Introduction
The epidermal growth factor receptor (EGFR) family,
also known as HER or ErbB, has a tyrosine kinase domain in its
intracellular region (1). The EGFR
family transduces extracellular to intracellular signals through
the activation of tyrosine kinase domain (1). By binding to the ligand, the
extracellular domain promotes the formation of homodimers or
heterodimers between the EGFR family receptors (2,3). This
dimerization is essential for the activation of the tyrosine kinase
domain and intracellular signaling pathways such as Ras/MAPK,
PI3K/Akt, and JAK/STAT (4,5).
The EGFR family consists of four members [EGFR
(HER1, ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4)] and
each member has different ligands: EGFR binds to seven ligands such
as EGF, TGF-α, and epigen; HER3 binds to neuregulin1 and
neuregulin2; HER4 binds to seven ligands such as heparin
binding-EGF, betacellulin, and epiregulin. In contrast, there is no
ligand for HER2 (6,7). Although HER3 has a tyrosine kinase
domain, its kinase activity is impaired (8,9).
Therefore, transphosphorylation by other members of the EGFR family
is required to activate HER3. HER3 can form an active heterodimer
with the other three members of the EGFR family (2,10–13).
The EGFR family plays an essential role in
regulating cell growth and in the differentiation, proliferation,
and survival of normal cells. Insufficient EGFR signaling is
associated with Alzheimer's disease and multiple sclerosis
(14), while the overexpression of
EGFR family is associated with the development of tumors (15–17). The
EGFR family has been found to be overexpressed in many cancers as
below: EGFR in breast, non-small cell lung, and prostate cancers
(18); HER2 in breast, colon, lung,
and pancreatic cancers (18); HER3 in
lung, breast, colon, prostate, and stomach cancers (2,19); HER4 in
non-small cell lung, and ovarian cancers (20,21).
Therefore, the EGFR family is thought to be a valid target for
candidates in cancer therapy.
High expression of HER3 is thought to be an
established negative prognostic factor in several solid tumors
including colorectal cancer (22,23).
Metastatic colorectal cancer is one of the most aggressive tumors,
associated with high mortality rates worldwide (24). In a previous study, 79% of primary
tumors were found to present a high HER3 expression and there was a
correlation between HER3 expression in primary tumors and
corresponding lymph node metastases in 236 colorectal cancer
patients (25). In addition, elevated
HER3 expression was associated with shorter overall survival and
disease-free survival in patients with colorectal cancer (25). Furthermore, HER3 downregulation in
colorectal cancer cell lines caused G2-M cell-cycle arrest, leading
to apoptosis and abrogated cell proliferation, migration, and
invasion (22). Altogether, these
results suggest that HER3 can be a potential therapeutic target for
colorectal cancer.
Several monoclonal antibodies (mAbs) have been
established as an innovative immunotherapy against tumors.
Programmed cell death 1 (PD-1) and cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4) are inhibitory receptors
for immune checkpoints, which are expressed on the surface of T
cells (26–29). Anti-PD-1 and anti-CTLA-4 mAbs have
been reported as potential anticancer drugs (30,31).
Nivolumab and pembrolizumab are anti-PD-1 mAbs, which were approved
by the US Food and Drug Administration (32–34). Both
mAbs activate the immune system to attack tumors, by blocking the
interaction between PD-1 and its ligand, PD-L1, which is expressed
in cancer cells. DNA mismatch repair deficient tumors have very
high levels of DNA microsatellite instability, and it is known that
microsatellite instable tumors have highly upregulated expression
of multiple immune checkpoint proteins, including PD-1 compared
with microsatellite stable tumors; therefore, nivolumab and
pembrolizumab are available for the treatment of DNA mismatch
repair deficiency and microsatellite instable subset of colorectal
cancer (35–37).
Several antibody drugs have been developed against
ligands, such as transforming growth factor (TGF)-α and EGF, or
receptors, such as EGFR (38). These
mAbs neutralize the interaction between ligands and receptors.
Antibody-drug conjugate (ADC) is a complex molecule, which is
composed of an antibody, linker, and an anticancer drug, and
delivers the anticancer drug to target cells (39). Moreover, some mAbs possess
antibody-dependent cellular cytotoxicity (ADCC) or
complement-dependent cytotoxicity (CDC). Cetuximab, a mouse/human
chimeric IgG1 against EGFR, binds to the ligand-binding
site of EGFR, and inhibits the activation and dimerization of EGFR
(38). Cetuximab has been used for
the treatment of metastatic colorectal cancer, metastatic non-small
cell lung cancer, and head and neck squamous cell carcinomas
(HNSCC) (38). Trastuzumab, a
humanized mAb against HER2, has been used to treat HER2-positive
cancers, such as breast cancers and gastric cancers (40,41).
Trastuzumab binds to the extracellular domain of HER2 and
downregulates activation of AKT (42). Moreover, trastuzumab exhibited ADCC in
a mouse model (43). However, it has
been shown that some types of cancers are resistant to cetuximab
and trastuzumab (44,45).
Most HNSCCs are resistant to cetuximab, because
cetuximab treatment induces HER2/HER3 dimerization and HER3
activation in HNSCC cell lines (44).
It has been reported that anti-HER3-ADC exerts antitumor effect on
breast cancer cells, which have resistance to trastuzumab (45). For this reason, the development of
anti-HER3 mAbs has been required for cancer therapy. Seribantumab
and lumretuzumab are anti-HER3 mAbs, which block HER3-neuregulin
interaction and inhibit HER3 heterodimerization and phosphorylation
(46,47). Lumretuzumab is also known to have ADCC
activity (46). Phase II and phase
Ib/II trial are now ongoing concerning seribantumab and
lumretuzumab, respectively (47,48). An
anti-HER3-ADC (U3-1402), composed of an anti-HER3 mAb (patritumab)
and a novel topoisomerase I inhibitor (DX-8951 derivative; DXd) has
entered phase I and II trials for the treatment of HER3-positive
non-small cell lung cancers (NCT04676477), metastatic breast
cancers (NCT02980341), and colorectal cancers (NCT04479436)
(49–51). Preliminary results demonstrate that
U3-1402 treatment appears to be safe and exhibits antitumor
activity, suggesting that HER3-targeting therapy may be effective
for HER3-overexpressing metastatic breast cancers (50).
It has been reported that one amino acid
substitution in EGFR in tumors causes acquisition of resistance to
gefitinib after gefitinib treatment (52,53);
therefore, HER3 may also acquire resistance to seribantumab and
lumretuzumab in the future. To characterize the HER3 and
HER3-targeting cancer therapy, the development of further anti-HER3
specific mAbs is required. In this study, we developed a novel
anti-HER3 mAb against colon cancers using a Cell-Based Immunization
and Screening (CBIS) method (54).
Furthermore, we investigated whether a novel anti-HER3 mAb shows
ADCC/CDC activities or antitumor activities for colon cancers.
Materials and methods
Construction of plasmids
The Genome Network Project clone IRAK174J18 (HER3)
was provided by the RIKEN BioResource Research Center through the
National BioResource Project of the MEXT and AMED agencies of
Japan. HER3 DNA plus N-terminal PA16 tag, recognized by NZ-1, was
subcloned into a pCAG-Ble vector (FUJIFILM Wako Pure Chemical
Corp.) and named pCAG/PA16-HER3. HER3 DNA plus C-terminal PA tag,
recognized by NZ-1, was subcloned into a pCAG-Neo vector (FUJIFILM
Wako Pure Chemical Corp.) and named pCAG/HER3-PA.
Cell lines
A mouse myeloma cell line (P3X63Ag8U.1; P3U1),
Chinese hamster ovary (CHO)-K1 cells, a glioblastoma cell line
(LN229), colorectal adenocarcinoma cell lines (Caco-2, LS 174T,
COLO 201, HCT-8, SW1116, and HT-29), and a colorectal carcinoma
cell line (HCT 116) were obtained from the American Type Culture
Collection. Colon adenocarcinoma cell lines (HCT-15, COLO 205, and
DLD-1) and a breast adenocarcinoma cell line (MCF7) were obtained
from the Cell Resource Center for Biomedical Research Institute of
Development, Aging and Cancer at Tohoku University. CHO/PA16-HER3
and CHO/HER3-PA were established by transfecting pCAG/PA16-HER3 and
pCAG/HER3-PA, respectively, into CHO-K1 cells using the Neon
Transfection System (Thermo Fisher Scientific, Inc.). A few days
after transfection, cells positive for anti-HER3 mAb (clone D22C5;
cat. no. 12708; Cell Signaling Technology, Inc.) were sorted using
a cell sorter (SH800; Sony Biotechnology Corp.). CHO/mock (Ble) and
CHO/mock (Neo) were established by transfection of the pCAG-Ble
vector and pCAG-Neo vector, respectively. Stable transfectants of
CHO/mock (Ble) and CHO/PA16-HER3 were cultured at 37°C for 14 days
on media containing 0.5 mg/ml of Zeocin (InvivoGen), and stable
transfectants of CHO/mock (Neo) and CHO/HER3-PA were cultured at
37°C for 14 days on media containing 0.5 mg/ml of G418 (Nacalai
Tesque, Inc.). BINDS-30 [MCF7/HER3-knockout (KO) cells] were
produced using CRISPR/Cas9 plasmids targeting human HER3
(http://www.med-tohoku-antibody.com/topics/001_paper_cell.htm).
