Introduction
Lung cancer is one of the most common malignant
tumors in the world (1). Previous
studies have shown that ~2.1 million individuals have been
diagnosed with lung cancer, accounting for 11.6% of all new cancer
cases in 2018. In addition, the number of deaths from lung cancer
is ranked first among cancer-associated deaths, accounting for
18.4% of the total cancer deaths in 2018 (1,2). Due to
untimely diagnoses and limited effective treatment options,
particularly for later stage cancers, the 5-year survival rate of
lung cancer is only 5% (3). Lung
adenocarcinoma accounts for >50% of all cases of lung cancer
(4). Mineral dust-induced gene
(mdig) is a newly discovered lung cancer-related gene, which
was first found in alveolar macrophages of coal miners (5,6). mdig
contains a Jumonji C domain with demethylase function of histone
H3K9me3, and can promote the activation of proto-oncogenes
(7,8).
mdig is also called myc-induced nuclear antigen with a molecular
weight of 53 kDa (MINA53) or nuclear protein 52 (NO52) (6,9,10). A previous study has shown that
compared with advanced lung cancer tissues, the expression levels
of mdig in early lung cancer tissues are significantly higher
(11). Therefore, it is speculated
that overexpression of mdig may be an early event in lung cancer
(6,11). It was reported that mdig possesses
oncogenic properties via antagonization of tri-methyl lysine 9 on
histone H3 and promoting ribosomal RNA synthesis (7). mdig can promote lung cancer cell
proliferation by accelerating cell cycle transition from the G1
phase to S phase (10,12), and it can also inhibit cell invasion
and migration via regulating the glycogen synthase
kinase-3β/β-catenin signaling pathway (13). However, the mechanism underlying this
paradoxical phenomenon of mdig is unclear.
The formation of tumors is typically divided into
two stages. First, normal cells are transformed into malignant
cells by the continuous activation of proto-oncogenes.
Subsequently, transformed malignant cells continue to proliferate
to form solid tumors (14).
Angiogenesis and lymphangiogenesis serve important roles in the
second stage, during which the interaction of several complex
signaling pathways is required. In addition, lack of nutrition and
oxygen within the tumors creates a hypoxic microenvironment as the
tumors continuously proliferate (15), further stimulating the activation of
proto-oncogenes, through which they synergistically regulate
angiogenesis and lymphangiogenesis (15,16).
Previous studies have shown that epidermal growth factor receptor
(EGFR) can induce angiogenesis by promoting the secretion of
vascular endothelial growth factor (VEGF)-A via both a
hypoxia-inducible factor-1α (HIF-1α)-dependent and
HIF-1α-independent manner (14,17,18). In
addition, studies have previously shown that VEGF-C and VEGF-D can
induce both angiogenesis and lymphangiogenesis (19,20).
Although it has been demonstrated that the effect of EGFR on HIF-1α
can occur under hypoxic and normoxic conditions (17,18,21–23),
the role and mechanism of mdig in tumor angiogenesis and
lymphangiogenesis have not been previously reported.
The aim of the present study was to explore the
effects of mdig on angiogenesis and lymphangiogenesis in lung
adenocarcinoma under normoxic and hypoxic conditions. The results
revealed that mdig is an oxygen-sensitive protein that promotes
tumor growth and angiogenesis by activating an
EGFR/p-EGFR/VEGF-A/VEGF-R1/R2 pathway, whilst also inhibits
lymphangiogenesis by blocking a HIF-1α/VEGF-C/D/VEGF-R3 signaling
pathway.
Materials and methods
Cell culture
Human lung adenocarcinoma cell lines A549 and H1299,
as well as 293T cells were purchased from the Cell Bank of the Type
Culture Collection of the Chinese Academy of Sciences. Human
umbilical vein endothelial cells (HUVECs) and human lymphatic
endothelial cells (HLECs) were purchased from the Cancer Institute
of Peking University Cell Bank. All the cell lines were tested for
mycoplasma using a MycoBlue™ Mycoplasma Detector according to the
manufacturer's protocol (Vazyme Biotech Co., Ltd.), which confirmed
that there was no mycoplasma contamination. All cell lines except
for 293T were cultured in RPMI-1640 medium (Hyclone; Cytiva)
supplemented with 10% FBS (Hyclone; Cytiva). 293T cells were
cultured in DMEM (Hyclone; Cytiva) containing 10% FBS. All cell
lines were maintained at 37°C with 5% CO2. Normoxic
conditions (21% O2) were achieved using an incubator
(Thermo Fisher Scientific, Inc.), whereas hypoxic conditions (1%
O2) were induced in a hypoxic chamber (model no. C-42;
Biospherix Oxycycler; BioSpherix, Ltd.). Signaling pathway agonists
and inhibitors used in the present study were all obtained from
Selleck Chemicals: EGFR agonist, NSC228155; EGFR inhibitor,
erlotinib; HIF-1α agonist, IOX2; and HIF-1α inhibitor, BAY
87-2243.
Lentivirus transfection
The mdig (accession no. NM_032778; GenBank)
overexpression lentiviral vector (LV-mdig) and its control vector
(LV-con), in addition to the mdig knockdown lentiviral vectors
(LV-mdig-RNAi 1, sequence, 5′-GGGTGATTTGTTGTACTTT-3′; LV-mdig-RNAi
2, sequence, 5′-AACGATTCAGTTTCACCAA-3′) and their control vector
(LV-mdig-RNAi-con, sequence, 5′-TTCTCCGAACGTGTCACGT-3′) were
purchased from Shanghai Genechem Co., Ltd. These vectors, which
were mixed with HitransG P transfection enhancement solution
(Shanghai Genechem Co., Ltd.), were transfected into A549, H1299
and 293T cells at multiplicities of infection of 50, 20 and 20,
respectively, in T12.5 flasks (Corning, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Ambion; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from cells. The
cDNA templates were then reverse transcribed using PrimeScript™ RT
reagent kit with gDNA Eraser according to the manufacturer's
protocol (Takara Bio, Inc.). The primers used for measuring the
expression levels of mdig, EGFR, HIF-1α and the normalization
control ACTB, were purchased from Takara Bio, Inc. The sequences of
the primers were: mdig forward, 5′-GCAACGATTCAGTTTCACCAACC-3′ and
reverse, 5′-ATGTACACATTCGAGCCAACCAAG-3′; EGFR forward,
5′-TGCATACAGTGCCACCCAGAG-3′ and reverse,
5′-GCACACTGGATACAGTTGTCTGGTC-3′; HIF-1α forward,
5′-CTCATCAGTTGCCACTTCCACATA-3′ and reverse,
5′-AGCAATTCATCTGTGCTTTCATGTC-3′; and ACTB forward,
5′-CCTGGCACCCAGCCAAT-3′ and reverse, 5′-GGGCCGGACTCGTCATAC-3′. qPCR
was subsequently performed using SYBR® Premix Ex Taq™
(Takara Bio, Inc.) in a LightCycler® 480 system (Roche
Diagnostics). The thermocycling conditions were as follows: Initial
denaturation for 5 sec, followed by 40 cycles of 95°C for 15 sec,
59°C for 30 sec and 70°C for 30 sec to detect the cycle threshold
value (Cq). The 2−ΔΔCq method was used to calculate the
relative ratio of genes, and expression was presented normalized to
ACTB expression (5,24–26).
Western blotting
Cells were lysed using RIPA lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.) containing 10% PMSF
(Beijing Solarbio Science & Technology Co., Ltd.). Equivalent
amounts (40 µg) of protein samples, which were quantified using a
bicinchoninic acid protein assay, were loaded on an 8% or 12%
SDS-gel, resolved using SDS-PAGE and subsequently transferred to
PVDF membranes (Millipore Co., Ltd.). Membranes were blocked for 2
h at room temperature using 5% nonfat dried milk, washed with TBST,
and then incubated with primary antibodies at 4°C overnight. The
primary antibodies (all at 1:1,000) used in the present study were:
Anti-mdig (mouse mAb; cat. no. sc-398521; Santa Cruz Biotechnology,
Inc.), anti-EGFR (rabbit mAb; cat. no. 4267; Cell Signaling
Technology, Inc.), anti-phospho (p)-EGF receptor (Tyr1068; rabbit
mAb; cat. no. 3777; Cell Signaling Technology, Inc.), anti-HIF-1α
(rabbit mAb; cat. no. 36169; Cell Signaling Technology, Inc.),
anti-VEGFA (mouse mAb; cat. no. ab1316; Abcam), anti-VEGFC (rabbit
polyclonal antibody; cat. no. ab9546; Abcam), anti-VEGFD (rabbit
mAb; cat. no. ab155288; Abcam), anti-VEGFR1 (rabbit mAb; cat. no.
ab32152; Abcam), anti-VEGFR2 (rabbit mAb; cat. no. 9698; Cell
Signaling Technology, Inc.), anti-VEGFR3 (rabbit mAb; cat. no.
33566; Cell Signaling Technology, Inc.), anti-histone H3 (rabbit
polyclonal antibody; cat. no. ab1791; Abcam) and anti-β-actin
(mouse mAb; cat. no. ab8224; Abcam). The membranes were then
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG (cat. no. 31460) or goat anti-mouse IgG (cat. no. 31430)
secondary antibodies (1:5,000; both from Thermo Fisher Scientific,
Inc.) at room temperature for 2 h. Clarity Western ECL Substrate
(Bio-Rad Laboratories, Inc.) was used to detect the bands in a
chemiluminescence detector (MicroChemi 4.2; DNR Bio-Imaging
Systems, Ltd.). Densitometry analysis was performed using ImageJ
version 1.8.0 (National Institutes of Health).
Co-immunoprecipitation
The mdig-overexpressing A549 cells cultured under
either normoxic or hypoxic conditions were lysed using
immunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology). Primary antibodies against mdig, EGFR or HIF-1α
were then added into the lysate to a final concentration of 5
µg/ml. In total, ~5 µl protein A/G immunoprecipitation magnetic
beads (cat. no. B23202; Bimake) were washed with
immunoprecipitation lysis buffer before being mixed with the
diluted antibodies. These mixtures were subsequently placed on a
mixer for incubation at 4°C overnight. The magnetic beads were
collected, and the proteins were denatured by the addition of 25 µl
1X SDS loading buffer. Western blotting was then performed to
assess mdig, EGFR and HIF-1α expression.
Nuclear and cytoplasmic
fractionation
The mdig-overexpressing cells were lysed using
cytoplasmic protein lysis buffer (Invent Biotechnologies, Inc.).
The lysate was centrifuged at 10,000 × g at 4°C for 5 min to
separate the proteins of the nuclear (pellet) and cytoplasmic
(supernatant) fractions. Nuclear protein lysis buffer (Invent
Biotechnologies, Inc.) was then used to lyse the precipitates,
before centrifuging again at 16,000 × g at 4°C for 30 sec, and the
supernatant was collected which contained the nuclear protein.
