Introduction
Cervical cancer (CC) is a commonly diagnosed tumor
among females, resulting in >500,000 new cases annually
worldwide (1–3). Investigating the mechanism underlying CC
pathogenesis is important for developing novel biomarkers and
therapeutic targets for CC.
Long non-coding RNAs (lncRNAs) were originally
considered to be transcriptional noise, but an increasing number of
studies have demonstrated that lncRNAs serve a significant
regulatory role in human diseases, including numerous types of
cancer, including gallbladder cancer, gastric cancer and lung
squamous cell carcinoma (4–7). lncRNA forkhead box D3 antisense RNA 1
(FOXD3-AS1) is abnormally expressed in neuroblastoma, breast cancer
and glioma, functioning as an oncogenic factor or tumor suppressor
(8–10). However, the functions and mechanism
underlying FOXD3-AS1 in CC are not completely understood.
MicroRNAs (miRNAs/miRs) can bind to the
3′-untranslated regions of target mRNAs, promoting mRNA degradation
or inhibiting mRNA translation (11).
miRNAs are involved in regulating a series of biological processes,
and dysregulated miRNA expression is a common event in
tumorigenesis and cancer progression (12,13).
miRNAs are also important regulators of CC pathogenesis (14–17). For
example, miR-20a regulates CC cell proliferation, apoptosis and
autophagy by targeting thrombospondin 2 (14). miR-15b upregulation is indicative of
poor prognosis in patients with CC (15). Additionally, it has been reported that
the expression of miR-128-3p is downregulated in CC tissues,
whereas miR-128-3p overexpression significantly inhibits CC cell
proliferation, migration and invasion (18). However, the mechanism underlying the
effects of miR-128-3p in CC requires further investigation.
LIM domain kinase 1 (LIMK1) is a serine/threonine
kinase that is involved in the activation of the cell division
cycle 42/myotonic dystrophy-Related Cdc42-binding kinase, Rac/p21
activated kinase 1 and ras homolog family member A/Rho associated
coiled-coil containing protein kinase 1 signaling pathways, and can
regulate cytoskeletal remodeling (19,20).
Recent studies have demonstrated that LIMK1 expression is
upregulated or abnormally activated in various types of cancer,
including breast, prostate and colorectal cancer (21–23).
Moreover, LIMK1 expression is also significantly upregulated in CC,
which is associated with unfavorable prognosis (24). However, the mechanism underlying
dysregulated expression of LIMK1 in CC is not completely
understood.
The present study aimed to explore the expression
and biological functions of FOXD3-AS1 in CC, and to determine its
regulatory effect on miR-128-3p and LIMK1.
Materials and methods
FOXD3-AS1 expression analysis
The Gene Expression Profiling Interactive Analysis
(GEPIA) database (gepia.cancer-pku.cn/) was used to predict the
expression level of FOXD3-AS1 in patients with CC.
Tissue samples
The present study was approved by the Ethics
Committee of Hengshui People's Hospital (approval no. H20150019).
Written informed consent was obtained from all patients. A total of
60 female patients with CC (age range, 29–67 years) who underwent
surgery at the Hengshui People's Hospital (Hebei, China) between
May 2015 and May 2019 were recruited. Paired cancerous tissue and
corresponding adjacent healthy tissue (distance from tumor margin,
>3 cm) samples were obtained. All samples were confirmed by
pathological diagnosis. Patients had not received treatment prior
to surgery. All tissue samples were preserved in liquid nitrogen at
−196°C until RNA extraction.
Cell culture and cell
transfection
Human CC cell lines (C33A, HeLa, HT-3 and SiHa) and
a normal cervical epithelial cell line (HCE 16/3) were obtained
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin (100 U/ml)/streptomycin
(100 µg/ml) at 37°C with 5% CO2.
Empty pcDNA3.1 plasmid, pcDNA3.1-FOXD3-AS1, small
interfering (si)RNA negative control (NC; si-NC;
5′-CCTCCAGTGACCGCCTAAG-3′) and si-FOXD3-AS1 (si-FOXD3-AS1-1,
5′-CTCCAAGATTTAACTTCCA-3′; si-FOXD3-AS1-2,
5′-GGAGTTCCGAGAGGAAATA-3′; si-FOXD3-AS1-3,
5′-GATGCTGGGATGTGGATTT-3′) were prepared by Guangzhou RiboBio Co.,
Ltd. miRNA mimics control (NC mimics, 5′-ACGUGACACGUUCGGAGAATT-3′),
miR-128-3p mimics (5′-AAGAGACCGUUCACUGUGA-3′), miRNA inhibitors
control (NC inhibitors, 5′-CUAACGCAUGCACAGUCGUACG-3′) and
miR-128-3p inhibitors (5′-UUUCUCUGGCCAAGUGACACU-3′) were obtained
from Shanghai GenePharma Co., Ltd. HeLa and C33A cells in the
logarithmic growth phase were harvested and resuspended in
antibiotic-free and serum-free medium. Subsequently, cell
suspension (5×105 cells/ml) was added to 6-well plates.
Cells were transfected with plasmids (1.5 µg/ml), siRNAs (100 nM)
or miRNAs (50 nM) using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 24 h. Cells were
cultured in complete medium supplemented with 10% FBS. Following
culture for 24 h, cells were harvested and transfection efficiency
was assessed.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA concentrations were determined using a NanoDrop1000
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA was reverse
transcribed into cDNA using a PrimeScript RT Master Mix kit (Takara
Bio, Inc.) according to the manufacturer's protocol. Subsequently,
cDNA was used for qPCR using SYBR Green MasterMix kit (Takara Bio,
Inc.) and an ABI 7900 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions used for qPCR were as follows: Initial denaturation at
94°C for 5 min; 40 cycles of denaturation at 94°C for 30 sec,
annealing at 62°C for 45 sec and extension at 72°C for 40 sec; and
final extension at 72°C for 7 min. The sequences of the primers
used for qPCR are presented in Table
I. FOXD3-AS1 and LIMK1 mRNA expression levels were normalized
to the internal reference gene GAPDH. miR-128-3p expression levels
were normalized to the internal reference gene U6. Gene expression
levels were quantified using the 2−ΔΔCq method (25).
 | Table I.Sequences of primers used for
quantitative PCR. |
Table I.
Sequences of primers used for
quantitative PCR.
| Gene | Sequence
(5′→3′) |
|---|
| FOXD3-AS1 | F:
GGTGGAGGAGGCGAGGATG |
|
| R:
GGTGGAGGAGGCGAGGATG |
| miR-128-3p | F:
GACTGCCGAGCGAGCG |
|
| R:
GACGCCGAGGCACTCTCTCCT |
| LIMK1 | F:
GGGGCATCATCAAGAGCA |
|
| R:
CCAGGCAGTTGTGGGAGT |
| GAPDH | F:
TGGGCTACACTGAGCACCAG |
|
| R:
GGGTGTCGCTGTTGAAGTCA |
| U6 | F:
CCATCGGAAGCTCGTATACGAAATT |
|
| R:
GGCCTCTCGAACTTGCGTGTCAG |
Isolation of cytoplasmic and nuclear
RNA
Subcellular isolation of RNA from HeLa and C33A
cells was performed using the Cytoplasmic and Nuclear RNA
Purification kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cytoplasmic and nuclear fraction
expression levels were determined via qPCR according to the
aforementioned protocol. U6 was utilized as nuclear RNA control and
GAPDH as cytoplasmic control.
Cell Counting Kit-8 (CCK-8) assay
Transfected HeLa and C33A cells were collected and
seeded (2×103 cells/well) into 96-well plates. Following
culture for 12, 24, 48, 72 or 96 h, 10 µl CCK-8 solution (Dojindo
Molecular Technologies, Inc.) was added to each well and incubated
for 2 h. The optical density was measured at a wavelength of 450 nM
using a microplate reader (BioRad Laboratories, Inc.).
BrdU assay
BrdU cell proliferation assay kit (Cell Signaling
Technology Inc.) was used for BrdU cell proliferation assay.