Using TruGuide gRNA tool, gRNA of HER3/ERBB3 (NM_001005915) was
selected from GeneArt predesigned gRNAs database (Thermo Fisher
Scientific, Inc.). gRNA sequence used was
GTCCCGTGAGCACAATCTCA(agg), which targeted exon 3 of HER3 (Assay ID:
CRISPR764358). Double strand gRNA sequence was subcloned into
GeneArt CRISPR Nuclease Vector with OFP Reporter (Thermo Fisher
Scientific, Inc.). P3U1, CHO-K1, CHO/PA16-HER3, CHO/HER3-PA, COLO
201, COLO 205, SW1116, DLD-1, MCF7, and BINDS-30 were cultured in
Roswell Park Memorial Institute (RPMI)-1640 media (Nacalai Tesque,
Inc.). LN229, Caco-2, HCT 116, HCT-15, HT-29, LS 174T, and HCT-8
were cultured in Dulbecco's modified Eagle's medium (DMEM; Nacalai
Tesque, Inc.). RPMI-1640 and DMEM were supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific
Inc.), 100 U/ml of penicillin (Nacalai Tesque, Inc.), 100 µg/ml
streptomycin (Nacalai Tesque, Inc.), and 0.25 µg/ml amphotericin B
(Nacalai Tesque, Inc.), and incubated at 37°C in a humidified
atmosphere containing 5% CO2.
Preparation of the purified
antibodies
Purified mouse IgG (cat. no. I8765) and mouse
IgG2a (cat. no. M7769) were purchased from
Sigma-Aldrich; Merck KGaA. An anti-HER3 mAb was purified using
Protein G-Sepharose (GE Healthcare BioSciences).
Hybridoma production
Female BALB/c mice (6 weeks old) were purchased from
CLEA Japan and kept under specific pathogen-free conditions. All
animal experiments were conducted in accordance with the relevant
guidelines and regulations in order to minimize animal suffering
and distress in the laboratory. The Animal Care and Use Committee
of Tohoku University approved all the animal experiments (permit
no. 2019NiA-001). Mice were euthanized by cervical dislocation
under inhalation anesthesia using 2% of isoflurane for both
induction and maintenance, and the death was verified to be
respiratory and cardiac arrest.
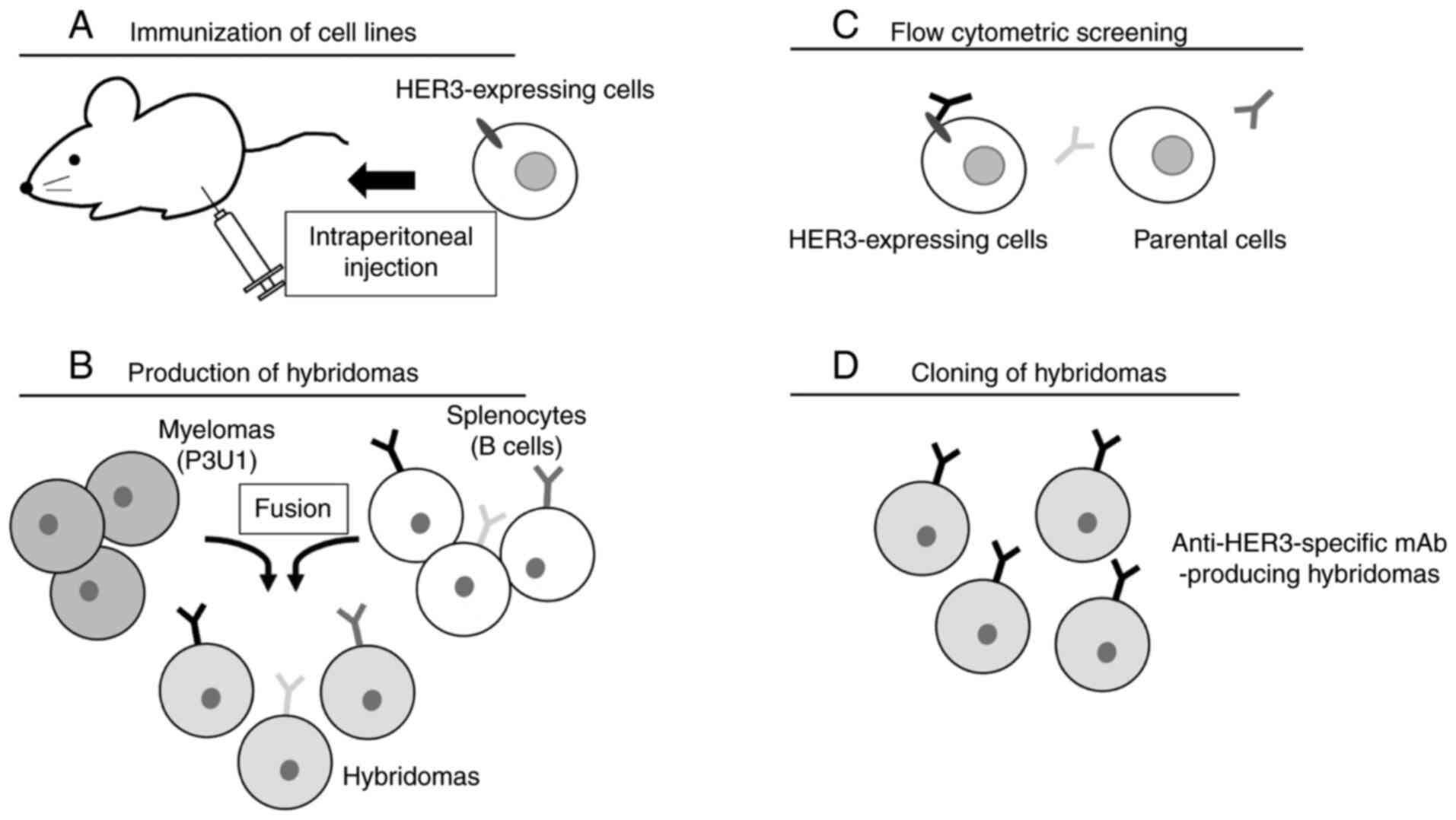
CBIS method was used as previously reported
(54) to develop mAbs against HER3
(Fig. 1). Two eight-week-old BALB/c
female mice were intraperitoneally (i.p.) immunized with
CHO/PA16-HER3 cells (1×108) along with Imject Alum
adjuvant (Thermo Fisher Scientific, Inc.) (Fig. 1A). The procedure included three
additional immunizations, followed by a final booster injection
administered i.p. two days before the spleen cell harvesting.
Spleen cells were then fused with P3U1 cells using PEG1500 (Roche
Diagnostics) (Fig. 1B). The developed
hybridomas were seeded into 96-well plates, and hybridomas were
grown at 37°C for 10 days in RPMI-1640 media with HAT Supplement
(50X) (cat. no. 21060017; Thermo Fisher Scientific, Inc.) for
selection. Supernatants positive for CHO/HER3-PA and negative for
CHO-K1 were selected by flow cytometry (Fig. 1C). After limiting dilution,
supernatants positive for LN229 were selected by flow cytometry.
Finally, anti-HER3 mAb-producing hybridomas were established
(Fig. 1D).
Western blot analysis
Cell pellets were resuspended in phosphate-buffered
saline (PBS; Nacalai Tesque, Inc.) with 1% Triton X-100 (cat. no.
168-11805; FUJIFILM Wako Pure Chemical Corp.) and 50 µg/ml
aprotinin (product no. 03346-84; Nacalai Tesque, Inc.). Cell debris
was removed by centrifugation at 21,880 × g for 10 min at 4°C.