Conditioned medium
The conditioned medium was prepared as described
previously (23). Transfected A549
and H1299 cells, which were cultured in either normoxic or hypoxic
conditions, were first washed twice with PBS. RPMI-1640 medium
without serum was used to culture the cells further for 24 h before
collecting the culture supernatants. The cell-conditioned media was
centrifuged at 1,000 × g at 4°C for 10 min, following which the
supernatant was subsequently used for culture of HUVECs and
HLECs.
Tube formation assays of angiogenesis
and lymphangiogenesis
Tube formation assays to assess angiogenesis and
lymphangiogenesis were performed using HUVECs and HLECs,
respectively, as described previously (17,23,27).
Subsequently, 96-well plates coated with cold Matrigel (50 µl/well;
cat. no. 356234; BD Biosciences) were incubated at 37°C in the
incubators for 30 min. HUVECs and HLECs (2×104
cells/well) suspended in conditioned media from the
mdig-overexpressing A549 cells were seeded into 96-well plates
pre-coated with Matrigel at 37°C for 4–6 h. Images of the tube-like
structures were taken at a magnification of ×100 using an inverted
fluorescence microscope (Carl Zeiss AG).
Xenograft tumor studies
Female athymic nu/nu mice (aged, 4–6 weeks) were
purchased from Charles River Laboratories, Inc. All mice (n=4
mice/group) were bred in independently ventilated cages and were
provided with sterilized food and water. mdig-overexpressing A549
cells, mdig-silenced A549 cells, mdig-overexpressing A549 cells
treated with EGFR inhibitor erlotinib and mdig-overexpressing A549
cells treated with HIF-1α agonist IOX2 (5×106
cells/mouse) were suspended in PBS and then injected subcutaneously
into the axilla of the mice. After 3 weeks, the mice were
sacrificed and tumors were harvested for subsequent experiments.
The experiments involving animals were performed in accordance with
the Ethical Guidelines for Animal Care of the Institutional Animal
Care and Use Committee of the China Medical University.
Immunohistochemistry
Xenograft tumor tissues were fixed in 4%
paraformaldehyde, embedded in paraffin and sectioned into 5-µm
thick sections. The sections were then deparaffinized in xylene
followed by hydration in a descending series of ethanol solutions
before being subsequently incubated in sodium citrate buffer and
blocked with endogenous peroxidase (cat. no. SP KIT-A1; Fuzhou
Maixin Biotech Co., Ltd.) and non-specific staining blocker (cat.
no. SP KIT-B1; Fuzhou Maixin Biotech Co., Ltd.) at room
temperature. These sections were incubated with primary antibodies
at 4°C overnight. The primary antibodies used for this experiment
included: Anti-VEGFA (rabbit polyclonal antibody; cat. no. ab39250;
1:100; Abcam), anti-VEGFC (rabbit polyclonal antibody; cat. no.
ab9546; 1:100; Abcam), anti-VEGFD (rabbit mAb; cat. no. ab155288;
1:100; Abcam), anti-CD31 (rabbit polyclonal antibody; cat. no.
ab28364; 1:50; Abcam), anti-LYVE1 (rabbit polyclonal antibody; cat.
no. ab33682; 1:100; Abcam) and anti-mdig (rabbit polyclonal
antibody; cat. no. ab126282; 1:100; Abcam). Biotinylated goat
anti-mouse/rabbit IgG (cat. no. SP KIT-C1; Fuzhou Maixin Biotech
Co., Ltd.) and streptavidin-peroxidase (cat. no. SP KIT-D1; Fuzhou
Maixin Biotech Co., Ltd.) were then applied for 10 min,
respectively, at room temperature to the sections, which were
stained with DAB solution and counterstained with hematoxylin at
room temperature. Images of the sections were taken in ≥3 random
fields of view at the magnification of ×200 and ×400 using an
upright light microscope (Carl Zeiss AG).
Statistical analysis
All experimental data are presented as the mean ±
standard deviation of ≥3 experimental repeats. Data were compared
using a Student's t-test or a one-way ANOVA followed by a Dunnett's
post-hoc test in GraphPad Prism version 7.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of hypoxia on the protein
expression levels of mdig, EGFR, HIF-1α and the VEGF family
A549, H1299 and 293T cells were cultured in either
normoxic (21% O2) or hypoxic (1% O2)
conditions for 24 h before the expression levels of these proteins
were measured by western blotting. The protein expression levels of
EGFR, HIF-1α and VEGF-A/C/D were found to be significantly higher
in the cells cultured under hypoxic conditions compared with those
in cells cultured under normoxic conditions (P<0.05; Fig. 1). By contrast, mdig protein expression
levels were significantly reduced by culturing under hypoxic
conditions compared with those in cells cultured under normoxic
conditions (P<0.05; Fig. 1).
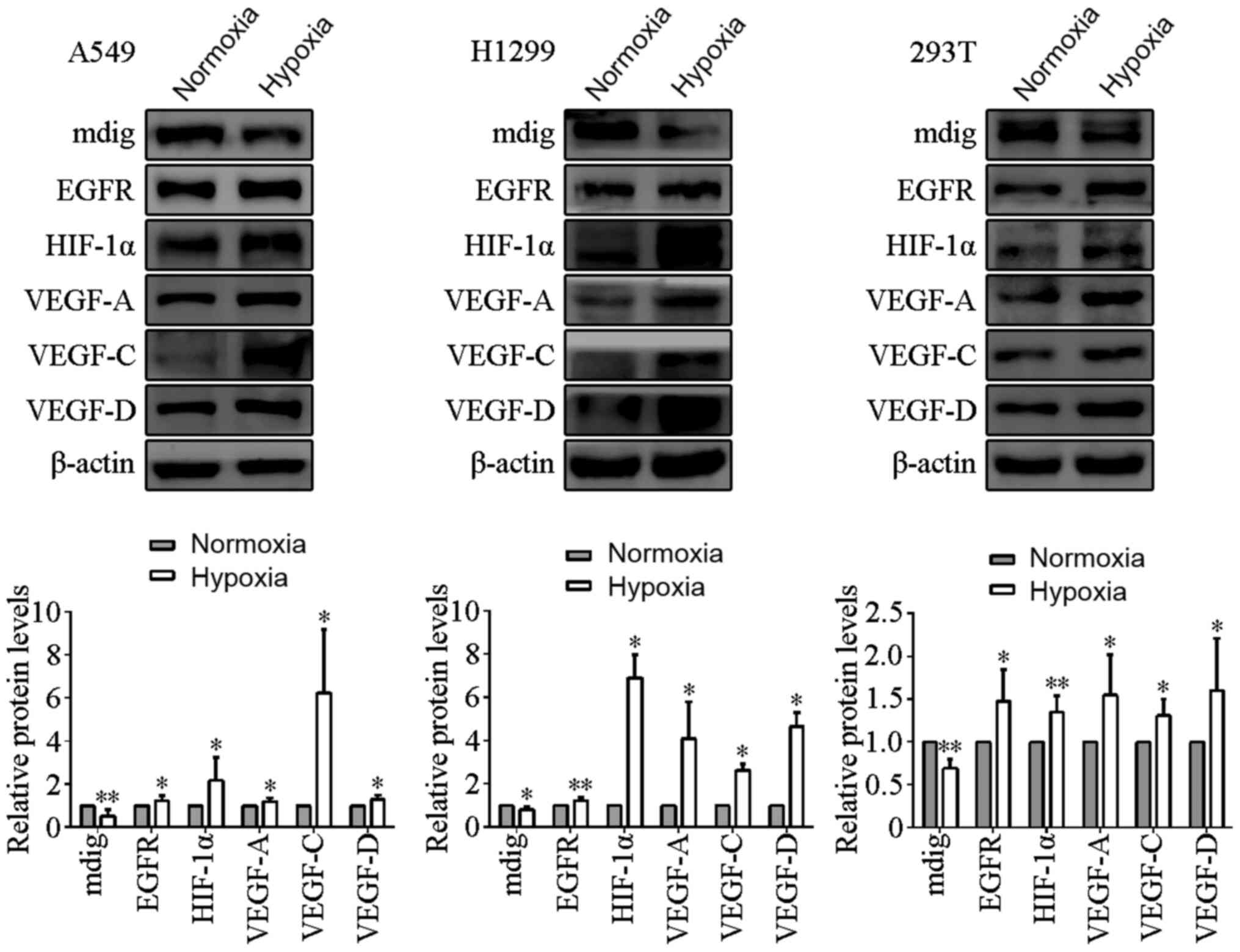 | Figure 1.Effects of hypoxia on the protein
expression levels of mdig, EGFR, HIF-1α and the VEGF family
members. A549, H1299 and 293T cells were cultured under normoxic
and hypoxic conditions. Hypoxia increased the protein expression
levels of EGFR, HIF-1α and the VEGF family members, but reduced the
expression of mdig. β-actin was used as the loading control.
*P<0.05, **P<0.01, normoxia vs. hypoxia. EGFR, epidermal
growth factor receptor; HIF-1α, hypoxia-inducible factor-1α; VEGF,
vascular endothelial growth factor; mdig, mineral dust-induced
gene. |
mdig promotes the protein expression
of EGFR and p-EGFR
To investigate the relationship between mdig and
EGFR, A549 cells were first transfected with mdig-overexpressing
LV-mdig and mdig-silencing LV-mdig-RNAi vectors, whereas H1299 and
293T cells were transfected with LV-mdig vector. The transfected
cells were then cultured under either normoxic or hypoxic
conditions. Western blotting results showed that the protein
expression levels of EGFR, in addition to the autophosphorylation
of one of its most important residues, Tyr1068 (28,29), were
shown to be significantly increased in the LV-mdig group compared
with those in the LV-con group (P<0.05). These two
aforementioned parameters were found to be significantly reduced in
the LV-mdig-RNAi group compared with those in the LV-mdig-RNAi-con
group (P<0.05; Figs. 2 and
3). However, these changes were not
significantly different between cells cultured under hypoxic and
normoxic conditions.