Transfected C33A and HeLa cells were seeded (5×103
cells/ml) into 96-well plates and cultured overnight. Subsequently,
20 µl BrdU solution (Beyotime Institute of Biotechnology) was added
to each well and incubated for 12 h. Subsequently, the culture
medium was discarded and cells were washed with PBS. Cells were
fixed with 4% paraformaldehyde for 30 min at room temperature and
washed again with PBS. Cells were incubated with anti-BrdU (cat.
no. ab6326; 1:2,000; Abcam) for 1 h at room temperature. Then, cell
nuclei were counterstained using Hoechst staining solution
(Beyotime Institute of Biotechnology) at room temperature for 30
min. BrdU+ cells were observed and counted using a
fluorescence microscope (CX41-32RFL; Olympus Corporation).
Transwell assay
Transwell chamber inserts (pore size, 8.0 µm; EMD
Millipore) were used to assess cell migration and invasion. To
assess cell invasion, the upper chambers of the Transwell inserts
were precoated with Matrigel (BD Biosciences) for 1 h at room
temperature. To assess cell migration, the upper chambers of the
Transwell inserts were not precoated with Matrigel. Transfected
HeLa and C33A cells (2×104 cells/well) resuspended in
serum-free DMEM were added to the upper chamber. The lower chamber
was filled with DMEM supplemented with 10% FBS. Following
incubation at 37°C for 24 h, a cotton swab was used to remove
non-migratory/invading cells in the upper chamber.
Migratory/invading cells were fixed using 4% paraformaldehyde for
30 min at room temperature and stained with 0.1% crystal violet
solution for 30 min at room temperature. Cells were counted using a
light microscope (Nikon Corporation).
Luciferase reporter assay
The possible target miRNAs of FOXD3-AS1 were
predicted using StarBase (starbase.sysu.edu.cn). The cDNA fragment containing
the wild-type (WT; FOXD3-AS1-WT) or mutant (MUT; FOXD3-AS1-MUT)
FOXD3-AS1 fragment was subcloned downstream of the luciferase gene
in psi-CHECK luciferase reporter vectors (Promega Corporation).
HeLa and C33A cells were co-transfected (4×104
cells/well) with 100 nM luciferase reporter vectors and 50 nM
miR-128-3p mimics or miRNA mimics control using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 24 h. Luciferase
activities were measured using a dual-luciferase reporter gene
system (Promega Corporation) according to the manufacturer's
protocol. Firefly luciferase activity was normalized to
Renilla luciferase activity.
RNA immunoprecipitation (RIP)
RIP analysis was performed using a Magna RIP RNA
binding protein immunoprecipitation kit (EMD Millipore). At 48 h
post-transfection, HeLa and C33A cells were lysed with RIP lysis
buffer (EMD Millipore). Cell lysate (2×107 cells) was
incubated with protein A magnetic beads (EMD Millipore) conjugated
to anti-argonaute RISC catalytic component 2 (Ago2; cat. no.
ab32381; 1:500; Abcam) or anti-IgG (cat. no. ab172730; 1:500;
Abcam) antibody. After 6 h, the beads (50 µl) were washed four
times with RIP buffer, and then incubated with 0.1% SDS/0.5 mg/ml
Proteinase K for 30 min at 55°C to remove proteins. After
purification, the enrichment of FOXD3-AS1 and miR-128-2p was
assessed via qPCR according to the aforementioned protocol.
Immunohistochemistry
CC tissues and corresponding adjacent healthy
tissues were fixed with 10% formalin for 60 min at room temperature
and embedded in paraffin. Paraffin sections (thickness, 4 µm) were
de-paraffinized with xylene and hydrated using a descending ethanol
series. Antigen retrieval was performed by heating (98°C) in 10 mM
sodium citrate-hydrochloric acid buffer (pH 8.0) for 30 min.
Following incubation in 3% H2O2 solution to
quench endogenous peroxidase activity, samples were incubated with
normal goat serum (cat. no. ab7481; 1:100; Abcam) for 20 min to
block non-specific binding at room temperature. Subsequently,
samples were incubated with a primary antibody targeted against
anti-LIMK1 (cat. no. 19699-1-AP; 1:100; ProteinTech Group, Inc.)
overnight at 4°C. Following washing with PBS, samples were
incubated with rabbit IgG monoclonal secondary antibody (cat. no.
ab172730; 1:500; Abcam) for 1 h at room temperature. Subsequently,
samples were washed with PBS again and then stained with
3,3-diaminobenzidine hydrochloride for 15 min at room temperature.
Stained sections were observed and scored by two independent
pathologists using a light microscope (Olympus Corporation) using a
double-blind method. Scoring criteria were based on staining
intensity and the percentage of stained cells. The expression score
was calculated according to the following formula: Percentage of
stained cells (0, no staining, 1, <25 positively stained cells;
2, 25–50% positively stained cells; and 3, >50% positively
stained cells) + staining intensity score (0, no staining; 1,
faint; 2, moderate; and 3, strong). The expression score was
categorized as negative (0–2) or positive (≥2).
Western blotting
Transfected C33A and HeLa cells were collected and
lysed using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein concentrations were determined using a
bicinchoninic assay kit (Bio-Rad Laboratories, Inc.). Proteins (15
µg) were separated via SDS-PAGE (10% separation gel and 4%
concentration gel) and transferred to PVDF membranes (EMD
Millipore). Following blocking with 5% skimmed milk for 2 h at room
temperature, the membranes were incubated with anti-LIMK1 (cat. no.
ab81046; 1:1,000; Abcam) or anti-GAPDH (cat. no. ab181602; 1:1,000;
Abcam) primary antibodies overnight at 4°C. Following washing with
TBST (0.1% Tween-20), the membranes were incubated with a
horseradish peroxidase-conjugated secondary rat monoclonal IgG
antibody (cat. no. sc-69916; 1:2,000; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Protein bands were visualized
using an ECL substrate kit (Amersham; Cytiva). GAPDH was used as
the loading control.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 17.0; SPSS Inc.). All experiments were performed
in triplicate. Data are presented as the mean ± SD. The paired
Student's t-test was used to analyze comparisons between paired
samples. The unpaired Student's t-test was used to analyze
comparisons between unpaired samples. Comparisons among multiple
groups were analyzed using one-way ANOVA followed by Tukey's post
hoc test. Categorical data were analyzed using the χ2
test. Kaplan-Meier survival analyses and log-rank tests were
performed to determine the association between overall survival
time and FOXD3-AS1 expression. Pearson's correlation coefficient
was used to analyze the correlation between FOXD3-AS1 and
miR-128-3p, LIMK1 and miR-128-3p, and LIMK1 and FOXD3-AS1.
P<0.05 was considered to indicate a statistically significant
difference.
