Introduction
Colorectal cancer (CRC) is the fourth most
frequently diagnosed cancer in the world (1), and the prognosis of patients with
advanced-stage CRC remains poor (2).
Despite the advances in colon cancer treatment, especially in
patients with non-metastatic colon cancer treated with curative
intent by colectomy, no effective therapy has been demonstrated for
patients with advanced stages of colon cancer (3–6).
Considerations of disease stage and pathologic features,
microsatellite instability status, possible efficacy and toxicity
profiles of the chosen treatment, along with patient age,
comorbidities, and personal preferences aid in the decision-making
regarding the use of effective chemotherapy in patients with colon
cancer (7–11). A better understanding of the
mechanisms involved in colon cancer development and progression
could improve the treatment outcomes in advanced-stage colon
cancer. Thus, there is a strong demand for new effective
therapeutic approaches for advanced stages of colon cancer.
Galectin-9 (Gal-9) belongs to the galectin protein
family which is a subset of lectins with carbohydrate recognition
domain (CRD) and subdivided into three groups: i) Prototype
galectins (galectin-1, −2, −7, −10, −13, and −14), ii) chimera-type
galectin (galectin-3), which have a single CRD, iii) and
tandem-repeat type galectins (galectin-4, −8, −9, and −12), which
have two CRDs joined by a flexible peptide linker (12–14). Gal-9
is known as a key molecule in eosinophil chemoattraction and
activation (15–17) and exerts various other cellular
functions, such as aggregation, adhesion, and apoptosis, in these
cells (18,19). Recent studies have demonstrated that
Gal-9 may have antitumor effects in breast cancer (20–22),
hepatocellular carcinoma (23,24), and
cholangiocarcinoma (25). In breast
cancer, Gal-9 expression induced tumor cell aggregation and made
tumor cells less aggressive, thus preventing metastasis and
prolonging patient survival (20,21).
However, the role of Gal-9 in colon cancer remains unknown.
MicroRNAs (miRNAs) are small interfering,
endogenous, noncoding RNAs that can modulate targeted protein
expression by inhibiting translational efficiency or the cleavage
of target mRNAs (6). Recently,
aberrant miRNA expression has been detected in various human
malignancies (9), and some reports
have revealed that specific miRNAs are expressed in colon cancer
(26). In addition, many studies,
including our own, have reported that miRNAs play an important role
in the antitumor effect of anticancer therapeutics (9,11,12). However, the relationships between the
anticancer effects of Gal-9 and miRNAs remain elusive.
Therefore, in the present study, we aimed to
ascertain whether Gal-9 is effective in suppressing the
proliferation of colon and colorectal cancer cells. Furthermore, we
explored the underlying mechanisms, including activation of
receptor tyrosine kinases, angiogenic profiles, and miRNA profiles
associated with the antitumor effect of Gal-9.
Materials and methods
Reagents and chemicals
Recombinant stable and mutant forms of human Gal-9
lacking the entire linker region were expressed and purified as
described in our previous report (27). Lactose, sucrose, and fetal bovine
serum (FBS) were purchased from Wako Chemicals. Cell Counting Kit-8
(CCK-8) was purchased from Dojindo Laboratories, and all other
chemicals were obtained from Sigma Chemical.
Cell lines and culture
The human colon cancer cell lines CACO-2, CW-2,
COLO-320, LoVo, and the colorectal cancer cell line WiDr were
obtained from the Japanese Cancer Research Resources Bank (Tokyo,
Japan). All the cell lines were certified by STRA and mycoplasma
testing was carried out for the cell lines used in our experiments.
The cells were cultured in RPMI-1640 (Gibco; Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated
FBS, and penicillin-streptomycin (100 µg/ml; Invitrogen; Thermo
Fisher Scientific, Inc.) in a humidified atmosphere with 5%
CO2 at 37°C.
Cell proliferation assay
We performed cell proliferation assays using the
CCK-8 assay according to the manufacturer's instructions. Each cell
line (1×104 cells per well) was seeded into the wells of
a 96-well plate and cultured in 100 µl of RPMI-1640 medium
supplemented with 10% FBS. After incubation for 24 h, the cells
were treated with 0.01, 0.03, 0.10, and 0.30 µM of Gal-9 added to
the culture medium and subsequently cultured for an additional 48
h. Furthermore, 30 mM of lactose was added to inhibit the
galactoside-binding of Gal-9, and sucrose was added as a control.
CCK-8 reagent (10 µl) was added to each well, and the plates were
incubated at 37°C for 3 h. The absorbance of each well was measured
at 450 nm using an auto-microplate reader.
Enzyme-linked immunosorbent (ELISA)
assay
Cell apoptosis assays that measured the amounts of
caspase-cleaved keratin-18 (CCK-18) were performed using the
M30-ApoptoSensek ELISA kit (Peviva AB) (28). Each cell type (5×103 cells
per well) was seeded into a 96-well plate and cultured in 100 µl of
culture medium for 24 h. Seeded cells were then treated with 0.3 µM
of Gal-9. The rest of the experiments were carried out according to
the manufacturer's instructions. The amount of antigen in the
controls and samples was calculated by interpolation from a
standard curve.
Flow cytometry analysis of the cell
cycle and apoptosis
We conducted a flow cytometric analysis using the
Cycle Phase Determination kit (Cayman Chemical Co.) to evaluate the
mechanism of growth inhibition by Gal-9. CW-2 cells were digested
with 0.25% trypsin and plated in 100-mm-diameter dishes at
1.0×106 cells per dish. After incubation for 24 h
without FBS, CW-2 cells were treated with 0.3 µM of Gal-9 or
dimethyl sulfoxide (DMSO; control) for another 24 h, then
harvested, washed with phosphate-buffered saline (PBS), suspended
in 500 µl of PBS plus 10 µl of RNase A (250 µg/ml) and 10 µl of
propidium iodide (PI) stain (100 µg/ml), and incubated for 30
min.
To determine the apoptosis rate of CW-2, CACO-2, and
WiDr cells, we used flow cytometry and the Annexin V-FITC Early
Apoptosis Detection Kit (Cell Signaling Technology, Inc.). CW-2,
CACO-2 and WiDr cells were plated in 100-mm-diameter dishes at
1.0×106 cells per dish and treated with 0.3 µM Gal-9 or
DMSO control for 24 h. After incubation for 24 h, CW-2, CACO-2 and
WiDr cells were harvested, and washed with PBS. Staining was
performed according to the manufacturer's protocol. After adding
Annexin V-FITC and PI, we analyzed apoptosis and necrotic cell
death. Flow cytometry was conducted with a Cytomics FC 500 flow
cytometer (Beckman Coulter) equipped with a 480-nm argon laser.
Cell percentages were analyzed with Kaluza software version 2.1
(Beckmann Coulter).
Cell lysate and tissue lysate
The lysates were prepared according to the methods
described in our previous report (29). All steps were carried out at 4°C.
Protein concentrations were measured using a dye-binding protein
assay based on the Bradford method (30).
Antibody arrays for phosphorylated
receptor tyrosine kinases
Human phosphorylated receptor tyrosine kinases
(p-RTKs) were assayed using Human phospho-RTK Array Kits (R&D
Systems), according to the manufacturer's instructions. Briefly,
p-RTK array membranes were blocked with 5% bovine serum
albumin/0.01 M Tris-HCl, pH 7.6 (TBS) for 1 h and then incubated
with 2 ml of the lysate prepared from cell lines after
normalization, so that the amounts of protein were equal. After
three washes for 10 min each with TBS plus 0.1% v/v Tween-20 and
two washes for 10 min with TBS alone to remove unbound material,
the membranes were incubated with anti-phospho-tyrosine-horseradish
peroxidase antibody for 2 h at room temperature. The unbound HRP
antibody was washed out with TBS plus 0.1% Tween-20. Finally, each
array membrane was exposed to an X-ray film using a
chemiluminescence detection system (Perkin-Elmer Co.).
Angiogenic profile analysis using an
antibody array
The RayBio Human Angiogenesis Antibody Array
(RayBiotech Inc.) was used according to the manufacturer's
protocol. This method is a dot-based assay enabling detection and
comparison of 20 angiogenesis-specific cytokines. Each array
membrane was exposed to an X-ray film using a chemiluminescence
detection system (PerkinElmer Co.).
Analysis of miRNA microarrays
The samples of cancer cell lines were processed for
total RNA extraction with the miRNeasy Mini Kit (Qiagen GmbH)
according to the manufacturer's instructions. Typically, RNA
samples showed A260/280 ratios of between 1.9 and
2.1, using an Agilent 2100 Bioanalyzer (Agilent Technologies,
Inc.).
After RNA measurement with an RNA 6000 Nano kit
(Agilent Technologies, Inc.), the samples were labeled using a
miRCURY Hy3/Hy5 Power labeling kit and were hybridized on a human
miRNA Oligo chip10, version 19.0 (Toray Industries). Scanning was
conducted with the 3D-Gene Scanner 3000 (Toray Industries). 3D-Gene
extraction version 1.2 software (Toray) was used to read the raw
intensity of the image. To determine the change in miRNA expression
between Gal-9-treated and control samples, the raw data were
analyzed via GeneSpring GX v 10.0 (Agilent Technologies, Inc.).
Samples were first normalized relative to 28S RNA, and the baseline
was corrected to the median of all samples.
Replicate data were consolidated into two groups,
those from Gal-9-treated cells and those from control cells, and
were organized by using the hierarchical clustering and ANOVA
functions in the GeneSpring GX v 10.0 software (Agilent
Technologies, Inc.). Hierarchical clustering was performed by
utilization of the clustering function (condition tree) and
Euclidean correlation as a distance metric. Two-way ANOVA analysis
and asymptotic P-value (<0.05) computation without any error
correction on the samples were performed to search for the miRNAs
that varied most prominently across the different groups. Only
changes >50% for at least one of the time points for each sample
were considered significant. All of the analyzed data were scaled
by global normalization. The statistical significance of
differentially expressed miRNAs was analyzed using Student's
t-test.
All our micoroarray data in this study were
submitted as a complete data set to the NCBI Gene Expression
Omnibus (GEO), no. GSE163790 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE163790).
