The accumulation of lactic acid and protons,
products of cancer metabolites together with acute and chronic
inflammatory diseases, reduces the extracellular pH level.
Inflammatory cells and tumour cells themselves usually show an
increase in metabolic activity, causing tissue hypoxia, leading to
glycolytic metabolism transition and subsequent lactic acid
accumulation (1,2). In addition, the disruption of the
vasculature due to hypoxia prevents protons from being efficiently
flushed from the extracellular space, exacerbating extracellular
acidification (3). Numerous studies
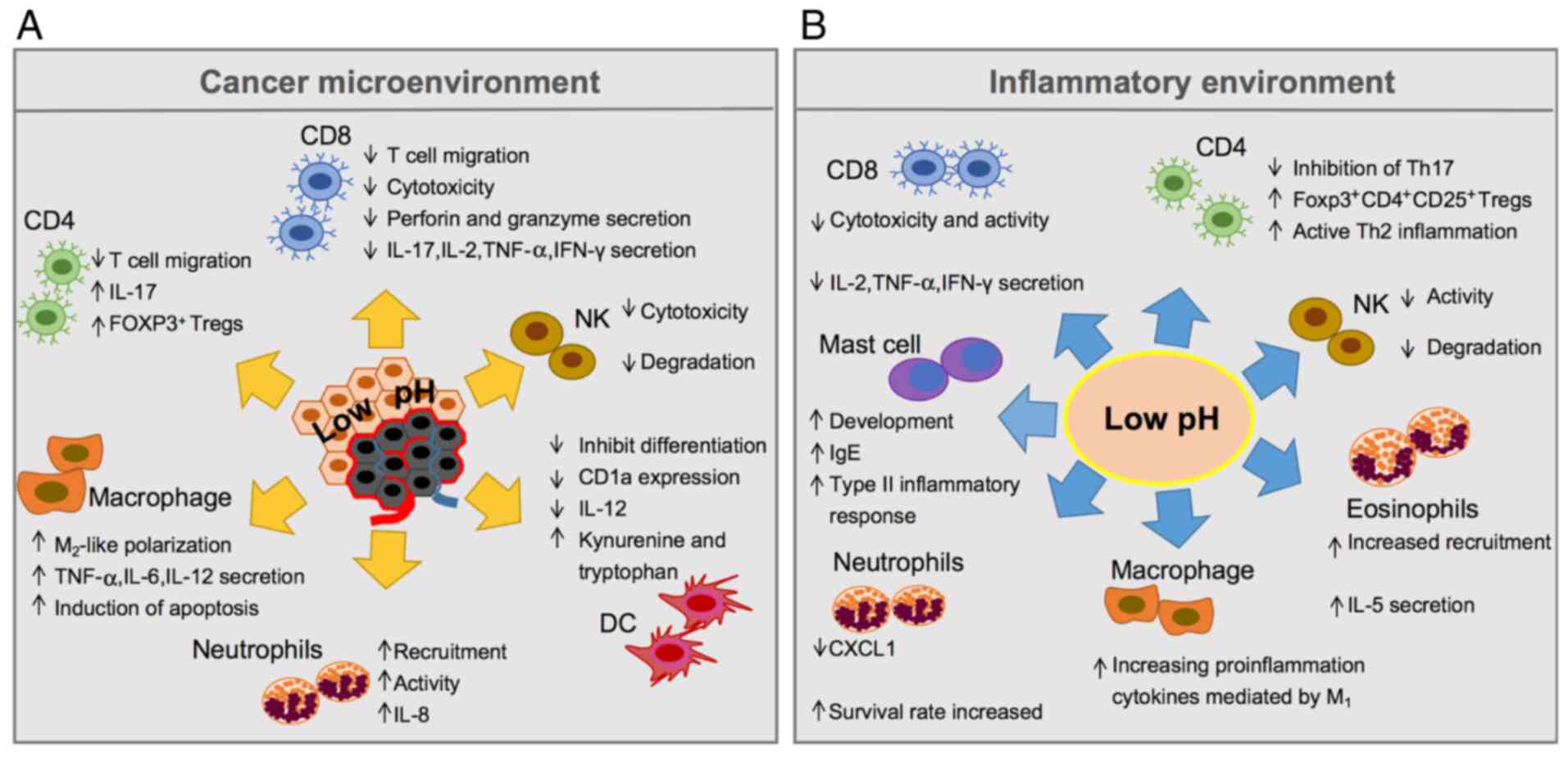
have shown that acidosis has a variety of effects on the
inflammation/immune response (4)
(Fig. 1). On the one hand, acidosis
drives T lymphocytes and natural killer (NK) cells toward
deprivation of their functions, and they remain in a reversible
paralysis condition followed by apoptosis and reduction of
interleukin (IL)-2, interferon (IFN)-γ, perforin and granzyme
secretion (5–7). On the other hand, the acidity of the
tumour microenvironment (TME) can change the differentiation of
dendritic cells (DCs) from haematopoietic stem cells and impair the
ability of both antigen presentation and induce specific T cell
responses by inhibiting the maturation and differentiation of DCs
(8,9).
Paradoxically, myeloid-derived suppressor cells (MDSCs) (10) and regulatory T cells (Tregs) (11) can be activated and recruited via
acidosis, as well as by neutrophils, to produce a series of
pro-inflammatory mediators (12).