Results
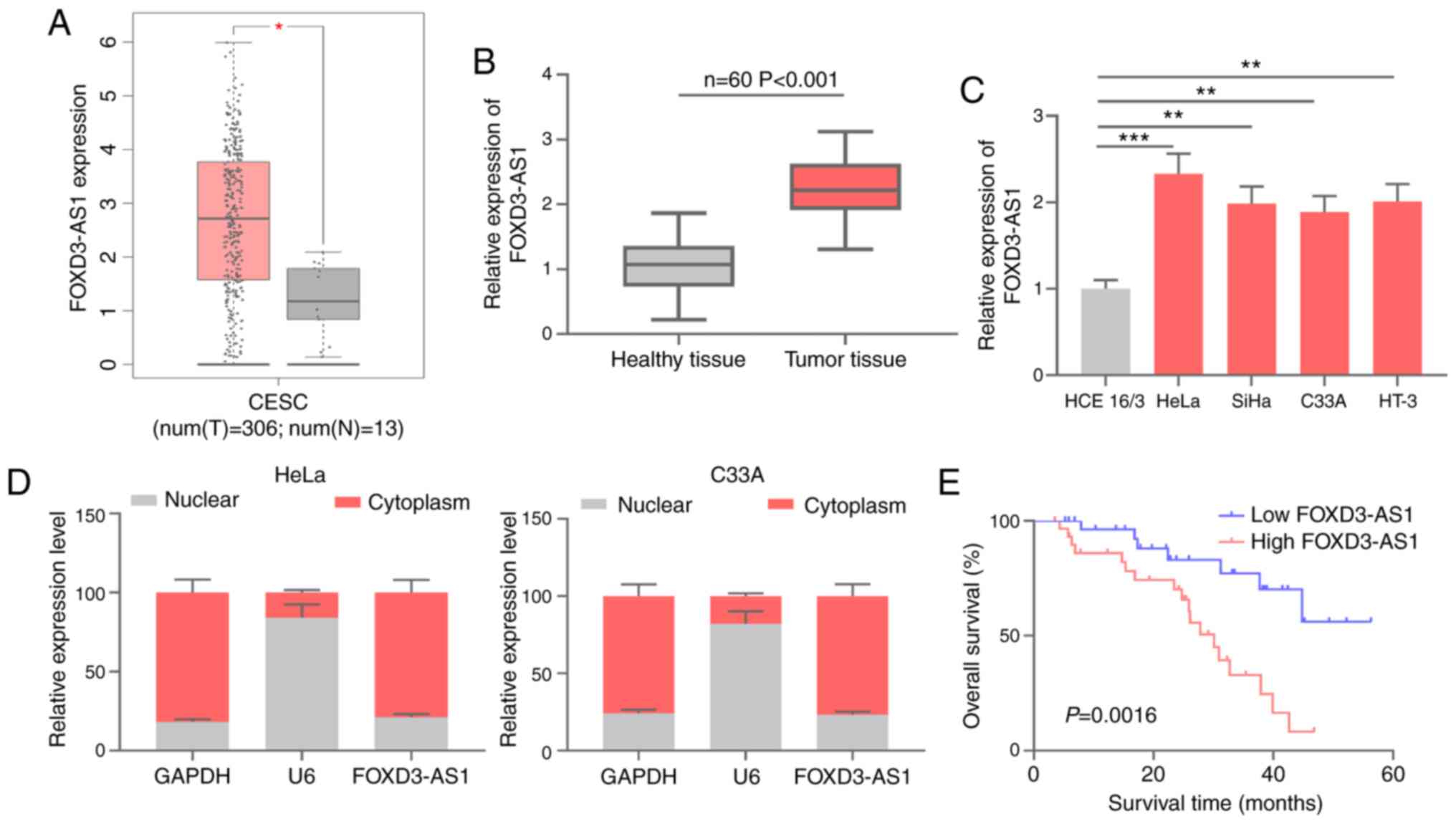
FOXD3-AS1 expression in CC and its
clinical significance
FOXD3-AS1 expression in CC samples was analyzed
using data obtained from GEPIA (gepia.cancer-pku.cn/). FOXD3-AS1
expression was significantly upregulated in CC tissues compared
with healthy cervical tissues (Fig.
1A). RT-qPCR was performed to measure FOXD3-AS1 expression
levels in CC tissue and corresponding adjacent healthy tissue
samples obtained from 60 patients with CC. Compared with
corresponding adjacent healthy tissues, FOXD3-AS1 expression levels
were significantly higher in CC tissues (Fig. 1B). Additionally, FOXD3-AS1 expression
levels in CC cells (HeLa, SiHa, C33A and HT-3) and normal cervical
epithelial cells (HCE 16/3) were determined. Consistent with the
tissue analysis, FOXD3-AS1 expression in CC cells was significantly
upregulated compared with HCE 16/3 cells (Fig. 1C). Subsequently, the subcellular
distribution of FOXD3-AS1 was assessed. The results demonstrated
that FOXD3-AS1 was primarily located in the cytoplasm of HeLa and
C33A cells compared with the nucleus (Fig. 1D). Kaplan-Meier survival analysis
demonstrated that the overall survival time of patients with CC
with high FOXD3-AS1 expression was significantly shorter compared
with patients with CC with low FOXD3-AS1 expression (Fig. 1E). To determine the clinical
significance of high FOXD3-AS1 expression in CC tissues, patients
were stratified according to the median expression level
(2.23±0.19) of FOXD3-AS1 in CC tissues. The results indicated that
high FOXD3-AS1 expression was significantly associated with larger
tumor size (P=0.0125), poorer tumor differentiation (P=0.0048) and
presence of lymph node metastasis (P=0.0195) (Table II). The aforementioned results
suggested that FOXD3-AS1 displayed a tumor-promoting effect on
CC.
 | Table II.Association between FOXD3-AS1
expression levels and clinical characteristics of patients with CC
(n=60). |
Table II.
Association between FOXD3-AS1
expression levels and clinical characteristics of patients with CC
(n=60).
|
|
| FOXD3-AS1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | n | High | Low | χ2 | P-value |
|---|
| Age (years) |
|
|
| 2.5837 | 0.1080 |
|
<40 | 22 | 8 | 14 |
|
|
|
≥40 | 38 | 22 | 16 |
|
|
|
Differentiation |
|
|
| 7.9365 | 0.0048b |
|
Poor | 42 | 16 | 26 |
|
|
| Well or
moderate | 18 | 14 | 4 |
|
|
| Tumor size
(cm) |
|
|
| 6.2388 | 0.0125a |
|
<4 | 19 | 14 | 5 |
|
|
| ≥4 | 41 | 16 | 25 |
|
|
| Histology |
|
|
| 0.1307 | 0.7177 |
|
Squamous | 51 | 26 | 25 |
|
|
|
Adenocarcinoma | 9 | 4 | 5 |
|
|
| HPV16/18 |
|
|
| 2.7000 | 0.1003 |
|
Negative | 40 | 17 | 23 |
|
|
|
Positive | 20 | 13 | 7 |
|
|
| Lymph node
metastasis |
|
|
| 5.4545 | 0.0195a |
|
Negative | 16 | 12 | 4 |
|
|
|
Positive | 44 | 18 | 26 |
|
|
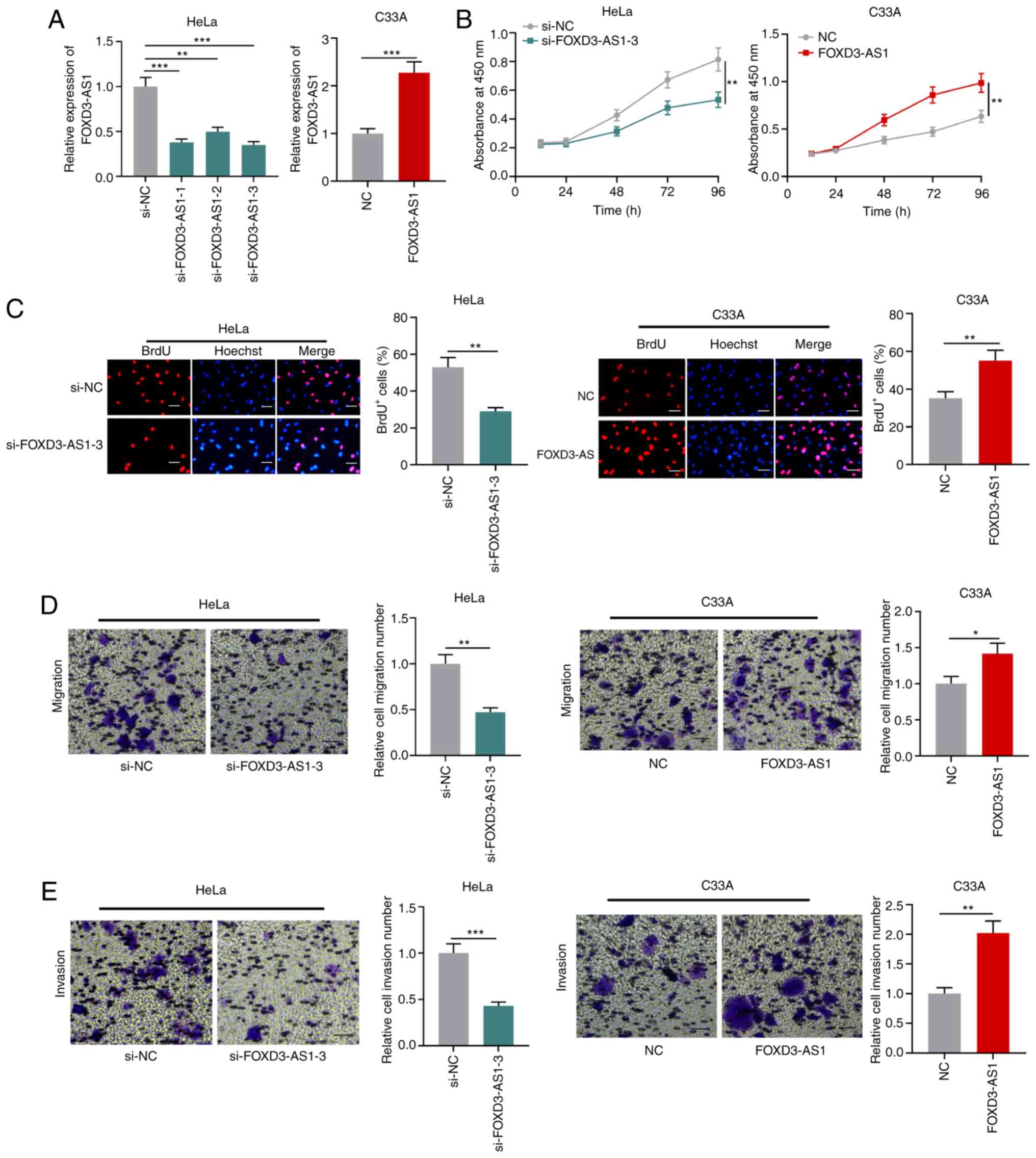
FOXD3-AS1 promotes CC cell
proliferation, migration and invasion
Among the four CC cell lines, the expression of
FOXD3-AS1 was lowest in C33A cells and highest in HeLa cells.
