92). Fibroblast activation
protein-α (FAP) is highly expressed in CAFs. The FAP single-chain
antibody is linked to an anti-human CD3 single-chain variable
region (scFv) and loaded into oncolytic adenovirus. FAP scFv, while
specifically recognizing and targeting CAFs, activates T cells and
enhances T-cell-mediated cytotoxic effects on tumor-associated
fibroblasts, thus weakening the cell barrier caused by CAFs and
enhancing oncolytic activity (24,53).
Blood vessels play a vital role in the development
of tumors, providing nutrition and metastasis channels for tumor
cells (93). The phenotype of
vascular endothelial cells changes in the TME. Tumor cells secrete
vascular endothelial growth factor (VEGF) and other endothelial
growth factors to promote the generation of tumor
neovascularization (94). Given that
the downstream target gene of microRNA (miRNA/miR)-126 is VEGF
(95), miR-126 is loaded into the
oncolytic adenovirus, namely ADCEAP-miR126/34A, which decreases the
generation of tumor blood vessels (96). Concurrently, the VEGF promoter is
inserted into the adenovirus vector, targeting the tumor vascular
endothelial cells via the same mechanism of action enhancing the
oncolysis of adenovirus (97). IL-24
is a tumor suppressor molecule with broad-spectrum antitumor
activity (98). It inhibits the
growth of tumor cells by inhibiting tumor angiogenesis (99). A previous study has demonstrated that
while expressing IL24, CRAd-IL24 significantly increased the
release of virus particles and enhanced their antitumor effect
(6).
Checkpoint molecules are regulatory molecules that
play an inhibitory role in the immune system and are critical to
maintain tolerance, prevent an autoimmune response and minimize
tissue damage by controlling the timing and intensity of the immune
response (100,101). The expression of immunological
checkpoint molecules on immune cells suppresses the immune cell
function as the host fails to produce an effective antitumor immune
response. There are numerous receptors on tumorigenic immune escape
T cells, including co-stimulatory signal receptors that can
stimulate T-cell proliferation, and co-inhibitory signal receptors
that can inhibit T-cell proliferation (102). Immune checkpoint molecules are
predominantly inhibitory molecules, in which the immune checkpoint
on T cells suppresses the immune function of T cells, causing tumor
escape (103). However, the clinical
benefits of monotherapy with immune checkpoint inhibitors, such as
anti-programmed death-1 antibody, are limited to small populations
(104). Zhang et al (105) designed an adenovirus (Ad5-PC) to
express a soluble fusion protein (programmed cell death protein
1/CD137L), which significantly increased the number of T
lymphocytes in the TME and effectively improved the survival rate
of tumor-bearing mice (105). After
loading anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)
antibody into the adenovirus vector, tumor cells were infected.
CTLA-4 antibody was significantly expressed in tumor cells and its
antitumor activity was significantly enhanced (106). Ad5/3-Δ24aCTLA4 can express CTLA-4
human intact monoclonal antibody, and in the normal donors and
patients with advanced solid tumors in the peripheral blood
mononuclear cells that were tested (107). Ad5/3-Δ24aCTLA4 significantly
enhanced the immune response and activated the pro-apoptotic effect
of T cells (107).
Recently, the transformation technology of oncolytic
adenoviruses has significantly progressed. However, increasing
evidence suggest concerns about the tumor-promoting effect of the
TME. Thus, the transformation of oncolytic viruses into TME has
become a research hot spot; however, the in vitro simulation
of the TME does not accurately reflect the human microenvironment.
Cytokines and growth factors were added to the cell culture, either
co-cultured with tumor cells and other cells, or with
three-dimensional scaffoldings to simulate the TME. However, due to
the complexity of the TME, it remains difficult to completely
simulate the human TME completely in vitro. Currently,
xenotransplantation of immunodeficient mice is the most commonly
used animal experimental method for studying human tumors. Briefly,
human tumor cells are inserted into mice; however, this fails to
fully reflect the human TME. Several factors affect the oncolytic
effect of an oncolytic virus. The strong immune response and severe
cytokine storm when the virus first enters the host may be fatal.
