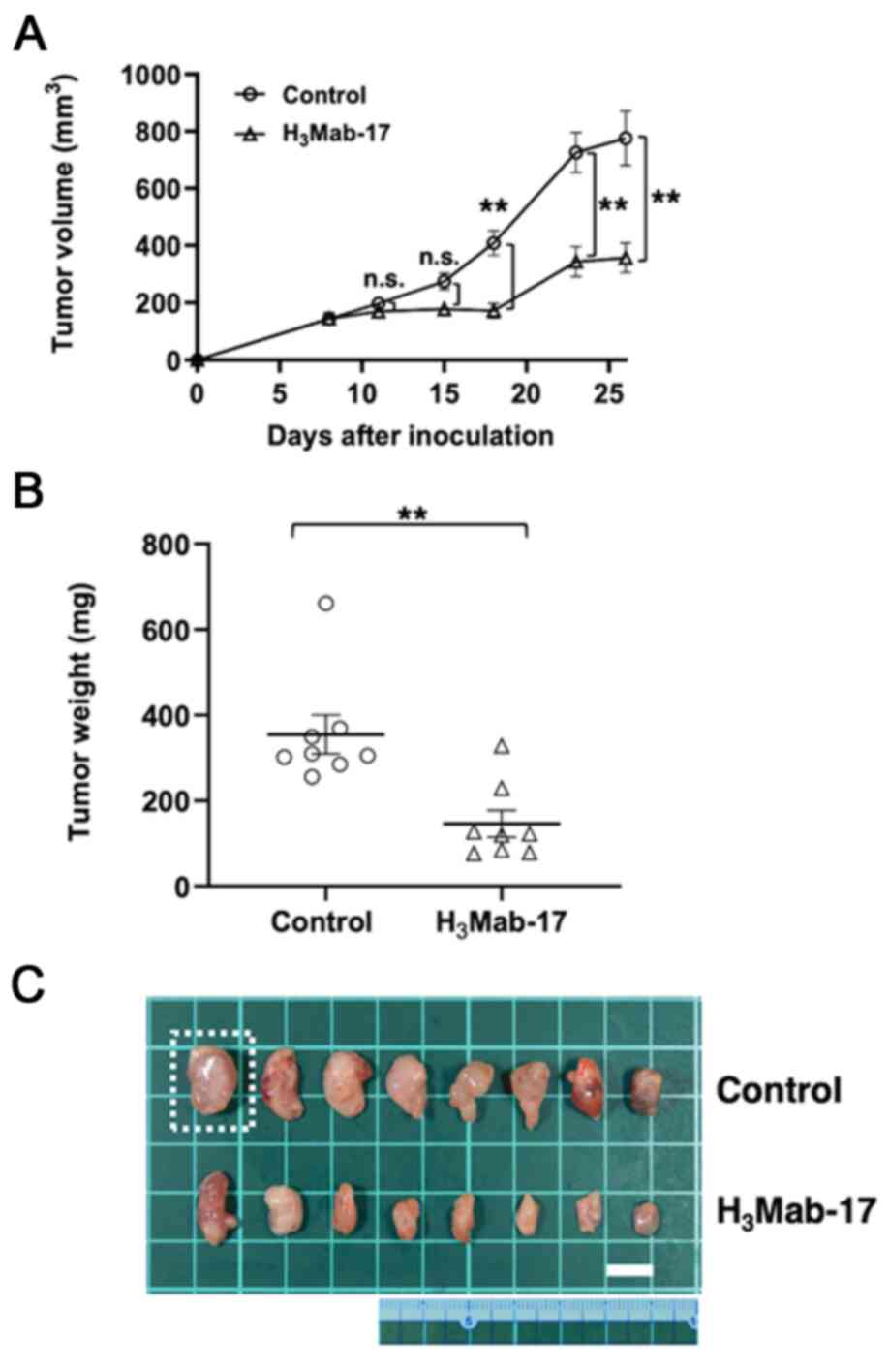
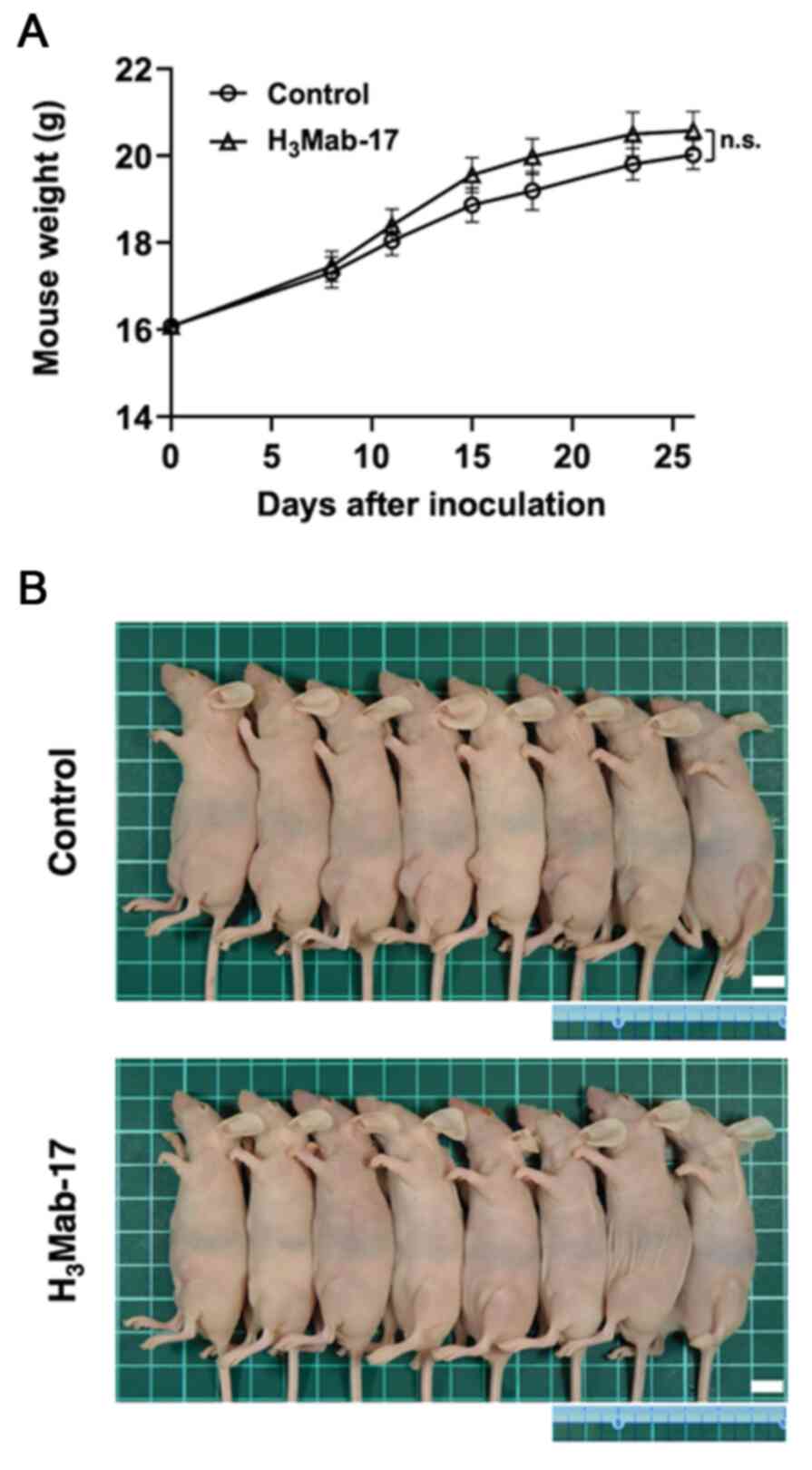
|
1
|
Gschwind A, Fischer OM and Ullrich A: The
discovery of receptor tyrosine kinases: Targets for cancer therapy.
Nat Rev Cancer. 4:361–370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schlessinger J: Ligand-induced,
receptor-mediated dimerization and activation of EGF receptor.