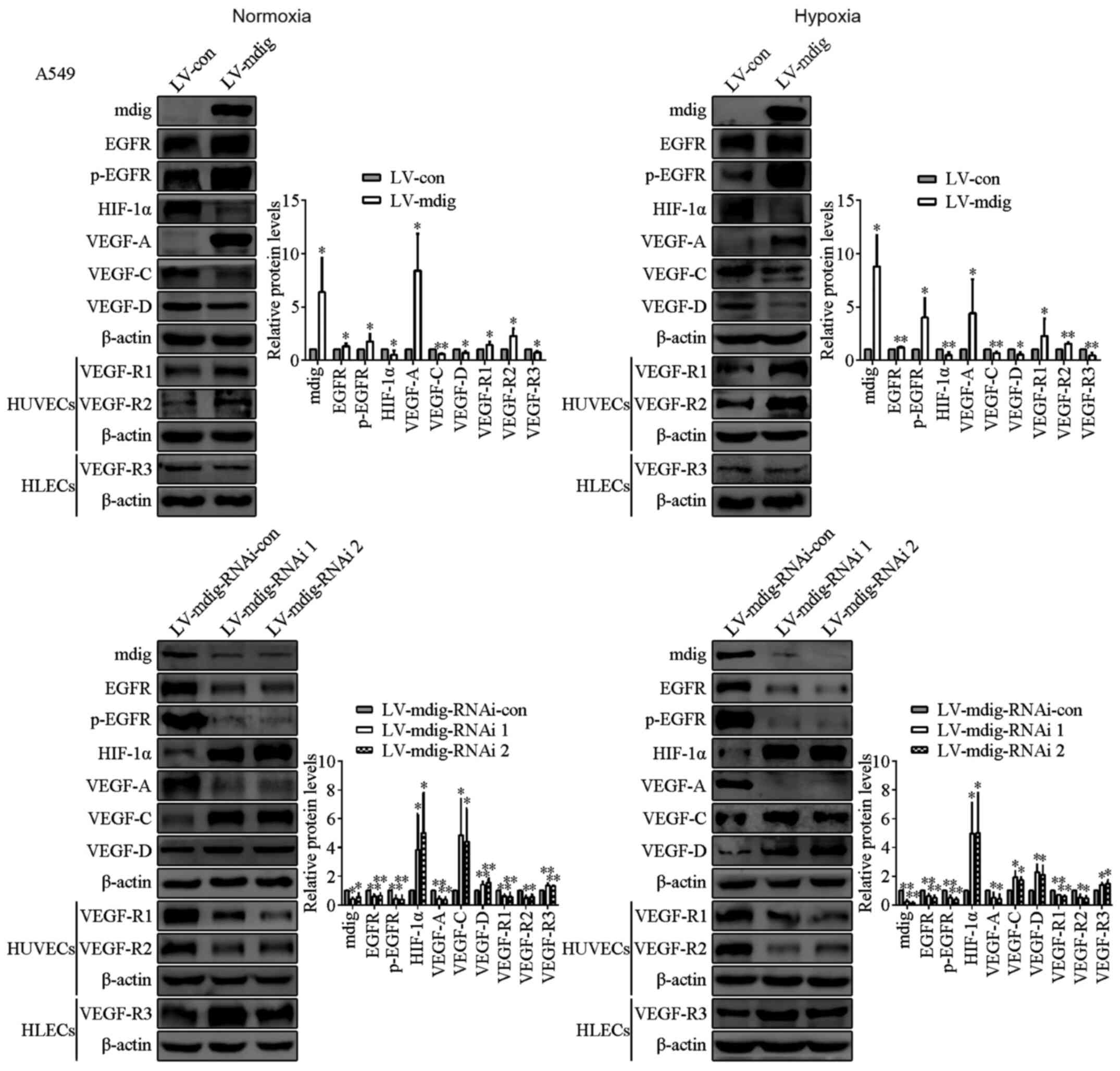 | Figure 2.Regulation of EGFR, p-EGFR, HIF-1α,
the VEGF family members and their receptors by mdig at the protein
levels in transfected A549 cells, and HUVECs and HLECs cultured
with conditioned media of LV-mdig A549 cells under normoxic and
hypoxic conditions. Mdig upregulated the expression of EGFR,
p-EGFR, VEGF-A and VEGF-R1/R2, and reduced the expression of
HIF-1α, VEGF-C/D and VEGF-R3. β-actin was used as the protein
loading control. *P<0.05, **P<0.01, LV-mdig vs. LV-con and
LV-mdig-RNAi vs. LV-mdig-RNAi-con. mdig, mineral dust-induced gene;
EGFR, epidermal growth factor receptor; HIF-1α, hypoxia-inducible
factor-1α; VEGF, vascular endothelial growth factor; p-, phospho;
LV, lentivirus; RNAi, RNA interference; con, control; HUVECs, human
umbilical vein endothelial cells; HLECs, human lymphatic
endothelial cells. |
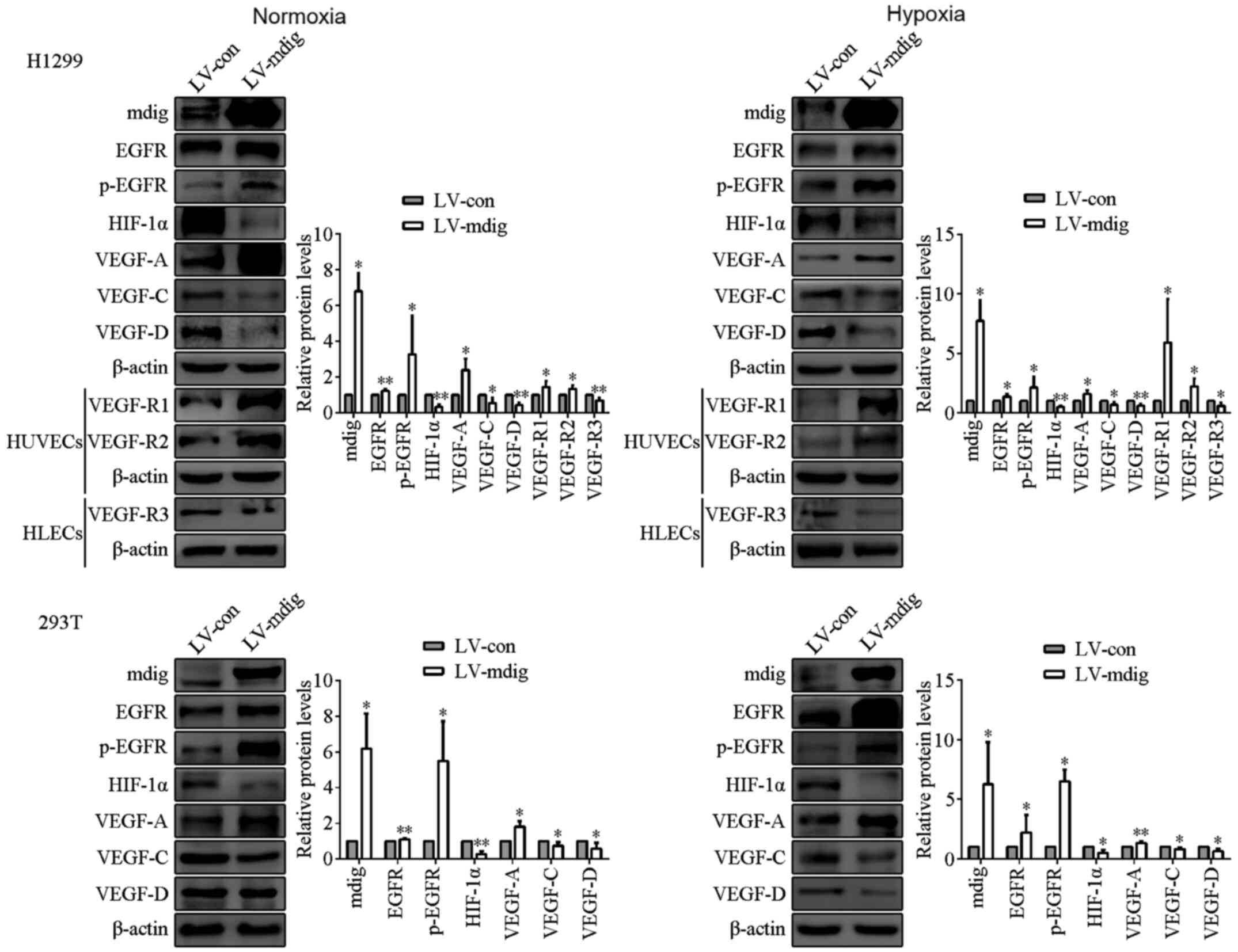 | Figure 3.Regulation of EGFR, p-EGFR, HIF-1α,
the VEGF family members and their receptors by mdig at the protein
levels in mdig-overexpressing H1299 and 293T cells, and HUVECs and
HLECs cultured with conditioned media of LV-mdig H1299 cells under
normoxic and hypoxic conditions. mdig upregulated the expression of
EGFR, p-EGFR, VEGF-A and VEGF-R1/R2, and reduced the expression of
HIF-1α, VEGF-C/D and VEGF-R3. β-actin was used as the protein
loading control. *P<0.05, **P<0.01, LV-mdig vs. LV-con. mdig,
mineral dust-induced gene; EGFR, epidermal growth factor receptor;
HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial
growth factor; p-, phospho; LV, lentivirus; con, control; HUVECs,
human umbilical vein endothelial cells; HLECs, human lymphatic
endothelial cells. |
Next, the mRNA expression levels of mdig and EGFR
were measured in the transfected cells using RT-qPCR. There were no
significant changes in EGFR mRNA expression levels in the LV-mdig
and LV-mdig-RNAi transfected cells compared with those in the
LV-con and LV-mdig-RNAi-con transfected cells, respectively, under
both normoxic and hypoxic conditions (Fig. S1A). Subsequently, the protein
expression levels of p-EGFR and EGFR were compared in the
mdig-overexpressing A549 and H1299 cells. The LV-mdig/LV-con ratio
of p-EGFR protein was significantly higher compared with that of
the EGFR protein (Fig. S1B).
Following the culturing of mdig-overexpressing A549 cells under
normoxic and hypoxic conditions, their lysates were subjected to
co-immunoprecipitation, and it was found that EGFR did not form
complexes with mdig (Fig. S1C).
mdig inhibits the protein expression
of HIF-1α and prevents its entry into the nucleus
To study the functional relationship between mdig
and HIF-1α, the protein expression levels of mdig and HIF-1α in
mdig-overexpressing and mdig-knockdown cells cultured under
normoxic and hypoxic conditions were measured by western blotting.
HIF-1α expression levels were significantly lower in the LV-mdig
group compared with those in the LV-con group (P<0.05), whereas
HIF-1α expression levels were significantly higher in the
LV-mdig-RNAi group compared with those in the LV-mdig-RNAi-con
group (P<0.05; Figs. 2 and
3). These changes were not
statistically significant between the cells cultured under hypoxic
and normoxic conditions. In addition, the mRNA expression levels of
mdig and HIF-1α were not statistically significant when comparing
the LV-mdig and LV-mdig-RNAi groups with LV-con and
LV-mdig-RNAi-con groups, under normoxic and hypoxic conditions
(Fig. S1A). Mdig-overexpressing A549
cells were also cultured under normoxic and hypoxic conditions
prior to co-immunoprecipitation analysis, and the results showed
that mdig did not interact with HIF-1α (Fig. S1C).
