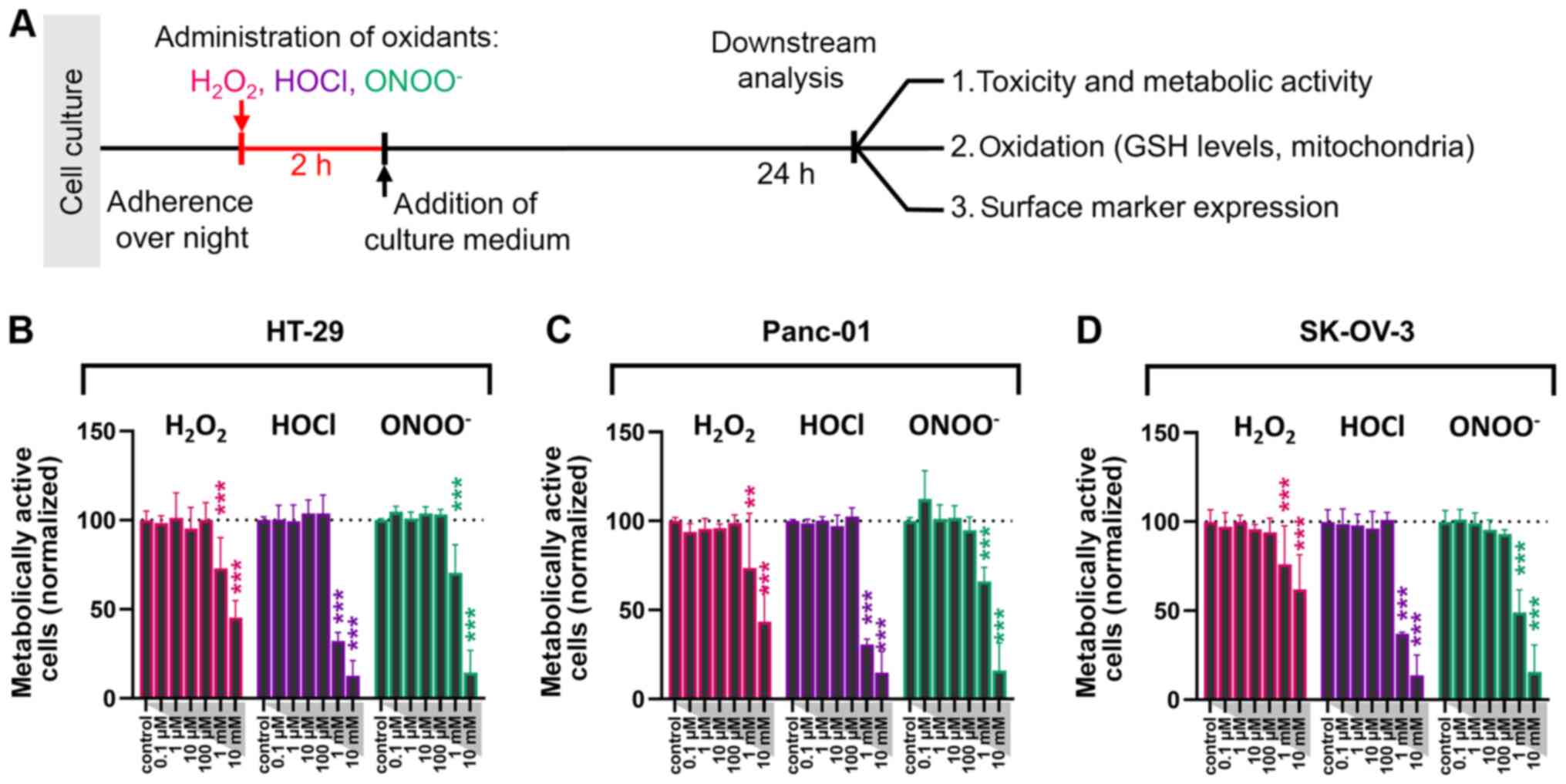
Introduction
Several types of tumor can metastasize on or into
the peritoneum (1). Rates of <30%
have been reported (2), making
peritoneal carcinomatosis (PC) a burden for patients and healthcare
systems alike. Pancreatic carcinoma often is diagnosed late and
shows aggressive growth and metastasization in the peritoneum,
limiting effective therapies (3,4).
Similarly, colorectal and ovarian carcinoma display widespread
localization in the abdominal cavity, which allows open field
metastasization and generation of PC before diagnosis (5–7). Curative
treatment is rarely achievable. For palliation, systemic
chemotherapy is of low efficacy and causes strong side effects
(8). The combination of cytoreductive
surgery with hyperthermic intraperitoneal chemotherapy (HIPEC;
peritoneal lavage with heated liquids containing chemotherapeutic
agents) has similar limitations (9).
For HIPEC, life expectancy and quality of life in patients with PC
are low (10). Alternative
treatments, such as laser-induced oxidation of peritoneal cancer,
may prolong survival but have been shown to be impractical and
cause severe side effects (11).
Reactive oxygen species (ROS) are increasingly
recognized as critical agents in anticancer therapy (12–15). For
instance, certain types of nanoparticle (such as metal oxides,
carbon nanotubes and silver nanoparticles) promote stress of the
endoplasmic reticulum and mitochondria via ROS (16–18).
Certain chemotherapeutic agents, such as 5-fluorouracil, can lead
to generation of intracellular oxidants, such as peroxynitrite
(ONOO−), in tumor cells (19). Anthracyclines also generate
intracellular ROS (20). Some
treatment strategies aim to directly generate ROS. These include
photodynamic therapy (PDT), which locally generates singlet Δ
oxygen via a photosensitizer (21).
PDT also promotes pro-immunogenic properties in tumor cells
(22). Similarly, gas plasma
treatment generates a multiple types of reactive oxygen and
nitrogen species (ROS/RNS) simultaneously (23–25). This
is not only toxic to tumor cells but also promotes their
immunogenicity via an increase in danger signals (26–28).
Specific pattern recognition receptors (PRR) are sensed by
evolutionary preserved structures, such as pathogen- and
damage-associated molecular patterns [including ATP, heat-shock
proteins, endoplasmic reticulum chaperon calreticulin (CRT)].
Innate immune cells are thus able to initiate quick and early
responses against infection or extensive cell death in tissue.
These mechanisms emphasize the important role of immunogenic cell
death (ICD) in initiating an anti-tumor immune response (29–31).
Since the award of the Nobel Prize for Physiology or
Medicine in 2018 for immune-checkpoint therapies, there has been an
increasing awareness of combination of conventional treatment
modalities with immunotherapy (32).
For PC treatment, standard therapy includes the intraperitoneal
administration of chemotherapeutics that directly reach tumor
lesions but cannot stimulate immunity via danger signals derived
from targeted cancer cells (33,34). This
limitation may be overcome by using liquids supplemented with ROS
to not only generate cytotoxic responses in tumor cells but also
render them more immunogenic by promoting the expression of danger
signals (35). To this end, the
present study compared three types of ROS, hydrogen peroxide
(H2O2), hypochlorous acid (HOCl) and
ONOO−, to investigate their ability to inactivate cancer
cells and promote expression of markers associated with ICD
(31).
Materials and methods
Cell culture
A total of three human abdominal cancer cell lines
(HT-29 colorectal, Panc-01 pancreatic and SK-OV-3 ovarian cancer
cells), as well as non-malignant HaCaT keratinocytes, were used.