Subsequent challenges arise at later stages during elimination of
oncolytic virus by the immune system of the host. It is
hypothesized that the selection of oncolytic viruses will be the
focus of future research, and it will be individualized, with the
intent that the oncolytic virus most suitable for each patient can
be selected, based on the characteristics of the tumor cells and
the TME.
Not applicable.
This review was funded by Programs for the National
Natural Scientific Foundation of China (grant no. 81430035), the
Major National Science and Technology Projects-Major New Drug
Creation (grant no. 2019ZX09301-132), the Changjiang Scholars and
Innovative Research Team in University (grant no. IRT_15R13), and
the Guangxi Science and Technology Base and Talent Special Project
(grant no. AD17129003).
Not applicable.
XW and YZ conceived the subject of the review. LZ
designed the review. XW wrote the manuscript, performed the
literature research as well as interpreted the relevant literature,
and prepared the figures. LZ analyzed the review critically for
important intellectual content. XW and LZ edited and revised the
manuscript. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Giraldo NA, Sanchez-Salas R, Peske JD,
Vano Y, Becht E, Petitprez F, Validire P, Ingels A, Cathelineau X,
Fridman WH and Sautès-Fridman C: The clinical role of the TME in
solid cancer. Br J Cancer. 120:45–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Liu J, Wang W, Xiang L, Wang J,
Liu S, Zhou H and Guo Z: High expression of hnRNPA1 promotes cell
invasion by inducing EMT in gastric cancer. Oncol Rep.
39:1693–1701. 2018.PubMed/NCBI
|
|
3
|
Choi J, Gyamfi J, Jang H and Koo JS: The
role of tumor-associated macrophage in breast cancer biology.
Histol Histopathol. 33:133–145. 2018.PubMed/NCBI
|
|
4
|
Sun Y: Tumor microenvironment and cancer
therapy resistance. Cancer Lett. 380:205–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akbulut H, Coleri A, Sahin G, Tang Y and
Icli F: A bicistronic adenoviral vector carrying cytosine deaminase
and granulocyte-macrophage colony-stimulating factor increases the
therapeutic efficacy of cancer gene therapy. Hum Gene Ther.
30:999–1007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashshi AM, El-Shemi AG, Dmitriev IP,
Kashentseva EA and Curiel DT: Combinatorial strategies based on
CRAd-IL24 and CRAd-ING4 virotherapy with anti-angiogenesis
treatment for ovarian cancer. J Ovarian Res. 9:382016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Athanasopoulos T, Munye MM and Yanez-Munoz
RJ: Nonintegrating gene therapy vectors. Hematol Oncol Clin North
Am. 31:753–770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salzman R, Cook F, Hunt T, Malech HL,
Reilly P, Foss-Campbell B and Barrett D: Addressing the value of
gene therapy and enhancing patient access to transformative
treatments. Mol Ther. 26:2717–2726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stasiak AC and Stehle T: Human adenovirus
binding to host cell receptors: A structural view. Med Microbiol
Immunol. 209:325–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greber UF and Flatt JW: Adenovirus entry:
From infection to immunity. Annu Rev Virol. 6:177–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoeben RC and Uil TG: Adenovirus DNA
replication. Cold Spring Harb Perspect Biol. 5:a0130032013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao J, Zhang W and Ehrhardt A: Expanding
the spectrum of adenoviral vectors for cancer therapy. Cancers
(Basel). 12:11392020. View Article : Google Scholar
|
|
13
|
Ip WH and Dobner T: Cell transformation by
the adenovirus oncogenes E1 and E4. FEBS Lett. 594:1848–1860. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bradley RR, Maxfield LF, Lynch DM,
Iampietro MJ, Borducchi EN and Barouch DH: Adenovirus serotype
5-specific neutralizing antibodies target multiple hexon
hypervariable regions. J Virol. 86:1267–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niemann J and Kuhnel F: Oncolytic viruses:
Adenoviruses. Virus Genes. 53:700–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Machitani M, Katayama K, Sakurai F, Matsui
H, Yamaguchi T, Suzuki T, Miyoshi H, Kawabata K and Mizuguchi H:
Development of an adenovirus vector lacking the expression of
virus-associated RNAs. J Control Release. 154:285–289. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Y, Stamminger T and Hearing P:
E2F/Rb family proteins mediate interferon induced repression of
adenovirus immediate early transcription to promote persistent
viral infection. PLoS Pathog. 12:e10054152016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamauchi S, Zhong B, Kawamura K, Yang S,
Kubo S, Shingyoji M, Sekine I, Tada Y, Tatsumi K, Shimada H, et al:
Cytotoxicity of replication-competent adenoviruses powered by an
exogenous regulatory region is not linearly correlated with the
viral infectivity/gene expression or with the E1A-activating
ability but is associated with the p53 genotypes. BMC Cancer.
