Introduction
Colorectal cancer (CRC) is one of the most
frequently occurring carcinomas, ranking third in terms of cancer
incidence and second terms of in cancer-related mortality worldwide
in 2020 (1). Globally, ~0.9 million
CRC-associated deaths are reported annually (2). The incidence of this disease has
increased over the past decades in a number of developing
countries, possibly owing to changes in lifestyle and aging
populations. Tumorigenesis is a complicated biological process
regulated by various factors, and requires timely and accurate
transport of functional molecules to their targets by motor
proteins. Kinesins, a group of motor proteins, act as transporters
along microtubules (MTs) (3) and have
vital roles in various physiological processes, such as protein
sorting and chromosome dynamics. Defects in kinesin function can
lead to dysregulated distribution of various proteins and
organelles within cells, and have been implicated in numerous
diseases, including left-right asymmetry, Alzheimer's disease and
amyotrophic lateral sclerosis (4–6). Studies
have also revealed a correlation between kinesins and CRC. For
example, elevated expression of kinesins positively influences
several clinical features, such as lymph node metastasis (7–11) and
tumor status (7,9,10,12–14), and
is relevant to the overall survival rate of patients with CRC
(7,9–13,15). The dysregulation of kinesins affects
cell proliferation, tumor formation and metastasis in CRC (7–18). Given
the importance of kinesins, the present review aims to illustrate
their roles in the prognosis of CRC and the potential underlying
regulatory mechanisms involved in CRC carcinogenesis, to provide
new targets for the clinical therapy of the disease.
Kinesins
Classification and structure
The kinesin superfamily in humans comprises 45
members classified into 14 families, Kinesin-1 to Kinesin-14,
according to their structural differences (19). Kinesin family members (KIFs) vary in
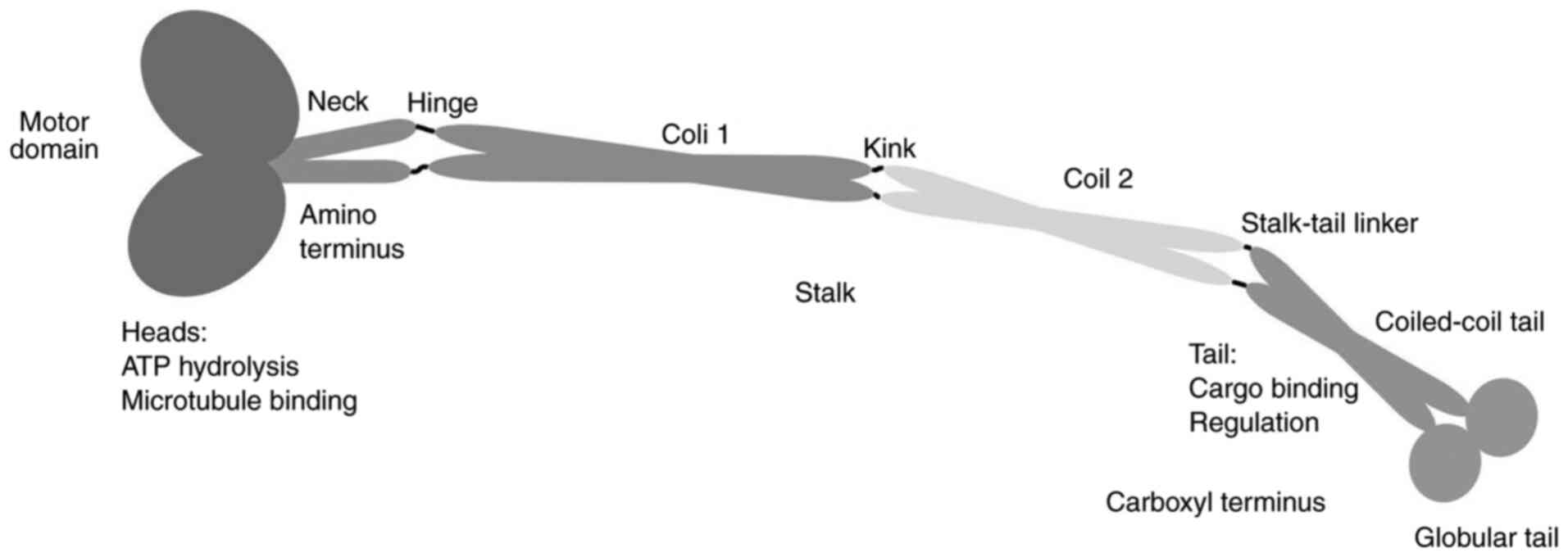
shape, but share three distinct domains, the head, stalk and tail,
which perform different functions during cargo transportation. The
head, which is an orbicular domain, is conserved among different
KIF members; this domain binds to the MT and regulates movement
through two binding sites: One binds the MT and is characterized by
a Rossman fold, while the other binds to ATP (20). There are three elements within the
ATP-binding site, Switch-I, Switch-II and the P-loop, which are
responsible for the hydrolysis of ATP and contribute to the
conformational changes of the MT-binding domain (21). The location of the head correlates
with the direction of kinesin movement along the MTs. The head
domain of Kinesin-1 to Kinesin-12 family members is found in the
NH2-terminal region and moves to the plus ends of MTs;
these are called the N-kinesins, whereas Kinesin-14A/B family
members are C-kinesins and contain the motor domain at the
COOH-terminus and the head for the minus end of the MTs. Kinesin-13
family members are M-kinesins, with the head domain situated in the
center of the molecule structure, and these are involved in
depolymerizing MTs (9).
In contrast to the conserved head domain, the amino
acid sequences in the stalk and tail domains are highly variable
(22). Most often, KIFs dimerize with
each other to form homo- or heterodimers through the stalk domain,
and the dimerization status is determined by the length of this
domain. KIFs can also function as monomers. KIFs bind with cargo
for its selective transport through the highly variable tail domain
and may be accompanied by light chain and/or associated proteins
(Fig. 1).