Xenograft model analysis
Animal experiments were performed according to the
guidelines of the Committee on Experimental Animals of Kagawa
University, Kagawa, Japan, following the National Institutes of
Health guide for the care and use of laboratory animals. Approval
Number of this animal experiment is HEISEI-25-164. We purchased 40
male athymic mice (BALB/c-nu/nu; 6 weeks old; 20–25 g) from Japan
SLC (Shizuoka, Japan). The mice were maintained under specific
pathogen-free conditions using a laminar airflow rack. The mice had
continuous free access to sterilized (γ-irradiated) food (CL-2;
CLEA Japan, Inc.) and autoclaved water. A total of 40 mice were
equally divided into two groups (CACO-2 group and CW-2 group). Each
mouse was subcutaneously inoculated with CACO-2 or CW-2 cells
(3×106 cells per animal) in the flank region.
Subsequently, when the xenografts were identifiable as masses with
a maximal diameter >3 mm, we randomly assigned the animals to
the following groups: i) CACO-2 only (n=5), ii) CACO-2 treated with
90 µg of Gal-9 (n=5), iii) CW-2 only (n=7), and iv) CW-2 treated
with 90 µg of Gal-9 (n=5). Our laboratory previously optimized the
concentration of galectin-9 for the treatment of various
gastroenterological cancers in mouse animal model (23,25,31). The
Gal-9-treated groups were intraperitoneally (i.p.) injected with
Gal-9 (90 µg) five times per week; the control groups were
administered 5% DMSO alone. The tumor growth was monitored daily by
the same investigators (AM and KN), and the tumor size was measured
two times per week. The tumor volume (mm3) was
calculated as the tumor length (mm) × tumor width
(mm)2/2 (32). All animals
were sacrificed on day 31 after the treatment, with all animals
remaining alive until this time point. Tumor-bearing mice were
euthanized using CO2 (20% of the volume of the chamber
per min) followed by cervical dislocation at the end of this
experiment.
Gene transfection
The gene transfection miR-1237 inhibitor and
negative control miRNA were obtained from Thermo Fisher Scientific,
Inc. CW-2 and WiDr cells were seeded into 6-well plates. After 24
h, the CW-2 and WiDr cells were transfected with the miR-1237
inhibitor or negative control miRNA at a final concentration of 0.3
µM using Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h of incubation, the cells were
harvested and washed with ice-cold PBS for subsequent analysis. The
integrity of miR-1237 transfection was confirmed using real-time
RT-PCR (Fig. S1). However, no
miR-1237 reduction was detected in both cancer cell lines after
microRNA inhibitor transfection. This inhibitor just binds to
specific microRNA and stops translation of target gene and
therefore, no reduction in specific miRNA was detected using
real-time RT-PCR. In addition, no obvious miR-1237 target was
published (no obvious downstream pathway). We demonstrated the
differences in phenotype using MTT assay after miR-1237
inhibition.
Statistical analysis
All analyses were conducted using the GraphPad Prism
8.4.2 software (GraphPad Software, Inc.). Two-tailed paired or
unpaired analysis between the groups was conducted using Student's
t-test. For comparison of multiple groups, Kruskal-Wallis test was
first performed, and then Dunnett test was conducted as a post hoc