Protein concentration was determined by BCA method. Cell lysates
(10 µg) were boiled in sodium dodecyl sulfate (SDS) sample buffer
(Nacalai Tesque, Inc.). Proteins were electrophoresed on 5–20%
polyacrylamide gels (FUJIFILM Wako Pure Chemical Corp.) and
transferred onto polyvinylidene difluoride (PVDF) membranes (Merck
KGaA). After blocking with 4% skim milk (Nacalai Tesque, Inc.) at
room temperature for 30 min, PVDF membranes were incubated with an
anti-HER3 mAb (diluted 1:1,000; clone D22C5) and anti-β-actin mAb
(1 µg/ml; clone AC-15; cat. no. A1978; Sigma-Aldrich; Merck KGaA)
at room temperature for 30 min, followed by incubation with
peroxidase-conjugated anti-rabbit immunoglobulins (diluted 1:1,000;
cat. no. P0448; Agilent Technologies Inc.) and
peroxidase-conjugated anti-mouse immunoglobulins (diluted 1:1,000;
cat. no. P0260; Agilent Technologies Inc.), respectively, at room
temperature for 30 min. Blots were developed using ImmunoStar LD
(cat. no. 290-69904; FUJIFILM Wako Pure Chemical Corp.) or Pierce™
ECL Plus Western Blotting Substrate (cat. no. 32132; Thermo Fisher
Scientific, Inc.) and imaged with a Sayaca-Imager (DRC Co., Ltd.).
Qcapture Pro software (DRC Co., Ltd) was used for the
densitometry.
Flow cytometry analyses
Cells (2×105 cells/ml) were harvested
after brief exposure to 0.25% trypsin in 1 mM
ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). After
being washed with 0.1% bovine serum albumin (BSA, Nacalai Tesque,
Inc.) in PBS, cells were treated with 1 µg/ml of anti-HER3 mAbs,
for 30 min at 4°C, and with Alexa Fluor 488-conjugated anti-mouse
IgG (1:1,000; cat. no. 4408; Cell Signaling Technology, Inc.).
Fluorescence data were collected using a flow cytometer: the EC800
Cell Analyzer (Sony Biotechnology Corp.).
Determination of the binding
affinity
Cells (2×105 cells/ml) were suspended in
100 µl of serially diluted anti-HER3 mAb (6 ng/ml-25 µg/ml),
followed by the addition of Alexa Fluor 488-conjugated anti-mouse
IgG (1:200). Fluorescence data were collected using a flow
cytometer: The BD FACSLyric (BD Biosciences). The dissociation
constant (KD) was calculated by fitting binding
isotherms to built-in one-site binding models in GraphPad Prism 8
(GraphPad Software, Inc.).
ADCC activity of an anti-HER3 mAb
ADCC inducement by HER3 was assayed as follows. Four
female five-week-old BALB/c nude mice (mean weight, 15±3 g) were
purchased from Charles River Laboratories, Inc. Mice were kept
under specific pathogen-free condition on an 11-h light/13-h dark
cycle at a temperature of 23±2°C and 55±5% humidity with food and
water supplied ad libitum during the experimental periods.
After euthanasia by cervical dislocation, spleens were removed
aseptically, and single-cell suspensions were obtained by forcing
spleen tissues through a sterile cell strainer (product no. 352360;
Corning, Inc.) with a syringe. Erythrocytes were lysed with a
10-sec exposure to ice-cold distilled water. The splenocytes were
washed with DMEM and resuspended in DMEM with 10% FBS; this
preparation was designated as effector cells. The target tumor
cells were labeled with 10 µg/ml Calcein-AM (Thermo Fisher
Scientific, Inc.) and resuspended in the same medium. The target
cells were then transferred to 96-well plates, at 2×104
cells/well, and mixed with effector cells at an effector-to-target
ratio of 100:1, along with 100 µg/ml of anti-HER3 antibodies or
control mouse IgG2a. After a 4.5-h incubation at 37°C,
Calcein release into the supernatant was measured for each well.
Fluorescence intensity was assessed using a microplate reader
(Power Scan HT; BioTek Instruments, Inc.) with an excitation
wavelength of 485 nm and an emission wavelength of 538 nm.
Cytolytic activity was measured as a percentage of lysis and
calculated using the equation: Percentage of lysis (%) =
(E-S)/(M-S) ×100, where E is the fluorescence measured in combined
cultures of target and effector cells, S is the spontaneous
fluorescence of the target cells, and M is the maximum fluorescence
measured after lysis of all cells with buffer containing 0.5%
Triton X-100, 10 mM Tris-HCl (pH 7.4), and 10 mM EDTA. Animal
studies for ADCC were approved by the Institutional Committee for
experiments of the Institute of Microbial Chemistry (permit no.
2020-024).
CDC activity of an anti-HER3 mAb
CDC inducement by HER3 was assayed as follows.
Target cells were labeled with 10 µg/ml Calcein-AM (Thermo Fisher
Scientific, Inc.), resuspended in medium and plated in 96-well
plates, at 2×104 cells/well, with 15% rabbit complement
(Low-Tox-M rabbit complement; Cedarlane Laboratories), 100 µg/ml of
anti-HER3 antibodies, or control IgG (mouse IgG2a) added
to each well. After 4.5 h of incubation at 37°C, Calcein release
into the supernatant was measured for each well. Fluorescence
intensity was calculated as described in the ADCC section
above.
Antitumor activity of an anti-HER3 mAb
in xenografts of colon cancers
Sixteen five-week-old female BALB/c nude mice (mean
weight, 15±3 g) were purchased from Charles River Laboratories,
Inc. All animal experiments were performed in accordance with
institutional guidelines and regulations to minimize animal
suffering and distress in the laboratory. The Institutional
Committee for experiments of the Institute of Microbial Chemistry
(permit no. 2020-024) approved the animal studies for antitumor
activity here described. Mice were maintained in a pathogen-free
environment on an 11-h light/13-h dark cycle at a temperature of
23±2°C and 55±5% humidity with food and water supplied ad
libitum throughout the experiments. Mice were monitored for
health and weight every three or five days. Experiments on mice
were conducted in four weeks. Weight loss exceeding 25% or tumor
volume exceeding 3,000 mm3 were identified as humane
endpoints for euthanasia. At humane and experimental endpoints,
mice were euthanized by cervical dislocation, and death was
verified by validating respiratory and cardiac arrest.
After a one-week acclimation period, these mice were
used in experiments at six weeks of age (mean weight, 16±2 g).
Caco-2 cells (0.3 ml of 1.33×108 cells/ml in DMEM) were
mixed with 0.5 ml BD Matrigel Matrix Growth Factor Reduced (BD
Biosciences), and 100 µl of this suspension (5×106
cells) was injected subcutaneously into the left flank of each
animal. On the eighth day post-inoculation, 16 mice were divided
into two groups (n=8 in each group) with equal mean tumor volume:
An anti-HER3 mAb group or a control mouse IgG group. Then, 100 µg
of an anti-HER3 mAb or control mouse IgG in 100 µl PBS was injected
i.p. Additional antibody inoculations were performed on days 15 and
23. Twenty-six days after cell implantation, all mice were
euthanized by cervical dislocation, and tumor diameters and volumes
were measured and recorded.
Statistical analyses
All data are expressed as mean ± standard error of
the mean (SEM). Statistical analysis was conducted with ANOVA and
Tukey's multiple comparisons tests for ADCC and CDC, ANOVA and
Sidak's multiple comparisons tests for tumor volume and mouse
weight, and Welch's t-test for tumor weight. All calculations were
performed with GraphPad Prism 8 (GraphPad Software, Inc.). A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Development of anti-HER3 mAbs
We employed the CBIS method to develop anti-HER3
mAbs using CHO/PA16-HER3 cells both for the immunization and flow
cytometry screening (Fig. 1). The
developed hybridomas were seeded into 96-well plates and cultivated
for 10 days. Supernatants positive for CHO/HER3-PA and negative for
CHO-K1 were selected by flow cytometry. After limiting dilution and
several additional screenings, an anti-HER3 mAb,
H3Mab-17 (mouse IgG2a, kappa), was finally
established.
Confirmation of HER3 expression by
western blot analysis
We established CHO/mock (Ble), CHO/PA16-HER3,
CHO/mock (Neo), and CHO/HER3-PA, and investigated whether HER3 was
overexpressed in those cell lines. As shown in Fig. 2A, overexpression of HER3 in
CHO/PA16-HER3 and CHO/HER3-PA was confirmed by western blot
analysis using an anti-HER3 mAb (clone D22C5). Endogenous HER3
expression in MCF7 cells was also detected by an anti-HER3 mAb. In
contrast, knockout of endogenous HER3 in BINDS-30 (MCF7/HER3-KO)
was confirmed by western blot analysis using an anti-HER3 mAb.