The HT-29 and Panc-01 cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.), while SK-OV-3 and HaCaT cells were
cultured in RPMI-1640 medium (PAN Biotech GmbH). Both media were
supplemented with 10% fetal bovine serum, 2%
penicillin-streptomycin and 1% glutamine (all Sigma-Aldrich; Merck
KGaA). Cells were cultured at 37°C, 5% CO2 and 95%
humidity in a cell culture incubator (Binder GmbH). Subculturing
was performed 2–3 times/week. In order to determine the cell count
for downstream experiments at high precision, cells were stained
with PI (Sigma-Aldrich; Merck KGaA) for 5 min at room temperature,
and absolute counts were obtained using acoustic focusing flow
cytometry (Attune Nxt; Thermo Fisher Scientific, Inc.) and Attune
Nxt Software v. 2.7.0 (Thermo Fisher Scientific, Inc.). Cells were
seeded at 1×105 cells in flat-bottom 24-well plates for
flow cytometry or 1×104 cells in 96-well plates (both
Eppendorf) for imaging experiments. The outer rim of each plate was
filled with double-distilled water to prevent surplus evaporation
in the edge wells.
Oxidant treatment
H2O2 (Alfa Aesar; Thermo
Fisher Scientific, Inc.), HOCl and ONOO−(both Carl Roth
GmbH & Co.) were diluted in PBS to obtain concentrations
between 10 mM and 0.1 µM. Cell culture medium was removed and 250
µl (per 1×105 cells in 24-well plates) or 25 µl (per
1×104 cells in 96-well plates) individual
oxidant-containing liquid was added to cells. After 2 h of
incubation at 37°C, 1,750 (for 24-well plates) or 175 µl culture
medium (for 96-well plates) was added to the cells to restore
preferred culture conditions with fully supplied nutrients. The
cells were allowed to culture for 22 h prior to further downstream
processing.
Metabolic activity
The total metabolic activity per well was analyzed
based on cell capability to metabolize
7-hydroxy-3H-phenoxazin-3-on-10-oxid (resazurin; Alfa Aesar; Thermo
Fisher Scientific, Inc.) to fluorescent resorufin. Resazurin was
added to wells at a final concentration of 100 µM and incubated for
2 h at 37°C before fluorescence was quantified using a multimode
plate reader (F200; Tecan Group Ltd.). The measurement was taken at
λex 535 and λem 590 nm.
Flow cytometry
Flow cytometry was performed to investigate cell
death and analyze surface marker expression levels. For the
collection of cells, cell culture supernatant and detached cells
(obtained using accutase; BioLegend, Inc.) were transferred to
v-bottom 96-well plates (Eppendorf) after centrifugation at 500 × g
for 5 min at room temperature. The plate was washed with PBS (PAN
Biotech GmbH) before one of the following mixes was added to the
wells: i) DAPI (BioLegend, Inc.) and active caspase-3/7 detection
reagent (Thermo Fisher Scientific, Inc.); or ii) DAPI, anti-CRT
monoclonal antibodies conjugated with phycoerythrin (Enzo Life
Sciences, Inc.; cat. no. ADI-SPA-601PE-F), anti-heat-shock protein
(HSP)70 monoclonal antibodies conjugated with Alexa
Fluor® (AF) 594 and anti-HSP90 monoclonal antibodies
conjugated with AF 700 (both Novus Biologicals LLC; cat. nos.
NBP1-77456AF594 and NB100-1972AF700, respectively). The staining
was performed for 30 min at 37°C using 20 ng antibodies per test
and DAPI and caspase-3/7 reagent at a final concentration of 1 µM.
Subsequently, the cells were washed twice and analyzed using a
CytoFLEX S (Beckman Coulter, Inc.) four-laser flow cytometer. The
excitation wavelength and bandpass filters for collecting
fluorescence emission were λex 405 nm/λem 450–45 nm and
λex 488 nm/λem 520–40 nm, respectively, for
master mix i); and λex 405 nm/λem 450-45 nm,
λex 561 nm/λem 585-42 nm, λex 561
nm/λem 610-20 nm and λex 638
nm/λem 712-25 nm for master mix ii). Forward and
side-scatter were also analyzed. Gating and quantification of cell
numbers and fluorescence intensities was performed using Kaluza
2.1.1 analysis software (Beckman Coulter, Inc.).
Quantitative high-content imaging
In order to determine cytosolic glutathione (GSH)
levels and mitochondrial redox status, cells were stained with 2 µM
GSH detection probe (Kerafast, Inc.) or 1 µM mitotracker orange
(MTO; Thermo Fisher Scientific, Inc.) for 90 min at 37°C. Following
exposure to oxidants, as aforementioned, cells were imaged using a
high content imaging device (Operetta CLS; PerkinElmer, Inc.). The
digital phase contrast (DPC) channel (pseudo-cytosolic signal), as
well as the fluorescence channels for bound (λex
390–420/λem 500–550 nm) or unbound GSH tracer or MTO
(λex 460–790/λem 570–650 nm), were imaged.
The experimental setup, as well as the software-based
quantification algorithms, were generated using Harmony
high-content imaging and analysis software 4.9 (PerkinElmer, Inc.).
For segmentation, cells were detected via DPC signal and the
fluorescence of both GSH tracer or MTO channels was quantified. For
algorithm-driven quantification, ≥1×104 individual cells
in 50 single images were quantified per treatment condition. The
intracellular GSH levels were calculated according to the formula:
GSH level=mean fluorescence intensity (MFI) of bound GSH tracer/MFI
of unbound GSH tracer.
Graphing and statistical analysis
Statistical analysis and graphing was performed
using Prism 8.4 (GraphPad Software, Inc.). For statistical
comparison between groups and controls, two-way analysis of
variance with Dunnett's post hoc test was used. All experiments
were performed ≥2 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
ROS decreases metabolic activity and
viability in a dose-dependent manner
The present study aimed to identify the effect of
different types of ROS on the viability and immunogenic properties
of abdominal cancer cells. Colorectal (HT-29), pancreatic (Panc-01)
and ovarian (SK-OV-3) cancer cells were exposed to either
H2O2, HOCl or ONOO− at different
doses. Following 2 h treatment with these ROS, cells were cultured
and analyzed 24 h post-treatment (Fig.
1A). Compared with untreated control cells, oxidant
concentrations between 0.1 µM and 100 µM did not significantly
decrease cancer cell metabolic activity (Fig. 1B-D). At concentrations >100 µM,
H2O2 showed the lowest capability in
decreasing the percentage of metabolically active tumor cells in
the three cancer cell lines tested. HOCl exhibited the most
substantial effects (Fig. 1B-D).
In order to analyze the cause of decreased metabolic
activity, flow cytometry was used to quantify viable
(DAPI−, active caspase−), early
(DAPI−, active caspase+) and late apoptotic
(DAPI+, active caspase+), and necrotic
(DAPI+, active caspase−) tumor cells. A
dose-dependent decrease in the number of viable cells was observed
in all cell lines (Fig. 2A-C). In
HT-29 cells, a significant decrease in the cell viability was found
at concentrations ≥10 µM for HOCl and ONOO−, and ≥100 µM
for H2O2 (Fig.
2A). In Panc-01 cells, a significant decrease in the number of
viable cells was observed at ≥10 µM ONOO−, ≥100 µM for
HOCl and ≥1 mM for H2O2 (Fig. 2B). Similar effects were also observed
in SK-OV-3 cells; the number of viable cells significantly
decreased at concentrations ≥1 mM H2O2 and
ONOO−, and 10 µM HOCl (Fig.
2C). Analysis of early and late apoptotic and necrotic cells
(Fig. 2D, H and L) demonstrated the
greatest extent of cell death occurred in HT-29 cells treated with
HOCl (Fig. 2E-G). The fraction of
necrotic cells remained small in all groups, except for
HOCl-treated HT-29 and SK-OV-3 cells, and ONOO−-treated
Panc-01 cells at higher concentrations (Fig. 2F, N and K). In most experimental
conditions, the fraction of late apoptotic cells was the largest of
the dead cell populations. All oxidants induced notable toxicity in
Panc-01 cells at high concentrations, while concentrations ≤10 µM
did not have a significant effect (Fig.