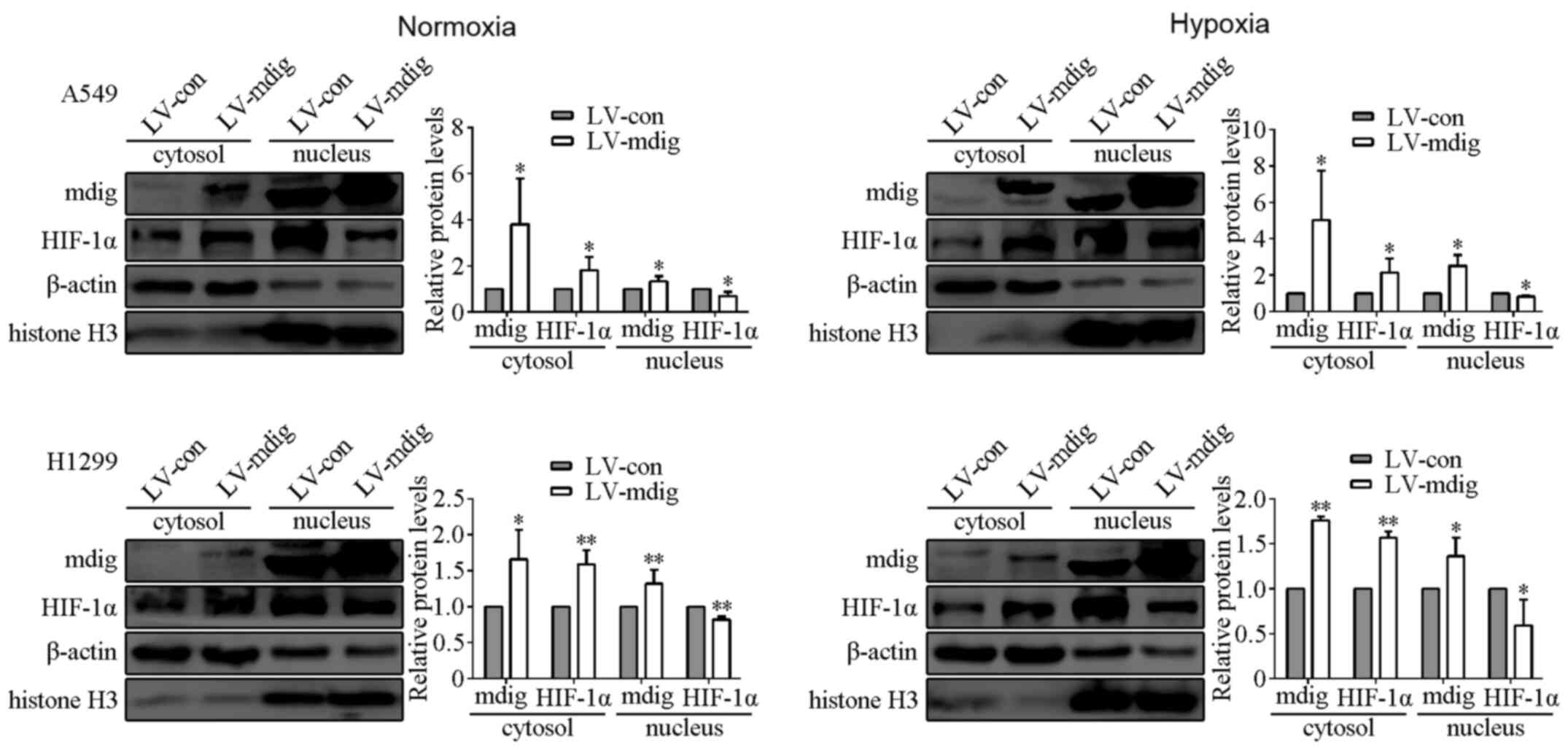
Since HIF-1α serves an important role in promoting
the transcription of a number of genes in the nucleus (27,30), the
nuclear and cytoplasmic proteins were isolated from A549 and H1299
cells overexpressing mdig, after culturing under normoxic and
hypoxic conditions. Protein expression of mdig was found to be
primarily distributed in the nucleus, with little localization
observed in the cytosol. HIF-1α was also primarily localized in the
nucleus, although the protein expression levels of HIF-1α in the
cytosol were significantly higher in cells in the LV-mdig group
compared with those in the LV-con group (P<0.05). By contrast,
the protein expression levels of HIF-1α in the nucleus of cells in
the LV-mdig group were significantly lower compared with those in
the LV-con group (P<0.05; Fig.
4).
mdig regulates the protein expression
of VEGF and VEGF receptors
To investigate the effects of mdig on tumor
angiogenesis and lymphangiogenesis, the influence of mdig on the
expression of VEGFs and their receptors was explored. The protein
expression levels of VEGFs and their receptors in the
mdig-overexpressing and mdig-silenced cells were measured by
western blotting. Under normoxic and hypoxic conditions, the
expression levels of VEGF-A were found to be significantly higher
in the LV-mdig group, whereas those of VEGF-C and VEGF-D were
significantly lower when compared with those in the LV-con group
(P<0.05). Opposite results were observed in the LV-mdig-RNAi
group compared with those in the LV-mdig group (Figs. 2 and 3).
Based on these observations, conditioned media were
obtained from transfected A549 and H1299 cells cultured under
normoxic and hypoxic conditions, which were then used to treat
HUVECs and HLECs for 24 h. Western blotting was subsequently
performed to measure the expression levels of VEGF-R1/R2 in HUVECs
and the expression levels of VEGF-R3 in HLECs. The expression
levels of VEGF-R1/R2 were shown to be significantly higher in the
HUVECs cultured with conditioned media of cells from the LV-mdig
group compared with those in HUVECs cultured with the conditioned
media of cells from the LV-con group (P<0.05). The expression
levels of VEGF-R3 were revealed to be significantly lower in the
HLECs cultured with the conditioned media of cells from the LV-mdig
group compared with those in the HLECs cultured with the
conditioned media of cells from the LV-con group (P<0.05). By
contrast, opposite observations were seen in HUVECs and HLECs
cultured with conditioned media of cells from the LV-mdig-RNAi
group compared with those in the LV-mdig group (Figs. 2 and 3).
mdig promotes tumor growth and
angiogenesis, and inhibits lymphangiogenesis in vitro and in
vivo
To verify the aforementioned findings, conditioned
media, obtained from the mdig-overexpressing A549 cells cultured
under normoxic and hypoxic conditions, were used to suspend HUVECs
and HLECs for the tube formation assay of angiogenesis and
lymphangiogenesis, respectively. The density of angiogenesis was
significantly increased in HUVECs cultured using the conditioned
media of cells from the LV-mdig group compared with that in HUVECs
cultured using the conditioned media of cells from the LV-con group
(P<0.05). However, the density of lymphangiogenesis was notably
decreased in HLECs cultured using conditioned media of cells in the
LV-mdig group compared with that in HLECs cultured using the
conditioned media of cells in the LV-con group (P<0.05; Fig. 5A).
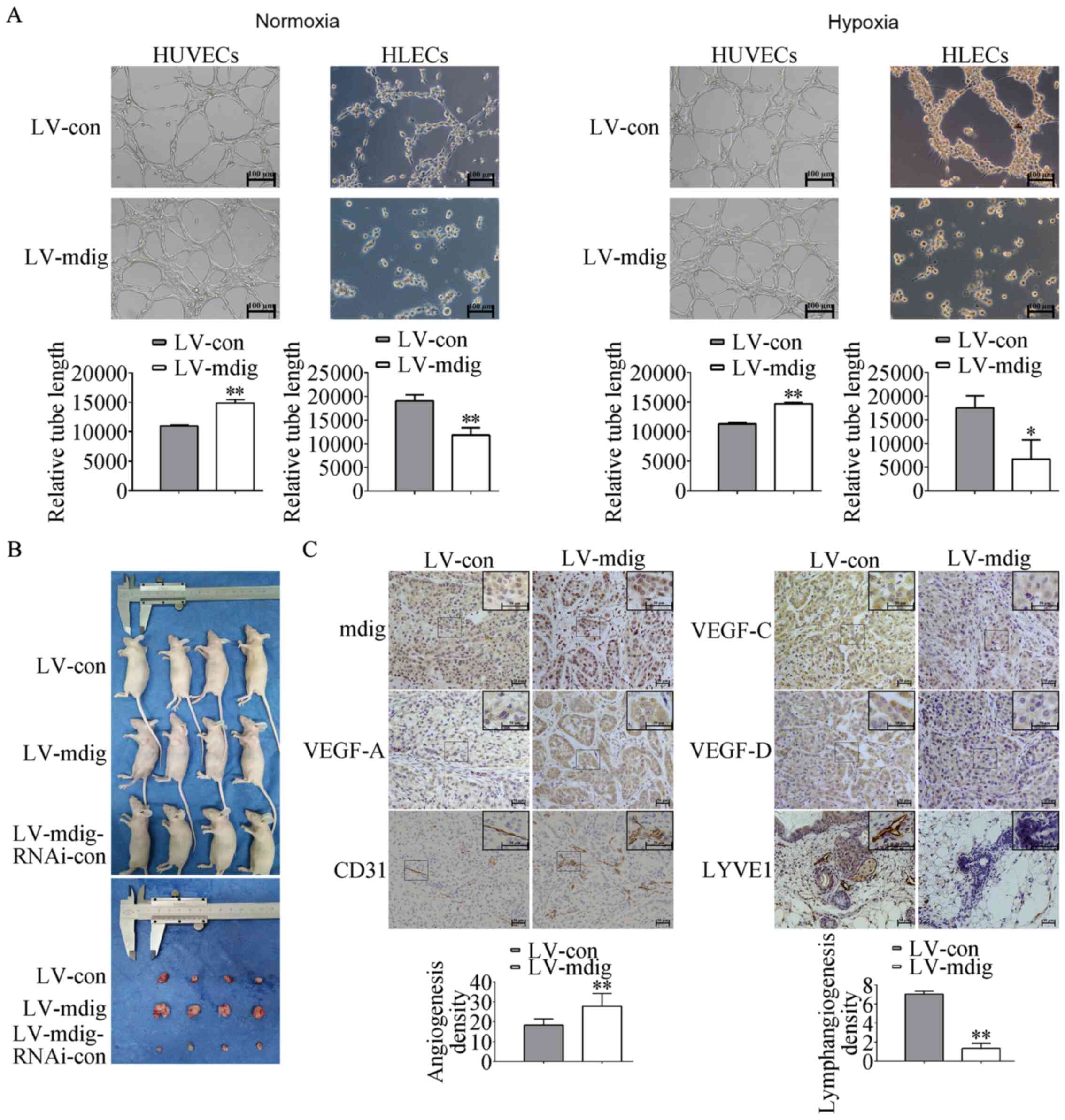 | Figure 5.Regulation of tumor growth,
angiogenesis and lymphangiogenesis by mdig in vitro and
in vivo. (A) Density of angiogenesis and lymphangiogenesis
regulated by mdig in tube formation assays. HUVECs and HLECs were
suspended in conditioned media obtained from the
mdig-overexpressing A549 cells cultured under normoxic and hypoxic
conditions for the tube formation assays. The length of
capillary-like tube structures was analyzed as the density of
angiogenesis and lymphangiogenesis. Magnification, ×100.