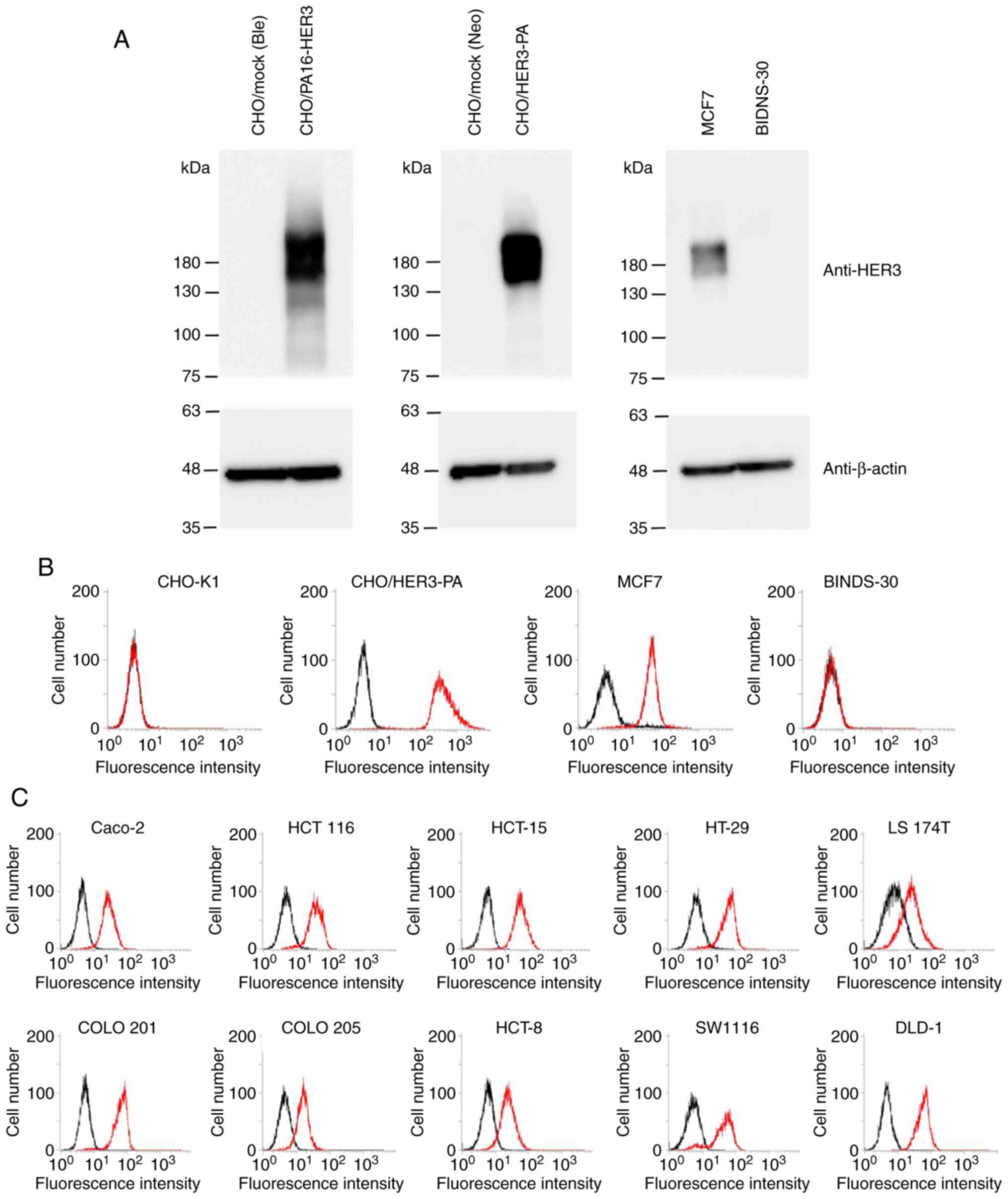 | Figure 2.Characterization of
H3Mab-17. (A) Confirmation of HER3 expression by western
blot analysis. Cell lysates were electrophoresed and transferred
onto PVDF membranes. After blocking, PVDF membranes were incubated
with an anti-HER3 mAb (clone D22C5) or anti-β-actin (clone AC-15),
followed by incubation with peroxidase-conjugated anti-rabbit
immunoglobulins or peroxidase-conjugated anti-mouse
immunoglobulins. Blots were developed using ImmunoStar LD or ECL
Plus Western Blotting Substrate and imaged with a Sayaca-Imager.
(B) Flow cytometry analysis. CHO-K1, CHO/HER3-PA, MCF7, and
BINDS-30 cells were treated with 1 µg/ml of H3Mab-17,
followed by treatment with Alexa Fluor 488-conjugated anti-mouse
IgG. Black line, negative control. (C) Flow cytometry analysis.
Colon cancer cell lines, such as Caco-2, HCT 116, HCT-15, HT-29, LS
174T, COLO 201, COLO 205, HCT-8, SW1116, and DLD-1 cells were
treated with 1 µg/ml of H3Mab-17, followed by treatment
with Alexa Fluor 488-conjugated anti-mouse IgG. Black line,
negative control. mAb, monoclonal antibody. |
Flow cytometry analyses of
H3Mab-17
We performed flow cytometry using
H3Mab-17 against CHO-K1, CHO/HER3-PA, MCF7, and BINDS-30
(MCF7/HER3-KO). H3Mab-17 recognized the CHO/HER3-PA
cells, but not the parental CHO-K1 cells (Fig. 2B). H3Mab-17 also recognized
the endogenous HER3 in MCF7 breast cancer cells (Fig. 2B). The reaction of H3Mab-17
to BINDS-30 was lost after the knockout of HER3 in MCF7 cells
(Fig. 2B), indicating the specificity
of H3Mab-17 for HER3.
Next, we investigated whether H3Mab-17
reacts with colon cancer cell lines. As shown in Fig. 2C, H3Mab-17 reacted with 10
colon cancer cell lines, Caco-2, HCT 116, HCT-15, HT-29, LS 174T,
COLO 201, COLO 205, HCT-8, SW1116, and DLD-1. Among them, Caco-2
was known to be useful for the mouse xenograft model (55). Therefore, we used Caco-2 cells for the
ADCC/CDC assay or in vivo xenograft models.
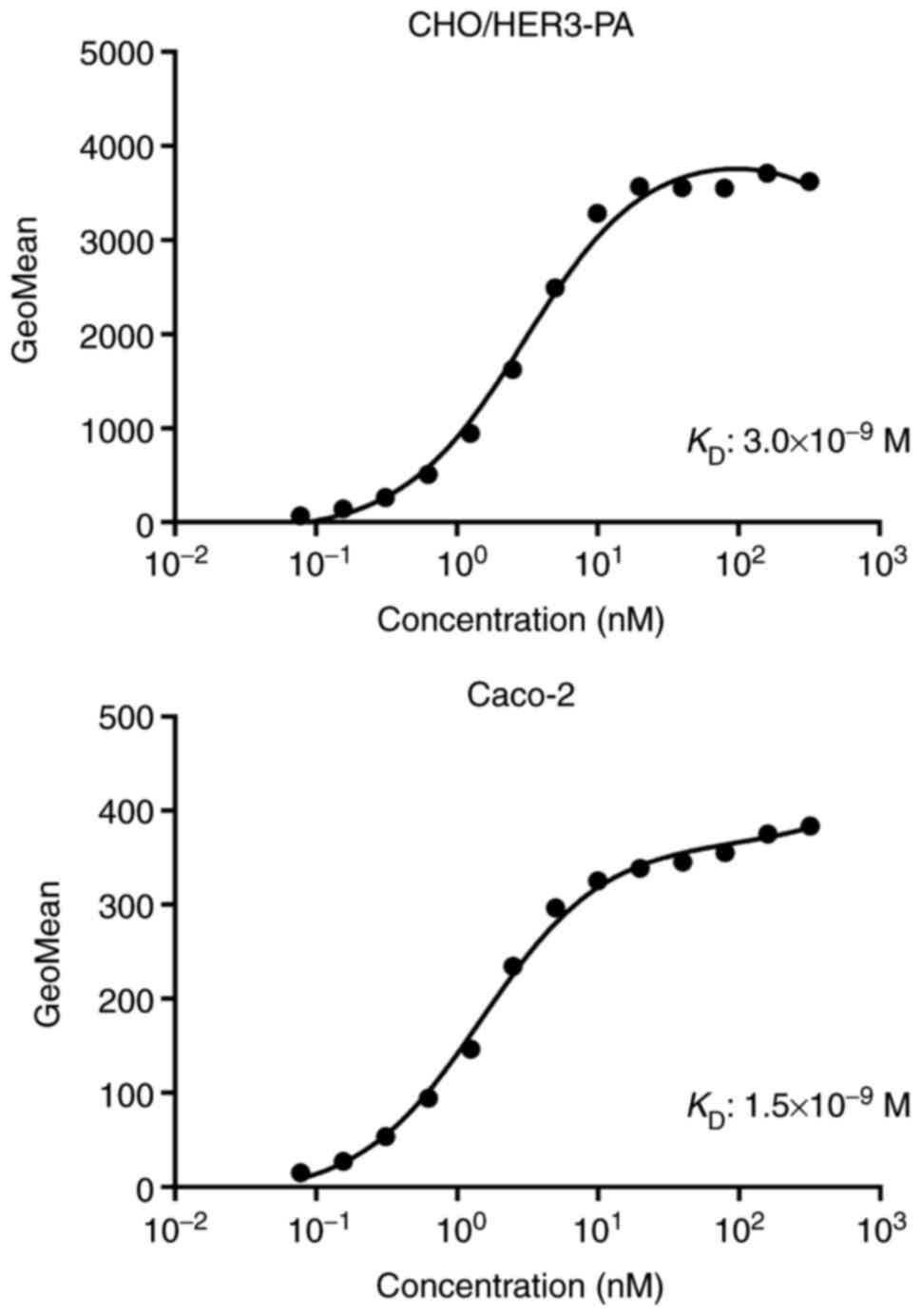
Determination of the binding affinity
of H3Mab-17
A kinetic analysis of the interactions of
H3Mab-17 with CHO/HER3-PA and Caco-2 cells was then
conducted using flow cytometry. The KD for
H3Mab-17 in CHO/HER3-PA and Caco-2 cells were
3.0×10−9 and 1.5×10−9 M, respectively
(Fig. 3), indicating high binding
affinity of H3Mab-17 against HER3-expressing cells.