2I-K). HOCl-treated cells (10 mM) were not detectable (data not
shown). SK-OV-3 cells exhibited notably elevated apoptotic cell
death in all oxidative regimens (except for 1 mM HOCl with more
necrotic cells); however, ONOO− at concentrations ≥1 mM
failed to generate single-cell data because of aberrant toxicity
(Fig. 2M-O).
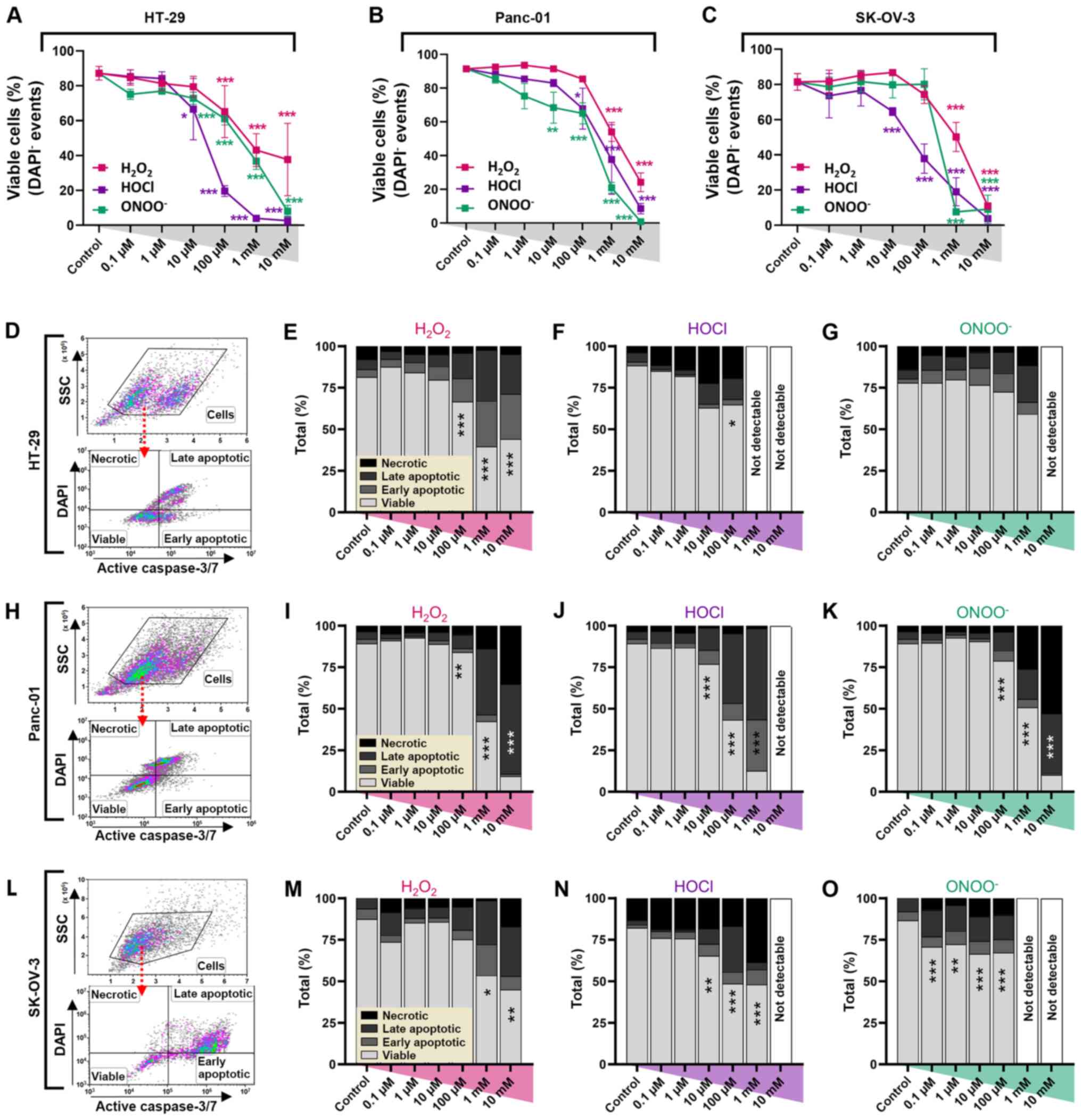 | Figure 2.Oxidant treatment decreases viability
of abdominal cancer cells in vitro. Percentage of viable (A)
HT-29, (B) Panc-01 and (C) SK-OV-3 carcinoma cells at 24 h
post-oxidant treatment. Data are presented as the mean ± SEM. (D)
Representative forward scatter and SSC and DAPI and active caspase
3/7 dot plots of HT-29 cells analyzed via flow cytometry. Cytotoxic
effects of (E) H2O2, (F) HOCl and (G)
ONOO− in HT-29 cells. (H) Representative dot-plots of
Panc-01 cells. Cytotoxic effects of (I) H2O2,
(J) HOCl and (K) ONOO− in Panc-01 cells. (L)
Representative dot-plots of SK-OV-3 cells. Cytotoxic effects of (M)
H2O2, (N) HOCl and (O) ONOO− in
SK-OV-3 cells. Data are from five (A-C) and 2–3 (D-O) independent
experiments. *P<0.05, **P<0.01, ***P<0.001 vs. control.
H2O2, hydrogen peroxide; HOCl, hypochlorous
acid; ONOO−, peroxynitrite; SSC, side scatter. |
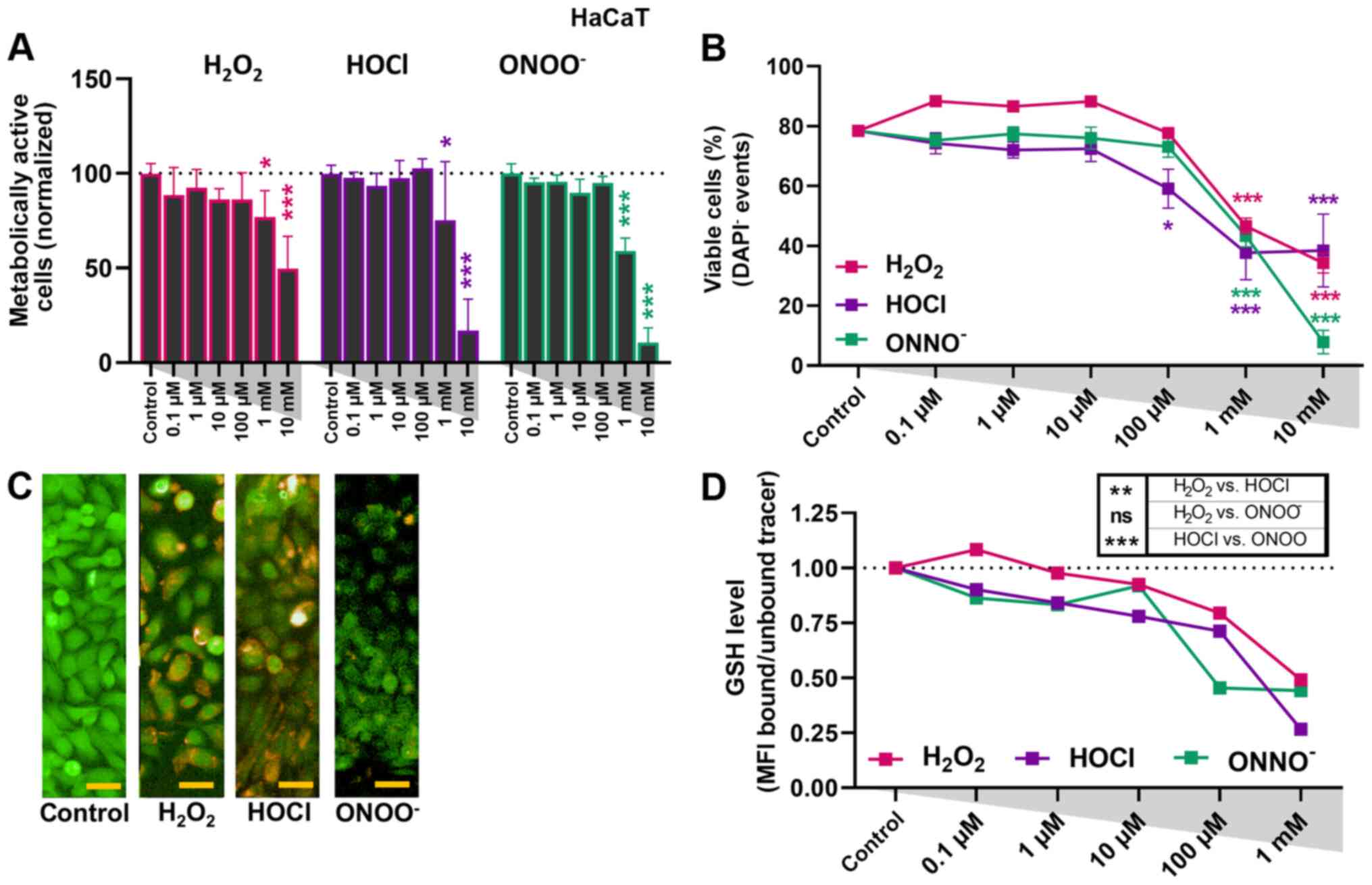
ROS treatment affects metabolic
activity and viability to a lesser extent in HaCaT cells than in
cancer cells
In order to investigate the effect of oxidants in
non-malignant cells, HaCaT keratinocytes were used as a control
cell line. Similar to the tumor cells, a decrease in metabolic
activity was observed (Fig. 3A).