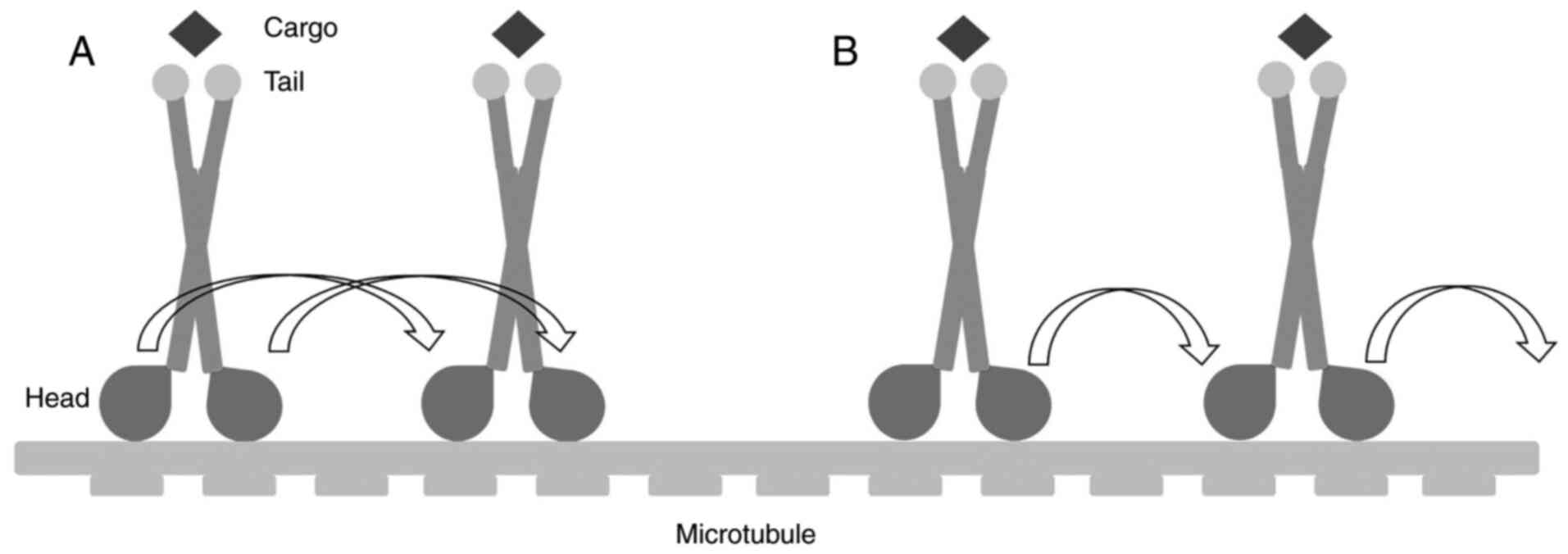
Two mechanisms have been suggested to describe the
movements of kinesins: The ‘hand-over-hand’ and ‘inchworm’ models
(23). In the ‘hand-over-hand’ model,
the front head alternates with the rear one on the MTs to forge
ahead, and every step consumes two molecules of ATP. In the
‘inchworm’ model, by contrast, the relative positions of the two
heads are maintained, enabling movements of 8 nm for the anterior
head (Fig. 2).
Biological activity of kinesins and
relevance to diseases
KIFs are responsible for the transport of cellular
organelles, functional proteins packed in vesicles and
macromolecules such as chromosomes to the required destinations;
thus, they are vital for protein sorting and appropriate
positioning of various biological molecules (24). Owing to these functions, KIFs are
involved in numerous diseases. Kinesin-1 has been reported to
interact with key molecules such as adenomatous polyposis coli
protein participating in the Wnt signaling pathway (25) and GluN2B, which regulates
N-methyl-D-aspartate receptor activity (26). Thus, dysregulated Kinesin-1
potentially causes neurological diseases (27). Selective knockout of KIF5B in
pancreatic β cells and adipocytes affects insulin and adipokine
secretion, which leads to metabolic disorders (28,29). For
organelle transport, KIF5B is found to regulate mitochondrial
localization and activity (30), and
contributes to pathological hypertrophic responses in
cardiomyocytes (31). Moreover,
during the cell cycle, kinesins are responsible for the formation
of the spindle apparatus and the alignment and detachment of
chromosomes (32). Modulations in KIF
activity could influence the cell cycle and alter the apoptosis
level of cells. The dysregulation of kinesins has also been
reported to affect cell multiplication and migration, with effects
on the carcinogenesis of various cancer types, including breast and
lung cancer (33,34).
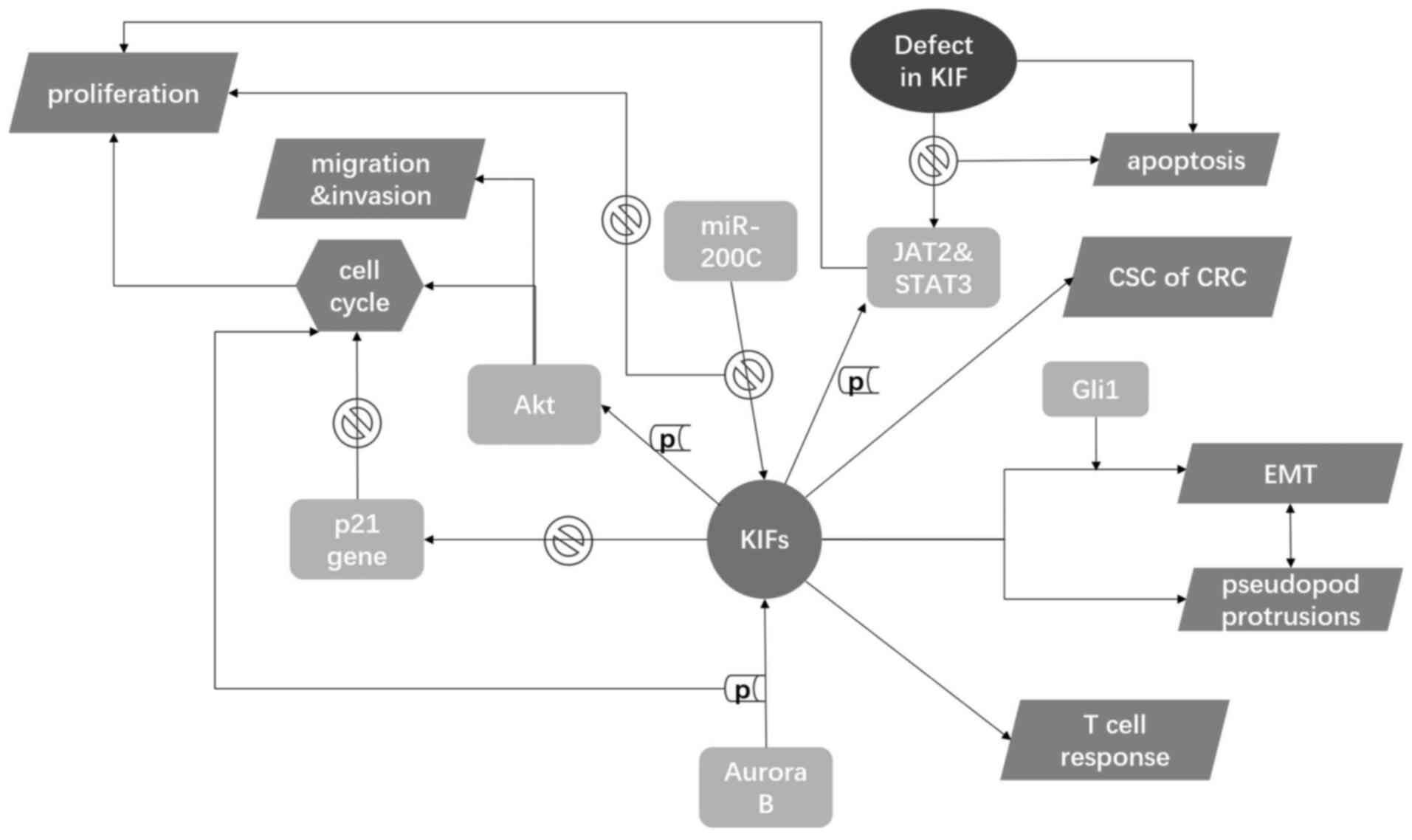
Kinesins in CRC
CRC occurs when epithelial cells in the colon and
rectum gain the ability for abnormal growth. To date, the
expression of 10 KIFs has been reported to be increased in CRC
tissue and has been associated with patient prognosis and CRC
metastasis (Table I). KIFs interact
with intracellular molecules to influence the activity of CRC
cells, with effects on proliferation, migration, invasion and the
immune reaction (Fig. 3).
 | Table I.Roles of various KIFs in colorectal
cancer. |
Table I.