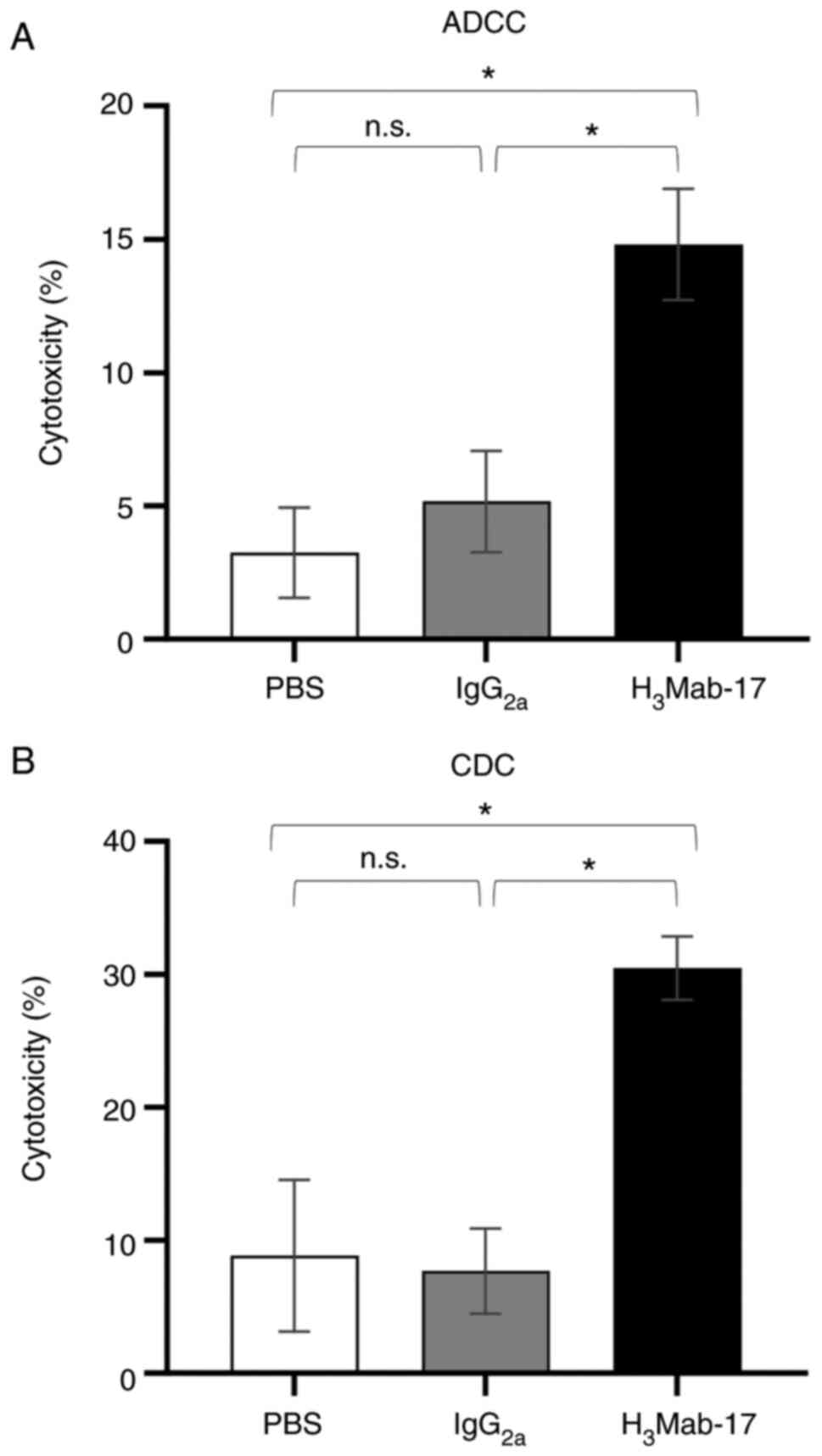
ADCC and CDC activities of
H3Mab-17 in colon cancer cell lines
We then examined whether H3Mab-17 (mouse
IgG2a) induced ADCC and CDC activity in HER3-expressing
Caco-2 colon cancer cell lines. H3Mab-17 exhibited
higher ADCC (14.8% cytotoxicity) in Caco-2 cells than that of
control mouse IgG2a (5.2% cytotoxicity; P<0.05) or
control PBS (3.2% cytotoxicity; P<0.05) treatment (Fig. 4A). H3Mab-17 was also
associated with a more robust CDC activity (30.4% cytotoxicity) in
Caco-2 cells than the control mouse IgG2a (7.7%
cytotoxicity; P<0.05) or the control with PBS treatment (8.8%
cytotoxicity; P<0.05) (Fig. 4B).
These favorable ADCC/CDC activities indicated that
H3Mab-17 may induce strong antitumor activity against
colon cancer cells in vivo as well as in vitro.
Antitumor effect of
H3Mab-17 in mouse xenografts of colon cancer cells
Tumor formation of 16 Caco-2-bearing mice was
observed on day eight. Then, these 16 Caco-2-bearing mice were
divided into an H3Mab-17-treated group and a control
group. On days 8, 15 and 23 after Caco-2 cell injections into the
mice, H3Mab-17 (100 µg) or control mouse IgG (100 µg)
were injected i.p. in the Caco-2 ×enograft model mice. The tumor
volume was measured on days 8, 11, 15, 18, 23 and 26 after the
Caco-2 cell injection. H3Mab-17-treated mice exhibited
significantly less tumor growth on day 18 (P<0.01), day 23
(P<0.01), and day 26 (P<0.01), compared with the IgG-treated
control mice (Fig. 5A). The reduction
in the volume of the tumors by H3Mab-17 treatment was of
54% on day 26. Tumors from the H3Mab-17-treated mice
weighed significantly less than tumors from the IgG-treated control
mice (59% reduction, P<0.01; Fig.