Therefore, C33A cells were used for FOXD3-AS1 overexpression and
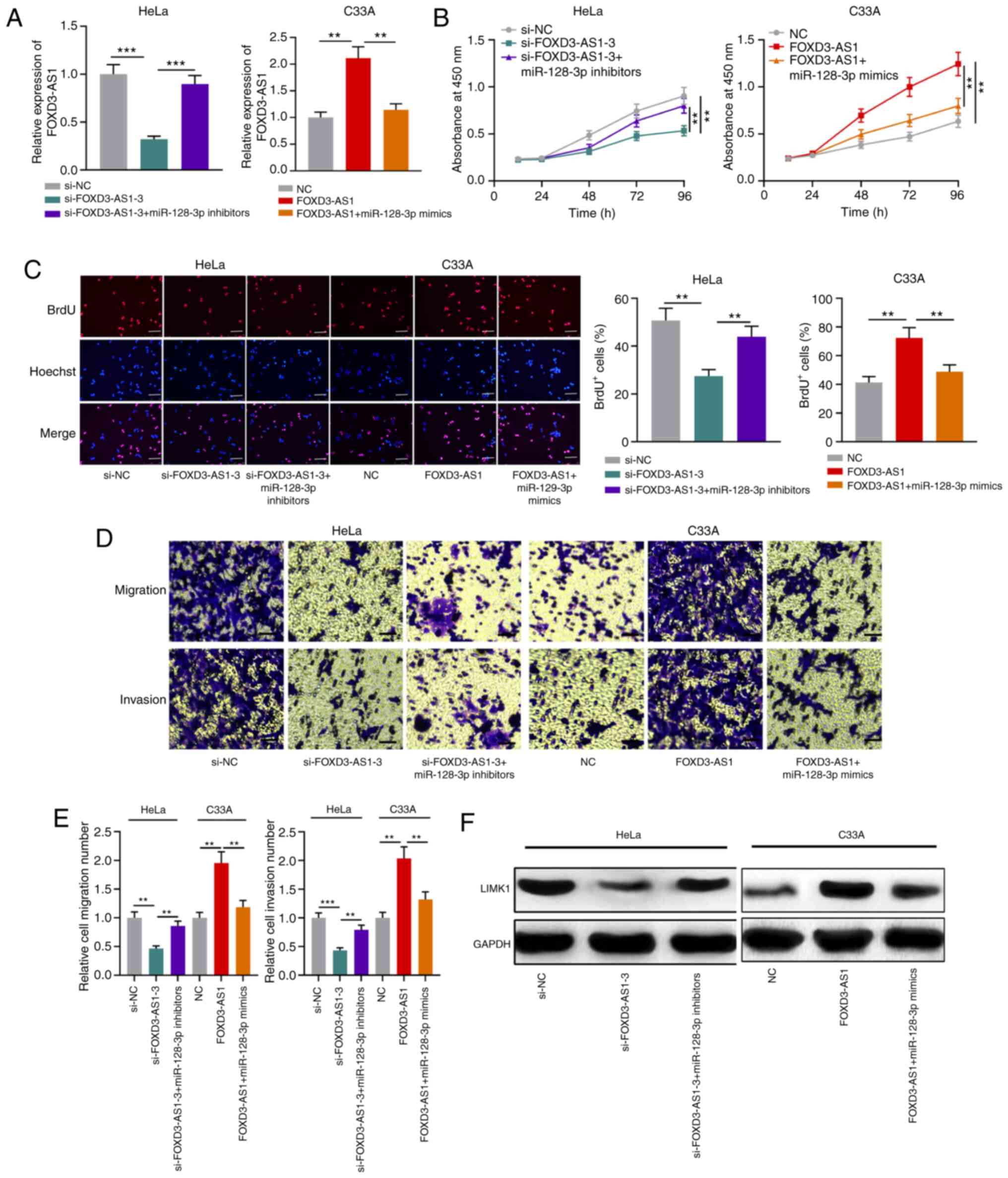
HeLa cells were used for FOXD3-AS1 knockdown (Fig. 2A). Subsequently, CCK-8 and BrdU assays
were performed to assess cell proliferation. FOXD3-AS1 knockdown
significantly inhibited HeLa cell proliferation compared with the
si-NC group, whereas FOXD3-AS1 overexpression significantly
increased C33A cell proliferation compared with the NC group
(Fig. 2B and C). Transwell assays
were conducted to assess CC cell migration and invasion. Compared
with the si-NC group, FOXD3-AS1 knockdown significantly suppressed
HeLa cell migration and invasion, whereas FOXD3-AS1 overexpression
significantly facilitated C33A cell migration and invasion compared
with the NC group (Fig. 2D and
E).
FOXD3-AS1 targets miR-128-3p
lncRNAs can regulate miRNAs by serving as
competitive endogenous RNAs (26).
StarBase (starbase.sysu.edu.cn) was used to
predict the target miRNAs of FOXD3-AS1. A potential binding site
between FOXD3-AS1 and miR-128-3p was identified (Figs. 3A and Table
SI). The dual-luciferase reporter assay results demonstrated
that miR-128-3p mimics significantly inhibited the luciferase
activity of the FOXD3-AS1-WT reporter compared with the NC group,
but did not display a significant effect on the luciferase activity
of the FOXD3-AS1-MUT reporter (Fig.
3B). The interaction between miR-128-3p and FOXD3-AS1 was
further verified by performing RIP assays. The results demonstrated
that FOXD3-AS1 and miR-128-3p were significantly enriched in
anti-Ago2 conditions compared with anti-IgG conditions (Fig. 3C). Furthermore, Pearson's correlation
coefficient demonstrated a negative correlation between miR-128-3p
and FOXD3-AS1 expression in CC tissues (Fig. 3D). miR-128-3p expression was
significantly decreased in CC tissues compared with adjacent
healthy tissues (Fig. 3E). miR-128-3p
expression was significantly lower in the four CC cell lines
compared with normal cervical epithelial cells (Fig. 3F). FOXD3-AS1 knockdown in HeLa cells
significantly increased miR-128-3p expression compared with the
si-NC group, whereas FOXD3-AS1 overexpression in C33A cells
significantly downregulated miR-128-3p expression compared with the
NC group (Fig. 3G). Collectively, the
results suggested that FOXD3-AS1 adsorbed miR-128-3p and negatively
regulated miR-128-3p expression in CC cells.