Cell. 110:669–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: A model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schreiber AB, Libermann TA, Lax I, Yarden
Y and Schlessinger J: Biological role of epidermal growth
factor-receptor clustering. Investigation with monoclonal
anti-receptor antibodies. J Biol Chem. 258:846–853. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linggi B and Carpenter G: ErbB receptors:
New insights on mechanisms and biology. Trends Cell Biol.
16:649–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris RC, Chung E and Coffey RJ: EGF
receptor ligands. Exp Cell Res. 284:2–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Citri A, Skaria KB and Yarden Y: The deaf
and the dumb: The biology of ErbB-2 and ErbB-3. Exp Cell Res.
284:54–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guy PM, Platko JV, Cantley LC, Cerione RA
and Carraway KL III: Insect cell-expressed p180erbB3 possesses an
impaired tyrosine kinase activity. Proc Natl Acad Sci USA.
91:8132–8136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holbro T, Beerli RR, Maurer F, Koziczak M,
Barbas CF 3rd and Hynes NE: The ErbB2/ErbB3 heterodimer functions
as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor
cell proliferation. Proc Natl Acad Sci USA. 100:8933–8938. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akhtar S, Chandrasekhar B, Attur S,
Dhaunsi GS, Yousif MH and Benter IF: Transactivation of ErbB family
of receptor tyrosine kinases is inhibited by angiotensin-(1–7) via
its mas receptor. PLoS One. 10:e01416572015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ceresa BP and Vanlandingham PA: Molecular
mechanisms that regulate epidermal growth factor receptor
inactivation. Clin Med Oncol. 2:47–61. 2008.PubMed/NCBI
|
|
13
|
Henriksen L, Grandal MV, Knudsen SL, van
Deurs B and Grøvdal LM: Internalization mechanisms of the epidermal
growth factor receptor after activation with different ligands.
PLoS One. 8:e581482013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bublil EM and Yarden Y: The EGF receptor
family: Spearheading a merger of signaling and therapeutics. Curr
Opin Cell Biol. 19:124–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yarden Y and Pines G: The ERBB network: At
last, cancer therapy meets systems biology. Nat Rev Cancer.
12:553–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hendler FJ and Ozanne BW: Human squamous
cell lung cancers express increased epidermal growth factor
receptors. J Clin Invest. 74:647–651. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kraus MH, Popescu NC, Amsbaugh SC and King
CR: Overexpression of the EGF receptor-related proto-oncogene
erbB-2 in human mammary tumor cell lines by different molecular
mechanisms. EMBO J. 6:605–610. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai WW, Chen FF, Wu MH, Chow NH, Su WC, Ma
MC, Su PF, Chen H, Lin MY and Tseng YL: Immunohistochemical
analysis of epidermal growth factor receptor family members in
stage I non-small cell lung cancer. Ann Thorac Surg. 72:1868–1876.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koutsopoulos AV, Mavroudis D, Dambaki KI,
Souglakos J, Tzortzaki EG, Drositis J, Delides GS, Georgoulias V
and Stathopoulos EN: Simultaneous expression of c-erbB-1, c-erbB-2,
c-erbB-3 and c-erbB-4 receptors in non-small-cell lung carcinomas:
Correlation with clinical outcome. Lung Cancer. 57:193–200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies S, Holmes A, Lomo L, Steinkamp MP,
Kang H, Muller CY and Wilson BS: High incidence of ErbB3, ErbB4,
and MET expression in ovarian cancer. Int J Gynecol Pathol.
33:402–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beji A, Horst D, Engel J, Kirchner T and
Ullrich A: Toward the prognostic significance and therapeutic
potential of HER3 receptor tyrosine kinase in human colon cancer.
Clin Cancer Res. 18:956–968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ocana A, Vera-Badillo F, Seruga B,
Templeton A, Pandiella A and Amir E: HER3 overexpression and
survival in solid tumors: A meta-analysis. J Natl Cancer Inst.
105:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledel F, Hallstrom M, Ragnhammar P,
Ohrling K and Edler D: HER3 expression in patients with primary
colorectal cancer and corresponding lymph node metastases related
to clinical outcome. Eur J Cancer. 50:656–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimura H, Minato N, Nakano T and Honjo
T: Immunological studies on PD-1 deficient mice: Implication of
PD-1 as a negative regulator for B cell responses. Int Immunol.