17:6222017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cervera-Carrascon V, Quixabeira DCA,
Havunen R, Santos JM, Kutvonen E, Clubb JHA, Siurala M, Heiniö C,
Zafar S, Koivula T, et al: Comparison of clinically relevant
oncolytic virus platforms for enhancing T cell therapy of solid
tumors. Mol Ther Oncolytics. 17:47–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang H, Liu Y, Liao W, Cao Y, Liu Q, Guo
Y, Lu Y and Xie Z: Oncolytic adenovirus programmed by synthetic
gene circuit for cancer immunotherapy. Nat Commun. 10:48012019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosewell Shaw A and Suzuki M: Recent
advances in oncolytic adenovirus therapies for cancer. Curr Opin
Virol. 21:9–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ran L, Tan X, Li Y, Zhang H, Ma R, Ji T,
Dong W, Tong T, Liu Y, Chen D, et al: Delivery of oncolytic
adenovirus into the nucleus of tumorigenic cells by tumor
microparticles for virotherapy. Biomaterials. 89:56–66. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tazawaa H, Kagawab S and Fujiwarab T:
Oncolytic adenovirus-induced autophagy: Tumor-suppressive effect
and molecular basis. Acta Med Okayama. 67:333–342. 2013.PubMed/NCBI
|
|
24
|
Freedman JD, Duffy MR, Lei-Rossmann J,
Muntzer A, Scott EM, Hagel J, Campo L, Bryant RJ, Verrill C,
Lambert A, et al: An oncolytic virus expressing a T-cell engager
simultaneously targets cancer and immunosuppressive stromal cells.
Cancer Res. 78:6852–6865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung KH, Choi IK, Lee HS, Yan HH, Son MK,
Ahn HM, Hong J, Yun CO and Hong SS: Oncolytic adenovirus expressing
relaxin (YDC002) enhances therapeutic efficacy of gemcitabine
against pancreatic cancer. Cancer Lett. 396:155–166. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yokoda RT, Nagalo BM and Borad MJ:
Oncolytic adenoviruses in gastrointestinal cancers. Biomedicines.
6:332018. View Article : Google Scholar
|
|
27
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belli C, Trapani D, Viale G, D'Amico P,
Duso BA, Della Vigna P, Orsi F and Curigliano G: Targeting the
microenvironment in solid tumors. Cancer Treat Rev. 65:22–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong S, Jeong JH, Chen Z, Chen Z and Luo
JL: Targeting tumor microenvironment by small-molecule inhibitors.
Transl Oncol. 13:57–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terren I, Orrantia A, Vitalle J,
Zenarruzabeitia O and Borrego F: NK cell metabolism and tumor
microenvironment. Front Immunol. 10:22782019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maimela NR, Liu S and Zhang Y: Fates of
CD8+ T cells in tumor microenvironment. Comput Struct
Biotechnol J. 17:1–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Najafi M, Farhood B and Mortezaee K:
Extracellular matrix (ECM) stiffness and degradation as cancer
drivers. J Cell Biochem. 120:2782–2790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo S and Deng CX: Effect of stromal cells
in tumor microenvironment on metastasis initiation. Int J Biol Sci.
14:2083–2093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jang I and Beningo KA: Integrins, CAFs and
mechanical forces in the progression of cancer. Cancers (Basel).
