Introduction
Liver cancer (LC) was the third leading cause of
cancer-associated deaths worldwide in 2016, demonstrating an
increasing incidence rate (1).
Notably, >50% of LC cases occur in China (2). Liver resection or transplantation is
available for early stage LC, while for patients who have reached a
stage beyond curative surgery, systematic chemotherapy is the
primary treatment option (3).
Tyrosine kinase inhibitors (TKIs), such as sorafenib, have been
widely used as first-line chemotherapy treatments for LC (4). Cisplatin (CDDP) is another frontline
chemotherapeutic drug used for the treatment of LC (5); it can induce the apoptosis of cancer
cells via intercalating base pairs of DNA strands and inhibiting
DNA/RNA synthesis (6,7). However, chemoresistance is one of the
greatest challenges for the chemotherapeutic treatment of LC,
leading to limited therapy efficiency and a poor prognosis
(8). Therefore, it remains a priority
to investigate the mechanisms involved in chemotherapy resistance
to overcome this resistance and increase the efficacies of
treatments.
The dysregulation of the Hippo signaling pathway has
been reported in various types of cancer, including prostate,
ovarian, colon, liver, lung and pancreatic cancer (9). Yes-associated protein (YAP) is the core
component of the Hippo signaling pathway and is highly conserved
from the fruit fly (Drosophila) to mammals (10). The upregulation of YAP expression has
been reported in several types of human tumor, such as breast
cancer (11), and has been associated
with a poor prognosis of cancer progression in breast and lung
cancer (12–14). Previous studies have indicated that
the dysregulation of the YAP and Hippo signaling pathway is
involved in the chemoresistance of cancer cells; for example, YAP
promotes epithelial-mesenchymal transition and chemoresistance in
pancreatic cancer cells (15), and it
regulates cellular quiescence to modulate chemoresistance and
cancer relapse in colon cancer cells (16). However, whether YAP is involved in the
chemoresistance of LC remains to be determined. Therefore, the
present study aimed to investigate the potential roles of YAP in LC
chemoresistance.
Materials and methods
Cell culture
The human LC cells, HepG2, Huh-6 and Huh-7, were
purchased from the American Type Culture Collection. Cells were
cultured in DMEM supplemented with 10% FBS (both Gibco; Thermo
Fisher Scientific, Inc.) and maintained in a 5% CO2
incubator at 37°C.
To generate CDDP-resistant LC cells, cells were
treated with increasing concentrations of CDDP (Sigma-Aldrich;
Merck KGaA) over 6 months, with a final concentration of 1 µM, as
reported previously (17,18). The resistant cells were named
HepG2/CDDP, Huh6/CDDP and Huh7/CDDP, respectively.
Cell proliferation assay
Cells were plated and cultured in 96-well plates in
100 µl medium at a density of 1×103 cells/well.
Following treatment with increasing concentrations (0, 0.5, 1, 5,
10, 20 and 50 µM) of CDDP for 48 h at room temperature, 10 µl Cell
Counting Kit-8 (Abmole Bioscience Inc.) reagent was added to each
well and incubated at 37°C for 2 h. In order to evaluate the effect
of YAP, HepG2/CDDP and Huh-7/CDDP cells were pre-treated with or
without 4 µM verteporfin (VP; Sigma-Aldrich; Merck KGaA; cat. no.
SML0534) for 90 min at room temperature and then further treated
with increasing concentrations of CDDP (0, 0.5, 1, 5, 10, 20 and 50
µM) for 48 h at room temperature. In order to investigate whether
IL-6 and TGF-β were involved in YAP-regulated chemoresistance of LC
cells, HepG2/CDDP cells were pre-treated with 100 ng/ml anti-IL-6
(cat. no. MAB206-SP; R&D Systems, Inc.) or anti-TGF-β (cat. no.
BE0057; Bio X Cell) for 2 h at room temperature and then further
treated with increasing concentrations of CDDP (0, 0.5, 1, 5, 10,
20 and 50 µM) for 48 h at room temperature. Additionally,
HepG2/CDDP or Huh-7/CDDP cells were pre-treated with VP (4 µM)
combined with recombinant (r)IL-6 (100 ng/ml; cat. no.
206-IL-010/CF; R&D Systems, Inc.) or rTGF-β (100 ng/ml; cat.
no. 240-B-002/CF; R&D Systems, Inc.) for 2 h at room
temperature, and then further treated with increasing
concentrations of CDDP (0–20 µM) for 48 h at room temperature. The
absorbance was measured at 450 nm using a microplate reader
(ENSIGHT; PerkinElmer, Inc.) according to the manufacturer's
protocol. The cell viability was calculated as the percentage of
the viability of untreated control cells. Experiments were repeated
≥3 times.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and treated with DNase I (Promega Corporation) to remove the
DNA contamination. RNA (1 µg) was reverse transcribed into cDNA
using the cDNA Synthesis SuperMix (Beijing TransGen Biotech Co.,
Ltd.) according to the manufacturer's protocol. qPCR was
subsequently performed using the SYBR Premix Ex Taq II kit (Takara
Biotechnology Co., Ltd.) and a Bio-Rad CFX96 system (Bio-Rad
Laboratories, Inc.). The following primer sequences were used: YAP
forward, 5′-GGCATACACCTACTCAACTACGG-3′ and reverse,
5′-TGGGCGGTGTAGAATCAGAGTC-3′; precursor-YAP forward,
5′-CCGGCTTGCTCTTATCAAAC-3′ and reverse, 5′-GTCATCGCTTCCCAAACATT-3′;
IL-6 forward, 5′-ACTCACCTCTTCAGAACGAATTG-3′ and reverse,
5′-CCATCTTTGGAAGGTTCAGGTTG-3′; IL-10 forward,
5′-TCTCCGAGATGCCTTCAGCAGA-3′ and reverse,
5′-TCAGACAAGGCTTGGCAACCCA-3′; IL-12 forward,
5′-TGCCTTCACCACTCCCAAAACC-3′ and reverse,
5′-CAATCTCTTCAGAAGTGCAAGGG-3′; TNF-α forward,
5′-CTCTTCTGCCTGCTGCACTTTG-3′ and reverse,
5′-ATGGGCTACAGGCTTGTCACTC-3′; TGF-β forward,
5′-TACCTGAACCCGTGTTGCTCTC-3′ and reverse,
5′-GTTGCTGAGGTATCGCCAGGAA-3′; MALAT1 forward,
5′-AAAGCAAGGTCTCCCCACAAG-3′ and reverse,
5′-GGTCTGTGCTAGATCAAAAGGCA-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG
3′. The PCR cycling conditions were 15 min at 95°C, followed by 40
cycles for 10 sec at 95°C, 30 sec at 60°C and 1 sec at 72°C, and 1
cycle of cooling for 30 sec at 50°C.
To analyze the expression levels of miRNAs, the
TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to generate cDNA according
to the manufacturer's protocol. The thermocycling conditions
included an initial denaturation at 95°C for 3 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 30 sec. The forward primer
is the exact sequence of the mature miRNA (http://www.mirbase.org/search.shtml). The forward
primer for U6 was 5′-TGCGGGTGCTCGCTTCGCAGC-3′. The reverse primer
was supplied by the aforementioned kit. GAPDH and U6 were used as
the internal reference genes for the normalization of mRNA and
miRNA, respectively. The gene expression levels were quantified
using the 2−ΔΔCq method (19). Each sample was analyzed in
triplicate.
