Introduction
The most common malignant tumor of nasopharyngeal
epithelial cells is nasopharyngeal carcinoma (NPC) (1). It is relatively rare in the world and
has a unique geographical distribution in Asia, particularly in
East and Southeast Asia. According to the records of the
International Agency for Research on Cancer, approximately 129,000
new cases of nasopharyngeal cancer occurred in 2018, accounting for
only 0.7% of all cancers diagnosed in 2018 (2). NPC pathogenesis is closely related to
Epstein-Barr virus (EBV) infection, human papillomavirus infection,
genetic susceptibility, and consumption of salted fish.
Furthermore, cancer-derived EBV DNA circulating in plasma has been
identified as a tumor marker for NPC with 96% sensitivity (3,4). The
anatomical location of NPC makes it difficult to access for surgery
and is highly sensitive to radiation. Therefore, radiotherapy (RT)
is the treatment of choice for non-metastatic NPC (5). Intensity-modulated radiation therapy is
the most commonly recommended radiation method because of its
excellent local control. For locally advanced NPC, concurrent RT
and chemotherapy is recommended as first-line treatment (6). Studies have indicated that simultaneous
chemotherapy and RT may increase treatment-related toxicity and
reduce willingness to undergo treatment, which may cause some
patients to stop using RT (7).
Therefore, providing appropriate treatment guidelines and reducing
drug toxicity can further increase the cure rate of cancer.
Natural compounds extracted from plants are used as
traditional medicines for treating various diseases, including
various cancer types. In addition, natural medicine use has
relatively low toxicity. Traditional Chinese pharmacopoeia has used
Picrasma quassioides (D. Don) Benn (PQ), a deciduous shrub
or small tree native to temperate regions of southern Asia, for the
treatment of inflammation, microbial infections, and fever. PQ
produces various compound types, such as alkaloids (mainly
β-carboline and cathinone alkaloids), bitter components, and
triterpenoids (8). The β-carboline
alkaloids extracted from PQ, which feature anti-inflammatory and
antitumor activity, are widely used in medical treatment (9–13).
Dehydrocrenatidine (DC) is a β-carboline alkaloid
abundantly present in PQ (14). Zhao
et al demonstrated that β-carboline alkaloids (the main
active ingredient of medicinal plants) exert anti-inflammatory
effects through the inhibition of the iNOS pathway (15). The β-carboline enantiomer extracted
from PQ was found to decrease cell viability and inhibit the
proliferation of various cancer cells, such as liver, cervical, and
breast cancer cells (10,14,16,17). Zhao
et al demonstrated that the analgesic effect of DC may be
achieved through the inhibition of neuronal excitability (14). Zhang et al showed that the
Janus kinase (JAK) inhibitor DC inhibits JAK2 in the tumorigenesis
of solid tumors constitutively activated through signal
transduction and by transcription activator 3 (18). However, the molecular targeting effect
of DC on human NPC cells is unclear. The present study aimed to
examine the cytotoxicity and biochemical role of DC in human NPC
cells.
Materials and methods
Cell culture
Human NPC cell lines [including NPC-039 and NPC-BM
(19)] were provided by Dr Jen-Tsun
Lin, Department of Hematology and Oncology, Changhua Christian
Hospital. RPMI-2650 head and neck squamous cell line was obtained
from Japanese Collection of Research Bioresources Cell Bank (JCRB
Cell Bank, Osaka, Japan) cultured in Eagle's Minimum Essential
Medium (Gibco BRL; Thermo Fisher Scientific, Inc.) with 10%
non-essential amino acids (Gibco BRL; Thermo Fisher Scientific,
Inc.) and 10% fetal bovine serum (FBS) (Gibco BRL; Thermo Fisher
Scientific, Inc.). NPC cell lines were cultured in RPMI-1640 medium
(Gibco BRL; Thermo Fisher Scientific, Inc.) and 10% FBS. All cell
lines were cultured under the same conditions (at 37°C and 5%
CO2 in a humid atmosphere) as described in previous
studies (20).
DC treatments
DC (purity ≥98%) was purchased from ChemFaces, and
the product was made into a 100 mM stock solution in dimethyl
sulfoxide (DMSO) and stored at −20°C. The final treatment
concentration in experiments with DMSO content was consistently
less than 0.1%. Various concentrations (0, 25, 50 and 100 µM) of DC
were prepared to treat NPC cells in subsequent experiments and were
incubated for 24 h.
Cell viability
The effect of DC on cell growth was determined using
the MTT method (20). First, NPC-BM,
NPC-039 and RPMI-2650 cell lines were seeded on a plate
(1×104 cells/well), treated with various concentrations
of DC, and cultured at 37°C for 24 h. Then, the culture medium was
removed, MTT reagent (final concentration of 0.5 mg/ml) was added
to each well, and cells were incubated in 5% (v/v) CO2
at 37°C for >4 h. After centrifugation, the supernatant was
removed. Then, DMSO was carefully added to each well to dissolve
formazan crystals for measurement. The absorbance was measured at
595 nm by using an ELISA microplate reader. Each experimental
condition was repeated three times, and the data were analyzed for
at least three independent experimental results.
Colony formation assays
In a suitable medium, the cell line was seeded on a
6-well cell culture plate at a concentration of 1×104
cells as described in a previous study (21). Then, the cells were evenly distributed
and incubated followed by culturing with various DC concentrations.
The incubation medium was changed twice a week, and the medium was
removed after two weeks. The colonies were further fixed with
formalin and stained with 0.5% crystal violet. Finally, a stereo
microscope was used to count the total number of colonies and
colonies consisting of >50 cells.
Cell cycle analysis
The cells seeded on the plate (1×104
cells/well) were treated with various DC concentrations and
cultured at 37°C for 24 h as previously described (22). Following the same drug treatment
method as specified in previous studies, after the cells were
collected through centrifugation and fixed in ethanol, the ethanol
was eliminated and the cells were suspended in Muse cell cycle kit
reagents and placed in the dark at room temperature. Finally, flow
cytometry was used to analyze the cell cycle distribution
results.
DAPI staining
The NPC cells (1×104 cells/well) were
grown on glass coverslips and then treated with various DC
concentrations for 24 h as described in previous research (20). The method used for cell processing was
the same as that in a previous study; according to fixation and
permeabilization, the DAPI dye was applied to stain cells in the
dark. Nuclear morphological changes associated with apoptosis were
evaluated in at least 500 cells. The resulting images were
immediately observed through a fluorescence microscope (Leica,
Bensheim, Germany).
Annexin V/PI double-staining
assay
Cell viability was determined following methods
described previously (22). The cells
(1×104 cells/well) were cultured in each well for 12 h
and further treated with various DC concentrations for 24 h. These
cells were collected and suspended in phosphate-buffered saline
(PBS) followed by incubation with reagents contained in the Muse
Annexin V and Dead Cell Kit (cat. no. MCH100105; Merck Millipore)
in the dark at room temperature. Results were analyzed using Muse
Cell Analyzer flow cytometry (Merck Millipore) and the data were
analyzed using Muse Cell Soft V1.4.0.0 Analyzer Assays (Merck
Millipore).
Mitochondrial membrane potential
evaluation
First, the cells were planted in a 6-well plate
(1×104 cells/well) and incubated with various DC
concentrations for 24 h as previously described (23). The collected cells were processed
under conditions previously studied (23). The obtained cells were added to Muse
Mitopotential Assay Kit (cat. no. MCH100110, Merck Millipore)
reaction, and the results were analyzed using Muse Cell Analyzer
flow cytometry and the data were analyzed using Muse Cell Soft
V1.4.0.0 Analyzer Assays (Merck Millipore).
Caspase-3/7 detection and
analysis
The analysis was performed as previously described
(24). The user guide of the Muse
Caspase-3/7 Kit (cat. no. MCH100108, Merck Millipore) describes the
caspase-3/7 detection method. After processing the DC, the cells
were obtained and stained with the reagent of Muse Caspase-3/7. The
experimental results were detected using a flow cytometer and
analyzed using Muse Cell Analyzer flow cytometry and the data were
analyzed using the Muse Cell Soft V1.4.0.0 Analyzer Assays (Merck
Millipore).
Reactive oxygen species (ROS)
assay
The user guide of the Muse Oxidative Stress Kit
(Cat. No. MCH100111, Merck Millipore) describes the oxidative
stress detection method. First, the cells were planted in a 6-well
plate (1×104 cells/well) and incubated with various DC
concentrations for 24 h as previously described (23). The collected cells were processed
under conditions previously studied (23). The obtained cells were added to the
Muse Oxidative Stress working solution reagent reaction at 37°C for
30 min, and the results were analyzed using Muse Cell Analyzer flow
cytometry and the data were analyzed using the Muse Cell Soft
V1.4.0.0 Analyzer Assays (Merck Millipore).
Protein extraction and western blot
analysis
NPC cell lines (NPC-039 and NPC-BM) were inoculated
into 6-well plates and cultured for 12 h; various DC concentrations
were added to each well, and the plate was incubated in an
incubator at 37°C for 24 h. Then, the cells were washed with PBS,
mixed with an inhibitor reagent, and lysed as described in a
previous study (20). BCA protein
assay (Pierce; Thermo Fisher Scientific, Inc.) was used to quantify
the proteins of the supernatant. All samples were analyzed through
sodium dodecyl sulfate polyacrylamide 10% or 12.5% gel
electrophoresis, and the separated proteins from the gel were
transferred to the PVDF membrane surface (EMD Millipore). The PVDF
membrane was reacted with 5% skimmed milk in TBST for 1 h. For
analysis, the primary antibody was used, which was described by the
antibody manufacturer [from Cell Signaling Technology, Inc. (CST),
1:1,000 dilution] as containing cell cycle related-proteins [cyclin
A (cat. no. #4656; 55 kDa), cyclin B (cat. no. #12231; 55 kDa),
cyclin D3 (cat. no. #2936; 31 kDa), cyclin-dependent kinase (CDK)4
(cat. no. #12790; 30 kDa), CDK6 (cat. no. #3136; 36 kDa),
phosphorylated (p)-cdc2 (cat. no. #4539; 34 kDa), Myt1 (cat. no.