test. P<0.05 was considered to indicate a significant difference
between groups.
Results
Gal-9 suppresses the proliferation of
human colon and colorectal cancer cells
To evaluate the effects of Gal-9 on the growth of
human colon and colorectal cancer cells in vitro, we
examined the effects of Gal-9 on the proliferation of the five
colon cancer and colorectal cell lines CACO-2, CW-2, COLO-320,
LoVo, and WiDr. Cells were grown in 10% FBS and treated with 0.01,
0.03, 0.10, and 0.30 µM of Gal-9 with untreated cells as controls.
The cell proliferation assay was conducted 48 h after the addition
of Gal-9. As shown in Fig. 1A and B,
Gal-9 led to a dose-dependent and strong inhibition of cell
proliferation in CACO-2 and CW-2, which are Gal-9-sensitive colon
cancer cell lines. However, a significant antiproliferative effect
of Gal-9 on COLO-320, LoVo, and WiDr cell lines was not detected
(Fig. 1C-E). These results are also
expressed as percentages of viable cells compared to control 48 h
after Gal-9 treatment (Fig. 1F).
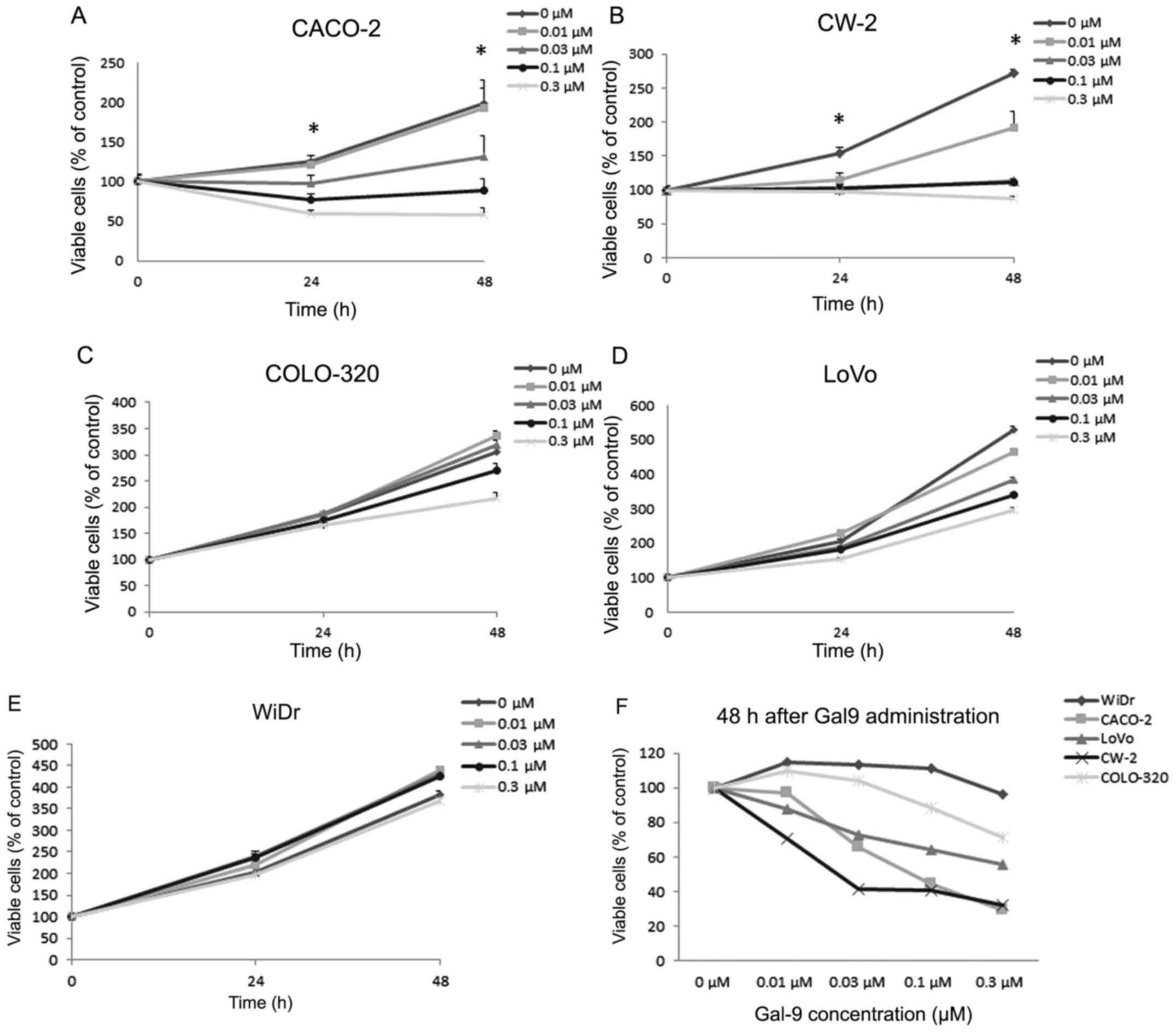 | Figure 1.Gal-9 inhibits the proliferation of
colon cancer cell lines. (A) CACO-2, (B) CW-2, (C) COLO-320, (D)
LoVo, and (E) WiDr cells were treated with varying concentrations
of Gal-9 (0.01, 0.03, 0.1, and 0.3 µM), and cell counts were
performed daily from time 0 to 48 h. The mean cell numbers from
three independent cultures are shown. (F) The results are also
expressed as percentages of viable cells compared to control 48 h
after Gal-9 treatment. In CACO-2 and CW-2 cells, the conditions at
24 and 48 h in cells treated with Gal-9 were significantly
different from those in the control (*P<0.05). Gal-9,
galectin-9. |
Gal-9 induces apoptosis in
Gal-9-sensitive CACO-2, CW-2 cells, but not in WiDr cell
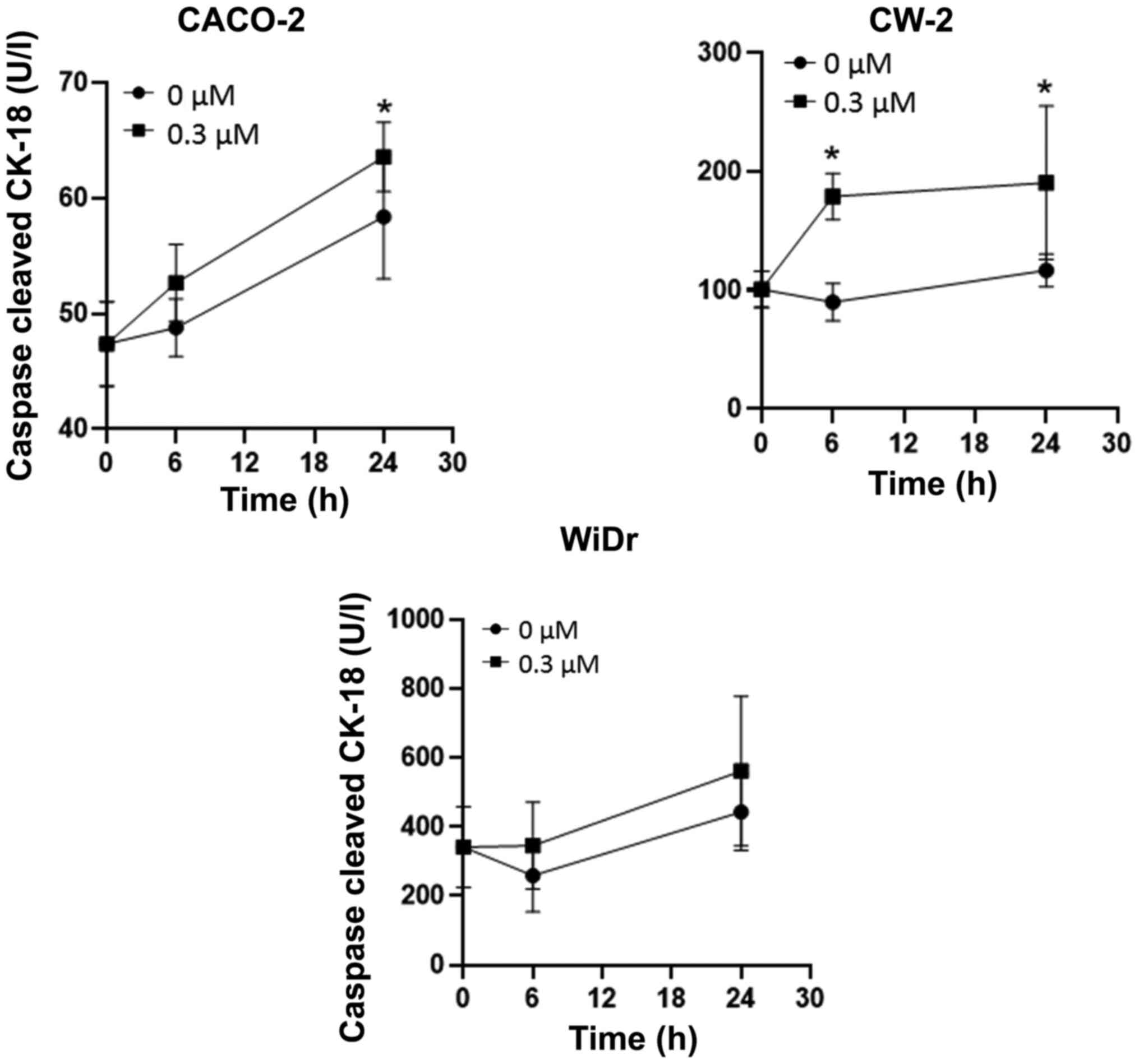
CCK-18 is produced specifically in apoptosis. We
performed ELISAs to determine the levels of CCK-18 in colorectal
cancer cells treated with 0.3 µM Gal-9. We found that Gal-9
significantly increased the CCK-18 levels in Gal-9-sensitive CACO-2
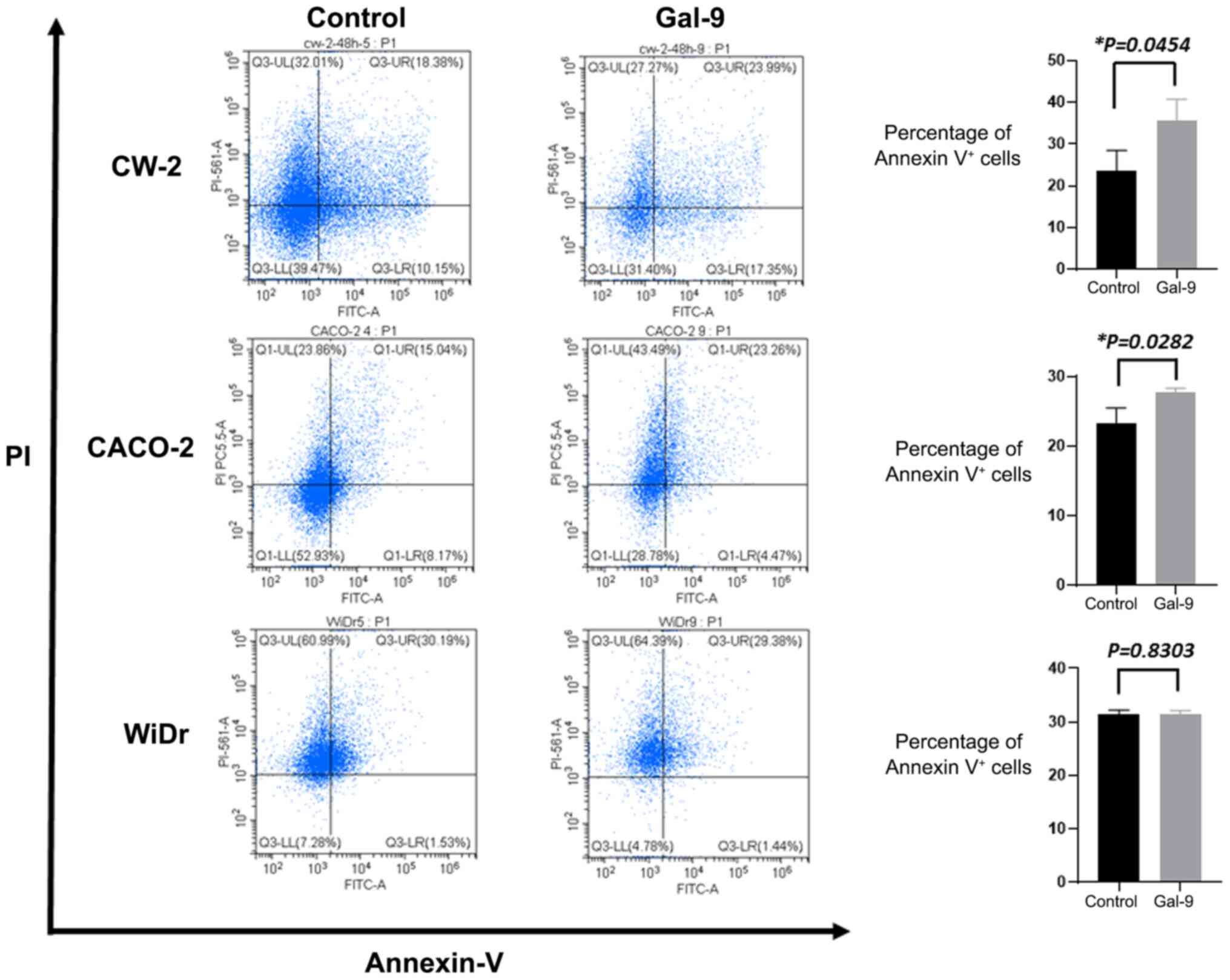
and CW-2 cells, but not in WiDr cells (Fig. 2). To further examine whether Gal-9
promotes apoptosis, we also assessed an apoptosis marker in CW-2,
CACO-2, and WiDr cells treated for 24 h with 0.3 µM Gal-9. In flow
cytometry measurements, the percentages of Annexin V+
cells were significantly increased in Gal-9-treated CW-2 and CACO-2
cells, but not in WiDr cells (Fig.
3). This suggests that Gal-9 administration induced apoptosis
in Gal-9-sensitive cell lines.
Differences in phosphorylated receptor
tyrosine kinases with and without Gal-9 treatment in
Gal-9-sensitive and -resistant colon cancer and colorectal cell
lines
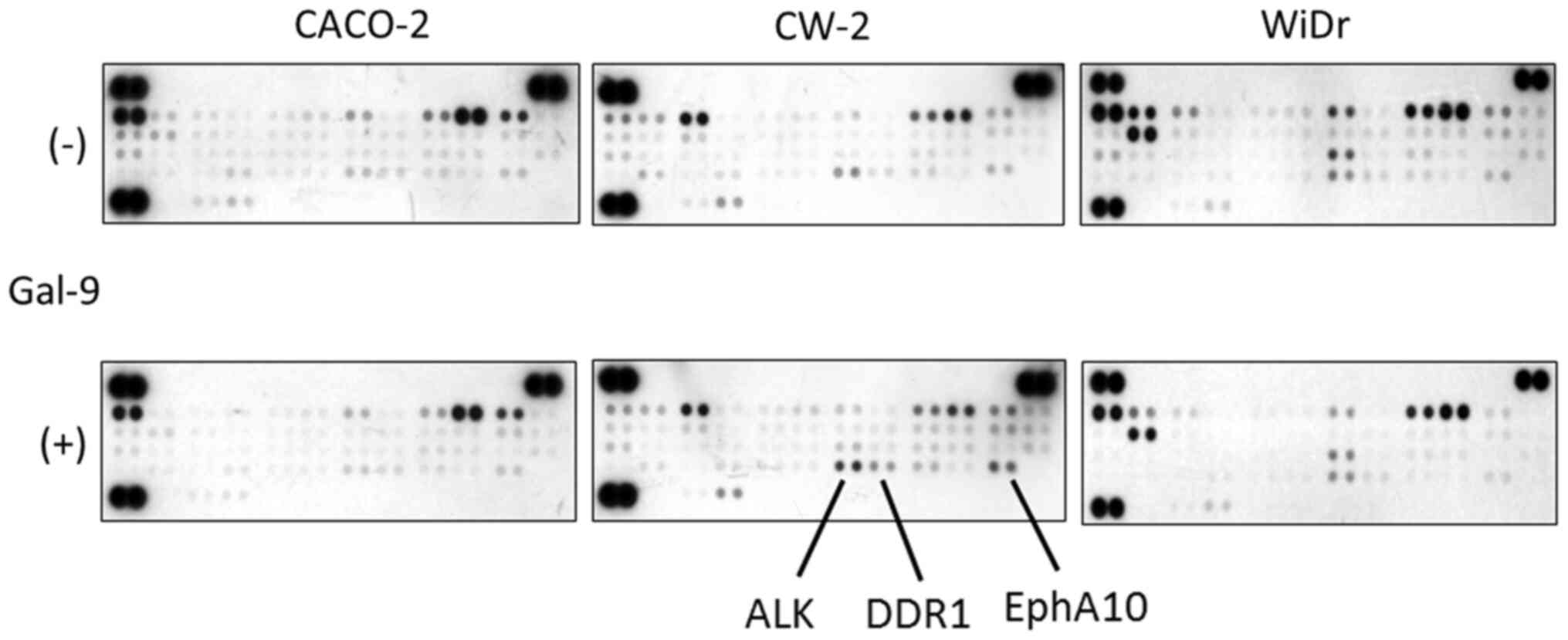
We next used a p-RTK array system to identify ‘key
RTKs’ associated with the antitumor effect of Gal-9. By using the
antibody array (Fig. 4), we
simultaneously screened the expression of 42 different activated
RTKs in CACO-2, CW-2, and WiDr cell lines with and without the
addition of 0.3 µM Gal-9. It was noted that Gal-9 enhanced the
expression of ALK, DDR1, and EphA10 in Gal-9-sensitive CW-2 cells
(Fig. 4). By contrast, CACO-2 cells
and Gal-9-resistant WiDr cells did not exhibit a change in the
expression of the activated RTKs.