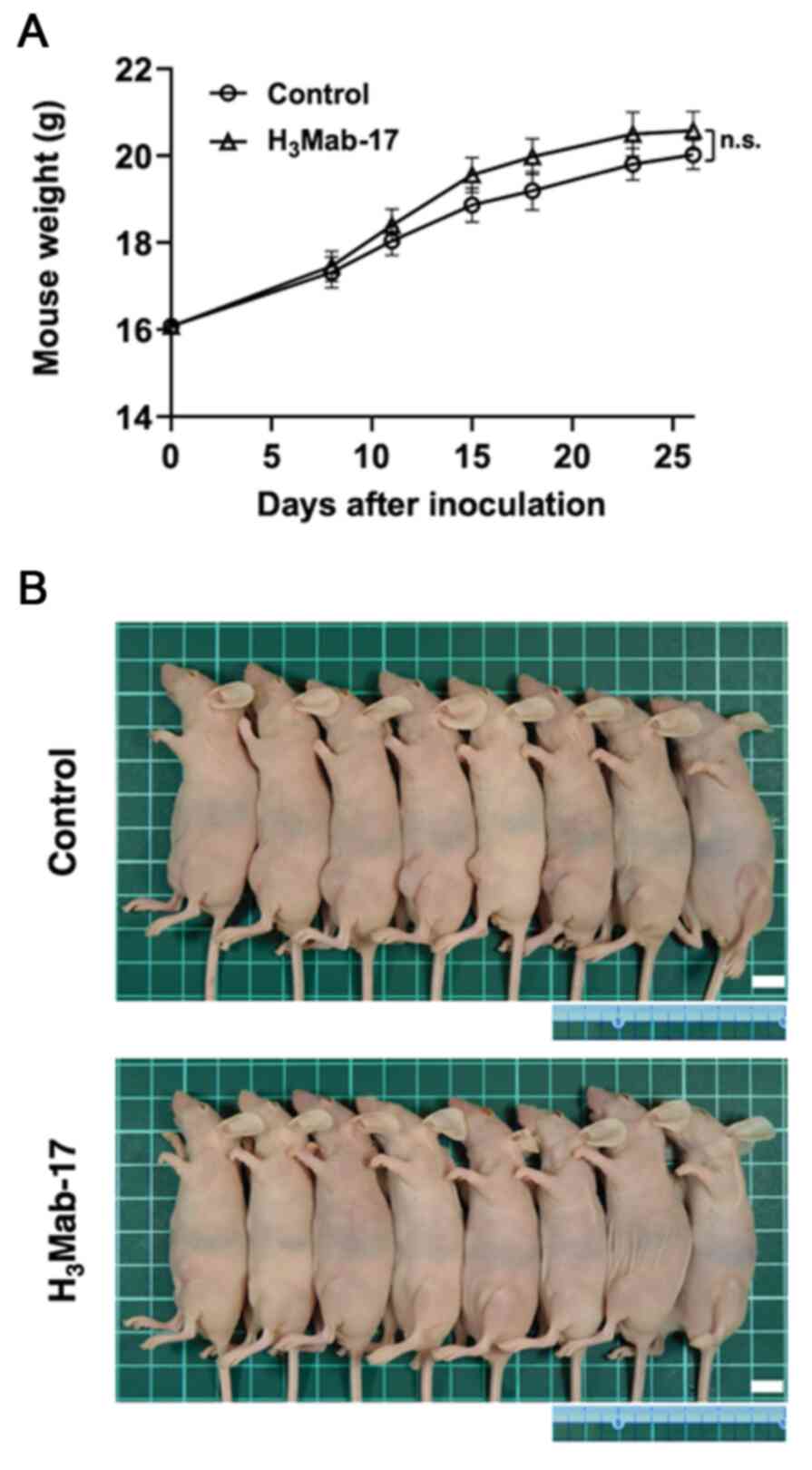
5B). Resected tumors on day 26 are presented in Fig. 5C. The total body weights did not
significantly differ between the treatment and control groups
(Fig. 6A and B). These results
indicated that H3Mab-17 reduced the growth of Caco-2
×enografts, without eliminating them completely.
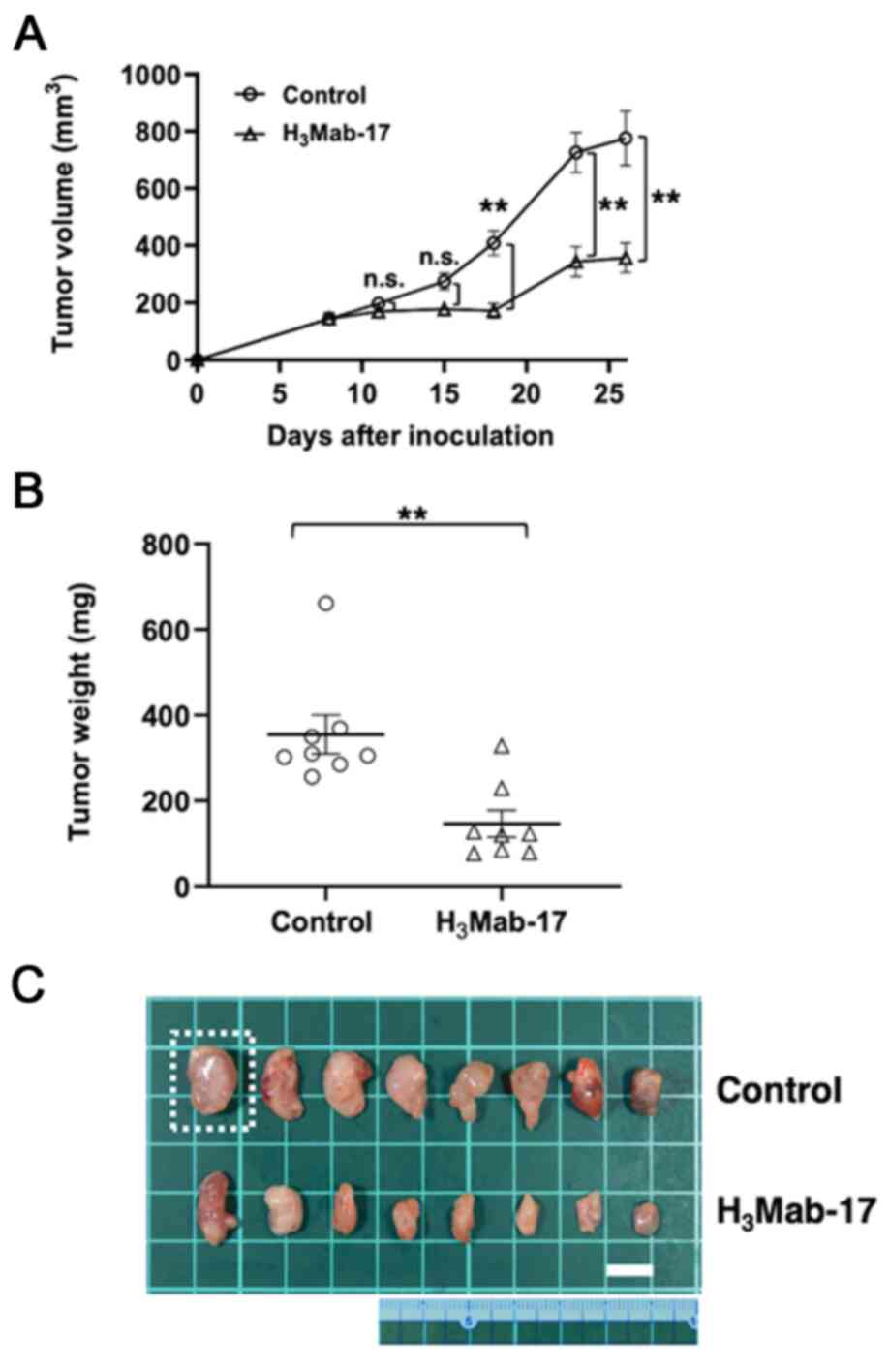 | Figure 5.Evaluation of antitumor activity of
H3Mab-17 in Caco-2 ×enografts. (A) Caco-2 cells
(5×106 cells) were injected subcutaneously into the left
flank. After day 8, 100 µg of H3Mab-17 and control mouse
IgG in 100 µl PBS were injected i.p. into the treated and control
mice, respectively. Additional antibodies were then injected on
days 15 and 23. Tumor volume was measured on days 8, 11, 15, 18, 23
and 26. Values are mean ± SEM. Asterisk indicates statistical
significance (**P<0.01; n.s., not significant, ANOVA and Sidak's
multiple comparisons test). ○, control; ∆, H3Mab-17. (B)
Tumors of Caco-2 ×enografts were resected from H3Mab-17
and control mouse IgG groups. Tumor weight on day 26 was measured
from excised xenografts. Values are mean ± SEM. Asterisk indicates
statistical significance (**P<0.01, Welch's t-test). ○, control;
∆, H3Mab-17. (C) Resected tumors of Caco-2 ×enografts
from H3Mab-17 and control mouse IgG groups on day 26.
The tumor in the square dotted region was the largest tumor in this
experiment. The vertical and horizontal lengths for Caco-2 cells
were 1.6 and 1.3 cm, respectively (estimated tumor volume, 1,352
mm3, tumor weight, 661 mg). Scale bar, 1 cm. |
Discussion
Many commercially available anti-HER3 mAbs have been
developed using recombinant HER3 protein, peptide or cDNA as an
immunogen. Seribantumab was developed by phage display (56,57) and
lumretuzumab was developed using recombinant HER3 extracellular
domain as an immunogen (46). In this
study, we succeeded in the development of an anti-HER3 mAb using
the CBIS method, which used HER3-expressed cells for both
immunization and screening. The CBIS method can help us effectively
develop mAbs that are useful in flow cytometry. We recently
succeeded in developing numerous useful mAbs that target membrane
proteins, including podoplanin (58–61), CD20
(62), CD44 (63), CD133 (54), and TROP2 (64,65).