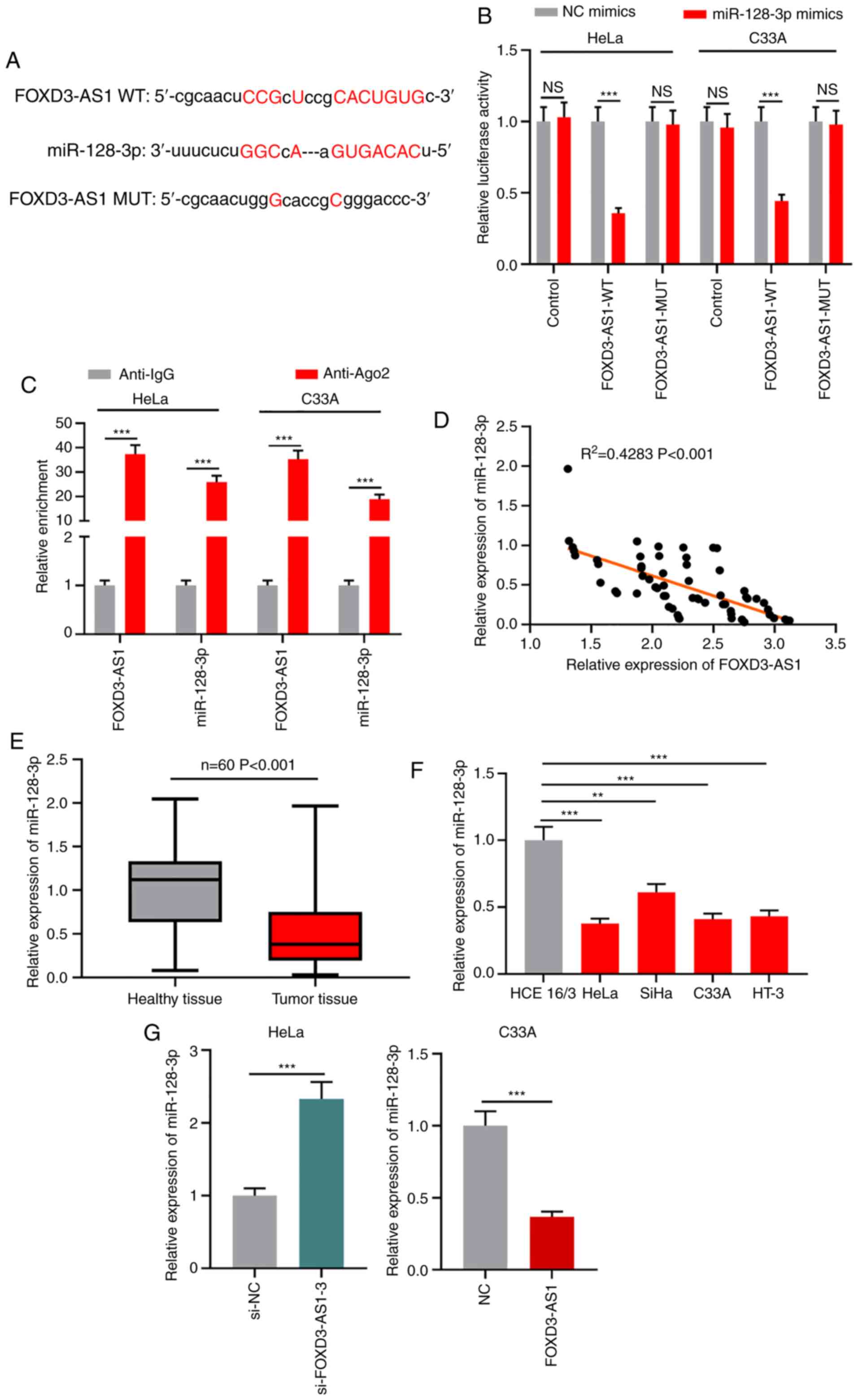 | Figure 3.FOXD3-AS1 targets miR-128-3p. (A)
Predicted binding site between FOXD3-AS1 and miR-128-3p. (B)
Dual-luciferase reporter assays were performed to verify the
interaction between FOXD3-AS1 and miR-128-3p. (C) Enrichment of
FOXD3-AS1 and miR-128-3p in anti-Ago2 or anti-IgG was determined by
conducting RNA immunoprecipitation assays. (D) Correlation between
FOXD3-AS1 expression and miR-128-3p expression in CC tissues.
RT-qPCR was performed to determine the expression levels of
miR-128-3p in (E) CC and adjacent healthy tissues, as well as in
(F) normal cervical epithelial and CC cell lines. (G) RT-qPCR was
performed to measure miR-128-3p expression levels in CC cells
following FOXD3-AS1 knockdown or overexpression. **P<0.01 and
***P<0.001. FOXD3-AS1, forkhead box D3 antisense RNA 1; miR,
microRNA; Ago2, argonaute RISC catalytic component 2; CC, cervical
cancer; RT-qPCR, reverse transcription-quantitative PCR; NC,
negative control; si, small interfering RNA; NS, not significant;
WT, wild-type; MUT, mutant. |
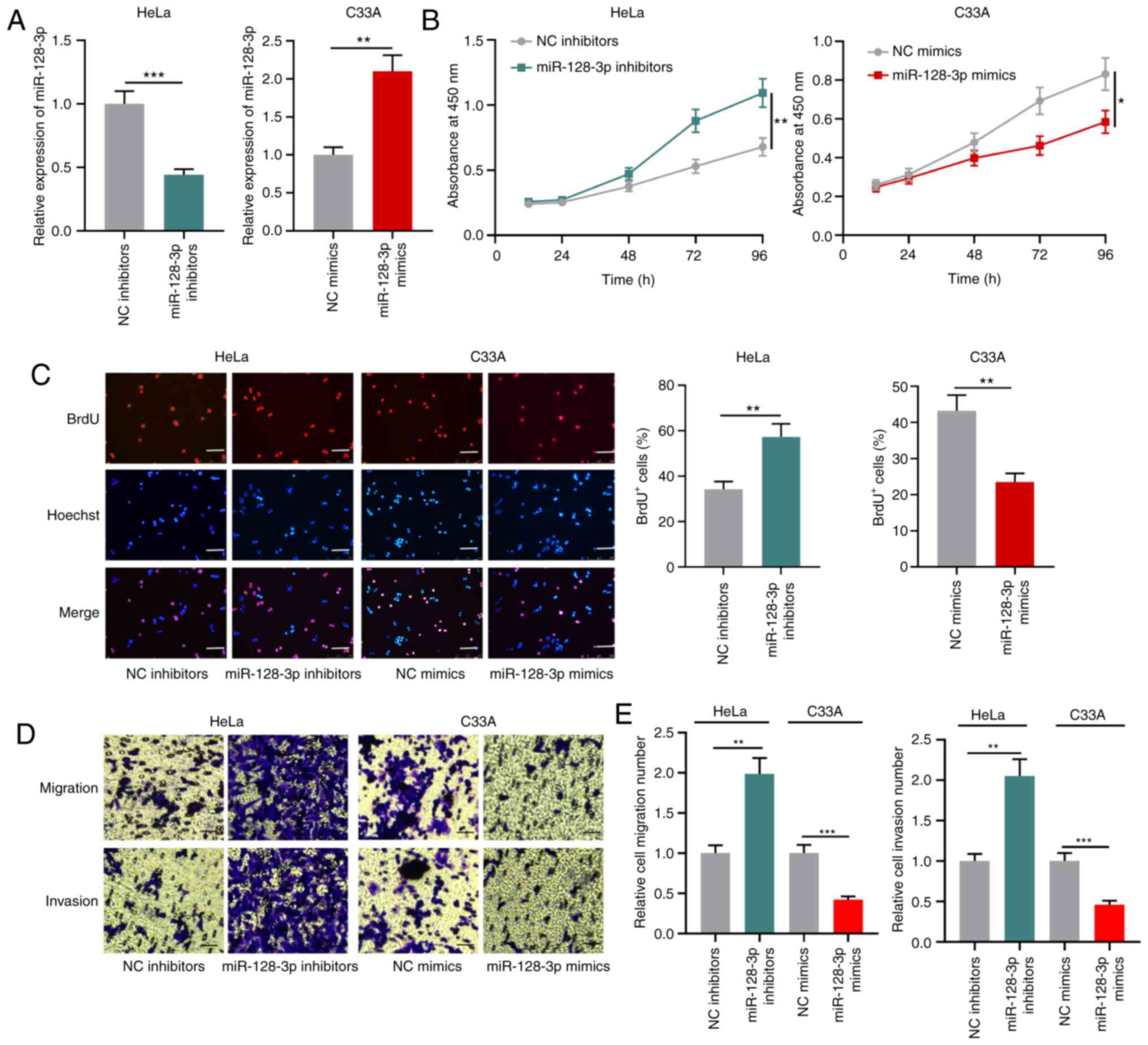
miR-128-3p suppresses CC cell
proliferation, migration and invasion
To investigate the biological function of miR-128-3p
in CC cells, HeLa cells were transfected with miR-128-3p inhibitors
and C33A cells were transfected with miR-128-3p mimics (Fig. 4A). The CCK-8 and BrdU assay results
demonstrated that miR-128-3p knockdown significantly promoted HeLa
cell proliferation compared with the NC inhibitors group, whereas
miR-128-3p overexpression significantly inhibited C33A cell
proliferation compared with the NC group (Fig. 4B and C). Furthermore, the Transwell
assay results indicated that miR-128-3p knockdown significantly
enhanced HeLa cell migration and invasion compared with the NC
inhibitors group, whereas miR-128-3p overexpression significantly
reduced C33A cell migration and invasion compared with the NC group
(Fig. 4D and E). The results
suggested that miR-128-3p functioned as a tumor suppressor in
CC.