11:7212019. View Article : Google Scholar
|
|
35
|
Jarosz-Biej M, Smolarczyk R, Cichon T and
Kulach N: Tumor microenvironment as A ‘Game Changer’ in cancer
radiotherapy. Int J Mol Sci. 20:32122019. View Article : Google Scholar
|
|
36
|
Nishide S, Uchida J, Matsunaga S, Tokudome
K, Yamaguchi T, Kabei K, Moriya T, Miura K, Nakatani T and Tomita
S: Prolyl-hydroxylase inhibitors reconstitute tumor blood vessels
in mice. J Pharmacol Sci. 143:122–126. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carretero R, Sektioglu IM, Garbi N,
Salgado OC, Beckhove P and Hammerling GJ: Eosinophils orchestrate
cancer rejection by normalizing tumor vessels and enhancing
infiltration of CD8(+) T cells. Nat Immunol. 16:609–617. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Tian X, He L, Li Y, Pu W, Liu Q,
Tang J, Wu J, Cheng X, Liu Y, et al: Apj+ vessels drive
tumor growth and represent a tractable therapeutic target. Cell
Rep. 25:1241–1254.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-Associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohashi T, Aoki M, Tomita H, Akazawa T,
Sato K, Kuze B, Mizuta K, Hara A, Nagaoka H, Inoue N and Ito Y:
M2-like macrophage polarization in high lactic acid-producing head
and neck cancer. Cancer Sci. 108:1128–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Urbanska K and Orzechowski A:
Unappreciated role of LDHA and LDHB to control apoptosis and
autophagy in tumor cells. Int J Mol Sci. 20:20852019. View Article : Google Scholar
|
|
44
|
Fleming V, Hu X, Weber R, Nagibin V, Groth
C, Altevogt P, Utikal J and Umansky V: Targeting myeloid-derived
suppressor cells to bypass tumor-induced immunosuppression. Front
Immunol. 9:3982018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hinshaw DC and Shevde LA: The tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Safarzadeh E, Orangi M, Mohammadi H,
Babaie F and Baradaran B: Myeloid-derived suppressor cells:
Important contributors to tumor progression and metastasis. J Cell
Physiol. 233:3024–3036. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dysthe M and Parihar R: Myeloid-Derived
suppressor cells in the tumor microenvironment. Adv Exp Med Biol.
1224:117–140. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ribatti D, Tamma R and Crivellato E: Cross
talk between natural killer cells and mast cells in tumor
angiogenesis. Inflamm Res. 68:19–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Albini A, Bruno A, Noonan DM and Mortara
L: Contribution to tumor angiogenesis from innate immune cells
within the tumor microenvironment: Implications for immunotherapy.
Front Immunol. 9:5272018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kabiraj A, Jaiswal R, Singh A, Gupta J,
Singh A and Samadi FM: Immunohistochemical evaluation of tumor
angiogenesis and the role of mast cells in oral squamous cell
carcinoma. J Cancer Res Ther. 14:495–502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shee K, Yang W, Hinds JW, Hampsch RA, Varn
FS, Traphagen NA, Patel K, Cheng C, Jenkins NP, Kettenbach AN, et
al: Therapeutically targeting tumor microenvironment-mediated drug
resistance in estrogen receptor-positive breast cancer. J Exp Med.
215:895–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de Sostoa J, Fajardo CA, Moreno R, Ramos
MD, Farrera-Sal M and Alemany R: Targeting the tumor stroma with an
oncolytic adenovirus secreting a fibroblast activation
protein-targeted bispecific T-cell engager. J Immunother Cancer.
7:192019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lang FF, Conrad C, Gomez-Manzano C, Yung
WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD,
et al: Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic
Adenovirus: Replication and immunotherapeutic effects in recurrent
malignant glioma. J Clin Oncol. 36:1419–1427. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Havunen R, Siurala M, Sorsa S,
Grönberg-Vähä-Koskela S, Behr M, Tähtinen S, Santos JM, Karell P,
Rusanen J, Nettelbeck DM, et al: Oncolytic adenoviruses armed with
tumor necrosis factor alpha and interleukin-2 enable successful
adoptive cell therapy. Mol Ther Oncolytics. 4:77–86. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Santos JM, Cervera-Carrascon V, Havunen R,
Zafar S, Siurala M, Sorsa S, Anttila M, Kanerva A and Hemminki A:
Adenovirus coding for interleukin-2 and tumor necrosis factor alpha
replaces lymphodepleting chemotherapy in adoptive T cell therapy.