Roles of various KIFs in colorectal
cancer.
| Family name | Kinesin | Kinesin type | Subcellular
localization | Reported roles in
CRC | Supposed
mechanisms | (Refs.) |
|---|
| Kinesin-3 | KIF14 | N-3 | Spindle, nucleus,
cytoplasm, midbody | Overexpressed in
CRC tissue. Upregulation is associated with CRC tumorigenesis. | Activates Akt and
expedites the cell cycle, which is negatively correlated with
miR-200c. | (17) |
| Kinesin-4 | KIF4A | N-5 | Spindle, nucleus
matrix, midbody, chromosome | Overexpressed in
CRC tissue. High expression leads to poor survival in human
patients with CRC and increased migration and invasion in CRC. | Negatively
modulates the promoter of p21 gene, which is downstream of the p53
gene and serves as a tumor suppressor regulating
G0/G1 arrest. | (8,9) |
| Kinesin-5 | KIF11 | N-2 | Spindle pole,
cytoplasm | Regulates cancer
cell stemness. | N/A | (16) |
| Kinesin-6 | KIF20A | N-6 | Spindle, Golgi
apparatus | Overexpressed in
CRC tissue. Elevated expression associated with poor survival,
increased cell proliferation and resistance to chemotherapy in
CRC. | N/A | (15) |
|
| KIF20B | N-6 | Nucleus, nucleolus,
nucleoplasm, centrosome, spindle, spindle pole, midbody, axon,
growth cone | Overexpressed in
CRC tissue. Associated with cancer metastasis and patient
prognosis. | Promotes the EMT
mediated by Gli1 and influences the formation of cell protrusions.
Activates the JAK/STAT3 pathway. | (12) |
| Kinesin-8 | KIF18A | N-8 | Centrosome,
nucleus, ruffle, cytoplasm | Overexpressed in
CRC tissue. Increased KIF18A expression is correlated with elevated
proliferation, migration and invasion of CRC cells. Loss of KIF18A
increases apoptosis. | Promotes CRC
progression and influences apoptosis by regulating Akt
signaling. | (10,51) |
| Kinesin-10 | KIF22 | N-8 | Nucleus,
cytoskeleton | Overexpressed in
colon cancer tissue. Elevated expression associated with tumor
grade and clinical stage. Suppression of KIF22 could repress cancer
cell proliferation and xenograft tumor growth. | N/A | (14) |
| Kinesin-11 | KIF26B | N-11 | Cytoskeleton,
cytoplasm | Overexpressed in
CRC tissue. High expression associated with tumor size, AJCC stage,
T stage, N stage and differentiation histology. An independent
prognostic factor of overall survival for patients with CRC. | N/A | (13) |
| Kinesin-13 | KIF2A | M | Centrosome,
spindle, pole, spindle, cytoplasm | Overexpressed in
CRC tissue. High expression associated with TNM stage and tumor
status, and indicates a poor prognosis in patients with CRC. | N/A | (7) |
|
| KIF2C/MCAK | M | Nucleus,
cytoskeleton, centromere, kinetochore | Overexpressed in
CRC tissue. Elevated expression associated with lymph node
metastasis, venous invasion, peritoneal dissemination, Dukes'
classification and the poor survival of patients with CRC. | Induces spontaneous
CD4(+) T-cell responses of the Th1-type. Aurora B phosphorylates
MCAK and regulates its catalytic ability, which influences the cell
cycle. | (11,18,62,65) |
Kinesin-3
The Kinesin-3 family is distinguished from
conventional kinesins by a peculiar neck region containing a
β-sheet and a helix (35), and by the
incorporation of a forkhead-associated domain in the tail region.
Kinesin-3 motors are essential for organelle transport and
cytokinesis, which suggest potential roles of kinesin-3 family
members in modulating the cell cycle (36).
KIF14, a member of the Kinesin-3 family, serves as a
mitotic kinesin and is encoded by a gene situated on chromosome
1q32.1 (37). In CRC, Wang et
al (17) found that elevated
KIF14 levels contributed to cell proliferation. Flow cytometry
results showed that high KIF14 expression could cause
G0/G1 arrest, indicating a role in the
modulation of the cell cycle. Additional experiments confirmed that
high KIF14 expression increased the phosphorylation of protein
kinase B (also known as Akt), thereby advancing the cell cycle to
promote tumorigenesis. This study also demonstrated that microRNA
such as miR-200c might directly bind to the 1402- to 1409-bp locus
of KIF14; thus, miR-200c could negatively regulate KIF14 function
to inhibit overgrowth of cells at the post-transcriptional
level.
Kinesin-4
Kinesin-4 motors participate in the transportation
of organelles and in chromosome dynamics, and are vital to the
regulation of cycle phase transitions during mitosis (38). In vertebrates, the Kinesin-4 family
has five members (KIF4, KIF7, KIF21, KIF27 and NcKIF21A) and KIF4A
plays a significant role in the cell cycle (32,38).
KIF4A forms part of the chromosome condensation and
separation machinery (38), and the
disordered function of KIF4A affects cell division and chromosome
integrity. Hou et al (9)
described the marked upregulation of KIF4A and its association with
the poor survival rate of patients with CRC. Using Transwell
assays, high expression of KIF4A increased the migration of CRC
cells and their invasion abilities. Given the role of KIF4A in
mitosis, cell cycle analysis was performed. The study showed an
accumulation of cells in the G0/G1 phase when
KIF4A expression was decreased. Subsequent assays confirmed that
KIF4A could accelerate the multiplication of cancer cells by
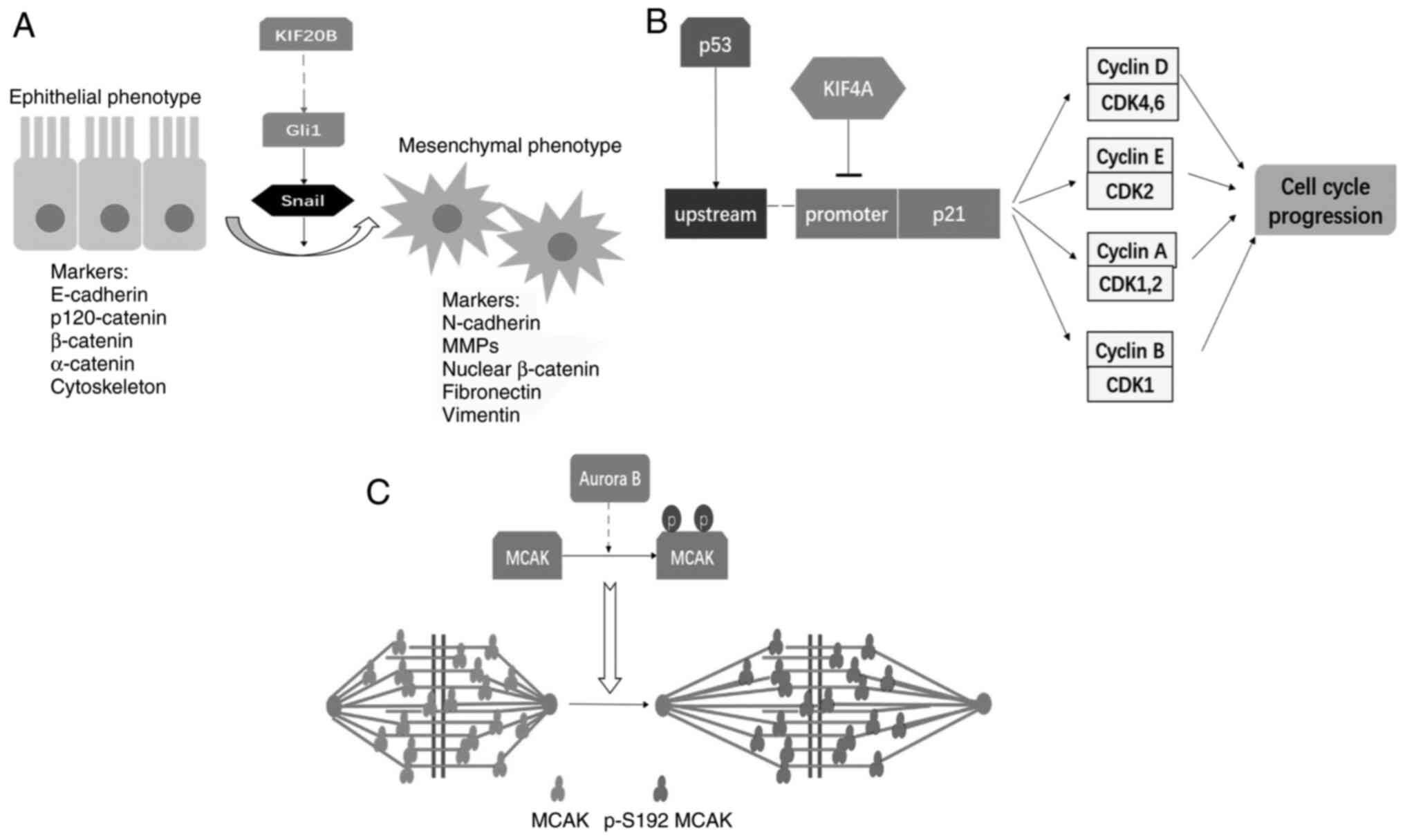
negatively modulating the promoter of the p21 gene (39). p21 is downstream of p53 and serves as
a tumor suppressor regulating G0/G1 arrest.