However, this was only notable at 1 mM. Moreover, viability data
from flow cytometry suggested these cells to be less sensitive to
oxidative treatment compared with cancer cells; a significant
decrease in the number of viable cells was observed only at
concentrations >1 mM, except for HOCl, which also exhibited a
significant effect at 100 µM (Fig.
3B). In order to identify the role of antioxidant defense, a
GSH tracer was used to assess the relative amounts of GSH in the
intracellular compartment (Fig. 3C).
A slight but non-significant decrease in cytosolic GSH levels was
observed at concentrations ≤10 µM (Fig.
3D). Among the three types of ROS investigated,
H2O2 exhibited the weakest effect on
cytosolic GSH levels in HaCaT keratinocytes.
ROS exposure is concomitant with
cytosolic and mitochondrial oxidation in cancer cells
Differences in oxidant capability to induce cell
death were observed. High concentrations of HOCl and
ONOO− exhibited the greatest toxicity and necrosis;
H2O2 exhibited lower toxicity, and late
apoptosis was observed at higher concentrations (Fig. 2). These differences indicated
different underlying mechanisms for each ROS. In order to
investigate this, intracellular oxidation was assessed. Cell
pseudo-cytosolic DPC signal was used to segment the cell area via a
software-based quantification tool (Fig.
4A) and the amount of bound and unbound GSH tracer, emitting at
characteristic fluorescence emission spectra (Fig. 4B), inside the segmented cell region
was quantified. Compared with untreated controls, ≥0.1 µM
H2O2 and HOCl decreased intracellular GSH
levels in HT-29 cancer cells (Fig. 4C and
D). ONOO− only notably decreased GSH levels at 1 mM
(Fig. 4D). In Panc-01 cells,
ONOO− and H2O2 decreased GSH
levels at ≥0.1 µM, but to a lesser extent compared with HOCl at
similar concentrations (Fig. 4F and
G). Similar results were observed in SK-OV-3 cancer cells
(Fig. 4I and J). Altogether, HOCl at
low concentrations induced a notable consistent decrease in
intracellular GSH levels in all three cancer cell lines, while
non-malignant HaCaT keratinocytes were less affected at equimolar
concentrations (Fig. 3D).
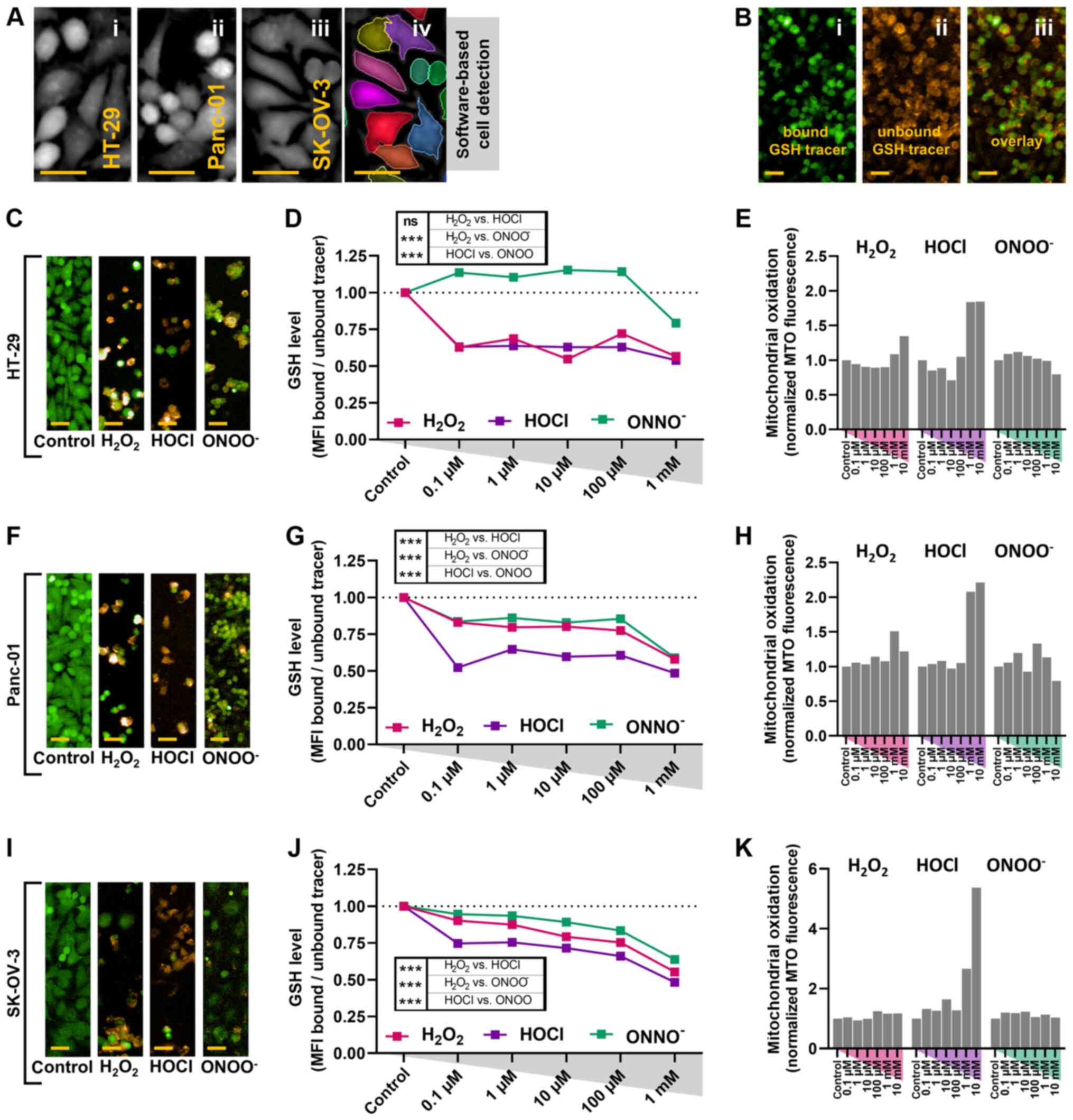 | Figure 4.Oxidant treatment decreases
intracellular GSH and increases mitochondrial ROS in cancer cells.