Subcellular fractionation
The cytoplasmic and nuclear fractions of cells were
prepared using the PARIS™ kit (Ambion; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The protein
expression levels within the cytoplasmic and nuclear fractions were
analyzed by western blotting. Aliquots of cytoplasmic and nuclear
fractions were also subjected to RNA isolation and RT-qPCR, as
aforementioned, to analyze the subcellular localization of YAP
mRNA. Transcripts of the housekeeping gene GAPDH were used for
normalization, while nuclear MALAT1 RNA was selected as endogenous
control for the nuclear RNA.
Western blotting
Total protein was extracted from cells using 1X RIPA
lysis buffer (50 mM Tris HCl, 150 mM NaCl and 1 mM EDTA) containing
a protease inhibitor cocktail (Roche Diagnostics). Total protein
was quantified using a bicinchoninic acid assay kit and 20 µg
protein/lane was separated by 10% SDS-PAGE. The separated proteins
were subsequently transferred onto a nitrocellulose membrane (EMD
Millipore) using a wet transfer apparatus. The membranes were
blocked with 5% skimmed milk at room temperature for 2 h. Following
the incubation with the primary antibodies at 4°C overnight, the
membranes were further incubated with the HRP-conjugated secondary
antibody (cat. no. ab7090; Abcam; 1:10,000) diluted in 5% skimmed
milk. Protein bands were then visualized in a gel imaging system
(MG8600; Bio-Rad Laboratories, Inc.). The following primary
antibodies (1:1,000; Abcam) were used: Anti-H2A.X (cat. no.
ab229914), anti-YAP (cat. no. ab56701), anti-TAZ (cat. no.
ab84927), anti-calpain (cat. no. ab39170) and anti-GAPDH (cat. no.
ab229914). GAPDH was used as the loading control for normalization.
The gray values were analyzed using ImageJ software (version 1.46;
National Institutes of Health).
Cell transfection and treatment
The small interfering RNA (siRNA/si) negative
control (si-NC; 5′-GCACAACAAGCCGAAUACA-3′), si-YAP (siYAP-1,
5′-GCGUAGCCAGUUACCAACA-3′; siYAP-2, 5′-CAGUGGCACCUAUCACUCU-3′),
miRNA control (miR, 5′-UUCUCCGAACGUGUCACGUTT-3′) and miR-375 mimics
(5′-UUUGUUCGUUCGGCUCGCGUGA-3′) were synthesized by Shanghai
GenePharma Co., Ltd.. Upon cells reaching 50–60% confluence, the
transfection was performed using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions with 20 µM of each construct or siRNA.
After transfection for 6 h at 37°C, the medium was replaced with
fresh complete medium. To investigate the effect of YAP on
chemosensitivity, HepG2/CDDP, Huh-6/CDDP and Huh-7/CDDP cells were
transfected with si-NC or si-YAP-1 for 12 h and then further
treated with increasing concentrations of CDDP (0, 0.5, 1, 5, 10,
20 and 50 µM) for 48 h.
mRNA and protein stability assay
To determine the mRNA stability, cells were treated
with 5 µg/ml actinomycin D (Act-D; cat. no. A9415; Sigma-Aldrich;
Merck KGaA) at 37°C for 0, 2, 4 or 8 h. Subsequently, total RNA was
collected and the target mRNA was analyzed using RT-qPCR, as
aforementioned. For the protein stability assay, cells were
incubated with 100 µg/ml cycloheximide (CHX) at 37°C for 0, 2, 6 or
12 h and then protein expression was analyzed using western
blotting, as aforementioned.
Immunofluorescence
Cells cultured on coverslips were washed with PBS
and fixed in 4% paraformaldehyde for 15 min at room temperature.
After blocking with 3% BSA in PBS containing 0.3% Triton X-100
solution at 37°C for 1 h, cells were incubated with a primary
antibody against YAP (cat. no. ab56701; 1:1,000; Abcam) overnight
at 4°C and then treated with an anti-Alexa Fluor 594 secondary
antibody (1:200; R&D Systems China Co., Ltd.; cat. no. IC1420T)
for 1 h at room temperature. Then, DAPI solution (5 µg/ml) was
added to stain the cell nuclei for 5 min at room temperature. The
fluorescence signal was observed under a confocal microscope
(TCS-SP5; Leica Microsystems GmbH; magnification, ×10).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc.) and presented as the mean ± SD. The
comparisons between two groups were analyzed using an unpaired
Student's t-test. All experiments were performed ≥3 times
independently. P<0.05 was considered to indicate a statistically
significant difference.
Results
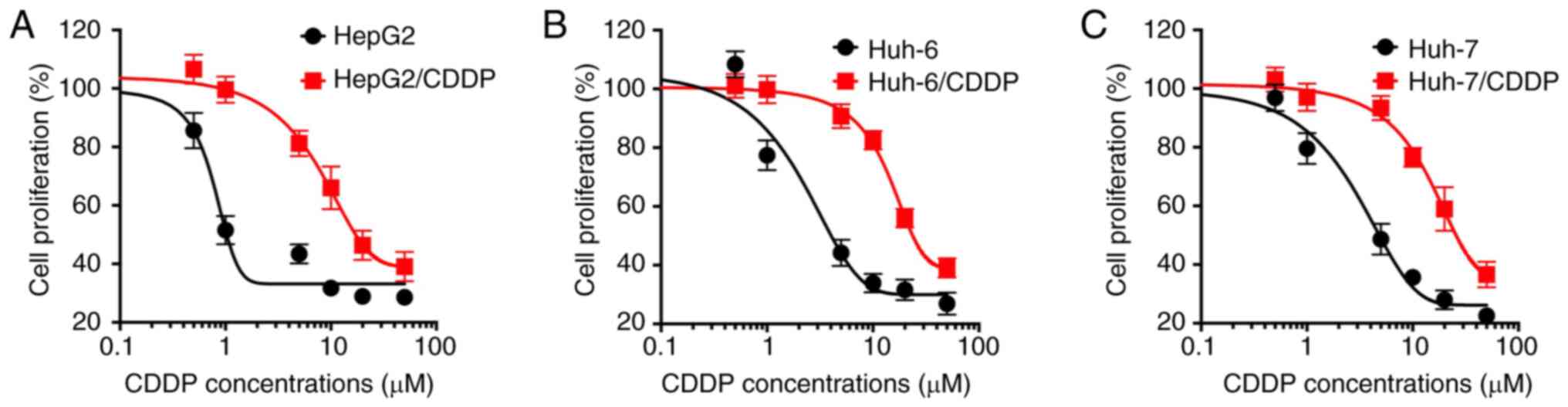
Establishment of LC/CDDP cells
The CDDP sensitivity of both resistant and parental
LC cells was investigated. The results revealed that the
established CDDP-resistant cells were more resistant to CDDP
treatment compared with their corresponding parental cells
(Fig. 1). The IC50 values
of CDDP for HepG2/CDDP and HepG2 cells were 22.8 and 3.45 µM,
respectively (Fig. 1A), those for
Huh-6/CDDP and Huh-6 cells were 30.6 and 5.05 µM, respectively
(Fig. 1B), while the IC50
values of CDDP for Huh-7/CDDP and Huh-7 cells were 30.5 and 6.51
µM, respectively (Fig. 1C). The
current data confirmed the successful establishment of LC/CDDP
cells.