Mol Ther. 26:2243–2254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee YS and Radford KJ: The role of
dendritic cells in cancer. Int Rev Cell Mol Biol. 348:123–178.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Parnas O, Jovanovic M, Eisenhaure TM,
Herbst RH, Dixit A, Ye CJ, Przybylski D, Platt RJ, Tirosh I,
Sanjana NE, et al: A genome-wide CRISPR screen in primary immune
cells to dissect regulatory networks. Cell. 162:675–686. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oh DS and Lee HK: Autophagy protein ATG5
regulates CD36 expression and anti-tumor MHC class II antigen
presentation in dendritic cells. Autophagy. 15:2091–2106. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen L, Hasni MS, Jondal M and Yakimchuk
K: Modification of anti-tumor immunity by tolerogenic dendritic
cells. Autoimmunity. 50:370–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Karnell JL, Rieder SA, Ettinger R and
Kolbeck R: Targeting the CD40-CD40L pathway in autoimmune diseases:
Humoral immunity and beyond. Adv Drug Deliv Rev. 141:92–103. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vitale LA, Thomas LJ, He LZ, O'Neill T,
Widger J, Crocker A, Sundarapandiyan K, Storey JR, Forsberg EM,
Weidlick J, et al: Development of CDX-1140, an agonist CD40
antibody for cancer immunotherapy. Cancer Immunol Immunother.
68:233–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zafar S, Sorsa S, Siurala M, Hemminki O,
Havunen R, Cervera-Carrascon V, Santos JM, Wang H, Lieber A, De
Gruijl T, et al: CD40L coding oncolytic adenovirus allows long-term
survival of humanized mice receiving dendritic cell therapy.
Oncoimmunology. 7:e14908562018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Eriksson E, Milenova I, Wenthe J, Moreno
R, Alemany R and Loskog A: IL-6 signaling blockade during
CD40-mediated immune activation favors antitumor factors by
reducing TGF-β, collagen type I, and PD-L1/PD-1. J Immunol.
202:787–798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Guo X, Ding C, Lu J, Zhou T, Liang T, Ji
Z, Xie P, Liu X and Kang Q: HP-NAP ameliorates OXA-induced atopic
dermatitis symptoms in mice. Immunopharmacol Immunotoxicol.
42:416–422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Codolo G, Fassan M, Munari F, Volpe A,
Bassi P, Rugge M, Pagano F, D'Elios MM and de Bernard M: HP-NAP
inhibits the growth of bladder cancer in mice by activating a
cytotoxic Th1 response. Cancer Immunol Immunother. 61:31–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
D'Elios MM, Amedei A, Cappon A, Del Prete
G and de Bernard M: The neutrophil-activating protein of
Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS
Immunol Med Microbiol. 50:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ramachandran M, Jin C, Yu D, Eriksson F
and Essand M: Vector-encoded Helicobacter pylori
neutrophil-activating protein promotes maturation of dendritic
cells with Th1 polarization and improved migration. J Immunol.
193:2287–2296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ubil E, Caskey L, Holtzhausen A, Hunter D,
Story C and Earp HS: Tumor-secreted Pros1 inhibits macrophage M1
polarization to reduce antitumor immune response. J Clin Invest.