(A) Representative images of the pseudo-cytosolic signal of i)
HT-29, ii) Panc-01 and iii) SK-OV-3 carcinoma cells, with iv)
representative software-based cell segmentation for subsequent
quantitative analysis. (B) Representative images of cancer cells
with i) bound and ii) unbound GSH tracer and iii) and overlay. (C)
Representative images of GSH fluorescence channels for HT-29 cells
in the presence or absence of 100 µM oxidants. (D) Quantification
of GSH levels of HT-29 cells at 24 h. (E) Quantification of MTO
fluorescence of HT-29 cells. (F) Representative images of GSH
fluorescence channels for Panc-01 cells in the presence or absence
of 100 µM oxidants. (G) Quantification of GSH levels of Panc-01
cells at 24 h. (H) Quantification of MTO fluorescence of Panc-01
cells. (I) Representative images of GSH fluorescence channels for
SK-OV-3 cells in the presence or absence of 100 µM oxidants. (J)
Quantification of GSH levels in SK-OV-3 cells at 24 h. (K)
Quantification of MTO fluorescence of SK-OV-3 cells. Scale bar, 30
µm. Data are representative of two independent experiments with ≥50
fields of view per treatment condition, concentration and cell
type. All data are normalized to untreated control.
H2O2, hydrogen peroxide; HOCl, hypochlorous
acid; ONOO−, peroxynitrite; GSH, glutathione; ROS,
reactive oxygen species; MTO, mitotracker orange; MFI, mean
fluorescence intensity. |
Following accumulation within the mitochondrial
membrane, MTO fluorescence is increased upon oxidation via ROS
(36). A notable increase in MTO
fluorescence was observed with higher HOCl concentrations in all
three cell lines (Fig. 4E, H and K).
By contrast, both ONOO− and H2O2
failed to induce notable mitochondrial oxidation, although a small
increase was seen with H2O2 at higher
concentrations. Among the three types of ROS compared at equimolar
concentrations, HOCl showed the most potent oxidative and cytotoxic
effects in the cancer cell lines investigated.
HOCl and H2O2 increase
inflammatory surface molecule expression levels in cancer cells. In
addition to cytotoxicity, the inflammatory and immune-stimulating
potential of anti-cancer agents is of interest in clinical and
pre-clinical research. Translocation of certain damage-associated
molecular patterns (DAMPs), such as CRT (the chaperon of the
endoplasmic reticulum), serves as an ‘eat-me’ signal to promote the
phagocytosis of dying tumor cells, leading to the promotion of
antitumor immunity (34). For HT-29
cells, the three types of ROS at concentrations ≥1 µM increased
expression levels of CRT on the membranes of viable cells (Fig. 5A and B). The most effective agent was
HOCl, which induced a 30-fold increase in CRT at 10 mM (Fig. 5B). In dead HT-29 cells,
H2O2 was most effective and significantly
increased CRT expression levels at concentrations ≥1 mM (Fig. 5C). Changes in CRT expression levels in
treated compared with untreated cells were smaller in dead compared
with viable HT-29 cells. In viable and dead Panc-01 cells, HOCl did
not significantly increase CRT expression levels. By contrast, both
ONOO− and H2O2 at high
concentrations promoted CRT levels, with H2O2
eliciting significantly elevated CRT levels, especially in dead
cells (Fig. 5D-F). In viable SK-OV-3
cells, upregulation of CRT on the membrane was observed in a
dose-dependent manner for all oxidative treatment regimens
(Fig. 5G and H). However, this was
only significant at 10 mM HOCl and ONOO−. In the
fraction of dead SK-OV-3 cells, significant CRT upregulation was
observed at H2O2 concentrations ≥1 mM
(Fig. 5I). Danger signals, such HSP70
and HSP90 (Fig. 5J and K), notably
increased in HOCl-treated HT-29 and
H2O2-treated Panc-01 cells. In dead SK-OV-3
cells, a notable increase in HSP90 was observed with all three
types of ROS.
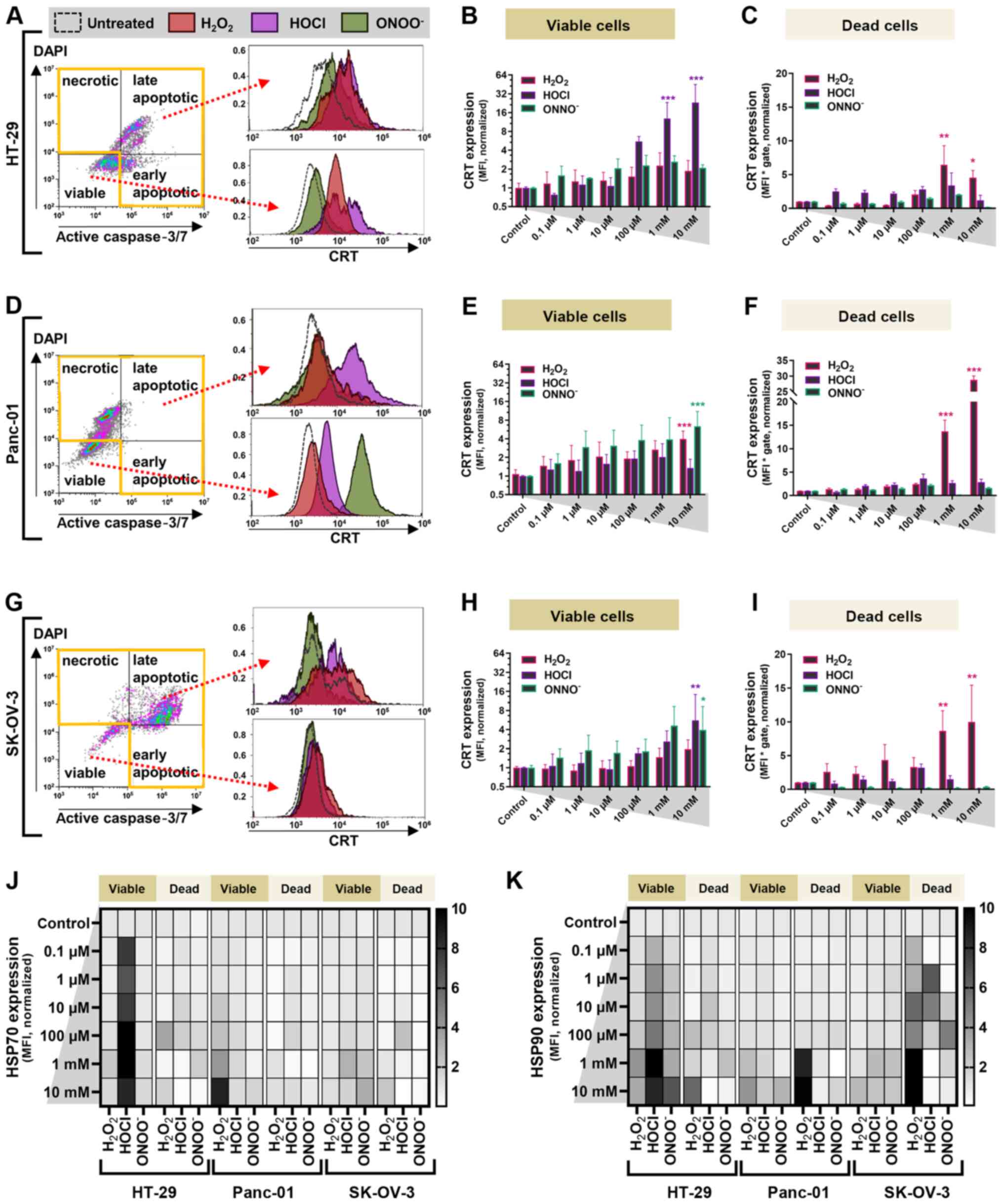 | Figure 5.Oxidant treatment induces CRT
expression in abdominal cancer cells. (A) Representative live-dead
cell gating and overlay of CRT fluorescence in HT-29 cells treated
with 100 µM H2O2 (red), HOCl (purple),
ONOO− (green) or untreated (dotted line). (B)
Fold-change in CRT expression levels in HT-29 cells. (C)
Quantification of CRT expression levels in dead HT-29 cells. (D)
Representative live-dead cell gating and overlay of CRT
fluorescence in Panc-01 cells treated with 100 µM oxidants. (E)
Fold-change in CRT expression levels in viable Panc-01 cells. (F)
Quantification of CRT expression in dead Panc-01 cells. (G)
Representative live-dead cell gating and overlay of CRT
fluorescence in SK-OV-3 cells treated with 100 µM oxidants. (H)
Fold-change of CRT expression levels in viable SK-OV-3 cells. (I)
Quantification of CRT expression levels in dead SK-OV-3 cells. Heat
map of expression levels of (J) HSP70 and (K) HSP90 in viable and
dead cancer cells at 24 h. Data are from 3–4 independent
experiments and are presented as the mean ± SD. All data are
normalized to untreated controls. *P<0.05, **P<0.01,
***P<0.001 vs. control. H2O2, hydrogen
peroxide; HOCl, hypochlorous acid; ONOO−, peroxynitrite;
CRT, calreticulin; HSP, heat-shock protein; MFI, mean fluorescence
intensity. |
Discussion
In order to investigate ROS as a putative supplement
to lavage treatment of PC, the present study assessed toxicity and
immune-relevant surface marker expression levels in three human
abdominal cancer cell lines (HT-29, Panc-01 and SK-OV-3) following
exposure to H2O2, HOCl or ONOO−.