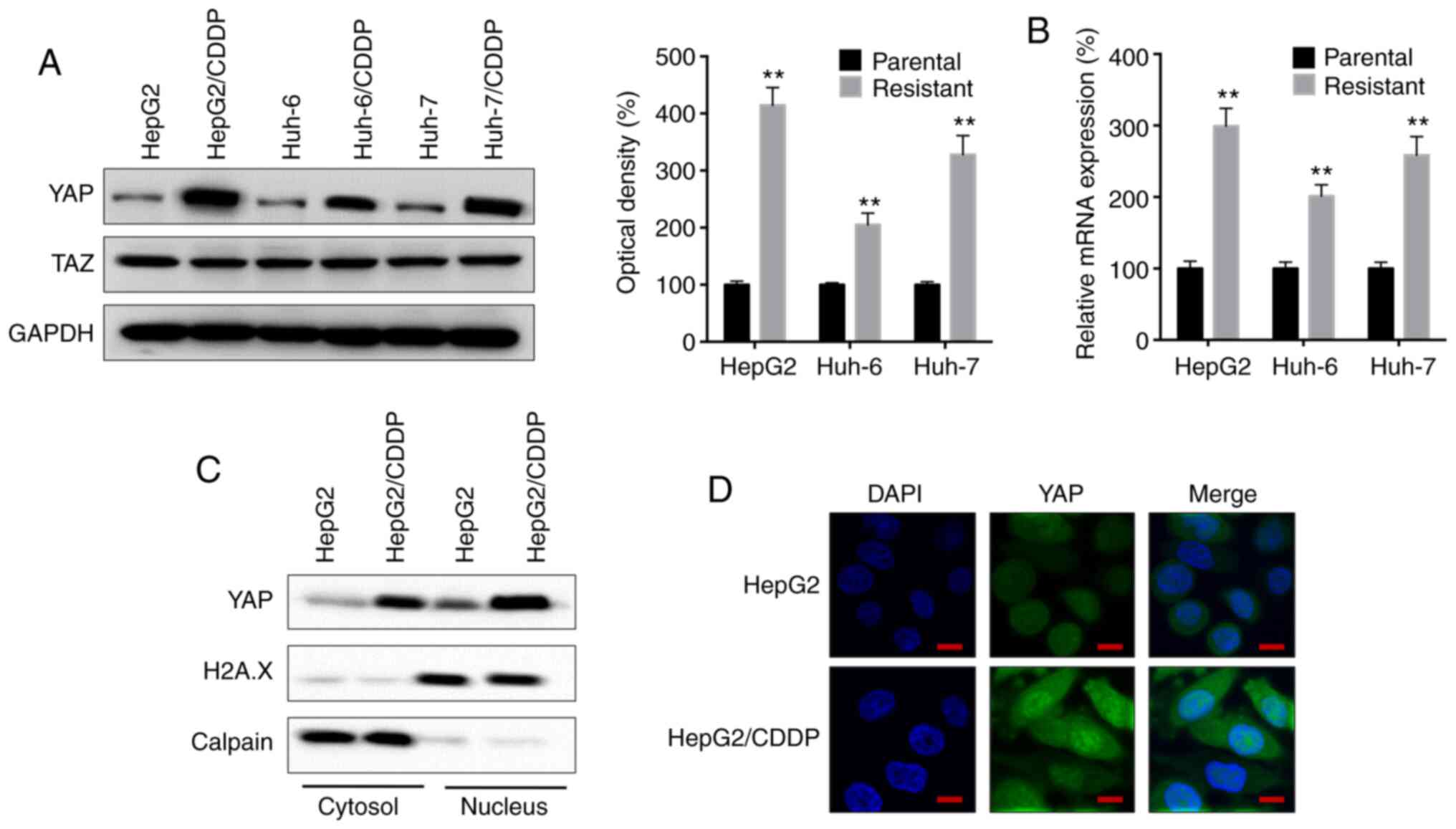
YAP expression is upregulated in
CDDP-resistant LC cells
It has been previously reported that the Hippo
signaling pathway regulates the progression of LC (20). Thus, the present study analyzed the
expression levels of YAP and the transcriptional coactivator with
PDZ-binding motif (TAZ), another important member of the Hippo
signaling pathway (20), in both
parental and CDDP-resistant LC cells. The protein expression levels
of YAP, but not TAZ, were significantly upregulated in the
HepG2/CDDP, Huh-6/CDDP and Huh-7/CDDP cells compared with in their
corresponding parental cells (Fig.
2A). Furthermore, RT-qPCR analysis revealed that the mRNA
expression levels of YAP were significantly upregulated in the
CDDP-resistant LC cells compared with in their respective parental
cells (Fig. 2B). In addition, the
amount of YAP localized in both the cytosol and nucleus was
increased in HepG2/CDDP cells compared with in HepG2 cells
(Fig. 2C), which was confirmed by
immunofluorescence staining (Fig.
2D).
YAP is involved in the CDDP resistance
of LC cells
To investigate whether YAP was involved in the
resistance to CDDP in LC cells, the CDDP-resistant LC cells were
transfected with si-YAP-1 and si-YAP-2 (Fig. 3A). si-YAP-1 was used for subsequent
experiments since it displayed increased efficiency. The results
revealed that si-YAP-1 markedly increased the CDDP sensitivity of
HepG2/CDDP (Fig. 3B), Huh-6/CDDP
(Fig. 3C) and Huh-7/CDDP (Fig. 3D) cells. Since the results revealed
that YAP expression was markedly increased in HepG2/CDDP and
Huh-7/CDDP cells, these cell lines were further treated with VP, a
suppressor of the YAP-TEAD complex (21). VP increased the sensitivity of CDDP in
HepG2/CDDP (Fig. 3E) and Huh-7/CDDP
(Fig. 3F) cells.
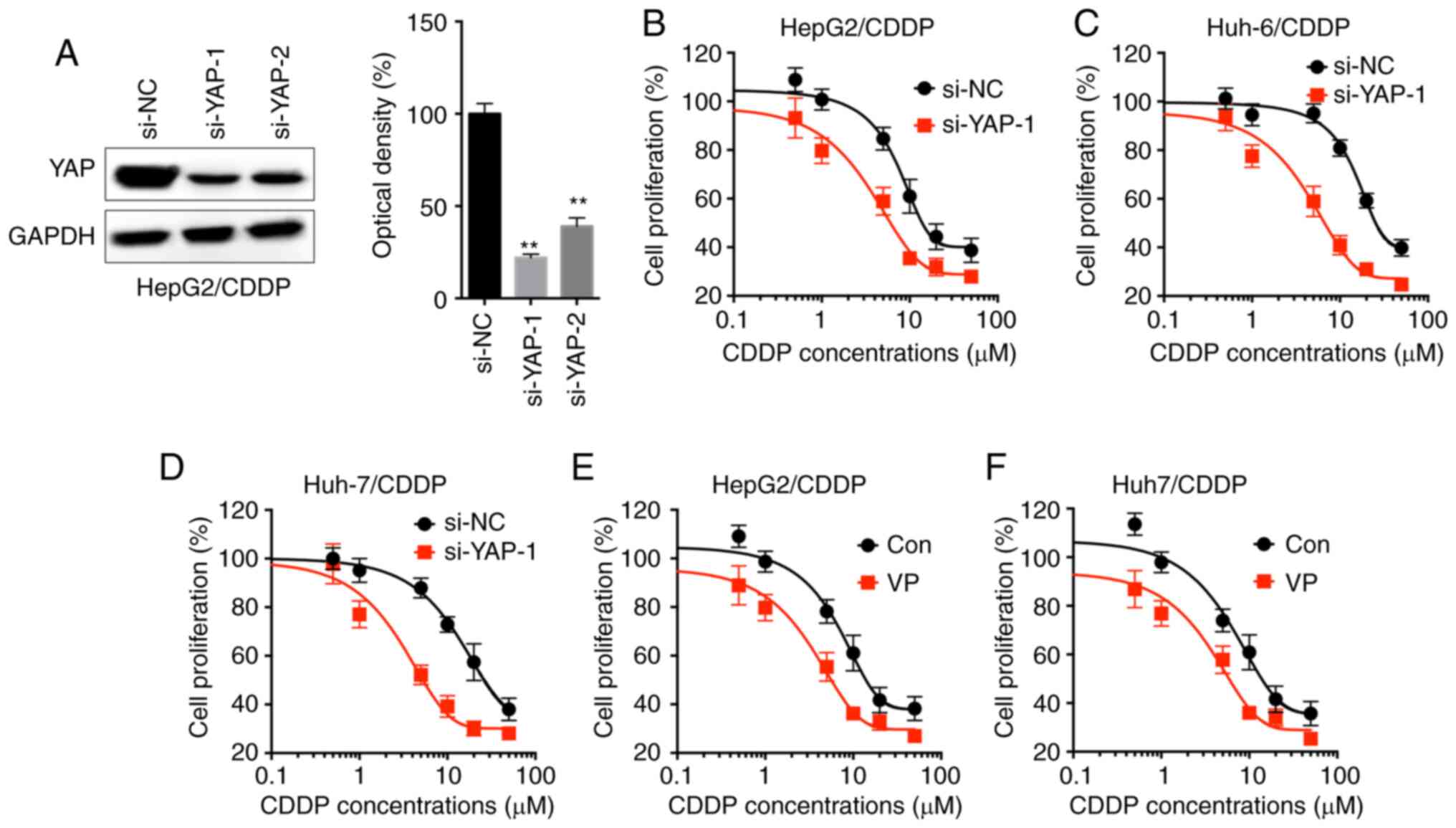 | Figure 3.YAP is involved in the CDDP
resistance of LC cells. (A) HepG2/CDDP cells were treated with
si-NC or si-YAP-1/2 for 24 h, and YAP expression was analyzed by
western blot analysis. Cell proliferation of (B) HepG2/CDDP, (C)
Huh-6/CDDP and (D) Huh-7/CDDP cells transfected with si-NC or
si-YAP-1 for 12 h and then further treated with increasing
concentrations of CDDP (0, 0.5, 1, 5, 10, 20 and 50 µM) for 48 h.