Extracellular acidosis also causes tumour-associated macrophages
(TAMs) to undergo M1 to M2 phenotype transformation induced by
hypoxia-inducible factor-1α (HIF1A, HIF-1α), and increases
the expression of M2-like phenotype-related genes, such as arginase
1 (ARG1), mannose receptor C-type 1 (MRC1) and
chitinase-3-like protein (CHI3L1) (13–15). To
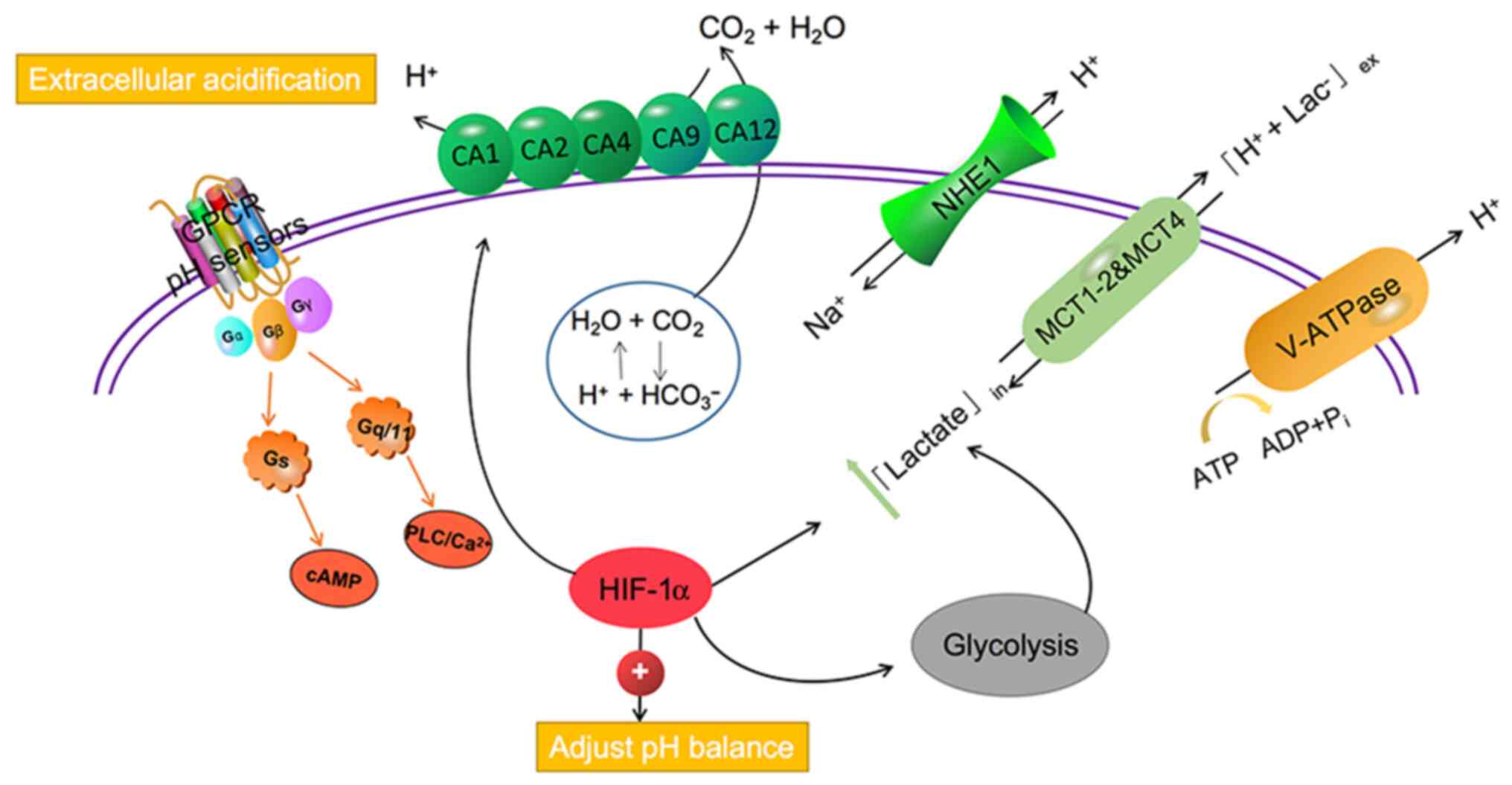
neutralise metabolic acid overload, immune cells use pH-sensing
proteins, transporters, and proton pumps and promote their survival
in an acidic environment (16). These
transporters or pumps include monocarboxylate transporters (MCTs),
such as MCT1-4, Na+/HCO3− co-transporters,
such as sodium-hydrogen exchanger 1 (NHE1), and carbonic anhydrase
(CA) family of proteins, such as CA1-2, CA4, CA9, and CA12, in
addition to vacuolar-ATPase (V-ATPase) that co-transports lactate
and protons (15,17). The proton-sensing G-protein coupled
receptor (GPCR) family has four members: GPR4, TDAG8 (GPR65), OGR1
(GPR68), and G2A (GPR132), which can be activated by acidic
extracellular pH. GPCRs are expressed on a variety of immune cells,
acting on the PLC/Ca2+ signaling pathway via Gq/11
proteins or cAMP signaling pathways through Gs (18,19). It is
worth noting that although pH-sensitive regulators are present in T
cells or NK cells, they are also widely expressed on cancer-related
myeloid cells, which means that immune cells may use pH
transporters to balance local acidic sites to survive in
uncongenial environments.
Although low pH is a common feature in inflammatory
environments and tumours, little attention has been paid to how pH
receptors modulate immune cell function in acidic environments.
Most of the current reviews focus on the mechanism of different pH
receptors regulating tumor metabolism in acidic environment. In
this review, we innovatively summarized the bidirectional
regulation mechanism of pH receptors on immune cells in the acidic
environment of tumours and inflammation, and listed the effects of
pH receptor inhibitors on the immune system in the preclinical
model, as well as the therapeutic efficacy and problems of the
latest clinical drugs at present. Interestingly, according to the
dissimilarities in the structure and expression levels of
pH-sensitive regulators on immune cells, immune evasion may be
driven by inactivating T lymphoctyes or NK cells, boosting the
accumulation and activity of pro-inflammatory factors, including
macrophages and neutrophils. Targeted drugs based on this
development have shown promising clinical applications and expanded
the number of people who benefit from immunotherapy (Table I).
MCTs consist of 14 members, and MCT1-4 promote the
passive transport of monocarboxylates, such as lactate, pyruvate,
and ketone bodies, as well as protons across the cell membrane
(20) (Fig.
2). As an important regulator of intracellular lactic acid and
pH, MCTs contribute to the production of lactic acid by
hyperglycolytic cells, such as cancer cells and immune cells
(21). Lymphocytes and NK cells may
be biased against immunosuppressive phenotypes and function at
lower pH values (22). As early as
2004, Merezhinskaya et al found the expression of three
monocarboxylate transporters (MCT1, MCT2, and MCT4) in isolated
human monocytes and lymphocytes, and assumed that leukocytes
express lactate transporters to promote their efflux under acidic
conditions to reduce intracellular acidosis (23,24).
Inhibition of MCT4 can help to enhance the cytotoxicity of NK cells
and their ability to kill tumour cells by inducing autophagy to
prevent lactic acid rejection by tumour cells (25). One of the characteristics of the
transformation of lymphocytes from primitive cells to effector ones
is aerobic glycolysis, which promotes the process of proliferation
and differentiation (5). Recent
studies indicate that this metabolic switch could cause memory
CD8+ T cells to undergo terminal differentiation,
increase lactate production and reduce mitochondrial consumed
oxygen (26). Therefore, suppression
of glycolysis can augment the number of memory CD8+ T
cells in conjunction with their antitumour function (26). Fischer et al proposed that
lymphocytes utilise glycolysis and produce lactic acid via MCT1
during activation (27). An acidic
environment may block the lactate transport by MCT1 on cytotoxic T
lymphocytes (CTLs), resulting in the impairment of their effector
function, causing an increased apoptosis rate and the decreased
production of IFN-γ, IL-2, perforin and granzyme B (28). It was later reported that high
concentrations of lactic acid are detected by MCT1 when
CD4+ and CD8+ T subsets enter the site of
inflammation. Moreover, it interferes with glycolysis by
downregulating hexokinase-1 (HK1) or inhibiting phosphofructokinase
(PFK), producing a large amount of IL-17 and losing its cytolytic
activity, which is adversely inhibited by T cell movement (29). A recent study using a variety of
tumour mouse models, reported that MCT1 inhibitors effectively
inhibit tumour growth by enhancing T cell infiltration and
reversing the immunosuppressive microenvironment of solid tumours
(30). In addition to MCT1, T
lymphocytes also express MCT2 and MCT4, which participate in lactic
acid transport by CTLs to inhibit proliferation and cytokine
production (23,27,28).