Matsumoto et al (8) showed
that KIF4A also affected the lymph node metastasis of CRC cells.
However, KIF4A was not involved in tumor status, venous invasion or
liver metastasis, and did not influence the overall survival
rate.
Kinesin-5
Homotetrameric kinesin-5 motors have vital roles in
the cell cycle via regulation of spindle formation (40). Thus, Kinesin-5 family members are
essential for cell growth and multiplication (41).
Cancer stem cells (CSCs) are involved in the
initiation of carcinoma cell growth and tumorigenesis (42). Imai et al (43) detected a significantly upregulated
level of KIF11 in gastric cancer (GC) and showed that KIF11 was
correlated with the activity of CSCs in GC. A subsequent study used
spheroid colony formation assays to measure how KIF11 expression
affected the stemness of CRC cells (16). In CRC cells, the KIF11 defect markedly
decreased the number and dimension of spheres, indicating an
important role of KIF11. However, phenotypic studies indicated that
there were no correlations between KIF11 and clinicopathological
characteristics.
Kinesin-6
Categorized as an N-type kinesin, Kinesin-6 family
members have a conventional structure and are involved in
cytokinesis and spindle arrangement (44). This family comprises three proteins,
KIF20A, KIF20B and KIF23, two of which have been reported to
correlate with CRC progression (12,15).
KIF20A is localized in the Golgi apparatus and is
responsible for the converse conveyance of Golgi membranes.
Upregulation of KIF20A has been observed in some malignant
carcinomas (45). With regard to CRC,
Xiong et al (15) demonstrated
that KIF20A was upregulated in CRC cells and promoted tumor growth
in vivo. From data extracted by the Gene Expression Omnibus
database (http://www.ncbi.nlm.nih.gov/geo/) and The Cancer
Genome Atlas (TCGA; http://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga),
it was found that elevated expression of KIF20A was associated with
the poor survival rate of patients with CRC. Furthermore, deficient
KIF20A expression could increase the apoptosis of cancer cells
after treatment with fluorouracil and oxaliplatin. By contrast, the
overexpression of KIF20A via transfection was associated with the
downregulation of apoptosis-related proteins, which provided
evidence that KIF20A affected the chemoresistance of CRC cells.
Notably, this study also showed that increased KIF20A expression
contributed to the increased phosphorylation of JAK2 and STAT3.
Interference with the JAK2/STAT3 pathway markedly prohibited CRC
cell proliferation and reversed the decrease in apoptosis of CRC
cells stimulated by the dysregulation of KIF20A. Thus, KIF20A was
proposed to promote CRC carcinogenesis by activating the JAK2/STAT3
signaling pathway.
Lin et al (12)
found that silencing KIF20B decreased the migration and invasion
abilities of cancer cells, and had effects on the expression of the
epithelial-mesenchymal transition (EMT)-associated transcription
factors Snail and Twist. Subsequent experiments showed that the
decrease in glioma-associated oncogene 1 (Gli1) expression was
associated with impaired KIF20B expression, while the
overexpression of Gli1 could relieve the loss of migration ability
of CRC cells resulting from the silencing of KIF20B. Gli1 can
activate EMT, thereby promoting cancer metastasis (46). Thus, KIF20B was speculated to
stimulate Gli1-mediated EMT. The latter experiments performed by
Lin et al (12) also revealed
the significance of KIF20B in the formation of cell pseudopod
protrusions and actin cytoskeleton dynamics. An evaluation of
clinical data found that KIF20B was associated with tumor status
and metastasis, and its high expression was correlated with the
poor overall survival rate of patients with CRC.
Kinesin-8
Kinesin-8 is indispensable for appropriate
allocation of chromosomes in several animal species (47,48).
Members of the Kinesin-8 family can modify spindle activity and
participate in the segregation of chromosomes (49).
A member of the Kinesin-8 family, KIF18A, serves as
an MT depolymerase and plays an important role in chromosome
agglutination (50). Nagahara et
al (10) showed that increased
KIF18A expression notably increased the proliferation, migration
and invasion abilities of CRC cells. Zhu et al (51) confirmed that overexpression of KIF18A
could accelerate tumor growth in chronic colitis. By contrast,
silencing the gene encoding KIF18A increased the apoptosis of tumor
cells, which was further confirmed by the observation of the
elevated expression of caspase-3 (51). Immunohistochemical assays showed that
the phosphorylation level of Akt was significantly decreased in
kif18a−/− dysplasia compared with that in the
wild-type (51). These data indicated
that KIF18A promotes CRC progression and influences apoptosis by
regulating Akt signaling.
Kinesin-10
The Kinesin-10 subfamily in humans has a single
member termed KIF22, which is also known as Kinesin-like 4
(KNSL4)/Kinesin-like DNA binding protein. KIF22 is a chromokinesin
that participates in regulating chromosome dynamics during mitosis
(52). KIF22 has two nuclear
localization sequences (53) and
possesses a helix-hairpin-helix DNA-binding motif feature, which
contributes to its activity as a transcription factor and the
regulation of gene expression (54).
The expression of KIF22 has been reported to be upregulated in CRC,
and is correlated with tumor stages, rather than with lymph node
metastasis or tumor differentiation (14). In addition, interference with KIF22
expression by short hairpin RNA inhibited cell proliferation in
vitro and xenograft tumor growth in in vivo models
(14). Since it has been reported
that KIF22 modulates the expression of CDC25C, a gene involved in
the regulation of CDK1 activity and control of mitosis (55), it is quite possible that KIF22
promotes CRC cell proliferation by regulating CDC25C/CDK1
activity.
Kinesin-11
Characterized by the presence of a divergent
catalytic core, Kinesin-11 proteins are distinct from other
kinesins and play a role in signal transduction pathways (56).