10:1563–1572. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engelhardt JJ, Sullivan TJ and Allison JP:
CTLA-4 overexpression inhibits T cell responses through a
CD28-B7-dependent mechanism. J Immunol. 177:1052–1061. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous Non-Small-Cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Golshani G and Zhang Y: Advances in
immunotherapy for colorectal cancer: A review. Therap Adv
Gastroenterol. 13:17562848209175272020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Y and Freeman GJ: The microsatellite
instable subset of colorectal cancer is a particularly good
candidate for checkpoint blockade immunotherapy. Cancer Discov.
5:16–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ,
Kussie P and Ferguson KM: Structural basis for inhibition of the
epidermal growth factor receptor by cetuximab. Cancer Cell.
7:301–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Flygare JA, Pillow TH and Aristoff P:
Antibody-drug conjugates for the treatment of cancer. Chem Biol
Drug Des. 81:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kute T, Lack CM, Willingham M, Bishwokama
B, Williams H, Barrett K, Mitchell T and Vaughn JP: Development of
Herceptin resistance in breast cancer cells. Cytometry A. 57:86–93.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Clynes RA, Towers TL, Presta LG and
Ravetch JV: Inhibitory Fc receptors modulate in vivo cytotoxicity
against tumor targets. Nat Med. 6:443–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang D, Qian G, Zhang H, Magliocca KR,
Nannapaneni S, Amin AR, Rossi M, Patel M, El-Deiry M, Wadsworth JT,
et al: HER3 targeting sensitizes HNSCC to Cetuximab by reducing
HER3 activity and HER2/HER3 dimerization: Evidence from cell line
and Patient-Derived xenograft models. Clin Cancer Res. 23:677–686.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gandullo-Sánchez L, Capone E, Ocaña A,
Iacobelli S, Sala G and Pandiella A: HER3 targeting with an
antibody-drug conjugate bypasses resistance to anti-HER2 therapies.
EMBO Mol Med. 12:e114982020. View Article : Google Scholar
|
|
46
|
Mirschberger C, Schiller CB, Schräml M,
Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G,
Kolm I, et al: RG7116, a therapeutic antibody that binds the
inactive HER3 receptor and is optimized for immune effector
activation. Cancer Res. 73:5183–5194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sequist LV, Gray JE, Harb WA, Lopez-Chavez
A, Doebele RC, Modiano MR, Jackman DM, Baggstrom MQ, Atmaca A,
Felip E, et al: Randomized Phase II trial of seribantumab in
combination with erlotinib in patients with EGFR wild-type
non-small cell lung cancer. Oncologist. 24:1095–1102. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cejalvo JM, Jacob W, Fleitas Kanonnikoff
T, Felip E, Navarro Mendivil A, Martinez Garcia M, Taus Garcia A,
Leighl N, Lassen U, Mau-Soerensen M, et al: A phase Ib/II study of
HER3-targeting lumretuzumab in combination with carboplatin and
paclitaxel as first-line treatment in patients with advanced or
metastatic squamous non-small cell lung cancer. ESMO Open.
4:e0005322019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koganemaru S, Kuboki Y, Koga Y, Kojima T,
Yamauchi M, Maeda N, Kagari T, Hirotani K, Yasunaga M, Matsumura Y
and Doi T: U3-1402, a Novel HER3-Targeting Antibody-Drug conjugate,
for the treatment of colorectal cancer. Mol Cancer Ther.
18:2043–2050. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Masuda N, Yonemori K, Takahashi S, Kogawa
T, Nakayama T, Iwase H, Takahashi M, Toyama T, Saeki T, Saji S, et
al: Abstract PD1-03: Single agent activity of U3-1402, a
HER3-targeting antibody-drug conjugate, in HER3-overexpressing
metastatic breast cancer: Updated results of a phase 1/2 trial.
Cancer Res. 79:2019.PubMed/NCBI
|
|
51
|
Hashimoto Y, Koyama K, Kamai Y, Hirotani
K, Ogitani Y, Zembutsu A, Abe M, Kaneda Y, Maeda N, Shiose Y, et
al: A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402,
exhibits potent therapeutic efficacy through the delivery of
cytotoxic payload by efficient internalization. Clin Cancer Res.