Cell proliferation of (E) HepG2/CDDP and (F) Huh-7/CDDP cells
pre-treated with or without 4 µM VP for 90 min and then further
treated with increasing concentrations of CDDP (0, 0.5, 1, 5, 10,
20 and 50 µM) for 48 h. Data are presented as the mean ± SD of
three independent experiments. **P<0.01 vs. si-NC. LC, liver
cancer; CDDP, cisplatin; LC/CDDP cells, CDDP-resistant LC cells;
YAP, Yes-associated protein; si-NC, siRNA negative control; VP,
verteporfin; Con, control. |
YAP regulates the expression levels of
IL-6 and TGF-β in LC/CDDP cells
It has been previously reported that YAP regulates
the expression levels of various cytokines to regulate cancer
progression (12–14). In the present study, an array of
cytokines was analyzed, including IL-6, IL-10, IL-12, TNF-α and
TGF-β, in si-YAP-1-transfected LC/CDDP cells. si-YAP-1
significantly downregulated the expression levels of IL-6 and TGF-β
in both HepG2/CDDP (Fig. 4A) and
Huh-7/CDDP (Fig. 4B) cells. In
addition, VP treatment significantly downregulated the expression
levels of IL-6 and TGF-β in both HepG2/CDDP (Fig. 4C) and Huh-7/CDDP (Fig. 4D) cells. On the other hand, the
expression levels of IL-6 and TGF-β in both HepG2/CDDP (Fig. 4E) and Huh-7/CDDP (Fig. 4F) cells were significantly upregulated
compared with in their corresponding control cells. The current
results suggested that YAP may regulate the expression levels of
IL-6 and TGF-β in LC/CDDP cells.
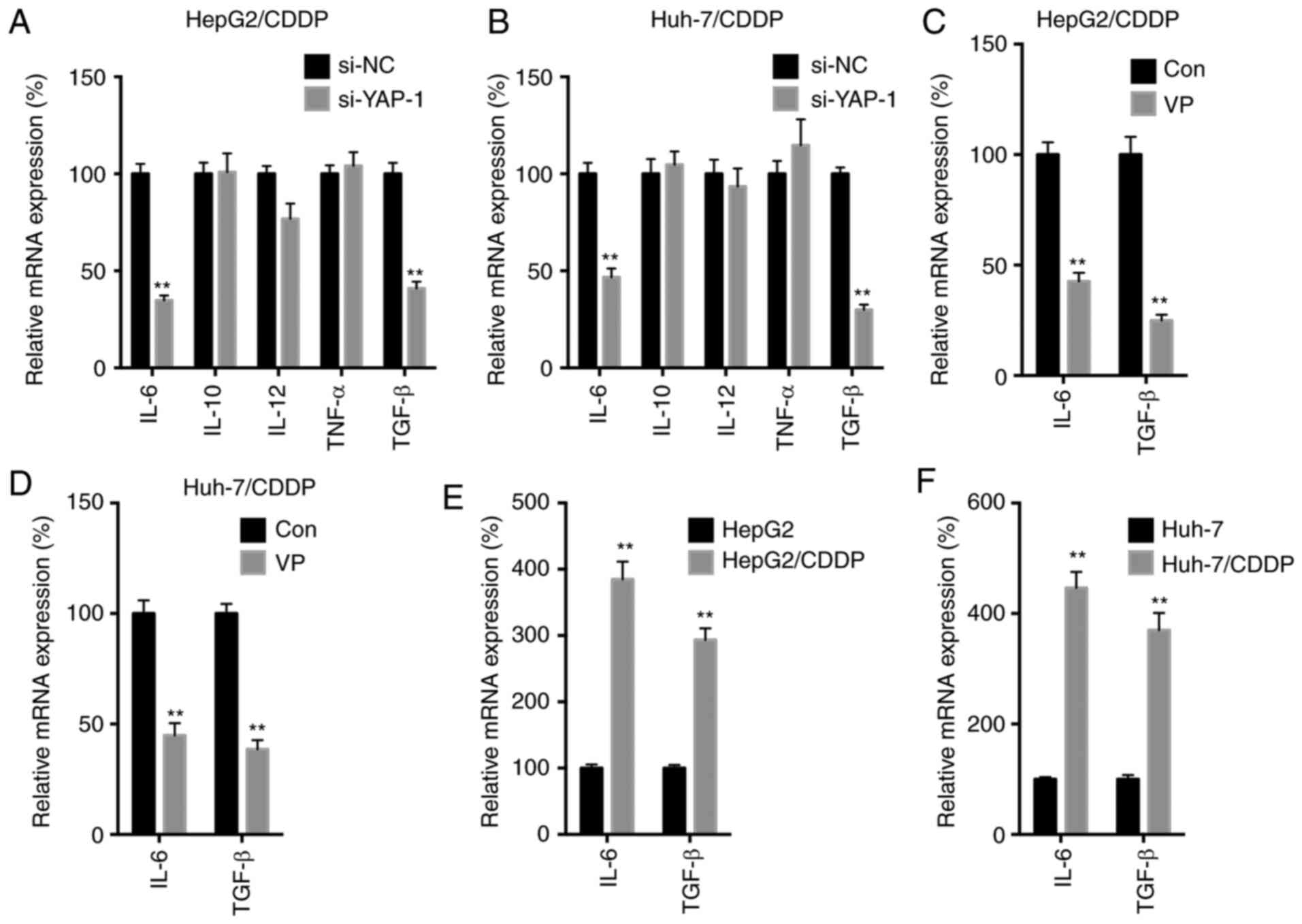 | Figure 4.YAP regulates the expression levels
of IL-6 and TGF-β in LC/CDDP cells. (A) HepG2/CDDP or (B)
Huh-7/CDDP cells were transfected with si-NC or si-YAP-1 for 24 h,
and the mRNA expression levels of different cytokines were measured
by RT-qPCR. (C) HepG2/CDDP or (D) Huh-7/CDDP cells were treated
with or without 4 µM VP for 24 h, and the mRNA expression levels of
IL-6 and TGF-β were measured by RT-qPCR. IL-6 and TGF-β expression
in (E) HepG2/CDDP and (F) Huh-7/CDDP cells and their corresponding
parental cells were measured by RT-qPCR. Data are presented as the
mean ± SD of three independent experiments. **P<0.01 vs. si-NC,
Con or parental cells. LC, liver cancer; CDDP, cisplatin; LC/CDDP
cells, CDDP-resistant LC cells; YAP, Yes-associated protein; si-NC,
siRNA negative control; VP, verteporfin; Con, control; RT-qPCR,
reverse transcription-quantitative PCR. |
IL-6 and TGF-β are involved in the
YAP-mediated chemoresistance of LC cells
The current study further analyzed whether IL-6 and
TGF-β were involved in the YAP-mediated chemoresistance of LC
cells. The data demonstrated that neutralization antibodies
anti-IL-6 (Fig. 5A) and anti-TGF-β
(Fig. 5B) significantly increased the
CDDP sensitivity of HepG2/CDDP cells. In addition, rIL-6 (Fig. 5C) and rTGF-β (Fig. 5D) significantly attenuated the
VP-induced CDDP sensitivity of HepG2/CDDP cells. All these data
indicated that IL-6 and TGF-β may be involved in the YAP-mediated
chemoresistance of LC cells.