Tumour-derived lactic acid is also an important
factor regulating DC phenotypes through MCT1 in the tumour
environment, which significantly inhibits the differentiation of
DCs characterised by low expression of CD1a and low secretion of
IL-12 (31,32). Extracellular lactic acid levels also
affect plasmacytoid DCs (pDCs) through the function of cytoplasmic
MCTs. Raychaudhuri et al found that MCT1 expression is
significantly elevated in human pDCs. Lactic acid weakens the
reaction of human pDCs to the TLR9 ligand, hinders TLR9-induced
glycolysis, which leads to the production of type I IFN, and
finally reduces the extracellular acidification significantly.
Meanwhile, lactate promotes kynurenine and tryptophan metabolism of
pDCs, which helps activate Foxp3+ CD4+ Tregs,
the main immunosuppressive immune cell subsets in the TME (31).
An increasing number of studies have shown that the
disruption of the glycolytic metabolism of inflammatory cells in
the TME is critical to the development and progression of cancer
(33). The ATP transfer mechanism of
MCTs in the tumour inflammatory microenvironment was studied by
co-culturing colorectal cancer cells with monocyte macrophages
(THP-1). The lactic acid in the microenvironment is absorbed by
THP-1 monocytes through MCT1, which promotes the positive
regulation of cyclooxygenase-2 and PEPCK transcription by HIF-1α,
which in turn accelerates tumour growth (34). Aerobic glycolysis also enhances and
mediates inflammatory responses in activated macrophages. To
understand the effects of tumour-derived lactic acid on the
functional polarisation of TAMs, Colegio et al established
lung and melanoma cancer mouse models and proved that MCTs are
involved in the cellular uptake of lactic acid, inducing vascular
endothelial growth factor (VEGF) and M2-like polarisation of TAMs
(35). A study found that MCT4 is
upregulated in lipopolysaccharide (LPS)-stimulated macrophages,
which is mediated by MyD88 in an NF-κB-dependent manner. MCT4
knockdown weakens the secretion of pro-inflammatory mediators, such
as tumor necrosis factor (TNF)-α, IL-6 and IL-12, in macrophages,
increases lactic acid accumulation and decreases glycolysis
(36). Similarly, lactate promotes
macrophage polarisation in gastric and cervical cancer through
MCT-HIF-1α signal transduction (37,38).
Microglia in the central nervous system upregulate MCT1 and MCT4
expression under LPS stimulation, which is similar to that of
macrophages in peripheral tissues. Studies have shown that MCT1 and
MCT4 may enhance glycolysis through HIF-1 and ultimately promote
microglial polarisation and pro-inflammatory effects (39,40).
However, some studies also found that MCT4, rather than the related
transporters MCT1 and MCT2, confers the ability of macrophages to
export lactic acid in a high lactic acid microenvironment (41). High expression of MCT4, rather than
MCT1, in TAMs is a marker of high metabolic heterogeneity between
Hodgkin's lymphoma and the TME (42).
The metabolic cooperation between tumour cells and
inflammatory/immune cells in the microenvironment is mediated by
MCT1 and MCT4 (43). MCT inhibitors
are considered an attractive therapeutic strategy. The lack of
targeted MCT specificity and associated toxicity in
first-generation MCT inhibitors is not feasible in clinical
treatment. However, the second-generation MCT1 inhibitor, AZD3965,
from AstraZeneca has displayed an effective result. AZD3965 was
initially used in the phenotypic screening of immunosuppression and
is currently being tested in a clinical trial. AZD3965 slows down
choline metabolism after the accumulation of lactic acid in
Burkitt's lymphoma and diffuse large B-cell lymphoma, and increases
the infiltration of monocytes, DCs, and NK cells (44). Oral administration of AZD3965 also
shows benefits in the treatment of Burkitt's lymphoma and diffuse
large B-cell lymphoma with low expression of MCT4 (45,46).
However, the efficacy of these agents in reducing lactate outflow
from tumour cells may be limited due to the co-expression of MCT4.
As the increase in glycolysis impair the effector function of T
cells, new drugs are needed to target not only MCT4, but also
immune cells to improve their metabolic function. The first-class
inhibitor of MCT1 and MCT4, 7-amino-carboxy coumarins (7ACC), has
recently been developed to prevent the influx but not the efflux of
lactic acid in tumour cells. 7ACC delayed cervical SIHA tumour
growth and inhibited tumour recurrence after cisplatin treatment.
Moreover, it was also found to inhibit the growth of colorectal
HCT116 tumours and in situ MCF-7 breast tumours (47). BAY-8002, as a new class of MCT1
inhibitors, significantly increased the lactate levels and
transient regulation of pyruvate levels in tumours, and provides a
new treatment for patients with MCT1 inhibitor resistance (48). Diclofenac, which blocks the activity
of MCT1 and MCT4 as well as lactate secretion in tumour cell lines
and primary T cells, can improve the killing effect of T
cell-mediated tumour cell death by increasing the number of
tumour-infiltrating leukocytes (CD45+) and T cell
subsets (CD3+, CD3+CD8+) (49). Furthermore, AS2495674, an MCT1
inhibitor, was found to impede the transportation of lactic acid in
CD4+ T lymphocytes, which inhibited the proliferation of
lymphocytes, thereby alleviating acute rejection and improving the
survival rate of allografts (50).
The combination of the targeted immune system and pH regulation can
inhibit tumour progression and drug resistance more effectively,
thereby, improving the therapeutic effect.
As a reversible reverse transporter, NHE1 belongs to
the solute carrier coupled transporter family 9A (slc9a) and uses
ATP provided by the Na+ gradient to excrete
H+ from the cytoplasm. NHE1 activity is very low under
neutral pH conditions but can be rapidly activated by cytoplasmic
acidification (51) (Fig. 2). NHE1 regulates intracellular pH and
cell volume, maintains the cavity microenvironment, and affects
nutrient absorption, but also plays an important role in cell
proliferation, migration, and apoptosis (52).