128:2356–2369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Myers KV, Amend SR and Pienta KJ:
Targeting Tyro3, Axl and MerTK (TAM receptors): Implications for
macrophages in the tumor microenvironment. Mol Cancer. 18:942019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Huang YJ, Yang CK, Wei PL, Huynh TT,
Whang-Peng J, Meng TC, Hsiao M, Tzeng YM, Wu AT and Yen Y:
Ovatodiolide suppresses colon tumorigenesis and prevents
polarization of M2 tumor-associated macrophages through YAP
oncogenic pathways. J Hematol Oncol. 10:602017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cho H, Seo Y, Loke KM, Kim SW, Oh SM, Kim
JH, Soh J, Kim HS, Lee H, Kim J, et al: Cancer-Stimulated CAFs
enhance monocyte differentiation and protumoral TAM activation via
IL6 and GM-CSF secretion. Clin Cancer Res. 24:5407–5421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kuryk L, Moller AW, Garofalo M, Cerullo V,
Pesonen S, Alemany R and Jaderberg M: Antitumor-specific T-cell
responses induced by oncolytic adenovirus ONCOS-102
(AdV5/3-D24-GM-CSF) in peritoneal mesothelioma mouse model. J Med
Virol. 90:1669–1673. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hayat SMG, Biancon V, Pirro M, Jaafari MR,
Hatamipour M and Sahebkar A: CD47 role in the immune system and
application to cancer therapy. Cell Oncol (Dordr.). 43:19–30. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huang Y, Lv SQ, Liu PY, Ye ZL, Yang H, Li
LF, Zhu HL, Wang Y, Cui LZ, Jiang DQ, et al: A SIRPα-Fc fusion
protein enhances the antitumor effect of oncolytic adenovirus
against ovarian cancer. Mol Oncol. 14:657–668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang KL, Li RP, Zhang BP, Gao ST, Li B,
Huang CJ, Cao R, Cheng JY, Xie XD, Yu ZH and Feng XY: Efficacy of a
new oncolytic adenovirus armed with IL-13 against oral carcinoma
models. Onco Targets Ther. 12:6515–6523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Scott EM, Jacobus EJ, Lyons B, Frost S,
Freedman JD, Dyer A, Khalique H, Taverner WK, Carr A, Champion BR,
et al: Bi- and tri-valent T cell engagers deplete tumour-associated
macrophages in cancer patient samples. J Immunother Cancer.
7:3202019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yao C, Ni Z, Gong C, Zhu X, Wang L, Xu Z,
Zhou C, Li S, Zhou W, Zou C and Zhu S: Rocaglamide enhances NK
cell-mediated killing of non-small cell lung cancer cells by
inhibiting autophagy. Autophagy. 14:1831–1844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Davis MR, Zhu Z, Hansen DM, Bai Q and Fang
Y: The role of IL-21 in immunity and cancer. Cancer Lett.
358:107–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li Y, Li YF, Si CZ, Zhu YH, Jin Y, Zhu TT,
Liu MY and Liu GY: CCL21/IL21-armed oncolytic adenovirus enhances
antitumor activity against TERT-positive tumor cells. Virus Res.
220:172–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rhode PR, Egan JO, Xu W, Hong H, Webb GM,
Chen X, Liu B, Zhu X, Wen J, You L, et al: Comparison of the
superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics
in animal models. Cancer Immunol Res. 4:49–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yan Y, Li S, Jia T, Du X, Xu Y, Zhao Y, Li
L, Liang K, Liang W, Sun H and Li R: Combined therapy with CTL
cells and oncolytic adenovirus expressing IL-15-induced enhanced
antitumor activity. Tumour Biol. 36:4535–4543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kubo N, Araki K, Kuwano H and Shirabe K:
Cancer-associated fibroblasts in hepatocellular carcinoma. World J
Gastroenterol. 22:6841–6850. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Feig C, Jones JO, Kraman M, Wells RJ,
Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL,
et al: Targeting CXCL12 from FAP-expressing carcinoma-associated
fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic
cancer. Proc Natl Acad Sci USA. 110:20212–20217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Erdogan B, Ao M, White LM, Means AL,
Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, et
al: Cancer-associated fibroblasts promote directional cancer cell
migration by aligning fibronectin. J Cell Biol. 216:3799–3816.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Erdogan B and Webb DJ: Cancer-associated
fibroblasts modulate growth factor signaling and extracellular
matrix remodeling to regulate tumor metastasis. Biochem Soc Trans.