Importantly, these mAbs are very useful for various experiments,
including not only flow cytometry, but also western blot analysis
and immunohistochemistry. Furthermore, those mAbs possess ADCC/CDC
activities and antitumor activities (61). Using the CBIS method, proteins for
immunogen expressed on cells maintain its native conformation and
glycosylation pattern. Previously, we successfully established a
cancer-specific mAb (CasMab) against podoplanin, which recognizes
the cancer cell-specific glycosylation of podoplanin (66). Therefore, we may develop CasMab
against HER3 using the CBIS method in the future. The CBIS method
is advantageous for the development for specific and sensitive mAbs
for antibody therapy.
New highly accurate therapeutic options are possible
to treat most solid tumors. In the case of colorectal cancer, HER3
overexpression is found in ~17-75%, although the definition of its
cutoff signals for HER3 expression are different in each
immunohistochemical study (67). It
has been reported that the incidence of HER3 overexpression in
metastatic colorectal cancer is much higher than that of HER2
(68). In this study, we developed an
anti-HER3 mAb, H3Mab-17, which specifically reacted with
endogenous HER3 in colorectal carcinoma cell lines in flow
cytometry. The KD for H3Mab-17 in
CHO/HER3-PA and Caco-2 cells were determined to be
3.0×10−9 and 1.5×10−9 M, respectively,
suggesting high binding affinity of H3Mab-17 for HER3.
In vitro experiments revealed strong ADCC and CDC inducement
against Caco-2 cells by H3Mab-17. In vivo
experiments on Caco-2 ×enografts revealed that the treatment with
H3Mab-17 significantly reduced the tumor growth,
compared with the control mouse IgG. Based on these findings,
H3Mab-17 may be useful in therapeutic approach for
patients with colorectal cancer.
Although H3Mab-17 recognizes both
overexpressed and endogenous HER3 by flow cytometric analyses, it
is not applicable to western blot and immunohistochemical analyses
(data not shown). H3Mab-17 did not recognize denatured
HER3, such as SDS-treated and formalin-fixed HER3 probably because
it might recognize the three-dimensional structure of HER3. Since
the antitumor activity mechanism of H3Mab-17 has not
been clarified, we need to identify the epitope of
H3Mab-17 and investigate the inhibitory activity of
HER3-neureglin interaction of H3Mab-17. Furthermore,
HER3-ADC and HER3-chimeric antigen receptor (CAR)-T should be
developed in future research.
Acknowledgements
We would like to thank Ms. Miyuki Yanaka, Ms. Saori
Handa, and Mr. Yu Komatsu (Department of Antibody Drug Development,
Tohoku University Graduate School of Medicine) for technical
assistance in the in vitro experiments, and Ms. Akiko
Harakawa [Institute of Microbial Chemistry (BIKAKEN), Numazu,
Microbial Chemistry Research Foundation] for technical assistance
in the animal experiments.
Funding
This research was supported in part by the Japan
Agency for Medical Research and Development (AMED) under grant nos.
JP21am0401013 (to YK) and JP21am0101078 (to YK), and by the Japan
Society for the Promotion of Science (JSPS) Grants-in-Aid for
Scientific Research (KAKENHI) grant nos. 21K15523 (to TA), 21K07168
(to MKK), 19K07705 (to YK) and 20K16322 (to MS).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TA, TO, TN, RN, HHo, TT, and MS performed the
experiments. JT and MKK analyzed the experimental data. HHa, MK,
and YK designed the present study. TA, TO, and YK wrote the
manuscript. All the authors read and approved the final manuscript
for publishing.
Ethics approval and consent to
participate
The Animal Care and Use Committee of Tohoku
University approved all the animal experiments (permit no.
2019NiA-001). Animal studies for ADCC and the antitumor activity
were approved by the Institutional Committee for experiments of the
Institute of Microbial Chemistry (permit no. 2020-024).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADC
|
antibody-drug conjugate
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
BSA
|
bovine serum albumin
|
|
CBIS
|
Cell-Based Immunization and
Screening
|
|
CDC
|
complement-dependent cytotoxicity
|
|
DMEM
|
Dulbecco's modified Eagle's
medium
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
FBS
|
fetal bovine serum
|
|
mAb
|
monoclonal antibody
|
|
PBS
|
phosphate-buffered saline
|
|
SEM
|
standard error of the mean
|
References
|
1
|
Gschwind A, Fischer OM and Ullrich A: The
discovery of receptor tyrosine kinases: Targets for cancer therapy.
Nat Rev Cancer. 4:361–370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schlessinger J: Ligand-induced,
receptor-mediated dimerization and activation of EGF receptor.
Cell. 110:669–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: A model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schreiber AB, Libermann TA, Lax I, Yarden
Y and Schlessinger J: Biological role of epidermal growth
factor-receptor clustering. Investigation with monoclonal
anti-receptor antibodies. J Biol Chem. 258:846–853. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linggi B and Carpenter G: ErbB receptors:
New insights on mechanisms and biology. Trends Cell Biol.
16:649–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris RC, Chung E and Coffey RJ: EGF
receptor ligands. Exp Cell Res. 284:2–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Citri A, Skaria KB and Yarden Y: The deaf
and the dumb: The biology of ErbB-2 and ErbB-3. Exp Cell Res.
284:54–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guy PM, Platko JV, Cantley LC, Cerione RA
and Carraway KL III: Insect cell-expressed p180erbB3 possesses an
impaired tyrosine kinase activity. Proc Natl Acad Sci USA.
91:8132–8136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holbro T, Beerli RR, Maurer F, Koziczak M,
Barbas CF 3rd and Hynes NE: The ErbB2/ErbB3 heterodimer functions
as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor
cell proliferation. Proc Natl Acad Sci USA. 100:8933–8938. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akhtar S, Chandrasekhar B, Attur S,
Dhaunsi GS, Yousif MH and Benter IF: Transactivation of ErbB family
of receptor tyrosine kinases is inhibited by angiotensin-(1–7) via
its mas receptor. PLoS One. 10:e01416572015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ceresa BP and Vanlandingham PA: Molecular
mechanisms that regulate epidermal growth factor receptor
inactivation. Clin Med Oncol. 2:47–61. 2008.PubMed/NCBI
|
|
13
|
Henriksen L, Grandal MV, Knudsen SL, van
Deurs B and Grøvdal LM: Internalization mechanisms of the epidermal
growth factor receptor after activation with different ligands.
PLoS One. 8:e581482013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bublil EM and Yarden Y: The EGF receptor
family: Spearheading a merger of signaling and therapeutics. Curr
Opin Cell Biol. 19:124–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yarden Y and Pines G: The ERBB network: At
last, cancer therapy meets systems biology. Nat Rev Cancer.
12:553–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hendler FJ and Ozanne BW: Human squamous
cell lung cancers express increased epidermal growth factor
receptors. J Clin Invest. 74:647–651. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kraus MH, Popescu NC, Amsbaugh SC and King
CR: Overexpression of the EGF receptor-related proto-oncogene
erbB-2 in human mammary tumor cell lines by different molecular
mechanisms. EMBO J. 6:605–610. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai WW, Chen FF, Wu MH, Chow NH, Su WC, Ma
MC, Su PF, Chen H, Lin MY and Tseng YL: Immunohistochemical
analysis of epidermal growth factor receptor family members in
stage I non-small cell lung cancer. Ann Thorac Surg. 72:1868–1876.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koutsopoulos AV, Mavroudis D, Dambaki KI,
Souglakos J, Tzortzaki EG, Drositis J, Delides GS, Georgoulias V
and Stathopoulos EN: Simultaneous expression of c-erbB-1, c-erbB-2,
c-erbB-3 and c-erbB-4 receptors in non-small-cell lung carcinomas:
Correlation with clinical outcome. Lung Cancer. 57:193–200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies S, Holmes A, Lomo L, Steinkamp MP,
Kang H, Muller CY and Wilson BS: High incidence of ErbB3, ErbB4,
and MET expression in ovarian cancer. Int J Gynecol Pathol.
33:402–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beji A, Horst D, Engel J, Kirchner T and
Ullrich A: Toward the prognostic significance and therapeutic
potential of HER3 receptor tyrosine kinase in human colon cancer.
Clin Cancer Res. 18:956–968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ocana A, Vera-Badillo F, Seruga B,
Templeton A, Pandiella A and Amir E: HER3 overexpression and
survival in solid tumors: A meta-analysis. J Natl Cancer Inst.
105:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledel F, Hallstrom M, Ragnhammar P,
Ohrling K and Edler D: HER3 expression in patients with primary
colorectal cancer and corresponding lymph node metastases related
to clinical outcome. Eur J Cancer. 50:656–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimura H, Minato N, Nakano T and Honjo
T: Immunological studies on PD-1 deficient mice: Implication of
PD-1 as a negative regulator for B cell responses. Int Immunol.
10:1563–1572. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engelhardt JJ, Sullivan TJ and Allison JP:
CTLA-4 overexpression inhibits T cell responses through a
CD28-B7-dependent mechanism. J Immunol. 177:1052–1061. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous Non-Small-Cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Golshani G and Zhang Y: Advances in
immunotherapy for colorectal cancer: A review. Therap Adv
Gastroenterol. 13:17562848209175272020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Y and Freeman GJ: The microsatellite
instable subset of colorectal cancer is a particularly good
candidate for checkpoint blockade immunotherapy. Cancer Discov.
5:16–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ,
Kussie P and Ferguson KM: Structural basis for inhibition of the
epidermal growth factor receptor by cetuximab. Cancer Cell.
7:301–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Flygare JA, Pillow TH and Aristoff P:
Antibody-drug conjugates for the treatment of cancer. Chem Biol
Drug Des. 81:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kute T, Lack CM, Willingham M, Bishwokama
B, Williams H, Barrett K, Mitchell T and Vaughn JP: Development of
Herceptin resistance in breast cancer cells. Cytometry A. 57:86–93.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Clynes RA, Towers TL, Presta LG and
Ravetch JV: Inhibitory Fc receptors modulate in vivo cytotoxicity
against tumor targets. Nat Med. 6:443–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang D, Qian G, Zhang H, Magliocca KR,
Nannapaneni S, Amin AR, Rossi M, Patel M, El-Deiry M, Wadsworth JT,
et al: HER3 targeting sensitizes HNSCC to Cetuximab by reducing
HER3 activity and HER2/HER3 dimerization: Evidence from cell line
and Patient-Derived xenograft models. Clin Cancer Res. 23:677–686.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gandullo-Sánchez L, Capone E, Ocaña A,
Iacobelli S, Sala G and Pandiella A: HER3 targeting with an
antibody-drug conjugate bypasses resistance to anti-HER2 therapies.
EMBO Mol Med. 12:e114982020. View Article : Google Scholar
|
|
46
|
Mirschberger C, Schiller CB, Schräml M,
Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G,
Kolm I, et al: RG7116, a therapeutic antibody that binds the
inactive HER3 receptor and is optimized for immune effector
activation. Cancer Res. 73:5183–5194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sequist LV, Gray JE, Harb WA, Lopez-Chavez
A, Doebele RC, Modiano MR, Jackman DM, Baggstrom MQ, Atmaca A,
Felip E, et al: Randomized Phase II trial of seribantumab in
combination with erlotinib in patients with EGFR wild-type
non-small cell lung cancer. Oncologist. 24:1095–1102. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cejalvo JM, Jacob W, Fleitas Kanonnikoff
T, Felip E, Navarro Mendivil A, Martinez Garcia M, Taus Garcia A,
Leighl N, Lassen U, Mau-Soerensen M, et al: A phase Ib/II study of
HER3-targeting lumretuzumab in combination with carboplatin and
paclitaxel as first-line treatment in patients with advanced or
metastatic squamous non-small cell lung cancer. ESMO Open.
4:e0005322019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koganemaru S, Kuboki Y, Koga Y, Kojima T,
Yamauchi M, Maeda N, Kagari T, Hirotani K, Yasunaga M, Matsumura Y
and Doi T: U3-1402, a Novel HER3-Targeting Antibody-Drug conjugate,
for the treatment of colorectal cancer. Mol Cancer Ther.
18:2043–2050. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Masuda N, Yonemori K, Takahashi S, Kogawa
T, Nakayama T, Iwase H, Takahashi M, Toyama T, Saeki T, Saji S, et
al: Abstract PD1-03: Single agent activity of U3-1402, a
HER3-targeting antibody-drug conjugate, in HER3-overexpressing
metastatic breast cancer: Updated results of a phase 1/2 trial.
Cancer Res. 79:2019.PubMed/NCBI
|
|
51
|
Hashimoto Y, Koyama K, Kamai Y, Hirotani
K, Ogitani Y, Zembutsu A, Abe M, Kaneda Y, Maeda N, Shiose Y, et
al: A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402,
exhibits potent therapeutic efficacy through the delivery of
cytotoxic payload by efficient internalization. Clin Cancer Res.
25:7151–7161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Itai S, Fujii Y, Nakamura T, Chang YW,
Yanaka M, Saidoh N, Handa S, Suzuki H, Harada H, Yamada S, et al:
Establishment of CMab-43, a sensitive and specific Anti-CD133
monoclonal antibody, for immunohistochemistry. Monoclon Antib
Immunodiagn Immunother. 36:231–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kato Y, Ohishi T, Yamada S, Itai S,
Furusawa Y, Sano M, Nakamura T, Kawada M and Kaneko MK: Anti-CD133
Monoclonal Antibody CMab-43 exerts antitumor activity in a mouse
xenograft model of colon cancer. Monoclon Antib Immunodiagn
Immunother. 38:75–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schoeberl B, Faber AC, Li D, Liang MC,
Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al: An
ErbB3 antibody, MM-121, is active in cancers with ligand-dependent
activation. Cancer Res. 70:2485–2494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schoeberl B, Pace EA, Fitzgerald JB, Harms
BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, et al:
Therapeutically targeting ErbB3: A key node in ligand-induced
activation of the ErbB receptor-PI3K axis. Sci Signal. 2:ra312009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Furusawa Y, Yamada S, Itai S, Nakamura T,
Yanaka M, Sano M, Harada H, Fukui M, Kaneko MK and Kato Y:
PMab-219: A monoclonal antibody for the immunohistochemical
analysis of horse podoplanin. Biochem Biophys Rep.
18:1006162019.PubMed/NCBI
|
|
59
|
Furusawa Y, Kaneko MK, Nakamura T, Itai S,
Fukui M, Harada H, Yamada S and Kato Y: Establishment of a
monoclonal antibody PMab-231 for tiger podoplanin. Monoclon Antib
Immunodiagn Immunother. 38:89–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Furusawa Y, Takei J, Sayama Y, Yamada S,
Kaneko MK and Kato Y: Development of an anti-bear podoplanin
monoclonal antibody PMab-247 for immunohistochemical analysis.
Biochem Biophys Rep. 18:1006442019.PubMed/NCBI
|
|
61
|
Furusawa Y, Yamada S, Itai S, Nakamura T,
Takei J, Sano M, Harada H, Fukui M, Kaneko MK and Kato Y:
Establishment of a monoclonal antibody PMab-233 for
immunohistochemical analysis against Tasmanian devil podoplanin.
Biochem Biophys Rep. 18:1006312019.PubMed/NCBI
|
|
62
|
Furusawa Y, Kaneko MK and Kato Y:
Establishment of C20Mab-11, a novel anti-CD20 monoclonal
antibody, for the detection of B cells. Oncol Lett. 20:1961–1967.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yamada S, Itai S, Nakamura T, Yanaka M,
Kaneko MK and Kato Y: Detection of high CD44 expression in oral
cancers using the novel monoclonal antibody, C44Mab-5.
Biochem Biophys Rep. 14:64–68. 2018.PubMed/NCBI
|
|
64
|
Sayama Y, Kaneko MK and Kato Y:
Development and characterization of TrMab-6, a novel anti-TROP2
monoclonal antibody for antigen detection in breast cancer. Mol Med
Rep. 23:922021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sayama Y, Kaneko MK, Takei J, Hosono H,
Sano M, Asano T and Kato Y: Establishment of a novel anti-TROP2
monoclonal antibody TrMab-29 for immunohistochemical analysis.
Biochem Biophys Rep. 25:1009022021.PubMed/NCBI
|
|
66
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4:59242014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y, Yang H and Duan G: HER3
over-expression and overall survival in gastrointestinal cancers.
Oncotarget. 6:42868–42878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stahler A, Heinemann V, Neumann J, Crispin
A, Schalhorn A, Stintzing S, Giessen-Jung C, Fischer von
Weikersthal L, Vehling-Kaiser U, Stauch M, et al: Prevalence and
influence on outcome of HER2/neu, HER3 and NRG1 expression in
patients with metastatic colorectal cancer. Anticancer Drugs.
28:717–722. 2017. View Article : Google Scholar : PubMed/NCBI
|