Cancer cells were more sensitive to ROS-induced toxicity compared
with non-malignant HaCaT keratinocytes and exhibited increased
levels of immuno-relevant surface markers. At equimolar
concentrations, HOCl appeared to be a particularly promising
candidate for adjuvant ROS therapy.
The three types of ROS notably decreased the
viability and metabolic activity in all three cancer cell lines,
especially at concentrations ≥100 µM. Mechanistically, ROS may act
via direct oxidation of intracellular proteins or nucleic acids,
which promotes regulated cell death and senescence (37–39), as
well as through ROS/reactive nitrogen species-redox signaling
events that drive apoptosis via downstream signaling (40–42). At
higher ROS concentrations, tumor cells were rendered inactivate but
were not eliminated completely. This has previously been observed
in H2O2-treated colorectal cancer cells
(43), and similar effects have been
detected in cells with functional ATM enzymes exposed to
ONOO− (44), as well as
HOCl in diabetes and liver disease (45). Here, HOCl and ONOO− were
slightly more effective than H2O2 in
decreasing the number of viable cancer cells, indicating different
mechanisms of action for these molecules. It was previously shown
that knockdown of the ROS-generating enzymes NADPH oxidase 1 (NOX1)
and dual oxidase 1 in cancer cells prevents apoptosis following
exposure to H2O2 (46). By contrast, knockdown of both NOX1 and
inducible nitric oxide synthase is required to abrogate
HOCl-mediated apoptosis (47). These
results suggest an underlying self-amplification mechanism of
intracellular and membrane-generated ROS that contributes to
ROS-induced cancer cell death. Moreover, ONOO− and
H2O2 have been implicated in
PI3K/Akt-mediated activation of the nuclear factor erythroid
2-related factor 2 pathway and microtubule-associated protein
1A/1B-light chain 3, indicating a role of antioxidant defense and
autophagy in ROS-mediated cell death (48,49). HOCl
is also produced endogenously by activated neutrophils via
myeloperoxidase during inflammation to promote anti-microbial
effects (50). In addition, HOCl
promotes tumor cell death via several mechanisms, such as as the
enhancement of antigen presentation and uptake, as well as the
induction of an anti-tumor response driven by cytotoxic T cells
(51).
The oxidation of cancer cells was here demonstrated
by decreased intracellular levels of the antioxidant GSH. The
availability of GSH provides information on the cellular
antioxidant defense capacity and is associated with redox signaling
processes (52). In the present
study, non-malignant HaCaT keratinocytes showed the lowest overall
decrease in GSH and the highest viability when exposed to different
types of ROS at equimolar concentrations compared with the cancer
cell lines tested. This may be because cancer cells naturally
exhibit high levels of endogenous oxidative stress, which leads to
increased susceptibility to ROS-induced cell death (14,53). The
greatest decrease in intracellular GSH levels was observed
following the administration of HOCl and
H2O2, compared with ONOO−. This
may be explained by the lower affinity of the latter to GSH, the
generation of S-nitroglutathione and the high affinity between
ONOO− and CO2 in reactions at high-rate
constants (54,55). Here, ONOO− was also shown
to have the lowest capacity to oxidize mitochondria. These findings
are consistent with a previous study (46) that suggested HOCl to be of particular
importance in the induction of cell death due to its ability of
auto-propagating ROS formation through mitochondria.
One of the critical hallmarks of antitumor immune
responses is the induction of ICD via increased danger signals
(such as DAMPs), including the chaperon of the endoplasmic
reticulum, CRT, on the tumor cell membrane (34,56). CRT
can act as a danger and ‘eat-me’ signal when recognized by innate
immune cells, such as dendritic cells (57). Other DAMPs include HSP70 and HSP90 as
markers for cellular stress, leading to their upregulation and
translocation to the cell membrane (58). ICD is associated with the upregulation
of these molecules, as previously shown using antitumor agents
tested for their immunogenicity in mice (34). In the present study, all types of ROS
tested increased the levels of CRT on tumor cells. Specific
responses, however, were dependent on the cell type and oxidant.
HOCl, and to a lesser extent ONOO−, gave promising
results in at least two of the three tumor cell lines investigated
by enhancing the presentation of pro-immunogenic molecules on their
membrane. Exposure to these ROS results in the formation of DAMPs,
which promote antitumor immunity (59,60),
although the present study did not directly investigate the
immunological consequences. Increased CRT levels were also observed
in dead tumor cells following treatment with high concentrations of
H2O2. H2O2 was
previously found to promote a pro-immunogenic phenotype in murine
colorectal cancer cells in vitro, as well as in mice with PC
(37); this was concomitant with
enhanced immune infiltration and activation. Moreover, ROS are
capable of shaping activation profiles in human myeloid cells
(37,61,62). ICD
was previously observed with photodynamic therapy, which generates
singlet oxygen to promote cellular oxidation (63,64),
supporting the findings of mitochondrial oxidation and decreased
cellular GSH levels in the present study. A previous report noted
that ONOO− affects major histocompatibility complex-I
recognition by T lymphocytes (65).
The present study identified HOCl as the most
promising candidate in terms of toxicity and induction of
immunogenic danger molecules (such as CRT) in cancer cells. HOCl
was previously shown to enhance the immunogenicity of colorectal
cancer cells in vivo, prevent distant metastasis of human
melanoma cells, and alter antigen-presenting machinery and the
cross-priming of tumor material (66–70). This
makes HOCl a promising candidate as an adjuvant in peritoneal
cancer therapy outside the current vaccination strategies against
ovarian cancer employed with this type of ROS (71).
Treatment with H2O2, HOCl and
ONOO− led to intracellular oxidation and notable
toxicity in all abdominal cancer cell lines tested. Non-malignant
HaCaT keratinocytes were less affected compared with cancer cells,
suggesting a degree of specificity to the ROS-induced cell death.
Treatment of cancer cells with these ROS also led to an
upregulation of molecules associated with activation of immune
cells. HOCl was the most promising therapeutic candidate, as it
exhibited the greatest ability to inactivate cancer cells and
upregulate danger molecules known to promote antitumor immunity.
Future studies may extend this concept to provide novel therapeutic
avenues in the treatment of PC.