KIF26B, encoded by a gene situated on the chromosome
region lq44 (57), is located
downstream of the zinc finger protein Stall1. Wang et al
(13) identified a marked
upregulation of KIF26B in CRC cells and reported that the
expression of KIF26B was associated with tumor size, tumor status
and histological differentiation. Survival analyses showed that a
high expression level of KIF26B led to low overall survival rate
and was a biomarker for a poor prognosis in patients with CRC.
Further, defects in KIF26B expression induced a decrease in the
expression of cyclin D1 and attenuated CRC cell proliferation.
Kinesin-13
Members of the Kinesin-13 family function as MT
depolymerases, participating in the formation and separation of
cilia, as well as the regulation of axon development and
rehabilitation (58,59). The proteins of this family are M-type
kinesins and include KIF2A, KIF2B, KIF2C/MCAK and KIF24 (35).
KIF2A functions as a MT depolymerase. In CRC, Fan
et al (7) analyzed the
expression of KIF2A in various tissue samples and observed its
significant upregulation in cancer tissues compared with that in
normal tissues. Furthermore, KIF2A was correlated with TNM stage
and tumor status. Specifically, higher expression of KIF2A
correlated with later TNM stages and higher levels of lymph node
metastasis. However, no correlations were found between KIF2A
expression and preoperative carcinoembryonic antigen level,
histological type, tumor location or differentiation. Survival
analyses also demonstrated the potential value of KIF2A in
predicting a poor prognosis in patients with CRC.
MCAK, encoded by a gene on the chromosomal region
1p34.1 (60), catalyzes the
disassembly of MTs from both ends by modulation of mitotic kinases
(61). Ishikawa et al
(11) found that the overexpression
of MCAK mRNA expression could predict lymph node metastasis and
could be used as an independent predictor of a poor prognosis in
patients with CRC. Furthermore, Ritter et al (18) showed that Aurora B, a vital kinase
involved in modulating mitosis, could induce the
serine-192-mediated phosphorylation of MCAK to influence its
catalytic ability and affect tumor metastasis. Interfering with
MCAK phosphorylation led to interference with the transition from
prometaphase to metaphase and induced abnormalities in chromosome
dynamics. Bioinformatics analysis of a variety of single-nucleotide
polymorphisms showed that an E403K mutation could also affect MCAK
activity and is crucial for CRC progression (62).
NY-CO-58, which is identical to MCAK and KNSL6
(63), is classified as a tumor
antigen and interacts with IgG antibodies in patients with CRC
(64). Gnjatic et al (65) observed significant upregulation of
NY-CO-58/MCAK expression in CRC samples. Notably, NY-CO-58/MCAK
also seemed to influence tumor growth, based on the detection of
Ki-67 expression, and could stimulate spontaneous T-cell responses
consisting mainly of CD4+ T cell-mediated immune
reactions. Cytoplasmatic staining indicated that CD4+ T
cells stimulated by NY-CO-58/MCAK secreted Th1-type cytokines to
evoke an immune response, under the regulation of T regulatory
cells.
Summary and perspectives
As the main MT-dependent cellular transporters,
kinesins have been studied for decades and have been shown to be
involved in a number of diseases. Studies have shown that
dysregulated kinesin expression and function could contribute to
the tumorigenesis and metastasis of several cancer types, including
breast, lung and colon cancer. In CRC, expression levels of KIF14,
KIF18A, KIF20A, KIF4A, KIF20B and MCAK have been reported to be
associated with tumor progression and prognosis via the regulation
of cell survival, the cell cycle, EMT and MT dynamics (Figs. 4 and 5).
KIF11, KIF22, KIF26B and KIF2A also contribute to the development
of CRC carcinogenesis; however, the underlying mechanisms remain to
be identified.
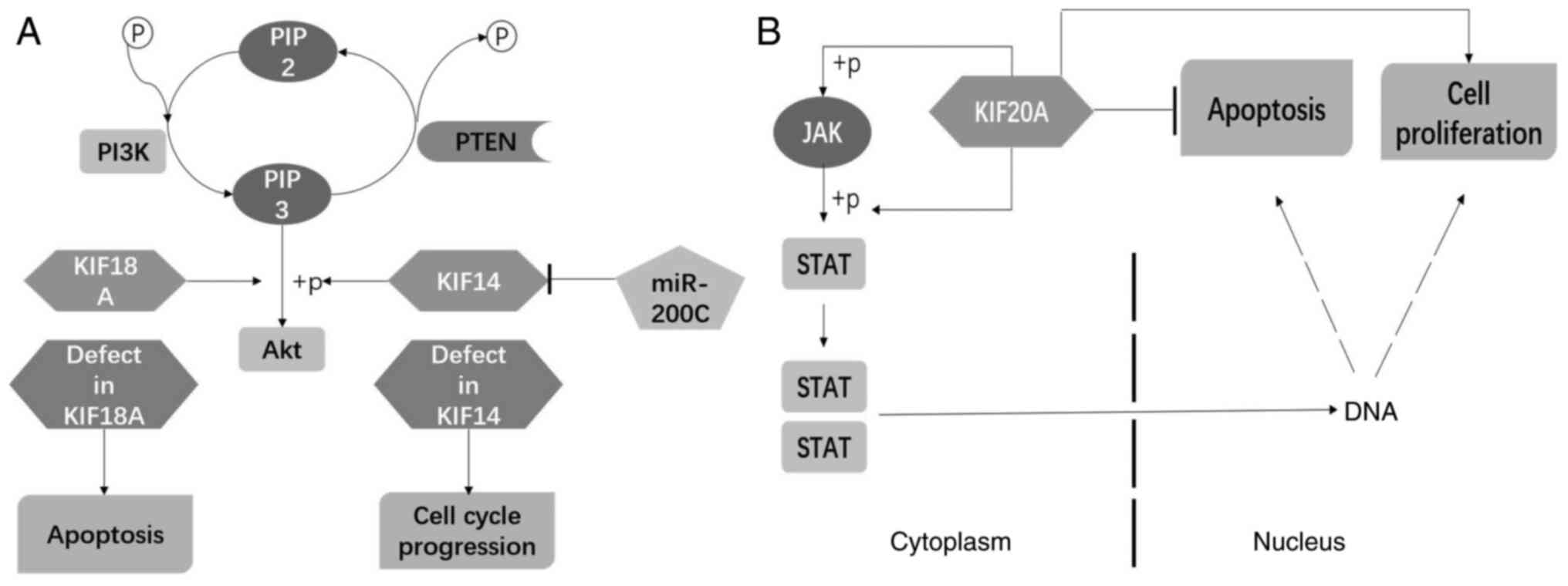
Recent studies have revealed that kinesins regulate
CRC cell survival via Akt and JAK2-STAT3 signaling pathways
(Fig. 4). Akt serves as a key
regulator of signal transduction in several classic pathways. In
the PI3K-Akt pathway, PI3K modulates the phosphorylation of Akt via
the induction of PIP3, stimulating a cascade able to activate
downstream signal molecules such as NF-κB and p53 to control cell
survival (66). Additional studies
have shown that KIF14 and KIF18A regulate CRC cell proliferation
and apoptosis by increased Akt signaling (Fig. 4A). With respect to the JAK2-STAT3
pathway, the activation of the expression of downstream genes such
as p53, Bcl-2 and Cyclin D1 is involved in regulating cell growth
and apoptosis (67). Xiong et
al (15) found that KIF20A
modulates the activity of JAK2 and STAT3 to affect CRC
tumorigenesis and chemoresistance (Fig.