Effects of Gal-9 on angiogenesis in
Gal-9-sensitive vs. -resistant colon and colorectal cancer
cells
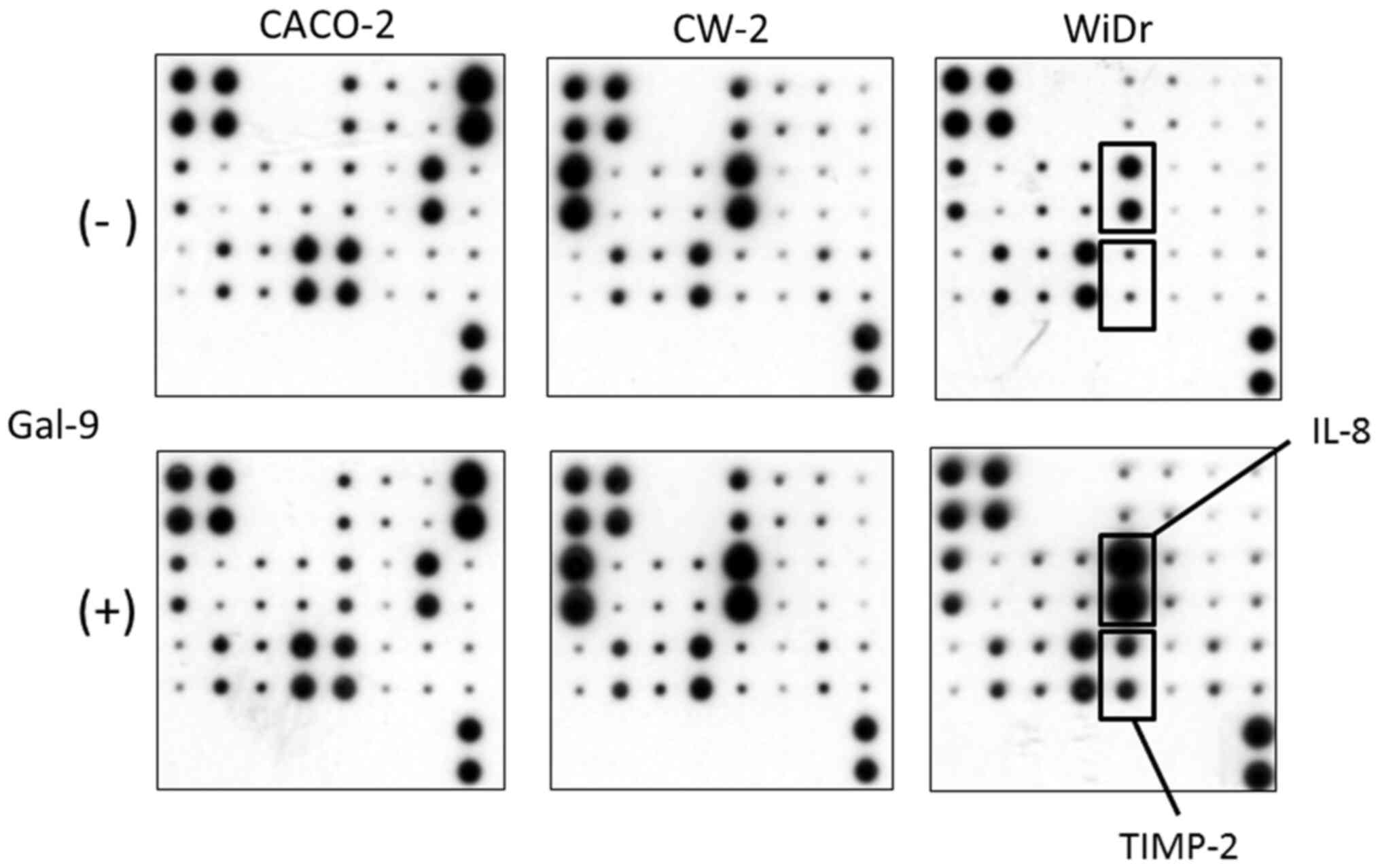
In order to examine the relationship between
angiogenesis and Gal-9, an angiogenesis antibody array system was
used to identify key angiogenesis-related molecules associated with
the antitumor effect of Gal-9 (Fig.
5). By using the antibody array, we simultaneously screened the
expression of 20 different angiogenesis-associated molecules in
CACO-2, CW-2, and WiDr cells with or without Gal-9 administration.
The expression levels of interleukin-8 (IL-8) and tissue inhibitor
of metalloproteinases-2 (TIMP-2) were induced by Gal-9 treatment in
Gal-9-resistant WiDr cells as detected by the protein array
(Fig. 5). In Gal-9-sensitive CACO-2
and CW-2 cells, the expression of angiogenesis molecules did not
change following treatment with Gal-9 (Fig. 5).
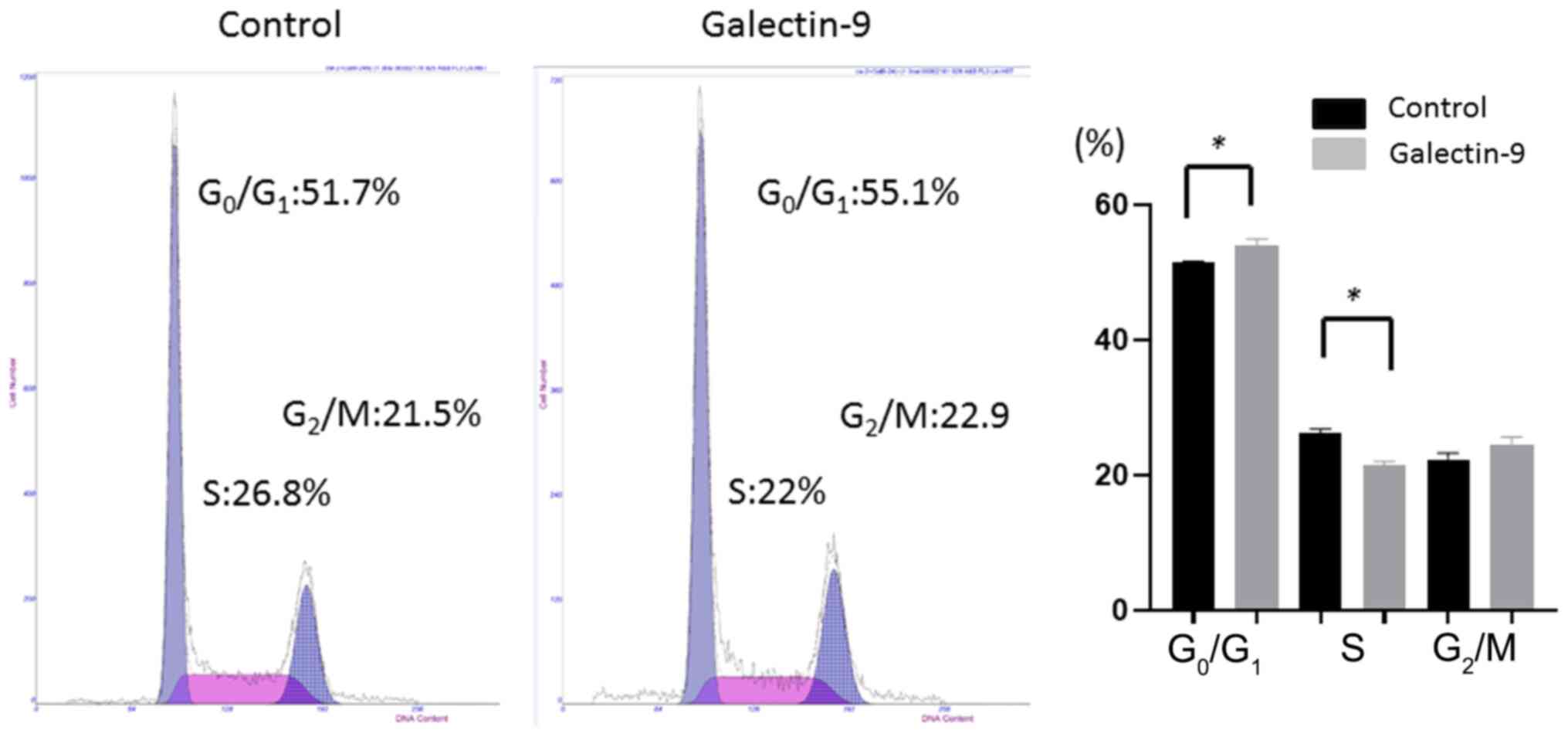
Gal-9-induced cell cycle arrest in the
G0/G1 phase
To examine whether growth inhibition was due to cell
cycle changes, we investigated the cell cycle profiles of CW-2
cells 24 h after treatment, with or without 0.3 µM Gal-9, using
flow cytometry. Treatment with 0.3 µM Gal-9 significantly increased
the percentage of cells in the G0/G1 phase
and significantly decreased the percentage of cells in the S phase
at 24 h after treatment (Fig. 6).
This result indicates that Gal-9 blocked the cell cycle progression
from the G0/G1 phase to the S phase and may
induce apoptosis.
Differences in cell cycle-related
protein expression in CACO-2 and CW-2 cells with and without Gal-9
treatment
To investigate the effects of Gal-9 administration
on Gal-9-sensitive cells, we examined the cell cycle-related
protein expression in CACO-2 and CW-2 cells using western blotting.
Cells were treated with 0 or 0.3 µM Gal-9 for 24–48 h. We observed
no obvious reduction in cyclin D1, which is one of the key proteins
involved in the transition from the G0 to the
G1 phase, due to Gal-9 treatment in CACO-2 and CW-2
cells (Fig. 7). Additionally, the
analysis of other proteins associated with
G0/G1 transition indicated that Cdk4 and
Cdk6, the catalytic subunits of cyclin D1, were not decreased at
any time point in CACO-2 cells after Gal-9 treatment (Fig. 7). Cyclin E, which is the key cyclin
for G1/S transition, was unchanged, and no pRB reduction
was detected in CACO-2 and CW-2 cells after Gal-9 treatment
(Fig. 7).