Acknowledgements
The authors would like to thank Felix Niessner
(Leibniz Institute for Plasma Science and Technology, INP
Greifswald) for technical support.
Funding
The present study was funded by the German Federal
Ministry of Education and Research (grant nos. 03Z22DN11 and
03Z22Di1) and Gerhard-Domagk Foundation (Greifswald, Germany).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EF and SB designed the study. LM performed the
experiments. EF, LM and SB confirm the authenticity of all the raw
data. EF, LM, MBS and SB analyzed the data, prepared the figures
and wrote and reviewed the manuscript. EF, LM, MBS and SB read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Dakwar GR, Shariati M, Willaert W, Ceelen
W, De Smedt SC and Remaut K: Nanomedicine-based intraperitoneal
therapy for the treatment of peritoneal carcinomatosis-mission
possible? Adv Drug Deliv Rev. 108:13–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piso P and Arnold D: Multimodal treatment
approaches for peritoneal carcinosis in colorectal cancer. Dtsch
Arztebl Int. 108:802–808. 2011.PubMed/NCBI
|
|
3
|
Oettle H, Neuhaus P, Hochhaus A, Hartmann
JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J,
Arning MB, et al: Adjuvant chemotherapy with gemcitabine and
long-term outcomes among patients with resected pancreatic cancer:
The CONKO-001 randomized trial. JAMA. 310:1473–1481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esposito I, Kleeff J, Bergmann F, Reiser
C, Herpel E, Friess H, Schirmacher P and Büchler MW: Most
pancreatic cancer resections are R1 resections. Ann Surg Oncol.
15:1651–1660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shida D, Tsukamoto S, Ochiai H and
Kanemitsu Y: Long-term outcomes after R0 resection of synchronous
peritoneal metastasis from colorectal cancer without cytoreductive
surgery or hyperthermic intraperitoneal chemotherapy. Ann Surg
Oncol. 25:173–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tentes AA, Pallas N, Karamveri C,
Kyziridis D and Hristakis C: Cytoreduction and HIPEC for peritoneal
carcinomatosis of pancreatic cancer. J BUON. 23:482–487.
2018.PubMed/NCBI
|
|
7
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pelz JO, Chua TC, Esquivel J, Stojadinovic
A, Doerfer J, Morris DL, Maeder U, Germer CT and Kerscher AG:
Evaluation of best supportive care and systemic chemotherapy as
treatment stratified according to the retrospective peritoneal
surface disease severity score (PSDSS) for peritoneal
carcinomatosis of colorectal origin. BMC Cancer. 10:6892010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goéré D, Souadka A, Faron M, Cloutier AS,
Viana B, Honoré C, Dumont F and Elias D: Extent of colorectal
peritoneal carcinomatosis: Attempt to define a threshold above
which HIPEC does not offer survival benefit: A comparative study.
Ann Surg Oncol. 22:2958–2964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solass W, Kerb R, Mürdter T, Giger-Pabst
U, Strumberg D, Tempfer C, Zieren J, Schwab M and Reymond MA:
Intraperitoneal chemotherapy of peritoneal carcinomatosis using
pressurized aerosol as an alternative to liquid solution: First
evidence for efficacy. Ann Surg Oncol. 21:553–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pinto A and Pocard M: Photodynamic therapy
and photothermal therapy for the treatment of peritoneal
metastasis: A systematic review. Pleura Peritoneum. 3:201801242018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farooqi AA, Li KT, Fayyaz S, Chang YT,
Ismail M, Liaw CC, Yuan SS, Tang JY and Chang HW: Anticancer drugs
for the modulation of endoplasmic reticulum stress and oxidative
stress. Tumour Biol. 36:5743–5752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galadari S, Rahman A, Pallichankandy S and
Thayyullathil F: Reactive oxygen species and cancer paradox: To
promote or to suppress? Free Radic Biol Med. 104:144–164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glasauer A and Chandel NS: Targeting
antioxidants for cancer therapy. Biochem Pharmacol. 92:90–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brigger I, Dubernet C and Couvreur P:
Nanoparticles in cancer therapy and diagnosis. Adv Drug Del Rev.
64:24–36. 2012. View Article : Google Scholar
|
|
17
|
Manshian BB, Poelmans J, Saini S, Pokhrel
S, Grez JJ, Himmelreich U, Mädler L and Soenen SJ:
Nanoparticle-induced inflammation can increase tumor malignancy.
Acta Biomater. 68:99–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manke A, Wang L and Rojanasakul Y:
Mechanisms of nanoparticle-induced oxidative stress and toxicity.
Biomed Res Int. 2013:9429162013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou JT, Wang B, Zhang Y, Cui B, Cao X,
Zhang M, Ye Y and Wang S: Observation of peroxynitrite
overproduction in cells during 5-fluorouracil treatment via a
ratiometric fluorescent probe. Chem Commun (Camb). 56:2759–2762.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kepp O, Menger L, Vacchelli E, Locher C,
Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M,
Senovilla L, et al: Crosstalk between ER stress and immunogenic
cell death. Cytokine Growth Factor Rev. 24:311–318. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garg AD and Agostinis P: ER stress,
autophagy and immunogenic cell death in photodynamic
therapy-induced anti-cancer immune responses. Photochem Photobiol
Sci. 13:474–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bekeschus S, Schmidt A, Niessner F,
Gerling T, Weltmann KD and Wende K: Basic research in plasma
medicine-a throughput approach from liquids to cells. J Vis Exp.
563312017.
|
|
24
|
Jablonowski H, Santos Sousa J, Weltmann
KD, Wende K and Reuter S: Quantification of the ozone and singlet
delta oxygen produced in gas and liquid phases by a non-thermal
atmospheric plasma with relevance for medical treatment. Sci Rep.
8:121952018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bekeschus S, Wende K, Hefny MM, Rödder K,
Jablonowski H, Schmidt A, Woedtke TV, Weltmann KD and Benedikt J:
Oxygen atoms are critical in rendering THP-1 leukaemia cells
susceptible to cold physical plasma-induced apoptosis. Sci Rep.
7:27912017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bekeschus S, Clemen R, Nießner F, Sagwal
SK, Freund E and Schmidt A: Medical gas plasma jet technology
targets murine melanoma in an immunogenic fashion. Adv Sci (Weinh).
7:19034382020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin A, Gorbanev Y, De Backer J, Van
Loenhout J, Van Boxem W, Lemière F, Cos P, Dewilde S, Smits E and
Bogaerts A: Non-thermal plasma as a unique delivery system of
short-lived reactive oxygen and nitrogen species for immunogenic
cell death in melanoma cells. Adv Sci (Weinh). 6:18020622019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bekeschus S, Clemena R and Metelmann HR:
Potentiating anti-tumor immunity with physical plasma. Clin Plas
Med. 12:17–22. 2018. View Article : Google Scholar
|
|
29
|
Garg AD, Nowis D, Golab J, Vandenabeele P,
Krysko DV and Agostinis P: Immunogenic cell death, DAMPs and
anticancer therapeutics: An emerging amalgamation. Biochim Biophys
Acta. 1805:53–71. 2010.PubMed/NCBI
|
|
30
|
Khalili M, Daniels L, Lin A, Krebs FC,
Snook AE, Bekeschus S, Bowne WB and Miller V: Non-thermal
plasma-induced immunogenic cell death in cancer: A topical review.