4B). These studies have provided insight into the
KIF-associated signaling pathways that modulate tumor cell
proliferation and survival, but further studies are still needed to
verify the mechanisms involved.
Dysregulation of cell cycle-related factors can
trigger disorders in the cell cycle and cause impairments in cell
proliferation (68). p21 is a
well-known cyclin-dependent kinases inhibitor (CDKI) that regulates
the activities of the CDKs, including cyclin D/CDK4 or CDK6, cyclin
E/CDK2, cyclin A/CDK1 (Cdc2) or CDK2, and cyclin B/CDK1, to
modulate the cell cycle, serving as a tumor suppressor (69). Hou et al (9) found that the nuclear localization of
KIF4A results in its direct binding on the p21 promoter and
negative regulation of the expression of p21. Overexpression of
KIF4A in CRC has been reported to downregulate p21 expression,
which further enhances cell proliferation by promoting cell cycle
progression, suggesting that KIF4A-targeted therapy could be
effective in inhibiting CRC tumor growth (Fig. 5B).
Epithelial cells undergo EMT to gain migrative and
invasive capacity, favoring the metastasis of cancer cells from the
primary tumor site to other tissues or organs (Fig. 5A). Tumor metastasis is associated with
the aggravation of cancer and a poor prognosis for patients. Lin
et al (12) found that KIF20B
in pseudopod protrusions promoted cell invasion and metastasis by
regulating actin cytoskeleton dynamics, and that knockdown of
KIF20B downregulated Gli1 expression and decreased the expression
levels of EMT marker proteins. Thus, targeting KIF20B could be a
promising treatment approach in cancer therapy (12).
MTs, as components of the cytoskeleton, play crucial
roles in cellular transport and spindle dynamics. Furthermore, MCAK
acts as a MT depolymerase to regulate the assembly of the mitotic
apparatus and disassembly of the sister-chromosome to affect the
cell cycle via MT dynamics (70).
Overexpression of MCAK has been correlated with several cancer
types (71). Ritter et al
(18) showed that in CRC, serine 192
was the key site regulating MCAK catalytic ability induced by
Aurora B (Fig. 5C). Thus, the
regulation of phosphorylation at serine 192 might be a novel way to
modulate the activity of MCAK for cancer therapy.
In addition to regulating cell survival, the cell
cycle, EMT and MT dynamics, kinesins are also involved in
regulating CRC chemoresistance. Fluorouracil and oxaliplatin are
anticancer agents that have been used in clinical trials to treat
CRC (72,73). Xiong et al (15) found that the overexpression of KIF20A
could attenuate the increase in the BAX/BCL-2 ratio induced by
fluorouracil and oxaliplatin to protect cancer cells against
apoptosis. After knocking down KIF20A, CRC cells underwent
increased apoptosis. The contribution of KIF20A to chemoresistance
in CRC indicates that kinesins might play a vital role in the
clinical therapy of CRC.
Given that a considerable number of kinesins have
been found to promote tumorigenesis and growth in CRC, targeting
this family of motor proteins seems a promising approach for cancer
treatment. Several inhibitors against KIFs have already been tested
for their efficacy in the treatment of cancer: Ispinesib, AZD4877,
ARRY-520, SB-743921, ARQ 621, LY2523355, MK-0731, EMD534085 and
4SC-205 targeting Eg5; GSK923295 targeting CENPE (KIF10); peptide
targeting MPP1 (KIF20B); AZ82 and SR31527 targeting KIFC1; and
lidocaine and tetracaine targeting KIF5C (74,75). In
CRC, studies have reported novel agents targeting KIF11 (also known
as Eg5, a kinesin spindle protein), which can repress tumor
progression. Zhang et al (76)
identified SRI35566 as a new inhibitor that could interact directly
with Eg5. More importantly, SRI35566 could prevent drug resistance,
which is common among agents targeting monastrol-binding sites.
K858 was shown by Nakai et al (77) to interfere with centrosome separation
and cause cell cycle arrest, effectively eliminating cancer cells
without damaging MT activity. Considering the crucial roles of KIFs
in CRC, more studies are needed to explore the mechanisms by which
kinesins influence tumor progression, to provide insight into using
kinesins as biomarkers for prediction of CRC progression, and to
identify therapeutic targets for efficient treatment of CRC in the
future.
Acknowledgements
Not applicable.
Funding
This study was supported, in part, by the National
Natural Science Foundation of China (grant nos. 82073264), the
Beijing Natural Science Foundation (grant no. 7154234) and the
Beijing Hospital Nova Project (grant no. BJ-2016-034). The Shenzhen
Peacock project (grant no. KQTD2015033-117210153) also provided
support.
Availability of data and materials
Not applicable.
Authors' contributions
DH performed the analysis and wrote the manuscript.
HY contributed to the conception of the study. JDH contributed to
the conception of the study and revised the manuscript. JPC
contributed to the conception of the study. JC contributed to the
conception of the study, manuscript preparation and revision. All
authors have read and approved the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.(Epub ahead
of print). doi: 10.3322/caac.21660. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vale RD and Milligan RA: The way things
move: Looking under the hood of molecular motor proteins. Science.
288:88–95. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nonaka S, Tanaka Y, Okada Y, Takeda S,
Harada A, Kanai Y, Kido M and Hirokawa N: Randomization of
left-right asymmetry due to loss of nodal cilia generating leftward
flow of extraembryonic fluid in mice lacking KIF3B motor protein.
Cell. 95:829–837. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldstein LS: Kinesin molecular motors:
Transport pathways, receptors, and human disease. Proc Natl Acad
Sci USA. 98:6999–7003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morel M, Héraud C, Nicaise C, Suain V and
Brion JP: Levels of kinesin light chain and dynein intermediate
chain are reduced in the frontal cortex in Alzheimer's disease:
Implications for axoplasmic transport. Acta neuropathol. 123:71–84.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan X, Wang X, Zhu H, Wang W, Zhang S and
Wang Z: KIF2A overexpression and its association with
clinicopathologic characteristics and unfavorable prognosis in
colorectal cancer. Tumour Biol. 36:8895–8902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumoto Y, Saito M, Saito K, Kanke Y,
Watanabe Y, Onozawa H, Hayase S, Sakamoto W, Ishigame T, Momma T,
et al: Enhanced expression of KIF4A in colorectal cancer is
associated with lymph node metastasis. Oncol Lett. 15:2188–2194.
2018.PubMed/NCBI
|
|
9
|
Hou PF, Jiang T, Chen F, Shi PC, Li HQ,
Bai J and Song J: KIF4A facilitates cell proliferation via
induction of p21-mediated cell cycle progression and promotes
metastasis in colorectal cancer. Cell Death Dis. 9:4772018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagahara M, Nishida N, Iwatsuki M,
Ishimaru S, Mimori K, Tanaka F, Nakagawa T, Sato T, Sugihara K,
Hoon DS and Mori M: Kinesin 18A expression: Clinical relevance to
colorectal cancer progression. Int J Cancer. 129:2543–2552. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishikawa K, Kamohara Y, Tanaka F,
Haraguchi N, Mimori K, Inoue H and Mori M: Mitotic
centromere-associated kinesin is a novel marker for prognosis and
lymph node metastasis in colorectal cancer. Br J Cancer.