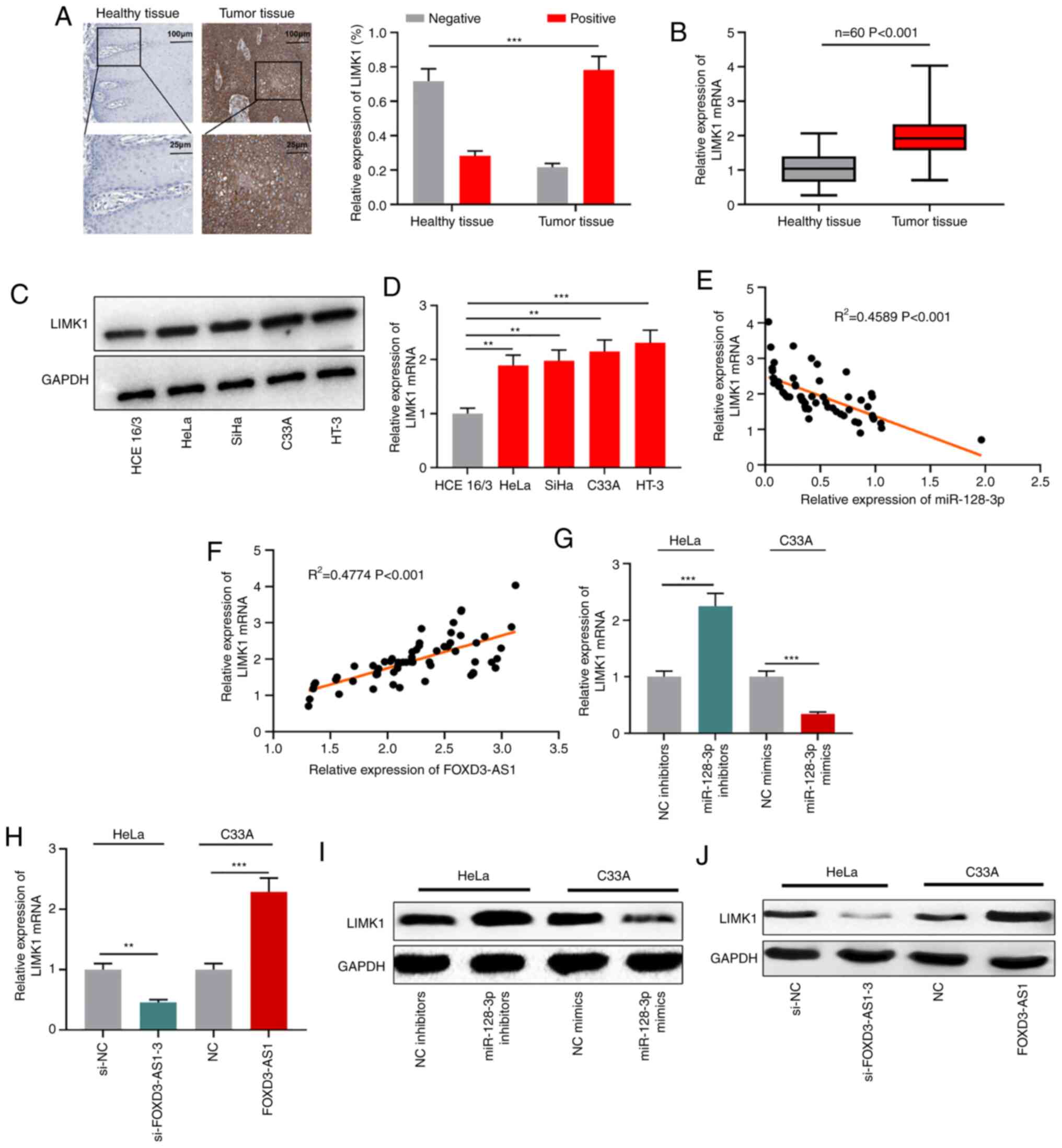
LIMK1 is regulated by the
FOXD3-AS1/miR-128-3p axis
It has been reported that LIMK1 is a downstream
target of miR-128-3p (27). In the
present study, immunohistochemistry was performed to assess LIMK1
expression in CC. The positive expression rate of LIMK1 in CC
tissues was significantly higher compared with adjacent healthy
tissues (Fig. 5A). RT-qPCR and
western blotting were performed to detect LIMK1 mRNA and protein
expression levels in CC tissues and cells. LIMK1 mRNA and protein
expression levels were notably upregulated in CC tissues and cells
compared with adjacent healthy tissues and normal cervical
epithelial cells, respectively (Fig.
5B-D). In CC tissue samples, LIMK1 mRNA expression was
negatively associated with miR-128-3p expression, but positively
associated with FOXD3-AS1 expression (Fig. 5E and F). Furthermore, miR-128-3p
knockdown markedly upregulated LIMK1 mRNA and protein expression
levels in HeLa cells compared with the NC inhibitors group, whereas
miR-128-3p overexpression notably downregulated LIMK1 mRNA and
protein expression levels in C33A cells compared with the NC group.
FOXD3-AS1 knockdown markedly reduced LIMK1 mRNA and protein
expression levels in HeLa cells compared with the si-NC group,
whereas FOXD3-AS1 overexpression obviously increased LIMK1 mRNA and
protein expression levels in C33A cells compared with the NC group
(Fig. 5G-J). The results indicated
that LIMK1 was regulated by the FOXD3-AS1/miR-128-3p axis in
CC.
miR-128-3p partially reverses the
effects of FOXD3-AS1
To further elucidate the role of the
FOXD3-AS1/miR-128-3p/LIMK1 axis in CC, miR-128-3p inhibitors were
transfected into FOXD3-AS1-knockdown HeLa cells and miR-128-3p
mimics were transfected into FOXD-AS1-overexpression C33A cells
(Fig. 6A). Functional experiments
demonstrated that miR-128-3p inhibitors significantly reversed
FOXD3-AS1 knockdown-mediated inhibitory effects on HeLa cell
proliferation, migration and invasion, whereas miR-128-3p
overexpression significantly inhibited FOXD3-AS1
overexpression-mediated promoting effects on C33A cell
proliferation, migration and invasion (Fig. 6B-E). Furthermore, miR-128-3p knockdown
markedly reversed FOXD3-AS1 knockdown-induced downregulation of
LIMK1 mRNA and protein expression levels, whereas miR-128-3p
overexpression obviously reversed FOXD3-AS1 overexpression-induced
upregulation of LIMK1 mRNA and protein expression levels (Fig. 6F). The results suggested that
FOXD3-AS1 participated in promoting CC progression via the
miR-128-3p/LIMK1 axis.