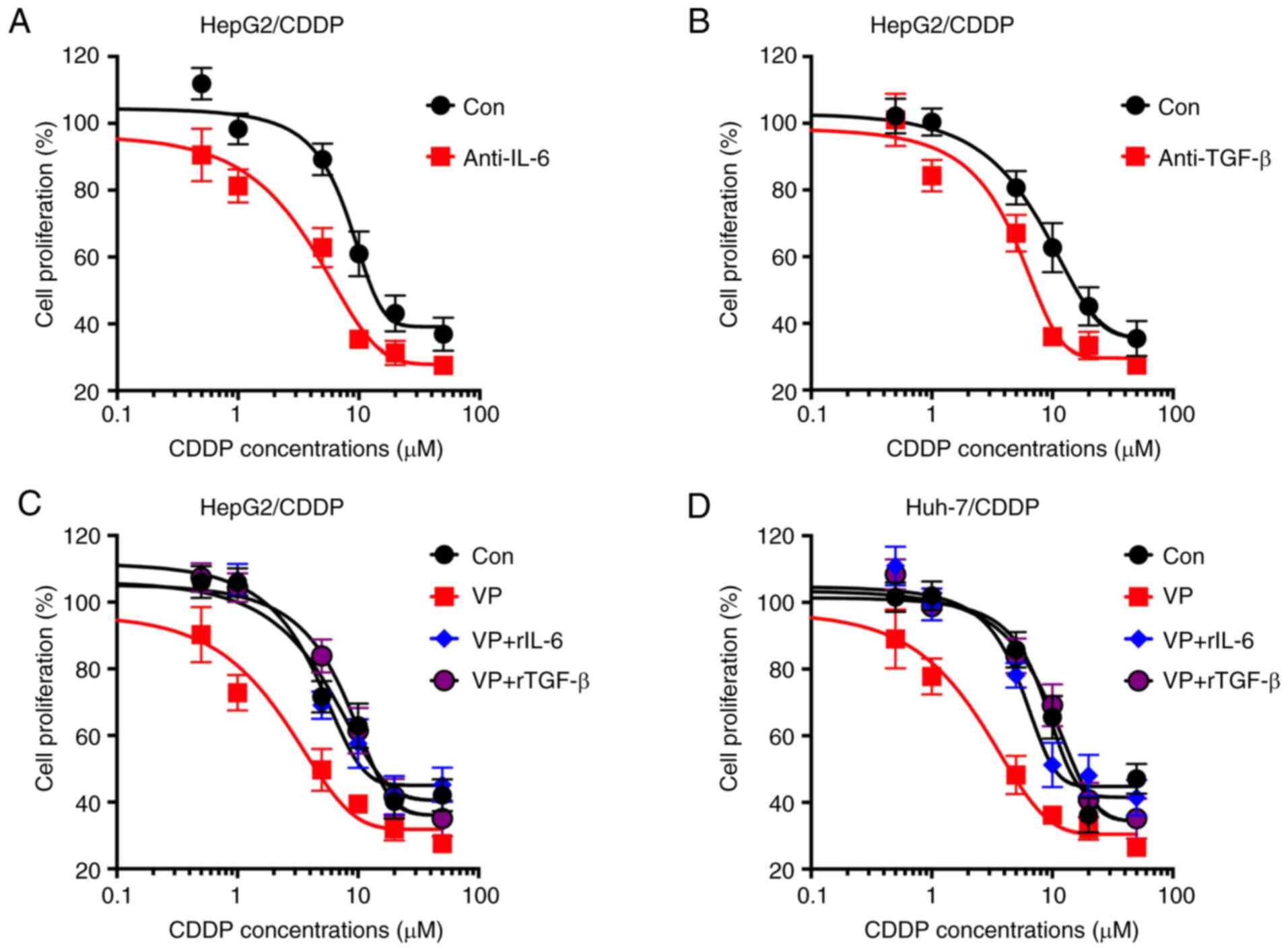 | Figure 5.IL-6 and TGF-β are involved in
YAP-regulated chemoresistance of LC cells. Cell proliferation of
HepG2/CDDP cells pre-treated with 100 ng/ml (A) anti-IL-6 or (B)
anti-TGF-β for 2 h and then further treated with increasing
concentrations of CDDP (0, 0.5, 1, 5, 10, 20 and 50 µM) for 48 h.
Cell proliferation of (C) HepG2/CDDP or (D) Huh-7/CDDP cells
pre-treated VP (4 µM) combined with rIL-6 (100 ng/ml) or rTGF-β
(100 ng/ml) for 2 h, and then further treated with increasing
concentrations of CDDP (0, 0.5, 1, 5, 10, 20 and 50 µM) for 48 h.
Data are presented as the mean ± SD of three independent
experiments. LC, liver cancer; CDDP, cisplatin; LC/CDDP cells,
CDDP-resistant LC cells; YAP, Yes-associated protein; VP,
verteporfin; Con, control; r, recombinant. |
mRNA stability is responsible for the
upregulation of YAP expression in LC/CDDP cells
The potential mechanisms responsible for the
upregulation of YAP expression in LC/CDDP cells were subsequently
investigated. The protein stability of YAP in HepG2 and HepG2/CDDP
cells following CHX treatment was similar to each other (Fig. 6A). Additionally, the expression levels
of the precursor mRNA of YAP, analyzed by RT-qPCR, were not
significantly different between HepG2 and HepG2/CDDP cells or
between Huh-7 and Huh-7/CDDP cells (Fig.
6B). In addition, the nuclear turnover rate of YAP was not
significantly different between HepG2 and HepG2/CDDP cells, as
analyzed by RT-qPCR (Fig. 6C).
However, the data revealed that the mRNA stability of YAP in
HepG2/CDDP cells following Act-D treatment was markedly increased
compared with in HepG2 cells (Fig.
6D). Consistently, the mRNA stability of YAP in Huh-7/CDDP
cells was also increased compared with in Huh-7 cells (Fig. 6E). These results indicated that
increased mRNA stability may be responsible for the upregulation of
YAP expression in LC/CDDP cells.