#4282; 60–70 kDa), p-WEE1 (cat. no. #4910; 95 kDa), and p-Rb (cat.
no. #8516; 110 kDa) (cat. no. #9301; 110 kDa)] death receptor
pathway-related proteins (FADD (cat. no. #2782; 28 kDa), TNF-R1
(cat. no. #3736; 55 kDa), DcR2 (cat. no. #8049; 45–60 kDa), cleaved
RIP (cat. no. #3493; 78 kDa), and DR5 (cat. no. #8074; 40, 48
kDa)), apoptosis-related proteins (cleaved PARP (cat. no. #9542;
89, 116 kDa), cleaved caspase-3 (cat. no. #9664; 17, 19 kDa),
cleaved caspase-8 (cat. no. #9496; 41, 43 kDa), cleaved caspase-9
(cat. no. #52873; 37 kDa), Bax (cat. no. #5023; 20 kDa), Bak (cat.
no. #12105; 25 kDa), t-Bid (cat. no. #2002; 15, 22 kDa), Bcl-xL
(cat. no. #2764; 30 kDa), and Bcl-2 (cat. no. #4223; 26 kDa), MAPK
pathway-related proteins (AKT (cat. no. #4685, 60 kDa), ERK1/2
(cat. no. #4695; 42, 44 kDa), p38 (cat. no. #8690; 40 kDa), JNK1/2
(cat. no. #9252; 46, 54 kDa), p-AKT (cat. no. #4060; 60 kDa),
p-ERK1/2 (cat. no. #4370; 42, 44 kDa), p-p38 (cat. no. #4511; 43
kDa), and p-JNK1/2 (cat. no. #4668; 46, 54 kDa)), and β-actin
(1:5000 dilution; cat. no. # NB600-501; 42 kDa; Novus Biologicals).
Finally, after washing with TBST, the PVDF membrane was incubated
with secondary anti-rabbit IgG (anti-rabbit IgG, #7074, 1:3,000) or
anti-mouse IgG (anti-mouse IgG, #7076, 1:3,000) (Cell Signaling
Technology, Inc.) attached to HRP for 1 h. The western blot was
observed using a chemiluminescence HRP substrate (Millipore), and
the photographic images observed by ImageQuant LAS 4000 mini (GE
Healthcare, USA) and relative density quantitated by ImageJ 1.47
version software (National Institutes of Health).
Statistical analysis
For the statistical analysis, GraphPad Prism
software (GraphPad Software, Inc.) was used, as in a previous study
(23). Statistical analysis of at
least three independent experimental results was performed, and the
calculated values are presented as mean ± standard deviation.
Statistical analysis methods used included ANOVA and Tukey's post
hoc test. A P-value <0.05 represented a valid significant
difference.
Results
DC causes cytotoxicity by inhibiting
the survival and proliferation of human NPC cell lines
We investigated the cell viability of the human NPC
cell lines (including NPC-039 and NPC-BM) and human head and neck
squamous cell carcinoma (RPMI-2650). These cells were treated with
various DC concentrations (0, 25, 50, and 100 µM) for 24, 48, and
72 h to measure DC cytotoxicity. MTT analysis revealed that the
cell viability of these cell lines was reduced by DC in a dose- and
time-dependent manner (P<0.05; Fig.
1A-C). The MTT analysis showed that DC induced approximately
40% of death by apoptosis in the NPC-BM and RPMI-2650 cell lines
and approximately 23% in the NPC-039 cell line at the highest
concentration. These results suggest that the nasopharyngeal cancer
cell lines were not resistant to the action of DC, and
nasopharyngeal cancer cells are also sensitive to the action of DC.
Moreover, to analyze the effect of DC against cell proliferation in
human NPC cell lines, the colony formation results were studied to
determine the effect of DC on both cell lines during long-term
treatment. Fig. 1D and E show that a
DC concentration of 25 µM significantly inhibited the colony
forming ability of both cell lines. Therefore, DC inhibited the
survival and proliferation of NPC-039, NPC-BM and RPMI-2650 cell
lines.
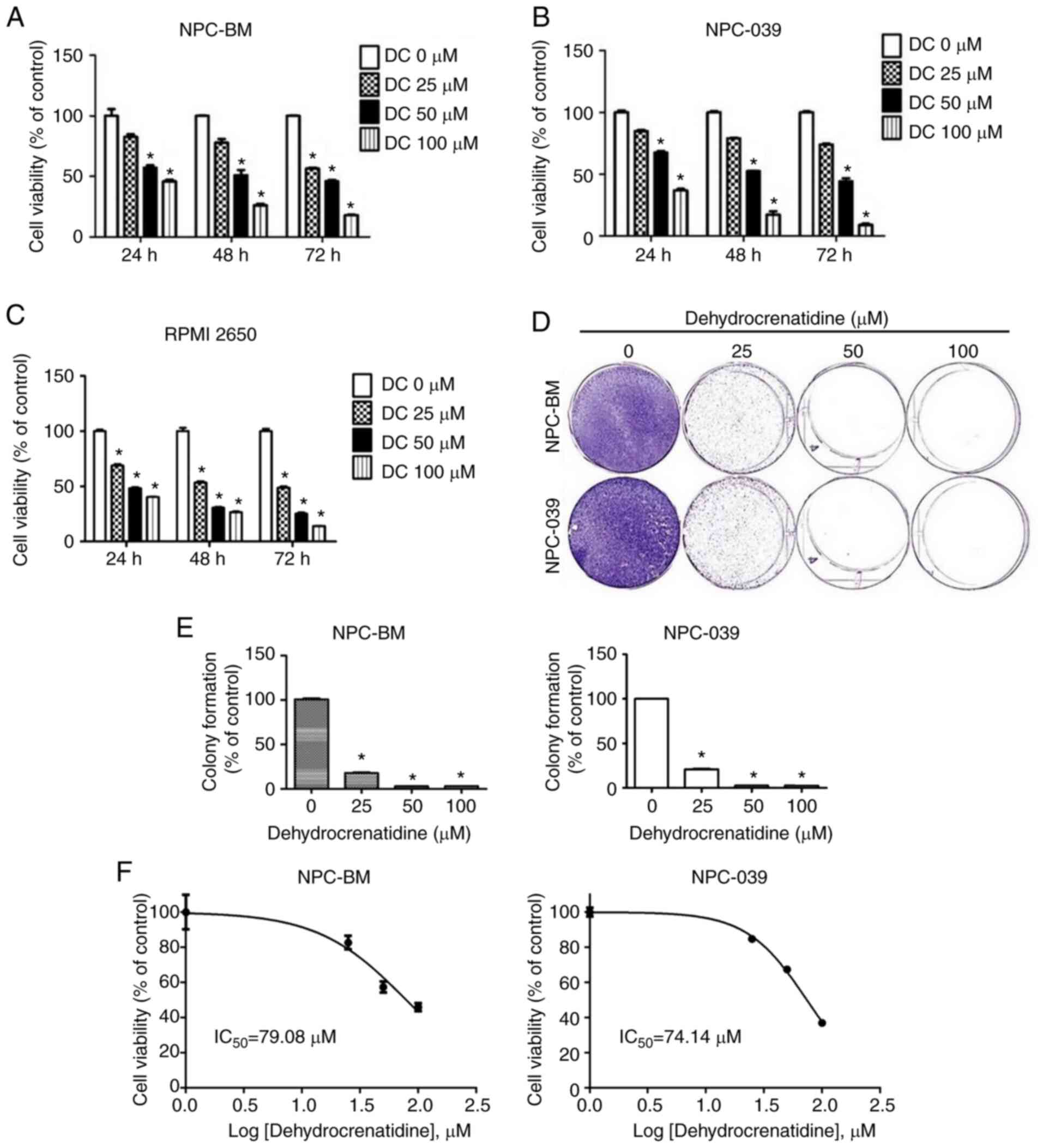 | Figure 1.DC causes cytotoxicity in human NPC
cells. (A-C) Human NPC cell lines (including NPC-039 and NPC-BM)
and RPMI-2650 were individually treated with various concentrations
(0, 25, 50, and 100 µM) of DC for 24, 48, and 72 h. Cell viability
was analyzed using MTT analysis. (D and E) In the colony formation
assay, NPC-039 and NPC-BM cell lines were evenly distributed and
incubated and then cultured with various DC concentrations (0, 25,
50, and 100 µM). The incubation medium was changed twice a week,
and the medium was removed after 2 weeks. (F) The IC50
values of NPC cells after treatment with DC. *P<0.05 vs. the
control group. NPC, nasopharyngeal carcinoma; DC,
dehydrocrenatidine; IC50, half maximal inhibitory
concentration. |
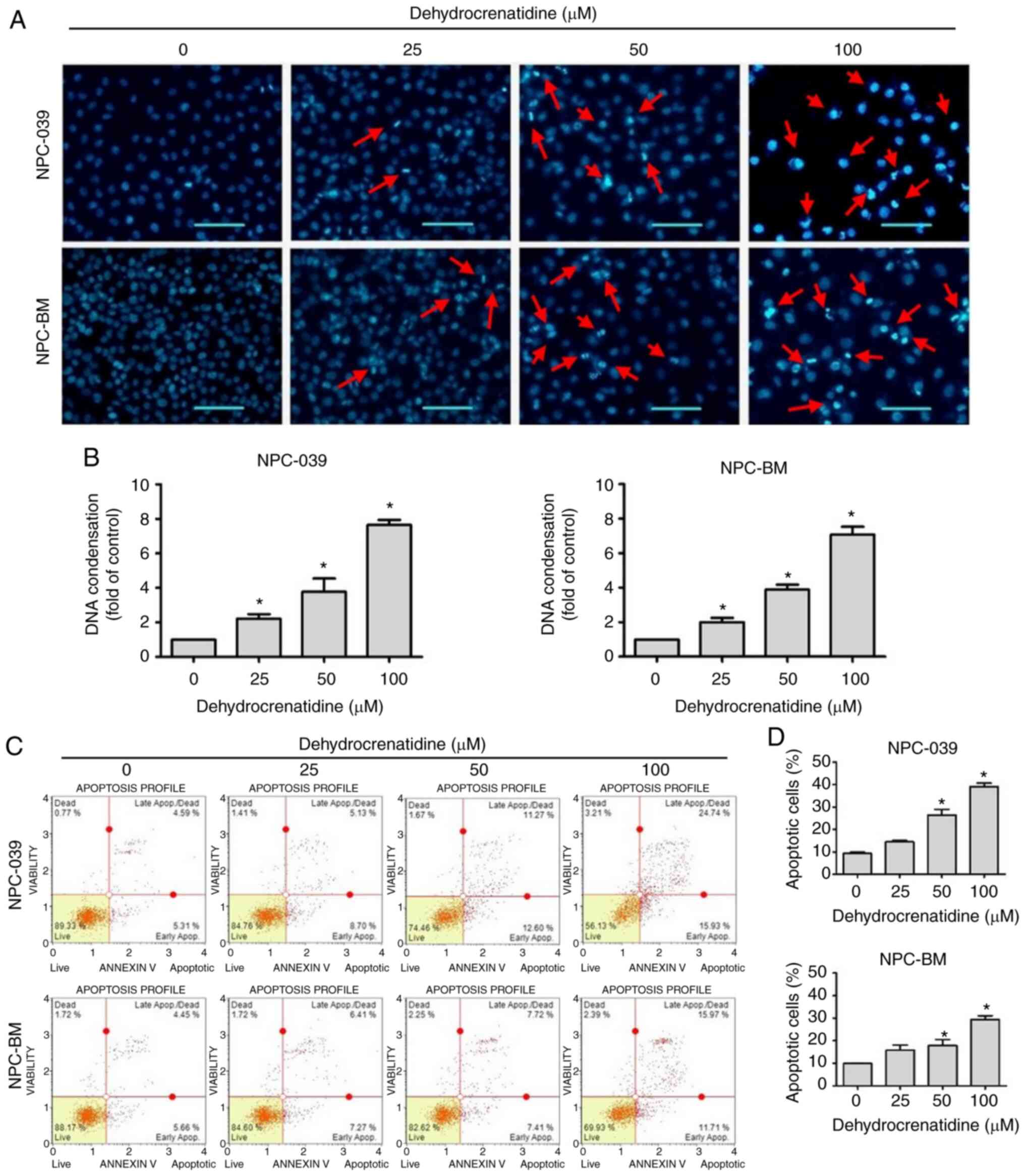
DC induces cell cycle arrest and
apoptosis of human NPC cells