*P<0.05, **P<0.01, LV-mdig vs. LV-con. (B) The volumes of the
tumors formed in the LV-mdig group were significantly larger
compared with those in the LV-con group 3 weeks after subcutaneous
injection of transfected A549 cells into nude mice. (C)
Immunohistochemistry analysis was performed using antibodies
against mdig, CD31, LYVE1 and VEGF-A/C/D. The density of
angiogenesis and the expression of mdig and VEGF-A in the LV-mdig
group were significantly increased compared with those in the
LV-con group. The density of lymphangiogenesis and the expression
levels of VEGF-C/D were reduced in the LV-mdig group compared with
those in the LV-con group. Magnification, ×200 and ×400.
**P<0.01, LV-mdig vs. LV-con. mdig, mineral dust-induced gene;
VEGF, vascular endothelial growth factor; LV, lentivirus; RNAi, RNA
interference; con, control; HUVECs, human umbilical vein
endothelial cells; HLECs, human lymphatic endothelial cells. |
To verify further the above findings in
vitro, mdig-overexpressing and mdig-silenced A549 cells were
subcutaneously injected into nude mice. A total of 3 weeks after
injection, the tumor volumes in the LV-mdig group were
significantly larger compared with those in the LV-con group, and
cells in the LV-mdig-RNAi group were not tumorigenic compared with
those in the LV-mdig-RNAi-con group (Fig.
5B). To assess the effects of mdig on tumor angiogenesis and
lymphangiogenesis, immunohistochemistry was performed on tissue
sections using the antibodies of angiogenesis endothelial cell
marker CD31, and lymphangiogenesis endothelial cell marker LYVE1.
Compared with the LV-con group, the density of angiogenesis in
tissues from the LV-mdig group was significantly increased, whereas
the density of lymphangiogenesis was significantly decreased
(P<0.05; Fig. 5C).
Subsequently, it was found that compared with cells
in the LV-con group, the expression of mdig was significantly
enhanced in the cells of the LV-mdig group, and expression was
primarily observed in the nucleus, with limited expression in the
cytosol. In addition, VEGF-A expression in the cytosol was
significantly increased, but the expression levels of VEGF-C and
VEGF-D in the cytosol were significantly reduced in cells in the
LV-mdig group compared with those in the LV-con group (Fig. 5C).
mdig induces angiogenesis via the
EGFR/p-EGFR/VEGF-A pathway
It was suggested that mdig lies upstream of EGFR,
p-EGFR (Tyr1068), VEGF-A and VEGF-R1/R2, and that it promotes the
expression of these proteins. Thus, the EGFR agonist NSC228155
[EGFR (+)] was therefore used in mdig-knockdown A549 cells, whereas
the EGFR inhibitor erlotinib [EGFR (−)] was used to treat
mdig-overexpressing A549 cells under normoxic and hypoxic
conditions. The protein expression levels of EGFR, p-EGFR and
VEGF-A were demonstrated to be significantly increased in the
mdig-silenced A549 cells following treatment with NSC228155 under
both normoxic and hypoxic conditions compared with those in the
LV-mdig-RNAi group (P<0.05). The expression levels of these
proteins were found to be significantly reduced in the
mdig-overexpressing A549 cells following treatment with erlotinib
compared with those in the LV-mdig group under normoxic and hypoxic
conditions (P<0.05; Fig. 6A).
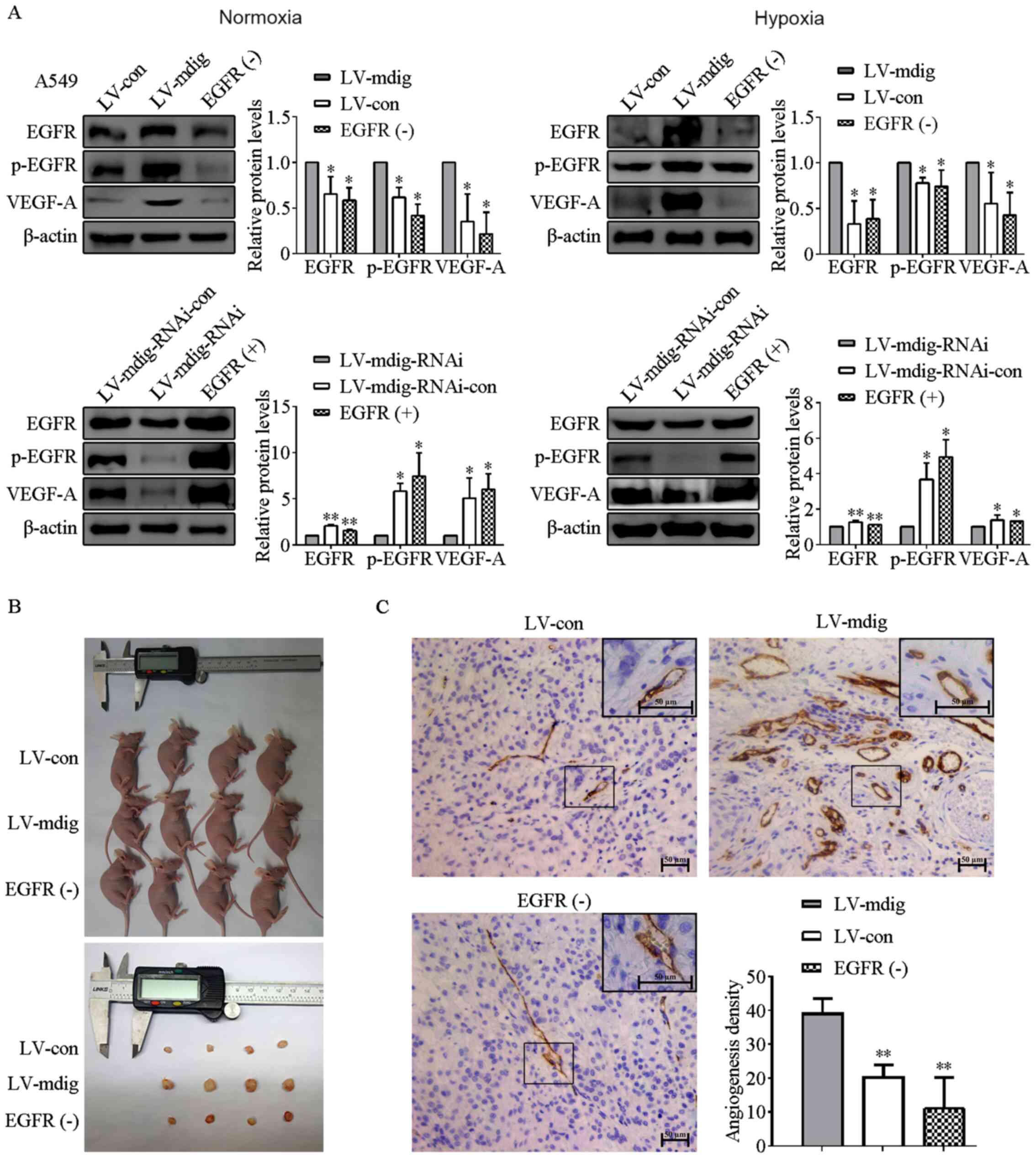 | Figure 6.Regulation of VEGF-A expression and
tumor angiogenesis by mdig via the EGFR pathway. (A) LV-mdig A549
cells were treated with the EGFR inhibitor [EGFR (−)] whereas
LV-mdig-RNAi A549 cells were treated with the EGFR agonist [EGFR
(+)]. The expression levels of EGFR, p-EGFR and VEGF-A were
measured by western blotting. β-actin was used as the loading
control. *P<0.05, **P<0.01, LV-mdig vs. LV-con; EGFR (−) vs.
LV-mdig; LV-mdig-RNAi vs. LV-mdig-RNAi-con; and EGFR (+) vs.
LV-mdig-RNAi. (B) Tumor volumes formed in the LV-mdig group treated
with EGFR inhibitor erlotinib [EGFR (−)] were significantly smaller
compared with those in the untreated LV-mdig group 3 weeks after
injection into nude mice. (C) Immunohistochemistry was performed
using antibodies against CD31. The density of angiogenesis in the
LV-mdig group treated with erlotinib was significantly decreased
compared with those in the untreated LV-mdig group. Magnification,
×200 and ×400. **P<0.01, LV-mdig vs. LV-con and EGFR (−) vs.
LV-mdig. Mdig, mineral dust-induced gene; EGFR, epidermal growth
factor receptor; p-, phospho; VEGF, vascular endothelial growth
factor; LV, lentivirus; RNAi, RNA interference; con, control. |
To verify these findings in vitro,
mdig-overexpressing A549 cells treated with/without the EGFR
inhibitor erlotinib were subcutaneously injected into nude mice. It
was found that the tumor volumes in the LV-mdig group treated with
erlotinib [EGFR (−)] were significantly smaller compared with those
in the LV-mdig group 3 weeks after injection (Fig. 6B). To assess the effects of EGFR on
tumor angiogenesis, immunohistochemistry was performed on tumor
tissue sections using CD31 antibody. It was found that the density
of angiogenesis in cancer tissues from the LV-mdig group treated
with erlotinib was significantly decreased compared with that in
the LV-mdig group (P<0.05; Fig.
6C).
mdig inhibits lymphangiogenesis by
blocking the HIF-1α/VEGF-C/D pathway
The above findings suggested that under both
normoxic and hypoxic conditions, mdig functions upstream of HIF-1α,
VEGF-C and VEGF-D to inhibit the expression of these proteins.
Previous studies have shown that HIF-1α upregulates the expression
of VEGF (21,23,27), such
that VEGF-C and VEGF-D serve important roles in tumor
lymphangiogenesis (19,20). Therefore, it was subsequently
hypothesized that mdig may inhibit the expression of HIF-1α,
thereby reducing the levels of VEGF-C and VEGF-D to ultimately
inhibit lymphangiogenesis in lung adenocarcinoma. To test this
hypothesis, mdig-knockdown A549 cells were treated with the HIF-1α
inhibitor BAY 87-2243 [HIF-1α (−)], while the mdig-overexpressing
A549 cells were treated with the HIF-1α agonist IOX2 [HIF-1α (+)].
Western blotting results showed that the expression levels of
HIF-1α, VEGF-C and VEGF-D were significantly reduced in cells in
the LV-mdig-RNAi group following treatment with BAY 87-2243
compared with those in cells in the LV-mdig-RNAi group (P<0.05).
Conversely, expression levels of these proteins were significantly
increased in the mdig-overexpressing cells treated with IOX2
compared with those in the LV-mdig group (P<0.05; Fig. 7A).