98:1824–1829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin WF, Lin XL, Fu SW, Yang L, Tang CT,
Gao YJ, Chen HY and Ge ZZ: Pseudopod-associated protein KIF20B
promotes Gli1-induced epithelial-mesenchymal transition modulated
by pseudopodial actin dynamic in human colorectal cancer. Mol
Carcinog. 57:911–925. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Cui F, Wang X, Xue Y, Chen J, Yu
Y, Lu H, Zhang M, Tang H and Peng Z: Elevated kinesin family member
26B is a prognostic biomarker and a potential therapeutic target
for colorectal cancer. J Exp Clin Cancer Res. 34:132015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Zhu FC, Yu SX, Liu SJ and Li BY:
Suppression of KIF22 inhibits cell proliferation and xenograft
tumor growth in colon cancer. Cancer Biother Radiopharm. 35:50–57.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong M, Zhuang K, Luo Y, Lai Q, Luo X,
Fang Y, Zhang Y, Li A and Liu S: KIF20A promotes cellular malignant
behavior and enhances resistance to chemotherapy in colorectal
cancer through regulation of the JAK/STAT3 signaling pathway. Aging
(Albany NY). 11:11905–11921. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imai T, Oue N, Sentani K, Sakamoto N,
Uraoka N, Egi H, Hinoi T, Ohdan H, Yoshida K and Yasui W: KIF11 is
required for spheroid formation by oesophageal and colorectal
cancer cells. Anticancer Res. 37:47–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang ZZ, Yang J, Jiang BH, Di JB, Gao P,
Peng L and Su XQ: KIF14 promotes cell proliferation via activation
of Akt and is directly targeted by miR-200c in colorectal cancer.
Int J Oncol. 53:1939–1952. 2018.PubMed/NCBI
|
|
18
|
Ritter A, Sanhaji M, Friemel A, Roth S,
Rolle U, Louwen F and Yuan J: Functional analysis of
phosphorylation of the mitotic centromere-associated kinesin by
Aurora B kinase in human tumor cells. Cell cycle. 14:3755–3767.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawrence CJ, Dawe RK, Christie KR,
Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV,
Hirokawa N, Howard J, et al: A standardized kinesin nomenclature. J
Cell Biol. 167:19–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirokawa N, Noda Y, Tanaka Y and Niwa S:
Kinesin superfamily motor proteins and intracellular transport. Nat
Rev Mol Cell Biol. 10:682–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woehlke G and Schliwa M: Walking on two
heads: The many talents of kinesin. Nat Rev Mol Cell Biol. 1:50–58.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sack S, Kull FJ and Mandelkow E: Motor
proteins of the kinesin family. Structures, variations, and
nucleotide binding sites. Eur J Biochem. 262:1–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hua W, Chung J and Gelles J:
Distinguishing inchworm and hand-over-hand processive kinesin
movement by neck rotation measurements. Science. 295:844–848. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vale RD: The molecular motor toolbox for
intracellular transport. Cell. 112:467–480. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruane PT, Gumy LF, Bola B, Anderson B,
Wozniak MJ, Hoogenraad CC and Allan VJ: Tumour suppressor
adenomatous polyposis Coli (APC) localisation is regulated by both
Kinesin-1 and Kinesin-2. Sci Rep. 6:274562016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin R, Duan Z, Sun H, Fung ML, Chen H,
Wang J, Lau CF, Yang D, Liu Y, Ni Y, et al: Kinesin-1 regulates
extrasynaptic targeting of NMDARs and neuronal vulnerability toward
excitotoxicity. iScience. 13:82–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirokawa N, Niwa S and Tanaka Y: Molecular
motors in neurons: Transport mechanisms and roles in brain
function, development, and disease. Neuron. 68:610–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui J, Wang Z, Cheng Q, Lin R, Zhang XM,
Leung PS, Copeland NG, Jenkins NA, Yao KM and Huang JD: Targeted
inactivation of kinesin-1 in pancreatic β-cells in vivo leads to
insulin secretory deficiency. Diabetes. 60:320–330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui J, Pang J, Lin YJ, Gong H, Wang ZH, Li
YX, Li J, Wang Z, Jiang P, Dai DP, et al: Adipose-specific deletion
of Kif5b exacerbates obesity and insulin resistance in a mouse
model of diet-induced obesity. FASEB J. 31:2533–2547. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka Y, Kanai Y, Okada Y, Nonaka S,
Takeda S, Harada A and Hirokawa N: Targeted disruption of mouse
conventional kinesin heavy chain, kif5B, results in abnormal
perinuclear clustering of mitochondria. Cell. 93:1147–1158. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tigchelaar W, de Jong AM, Bloks VW, van
Gilst WH, de Boer RA and Silljé HH: Hypertrophy induced KIF5B
controls mitochondrial localization and function in neonatal rat
cardiomyocytes. J Mol Cell Cardiol. 97:70–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu C, Zhao J, Bibikova M, Leverson JD,
Bossy-Wetzel E, Fan JB, Abraham RT and Jiang W: Functional analysis
of human microtubule-based motor proteins, the kinesins and
dyneins, in mitosis/cytokinesis using RNA interference. Mol Biol
Cell. 16:3187–3199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lukong KE and Richard S: Breast tumor
kinase BRK requires kinesin-2 subunit KAP3A in modulation of cell
migration. Cell Signal. 20:432–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corson TW, Zhu CQ, Lau SK, Shepherd FA,
Tsao MS and Gallie BL: KIF14 messenger RNA expression is
independently prognostic for outcome in lung cancer. Clin Cancer
Res. 13:3229–3234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miki H, Okada Y and Hirokawa N: Analysis
of the kinesin superfamily: Insights into structure and function.
Trends Cell Biol. 15:467–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Siddiqui N and Straube A: Intracellular
cargo transport by Kinesin-3 motors. Biochemistry (Mosc).
82:803–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Corson TW, Huang A, Tsao MS and Gallie BL:
KIF14 is a candidate oncogene in the 1q minimal region of genomic
gain in multiple cancers. Oncogene. 24:4741–4753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mazumdar M, Sundareshan S and Misteli T:
Human chromokinesin KIF4A functions in chromosome condensation and
segregation. J Cell Biol. 166:613–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karimian A, Ahmadi Y and Yousefi B:
Multiple functions of p21 in cell cycle, apoptosis and
transcriptional regulation after DNA damage. DNA Repair (Amst).
42:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Waitzman JS and Rice SE: Mechanism and
regulation of kinesin-5, an essential motor for the mitotic
spindle. Biol Cell. 106:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang M, Zhuang H, Xia R, Gan L, Wu Y, Ma
J, Sun Y and Zhuang Z: KIF11 is required for proliferation and
self-renewal of docetaxel resistant triple negative breast cancer
cells. Oncotarget. 8:92106–92118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takaishi S, Okumura T and Wang TC: Gastric
cancer stem cells. J Clin Oncol. 26:2876–2882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Imai T, Oue N, Nishioka M, Mukai S, Oshima
T, Sakamoto N, Sentani K, Matsusaki K, Yoshida K and Yasui W:
Overexpression of KIF11 in gastric cancer with intestinal mucin
phenotype. Pathobiology. 84:16–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cesario JM, Jang JK, Redding B, Shah N,
Rahman T and McKim KS: Kinesin 6 family member Subito participates
in mitotic spindle assembly and interacts with mitotic regulators.