Previous studies in human lymphocytes demonstrated
that IL-2 stimulation increases the abundance of pHi and NHE1,
thereby affecting cell proliferation and cytokine production. In
contrast, inhibiting the activity of NHE1 results in the rapid
acidification of T cells which leads to apoptosis (53,54). It
has been further proposed that glucocorticoid and progesterone can
inhibit T cell activation through intracellular acidification,
increase in free calcium concentration
([Ca2+]i) and rapid non-genomic inhibition of
membrane NHE1 activity. Mifepristone (RU486), an antagonist of
glucocorticoids in T cells, reportedly restrains the rapid decline
of NHE1 activity induced by glucocorticoids and blocks
PHA-stimulated T cells at the G0/G1 phase, indicating that RU486
antagonises NHE1 in the plasma membrane of T cells (55–57). As a
subtype of CD4+ T cells, Th9 cells highly express NHE1.
siRNA-silencing of NHE1 was found to downregulate the production of
IL-9 and ATP, and the increased activity of the
Na+/H+ exchanger depends on Akt/rictor/mTOR
signal transduction, which can protect the acidic environment
(58). Similarly, inhibition of NHE1
activity in DCs resulted in cell swelling and oxidative burst with
ROS formation, which is dependent on phosphoinositide 3 kinase
(PI3K) activity and directly proportional to Akt phosphorylation
(59–61).
NHE1 plays a significant role in the regulation of
pHi homeostasis, as well as activation and migration of microglia
(62). NHE1 is expressed in
microglia/TAMs and participates in the pretumour communication
between glioblastoma and TAMs. Co-culturing glioma-conditioned
medium with microglia stimulates the activity of NHE1 on microglia
and promotes the proliferation and migration of glioma by
regulating microglia-derived factors, such as matrix
metalloproteinase (MMP)-9, inducible nitric oxide synthase (iNOS),
tumour growth factor (TGF)-β and IL-6. The NHE1-specific inhibitor,
HOE642, stimulates the pro-inflammatory polarisation of microglia
by downregulating iNOS and Arg1. Moreover, it also increases the
infiltration of CD8+, CD4+ T cells, and Th1
cells in the tumour core and margin, while decreasing the
infiltration of Treg cells, thus improving the microenvironment of
immunosuppression in glioma (62).
However, in one study, it was found that selective NHE1-knockout
mice did not have inhibited microglia/macrophages pro-inflammatory
response, but had improved neural repair function after ischaemic
stroke (63). In addition to nervous
system tumours, NHE1 also regulates macrophage function in other
diseases. For example, in atherosclerotic disease, the activation
of NHE1 on macrophages by IgE can reduce the extracellular pH value
and induce apoptosis of macrophages, which leads to an increase in
apoptosis in atherosclerotic lesions (64). A study examined NHE1 expression in
macrophages of inflammatory diseases. Long-term inflammatory
stimulation, such as LPS exposure, can activate TLR4 on intestinal
macrophages, leading to inflammation through the MYD88-dependent
pathway, thereby accelerating intracellular degranulation of NHE1
mediated by the ubiquitin-proteasome system (65).
Amilolol was the first NHE inhibitor developed to
reduce VEGF production and the activity of MMPs, as well as other
proteases which aid tumour metastasis, and notably increase the
infiltration of T cells into the tumour core (66). It is safe and well-tolerated when used
for chronic disease treatment in pharmacological dosages with the
common side effect of an occasional increase in plasma
K+ levels (67). Potential
analogues of amiloride have been prepared, including ethyl
isopropyl amiloride, hexamethyl amiloride (HMA), and dimethyl
amiloride (DMA) (67). The only
clinically tested amiloride with strong NHE1 inhibitory activity is
cariporide, which is useful in overcoming cancer and cardiovascular
diseases. Some studies have shown that treatment with cariporide
(HOE642) inhibits microglial activation and pro-inflammatory
responses in the brain tissue after transient ischaemic stroke
(68,69). NHE1 inhibition can change the glioma
microenvironment by stimulating the pro-inflammatory polarisation
of TAMs, increasing the activation of cytotoxic T cells, and
reducing the number of Treg cells. The combination of anti-PD-1
therapy with cariporide minimised GL26 glioma volume and improved
the survival rate of animals with glioma (62,70).
Cariporide also diminished hypoxia-mediated tumour invasion in
human tongue squamous cell carcinoma (67). Similarly, inhibition of NHE1
noticeably downregulated CCAAT enhancer-binding protein (C/EBPα)
expression under hypoxic conditions via the pharmacological
suppression of p38 mitogen-activated protein kinase (MAPK),
suggesting that NHE1 may be a target for leukaemia treatment in a
hypoxic microenvironment (71,72). It
was found equally important in heart disease and
ischaemia-reperfusion injury, and was used in 1,590 patients with
unstable angina pectoris or myocardial infarction to gain clinical
benefits in the early stage of the disease and improve the 6-month
survival rate (64).
Cancer cells secrete an a2V peptide (a2NTD), which
has vital immunomodulatory properties in the TME. It was found to
increase the recruitment of neutrophils at the tumour site and
promote the tumourigenicity of neutrophils and macrophages
(84,85). In addition, it can enhances tumour
angiogenesis and cancer cell invasion (86,87).
Recent studies have shown that a2NTD activates the NF-κB pathway in
neutrophils, leading to increased secretion of IL-8, which, in
turn, prolongs the life of neutrophils and stimulates their
migration to tumour sites via autocrine signaling (84,87).
Granule-associated a2V subtypes play a role in maintaining the pH
gradient between cytoplasmic and granular neutrophils and may serve
as a biomarker for activated neutrophils (88).
A V-ATPase, ATP6V0D2/subunit d2, is a key component
of the macrophage-specific autophagy-lysosome fusion mechanism,
which can maintain the homeostasis of macrophage organelles, thus
limiting inflammation and bacterial infection (89). In an established macrophage cell line
lacking ATP6V0D2, the expression of TLR4 on the cell surface was
prolonged, which enhanced the inflammatory response to LPS and
decreased the response of I–IFN (90). In addition, ATP6V0D2 deficiency can
lead to the accumulation of damaged mitochondria in macrophages. As
shown in a previous study, deficiency of ATP6V0D2 resulted in
decreased Salmonella clearance and increased DSS-induced
colitis in vivo (89).
V-ATPase is the core for the transport and secretion of
inflammatory cytokines, and its activation is essential for the
polarization of macrophages toward the M2-like phenotype and is
required for the suppression of innate immune response (91–93). The
inhibition of V-ATPase was found to selectively upregulate the
production of TNF-α and the activation of NF-κB as well as SAPK/JNK
in macrophages, which led to the M1-like phenotype (94).