We explored whether various DC concentrations (0,
25, 50, and 100 µM) affect cell viability and apoptosis within 24 h
and analyzed the cell cycle distribution of NPC-039 and NPC-BM cell
lines by using PI staining and flow cytometry. Fig. 2A and B demonstrate that in both cell
lines, DC at 100 µM resulted in a significant increase in the
sub-G1 phase and decreased the number of cells in the G0/G1 phase
for both cell lines (P<0.05). We understand that sub-G1 and G2/M
phases block cell cycle binding of the cyclin-CDK complex induced
by DC. We further explored whether DC regulates the expression of
G2/M cell cycle regulators and then checked the level of G2/M cell
cycle regulators by using western blot analysis. As shown in
Fig. 2C and D, in both cell lines,
the expression levels of cyclin A and B were decreased (DC, 100
µM), and regulatory proteins p-cdc2 (Tyr15), Myt1, and p-WEE1
(Ser642) were decreased at high DC concentrations. Furthermore, we
investigated DC regulation of the expression of the G0/G1 cell
cycle regulator and analyzed the level of the G0/G1 cell cycle
regulator. Fig. 2C and D show that
the expression levels of cyclin D3, CDK4, and CDK6 were decreased
after NPC-039 and NPC-BM cells were treated with a high DC
concentration, thereby reducing the effect of regulatory protein
p-Rb. Next, we determined whether the growth inhibitory effect of
the NPC-BM and NPC-039 cell lines treated with DC leads to
apoptosis. After DAPI staining, DC-treated NPC-BM and NPC-039 cell
lines were examined using a fluorescence microscope. Cells treated
with DC showed significant morphological changes compared with
control cells, leading to nuclear bleb formation in both cell lines
(Fig. 3A). Next, we examined whether
NPC-BM and NPC-039 cell lines treated with DC showed a significant
increase in dose-dependent DNA condensation folding compared with
the control (Fig. 3B; P<0.05). To
further clarify whether apoptosis was affected by treating NPC-BM
and NPC-039 cell lines with DC, we used Annexin V/PI double
staining to check cell morphology and measured the results with
flow cytometry. Notably, compared with the control, the apoptotic
rate of DC-treated cells was significantly increased (DC, 50 and
100 µM) (Fig. 3C and D; P<0.05).
These results confirmed that DC can reduce the viability of human
NPC cell lines.
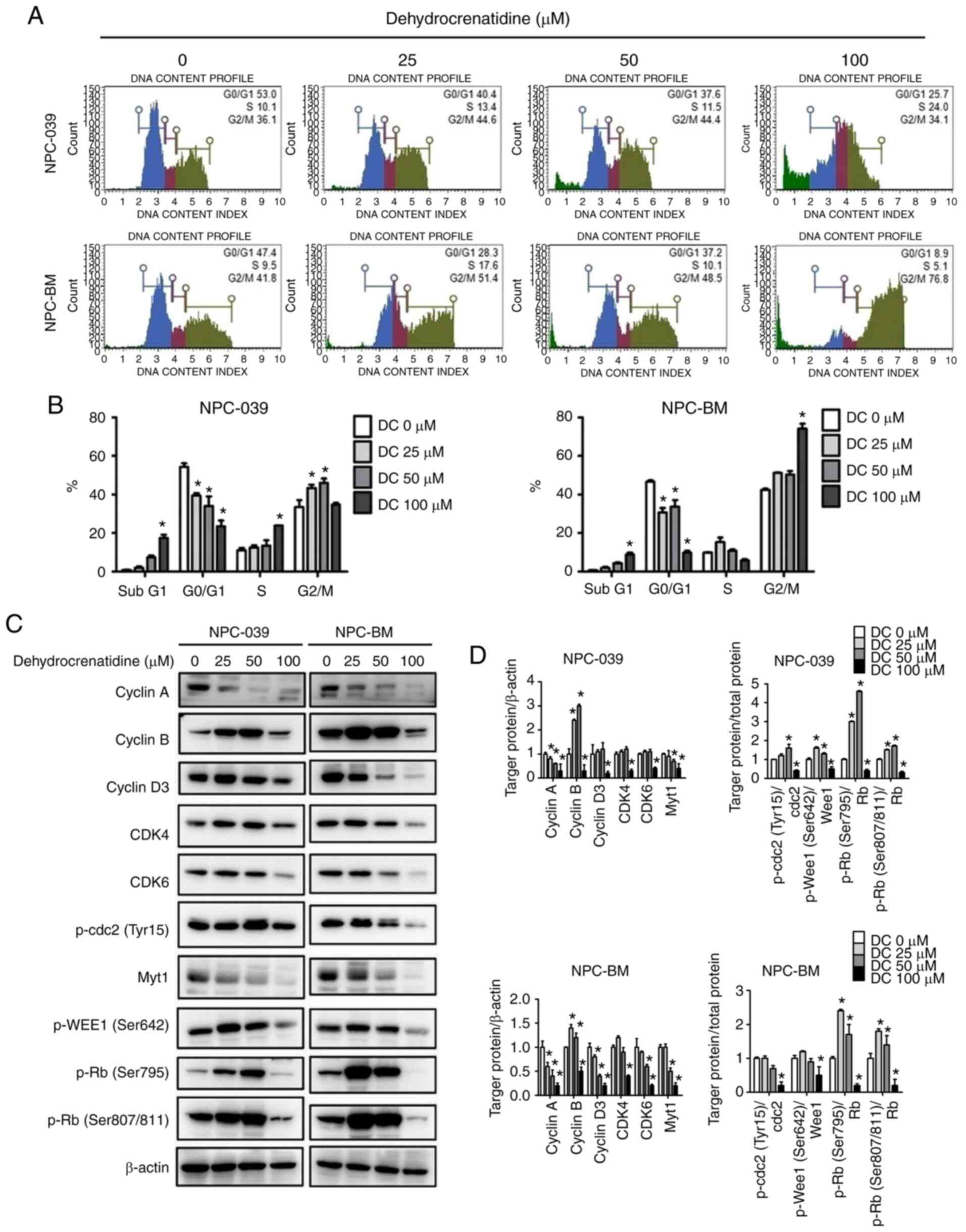 | Figure 2.DC-mediated cell cycle arrest in
human NPC cell lines (including NPC-039 and NPC-BM). (A) NPC-039
and NPC-BM cell lines were treated with DC (0, 25, 50, and 100 µM)
to measure and predict the phase distribution of the cell cycle
(G0/G1, S, and G2/M) by using flow cytometry. (B) Distribution of
cell cycle phases in the NPC-039 and NPC-BM cell lines was detected
and compared with the control group. (C and D) Western blotting for
the determination of cell cycle marker-related proteins showed the
levels of cyclin A, cyclin B, cyclin D3, CDK4, CDK6, p-cdc2, Myt1,
p-WEE1, and p-Rb. β-actin was used as an internal standard for
protein expression, and the results of protein levels were
normalized to β-actin for quantification. *P<0.05 vs. the
control group. NPC, nasopharyngeal carcinoma; DC,
dehydrocrenatidine; CDK, cyclin-dependent kinase; Myt1, myelin
transcription factor 1; WEE1, WEE1 G2 checkpoint kinase; Rb,
retinoblastoma protein; p-, phosphorylated. |
Apoptosis of human NPC cells induced
by DC is related to the extrinsic and intrinsic apoptosis
pathway
To determine the apoptotic mechanism in NPC-039 and
NPC-BM cells induced by DC, the results were analyzed using Muse's
flow cytometer and software. Treatments to increase the DC
concentration in both cell lines significantly enhanced the
depolarization of the mitochondrial membrane compared with the
untreated cells. Furthermore, DC (50 and 100 µM) significantly
increased depolarized cells in a dose-dependent manner (Fig. 4A; P<0.05). In addition, we
conducted a study on the role of DC in the death receptor pathway
of NPC-039 and NPC-BM through western blot analysis. As illustrated
in Fig. 4B and C, western blot
analysis revealed that human NPC cell lines treated with DC
exhibited increased protein expression levels of FADD, TNF-R1,
DcR2, cleaved RIP, and DR5 (P<0.05).