25:7151–7161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Itai S, Fujii Y, Nakamura T, Chang YW,
Yanaka M, Saidoh N, Handa S, Suzuki H, Harada H, Yamada S, et al:
Establishment of CMab-43, a sensitive and specific Anti-CD133
monoclonal antibody, for immunohistochemistry. Monoclon Antib
Immunodiagn Immunother. 36:231–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kato Y, Ohishi T, Yamada S, Itai S,
Furusawa Y, Sano M, Nakamura T, Kawada M and Kaneko MK: Anti-CD133
Monoclonal Antibody CMab-43 exerts antitumor activity in a mouse
xenograft model of colon cancer. Monoclon Antib Immunodiagn
Immunother. 38:75–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schoeberl B, Faber AC, Li D, Liang MC,
Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al: An
ErbB3 antibody, MM-121, is active in cancers with ligand-dependent
activation. Cancer Res. 70:2485–2494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schoeberl B, Pace EA, Fitzgerald JB, Harms
BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, et al:
Therapeutically targeting ErbB3: A key node in ligand-induced
activation of the ErbB receptor-PI3K axis. Sci Signal. 2:ra312009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Furusawa Y, Yamada S, Itai S, Nakamura T,
Yanaka M, Sano M, Harada H, Fukui M, Kaneko MK and Kato Y:
PMab-219: A monoclonal antibody for the immunohistochemical
analysis of horse podoplanin. Biochem Biophys Rep.
18:1006162019.PubMed/NCBI
|
|
59
|
Furusawa Y, Kaneko MK, Nakamura T, Itai S,
Fukui M, Harada H, Yamada S and Kato Y: Establishment of a
monoclonal antibody PMab-231 for tiger podoplanin. Monoclon Antib
Immunodiagn Immunother. 38:89–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Furusawa Y, Takei J, Sayama Y, Yamada S,
Kaneko MK and Kato Y: Development of an anti-bear podoplanin
monoclonal antibody PMab-247 for immunohistochemical analysis.
Biochem Biophys Rep. 18:1006442019.PubMed/NCBI
|
|
61
|
Furusawa Y, Yamada S, Itai S, Nakamura T,
Takei J, Sano M, Harada H, Fukui M, Kaneko MK and Kato Y:
Establishment of a monoclonal antibody PMab-233 for
immunohistochemical analysis against Tasmanian devil podoplanin.
Biochem Biophys Rep. 18:1006312019.PubMed/NCBI
|
|
62
|
Furusawa Y, Kaneko MK and Kato Y:
Establishment of C20Mab-11, a novel anti-CD20 monoclonal
antibody, for the detection of B cells. Oncol Lett. 20:1961–1967.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yamada S, Itai S, Nakamura T, Yanaka M,
Kaneko MK and Kato Y: Detection of high CD44 expression in oral
cancers using the novel monoclonal antibody, C44Mab-5.
Biochem Biophys Rep. 14:64–68. 2018.PubMed/NCBI
|
|
64
|
Sayama Y, Kaneko MK and Kato Y:
Development and characterization of TrMab-6, a novel anti-TROP2
monoclonal antibody for antigen detection in breast cancer. Mol Med
Rep. 23:922021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sayama Y, Kaneko MK, Takei J, Hosono H,
Sano M, Asano T and Kato Y: Establishment of a novel anti-TROP2
monoclonal antibody TrMab-29 for immunohistochemical analysis.
Biochem Biophys Rep. 25:1009022021.PubMed/NCBI
|
|
66
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4:59242014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y, Yang H and Duan G: HER3
over-expression and overall survival in gastrointestinal cancers.
Oncotarget. 6:42868–42878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stahler A, Heinemann V, Neumann J, Crispin
A, Schalhorn A, Stintzing S, Giessen-Jung C, Fischer von
Weikersthal L, Vehling-Kaiser U, Stauch M, et al: Prevalence and
influence on outcome of HER2/neu, HER3 and NRG1 expression in
patients with metastatic colorectal cancer. Anticancer Drugs.
28:717–722. 2017. View Article : Google Scholar : PubMed/NCBI
|