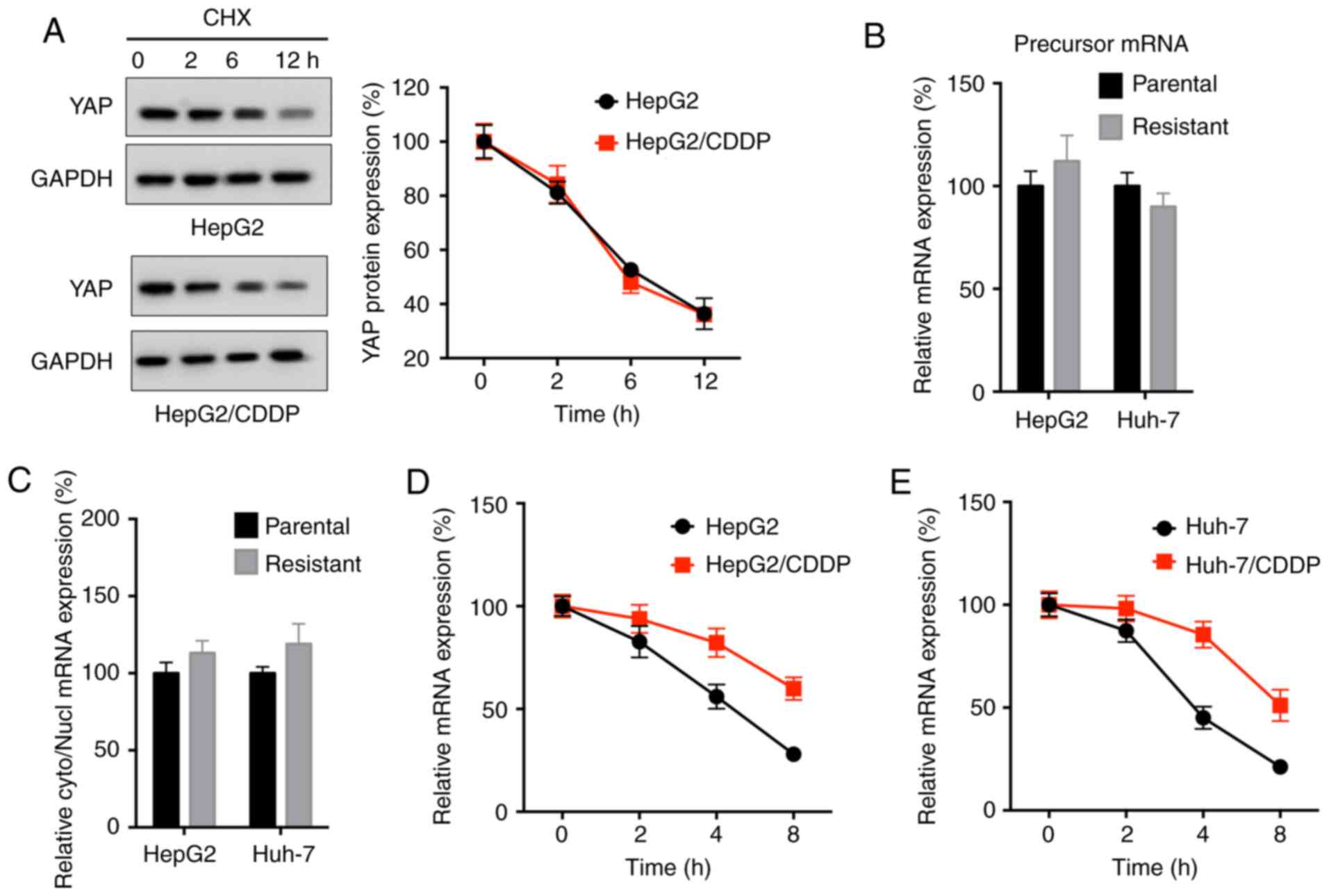 | Figure 6.mRNA stability is responsible for the
upregulation of YAP expression in LC/CDDP cells. (A) HepG2 and
HepG2/CDDP cells were treated with CHX (100 µg/ml) for the
indicated time periods, and YAP protein expression was analyzed by
western blot analysis (left) and quantitatively analyzed (right).
(B) Expression levels of the precursor mRNA of YAP in LC and
LC/CDDP cells were measured via RT-qPCR. (C) Relative cyto/nucl
levels of YAP mRNA expression in LC and LC/CDDP cells were measured
via RT-qPCR. (D) HepG2/CDDP or (E) Huh-7/CDDP cells and their
corresponding parental cells were treated with actinomycin D for
the indicated time periods, and YAP mRNA expression was analyzed
via RT-qPCR. Data are presented as the mean ± SD of three
independent experiments. LC, liver cancer; CDDP, cisplatin; LC/CDDP
cells, CDDP-resistant LC cells; YAP, Yes-associated protein;
RT-qPCR, reverse transcription-quantitative PCR; CHX,
cycloheximide; cyto, cytoplasm; nucl, nucleus. |
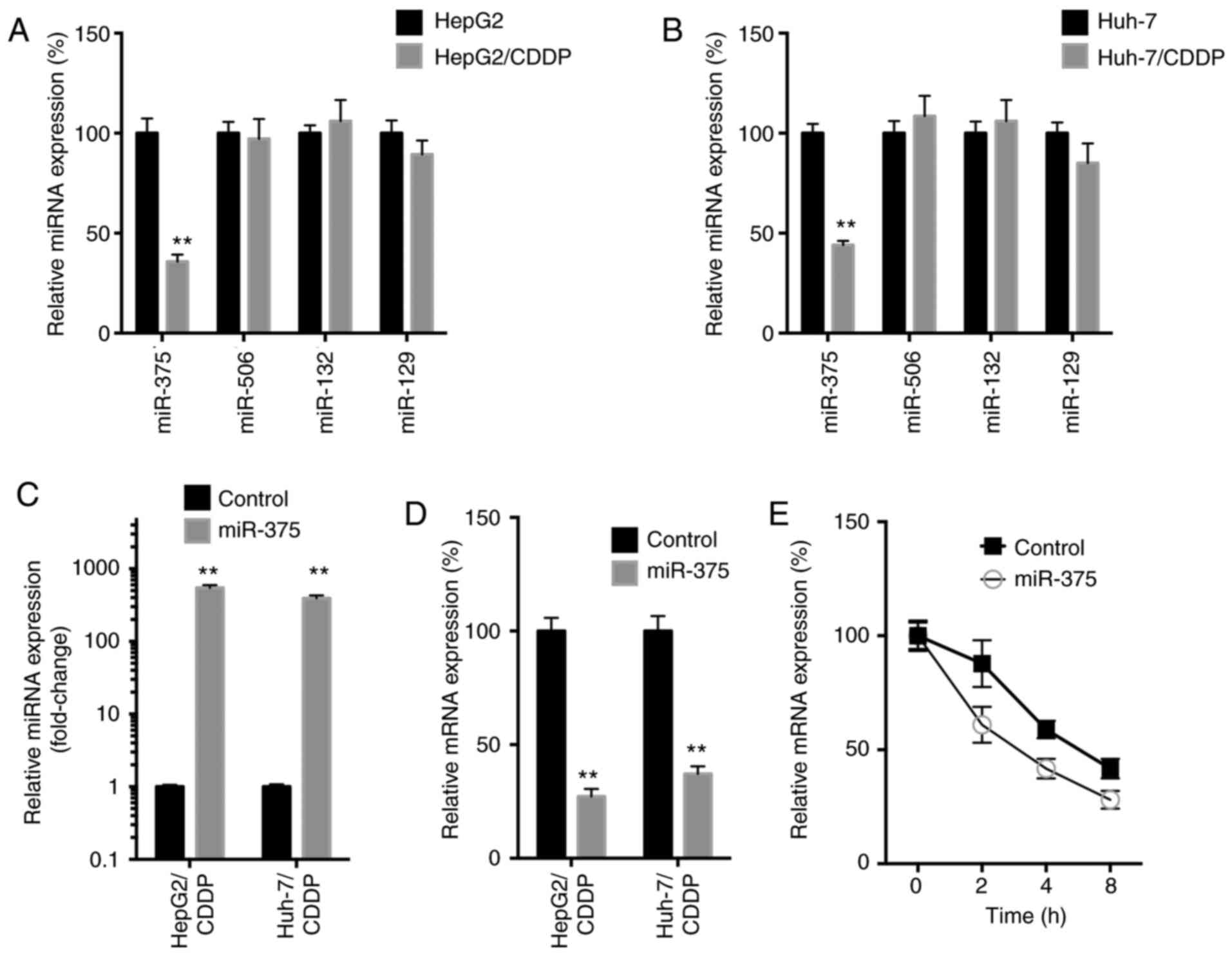
miR-375 decreases the mRNA stability
of YAP in LC/CDDP cells
miRNAs can decrease mRNA stability via binding to
the 3′-untranslated regions of mRNA (22). It has been revealed that miR-375
(23), miR-506 (24), miR-132 (25) and miR-129 (26) directly target YAP mRNA to downregulate
its expression. Thus, the expression levels of these miRNAs in both
LC/CDDP and LC cells were subsequently analyzed. The data revealed
that, among all miRNAs, only the expression levels of miR-375 were
significantly downregulated in both HepG2/CDDP (Fig. 7A) and Huh-7/CDDP (Fig. 7B) cells. Furthermore, the
overexpression of miR-375 (Fig. 7C)
using miR-375 mimics significantly downregulated the mRNA
expression levels of YAP in both HepG2/CDDP and Huh-7/CDDP cells
(Fig. 7D). This was due to the fact
that miR-375 decreased the mRNA stability of YAP (Fig. 7E).