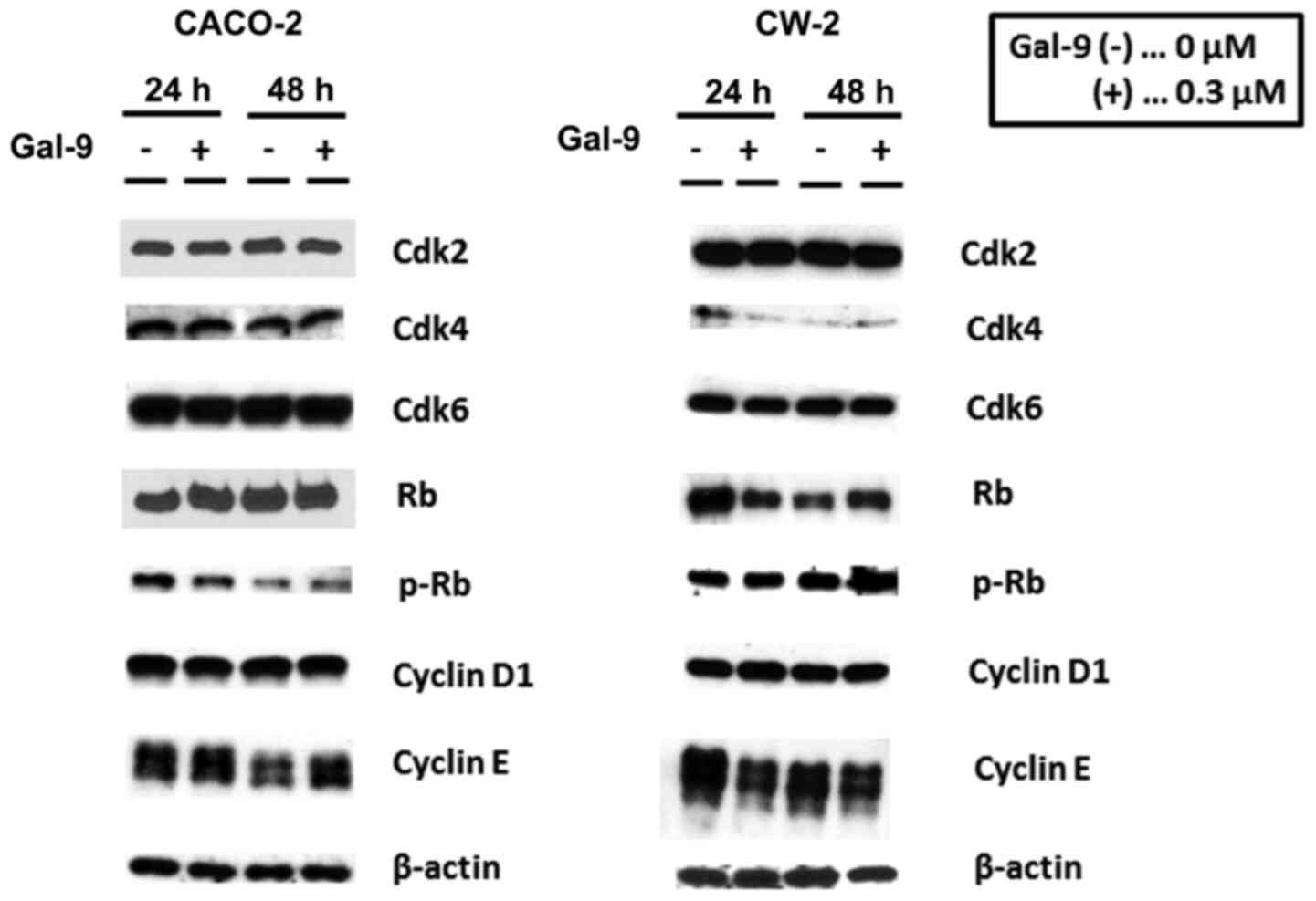 | Figure 7.Effects of Gal-9 on cell cycle
regulatory molecules in CACO-2 and CW-2 cells. Western blotting for
Cdk2, Cdk4, Cdk6, Rb, p-Rb, cyclin D1, and cyclin E in CACO-2 and
CW-2 cells after 24 or 48 h of 0.3 µM Gal-9 treatment. At both 24
and 48 h, cyclin D1 was not decreased in Gal-9-treated cells
compared to untreated cells. In CACO-2 cells, the quantities of
cyclin D1, Cdk4, and Cdk6 did not differ between treated and
untreated cells at any time point. The p-Rb expression levels were
not decreased in the CACO-2 and CW-2 cells following Gal-9
treatment. β-actin was used as the loading control. Gal-9,
galectin-9. |
These findings suggest that Gal-9 is not involved in
the regulation of cell cycle-related molecules in Gal-9-sensitive
cells.
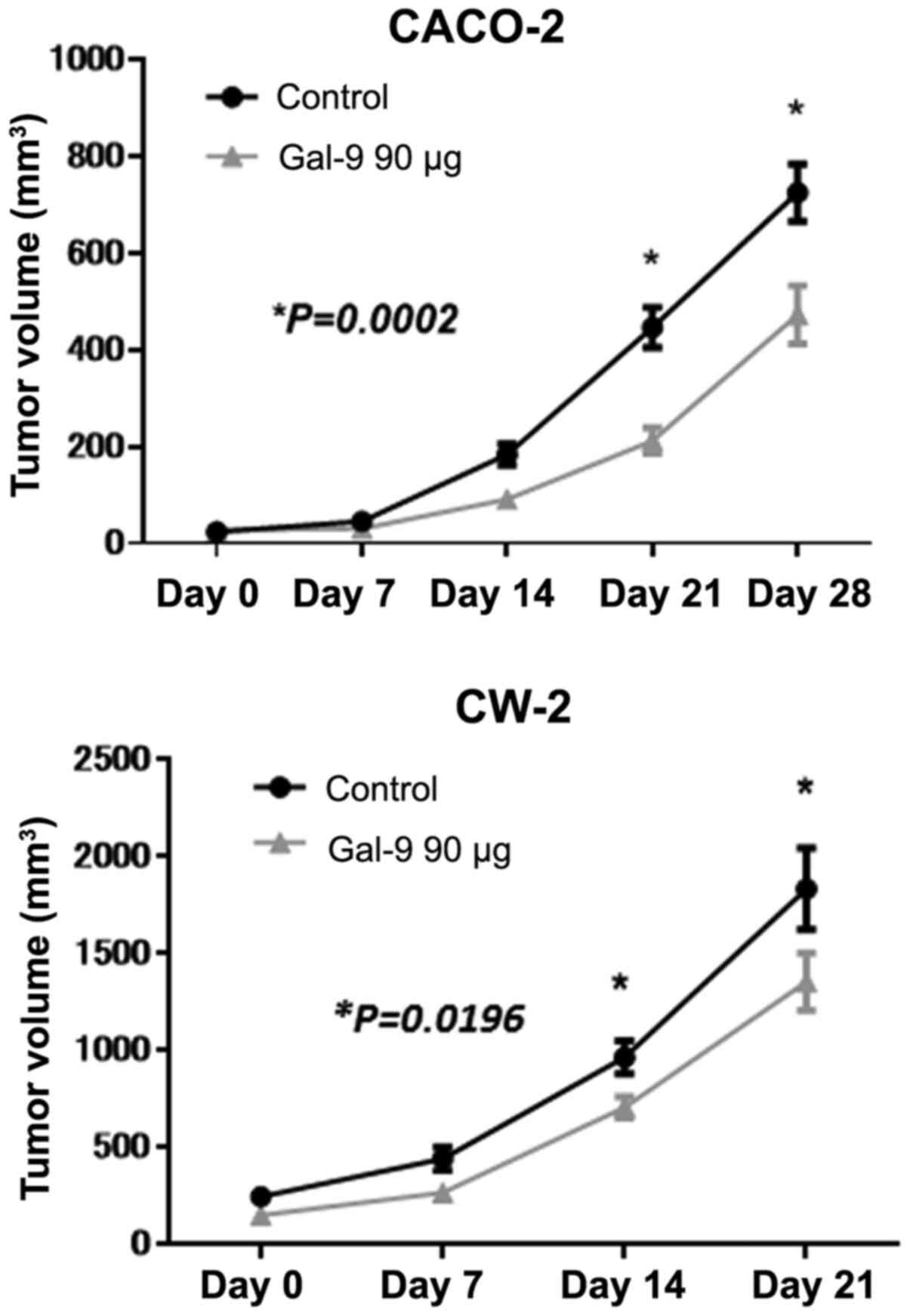
Gal-9 inhibits tumor proliferation in
vivo
To determine whether Gal-9 can affect tumor growth
in vivo, we subcutaneously inoculated nude mice with CACO-2
and CW-2 cells, followed by i.p. injection of Gal-9. The Gal-9
treatment at a dose of 90 µg significantly inhibited tumor growth
in both CACO-2 and CW-2 implanted tumors compared to untreated
control mice, as determined by integrated tumor growth curves
(Student's t-test, *P<0.05; Fig.
8). Gal-9 had no apparent toxic effects on the mice including
their body weight during the study (data not shown). Furthermore,
all animals survived until the end of the experiment.
miRNA profiles of the cell lines with
and without Gal-9 treatment
Using a custom microarray platform, we analyzed the
expression levels of 2,555 human miRNA probes in colon cancer cell
lines in vitro and in vivo with and without Gal-9
treatment. As shown in Fig. 9, when
the expression of miRNAs was studied in CACO-2 cells with and
without Gal-9 treatment in vitro and in vivo, only
hsa-miR-1246 was found to be commonly upregulated 24 h after Gal-9
treatment (P=0.00000689 and P=0.000804, respectively), whereas
hsa-miR-15b-5p and hsa-miR-1237 expression levels were
significantly changed in CW-2 cells in vitro (P=0.00956 and
P=0.0033, respectively) and in vivo (P=0.00582 and
P=0.000289, respectively). In addition, only hsa-miR-1237 was
commonly altered in CACO-2 cells in vitro, CW-2 cells in
vitro, and CW-2 cells in vivo.