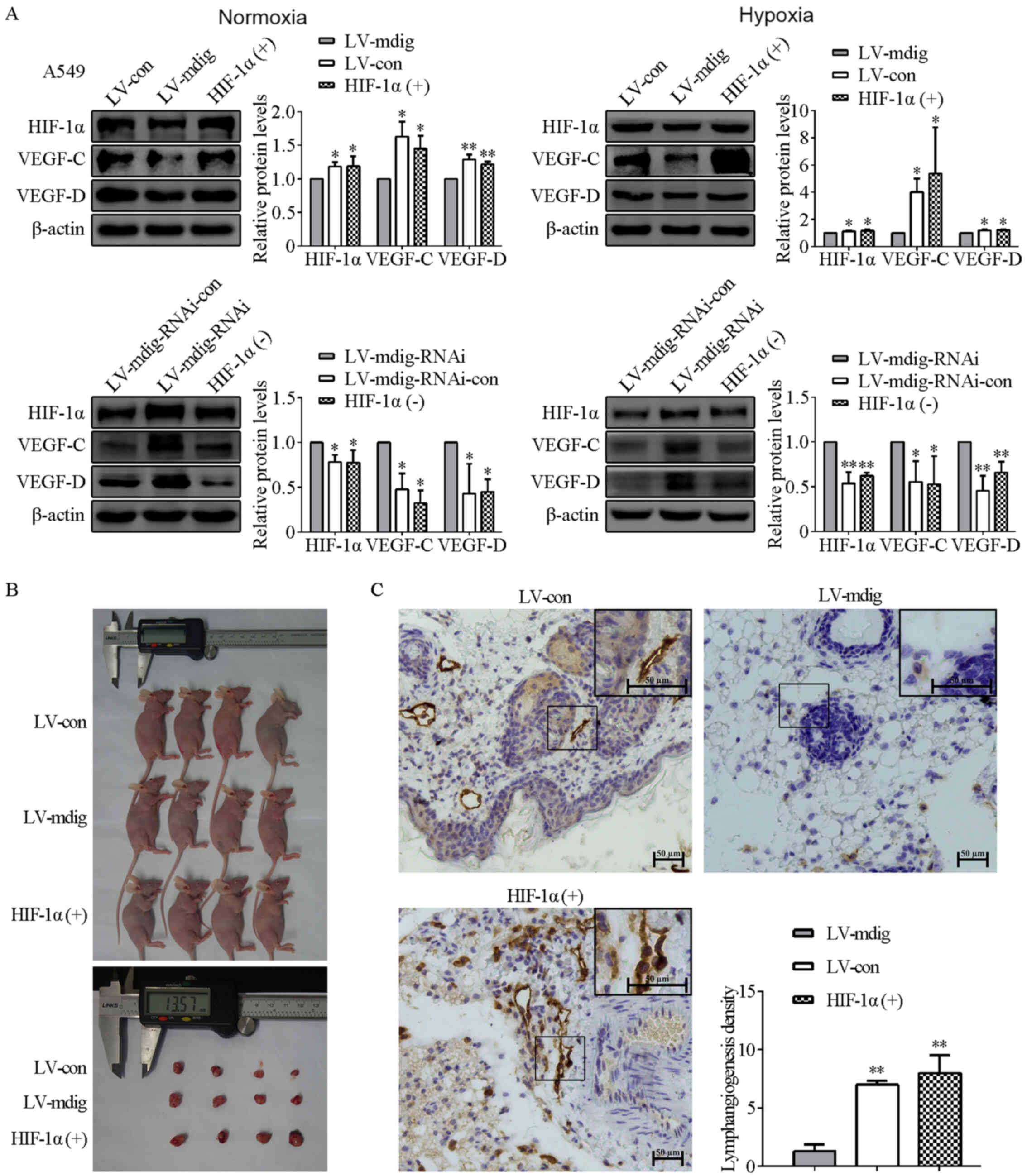 | Figure 7.Regulation of VEGF-C/D expression and
lymphangiogenesis by mdig via the HIF-1α pathway. (A) LV-mdig A549
cells were treated with the HIF-1α agonist [HIF-1α (+)] whereas
LV-mdig-RNAi A549 cells were treated with the HIF-1α inhibitor
[HIF-1α (−)]. The expression levels of HIF-1α and VEGF-C/D were
measured by western blotting, with β-actin used as the loading
control. *P<0.05, **P<0.01, LV-mdig vs. LV-con; HIF-1α (+)
vs. LV-mdig; LV-mdig-RNAi vs. LV-mdig-RNAi-con; and HIF-1α (−) vs.
LV-mdig-RNAi. (B) Tumor volumes formed in the LV-mdig group treated
with HIF-1α agonist [HIF-1α (+)] 3 weeks after subcutaneous
injection into nude mice. (C) Immunohistochemistry was performed
using antibodies against LYVE1. The density of lymphangiogenesis in
the LV-mdig group treated with HIF-1α agonist [HIF-1α (+)] was
significantly increased compared with that in the untreated LV-mdig
group. Magnification, ×200 and ×400. **P<0.01, LV-mdig vs.
LV-con and HIF-1α (+) vs. LV-mdig. mdig, mineral dust-induced gene;
HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial
growth factor; LV, lentivirus; RNAi, RNA interference; con,
control. |
To verify these findings in vitro,
mdig-overexpressing A549 cells treated with/without the HIF-1α
agonist IOX2 were subcutaneously injected into nude mice (Fig. 7B). Immunohistochemistry was performed
on tissue sections using LYVE1 antibody. It was found that the
density of lymphangiogenesis in tissues from the LV-mdig group
treated with IOX2 [HIF-1α (+)] was significantly increased compared
with that in the LV-mdig group (P<0.05; Fig. 7C).
Discussion
Upregulation of mineral dust-induced (mdig) mRNA and
protein is a common feature of all types of lung cancer clinically,
particularly in the early stages (6).
Previous studies have found that mdig can promote tumor cell
proliferation (6,12), but it inhibits tumor cell invasion and
migration (9,13). The mechanism of this paradoxical
phenomenon is unclear, which suggests that the role of mdig is
different in different stages of carcinogenesis. Hence, the role of
mdig in the regulation of lung adenocarcinoma requires further
study.
Tumor angiogenesis is a prerequisite for tumor
growth, which not only provides oxygen and nutrition for tumor cell
growth in the early stages, but also activates a pathway together
with lymphangiogenesis for tumor cell metastasis in the advanced
stages. With the rapid proliferation of tumor cells, oxygen
consumption increases, eventually leading to hypoxic conditions in
the local microenvironment of the tumor, which further stimulates
tumor angiogenesis (31–33). It has been confirmed that epidermal
growth factor receptor (EGFR), hypoxia-inducible factor-1α (HIF-1α)
and the vascular endothelial growth factor (VEGF) family serve very
important roles in tumor angiogenesis and lymphangiogenesis
(17,21,34).
However, the role and molecular mechanism of mdig in tumor
angiogenesis and lymphangiogenesis have not been reported
previously.
The present study first investigated the effects of
hypoxia on the protein expression levels of mdig, EGFR, HIF-1α and
the VEGF family. A549, H1299 and 293T cells were cultured under
normoxic (21% O2) and hypoxic (1% O2)
conditions, and subsequently, protein expression levels were
determined by western blotting. It was found that the protein
expression levels of EGFR, HIF-1α and VEGF-A/C/D in each group of
cells cultured under hypoxic conditions were significantly
increased compared with those in cells cultured under normoxic
conditions, which were consistent with previous studies (34–36). These
results further confirmed that hypoxia can upregulate the protein
expression levels of EGFR, HIF-1α and VEGF-A/C/D. In addition, it
was observed in the present study that the protein expression
levels of mdig in cells cultured under hypoxic conditions were
significantly lower compared with those in cells cultured under
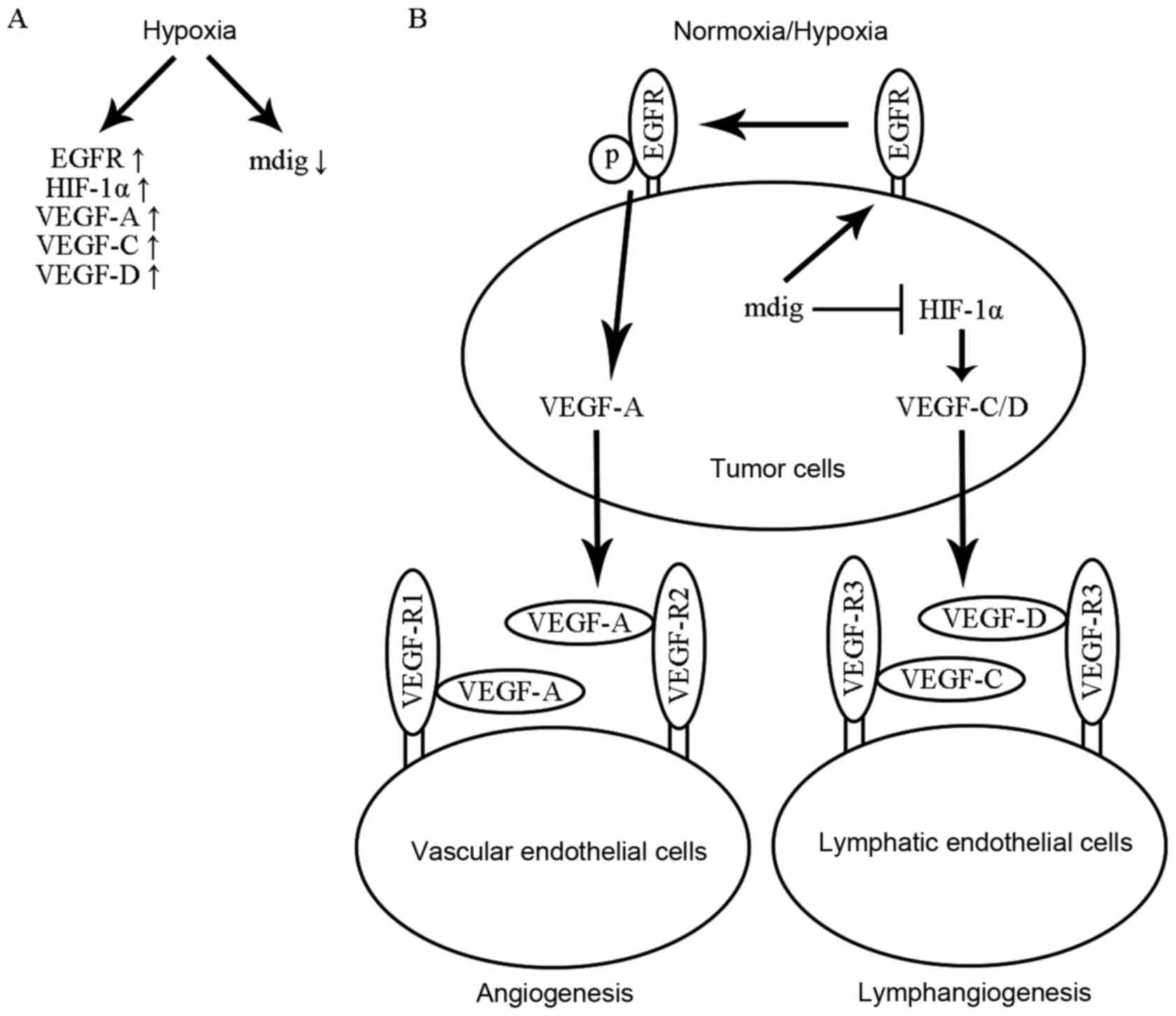
normoxic conditions, suggesting that mdig is an oxygen-sensitive
protein, and its expression is negatively regulated by hypoxia
(Fig. 8A). However, the regulatory
mechanism remains unclear.