J Cell Sci. 119:4770–4780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Imai K, Hirata S, Irie A, Senju S, Ikuta
Y, Yokomine K, Harao M, Inoue M, Tomita Y, Tsunoda T, et al:
Identification of HLA-A2-restricted CTL epitopes of a novel
tumour-associated antigen, KIF20A, overexpressed in pancreatic
cancer. Br J Cancer. 104:300–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang C, Wang Y, Feng Y, Zhang Y, Ji B,
Wang S and Sun Y, Zhu C, Zhang D and Sun Y: Gli1 promotes
colorectal cancer metastasis in a Foxm1-dependent manner by
activating EMT and PI3K-AKT signaling. Oncotarget. 7:86134–86147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pinder C, Matsuo Y, Maurer SP and Toda T:
Kinesin-8 and Dis1/TOG collaborate to limit spindle elongation from
prophase to anaphase A for proper chromosome segregation in fission
yeast. J Cell Sci. 132:jcs2323062019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Edzuka T and Goshima G: Drosophila
kinesin-8 stabilizes the kinetochore-microtubule interaction. J
Cell Biol. 218:474–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gardner MK, Odde DJ and Bloom K: Kinesin-8
molecular motors: Putting the brakes on chromosome oscillations.
Trends Cell Biol. 18:307–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mayr MI, Hümmer S, Bormann J, Grüner T,
Adio S, Woehlke G and Mayer TU: The human kinesin Kif18A is a
motile microtubule depolymerase essential for chromosome
congression. Curr Biol. 17:488–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu H, Xu W, Zhang H, Liu J, Xu H, Lu S,
Dang S, Kuang Y, Jin X and Wang Z: Targeted deletion of Kif18a
protects from colitis-associated colorectal (CAC) tumors in mice
through impairing Akt phosphorylation. Biochem Biophys Res Commun.
438:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Levesque AA and Compton DA: The
chromokinesin Kid is necessary for chromosome arm orientation and
oscillation, but not congression, on mitotic spindles. J Cell Biol.
154:1135–1146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tahara K, Takagi M, Ohsugi M, Sone T,
Nishiumi F, Maeshima K, Horiuchi Y, Tokai-Nishizumi N, Imamoto F,
Yamamoto T, et al: Importin-beta and the small guanosine
triphosphatase Ran mediate chromosome loading of the human
chromokinesin Kid. J Cell Biol. 180:493–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tokai N, Fujimoto-Nishiyama A, Toyoshima
Y, Yonemura S, Tsukita S, Inoue J and Yamamota T: Kid, a novel
kinesin-like DNA binding protein, is localized to chromosomes and
the mitotic spindle. EMBO J. 15:457–467. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yu Y, Wang XY, Sun L, Wang YL, Wan YF, Li
XQ and Feng YM: Inhibition of KIF22 suppresses cancer cell
proliferation by delaying mitotic exit through upregulating CDC25C
expression. Carcinogenesis. 35:1416–1425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou R, Niwa S, Homma N, Takei Y and
Hirokawa N: KIF26A is an unconventional kinesin and regulates
GDNF-Ret signaling in enteric neuronal development. Cell.
139:802–813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Orellana C, Roselló M, Monfort S, Oltra S,
Quiroga R, Ferrer I and Martínez F: Corpus callosum abnormalities
and the controversy about the candidate genes located in 1q44.
Cytogenet Genome Res. 127:5–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Miyamoto T, Hosoba K, Ochiai H, Royba E,
Izumi H, Sakuma T, Yamamoto T, Dynlacht BD and Matsuura S: The
Microtubule-Depolymerizing activity of a mitotic kinesin protein
KIF2A drives primary cilia disassembly coupled with cell
proliferation. Cell Rep. 10:664–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vasudevan KK, Jiang YY, Lechtreck KF,
Kushida Y, Alford LM, Sale WS, Hennessey T and Gaertig J:
Kinesin-13 regulates the quantity and quality of tubulin inside
cilia. Mol Biol Cell. 26:478–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wordeman L and Mitchison TJ:
Identification and partial characterization of mitotic
centromere-associated kinesin, a kinesin-related protein that
associates with centromeres during mitosis. J Cell Biol.
128:95–104. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Helenius J, Brouhard G, Kalaidzidis Y,
Diez S and Howard J: The depolymerizing kinesin MCAK uses lattice
diffusion to rapidly target microtubule ends. Nature. 441:115–119.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kumar A, Rajendran V, Sethumadhavan R and
Purohit R: Evidence of colorectal cancer-associated mutation in
MCAK: A computational report. Cell Biochem Biophys. 67:837–851.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Aoki S, Ohta K, Yamazaki T, Sugawara F and
Sakaguchi K: Mammalian mitotic centromere-associated kinesin
(MCAK): A new molecular target of sulfoquinovosylacylglycerols
novel antitumor and immunosuppressive agents. FEBS J.
272:2132–2140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Scanlan MJ, Welt S, Gordon CM, Chen YT,
Gure AO, Stockert E, Jungbluth AA, Ritter G, Jäger D, Jäger E, et
al: Cancer-related serological recognition of human colon cancer:
Identification of potential diagnostic and immunotherapeutic
targets. Cancer Res. 62:4041–4047. 2002.PubMed/NCBI
|
|
65
|
Gnjatic S, Cao Y, Reichelt U, Yekebas EF,
Nölker C, Marx AH, Erbersdobler A, Nishikawa H, Hildebrandt Y,
Bartels K, et al: NY-CO-58/KIF2C is overexpressed in a variety of
solid tumors and induces frequent T cell responses in patients with
colorectal cancer. Int J Cancer. 127:381–393. 2010.PubMed/NCBI
|
|
66
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers (Basel). 6:897–925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kramer HB, Lai CF, Patel H, Periyasamy M,
Lin ML, Feller SM, Fuller-Pace FV, Meek DW, Ali S and Buluwela L:
LRH-1 drives colon cancer cell growth by repressing the expression
of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res.
44:582–594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Braun A, Dang K, Buslig F, Baird MA,
Davidson MW, Waterman CM and Myers KA: Rac1 and Aurora A regulate
MCAK to polarize microtubule growth in migrating endothelial cells.
J Cell Biol. 206:97–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shimo A, Tanikawa C, Nishidate T, Lin ML,
Matsuda K, Park JH, Ueki T, Ohta T, Hirata K, Fukuda M, et al:
Involvement of kinesin family member 2C/mitotic
centromere-associated kinesin overexpression in mammary
carcinogenesis. Cancer Sci. 99:62–70. 2008.PubMed/NCBI
|
|
72
|
Yamada Y, Takahari D, Matsumoto H, Baba H,
Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y,
et al: Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab
versus S-1 and oxaliplatin plus bevacizumab in patients with
metastatic colorectal cancer (SOFT): An open-label,
non-inferiority, randomised phase 3 trial. Lancet Oncol.
14:1278–1286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lucanus AJ and Yip GW: Kinesin
superfamily: Roles in breast cancer, patient prognosis and
therapeutics. Oncogene. 37:833–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rath O and Kozielski F: Kinesins and
cancer. Nat Rev Cancer. 12:527–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang W, Zhai L, Lu W, Boohaker RJ,
Padmalayam I and Li Y: Discovery of novel allosteric Eg5 Inhibitors
through structure-based virtual screening. Chem Biol Drug Des.
88:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nakai R, Iida S, Takahashi T, Tsujita T,
Okamoto S, Takada C, Akasaka K, Ichikawa S, Ishida H, Kusaka H, et
al: K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor
agent, induces cell death in cancer cells. Cancer Res.
69:3901–3909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zong H, Carnes SK, Moe C, Walczak CE and
Ems-McClung SC: The far C-terminus of MCAK regulates its
conformation and spindle pole focusing. Mol Biol Cell.
27:1451–1464. 2016. View Article : Google Scholar : PubMed/NCBI
|