Bafilomycin A1 (Baf-A1) is a specific inhibitor of
the V-ATPase C subunit. Baf-A1 has been used for the treatment of
osteoporosis and antiviral infection in the clinic (95). Although it is still in the primary
research stage for cancer treatment, many studies have reported
that Baf-A1 can inhibit the proliferation and metastasis of cancer
cells. For example, high concentrations of Baf-A1 were found to
inhibit cell growth in prostate cancer (PC3), liver cancer
(BEL-7402), and ovarian cancer (HO-8910), and to induce the
apoptosis of glioblastoma (U87MG) (96,97).
Recent research has demonstrated that Baf-A1 is a promising
candidate for the treatment of B-cell acute lymphoblastic leukaemia
(B-ALL) and mantle cell lymphoma (MCL) (98,99). The
reason why it has not been used in the clinic is that a high
concentration of Baf-A1 may act on all vesicular V-ATPases in
eukaryotic cells, thus acidosis and oxygen deficiency may occur
under normal physiological conditions, resulting in strong side
effects in vivo.
Glutamine metabolism promotes acidosis and produces
carbon dioxide under hypoxia (106).
Carbon dioxide is hydrated by CA and converted into bicarbonate and
protons to acidify the extracellular environment. At the same time,
hypoxia induces HCO3− ions to combine with
intracellular acids, resulting in the diffusion of CO2
out of the cell, which is maintained by CA (2,107)
(Fig. 2). CAs and metabolic acidosis
are further known to modulate immune cell activation. The human
asthmatic airway is an acidic microenvironment, in which
infiltrated leukocytes expressing surface CAs may regulate local pH
(108). When comparing eosinophils
from lung samples exposed to allergens or saline (control) using
genome-wide mRNA microarray analysis, it was found that CA4 is
overexpressed on the plasma membrane of IL-5-activated eosinophils,
which also regulate the lung transcriptome associated with allergic
airway inflammation. Therefore, CA4 has a potential value in
diagnosing and monitoring the eosinophilic reaction (109). In addition, allergic inflammation is
also associated with type 2 cytokine responses, in which elevated
levels of CA are expressed (110).
Henry et al reported that CA1 and CA2 are significantly
upregulated in mature mast cells. Methimazole (MZ), an inhibitor of
CA1 and Ca2, was used to reduce the development of mast cells in
stem cells and type 2 inflammatory response (111). Furthermore, a mouse model of food
allergy-like disease caused by chicken ovalbumin was treated with
MZ, which significantly reduced intestinal mastocytosis and
effectively halted the allergic response (112). In conclusion, these data suggest
that CA1 and CA2 are positive regulators of mast cell development
and targeting them may prove to be effective in the treatment of
mast cell-mediated inflammation. Currently, there are patent
applications for CA inhibitors for the treatment of allergic
diseases, bacterial infections, virus infections, mastocytosis, and
mast cell-mediated inflammation (113–115).
The change in pH is usually accompanied by
infectious and inflammatory injury, and CAs may also serve as a
sensory mechanism for immune cell haematopoiesis under inflammatory
conditions. CA2 and CA4 are related to the pathogenesis of diarrhea
(116) and pulmonary diseases caused
by infection. CA1 can induce antigen-specific immune tolerance by
producing Foxp3+CD4+CD25+ Tregs
and inhibiting Th17 cells, resulting in a negative effect on the
pathogenesis of inflammatory bowel disease (117). CA4 was identified by genome-wide
assays as a specific regulatory element expressed in lung
macrophages, which can be used to distinguish different
tissue-resident macrophages and is essential for understanding
their role in alerting the immune system to lung disease (118).
In addition to CA1, CA2 and CA4, CA9 and CA12, which
support the pH regulation mechanism, are considered as therapeutic
targets. Lounnas et al observed that CA12 is overexpressed
in T-cell acute lymphoblastic leukaemia/lymphoma (T-ALL/LL) cells,
and its inhibitor was found to reduce cell proliferation and induce
cell death in a T-lymphoma cell line (119). The analysis of some solid tumours
has confirmed that the tumour margin has higher proliferation,
lower apoptosis rate, and more immune cells infiltration than the
core area. Cells at the edge of invasion also expressed more CA9
and less CA12 (120). In a cohort of
449 patients with metastatic melanoma and basal-like breast cancer,
hypoxia-induced expression of the pH regulator CA9 was associated
with poor overall survival. The ureido-sulphonamide CA9 inhibitor
(SLC-0111) was found to reduce tumour glycolytic metabolism and
extracellular acidification, increase the conversion of T cells to
cytotoxic phenotypes, reduce the number of Tregs, and eliminate
Th17 cells (121). SLC-0111 has been
used in the phase 1 evaluation of 17 patients with advanced solid
tumours and shown safe and effective results (122). Nasu et al also found that CA9
enhanced the tumourigenicity of ST1 cells isolated from human T
cell leukaemia/lymphoma (ATL), and successfully established a
xenograft model of leukaemia (123).
Similarly, B-cell lymphoma cells also express CA9, which is
associated with extracellular acidosis in xenograft tumours
(124).
Research on CA9 has now been extended to the field
of tumour immunotherapy including the production of vaccines and
adoptive chimeric antigen receptor (CAR) T or NK cell therapy. DNA
vaccination can be used as a potential method to induce
antigen-specific T cell immune responses to enhance anti-tumour
therapy (125,126). CA9 has obvious tumour specificity
and can be used as an ideal target for the immunotherapy of renal
cell carcinoma (127). To date,
there have been many studies on the vaccines of renal cell
carcinoma based on CA9 antigens, including peptide vaccines, DCs
containing CA9 antigen, and DNA vaccine (128). A new study designed a DNA vaccine
containing CA9 antigen with the result of a definite inhibition of
CA9-Renca tumour compared with the control group. The vaccine
activated CTL responses, and activated CD8+ T cells with
the expression of IFN-γ, IL-2, and TNF-α (129). In addition, CA9-specific CAR was
transduced into T or NK92 cells, which showed specific cytotoxicity
in vitro and in vivo, including the release of IFN-γ,
granzyme B and perforin (130,131).
Antigen-dependent cellular cytotoxicity (ADCC) induces the
production of NK cells. The monoclonal antibody, girentuximab, can
specifically bind to CA9 expressed in tumour cells and trigger the
ADCC immune response to kill tumour cells. This monoclonal antibody
is currently in phase III clinical trials for patients with clear
cell renal cell carcinoma (132,133).