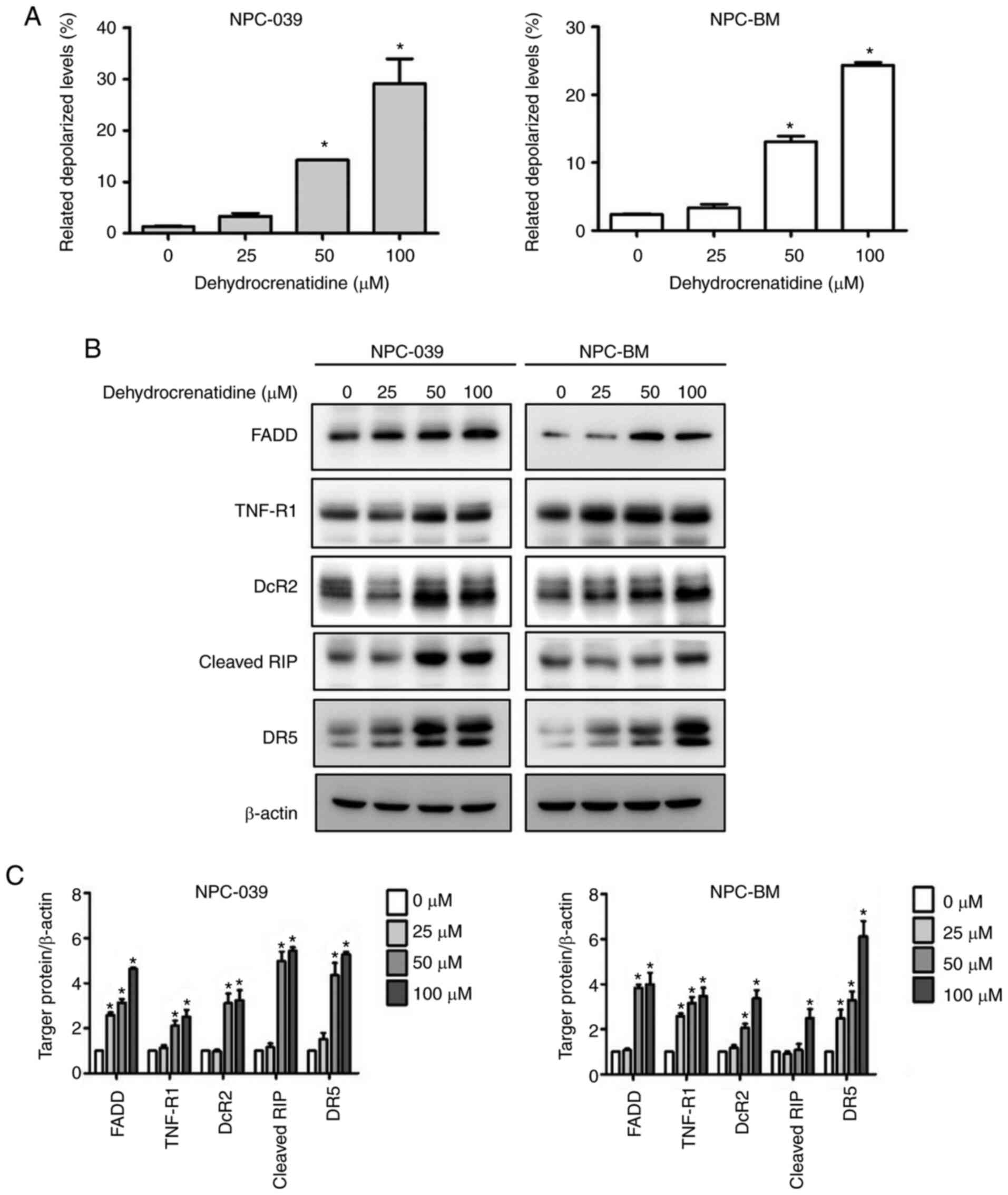 | Figure 4.DC treatment leads to the apoptosis
of human NPC cell lines (including NPC-039 and NPC-BM) through the
mitochondrial and death receptor pathways. (A) Mitochondrial
membrane potential was analyzed after treatment with DC (0, 25, 50,
and 100 µM) by using the Muse Cell Analyzer. Quantitative results
were analyzed using Muse Cell software and compared with the
control group. (B and C) Protein levels of the Fas pathway and the
TNF pathway including FADD, TNF-R1, DcR2, cleaved-RIP, and DR5 were
detected by western blotting. β-actin was used as an internal
standard for protein expression, and the results of protein levels
were normalized to β-actin for quantification. *P<0.05 vs. the
control group. NPC, nasopharyngeal carcinoma; DC,
dehydrocrenatidine; FADD, FAS-associated death domain protein;
TNF-R1, tumor necrosis factor receptor 1; DcR2, decoy receptor 2;
RIP, ribosome-inactivating protein; DR5, death receptor 5. |
DC induces apoptosis of human NPC
cells through the cell signaling pathway in the induction of the
extrinsic and intrinsic apoptosis pathway
The cell signal transduction pathway of DC in the
activation of the extrinsic and intrinsic apoptotic pathways was
evaluated. To investigate the role of caspase in the process of
DC-induced apoptosis, we tested the effect of DC-activated caspase
using flow cytometry and western blot assay. Fig. 5A and B demonstrate that DC (50 and 100
µM) treatment increased the cell levels in caspase-3 and caspase-7
in both cell lines and achieved a significant dose-dependent
increase in fold activation expression compared with the control
(P<0.05). The expression levels of cleaved caspase-3, −8, and −9
and cleaved PARP were all significantly enhanced in human NPC cells
treated with DC at high concentrations based on western blot
analysis (Fig. 5C and D; P<0.05).
Thus, DC can significantly provoke caspase activation in human NPC
cells. The related expression levels of the apoptosis-regulating
proteins Bax, Bak, and t-Bid (truncated BID, cleaved at Asp60 by
caspase-8 during Fas signaling) were significantly increased in
both cell lines, whereas Bcl-xL expression was decreased in a
dose-dependent manner. Bcl-2 expression changes were not seen at
all concentrations and for both cell lines (Fig. 5E and F; P<0.05). The expression
levels of cleaved caspase-3, −8, and −9 and cleaved PARP were
significantly decreased in human NPC cells following combined
treatment with DC and Z-VAD-FMK (caspase inhibitor) based on
western blot analysis (Fig. 5G and
H).