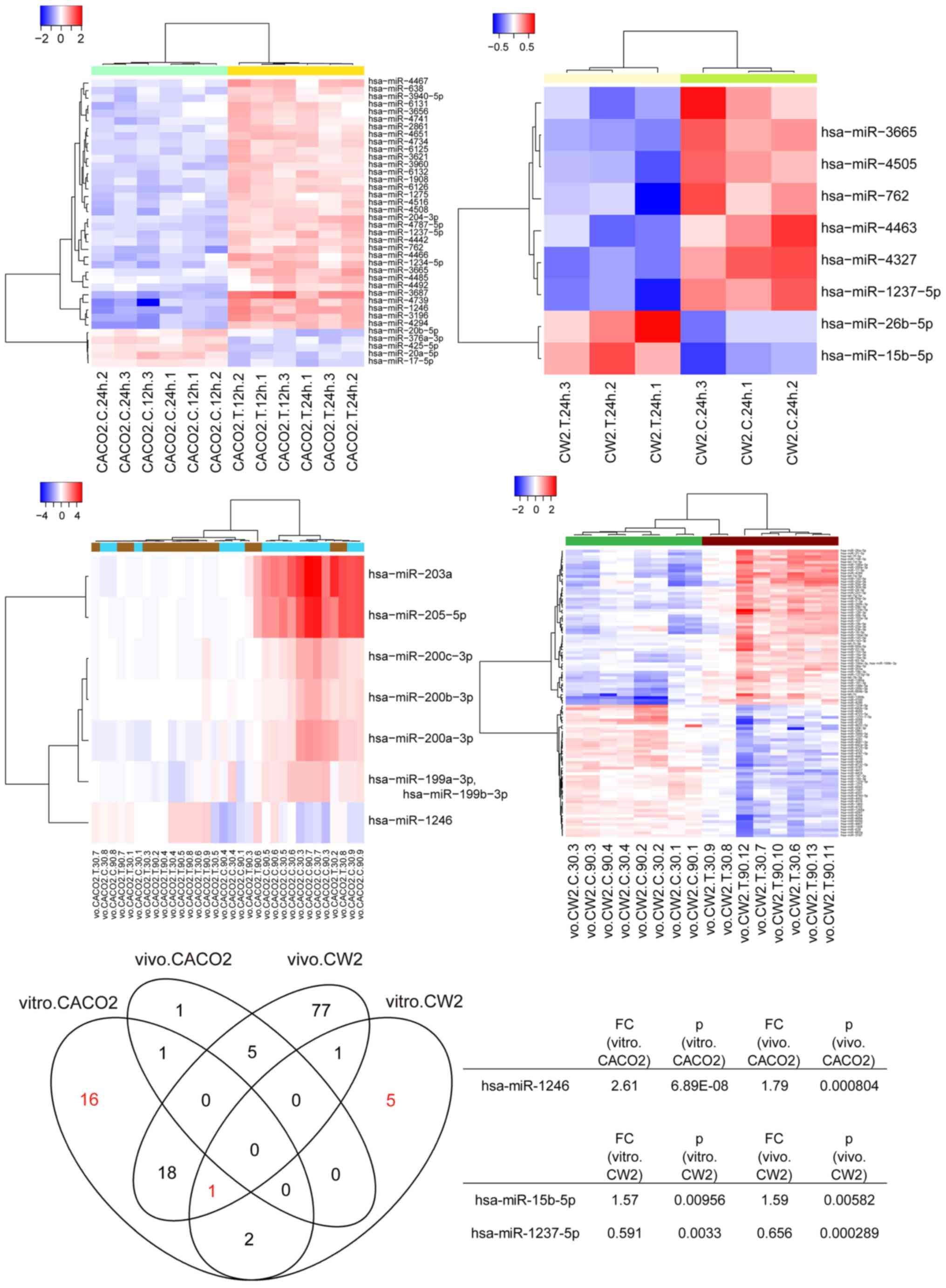 | Figure 9.Hierarchical clustering of CACO-2,
CW-2, and WiDr cells with and without Gal-9 treatment. CACO-2 cells
were clustered according to the expression profiles that were
differentially expressed between treated and untreated CACO-2 cells
in vitro. CW-2 cells were clustered separately according to
treated and untreated CW-2 cells in vitro and in
vivo. The analyzed samples are in columns, and the miRNAs are
presented in rows. The miRNA clustering tree is shown on the left,
and the sample-clustering tree appears at the top. The color scale
shown at the top illustrates the relative expression level of
miRNAs; red represents a high expression level, and blue represents
a low expression level. Only hsa-miR-1246 was detected to be
commonly upregulated 24 h after Gal-9 treatment in CACO-2 cells
(P=0.00000689 and P=0.000804), whereas hsa-miR-15b-5p and
hsa-miR-1237 expression levels were significantly changed in CW-2
cells in vitro (P=0.00956 and P=0.0033) and in vivo
(P=0.00582 and P=0.000289). In addition, only hsa-miR-1237 was
commonly altered in CACO-2 cells in vitro, CW-2 cells in
vitro, and CW-2 cells in vivo. Gal-9, galectin-9. |
Unsupervised hierarchical clustering analysis with
Pearson's correlation showed that CACO-2 cells in vitro,
CW-2 cells in vitro, and CW-2 cells in vivo treated
with Gal-9 clustered together and separately from untreated CACO-2
and CW-2 cells (Fig. 9).
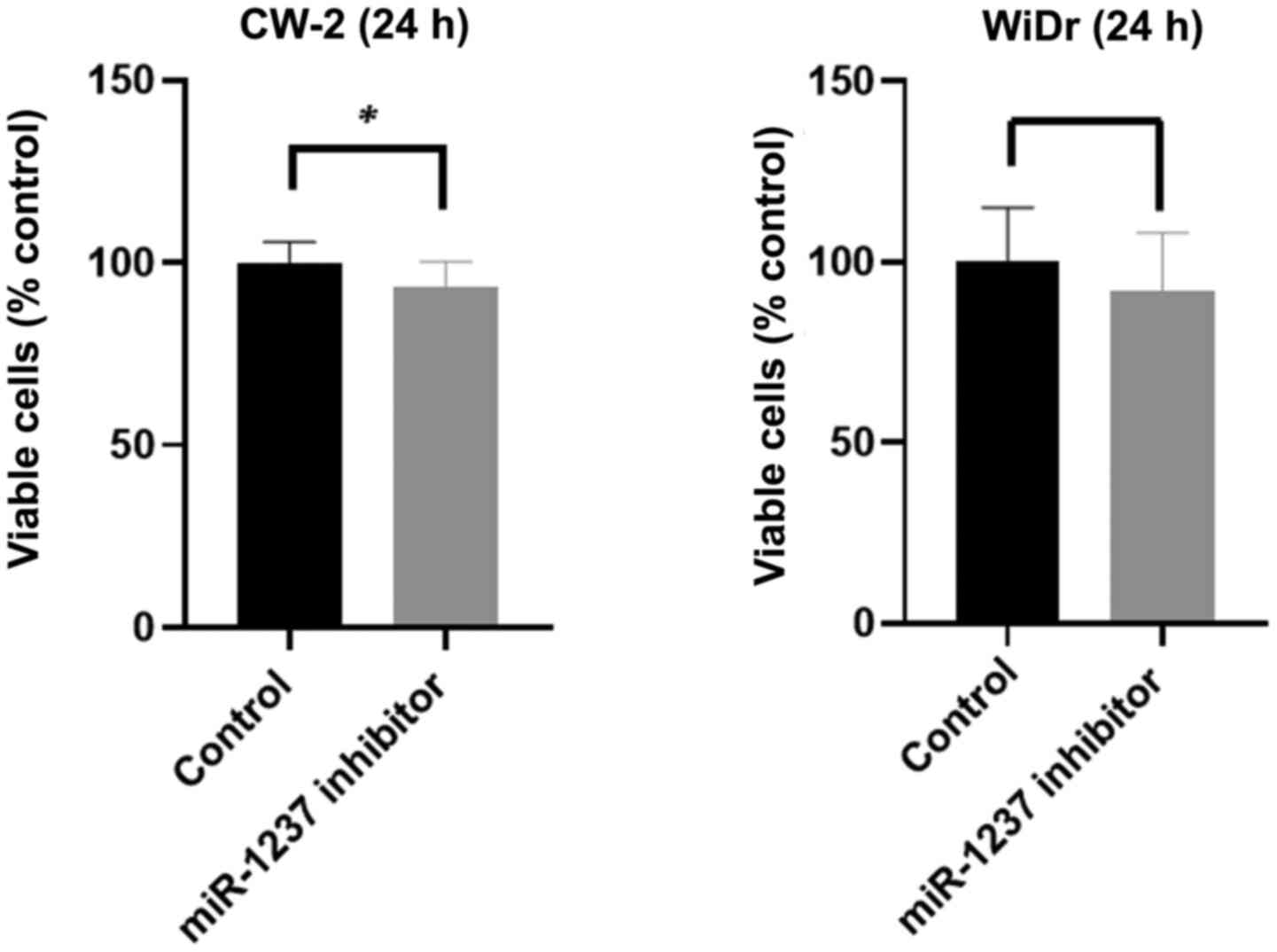
Inhibition of miR-1237 reduces cell
proliferation in colon cancer cells
Our in vitro findings led us to hypothesize
that Gal-9 might inhibit the proliferation of colon and colorectal
cancer cells via miR-1237 downregulation. To examine this
hypothesis, we examined CW-2 and WiDr cells transfected with
miR-1237 inhibitor or negative control miRNA. After 24 h of
incubation, we examined the cell proliferation with or without
miR-1237. Interestingly, the percentages of viable cells were
significantly diminished 24 h after miR-1237 inhibitor transfection
in the colon cancer CW-2 cells, but not in the colorectal cancer
WiDr cells (Fig. 10). This revealed
that miR-1237 inhibited by Gal-9 suppressed the cell proliferation
in one of the Gal-9-sensitive colon cancer cell lines.
Discussion
Colorectal cancer (CRC) is a commonly disease
worldwide, being the third and second commonly diagnosed cancer in
men and women, respectively (33).
Although the overall incidence and mortality have dramatically
declined over the last few decades, it remains a major health issue
(3–6).
Currently, CRC treatment is mainly based on surgical removal of
tumor tissue, chemotherapy, and radiotherapy, with the recent
addition of immunotherapy (34).
However, the overall survival rate of advanced CRC patients remains
poor (35). Thus, there is a strong
demand for new therapeutic approaches to CRC therapy.
Nobumoto et al reported that Gal-9 suppresses
tumor metastasis by inhibiting adhesion between endothelium and
extracellular matrix (36).
Additionally, reduced expression of Gal-9 is related to poor
outcome in colon cancer (37).
However, the antitumor effect of recombinant Gal-9 on colon cancer
and colorectal cells in vitro is unknown. In the present
study, we found that Gal-9 inhibited the proliferation of colon
cancer cell lines and we the miRNA associated with the antitumor
effect of Gal-9 in colon cancer.