Subsequently, the relationship between mdig and EGFR
in normoxia and hypoxia were explored in the present study. The
results showed that under both normoxic and hypoxic conditions,
mdig promoted the protein expression of EGFR and phosphorylated
(p)-EGFR, indicating that mdig exerts its effects upstream of EGFR.
However, mdig had no effect on mRNA levels of EGFR. Therefore,
these results suggested that mdig regulates the expression of EGFR
at the protein level, but not at a transcriptional level. Changes
in p-EGFR and EGFR protein expression regulated by mdig were
subsequently compared. It was found that the LV-mdig/LV-con ratio
of p-EGFR protein was significantly higher compared with that of
the EGFR protein. These observations suggested that mdig
upregulated the protein expression of not only EGFR, but also the
phosphorylation of its Tyr1068 residue, indicating that mdig
regulates the expression of EGFR by post-translational modification
at Tyr1068. To further explore the relationship between mdig and
EGFR, co-immunoprecipitation analysis was performed. The results
showed that no direct interactions were observed between mdig and
EGFR. Combined with the results from RT-qPCR, these results
indicated that mdig may increase the protein expression of EGFR
through post-translational modification or signaling pathways.
HIF-1α serves a pivotal role in tumor angiogenesis
and lymphangiogenesis (14,17,27,34).
Studies have previously shown that HIF-1α functions downstream of
EGFR. However, EGFR can promote tumor angiogenesis and
lymphangiogenesis in both a HIF-1α-dependent and -independent
manner (17,18,22).
Therefore, the relationship between mdig and HIF-1α under normoxic
and hypoxic conditions was also explored in the present study. The
results showed that mdig significantly inhibited HIF-1α protein
expression under both normoxic and hypoxic conditions, but had no
effect on mRNA levels of HIF-1α. In the subsequent
co-immunoprecipitation experiment, no direct interactions were
found between mdig and HIF-1α. These results suggested that mdig
may inhibit HIF-1α protein expression by modulation of signaling
pathways or by post-translational modification. In the nuclear and
cytoplasmic fractionation experiment, the expression levels of
HIF-1α protein were shown to be significantly reduced in the
nucleus but were significantly increased in the cytosol following
overexpression of mdig, suggesting that mdig prevents HIF-1α
entering the nucleus from the cytosol, blocking HIF-1α function in
the nucleus.
The VEGF family serves an important role in tumor
angiogenesis and lymphangiogenesis (19,20,35).
Specifically, VEGF-A has been reported to serve a major role in
tumor angiogenesis (14,17,36),
whereas VEGF-C/D is primarily associated with lymphangiogenesis
(19,20). The relationship between mdig and VEGF
family was also explored in the present study. It was found that
mdig promoted the expression of VEGF-A and VEGF-R1/R2 protein but
inhibited the expression of VEGF-C/D and VEGF-R3 protein both under
normoxic and hypoxic conditions. These results suggested that mdig
serves functionally distinct regulatory roles on tumor angiogenesis
and lymphangiogenesis by regulating different members of the VEGF
family and their receptors; namely, mdig promotes tumor
angiogenesis by inducing protein expression of VEGF-A and
VEGF-R1/R2, and suppresses lymphangiogenesis by reducing the
expression of VEGF-C/D and VEGF-R3 protein. To confirm this
hypothesis, tube formation assays of angiogenesis and
lymphangiogenesis were also performed. The results showed that mdig
significantly increased the density of angiogenesis but
significantly decreased the density of lymphangiogenesis,
suggesting that mdig promotes tumor angiogenesis but suppresses
lymphangiogenesis.
Previous studies have shown that the EGF/RHIF-1α/
VEGF (17), HIF-1α/Notch1/VEGF-A
(22), WISP-1/FAK/c-Src/VEGF-A
(37), EGFR/p38/MMP-1 (14) and c-myc/HIF-1α/ VEGF-A (38) signaling pathways can affect tumor
angiogenesis, and HIF-1α/PDGF-B/PDGFRβ (34,39),
CCL21/CCR7/ERK/VEGF-D (40),
EGFR/HIF-1α/VEGF (41) and
CXCL12/CXCR4 (42) signaling pathways
can regulate tumor lymphangiogenesis. Because the EGFR/HIF-1α/VEGF
signaling pathway is one of the classical pathways associated with
tumor angiogenesis and lymphangiogenesis (14,17,41), it
was explored in the present study. An EGFR agonist/inhibitor and a
HIF-1α agonist/inhibitor were used in the present study to assess
whether mdig also regulated tumor angiogenesis and
lymphangiogenesis via the EGFR/HIF-1α/VEGF signaling pathway. The
results showed that the regulatory role of mdig on EGFR, p-EGFR and
VEGF-A protein expression was significantly reversed by the EGFR
agonist/inhibitor, suggesting that mdig increased the secretion of
VEGF-A by promoting EGFR expression, indicating that mdig promotes
angiogenesis in lung adenocarcinoma via the
EGFR/p-EGFR/VEGF-A/VEGF-R1/R2 pathway. The present study also
showed that the regulatory role of mdig on HIF-1α and VEGF-C/D
protein expression was reversed by HIF-1α agonist/inhibitor,
suggesting that mdig reduces the secretion of VEGF-C/D by
inhibiting HIF-1α expression, indicating that mdig can inhibit
lymphangiogenesis by blocking the HIF-1α/VEGF-C/D/VEGF-R3
pathway.
Previous studies have shown that EGFR can upregulate
VEGF-A expression not only in a HIF-1α-dependent manner (17,43), but
also in a HIF-1α-independent manner, including via the PI3K/Akt
(18,44), Akt/NF-κB (45,46) and
K-Ras (47) signaling pathways. The
results of the present study showed that mdig upregulated the
protein expression levels of EGFR, VEGF-A and VEGF-R1/R2, but
downregulated the protein expression levels of HIF-1α, indicating
that mdig may promote angiogenesis through increasing the
expression of VEGF-A and VEGF-R1/R2 by EGFR in a HIF-1α-independent
manner in lung adenocarcinoma.
To confirm these aforementioned findings in
vitro, mdig-transfected A549 cells were subsequently injected
into nude mice. The results showed that mdig promoted tumor growth
and tumor angiogenesis, but suppressed tumor lymphangiogenesis. In
addition, the changes in expression of VEGF-A and VEGF-C/D in
vivo were consistent with those observed in vitro,
further supporting the conclusion that mdig promotes tumor
angiogenesis by increasing the expression of VEGF-A, and inhibits
lymphangiogenesis by suppressing the expression of VEGF-CD.
To further verify the results that mdig promotes
tumor growth and angiogenesis via the EGFR signaling pathway and
suppresses tumor lymphangiogenesis via the HIF-1α signaling pathway
in vitro, an EGFR inhibitor (erlotinib) and a HIF-1α agonist
(IOX2) were also used in the xenograft tumor experiments in
vivo. The results showed that both tumor volumes and
angiogenesis density were significantly decreased by the EGFR
inhibitor erlotinib, and that the lymphangiogenesis density was
significantly increased by the HIF-1α agonist IOX2. These results
further confirmed that mdig promotes tumor growth and angiogenesis
via the EGFR signaling pathway, and inhibits lymphangiogenesis by
blocking the HIF-1α signaling pathway (Fig. 8B).
In conclusion, the present study confirmed that mdig
is an oxygen-sensitive protein and hypoxia can inhibit the
expression of mdig, that mdig induces tumor growth and angiogenesis
by activating the EGFR/p-EGFR/VEGF-A/VEGF-R1/R2 pathway, and that
mdig inhibits tumor lymphangiogenesis by blocking the
HIF-1α/VEGF-C/D/VEGF-R3 pathway, as well as suppresses the
translocation of HIF-1α from the cytosol to the nucleus. The
present study lays the foundation for future research of lung
adenocarcinoma angiogenesis and lymphangiogenesis, and highlights
potentially novel targets for the development of novel therapies.
In addition, previous studies have demonstrated that varieties of
tyrosine kinase receptors can regulate tumor angiogenesis and
lymphangiogenesis, such as MET, IGFR and the FGFR family (48–51). The
effects of mdig on angiogenesis and lymphangiogenesis through other
tyrosine kinase receptors will be explored in our subsequent
studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472194) and by the
Project of Liaoning Distinguished Professor [grant no. (2013)204]
to ZHW.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and HZ designed the study and wrote the
manuscript. HZ, FG, YC, JD, XZ and BL performed the experiments and
collected the data. HZ, HH and DS performed the statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of the China Medical
University (approval no. 2019135).
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Lin HT, Liu FC, Wu CY, Kuo CF, Lan WC and
Yu HP: Epidemiology and survival outcomes of lung cancer: A
population-based study. BioMed Res Int. 2019:81481562019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang B, Li J, Chen J, Xiang X, Xiong J
and Deng J: Aortic dissection in a patient treated with anlotinib
for metastatic lung squamous cell carcinoma. Thorac Cancer.
11:461–464. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zang R, Wang X, Jin R, Lei Y, Huang J, Liu
C, Zheng S, Zhou F, Wu Q, Sun N, et al: Translational value of IDH1
and DNA methylation biomarkers in diagnosing lung cancers: A novel
diagnostic panel of stage and histology-specificity. J Transl Med.
17:4302019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao Y, Zhu W, Chen W, Wu J, Hou G and Li
Y: Prognostic value of BIRC5 in lung adenocarcinoma lacking EGFR,
KRAS, and ALK mutations by integrated bioinformatics analysis. Dis
Markers. 2019:54512902019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Q, Geng F, Zhou H, Chen Y, Du J,
Zhang X, Song D and Zhao H: MDIG promotes cisplatin resistance of
lung adenocarcinoma by regulating ABC transporter expression via
activation of the WNT/β-catenin signaling pathway. Oncol Lett.
18:4294–4307. 2019.PubMed/NCBI
|
|
6
|
Thakur C and Chen F: Current understanding
of mdig/MINA in human cancers. Genes Cancer. 6:288–302. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Y, Chang Q, Zhang Y, Beezhold K,
Rojanasakul Y, Zhao H, Castranova V, Shi X and Chen F: Lung
cancer-associated JmjC domain protein mdig suppresses formation of
tri-methyl lysine 9 of histone H3. Cell Cycle. 8:2101–2109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J and Zhao H: From biological
mechanisms to clinical implications: The role of mineral
dust-induced gene in lung cancers. J Thorac Dis. 9:1178–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu M, Sun J, Thakur C, Chen B, Lu Y, Zhao
H and Chen F: Paradoxical roles of mineral dust induced gene on
cell proliferation and migration/invasion. PLoS One. 9:e879982014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun J, Yu M, Lu Y, Thakur C, Chen B, Qiu
P, Zhao H and Chen F: Carcinogenic metalloid arsenic induces
expression of mdig oncogene through JNK and STAT3 activation.