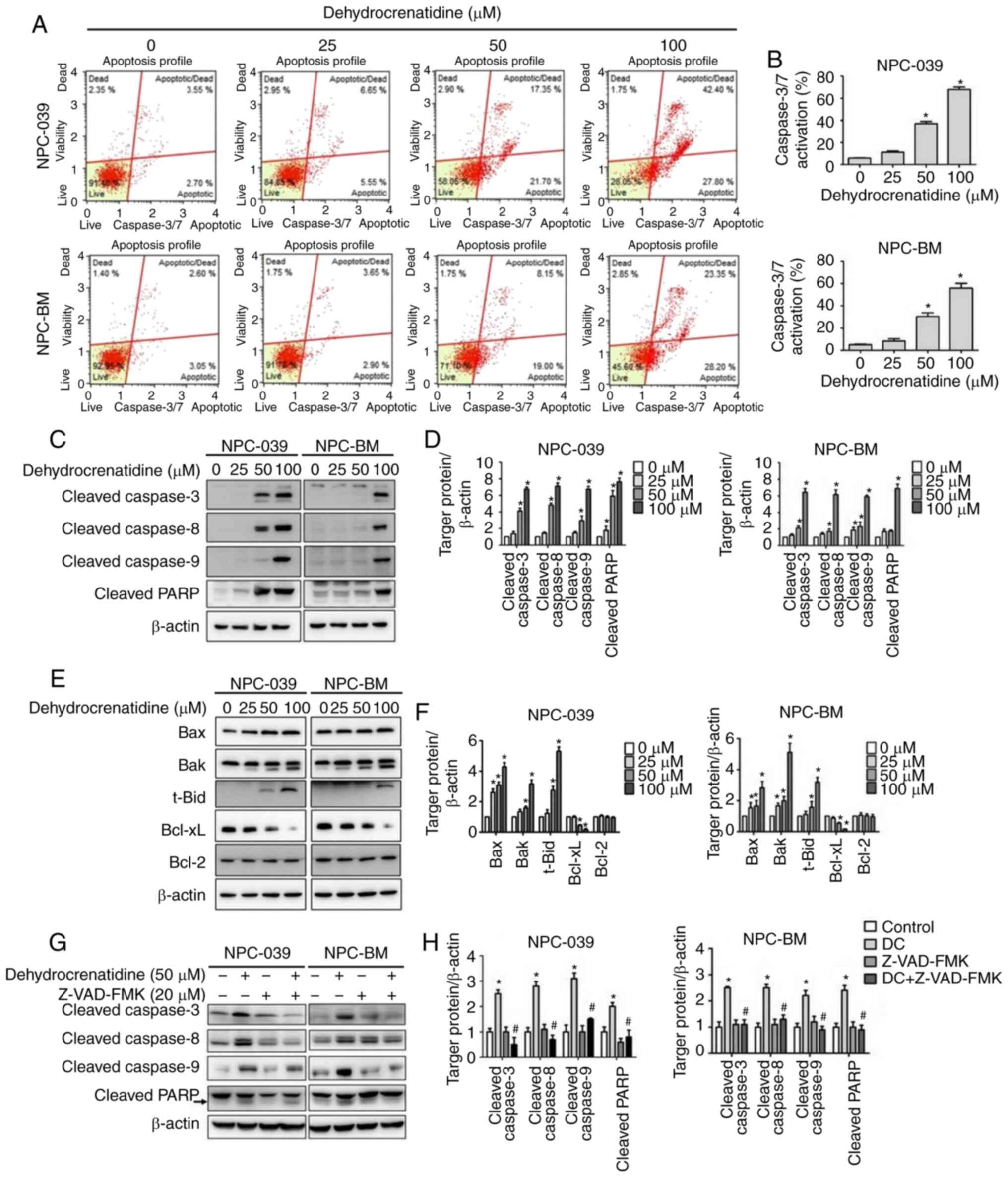 | Figure 5.DC promotes apoptosis through the
regulation of apoptosis-related proteins in human NPC cell lines
(including NPC-039 and NPC-BM) through extrinsic and intrinsic
caspase cell signaling pathways. (A and B) After treatment of
NPC-039 and NPC-BM cell lines for 24 h with DC, caspase-3/7 was
detected using the Muse caspase-3/7 kit. The level of caspase-3/7
activation was quantitatively analyzed in the treatment group and
compared with the control group. (C and D) The activated form of
apoptosis proteins was detected through western blotting, including
cleaved caspase-3, −8, and −9 and cleaved PARP proteins. (E and F)
Expression levels of related proteins, including Bax, Bak, t-Bid,
Bcl-xL, and Bcl-2 proteins, were determined and quantified through
Western blotting. *P<0.05 vs. the control group. (G and H) Cell
lines were pre-treated with Z-VAD-FMK (20 µM) for 1 h, then with
treated DC (50 µM) for 24 h. The activated form of apoptosis
proteins was detected through western blotting, including cleaved
caspase-3, −8, and −9 and cleaved PARP proteins. Protein levels
were determined through densitometry, with β-actin as an internal
standard for protein expression. Results of all protein levels were
normalized to β-actin for quantification compared with the control,
*P<0.05 vs. the control group; #P<0.05 vs. DC
treatment alone group. NPC, nasopharyngeal carcinoma; DC,
dehydrocrenatidine; Bax, BCL2 associated X, apoptosis regulator;
Bak, BCL2 antagonist/killer 1; Bid, BH3-interacting domain death
agonist; Bcl-xL, B-cell lymphoma-extra large; Bcl-2, B-cell
lymphoma 2; PARP, poly(ADP-ribose) polymerase. |
DC regulates the protein expression of
the MAPK pathway in human NPC cells
To clarify the role of protein expression levels in
the MAPK pathway and to confirm the mechanism related to
DC-mediated apoptosis, western blot analysis was used. In
particular, western blot analysis revealed that the DC-treated
human NPC cells exhibited upregulated phosphorylated (p)-AKT and
p-ERK1/2 and downregulated p-JNK1/2 (Fig.
6A and B; P<0.05). In addition, p-p38 activation remained
unchanged after treatment with DC in both cell lines, and DC
treatment increased the expression of p-AKT and p-ERK1/2 in both
cell lines and decreased the expression of p-JNK1/2. To explore
whether the MAPK pathway directly mediates DC-induced apoptosis, we
pretreated human NPC cells with AKT inhibitor (LY294002), ERK
inhibitor (U0126), and JNK1/2 inhibitor (SP600125) for 24 h before
DC treatment. The results in Fig.
6E-H demonstrate that the co-treatment with the ERK1/2
inhibitor resulted in a significantly higher reduction of
apoptosis-related proteins in both cell lines as compared to the DC
treatment alone (P<0.05), whereas the co-treatment with JNK1/2
inhibitor resulted in a significantly higher induction of
apoptosis-related proteins in both cells as compared to the DC
treatment alone (P<0.05). Notably, Fig. 6C and D illustrate that in the presence
of an AKT inhibitor, the expression of apoptosis-related proteins
of human NPC cells treated with DC remained unaffected compared
with treatment with DC alone (P<0.05).
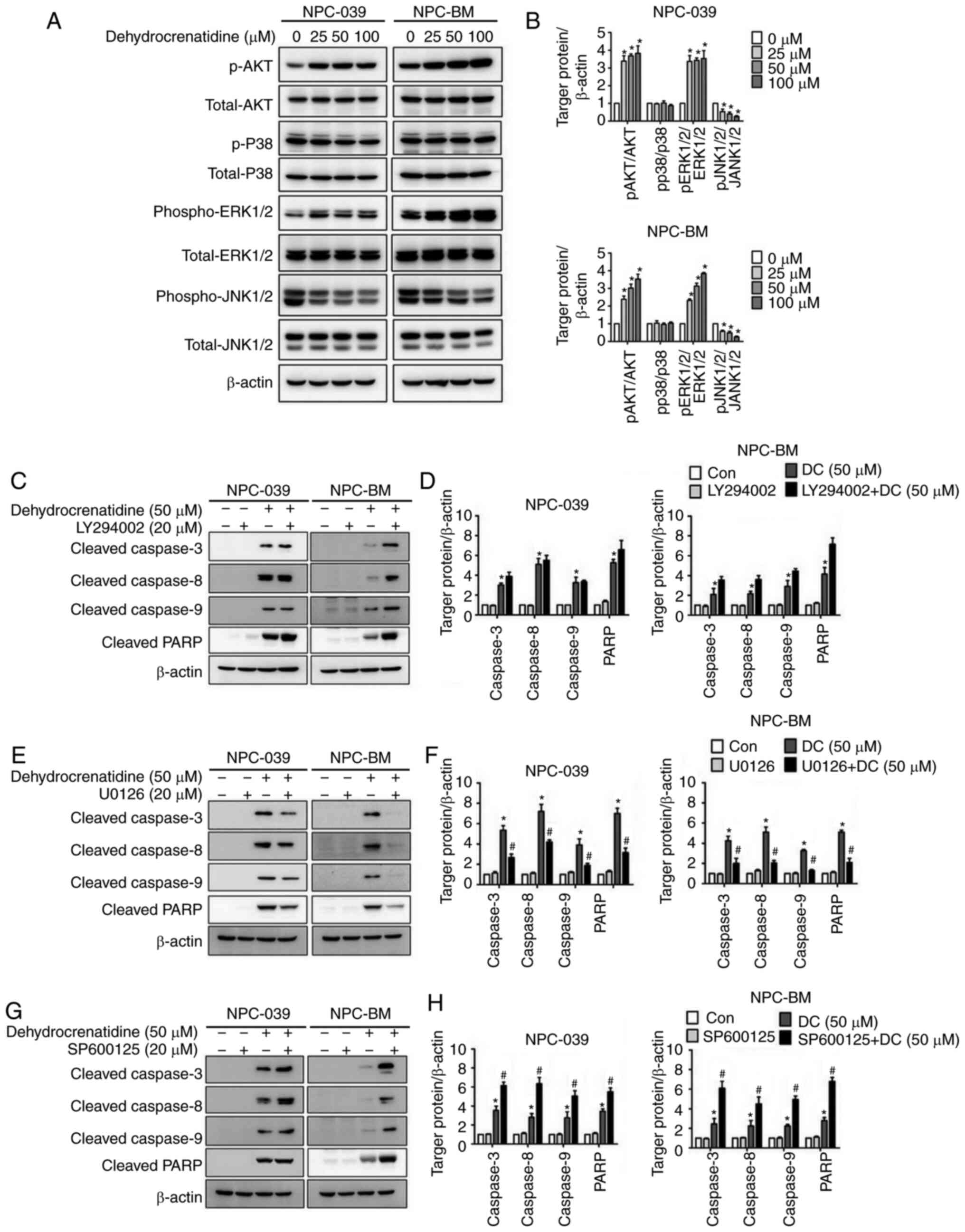 | Figure 6.DC regulates the protein expression
of the MAPK pathway in human NPC cell lines (including NPC-039 and
NPC-BM). (A) Analysis and quantification of expression levels of
AKT, p38, ERK1/2, and JNK1/2 proteins through western blotting. (B)
Protein levels were analyzed through densitometry; β-actin was used
as an internal standard for protein expression, and all proteins
were normalized to β-actin. *P<0.05 vs. the control group. (C-H)
NPC-039 and NPC-BM cell lines were pretreated with each MAPK
inhibitor, including AKT inhibitor (LY294002), ERK1/2 inhibitor
(U0126), and JNK1/2 inhibitor (SP600125), for 1 h and then
cotreated with or without DC for 23 h. Analysis and quantitative
regulation of protein expression, including cleaved caspase-3, −8,
and −9 and cleaved PARP protein, through western blotting. Protein
levels were determined through densitometry, with β-actin as an
internal standard for protein expression. Results of all protein
levels were normalized to β-actin for quantification compared with
control, *P<0.05 vs. the control group; #P<0.05
vs. DC treatment alone group. NPC, nasopharyngeal carcinoma; DC,
dehydrocrenatidine; PARP, poly(ADP-ribose) polymerase; p-,
phosphorylated; AKT, protein kinase B (PKB); p38, p38
mitogen-activated protein kinases; ERK, extracellular
signal-regulated kinases; JNK, c-Jun N-terminal kinase. |
Discussion
Nasopharyngeal carcinoma (NPC) is one of the five
main head and neck malignancies that develop in the lining of the
nasopharyngeal epithelium. However, the causes of NPC and treatment
strategies are different from those of other head and neck cancers
(25). The prognosis of NPC patients
has considerably improved with the combined use of magnetic
resonance imaging, intensity-modulated radiotherapy (RT), and
concurrent chemoradiation (26,27). NPC
is highly sensitive to RT and chemotherapy. Due to local recurrence
and distant metastasis, prognosis is poor in approximately 15–60%
of cases (25). Approximately 30% of
NPC patients present recurrence or distant metastasis, resulting in
poor treatment of these patients (28). Head and neck squamous cell carcinoma
(HNSCC) arises from the mucosal epithelium of the oral cavity,
nasopharynx, oropharynx, hypopharynx and larynx. However, it is
difficult to obtain important NPC cell lines from research
institutions. Only RPMI-2650 head and neck squamous cell carcinoma
was obtained from the Japanese Collection of Research Bioresources
Cell Bank.