Recombinant Gal-9 was found to inhibit proliferation
in various types of cancers such as hepatocellular carcinoma by
inducing apoptosis (23). Our study
results revealed that Gal-9 suppresses cell proliferation in human
colon and colorectal cancer cell lines in vitro. Gal-9 led
to a dose-dependent and strong inhibition of cell proliferation in
CACO-2 and CW-2 cell lines, but not in COLO-320, LoVo, and WiDr
cell lines. Ahmed et al demonstrated that TP53 is mutated in
the CACO-2 cell line and BRAF, PIK3CA, and TP53 are mutated in the
WiDr cell line (38). In addition,
LoVo cells have a KRAS mutation (38). These results indicate that colon and
colorectal cancer cell lines with more mutations of genes critical
for cancer may be resistant to Gal-9.
Caspase cleavage of cytokeratin (CCK-18)
upregulation occurs as an early event during apoptosis following
activation of apoptosis executioners, particularly effector
caspases, yet its levels remain unchanged during other types of
cell death such as autophagy or necrosis (39). In our study, Gal-9 increased the
levels of CCK-18 in CACO-2 and CW-2 cell lines which are sensitive
to Gal-9, but not in the WiDr cell line which is resistant to
Gal-9. Moreover, flow cytometry experiments revealed that the
percentages of Annexin V+ CW-2 cells were significantly
increased after Gal-9 treatment, indicating that the presence of
Gal-9 caused apoptosis in these cells. These data suggest that
Gal-9 suppresses the cell proliferation of Gal-9-sensitive colon
and colorectal cancer cells by inducing apoptosis.
Since the discovery of receptor tyrosine kinases
(RTKs), their functions have been examined over the years as key
regulators of proliferation, differentiation, and metastasis in
gastric cancer (40) and colon cancer
(41). Using p-RTK arrays, our study
showed increased activation of ALK, DDR1, and EphA10 following
Gal-9 treatment in Gal-9-sensitive cells (CW-2) but not in
Gal-9-resistant cells (WiDr). These RTKs are expressed in various
cancers and are involved in cell proliferation and the prevention
of apoptosis (42–44). In Gal-9-sensitive cells, these p-RTKs
were not activated in CACO-2 cells. Gal-9 might have other pathways
to inhibit cell proliferation and induce apoptosis. These results
suggest that the activation of these identified RTKs induced by
Gal-9 may be associated with the predisposition of a type of
Gal-9-sensitive cell lines to induce cell growth inhibition and
apoptosis.
Galectins are important regulators of tumor
progression that influence tumor cell transformation, tumor immune
escape, and tumor angiogenesis (12–14). Using
an angiogenesis-related protein assay, we discovered that IL-8 and
TIMP-2 were stimulated in WiDr cells which is a Gal-9-resistant
cell line. Specifically, high levels of IL-8 may be associated with
poor prognosis, as judged by stage and histology, and may be
indicative of a more aggressive cancer type (45–47). These
data suggest that the Gal-9-resistant colorectal cancer cells
escaping from the antitumor effect of Gal-9 may produce
angiogenesis-related proteins. Nevertheless, the effect of Gal-9 on
angiogenesis in colon cancer remains controversial.
miRNAs, small noncoding RNA sequences, have been
shown to regulate the development and progression of various
cancers (48). Using miRNA expression
arrays to identify the miRNAs associated with the antitumor effect
of Gal-9, we determined the variations in the miRNA profiles in
CACO-2 and CW-2 cell lines in vitro and in vivo with
or without Gal-9 treatment. The cluster analysis clearly
demonstrated that Gal-9 treatment affected the expression of
numerous miRNAs. In the analysis, we selected sets of miRNAs that
displayed a significant alteration in their expression levels
following Gal-9 treatment. These altered miRNAs may provide clues
to the molecular basis of the anticancer effects of Gal-9 in colon
cancer. Our data showed that miR-1246 was upregulated in CACO-2
cells in vitro and in vivo and that miR-1237-5p was
downregulated in CW-2 cells in vitro and in vivo
after treatment with Gal-9. To determine whether the loss of
miR-1237 can inhibit cell proliferation, we further assayed the
effect of miR-1237 inhibition on the cell proliferation of CW-2 and
WiDr cells. Remarkably, miR-1237 inhibition suppressed cell
proliferation in CW-2, but not in WiDr cells. Thus, Gal-9 inhibited
cell proliferation through miR-1237 in Gal-9-sensitive CW-2 cells.
In addition, miR-15b-5p was also previously found to be
significantly upregulated in CW-2 cells in vitro and in
vivo by Gal-9 treatment. miR-1246 targets CADM1 in
hepatocellular carcinoma (49), CXCR4
in lung cancer cells (50), and
thrombospondin-2 in cervical cancer cells (51) leading to apoptosis and cell cycle
arrest. On the other hand, miR-1246 has been reported to be a
target of the p53 family, and inhibits Down syndrome-associated
DYRK1A, consequently activating NFTA1c and inducing apoptosis
(46). In addition, miR-15b-5p
targets SIRT1 in colorectal cancer (52) and Axin2 in liver cancer (53). Our previous studies revealed that
Gal-9 treatment leads to the upregulation of miR-1246 in
hepatocellular carcinoma (23) and
cholangiocarcinoma (25), suggesting
that miR-1246 may be associated with the antitumor effect of Gal-9
in various cancer cells. In addition, Tang et al
demonstrated that miR-1237 was associated with EMT and cancer
metastasis in nasopharyngeal carcinoma (54). These reports suggest that several
microRNAs might be key molecules for Gal-9-induced inhibition of
tumor growth.
In conclusion, our results revealed that Gal-9
suppresses human colon cancer cell proliferation, possibly by
inducing apoptosis through the alteration of miRNAs. These findings
suggest that Gal-9 may be a candidate for a new therapeutic
approach to the treatment of colon cancer.
Supplementary Material
Supporting Data
Acknowledgements
We thank Ms. Kayo Hirose for providing technical
assistance.
Funding
This study received no funding from external
sources.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AM designed the concept of the present study. AM, MH
and TM designed this study. AM, KN, JT, KF, KT, MN, TT, KO, TC and
SF performed the in vitro experiments. AM and KN performed
the in vivo experiments. AM, HI, TN, AN and TH analyzed the
microRNA profiles. AM wrote the draft of the manuscript, and TN,
MH, AN, TH and TM reviewed it. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Committee on
Experimental Animals of Kagawa University (approval no.
HEISEI25-164).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCK-18
|
caspase-cleaved keratin-18
|
|
CCK-8
|
Cell Counting Kit-8
|
|
CRC
|
colorectal cancer
|
|
CRD
|
carbohydrate-recognition domain
|
|
DMSO
|
dimethyl sulfoxide
|
|
ELISA
|
enzyme-linked immunosorbent
|
|
FBS
|
fetal bovine serum
|
|
Gal-9
|
galectin-9
|
|
IL-8
|
interleukin-8
|
|
miRNA/miR
|
microRNA
|
|
p-RTK
|
phosphorylated receptor tyrosine
kinase
|
|
PBS
|
phosphate-buffered saline
|
|
TIMP-2
|
tissue inhibitor of
metalloproteinases-2
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: NCCN Guidelines insights: Colon cancer, version
2.2018. J Natl Compr Canc Netw. 16:359–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boland GM, Chang GJ, Haynes AB, Chiang YJ,
Chagpar R, Xing Y, Hu CY, Feig BW, You YN and Cormier JN:
Association between adherence to National Comprehensive Cancer
Network treatment guidelines and improved survival in patients with
colon cancer. Cancer. 119:1593–1601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Booth CM, Nanji S, Wei X, Peng Y, Biagi
JJ, Hanna TP, Krzyzanowska MK and Mackillop WJ: Use and
effectiveness of adjuvant chemotherapy for stage III colon cancer:
A population-based study. J Natl Compr Canc Netw. 14:47–56. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casadaban L, Rauscher G, Aklilu M,
Villenes D, Freels S and Maker AV: Adjuvant chemotherapy is
associated with improved survival in patients with stage II colon
cancer. Cancer. 122:3277–3287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hines RB, Barrett A, Twumasi-Ankrah P,
Broccoli D, Engelman KK, Baranda J, Ablah EA, Jacobson L, Redmond
M, Tu W and Collins TC: Predictors of guideline treatment
nonadherence and the impact on survival in patients with colorectal
cancer. J Natl Compr Canc Netw. 13:51–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sargent DJ, Marsoni S, Monges G, Thibodeau
SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri
V, et al: Defective mismatch repair as a predictive marker for lack
of efficacy of fluorouracil-based adjuvant therapy in colon cancer.
J Clin Oncol. 28:3219–3226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JE, Hong YS, Kim HJ, Kim KP, Lee JL,
Park SJ, Lim SB, Park IJ, Kim CW, Yoon YS, et al: Defective
mismatch repair status was not associated with DFS and OS in stage
II colon cancer treated with adjuvant chemotherapy. Ann Surg Oncol.
22 (Suppl 3):S630–S637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McCleary NJ, Meyerhardt JA, Green E,
Yothers G, de Gramont A, Van Cutsem E, O'Connell M, Twelves CJ,
Saltz LB, Haller DG and Sargent DJ: Impact of age on the efficacy
of newer adjuvant therapies in patients with stage II/III colon
cancer: Findings from the ACCENT database. J Clin Oncol.
31:2600–2606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yothers G, O'Connell MJ, Allegra CJ,
Kuebler JP, Colangelo LH, Petrelli NJ and Wolmark N: Oxaliplatin as
adjuvant therapy for colon cancer: Updated results of NSABP C-07
trial, including survival and subset analyses. J Clin Oncol.
29:3768–3774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tournigand C, André T, Bonnetain F,
Chibaudel B, Lledo G, Hickish T, Tabernero J, Boni C, Bachet JB,
Teixeira L and de Gramont A: Adjuvant therapy with fluorouracil and
oxaliplatin in stage II and elderly patients (between ages 70 and
75 years) with colon cancer: Subgroup analyses of the multicenter
international study of oxaliplatin, fluorouracil, and leucovorin in
the adjuvant treatment of colon cancer trial. J Clin Oncol.
30:3353–3360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thijssen VL, Heusschen R, Caers J and
Griffioen AW: Galectin expression in cancer diagnosis and
prognosis: A systematic review. Biochim Biophys Acta. 1855:235–247.