Discussion
In recent years, several studies on lncRNAs have
provided a theoretical basis for the progression of CC (28,29). For
example, lncRNA-transthyretin expression is upregulated in CC,
where it promotes CC cell malignant biological behaviors by
regulating TGF-β signaling, and upregulated lncRNA-transthyretin
expression is indicative of poor prognosis in patients with CC
(30). Long intergenic non-coding RNA
(LINC)00675 promotes CC cell proliferation, migration and invasion,
and inhibits apoptosis via regulation of the Wnt/β-catenin
signaling pathway (31). FOXD3-AS1
serves a crucial role in certain types of cancer. For example,
FOXD3-AS1 represses neuroblastoma progression by inhibiting
poly(ADP-ribose) polymerase 1-mediated CCCTC-binding factor
activation (8). In breast cancer,
FOXD3-AS1 knockdown inhibits cancer cell proliferation, migration
and invasion (9). To the best of our
knowledge, the present study was the first to demonstrate that
FOXD3-AS1 expression in CC tissues and cell lines was significantly
upregulated compared with adjacent healthy cervical tissues and
normal cervical epithelial cells, respectively. Moreover,
upregulated FOXD3-AS1 expression was significantly associated with
poor tumor differentiation, increased tumor size and lymph node
metastasis in patients with CC. FOXD3-AS1 knockdown significantly
inhibited CC cell malignant behaviour compared with the si-NC
group, whereas FOXD3-AS1 overexpression displayed the opposite
effect compared with the NC group. The aforementioned results
indicated that FOXD3-AS1 displayed a tumor-promoting effect in
CC.
miRNAs are important regulators involved in numerous
aspects of cancer biology (32,33).
miR-128-3p is abnormally expressed in various types of cancer,
where it is associated with promoting or inhibiting tumor
progression. In glioma, miR-128-3p targets neuronal pentraxin 1 and
regulates the Insulin receptor substrate 1/PI3K/AKT signaling
pathway to repress glioma cell proliferation (34). Additionally, miR-128-3p suppresses
drosha ribonuclease III to facilitate lung cancer cell migration
(35). miR-128-3p inhibits liver
cancer cell proliferation by repressing phosphoinositide-3-kinase
regulatory subunit 1 (36). The
present study demonstrated that miR-128-3p expression was
significantly reduced in CC tissues and cell lines compared with
adjacent healthy tissues and normal cervical epithelial cells,
respectively. Moreover, compared with the NC inhibitors group,
miR-128-3p knockdown significantly facilitated CC cell
proliferation, migration and invasion, suggesting that miR-128-3p
displayed a suppressive effect on CC progression, which was
consistent with a previous study (18). lncRNAs can directly interact with
miRNAs and regulate the availability of their target miRNAs by
serving as competing endogenous RNAs (26), a mechanism that has received
increasing attention in cancer research. For example, lncRNA HOXA
cluster antisense RNA 2 facilitates non-small cell lung cancer
progression by sponging miR-520a-3p (37). lncRNA double homeobox A pseudogene 8
promotes renal cell cancer progression by repressing miR-126
(38). The present study identified a
binding site between FOXD3-AS1 and miR-128-3p. Moreover, the
results indicated that miR-128-3p was negatively regulated by
FOXD3-AS1 in CC cells, confirming that the biological function of
FOXD3-AS1 in CC was partly dependent on miR-128-3p.
LIMK1 phosphorylation may result in dynamic
alterations to the cytoskeleton, thereby promoting cancer cell
migration and invasion (20,21). In ovarian cancer, LIMK1 knockdown
results in decreased cancer cell migration and invasion (39). Additionally, LIMK1 interacts with
myosin-9 and α-actinin-4, activating the PI3K/AKT signaling pathway
and driving epithelial-to-mesenchymal transition of colorectal
cancer cells, thus facilitating cancer progression (40). In prostate cancer, LIMK1 regulates
actin polymerization via phosphorylation and inactivation of
cofilin, and modulating cancer cell proliferation, cell cycle
progression, migration, invasion and androgen receptor nuclear
translocation (41). By performing
immunohistochemical staining, the present study demonstrated that
LIMK1 expression was significantly higher in CC tissues compared
with adjacent healthy cervical tissues. Additionally, LIMK1 mRNA
expression levels were notably higher in CC cells and tissues
compared with the normal cervical cell line and adjacent healthy
tissues, respectively. It has been reported that LIMK1 is a target
of miR-128-3p in breast cancer (27).
The present study demonstrated that LIMK1 mRNA expression was
negatively associated with miR-128-3p expression, but positively
associated with FOXD3-AS1 expression in CC tissues. LIMK1 was
negatively regulated by miR-128-3p, which indirectly upregulated
FOXD3-AS1 expression. The results suggested that FOXD3-AS1 may
enhance LIMK1 expression by adsorbing miR-128-3p, thereby promoting
the malignant phenotype of CC cells. However, there were a number
of limitations to the present study, including the lack of in
vivo experiments.
In summary, the results of the present study
demonstrated that FOXD3-AS1 upregulated LIMK1 expression by
targeting miR-128-3p, thereby facilitating CC cell proliferation,
migration and invasion. Therefore, FOXD3-AS1 may serve as a
potential therapeutic target and prognostic biomarker for patients
with CC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and HD conceived and designed the study. XY, WB
and QL performed the experiments. HS and QL analyzed the data. YX,
DH and WB drafted the manuscript. XY and HD confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of Hengshui People's Hospital (approval no. H20150019).
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maździarz A, Wyględowski J, Osuch B,
Jagielska B and Śpiewankiewicz B: New directions in cervical cancer
prophylaxis worldwide and in Poland-Case study of the polish rural
female population. Ann Agric Environ Med. 24:592–595. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Yu Y, Li H, Hu Q, Chen X, He Y,
Xue C, Ren F, Ren Z, Li J, et al: Long non-coding RNA PVT1 promotes
tumor progression by regulating the miR-143/HK2 axis in gallbladder
cancer. Mol Cancer. 18:332019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou C, Zhao J, Liu J, Wei S, Xia Y, Xia
W, Bi Y, Yan Z and Huang H: LncRNA SNHG16 promotes epithelial-
mesenchymal transition via down-regulation of DKK3 in gastric
cancer. Cancer Biomark. 26:393–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu Y, Li J, Wang P, Zhang Z and Wang X:
LncRNA HULC promotes lung squamous cell carcinoma by regulating
PTPRO via NF-κB. J Cell Biochem. 120:19415–19421. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tong W, Han TC, Wang W and Zhao J: LncRNA
CASC11 promotes the development of lung cancer through targeting
microRNA-302/CDK1 axis. Eur Rev Med Pharmacol Sci. 23:6539–6547.
2019.PubMed/NCBI
|
|
8
|
Zhao X, Li D, Huang D, Song H, Mei H, Fang
E, Wang X, Yang F, Zheng L, Huang K and Tong Q: Risk-associated
long noncoding RNA FOXD3-AS1 inhibits neuroblastoma progression by
repressing PARP1-mediated activation of CTCF. Mol Ther. 26:755–773.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan Y, Bhandari A, Xia E, Yang F, Xiang J
and Wang O: lncRNA FOXD3-AS1 is associated with clinical
progression and regulates cell migration and invasion in breast
cancer. Cell Biochem Funct. 37:239–244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen ZH, Hu HK, Zhang CR, Lu CY, Bao Y,
Cai Z, Zou YX, Hu GH and Jiang L: Down-regulation of long
non-coding RNA FOXD3 antisense RNA 1 (FOXD3-AS1) inhibits cell
proliferation, migration, and invasion in malignant glioma cells.