The compounds of natural plants or traditional
Chinese medicine have anticancer effects. For example, β-carboline
belongs to the class of indole alkaloids. These compounds have
attracted much attention owing to their various biological
activities. In particular, these compounds have been shown to
insert themselves into DNA and consequently inhibit CDK,
topoisomerase, and monoamine oxidase (29). In addition, β-carboline derivatives
exhibit a wide range of pharmacological properties, leading to
cytotoxic and antiproliferative effects on other cancer cells,
including fibrosarcoma, prostate cancer, lung cancer, melanoma,
colorectal cancer, liver cancer, breast cancer, and cervical cancer
(30–32). The β-carboline alkaloid derivative
dehydrocrenatidine (DC) is predominantly isolated from Picrasma
quassioides (D. Don) Benn (PQ). Studies have shown that the
toxic properties of β-carboline alkaloid derivatives in PQ lead to
apoptosis in HepG2 cells (8). Our
study revealed that DC cytotoxicity in NPC cell lines was dose- and
time-dependent.
The cell cycle is a conservative biological
mechanism that controls the growth, development, and
differentiation of cells. The cell cycle is mainly adjusted by the
cyclin-CDK complex, checkpoint kinase, and CDK inhibitor. Cell
cycle disorder is a sign of transformation of normal cells into
tumor cells (33). Cyclin A is
particularly crucial in cyclins because it participates in the S
phase and mitosis, which are related to CDC2 (also known as CDK1)
and CDK2; moreover, its expression is increased in many tumors
(34). Cyclin A/CDK1 kinase is a
factor that triggers mitosis. Vigneron et al confirmed that
Bora phosphorylation of cyclin A/cdk1 is both necessary and
sufficient for mitosis formation (35). The nuclear translocation of cyclin B
plays a crucial role in promoting mitosis. The cyclin B/CDK1
complex controls the G2-M phase transition and is essential for
initiating mitosis in patients with breast cancer (36). During G2, CDK1 bind to cyclin B mainly
through the activation of the complex, which requires cdc25c
phosphatase to dephosphorylate cdc2 at the Tyr15 site. Furthermore,
cyclin B/CDK1 remains inactivated by WEE1/Myt1-dependent
phosphorylation of Tyr15 of cdk1 (37–39). Mota
et al demonstrated that harmine is a β-carboline alkaloid,
which was confirmed to be a specific inhibitor of CDK1/cyclin B and
CDK2/cyclin A, which may explain the significant reduction of cells
in the S phase and cell cycle arrest in the G2/M phase (40). Notably, in our study, DC induced G2/M
blockage of NPC-039 and NPC-BM cell apoptosis by reducing the
expression of cyclin A and B and phosphorylated cdc2. However, DC
was found to directly lead to the reduction of phosphorylated WEE1
and Myt1 proteins. In the G1 phase, CDK4/6-cyclin D initiates cell
cycle progression through RB phosphorylation and chelation of p21
and p27 to release CDK2-cyclin E complex and promote CDK2 kinase
activity (33). The aforementioned
kinase complexes can phosphorylate RB1 together to release E2F to
mediate the transition to the S phase (41). As demonstrated by Ahmad et al,
β-carboline alkaloids that inhibit G0/G1 transition in cancer cells
are believed to inhibit cyclin D1/D3 and reduce CDK4, CDK6, and
cyclin E expression in HeLa cells (42). Similarly, our study revealed that DC
induced apoptosis by reducing the expression of complex
CDK4/6-cyclin D3 protein, thereby inhibiting the expression of RB
phosphorylation. Consistent with our results, Cao et al
indicated that β-carboline alkaloid derivatives and harmine altered
the cell cycle distribution by reducing the ratio of cells in the
G0/G1 and increasing the ratio in the S and G2/M phases (43). Further mechanistic studies by
Abdelsalam et al showed that β-carboline alkaloid
derivatives can trigger sub-G1 upregulation and cause MDA-MB435
cell cycle arrest (44). In
particular, our results showed that DC induced the number of cells
in the sub-G1 phase and led to apoptosis. In this study, it was
found that DC inhibited the expression of cell cycle check point
proteins. However, this was only evident at 100 µM, under which
condition the cells barely survived. To note, when treated with DC
at 25 and 50 µM, some proteins (including cyclin B, cyclinD3,
p-WEE1 and p-Rb) were upregulated in the two cell lines. We
hypothesize that this situation was due to cell cycle arrest at
different stages of the cell cycle when the cells were treated with
low concentrations (25 or 50 µM) of DC at the same time point,
resulting in different expression of cell cycle regulatory
proteins. This situation lacked consistency across doses, cell
lines and time points.
Apoptosis is the main type of cell death that occurs
when DNA repair is irreversible and includes external and internal
pathways. According to the present data, DNA damage was induced
after DC treatment in the NPC cell lines. The extrinsic pathway is
mediated by a subgroup of the tumor necrosis factor receptor (TNFR)
superfamily, including TNFR, Fas, and TRAIL (45,46). TRAIL
induces apoptosis through interaction with its receptors to induce
membrane protein receptors, including DR4, DR5, DcR1, DcR2, and
osteoprotegerin (47,48). The apoptotic signal transduction
mechanism of TNFR1 is similar to Fas, mainly through the
combination of a complex (FADD, caspase-8 and RIP cleavage), which
are essential for the apoptotic signal transduction of Fas and
TNF-R1 (49,50). However, FADD, TNF-R1, DcR2, cleaved
RIP, and DR5 were increased in DC-induced apoptosis, a similar
finding as in other studies (51–53). The
Bcl-2 family proteins play a key role in adjusting the
mitochondrial pathway, with particular effect on the antiapoptotic
members (Bcl-2 and Bcl-xL) and proapoptotic molecules (Bax, Bak,
Bad, and BH3 domains). However, these proteins are connected to the
mitochondrial pathway (t-Bid, Bim, Puma, and Noxa) through the
death receptor pathway (54,55). Weber indicated that some traditional
Chinese medicine compounds whose therapeutic mechanisms are
relatively well characterized kill tumor cells through apoptosis
induction (55). The β-carboline
derivative, harmine, significantly increased the level of active
proteins, including caspase-3, −8, and −9, PARP, and Bax, and
reduces Bcl-xL expression in different cancers (56–59). The
present results showed that DC significantly enhanced the
expression of proapoptotic regulatory proteins, including Bax, Bak,
and t-Bid, and reduced the expression of antiapoptotic factor
protein Bcl-xL. DC increased the expression of caspase-3, −8, and
−9 and PARP protein in a dose-dependent manner and promoted cell
apoptosis in NPC-039 and NPC-BM cells. Thus, DC triggers apoptosis
through the activation of the caspase pathway and induces the
expression of proapoptotic and antiapoptotic proteins.