Cancer Lett. 346:257–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Komiya K, Sueoka-Aragane N, Sato A,
Hisatomi T, Sakuragi T, Mitsuoka M, Sato T, Hayashi S, Izumi H,
Tsuneoka M, et al: Expression of Mina53, a novel c-Myc target gene,
is a favorable prognostic marker in early stage lung cancer. Lung
Cancer. 69:232–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma D, Guo D, Li W and Zhao H: Mdig, a lung
cancer-associated gene, regulates cell cycle progression through
p27(KIP1). Tumour Biol. 36:6909–6917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng F, Jiang Z, Song X, Zhou H and Zhao
H: Mdig suppresses epithelial-mesenchymal transition and inhibits
the invasion and metastasis of non small cell lung cancer via
regulating GSK-3β/β-catenin signaling. Int J Oncol. 51:1898–1908.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim D, Dai J, Park YH, Fai LY, Wang L,
Pratheeshkumar P, Son YO, Kondo K, Xu M, Luo J, et al: Activation
of epidermal growth factor receptor/p38/hypoxia-inducible factor-1α
is pivotal for angiogenesis and tumorigenesis of malignantly
transformed cells induced by hexavalent chromium. J Biol Chem.
291:16271–16281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei H, Li D, Yang X, Shang H, Fan S, Li Y
and Song D: Design and synthesis of vandetanib derivatives
containing nitroimidazole groups as tyrosine kinase inhibitors in
normoxia and hypoxia. Molecules. 21:1693–1707. 2016. View Article : Google Scholar
|
|
16
|
Dong XF, Liu TQ, Zhi XT, Zou J, Zhong JT,
Li T, Mo XL, Zhou W, Guo WW, Liu X, et al: COX-2/PGE2 axis
regulates HIF2a activity to promote hepatocellular carcinoma
hypoxic response and reduce the sensitivity of sorafenib treatment.
Clin Cancer Res. 24:3204–3216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao H, Tong R, Ding C, Lv Z, Du C, Peng
C, Cheng S, Xi H, Zhou L, Wu J, et al: γ-H2AX promotes
hepatocellular carcinoma angiogenesis via EGFR/HIF-1α/VEGF pathways
under hypoxic condition. Oncotarget. 6:2180–2192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pore N, Jiang Z, Gupta A, Cerniglia G, Kao
GD and Maity A: EGFR tyrosine kinase inhibitors decrease VEGF
expression by both hypoxia-inducible factor (HIF)-1-independent and
HIF-1-dependent mechanisms. Cancer Res. 66:3197–3204. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang HF, Wang YL, Tan YZ, Wang HJ, Tao P
and Zhou P: Enhancement of cardiac lymphangiogenesis by
transplantation of CD34+VEGFR-3+ endothelial
progenitor cells and sustained release of VEGF-C. Basic Res
Cardiol. 114:432019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wen Jun L, Pit Foong C and Abd Hamid R:
Ardisia crispa root hexane fraction suppressed angiogenesis in
human umbilical vein endothelial cells (HUVECs) and in vivo
zebrafish embryo model. Biomed Pharmacother. 118:109221–109233.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rigiracciolo DC, Scarpelli A, Lappano R,
Pisano A, Santolla MF, De Marco P, Cirillo F, Cappello AR, Dolce V,
Belfiore A, et al: Copper activates HIF-1α/GPER/VEGF signalling in
cancer cells. Oncotarget. 6:34158–34177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang WM, Zhao ZL, Ma SR, Yu GT, Liu B,
Zhang L, Zhang WF, Kulkarni AB, Sun ZJ and Zhao YF: Epidermal
growth factor receptor inhibition reduces angiogenesis via
hypoxia-inducible factor-1α and Notch1 in head neck squamous cell
carcinoma. PLoS One. 10:1–17. 2015.
|
|
23
|
De Francesco EM, Pellegrino M, Santolla
MF, Lappano R, Ricchio E, Abonante S and Maggiolini M: GPER
mediates activation of HIF1α/VEGF signaling by estrogens. Cancer
Res. 74:4053–4064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su D, Liao Z, Feng B, Wang T, Shan B, Zeng
Q, Song J and Song Y: Pulsatilla chinensis saponins cause liver
injury through interfering ceramide/sphingomyelin balance that
promotes lipid metabolism dysregulation and apoptosis.
Phytomedicine. 76:1532652020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shan B, Ai Z, Zeng S, Song Y, Song J, Zeng
Q, Liao Z, Wang T, Huang C and Su D: Gut microbiome-derived lactate
promotes to anxiety-like behaviors through GPR81 receptor-mediated
lipid metabolism pathway. Psychoneuroendocrinology. 117:1046992020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Q, Chen X, Xu Y, Liang J, Wang F and
Liu J: CEACAM1 resists hypoxia-induced inhibition of tube formation
of human dermal lymphatic endothelial cells. Cell Signal.
45:145–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Henson ES, Xiao W, Huang D,
McMillan-Ward EM, Israels SJ and Gibson SB: Tyrosine kinase
receptor EGFR regulates the switch in cancer cells between cell
survival and cell death induced by autophagy in hypoxia. Autophagy.
12:1029–1046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka T, Ozawa T, Oga E, Muraguchi A and
Sakurai H: Cisplatin-induced non-canonical endocytosis of EGFR via
p38 phosphorylation of the C-terminal region containing Ser-1015 in
non-small cell lung cancer cells. Oncol Lett. 15:9251–9256.
2018.PubMed/NCBI
|
|
30
|
Song X, Yao J, Wang F, Zhou M, Zhou Y,
Wang H, Wei L, Zhao L, Li Z, Lu N, et al: Wogonin inhibits tumor
angiogenesis via degradation of HIF-1α protein. Toxicol Appl
Pharmacol. 271:144–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Flegg JA, Menon SN, Byrne HM and McElwain
DL: A current perspective on wound healing and tumour-induced
angiogenesis. Bull Math Biol. 82:232020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schito L: Hypoxia-dependent angiogenesis
and lymphangiogenesis in cancer. Adv Exp Med Biol. 1136:71–85.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morfoisse F, Renaud E, Hantelys F, Prats
AC and Garmy-Susini B: Role of hypoxia and vascular endothelial
growth factors in lymphangiogenesis. Mol Cell Oncol. 1:e299072014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Devi L, Pothana L and Goel S:
Dysregulation of angiogenesis-specific signalling in adult testis
results in xenograft degeneration. Sci Rep. 7:26052017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zepeda-Orozco D, Wen HM, Hamilton BA,
Raikwar NS and Thomas CP: EGF regulation of proximal tubule cell
proliferation and VEGF-A secretion. Physiol Rep. 5:1–14. 2017.
View Article : Google Scholar
|
|
37
|
Chuang JY, Chen PC, Tsao CW, Chang AC,
Lein MY, Lin CC, Wang SW, Lin CW and Tang CH: WISP-1 a novel
angiogenic regulator of the CCN family promotes oral squamous cell
carcinoma angiogenesis through VEGF-A expression. Oncotarget.
6:4239–4252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JG and Wu R: Erlotinib-cisplatin
combination inhibits growth and angiogenesis through c-MYC and
HIF-1α in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia.
17:190–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyazaki H, Yoshimatsu Y, Akatsu Y,
Mishima K, Fukayama M, Watabe T and Miyazono K: Expression of
platelet-derived growth factor receptor β is maintained by Prox1 in
lymphatic endothelial cells and is required for tumor
lymphangiogenesis. Cancer Sci. 105:1116–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun L, Zhang Q, Li Y, Tang N and Qiu X:
CCL21/CCR7 up-regulate vascular endothelial growth factor-D
expression via ERK pathway in human non-small cell lung cancer
cells. Int J Clin Exp Pathol. 8:15729–15738. 2015.PubMed/NCBI
|
|
41
|
Zhang Y, Yang X, Liu H, Cai M and Shentu
Y: Inhibition of tumor lymphangiogenesis is an important part that
EGFR-TKIs play in the treatment of NSCLC. J Cancer. 11:241–250.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Du LL and Liu P: CXCL12/CXCR4 axis
regulates neovascularization and lymphangiogenesis in sutured
corneas in mice. Mol Med Rep. 13:4987–4994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jeong YJ, Cho HJ, Magae J, Lee IK, Park KG
and Chang YC: Ascofuranone suppresses EGF-induced HIF-1α protein
synthesis by inhibition of the Akt/mTOR/p70S6K pathway in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
273:542–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi SB, Park JB, Song TJ and Choi SY:
Molecular mechanism of HIF-1-independent VEGF expression in a
hepatocellular carcinoma cell line. Int J Mol Med. 28:449–454.
2011.PubMed/NCBI
|
|
45
|
DeNiro M, Al-Mohanna FH, Alsmadi O and
Al-Mohanna FA: The nexus between VEGF and NFκB orchestrates a
hypoxia-independent neovasculogenesis. PLoS One. 8:e590212013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kiriakidis S, Andreakos E, Monaco C,
Foxwell B, Feldmann M and Paleolog E: VEGF expression in human
macrophages is NF-kappaB-dependent: Studies using adenoviruses
expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a
kinase-defective form of the IkappaB kinase 2. J Cell Sci.
116:665–674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mizukami Y, Li J, Zhang X, Zimmer MA,
Iliopoulos O and Chung DC: Hypoxia-inducible factor-1-independent
regulation of vascular endothelial growth factor by hypoxia in
colon cancer. Cancer Res. 64:1765–1772. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mohamady S, Galal M, Eldehna WM, Gutierrez
DC, Ibrahim HS, Elmazar MM and Ali HI: Dual targeting of VEGFR2 and
C-Met kinases via the design and synthesis of substituted
3-(Triazolo-thiadiazin-3-yl)indolin-2-one derivatives as
angiogenesis inhibitors. ACS Omega. 5:18872–18886. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim HJ, Baek MJ, Kang DH, Lee SC, Bae SB,
Lee KT, Lee N, Kim H, Jeong D, Ahn TS, et al: Association between
c-met and lymphangiogenic factors in patients with colorectal
cancer. Ann Coloproctol. 34:88–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Montico F, Kido LA, Hetzl AC, Lorencini
RM, Cândido EM and Cagnon VH: Antiangiogenic therapy effects on
age-associated matrix metalloproteinase-9 (MMP-9) and insulin-like
growth factor receptor-1 (IGFR-1) responses: A comparative study of
prostate disorders in aged and TRAMP mice. Histochem Cell Biol.
142:269–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Larrieu-Lahargue F, Welm AL, Bouchecareilh
M, Alitalo K, Li DY, Bikfalvi A and Auguste P: Blocking Fibroblast
Growth Factor receptor signaling inhibits tumor growth,
lymphangiogenesis, and metastasis. PLoS One. 7:e395402012.
View Article : Google Scholar : PubMed/NCBI
|