Am J Transl Res. 8:4106–4119. 2016.PubMed/NCBI
|
|
11
|
Benz F, Roy S, Trautwein C, Roderburg C
and Luedde T: Circulating MicroRNAs as biomarkers for sepsis. Int J
Mol Sci. 17:782016. View Article : Google Scholar
|
|
12
|
Zhou Y, Wang B, Wang Y, Chen G, Lian Q and
Wang H: miR-140-3p inhibits breast cancer proliferation and
migration by directly regulating the expression of tripartite motif
28. Oncol Lett. 17:3835–3841. 2019.PubMed/NCBI
|
|
13
|
Zhu X, Ma SP, Yang D, Liu Y, Wang YP, Lin
T, Li YX, Yang SH, Zhang WC and Wang XL: miR-142-3p suppresses cell
growth by targeting CDK4 in colorectal cancer. Cell Physiol
Biochem. 51:1969–1981. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Q, Dong J, Luo R, Zhou X, Wang J and
Chen F: MicroRNA-20a regulates cell proliferation, apoptosis and
autophagy by targeting thrombospondin 2 in cervical cancer. Eur J
Pharmacol. 844:102–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wen F, Xu JZ and Wang XR: Increased
expression of miR-15b is associated with clinicopathological
features and poor prognosis in cervical carcinoma. Arch Gynecol
Obstet. 295:743–749. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang H, Luo R, Chen X, Zhao Y and Tan A:
miR-187 inhibits the growth of cervical cancer cells by targeting
FGF9. Oncol Rep. 38:1977–1984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Juan C, Hua Q, Ruping Z and Tingting W:
miRNA-489 as a biomarker in diagnosis and treatment of cervical
cancer. Bratisl Lek Listy. 119:278–283. 2018.PubMed/NCBI
|
|
18
|
Wang R, Liu L, Jiao J and Gao D: Knockdown
of MIR4435-2HG suppresses the proliferation, migration and invasion
of cervical cancer cells via regulating the miR-128-3p/MSI2 axis in
vitro. Cancer Manag Res. 12:8745–8756. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scott RW and Olson MF: LIM kinases:
Function, regulation and association with human disease. J Mol Med
(Berl). 85:555–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Ji J, Hong F, Zhuang J and Wang L:
Maternal exposure to nanoparticulate titanium dioxide causes
inhibition of hippocampal development involving dysfunction of the
Rho/NMDAR signaling pathway in offspring. J Biomed Nanotechnol.
15:839–847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong H, Zhou L, Khelfat L, Qiu G, Wang Y,
Mao K and Chen W: Rho-associated protein kinase (ROCK) promotes
proliferation and migration of PC-3 and DU145 prostate cancer cells
by targeting LIM Kinase 1 (LIMK1) and matrix Metalloproteinase-2
(MMP-2). Med Sci Monit. 25:3090–3099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu J, Yu J, Chen J, Xu H, Luo Y and Lu H:
In vitro inhibitory properties of sesquiterpenes from Chloranthus
serratus on cell motility via down-regulation of LIMK1 activation
in human breast cancer. Phytomedicine. 49:23–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Li A, Shi J, Fang Y, Gu C, Cai J,
Lin C, Zhao L and Liu S: Imbalanced LIMK1 and LIMK2 expression
leads to human colorectal cancer progression and metastasis via
promoting β-catenin nuclear translocation. Cell Death Dis.
9:7492018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chhavi, Saxena M, Singh S, Negi MP,
Srivastava AK, Trivedi R, Singh U, Pant MC and Bhatt ML: Expression
profiling of G2/M phase regulatory proteins in normal, premalignant
and malignant uterine cervix and their correlation with survival of
patients. J Cancer Res Ther. 6:167–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Zhang W, Liu K and Liu Y: LncRNA
SNHG16 promotes tumor growth of pancreatic cancer by targeting
miR-218-5p. Biomed Pharmacother. 114:1088622019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao J, Li D and Fang L: miR-128-3p
suppresses breast cancer cellular progression via targeting LIMK1.
Biomed Pharmacother. 115:1089472019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding X, Jia X, Wang C, Xu J, Gao SJ and Lu
C: A DHX9-lncRNA-MDM2 interaction regulates cell invasion and
angiogenesis of cervical cancer. Cell Death Differ. 26:1750–1765.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang H, Liang M, Jiang Y, Zhang T, Mo K,
Su S, Wang A, Zhu Y, Huang G and Zhou R: The lncRNA TDRG1 promotes
cell proliferation, migration and invasion by targeting miR-326 to
regulate MAPK1 expression in cervical cancer. Cancer Cell Int.
19:1522019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng S, Liu W, Bai X, Pan W, Jia Z, Zhang
S, Zhu Y and Tan W: LncRNA-CTS promotes metastasis and
epithelial-to-mesenchymal transition through regulating
miR-505/ZEB2 axis in cervical cancer. Cancer Lett. 465:105–117.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma S, Deng X, Yang Y, Zhang Q, Zhou T and
Liu Z: The lncRNA LINC00675 regulates cell proliferation,
migration, and invasion by affecting Wnt/β-catenin signaling in
cervical cancer. Biomed Pharmacother. 108:1686–1693. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Li W, Pang Y, Zhou Z and Liu S,
Cheng K, Qin Q, Jia Y and Liu S: SF3B4 is regulated by
microRNA-133b and promotes cell proliferation and metastasis in
hepatocellular carcinoma. EBioMedicine. 38:57–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu C, Jian M, Qi H and Mao WZ: MicroRNA
495 inhibits proliferation and metastasis and promotes apoptosis by
targeting Twist1 in gastric cancer cells. Oncol Res. 27:389–397.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huo L, Wang B, Zheng M, Zhang Y, Xu J,
Yang G and Guan Q: miR-128-3p inhibits glioma cell proliferation
and differentiation by targeting NPTX1 through IRS-1/PI3K/AKT
signaling pathway. Exp Ther Med. 17:2921–2930. 2019.PubMed/NCBI
|
|
35
|
Frixa T, Sacconi A, Cioce M, Roscilli G,
Ferrara FF, Aurisicchio L, Pulito C, Telera S, Carosi M, Muti P, et
al: MicroRNA-128-3p-mediated depletion of Drosha promotes lung
cancer cell migration. Carcinogenesis. 39:293–304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang CY, Huang XP, Zhu JY, Chen ZG, Li
XJ, Zhang XH, Huang S, He JB, Lian F, Zhao YN and Wu GB: miR-128-3p
suppresses hepatocellular carcinoma proliferation by regulating
PIK3R1 and is correlated with the prognosis of HCC patients. Oncol
Rep. 33:2889–2898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Lin X, Zhou S, Zhang P, Shao G and
Yang Z: Long noncoding RNA HOXA-AS2 promotes non-small cell lung
cancer progression by regulating miR-520a-3p. Biosci Rep.
39:BSR201902832019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang T, Wang X, Yang X, Ji J, Wang Q, Yue
X and Dong Z: Long non-coding RNA DUXAP8 enhances renal cell
carcinoma progression via downregulating miR-126. Med Sci Monit.
24:7340–7347. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen P, Zeng M, Zhao Y and Fang X:
Upregulation of Limk1 caused by microRNA-138 loss aggravates the
metastasis of ovarian cancer by activation of Limk1/cofilin
signaling. Oncol Rep. 32:2070–2076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liao Q, Li R, Zhou R, Pan Z, Xu L, Ding Y
and Zhao L: LIM kinase 1 interacts with myosin-9 and
alpha-actinin-4 and promotes colorectal cancer progression. Br J
Cancer. 117:563–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mardilovich K, Gabrielsen M, McGarry L,
Orange C, Patel R, Shanks E, Edwards J and Olson MF: Elevated LIM
kinase 1 in nonmetastatic prostate cancer reflects its role in
facilitating androgen receptor nuclear translocation. Mol Cancer
Ther. 14:246–258. 2015. View Article : Google Scholar : PubMed/NCBI
|