The MAPK signaling pathway regulates various
biological processes through various cellular mechanisms, including
the main proapoptotic and antiapoptotic mechanisms regulated by
MAPK (60,61). Studies have shown that compounds of
traditional herbal medicines induce apoptosis in different cancers
through the JNK/ERK/MAPK signaling pathway (62,63). Lee
et al demonstrated that PFHxS activation at different times
increased the activation of ERK1/2, JNK, and p38 MAPK. Notably, ERK
inhibitors significantly reduced apoptosis, whereas JNK inhibitors
increased apoptosis (64). Our
results showed that the co-treatment with ERK1/2 inhibitor resulted
in a significantly higher reduction of apoptosis-related proteins
in both cell lines as compared to the DC treatment alone, whereas
the co-treatment with JNK1/2 inhibitor resulted in a significantly
higher induction of apoptosis-related proteins in both cells as
compared to the DC treatment alone. Thus, DC-mediated MAPK signal
transduction induced apoptosis of human NPC cells. Given that
radiation therapy and chemotherapy are the cornerstone in NPC
treatment. Further check the potential synergistic of DC with
radiation therapy and chemotherapy will increase the value of
research. According to the ROS data (Fig. S1), DC treatment did not increase ROS
production. Therefore, DC induces DNA damage and then causes
apoptosis. In this study, our results suggested that DC induces
G2/M cell cycle arrest. Previous studies have shown that DNA damage
cues activate the sensory DNA-PK/ATM/ATR kinases (65,66), which
relay inhibits progression into mitosis and involves
phosphorylation of p53 (67,68) that ultimately serve to inactivate the
cyclin B-cdc2 complex. Therefore, ATM/ATR and p53 may be potential
targets of DC.
In conclusion, the study results showed that DC
inhibited the proliferation of human NPC cells through induced DNA
damage, caused an increase in death receptor expression and
mitochondrial membrane depolarization, adjustment of the MAPK
pathway, induction of cell cycle arrest and apoptosis. Notably,
this is the first anti-nasopharyngeal cancer study on the natural
Chinese herbal medicine β-hydrocarbon alkaloid DC against NPC. The
lack of in vivo experiments was a potential limitation to
the present study. However, many published article have suggested
the antitumor functions and its biological relevance of pure
compounds in nasopharyngeal carcinoma in vitro and in
vivo (69–71). These in vivo study groups
received a daily intraperitoneal injection in animal model,
therefore, it can eliminate the problem of poor bioavailability of
natural compounds. We deduce that DC may be a promising anticancer
drug.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from National
Science Council, Taiwan (grant no. MOST 109-2314-B-371-005) and
Changhua Christian Hospital (grant no. 109-CCH-MST-161).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MCH, MJH and JTL conceptualized and designed the
study. CCL, YCC, YSL and HYH. acquired, analyzed and interpreted
the data. MCH, CCL and MJH drafted and revised the manuscript. MJH
and JTL had overall responsibility for the published work. MJH and
CCL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du T, Xiao J, Qiu Z and Wu K: The
effectiveness of intensity-modulated radiation therapy versus 2D-RT
for the treatment of nasopharyngeal carcinoma: A systematic review
and meta-analysis. PLoS One. 14:e02196112019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YP, Chan AT, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan KC, Woo JK, King A, Zee BC, Lam WK,
Chan SL, Chu SW, Mak C, Tse IO, Leung SY, et al: Analysis of plasma
Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl
J Med. 377:513–522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsang RK: Nasopharyngeal
carcinoma-improving cure with technology and clinical trials. World
J Otorhinolaryngol Head Neck Surg. 6:1–3. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun XS, Li XY, Chen QY, Tang LQ and Mai
HQ: Future of radiotherapy in nasopharyngeal carcinoma. Br J
Radiol. 92:201902092019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo SS, Hu W, Chen QY, Li JM, Zhu SH, He
Y, Li JW, Xia L, Ji L, Lin CY, et al: Pretreatment quality of life
as a predictor of survival for patients with nasopharyngeal
carcinoma treated with IMRT. BMC Cancer. 18:1142018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu LT, Liang YJ, Guo SS, Mo HY, Guo L,
Wen YF, Xie HJ, Tang QN, Sun XS, Liu SL, et al: Induction
chemotherapy followed by radiotherapy versus concurrent
chemoradiotherapy in the treatment of different risk locoregionally
advanced nasopharyngeal carcinoma. Ther Adv Med Oncol.
12:17588359209282142020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao WY, Shang XY, Zhao L, Yao GD, Sun Z,
Huang XX and Song SJ: Bioactivity-guided isolation of β-carboline
alkaloids with potential anti-hepatoma effect from Picrasma
quassioides (D. Don) Benn. Fitoterapia. 130:66–72. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HE, Choi ES, Shin JA, Kim LH, Cho NP
and Cho SD: Apoptotic effect of methanol extract of Picrasma
quassioides by regulating specificity protein 1 in human
cervical cancer cells. Cell Biochem Funct. 32:229–235. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie DP, Gong YX, Jin YH, Ren CX, Liu Y,
Han YH, Jin MH, Zhu D, Pan QZ, Yu LY, et al: Anti-tumor properties
of Picrasma quassioides extracts in H-RasG12V
liver cancer are mediated through ROS-dependent mitochondrial
dysfunction. nticancer Res. 40:3819–3830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin NR, Shin IS, Jeon CM, Hong JM, Oh SR,
Hahn KW and Ahn KS: Inhibitory effects of Picrasma
quassioides (D.Don) Benn. On airway inflammation in a murine
model of allergic asthma. Mol Med Rep. 10:1495–1500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Y and Wink M: The beta-carboline
alkaloid harmine inhibits BCRP and can reverse resistance to the
anticancer drugs mitoxantrone and camptothecin in breast cancer
cells. Phytother Res. 24:146–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao F, Tang Q, Xu J, Wang S, Li S, Zou X
and Cao Z: Dehydrocrenatidine inhibits voltage-gated sodium
channels and ameliorates mechanic allodia in a rat model of
neuropathic pain. Toxins (Basel). 11:2292019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao WY, Chen JJ, Zou CX, Zhou WY, Yao GD,
Wang XB, Lin B, Huang XX and Song SJ: Effects of enantiomerically
Pure β-carboline alkaloids from Picrasma quassioides on
human hepatoma cells. Planta Med. 85:648–656. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao F, Gao Z, Jiao W, Chen L, Chen L and
Yao X: In vitro anti-inflammatory effects of beta-carboline
alkaloids, isolated from Picrasma quassioides, through
inhibition of the iNOS pathway. Planta Med. 78:1906–1911. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao WH, Chen GD, Gao H, Li J, Gu BB, Xu
TT, Yu HB, Shi GH, Yang F, Yao XS and Lin HW: (±)-Quassidines I and
J, two pairs of cytotoxic bis-β-carboline alkaloid enantiomers from
Picrasma quassioides. J Nat Prod. 78:125–130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong YX, Liu Y, Jin YH, Jin MH, Han YH, Li
J, Shen GN, Xie DP, Ren CX, Yu LY, et al: Picrasma
quassioides extract elevates the cervical cancer cell apoptosis
through ROS-mitochondrial axis activated p38 MAPK signaling
pathway. In Vivo. 34:1823–1833. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Zhu N, Du Y, Bai Q, Chen X, Nan
J, Qin X, Zhang X, Hou J, Wang Q and Yang J: Dehydrocrenatidine is
a novel janus kinase inhibitor. Mol Pharmacol. 87:572–581. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao SK, Perng YP, Shen YC, Chung PJ,
Chang YS and Wang CH: Chromosomal abnormalities of a new
nasopharyngeal carcinoma cell line (NPC-BM1) derived from a bone
marrow metastatic lesion. Cancer Genet Cytogenet. 103:52–58. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu YT, Chuang YC, Lo YS, Lin CC, His YT,
Hsieh MJ and Chen MK: Asiatic acid, extracted from centella
asiatica and induces apoptosis pathway through the phosphorylation
p38 mitogen-activated protein kinase in cisplatin-resistant
nasopharyngeal carcinoma cells. Biomolecules. 10:1842020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jana A, Das A, Krett NL, Guzman G, Thomas
A, Mancinelli G, Bauer J, Ushio-Fukai M, Fukai T and Jung B:
Nuclear translocation of Atox1 potentiates activin A-induced cell
migration and colony formation in colon cancer. PLoS One.
15:e02279162020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YT, Hsieh MJ, Chen PN, Weng CJ, Yang
SF and Lin CW: Erianin induces apoptosis and autophagy in oral
squamous cell carcinoma cells. Am J Chin Med. 48:183–200. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsieh MY, Hsieh MJ, Lo YS, Lin CC, Chuang
YC, Chen MK and Chou MC: Modulating effect of coronarin D in
5-fluorouracil resistance human oral cancer cell lines induced
apoptosis and cell cycle arrest through JNK1/2 signaling pathway.