Discussion
Chemotherapy is an important treatment for patients
with LC, especially for those with advanced LC (27). Cisplatin has been widely used as a
therapeutic agent for patients with LC; however, its application
has been significantly limited due to the development of
chemoresistance (28). To the best of
our knowledge, the molecular mechanisms involved in LC
chemoresistance to CDDP are not fully understood. The results of
the present study suggested that YAP, an important downstream
signaling protein of the Hippo signaling pathway, may mediate the
CDDP resistance of LC cells via upregulating IL-6 and TGF-β
expression. In addition, the downregulation of miR-375 expression
in LC/CDDP cells was responsible for the upregulation of YAP
expression. Collectively, these results suggested that the
miR-375/YAP axis-induced expression of IL-6 and TGF-β may be
critical for the CDDP resistance of LC cells.
The present study discovered that YAP was involved
in the CDDP resistance of LC cells. It has been previously revealed
that YAP upregulation is strongly associated with the
carcinogenesis of LC (29,30). The activation of YAP suppresses the
sensitivity of cancer cells to various drugs, such as anti-tubulin
drugs and DNA-damaging agents (31–34). In LC
cells, it has been reported that YAP upregulation confers
resistance to doxorubicin (35) and
the topoisomerase I inhibitor SN38 (36). The data of the present study
illustrated that the expression levels and nuclear localization of
YAP were increased in LC/CDDP cells. In addition, the targeted
inhibition of YAP via siRNA or an inhibitor restored the CDDP
sensitivity of LC cells, which indicated that YAP may be involved
in the chemoresistance of LC cells.
The data of the current study also demonstrated that
IL-6 and TGF-β were involved in the YAP-mediated chemoresistance of
LC cells. It has been previously reported that the activation of
YAP stimulates IL-6 gene transcription during colonic tumorigenesis
(37). In LC cells, YAP induces IL-6
expression to recruit tumor-associated macrophages (38). Additionally, a recent study has
confirmed that YAP can directly bind to the promoter of IL-6 to
regulate its transcription (39). As
to TGF-β, it has been reported that YAP promotes the TGF-β-induced
tumorigenic phenotype in breast cancer cells (40). In addition, YAP/TAZ regulate
TGF-β/Smad3 signaling through the induction of Smad7 via activator
protein 1 in human skin dermal fibroblasts (41). However, whether YAP can directly
activate the transcription of TGF-β requires further
investigation.
Furthermore, the present study indicated that the
downregulation of miR-375 expression may be responsible for the
upregulation of YAP expression in LC/CDDP cells, indicated by the
fact that YAP mRNA stability was increased, while miR-375
expression was downregulated, in LC/CDDP cells compared with in LC
cells. In gastric cancer cells, the upregulation of miR-375
expression increases the CDDP sensitivity via the regulation of
ERBB2 (42). miR-375 is induced in
CDDP nephrotoxicity to repress hepatocyte nuclear factor-1β
(43). Furthermore, miR-375 can
target YAP in LC to inhibit cancer cell viability (23,44).
Similarly, miR-375 suppresses YAP expression in lung cancer
(45) and mouse pancreatic progenitor
(46) cells. All these data suggested
that miR-375 may be involved in the CDDP resistance and progression
of LC.
In conclusion, the results of the present study
revealed that the miR-375/YAP axis may regulate the CDDP resistance
of LC via the regulation of IL-6 and TGF-β. Therefore, the targeted
inhibition of this axis and signaling pathway may be useful in
overcoming the CDDP resistance and enhancing the clinical treatment
of patients with LC. Whether the miR-375/YAP axis-induced
expression of IL-6 and TGF-β is involved in the TKI resistance of
LC requires further investigation in future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KY, KW and HL conceived and designed the study. ZJ,
HJH, HCH, YZ and KW acquired the data. KY, KW, HL, ZJ and YZ
analyzed and interpreted the data. KY, HCH, YZ and KW wrote and
revised the manuscript. The authenticity of the raw data has been
assessed by all authors. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raoul JL, Kudo M, Finn RS, Edeline J, Reig
M and Galle PR: Systemic therapy for intermediate and advanced
hepatocellular carcinoma: Sorafenib and beyond. Cancer Treat Rev.
68:16–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grothey A, Blay JY, Pavlakis N, Yoshino T
and Bruix J: Evolving role of regorafenib for the treatment of
advanced cancers. Cancer Treat Rev. 86:1019932020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Korita PV, Wakai T, Shirai Y, Matsuda Y,
Sakata J, Takamura M, Yano M, Sanpei A, Aoyagi Y, Hatakeyama K and
Ajioka Y: Multidrug resistance-associated protein 2 determines the
efficacy of cisplatin in patients with hepatocellular carcinoma.
Oncol Rep. 23:965–972. 2010.PubMed/NCBI
|
|
6
|
Plimack ER, Dunbrack RL, Brennan TA,
Andrake MD, Zhou Y, Serebriiskii IG, Slifker M, Alpaugh K, Dulaimi
E, Palma N, et al: Defects in DNA repair genes predict response to
neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder
cancer. Eur Urol. 68:959–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zamble DB and Lippard SJ: Cisplatin and
DNA repair in cancer chemotherapy. Trends Biochem Sci. 20:435–439.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu AX: Systemic therapy of advanced
hepatocellular carcinoma: How hopeful should we be? Oncologist.
11:790–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Camargo FD, Gokhale S, Johnnidis JB, Fu D,
Bell GW, Jaenisch R and Brummelkamp TR: YAP1 increases organ size
and expands undifferentiated progenitor cells. Curr Biol.
17:2054–2060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan KLL: Regulation and function of the
Hippo-YAP pathway in organ size, tumorigenesis, and metastasis.