The proton-sensitive GPCR family has four members:
GPR4, TDAG8 (GPR65), OGR1 (GPR68), and G2A (GPR132), which help
cells respond to extracellular acidosis (134) (Fig.
2). A growing body of research suggests that proton-sensing
GPCRs are expressed on a variety of immune cells. As
lysophosphatidic receptors, they can be activated in a pH range of
6.4 to 6.8, via the protonation of histidine residues located in
the extracellular domain (135,136).
Their activated signaling pathways such as activation of the
protein kinase A/ERK signaling pathway and stimulation of
phospholipase C- and adenylate cyclase-induced cAMP accumulation,
enable them to regulate the role of immune cells in inflammation,
allergic reaction and tumour biology (137–139).
Inflammation is attributed to an increase in local proton
concentration, lactic acid production, and subsequent
pro-inflammatory cytokine production, while acidic environments, in
turn, influence the progression and regression of inflammation
(140).
Upon activation of NF-κB by TNF, myeloid cells,
including monocytes and macrophages, overexpress OGR1, which
indicates the pathological role of the pH-sensitive receptor OGR1
in precancerous mucositis. Many studies have shown that an acidic
environment can trigger the production of pro-inflammatory
cytokines (including TNF, IL-6, IFN-γ, and IL-1β) in macrophages by
increasing proton concentration and lactate production. In such an
environment, OGR1 can induce extensive activation, including
phospholipase C, and the formation of inositol triphosphate and
Ca2+. Interestingly, TNF induces OGR1 expression in
monocytes, thus playing a key role in chronic colitis, and OGR1
deficiency was found to prevent spontaneous inflammation in
preclinical models (141).
Similarly, GPR4 knockout was found to effectively alleviate colitis
in mice (142). In a
G2A−/− mouse model of enteritis, it was found that
monocyte and eosinophil recruitment to the injured colon was
increased, while the number of CD4+ lymphocytes, such as
IFN-γ, were decreased (143).
Similarly, the G2A−/− sepsis mouse model showed higher
lethality, as well as lower plasma cytokine levels and bacterial
scavenging capacity (144). G2A also
acts as a threshold regulator of neurons. In nerve injury sites,
G2A overexpression reduced the release of pro-inflammatory
cytokines and growth factors to alleviate the inflammatory
reaction, thereby alleviating hyperalgesia (145,146).
In acute inflammation, G2A and TDAG8 indirectly regulate the M1/M2
polarisation by increasing proinflammatory cytokine levels
(produced by M1 macrophages) and reducing anti-inflammatory
cytokine levels (produced by M2 macrophages) to relieve joint
inflammation and ease pain (147–149).
In other inflammatory diseases, such as acute lung injury with
large neutrophil infiltration, TDAG8, as a negative regulator of
lung neutrophilic inflammation and injury, significantly reduced
the expression of CXCL1 (150).
However, the role of TDAG8 in neutrophil survival was found to
augment, rather than attenuate, intestinal inflammation in an
adoptive transfer colitis model (151).
Furthermore, in the TME, G2A on macrophages can
sense and respond to the lactic acid signal from tumour cells and
induce M2-like macrophage polarisation, thereby increasing the risk
of breast cancer metastasis (152).
OGR1 expression in bone marrow-derived CD11b+
GR1+ DP cells can promote M2 type macrophage
polarisation and inhibit the infiltration of T cells, which is
essential for tumour cell-induced immunosuppression and tumour
development (153). The above data
support the hypothesis that pH sensors expressed by immune cells
may be involved in maintaining precancerous inflammation at an
early stage, and thus represent a potential target for tumour
prevention based on recurrent inflammation-induced
carcinogenesis.
GPCRs also play a variety of roles in airway
response. Allergic asthma is an inflammatory airway disease that is
mediated by Th2 lymphocytes. Due to the stimulation of glycolysis
and respiratory burst, lactic acid is produced around the bronchial
tube where inflammatory cells aggregate (154). In the ovalbumin (OVA)-induced asthma
model, OGR1 regulates the migration of DCs to draining lymph nodes
after exposure to antigens and stimulates Th2 phenotype changes,
which subsequently induces airway inflammation and airway
hyperresponsiveness (155). TDAG8 is
expressed on eosinophils in two different allergic asthma models
and regulates allergen-induced airway eosinophilia (156).
The proton-sensitive GPCR family as orphan GPCRs,
whose endogenous ligands are yet to be discovered, can be used as a
potential new target for the treatment of various indications. For
example, G2A acts as a threshold regulator of neurons for treatment
of intestinal inflammation. TDAG8 was used as an antispasmodic
target for the treatment of allergic inflammation and OGR1 was used
for the treatment of melanoma and prostate cancer. Only GPR4 has a
small molecule inhibitor, GPR4 antagonist 13 (NE-52-QQ57), which
can reduce the ability of neutrophils, macrophages, and T cells to
infiltrate into the inflammatory site, thereby reducing intestinal
and joint inflammation (157–159).
Compound 3b is also a selective antagonist of GPR4. It is a
suitable pharmacological tool for in vitro and in
vivo studies on the role of GPR4 in tissue acidosis and
consequential pathological tissue damage (160). Although OGR1 inhibitors have not
been explored, dibenzazine derivatives, a GPR4 antagonist, have
been reported to have antagonistic effects on OGR1 at high
concentrations (160).
As mentioned above, extracellular acidosis is
associated with the duration and severity of tumours, allergies,
and infectious diseases. Targeting extracellular acidosis can not
only deprive tumours of local progression, metastasis, and
resistance to cytotoxic drugs, but also may serve as a key factor
in reversing immune cell effector function.
V-ATPase inhibitors can improve the acidity of the
extracellular microenvironment and lysosomal compartment so that
the acidity gradient inside and outside the cell is unbalanced, to
achieve tumour cell death. PPIs are widely used in clinical
practice. They have low toxicity and improve the recruitment of the
host immune system to control tumours better (171). The same effect can be achieved by CA
to regulate the extracellular pH gradient. Small molecule
inhibitors of CA, such as sulphonamide U-104 and SLC-0111, are in
preclinical evaluation for solid tumours and metastases with CA9
overexpression (172). It is worth
noting that, compared with small-molecule inhibitors, antibodies
are currently the main drugs in clinical development. For example,
the monoclonal antibody, girentuximab, not only inhibits the growth
of primary and metastatic tumours by reversing tumour
acidification, but also circumvents some toxicity and selectivity
problems encountered by small-molecule compounds. It is clinically
used to treat kidney cancer, brain metastases or arthritis
(172).