Biomed Pharmacother. 128:1103182020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tunc D, Dere E, Karakas D, Cevatemre B,
Yilmaz VT and Ulukaya E: Cytotoxic and apoptotic effects of the
combination of palladium (II) 5,5-diethylbarbiturate complex with
bis(2-pyridylmethyl)amine and curcumin on non small lung cancer
cell lines. Bioorg Med Chem. 25:1717–1723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Fong AH, Liu N and Cho WC:
Molecular subtyping of nasopharyngeal carcinoma (NPC) and a
microRNA-based prognostic model for distant metastasis. J Biomed
Sci. 25:162018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun PY, Chen YH, Feng XB, Yang CX, Wu F
and Wang RS: High-dose static and dynamic intensity-modulated
radiotherapy combined with chemotherapy for patients with locally
advanced nasopharyngeal carcinoma improves survival and reduces
brainstem toxicity. Med Sci Monit. 24:8849–8859. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Jiang C, Wang L, Yan F, Sun Q, Ye
Z, Liu T, Fu Z and Jiang Y: Influence of concurrent chemotherapy on
locoregionally advanced nasopharyngeal carcinoma treated with
neoadjuvant chemotherapy plus intensity-modulated radiotherapy: A
retrospective matched analysis. Sci Rep. 10:24892020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL,
Lv JW, Huang XD, Liu RQ, Chen F, He XJ, et al: Long noncoding RNA
FAM225A promotes nasopharyngeal carcinoma tumorigenesis and
metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and
upregulate ITGB3. Cancer Res. 79:4612–4626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jain CK, Majumder HK and Roychoudhury S:
Natural compounds as anticancer agents targeting DNA
topoisomerases. Curr Genomics. 18:75–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao R, Peng W, Wang Z and Xu A:
beta-Carboline alkaloids: Biochemical and pharmacological
functions. Curr Med Chem. 14:479–500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mansoor TA, Ramalho RM, Mulhovo S,
Rodrigues CM and Ferreira MJ: Induction of apoptosis in HuH-7
cancer cells by monoterpene and beta-carboline indole alkaloids
isolated from the leaves of tabernaemontana elegans. Bioorg Med
Chem Lett. 19:4255–4258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bemis DL, Capodice JL, Gorroochurn P, Katz
AE and Buttyan R: Anti-prostate cancer activity of a beta-carboline
alkaloid enriched extract from rauwolfia vomitoria. Int J Oncol.
29:1065–1073. 2006.PubMed/NCBI
|
|
33
|
Bai J, Li Y and Zhang G: Cell cycle
regulation and anticancer drug discovery. Cancer Biol Med.
14:348–362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yam CH, Fung TK and Poon RY: Cyclin A in
cell cycle control and cancer. Cell Mol Life Sci. 59:1317–1326.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vigneron S, Sundermann L, Labbé JC,
Pintard L, Radulescu O, Castro A and Lorca T: Cyclin
A-cdk1-dependent phosphorylation of bora is the triggering factor
promoting mitotic entry. Dev Cell. 45:637–650.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun X, Zhangyuan G, Shi L, Wang Y, Sun B
and Ding Q: Prognostic and clinicopathological significance of
cyclin B expression in patients with breast cancer: A
meta-analysis. Medicine (Baltimore). 96:e68602017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ,
Golkar L, Milam B, Talamonti MS, Bell RH Jr, Iwamura T and Adrian
TE: Apigenin inhibits pancreatic cancer cell proliferation through
G2/M cell cycle arrest. Mol Cancer. 5:762006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pomerening JR, Kim SY and Ferrell JE Jr:
Systems-level dissection of the cell-cycle oscillator: Bypassing
positive feedback produces damped oscillations. Cell. 122:565–578.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sha W, Moore J, Chen K, Lassaletta AD, Yi
CS, Tyson JJ and Sible JC: Hysteresis drives cell-cycle transitions
in xenopus laevis egg extracts. Proc Natl Acad Sci USA.
100:975–980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mota NSRS, Kviecinski MR, Felipe KB,
Grinevicius VMAS, Siminski T, Almeida GM, Zeferino RC, Pich CT,
Filho DW and Pedrosa RC: β-carboline alkaloid harmine induces DNA
damage and triggers apoptosis by a mitochondrial pathway: Study in
silico, in vitro and in vivo. Int J Funct Nutr. 1:12020.
|
|
41
|
Johnson J, Thijssen B, McDermott U,
Garnett M, Wessels LF and Bernards R: Targeting the RB-E2F pathway
in breast cancer. Oncogene. 35:4829–4835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahmad I, Fakhri S, Khan H, Jeandet P,
Aschner M and Yu ZL: Targeting cell cycle by β-carboline alkaloids
in vitro: Novel therapeutic prospects for the treatment of cancer.
Chem Biol Interact. 330:1092292020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao MR, Li Q, Liu ZL, Liu HH, Wang W, Liao
XL, Pan YL and Jiang JW: Harmine induces apoptosis in HepG2 cells
via mitochondrial signaling pathway. Hepatobiliary Pancreat Dis
Int. 10:599–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdelsalam MA, AboulWafa OM, Badawey EA,
El-Shoukrofy MS, El-Miligy MM and Gouda N: Design and synthesis of
some β-carboline derivatives as multi-target anticancer agents.
Future Med Chem. 10:2791–2814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Elrod HA and Sun SY: Modulation of death
receptors by cancer therapeutic agents. Cancer Biol Ther.
7:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kischkel FC, Lawrence DA, Chuntharapai A,
Schow P, Kim KJ and Ashkenazi A: Apo2L/TRAIL-dependent recruitment
of endogenous FADD and caspase-8 to death receptors 4 and 5.
Immunity. 12:611–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin Y, Devin A, Rodriguez Y and Liu ZG:
Cleavage of the death domain kinase RIP by caspase-8 prompts
TNF-induced apoptosis. Genes Dev. 13:2514–2526. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deng Z, Gao P, Yu L, Ma B, You Y, Chan L,
Mei C and Chen T: Ruthenium complexes with phenylterpyridine
derivatives target cell membrane and trigger death
receptors-mediated apoptosis in cancer cells. Biomaterials.
129:111–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Derakhshan A, Chen Z and Van Waes C:
Therapeutic small molecules target inhibitor of apoptosis proteins
in cancers with deregulation of extrinsic and intrinsic cell death
pathways. Clin Cancer Res. 23:1379–1387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han NN, Zhou Q, Huang Q and Liu KJ:
Carnosic acid cooperates with tamoxifen to induce apoptosis
associated with caspase-3 activation in breast cancer cells in
vitro and in vivo. Biomed Pharmacother. 89:827–837. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Debatin KM: Apoptosis pathways in cancer
and cancer therapy. Cancer Immunol Immunother. 53:153–159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li-Weber M: Targeting apoptosis pathways
in cancer by Chinese medicine. Cancer Lett. 332:304–312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li C, Wang Y, Wang C, Yi X, Li M and He X:
Anticancer activities of harmine by inducing a pro-death autophagy
and apoptosis in human gastric cancer cells. Phytomedicine.
28:10–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hamsa TP and Kuttan G: Harmine activates
intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma.
Chin Med. 6:112011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zaid H, Silbermann M, Amash A, Gincel D,
Abdel-Sattar E and Sarikahya NB: Medicinal plants and natural
active compounds for cancer chemoprevention/chemotherapy. Evid
Based Complement Alternat Med. 2017:79524172017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang P, Huang CR, Wang W, Zhang XK, Chen
JJ, Wang JJ, Lin C and Jiang JW: Harmine hydrochloride triggers G2
phase arrest and apoptosis in MGC-803 cells and SMMC-7721 cells by
upregulating p21, activating caspase-8/Bid, and downregulating
ERK/bad pathway. Phytother Res. 30:31–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yue J and López JM: Understanding MAPK
signaling pathways in apoptosis. Int J Mol Sci. 21:23462020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fan Y, Patima A, Chen Y, Zeng F, He W, Luo
L, Jie Y, Zhu Y, Zhang L, Lei J, et al: Cytotoxic effects of
β-carboline alkaloids on human gastric cancer SGC-7901 cells. Int J
Clin Exp Med. 8:12977–12982. 2015.PubMed/NCBI
|
|
63
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamine-induced apoptosis and G2/M arrest in human colorectal
carcinoma cells: A structure-activity study of evodiamine. PLoS
One. 9:e997292014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee YJ, Choi SY and Yang JH: NMDA
receptor-mediated ERK 1/2 pathway is involved in PFHxS-induced
apoptosis of PC12 cells. Sci Total Environ. 491-492:227–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Al-Ejeh F, Kumar R, Wiegmans A, Lakhani
SR, Brown MP and Khanna KK: Harnessing the complexity of DNA-damage
response pathways to improve cancer treatment outcomes. Oncogene.
29:6085–6098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Boutros R, Lobjois V and Ducommun B: CDC25
phosphatases in cancer cells: Key players? Good targets? Nat Rev
Cancer. 7:495–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Freed-Pastor WA, Mizuno H, Zhao X,
Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li
W, Polotskaia A, et al: Mutant p53 disrupts mammary tissue
architecture via the mevalonate pathway. Cell. 148:244–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Freed-Pastor WA and Prives C: Mutant p53:
One name, many proteins. Genes Dev. 26:1268–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fang EF, Zhang CZ, Ng TB, Wong JH, Pan WL,
Ye XJ, Chan YS and Fong WP: Momordica Charantia lectin, a type II
ribosome inactivating protein, exhibits antitumor activity toward
human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer
Prev Res (Phila). 5:109–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Song Y, Yang J, Bai WL and Ji WY:
Antitumor and immunoregulatory effects of astragalus on
nasopharyngeal carcinoma in vivo and in vitro. Phytother Res.
25:909–915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zeng M, Wu X, Li F, She W, Zhou L, Pi B,
Xu Z and Huang X: Laminaria japonica polysaccharides effectively
inhibited the growth of nasopharyngeal carcinoma cells in vivo and
in vitro study. Exp Toxicol Pathol. 69:527–532. 2017. View Article : Google Scholar : PubMed/NCBI
|