J Phys D Appl Phys. 52:4230012019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunogenic cell death in cancer and infectious
disease. Nat Rev Immunol. 17:97–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ledford H, Else H and Warren M: Cancer
immunologists scoop medicine nobel prize. Nature. 562:20–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leebmann H and Piso P: PIPAC and
HIPEC-competing or supplementary therapeutic procedures for
peritoneal metastases. Chirurg. 89:693–698. 2018.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Freund E and Bekeschus S: Gas
plasma-oxidized liquids for cancer treatment: Pre-clinical
relevance, immuno-oncology, and clinical obstacles. IEEE Trans
Radiat Plasma Med Sci. 1. 2020. View Article : Google Scholar
|
|
36
|
Kholmukhamedov A, Schwartz JM and
Lemasters JJ: Isolated mitochondria infusion mitigates
ischemia-reperfusion injury of the liver in rats: Mitotracker
probes and mitochondrial membrane potential. Shock. 39:5432013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Freund E, Liedtke KR, van der Linde J,
Metelmann HR, Heidecke CD, Partecke LI and Bekeschus S: Physical
plasma-treated saline promotes an immunogenic phenotype in CT26
colon cancer cells in vitro and in vivo. Sci Rep. 9:6342019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamao T, Yamashita YI, Yamamura K, Nakao
Y, Tsukamoto M, Nakagawa S, Okabe H, Hayashi H, Imai K and Baba H:
Cellular senescence, represented by expression of caveolin-1, in
cancer-associated fibroblasts promotes tumor invasion in pancreatic
cancer. Ann Surg Oncol. 26:1552–1559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5:142006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu FX and Guan KL: The hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Freund E, Liedtke KR, Miebach L, Wende K,
Heidecke A, Kaushik NK, Choi EH, Partecke LI and Bekeschus S:
Identification of two kinase inhibitors with synergistic toxicity
with low-dose hydrogen peroxide in colorectal cancer cells in
vitro. Cancers (Basel). 12:1222020. View Article : Google Scholar
|
|
44
|
Bagheri M, Nair RR, Singh KK and Saini DK:
ATM- ROS-iNOS axis regulates nitric oxide mediated cellular
senescence. Biochim Biophys Acta Mol Cell Res. 1864:177–190. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Acosta JC, Banito A, Wuestefeld T,
Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka
F, Andrulis M, et al: A complex secretory program orchestrated by
the inflammasome controls paracrine senescence. Nat Cell Biol.
15:978–990. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Weyemi U, Redon CE, Parekh PR, Dupuy C and
Bonner WM: NADPH oxidases NOXs and DUOXs as putative targets for
cancer therapy. Anticancer Agents Med Chem. 13:502–514. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bauer G: Central signaling elements of
intercellular reactive oxygen/nitrogen species-dependent induction
of apoptosis in malignant cells. Anticancer Res. 37:499–513. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Navarro-Yepes J, Burns M, Anandhan A,
Khalimonchuk O, del Razo LM, Quintanilla-Vega B, Pappa A,
Panayiotidis MI and Franco R: Oxidative stress, redox signaling,
and autophagy: Cell death versus survival. Antioxid Redox Signal.
21:66–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Speckmann B, Steinbrenner H, Grune T and
Klotz LO: Peroxynitrite: From interception to signaling. Arch
Biochem Biophys. 595:153–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Perkins A, Tudorica DA, Amieva MR,
Remington SJ and Guillemin K: Helicobacter pylori senses bleach
(HOCl) as a chemoattractant using a cytosolic chemoreceptor. PLoS
Biol. 17:e30003952019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vandenberk L, Belmans J, Van Woensel M,
Riva M and Van Gool SW: Exploiting the immunogenic potential of
cancer cells for improved dendritic cell vaccines. Front Immunol.
6:6632016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bansal A and Simon MC: Glutathione
metabolism in cancer progression and treatment resistance. J Cell
Biol. 217:2291–2298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Acharya A, Das I, Chandhok D and Saha T:
Redox regulation in cancer: A double-edged sword with therapeutic
potential. Oxid Med Cell Longev. 3:23–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Balazy M, Kaminski PM, Mao K, Tan J and
Wolin MS: S-nitroglutathione, a product of the reaction between
peroxynitrite and glutathione that generates nitric oxide. J Biol
Chem. 273:32009–32015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Padmaja S, Squadrito GL and Pryor WA:
Inactivation of glutathione peroxidase by peroxynitrite. Arch
Biochem Biophys. 349:1–6. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tesniere A, Apetoh L, Ghiringhelli F, Joza
N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L and Kroemer G:
Immunogenic cancer cell death: A key-lock paradigm. Curr Opinc
Immunol. 20:504–511. 2008. View Article : Google Scholar
|
|
57
|
Fucikova J, Kasikova L, Truxova I, Laco J,
Skapa P, Ryska A and Spisek R: Relevance of the chaperone-like
protein calreticulin for the biological behavior and clinical
outcome of cancer. Immunol Lett. 193:25–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Adkins I, Sadilkova L, Hradilova N, Tomala
J, Kovar M and Spisek R: Severe, but not mild heat-shock treatment
induces immunogenic cell death in cancer cells. Oncoimmunology.
6:e13114332017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Panaretakis T, Kepp O, Brockmeier U,
Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N,
Pierron G, van Endert P, et al: Mechanisms of pre-apoptotic
calreticulin exposure in immunogenic cell death. EMBO J.
28:578–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gordon S: The role of the macrophage in
immune regulation. Res Immunol. 149:685–688. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liedtke KR, Freund E, Hackbartha C,
Heideckea CD, Parteckea LI and Bekeschus S: A myeloid and lymphoid
infiltrate in murine pancreatic tumors exposed to plasma-treated
medium. Clin Plas Med. 11:10–17. 2018. View Article : Google Scholar
|
|
62
|
Freund E, Moritz J, Stope M, Seebauer C,
Schmidt A and Bekeschus S: Plasma-derived reactive species shape a
differentiation profile in human monocytes. Appl Sci. 9:25302019.
View Article : Google Scholar
|
|
63
|
Panzarini E, Inguscio V and Dini L:
Immunogenic cell death: Can it be exploited in photodynamic therapy
for cancer? Biomed Res Int. 2013:4821602013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Garg AD, Krysko DV, Vandenabeele P and
Agostinis P: Hypericin-based photodynamic therapy induces surface
exposure of damage-associated molecular patterns like HSP70 and
calreticulin. Cancer Immunol Immunother. 61:215–221. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tcyganov E and Gabrilovich DI:
Peroxynitrite affects MHC I peptide repertoire presented on tumor
cells and impedes the efficacy of antitumor cytotoxic
T-lymphocytes. J Immunol. 202 (Suppl 1):S137.12. 2019.
|
|
66
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Palmer LJ, Cooper PR, Ling MR, Wright HJ,
Huissoon A and Chapple IL: Hypochlorous acid regulates neutrophil
extracellular trap release in humans. Clin Exp Immunol.
167:261–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Prokopowicz ZM, Arce F, Biedroń R, Chiang
CL, Ciszek M, Katz DR, Nowakowska M, Zapotoczny S, Marcinkiewicz J
and Chain BM: Hypochlorous acid: A natural adjuvant that
facilitates antigen processing, cross-priming, and the induction of
adaptive immunity. J Immunol. 184:824–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhou R, Huang WJ, Ma C, Zhou Y, Yao YQ,
Wang YX, Gou LT, Yi C and Yang JL: HOCl oxidation-modified CT26
cell vaccine inhibits colon tumor growth in a mouse model. Asian
Pac J Cancer Prev. 13:4037–4043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chiang CL, Kandalaft LE, Tanyi J, Hagemann
AR, Motz GT, Svoronos N, Montone K, Mantia-Smaldone GM, Smith L,
Nisenbaum HL, et al: A dendritic cell vaccine pulsed with
autologous hypochlorous acid-oxidized ovarian cancer lysate primes
effective broad antitumor immunity: From bench to bedside. Clin
Cancer Res. 19:4801–4815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tanyi JL, Bobisse S, Ophir E, Tuyaerts S,
Roberti A, Genolet R, Baumgartner P, Stevenson BJ, Iseli C, Dangaj
D, et al: Personalized cancer vaccine effectively mobilizes
antitumor T cell immunity in ovarian cancer. Sci Transl Med.
10:eaao59312018. View Article : Google Scholar : PubMed/NCBI
|