2015.PubMed/NCBI
|
|
13
|
Wiersma VR, de Bruyn M, Helfrich W and
Bremer E: Therapeutic potential of Galectin-9 in human disease. Med
Res Rev. 33 (Suppl 1):E102–E126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujihara S, Mori H, Kobara H, Rafiq K,
Niki T, Hirashima M and Masaki T: Galectin-9 in cancer therapy.
Recent Pat Endocr Metab Immune Drug Discov. 7:130–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirashima M: Ecalectin/galectin-9, a novel
eosinophil chemoattractant: Its function and production. Int Arch
Allergy Immunol. 122 (Suppl 1):S6–S9. 2000. View Article : Google Scholar
|
|
16
|
Matsumoto R, Matsumoto H, Seki M, Hata M,
Asano Y, Kanegasaki S, Stevens RL and Hirashima M: Human ecalectin,
a variant of human galectin-9, is a novel eosinophil
chemoattractant produced by T lymphocytes. J Biol Chem.
273:16976–16984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsushita N, Nishi N, Seki M, Matsumoto
R, Kuwabara I, Liu FT, Hata Y, Nakamura T and Hirashima M:
Requirement of divalent galactoside-binding activity of
ecalectin/galectin-9 for eosinophil chemoattraction. J Biol Chem.
275:8355–8360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saita N, Goto E, Yamamoto T, Cho I,
Tsumori K, Kohrogi H, Maruo K, Ono T, Takeya M, Kashio Y, et al:
Association of galectin-9 with eosinophil apoptosis. Int Arch
Allergy Immunol. 128:42–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asakura H, Kashio Y, Nakamura K, Seki M,
Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T, et al:
Selective eosinophil adhesion to fibroblast via IFN-gamma-induced
galectin-9. J Immunol. 169:5912–5918. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H and
Hirashima M: Galectin-9 as a prognostic factor with antimetastatic
potential in breast cancer. Clin Cancer Res. 11:2962–2968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamauchi A, Kontani K, Kihara M, Nishi N,
Yokomise H and Hirashima M: Galectin-9, a novel prognostic factor
with antimetastatic potential in breast cancer. Breast J. 12 (Suppl
2):S196–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yasinska IM, Sakhnevych SS, Pavlova L, Teo
Hansen Selnø A, Teuscher Abeleira AM, Benlaouer O, Gonçalves Silva
I, Mosimann M, Varani L, Bardelli M, et al: The Tim-3-Galectin-9
pathway and its regulatory mechanisms in human breast cancer. Front
Immunol. 10:15942019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujita K, Iwama H, Sakamoto T, Okura R,
Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et
al: Galectin-9 suppresses the growth of hepatocellular carcinoma
via apoptosis in vitro and in vivo. Int J Oncol.
46:2419–2430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong F, Jin M, Cao D, Jia Z, Liu Y and
Jiang J: Galectin-3 not Galectin-9 as a candidate prognosis marker
for hepatocellular carcinoma. PeerJ. 8:e99492020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi K, Morishita A, Iwama H, Fujita
K, Okura R, Fujihara S, Yamashita T, Fujimori T, Kato K, Kamada H,
et al: Galectin-9 suppresses cholangiocarcinoma cell proliferation
by inducing apoptosis but not cell cycle arrest. Oncol Rep.
34:1761–1770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muthusami S, Ramachandran I,
Krishnamoorthy S, Sambandam Y, Ramalingam S, Queimado L, Chaudhuri
G and Ramachandran IK: Regulation of microRNAs in
inflammation-associated colorectal cancer: A mechanistic approach.
Endocr Metab Immune Disord Drug Targets. 21:67–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishi N, Itoh A, Fujiyama A, Yoshida N,
Araya S, Hirashima M, Shoji H and Nakamura T: Development of highly
stable galectins: Truncation of the linker peptide confers
protease-resistance on tandem-repeat type galectins. FEBS Lett.
579:2058–2064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schutte B, Henfling M, Kölgen W, Bouman M,
Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et
al: Keratin 8/18 breakdown and reorganization during apoptosis. Exp
Cell Res. 297:11–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masaki T, Tokuda M, Yoshida S, Nakai S,
Morishita A, Uchida N, Funaki T, Kita Y, Funakoshi F, Nonomura T,
et al: Comparison study of the expressions of myristoylated
alanine-rich C kinase substrate in hepatocellular carcinoma, liver
cirrhosis, chronic hepatitis, and normal liver. Int J Oncol.
26:661–671. 2005.PubMed/NCBI
|
|
30
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takano J, Morishita A, Fujihara S, Iwama
H, Kokado F, Fujikawa K, Fujita K, Chiyo T, Tadokoro T, Sakamoto T,
et al: Galectin-9 suppresses the proliferation of gastric cancer
cells in vitro. Oncol Rep. 35:851–860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
D'Incalci M, Colombo T, Ubezio P,
Nicoletti I, Giavazzi R, Erba E, Ferrarese L, Meco D, Riccardi R,
Sessa C, et al: The combination of yondelis and cisplatin is
synergistic against human tumor xenografts. Eur J Cancer.
39:1920–1926. 2003. View Article : Google Scholar
|
|
33
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, Regional, and National Cancer Incidence, Mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mishra J, Drummond J, Quazi SH, Karanki
SS, Shaw JJ, Chen B and Kumar N: Prospective of colon cancer
treatments and scope for combinatorial approach to enhanced cancer
cell apoptosis. Crit Rev Oncol Hematol. 86:232–250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li C, Zuo D, Liu T, Yin L, Li C and Wang
L: Prognostic and clinicopathological significance of MUC family
members in colorectal cancer: A systematic review and
meta-analysis. Gastroenterol Res Pract. 2019:23916702019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nobumoto A, Nagahara K, Oomizu S, Katoh S,
Nishi N, Takeshita K, Niki T, Tominaga A, Yamauchi A and Hirashima
M: Galectin-9 suppresses tumor metastasis by blocking adhesion to
endothelium and extracellular matrices. Glycobiology. 18:735–744.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Sun J, Ma C, Gao W, Song B, Xue H,
Chen W, Chen X, Zhang Y, Shao Q, et al: Reduced expression of
Galectin-9 contributes to a poor outcome in colon cancer by
inhibiting NK cell chemotaxis partially through the Rho/ROCK1
signaling pathway. PLoS One. 11:e01525992016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahmed D, Eide PW, Eilertsen IA, Danielsen
SA, Eknæs M, Hektoen M, Lind GE and Lothe RA: Epigenetic and
genetic features of 24 colon cancer cell lines. Oncogenesis.
2:e712013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kramer G, Erdal H, Mertens HJ, Nap M,
Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC and Linder
S: Differentiation between cell death modes using measurements of
different soluble forms of extracellular cytokeratin 18. Cancer
Res. 64:1751–1756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morishita A, Gong J and Masaki T:
Targeting receptor tyrosine kinases in gastric cancer. World J
Gastroenterol. 20:4536–4545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morishita A, Gong J, Nomura T, Yoshida H,
Izuishi K, Suzuki Y, Kushida Y, Haba R, D'Armiento J and Masaki T:
The use of protein array to identify targetable receptor tyrosine
kinases for treatment of human colon cancer. Int J Oncol.
37:829–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aubry A, Galiacy S and Allouche M:
Targeting ALK in cancer: Therapeutic potential of proapoptotic
peptides. Cancers (Basel). 11:2752019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Azizi R, Salemi Z, Fallahian F and Aghaei
M: Inhibition of didscoidin domain receptor 1 reduces
epithelial-mesenchymal transition and induce cell-cycle arrest and
apoptosis in prostate cancer cell lines. J Cell Physiol.
234:19539–19552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagano K, Yamashita T, Inoue M,
Higashisaka K, Yoshioka Y, Abe Y, Mukai Y, Kamada H, Tsutsumi Y and
Tsunoda S: Eph receptor A10 has a potential as a target for a
prostate cancer therapy. Biochem Biophys Res Commun. 450:545–549.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamada S, Kato S, Matsuhisa T,
Makonkawkeyoon L, Yoshida M, Chakrabandhu T, Lertprasertsuk N,
Suttharat P, Chakrabandhu B, Nishiumi S, et al: Predominant mucosal
IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer.
World J Gastroenterol. 19:2941–2949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH,
Song SK and Kim HS: Relationship between urokinase-type plasminogen
receptor, interleukin-8 gene expression and clinicopathological
features in gastric cancer. Oncology. 66:210–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee KE, Khoi PN, Xia Y, Park JS, Joo YE,
Kim KK, Choi SY and Jung YD: Helicobacter pylori and
interleukin-8 in gastric cancer. World J Gastroenterol.
19:8192–8202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morishita A and Masaki T: miRNA in
hepatocellular carcinoma. Hepatol Res. 45:128–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun Z, Meng C, Wang S, Zhou N, Guan M, Bai
C, Lu S, Han Q and Zhao RC: MicroRNA-1246 enhances migration and
invasion through CADM1 in hepatocellular carcinoma. BMC Cancer.
14:6162014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu X, Cao L, Zhang Y, Lian H, Sun Z and
Cui Y: MicroRNA-1246 inhibits cell invasion and epithelial
mesenchymal transition process by targeting CXCR4 in lung cancer
cells. Cancer Biomark. 21:251–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Du P, Lai YH, Yao DS, Chen JY and Ding N:
Downregulation of microRNA-1246 inhibits tumor growth and promotes
apoptosis of cervical cancer cells by targeting thrombospondin-2.
Oncol Lett. 18:2491–2499. 2019.PubMed/NCBI
|
|
52
|
Sun LN, Zhi Z, Chen LY, Zhou Q, Li XM, Gan
WJ, Chen S, Yang M, Liu Y, Shen T, et al: SIRT1 suppresses
colorectal cancer metastasis by transcriptional repression of
miR-15b-5p. Cancer Lett. 409:104–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dong Y, Zhang N, Zhao S, Chen X, Li F and
Tao X: miR-221-3p and miR-15b-5p promote cell proliferation and
invasion by targeting Axin2 in liver cancer. Oncol Lett.
18:6491–6500. 2019.PubMed/NCBI
|
|
54
|
Tang T, Yang L, Cao Y, Wang M, Zhang S,
Gong Z, Xiong F, He Y, Zhou Y, Liao Q, et al: LncRNA AATBC
regulates Pinin to promote metastasis in nasopharyngeal carcinoma.
Mol Oncol. 14:2251–2270. 2020. View Article : Google Scholar : PubMed/NCBI
|