Cancer Res. 72 (Suppl 8):SY29–03. 2012.PubMed/NCBI
|
|
12
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Yue T and Jiang J: Hippo
signaling pathway and organ size control. Fly (Austin). 3:68–73.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan Y, Li D, Li H, Wang L, Tian G and
Dong Y: YAP overexpression promotes the epithelial-mesenchymal
transition and chemoresistance in pancreatic cancer cells. Mol Med
Rep. 13:237–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corvaisier M, Bauzone M, Corfiotti F,
Renaud F, El Amrani M, Monté D, Truant S, Leteurtre E, Formstecher
P, Van Seuningen I, et al: Regulation of cellular quiescence by
YAP/TAZ and Cyclin E1 in colon cancer cells: Implication in
chemoresistance and cancer relapse. Oncotarget. 7:56699–56712.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin J, Luo M, Qian H and Chen W:
Upregulated miR-182 increases drug resistance in cisplatin-treated
HCC cell by regulating TP53INP1. Gene. 538:342–347. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F
and Xia Q: Cisplatin-induced downregulation of miR-199a-5p
increases drug resistance by activating autophagy in HCC cell.
Biochem Biophys Res Commun. 423:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng T, Wang J, Jiang H and Liu L: Hippo
signaling in oval cells and hepatocarcinogenesis. Cancer Lett.
302:91–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu-Chittenden Y, Huang B, Shim JS, Chen
Q, Lee SJ, Anders RA, Liu JO and Pan D: Genetic and pharmacological
disruption of the TEAD-YAP complex suppresses the oncogenic
activity of YAP. Genes Dev. 26:1300–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uddin A and Chakraborty S: Role of miRNAs
in lung cancer. J Cell Physiol. Apr 20–2018.(Online ahead of
print). View Article : Google Scholar
|
|
23
|
Liu AM, Poon RT and Luk JM: MicroRNA-375
targets Hippo-signaling effector YAP in liver cancer and inhibits
tumor properties. Biochem Biophys Res Commun. 394:623–627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Cui M, Sun BD, Liu FB, Zhang XD
and Ye LH: MiR-506 suppresses proliferation of hepatoma cells
through targeting YAP mRNA 3′UTR. Acta Pharmacol Sin. 35:1207–1214.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei CJ, Li L, Gao X, Zhang J, Pan QY, Long
HC, Chen CZ, Ren DF and Zheng G: Hsa-miR-132 inhibits proliferation
of hepatic carcinoma cells by targeting YAP. Cell Biochem Funct.
33:326–333. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan G, Cao X, Dai Q, Zhang B, Huang J,
Xiong S, Zhang YY, Chen W, Yang J and Li H: A novel role for
microRNA-129-5p in inhibiting ovarian cancer cell proliferation and
survival via direct suppression of transcriptional co-activators
YAP and TAZ. Oncotarget. 6:8676–8686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pratama MY, Pascut D, Massi MN and
Tiribelli C: The role of microRNA in the resistance to treatment of
hepatocellular carcinoma. Ann Transl Med. 7:5772019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perra A, Kowalik MA, Ghiso E,
Ledda-Columbano GM, Di Tommaso L, Angioni MM, Raschioni C, Testore
E, Roncalli M, Giordano S and Columbano A: YAP activation is an
early event and a potential therapeutic target in liver cancer
development. J Hepatol. 61:1088–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Wang J, Zhang Y, Zhang Y, Ma L, Weng
W, Qiao Y, Xiao W, Wang H, Yu W, et al: MEK1 promotes YAP and their
interaction is critical for tumorigenesis in liver cancer. FEBS
Lett. 587:3921–3927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Khanal P, Savage P, She YM, Cyr TD
and Yang X: YAP-induced resistance of cancer cells to antitubulin
drugs is modulated by a Hippo-independent pathway. Cancer Res.
74:4493–4503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia Y, Zhang YL, Yu C, Chang T and Fan HY:
YAP/TEAD co-activator regulated pluripotency and chemoresistance in
ovarian cancer initiated cells. PLoS One. 9:e1095752014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshikawa K, Noguchi K, Nakano Y, Yamamura
M, Takaoka K, Hashimoto-Tamaoki T and Kishimoto H: The Hippo
pathway transcriptional co-activator, YAP, confers resistance to
cisplatin in human oral squamous cell carcinoma. Int J Oncol.
46:2364–2370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Y and Yang X: The Hippo pathway in
chemotherapeutic drug resistance. Int J Cancer. 137:2767–2773.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huo X, Zhang Q, Liu AM, Tang C, Gong Y,
Bian J, Luk JM, Xu Z and Chen J: Overexpression of Yes-associated
protein confers doxorubicin resistance in hepatocellullar
carcinoma. Oncol Rep. 29:840–846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dai XY, Zhuang LH, Wang DD, Zhou TY, Chang
LL, Gai RH, Zhu DF, Yang B, Zhu H and He QJ: Nuclear translocation
and activation of YAP by hypoxia contributes to the chemoresistance
of SN38 in hepatocellular carcinoma cells. Oncotarget. 7:6933–6947.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taniguchi K, Moroishi T, de Jong PR,
Krawczyk M, Grebbin BM, Luo H, Xu RH, Golob-Schwarzl N, Schweiger
C, Wang K, et al: YAP-IL-6ST autoregulatory loop activated on APC
loss controls colonic tumorigenesis. Proc Natl Acad Sci USA.
114:1643–1648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou TY, Zhou YL, Qian MJ, Fang YZ, Ye S,
Xin WX, Yang XC and Wu HH: Interleukin-6 induced by YAP in
hepatocellular carcinoma cells recruits tumor-associated
macrophages. J Pharmacol Sci. 138:89–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Song T, Zhou S and Kong X: YAP
promotes the malignancy of endometrial cancer cells via regulation
of IL-6 and IL-11. Mol Med. 25:322019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hiemer SE, Szymaniak AD and Varelas X: The
transcriptional regulators TAZ and YAP direct transforming growth
factor β-induced tumorigenic phenotypes in breast cancer cells. J
Biol Chem. 289:13461–13474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L, Lee W, Oh JY, Cui YR, Ryu B and
Jeon YJ: Protective effect of sulfated polysaccharides from
celluclast-assisted extract of Hizikia fusiforme against
ultraviolet B-induced skin damage by regulating NF-κB, AP-1, and
MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar
Drugs. 16:2392018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou N, Qu Y, Xu C and Tang Y:
Upregulation of microRNA-375 increases the cisplatin-sensitivity of
human gastric cancer cells by regulating ERBB2. Exp Ther Med.
11:625–630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hao J, Lou Q, Wei Q, Mei S, Li L, Wu G, Mi
QS, Mei C and Dong Z: MicroRNA-375 is induced in cisplatin
nephrotoxicity to repress hepatocyte nuclear factor 1-β. J Biol
Chem. 292:4571–4582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang Y, Yan W, He X, Zhang L, Li C, Huang
H, Nace G, Geller DA, Lin J and Tsung A: miR-375 inhibits autophagy
and reduces viability of hepatocellular carcinoma cells under
hypoxic conditions. Gastroenterology. 143:177–187.e8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nishikawa E, Osada H, Okazaki Y, Arima C,
Tomida S, Tatematsu Y, Taguchi A, Shimada Y, Yanagisawa K, Yatabe
Y, et al: miR-375 is activated by ASH1 and inhibits YAP1 in a
lineage-dependent manner in lung cancer. Cancer Res. 71:6165–6173.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang ZW, Men T, Feng RC, Li YC, Zhou D
and Teng CB: miR-375 inhibits proliferation of mouse pancreatic
progenitor cells by targeting YAP1. Cell Physiol Biochem.
32:1808–1817. 2013. View Article : Google Scholar : PubMed/NCBI
|