45:229–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Arwert EN, Milford EL, Rullan A, Derzsi S,
Hooper S, Kato T, Mansfield D, Melcher A, Harrington KJ and Sahai
E: STING and IRF3 in stromal fibroblasts enable sensing of genomic
stress in cancer cells to undermine oncolytic viral therapy. Nat
Cell Biol. 22:758–766. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Puig-Saus C, Gros A, Alemany R and
Cascallo M: Adenovirus i-leader truncation bioselected against
cancer-associated fibroblasts to overcome tumor stromal barriers.
Mol Ther. 20:54–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jing Y, Chavez V, Ban Y, Acquavella N,
El-Ashry D, Pronin A, Chen X and Merchan JR: Molecular effects of
stromal-selective targeting by uPAR-retargeted oncolytic virus in
breast cancer. Mol Cancer Res. 15:1410–1420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Czekierdowska S, Stachowicz N, Chrosciel M
and Czekierdowski A: Proliferation and maturation of intratumoral
blood vessels in women with malignant ovarian tumors assessed with
cancer stem cells marker nestin and platelet derived growth factor
PDGF-B. Ginekol Pol. 88:120–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Feng SD, Mao Z, Liu C, Nie YS, Sun B, Guo
M and Su C: Simultaneous overexpression of miR-126 and miR-34a
induces a superior antitumor efficacy in pancreatic adenocarcinoma.
Onco Targets Ther. 10:5591–5604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Harada A, Uchino J, Harada T, Nakagaki N,
Hisasue J, Fujita M and Takayama K: Vascular endothelial growth
factor promoter-based conditionally replicative adenoviruses
effectively suppress growth of malignant pleural mesothelioma.
Cancer Sci. 108:116–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen QN, Chen X, Chen ZY, Nie FQ, Wei CC,
Ma HW, Wan L, Yan S, Ren SN and Wang ZX: Long intergenic non-coding
RNA 00152 promotes lung adenocarcinoma proliferation via
interacting with EZH2 and repressing IL24 expression. Mol Cancer.
16:172017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhuo B, Wang R, Yin Y, Zhang H, Ma T, Liu
F, Cao H and Shi Y: Adenovirus arming human IL-24 inhibits
neuroblastoma cell proliferation in vitro and xenograft tumor
growth in vivo. Tumour Biol. 34:2419–2426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang Y and Zheng J: Functions of immune
checkpoint molecules beyond immune evasion. Adv Exp Med Biol.
1248:201–226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li K and Tian H: Development of
small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new
therapeutic strategy for tumour immunotherapy. J Drug Target.
27:244–256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kurachi M, Barnitz RA, Yosef N, Odorizzi
PM, DiIorio MA, Lemieux ME, Yates K, Godec J, Klatt MG, Regev A, et
al: The transcription factor BATF operates as an essential
differentiation checkpoint in early effector CD8+ T cells. Nat
Immunol. 15:373–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Deng J, Wang ES, Jenkins RW, Li S, Dries
R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, et al: CDK4/6
inhibition augments antitumor immunity by enhancing T-cell
activation. Cancer Discov. 8:216–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Johnson DB, Sullivan RJ and Menzies AM:
Immune checkpoint inhibitors in challenging populations. Cancer.
123:1904–1911. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang Y, Zhang H, Wei M, Mou T, Shi T, Ma
Y, Cai X, Li Y, Dong J and Wei J: Recombinant adenovirus expressing
a soluble fusion protein PD-1/CD137L subverts the suppression of
CD8+ T cells in HCC. Mol Ther. 27:1906–1918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Du T, Shi G, Li YM, Zhang JF, Tian HW, Wei
YQ, Deng H and Yu DC: Tumor-specific oncolytic adenoviruses
expressing granulocyte macrophage colony-stimulating factor or
anti-CTLA4 antibody for the treatment of cancers. Cancer Gene Ther.
21:340–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Dias JD, Hemminki O, Diaconu I, Hirvinen
M, Bonetti A, Guse K, Escutenaire S, Kanerva A, Pesonen S, Löskog
A, et al: Targeted cancer immunotherapy with oncolytic adenovirus
coding for a fully human monoclonal antibody specific for CTLA-4.
Gene Ther. 19:988–998. 2012. View Article : Google Scholar : PubMed/NCBI
|