Acidosis is commonly developed in several diseases
via multiple pathways, such as an acidic TME caused by inadequate
hemoperfusion, hypoxia and fatty acid metabolism, a respiratory
anaphylactic reaction caused by airway vapour condensate
acidification, and accumulation of lactic acid secreted by
inflammatory mediators in inflammation. Importantly, acidosis
affects the phenotype and activity of immune cells, and
pH-sensitive transporters have become excellent targets for
regulating immune cell function (Fig.
1). However, the cellular composition and functional state of
tumor-infiltrating immunocytes show significant differences in
different tumors. For example, brain tumors show the lowest level
of immunocyte infiltration, with macrophages as the dominant cells
over lymphocytes and NK cells, which might be the reason for the
limited response to immune checkpoint blockade. Therefore, NHE1
inhibitor, cariporide, which impells the polarization of TAMs
towards pro-inflammatory M1-type has become an effective treatment.
Malignant tumors containing a higher proportion of immune cells
including lung cancer, renal cancer, and skin melanoma, are the
ones most sensitive to immunotherapy. They show a high frequency of
T cells in the immunology compartment, for which the combination
with pH receptor inhibitors may further enhance the effect of
immunotherapy (173).
The main purpose of this review was to clarify the
importance of the molecular mechanisms involved in pH dysregulation
in different diseases, as well as the recent advances and
applications of pH regulator inhibitors. It is suggested that
targeting extracellular acidosis in combination with other
conventional therapies can selectively sensitise cancer cells to
combination therapies, thereby reducing drug resistance. This could
improve the survival rates of patients with cancer. To understand
the clinical application of pH-regulator-related inhibitors better
and facilitate the development of modern cancer research in this
new field, improvement in the current chemotherapy, surgery, and
radiotherapy models is warranted.
We thank all the individuals who took part in this
research.
Our work was supported by grants from the CAMS
initiative for Innovative Medicine of China (2016-12M-3-019), and
the National Mega Projects of China for Major Infectious Diseases
(2017ZX10304402).
All information provided in this review is
documented by relevant references.
Conceptualization of the review was accomplished by
LC, RG and XC. Data curation was carried out by LC, WL and TH.
Formal analysis was conducted by LC, TH, WZ, XY and ML. Funding
acquisition was the responsibility of RG. Writing of original draft
was carried out by LC and TH. Writing, review and editing were the
responsibility of LC, WL and TH. Project administration was the
responsibility of RG and XC.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Imtiyaz HZ and Simon MC: Hypoxia-inducible
factors as essential regulators of inflammation. Curr Top Microbiol
Immunol. 345:105–120. 2010.PubMed/NCBI
|
|
2
|
Jing X, Yang F, Shao C, Wei K, Xie M, Shen
H and Shu Y: Role of hypoxia in cancer therapy by regulating the
tumor microenvironment. Mol Cancer. 18:1572019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koltai T: The Ph paradigm in cancer. Eur J
Clin Nutr. 74 (Suppl 1):S14–S19. 2020. View Article : Google Scholar
|
|
4
|
Jancic CC, Cabrini M, Gabelloni ML,
Rodríguez Rodrigues C, Salamone G, Trevani AS and Geffner J: Low
extracellular pH stimulates the production of IL-1beta by human
monocytes. Cytokine. 57:258–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang CH, Curtis JD, Maggi LB Jr, Faubert
B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih
J, Qiu J, et al: Posttranscriptional control of T cell effector
function by aerobic glycolysis. Cell. 153:1239–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakagawa Y, Negishi Y, Shimizu M,
Takahashi M, Ichikawa M and Takahashi H: Effects of extracellular
pH and hypoxia on the function and development of antigen-specific
cytotoxic T lymphocytes. Immunol Lett. 167:72–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chalmin F, Bruchard M, Vegran F and
Ghiringhelli F: Regulation of T cell antitumor immune response by
tumor induced metabolic stress. Cell Stress. 3:9–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hargadon KM: Tumor-altered dendritic cell
function: Implications for anti-tumor immunity. Front Immunol.
4:1922013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cramer T, Yamanishi Y, Clausen BE, Förster
I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V,
et al: HIF-1alpha is essential for myeloid cell-mediated
inflammation. Cell. 112:645–657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JH, Elly C, Park Y and Liu YC: E3
ubiquitin ligase VHL regulates hypoxia-inducible factor-1α to
maintain regulatory T cell stability and suppressive capacity.
Immunity. 42:1062–1074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martínez D, Vermeulen M, Trevani A,
Ceballos A, Sabatté J, Gamberale R, Alvarez ME, Salamone G, Tanos
T, Coso OA and Geffner J: Extracellular acidosis induces neutrophil
activation by a mechanism dependent on activation of
phosphatidylinositol 3-kinase/Akt and ERK pathways. J Immunol.
176:1163–1171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franklin RA, Liao W, Sarkar A, Kim MV,
Bivona MR, Liu K, Pamer EG and Li MO: The cellular and molecular
origin of tumor-associated macrophages. Science. 344:921–925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erra Díaz F, Dantas E and Geffner J:
Unravelling the interplay between extracellular acidosis and immune
cells. Mediators Inflamm. 2018:12182972018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roma-Rodrigues C, Mendes R, Baptista PV
and Fernandes AR: Targeting tumor microenvironment for cancer
therapy. Int J Mol Sci. 20:8402019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huber V, Camisaschi C, Berzi A, Ferro S,
Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C and
Rivoltini L: Cancer acidity: An ultimate frontier of tumor immune
escape and a novel target of immunomodulation. Semin Cancer Biol.
43:74–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDonald PC, Chafe SC and Dedhar S:
Overcoming hypoxia-mediated tumor progression: Combinatorial
approaches targeting pH regulation, angiogenesis and immune
dysfunction. Front Cell Dev Biol. 4:272016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Guo B, Wang J, Cheng X, Xu Y and
Sang J: Ovarian cancer G protein coupled receptor 1 suppresses cell
migration of MCF7 breast cancer cells via a Gα12/13-Rho-Rac1
pathway. J Mol Signal. 8:62013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiley SZ, Sriram K, Liang W, Chang SE,
French R, McCann T, Sicklick J, Nishihara H, Lowy AM and Insel PA:
GPR68, a proton-sensing GPCR, mediates interaction of
cancer-associated fibroblasts and cancer cells. FASEB J.
32:1170–1183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pérez-Escuredo J, Van Hée VF, Sboarina M,
Falces J, Payen VL, Pellerin L and Sonveaux P: Monocarboxylate
transporters in the brain and in cancer. Biochim Biophys Acta.
1863:2481–2497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halestrap AP and Wilson MC: The
monocarboxylate transporter family-role and regulation. IUBMB Life.
64:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alfarouk KO: Tumor metabolism, cancer cell
transporters, and microenvironmental resistance. J Enzyme Inhib Med
Chem. 31:859–866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Merezhinskaya N, Ogunwuyi SA, Mullick FG
and Fishbein WN: Presence and localization of three lactic acid
transporters (MCT1, −2, and −4) in separated human granulocytes,
lymphocytes, and monocytes. J Histochem Cytochem. 52:1483–1493.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Merezhinskaya N, Ogunwuyi SA and Fishbein
WN: Expression of monocarboxylate transporter 4 in human platelets,
leukocytes, and tissues assessed by antibodies raised against
terminal versus pre-terminal peptides. Mol Genet Metab. 87:152–161.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long Y, Gao Z, Hu X, Xiang F, Wu Z, Zhang
J, Han X, Yin L, Qin J, Lan L, et al: Downregulation of MCT4 for
lactate exchange promotes the cytotoxicity of NK cells in breast
carcinoma. Cancer Med. 7:4690–4700. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sukumar M, Liu J, Ji Y, Subramanian M,
Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly
ED, et al: Inhibiting glycolytic metabolism enhances CD8+ T cell
memory and antitumor function. J Clin Invest. 123:4479–4488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murray CM, Hutchinson R, Bantick JR,
Belfield GP, Benjamin AD, Brazma D, Bundick RV, Cook ID, Craggs RI,
Edwards S, et al: Monocarboxylate transporter MCT1 is a target for
immunosuppression. Nat Chem Biol. 1:371–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Végran F, Boidot R, Michiels C, Sonveaux P
and Feron O: Lactate influx through the endothelial cell
monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway
that drives tumor angiogenesis. Cancer Res. 71:2550–2560. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang T, Feng Q, Wang Z, Li W, Sun Z,
Wilhelm J, Huang G, Vo T, Sumer BD and Gao J: Tumor-targeted
inhibition of monocarboxylate transporter 1 improves T-cell
immunotherapy of solid tumors. Adv Healthc Mater. 10:e20005492021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raychaudhuri D, Bhattacharya R, Sinha BP,
Liu CSC, Ghosh AR, Rahaman O, Bandopadhyay P, Sarif J, D'Rozario R,
Paul S, et al: Lactate induces pro-tumor reprogramming in
intratumoral plasmacytoid dendritic cells. Front Immunol.
10:18782019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonuccelli G, Whitaker-Menezes D,
Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK,
Vander Heiden MG, Migneco G, Chiavarina B, et al: The reverse
Warburg effect: Glycolysis inhibitors prevent the tumor promoting
effects of caveolin-1 deficient cancer associated fibroblasts. Cell
Cycle. 9:1960–1971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei L, Zhou Y, Yao J, Qiao C, Ni T, Guo R,
Guo Q and Lu N: Lactate promotes PGE2 synthesis and gluconeogenesis
in monocytes to benefit the growth of inflammation-associated
colorectal tumor. Oncotarget. 6:16198–16214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan Z, Xie N, Banerjee S, Cui H, Fu M,
Thannickal VJ and Liu G: The monocarboxylate transporter 4 is
required for glycolytic reprogramming and inflammatory response in
macrophages. J Biol Chem. 290:46–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L and Li S: Lactic acid promotes
macrophage polarization through MCT-HIF1α signaling in gastric
cancer. Exp Cell Res. 388:1118462020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stone SC, Rossetti RA, Alvarez KL,
Carvalho JP, Margarido PF, Baracat EC, Tacla M, Boccardo E, Yokochi
K, Lorenzi NP and Lepique AP: Lactate secreted by cervical cancer
cells modulates macrophage phenotype. J Leukoc Biol. 105:1041–1054.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong L, Wang Z, Liang X, Wang Y, Gao L and
Ma C: Monocarboxylate transporter 1 promotes classical microglial
activation and pro-inflammatory effect via
6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3. J
Neuroinflammation. 16:2402019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Silva LS, Poschet G, Nonnenmacher Y,
Becker HM, Sapcariu S, Gaupel AC, Schlotter M, Wu Y, Kneisel N,
Seiffert M, et al: Branched-chain ketoacids secreted by
glioblastoma cells via MCT1 modulate macrophage phenotype. EMBO
Rep. 18:2172–2185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Contreras-Baeza Y, Sandoval PY, Alarcón R,
Galaz A, Cortés-Molina F, Alegría K, Baeza-Lehnert F, Arce-Molina
R, Guequén A, Flores CA, et al: Monocarboxylate transporter 4
(MCT4) is a high affinity transporter capable of exporting lactate
in high-lactate microenvironments. J Biol Chem. 294:20135–20147.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mikkilineni L, Whitaker-Menezes D,
Domingo-Vidal M, Sprandio J, Avena P, Cotzia P, Dulau-Florea A,
Gong J, Uppal G, Zhan T, et al: Hodgkin lymphoma: A complex
metabolic ecosystem with glycolytic reprogramming of the tumor
microenvironment. Semin Oncol. 44:218–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Afonso J, Pinto T, Simões-Sousa S, Schmitt
F, Longatto-Filho A, Pinheiro C, Marques H and Baltazar F: Clinical
significance of metabolism-related biomarkers in non-Hodgkin
lymphoma-MCT1 as potential target in diffuse large B cell lymphoma.
Cell Oncol (Dordr). 42:303–318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beloueche-Babari M, Casals Galobart T,
Delgado-Goni T, Wantuch S, Parkes HG, Tandy D, Harker JA and Leach
MO: Monocarboxylate transporter 1 blockade with AZD3965 inhibits
lipid biosynthesis and increases tumour immune cell infiltration.
Br J Cancer. 122:895–903. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Noble RA, Bell N, Blair H, Sikka A, Thomas
H, Phillips N, Nakjang S, Miwa S, Crossland R, Rand V, et al:
Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel
therapeutic approach for diffuse large B-cell lymphoma and Burkitt
lymphoma. Haematologica. 102:1247–1257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Braga M, Kaliszczak M, Carroll L, Schug
ZT, Heinzmann K, Baxan N, Benito A, Valbuena GN, Stribbling S,
Beckley A, et al: Tracing nutrient flux following monocarboxylate
transporter-1 inhibition with AZD3965. Cancers (Basel).
12:17032020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Draoui N, Schicke O, Seront E, Bouzin C,
Sonveaux P, Riant O and Feron O: Antitumor activity of
7-aminocarboxycoumarin derivatives, a new class of potent
inhibitors of lactate influx but not efflux. Mol Cancer Ther.
13:1410–1418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Quanz M, Bender E, Kopitz C, Grünewald S,
Schlicker A, Schwede W, Eheim A, Toschi L, Neuhaus R, Richter C, et
al: Preclinical efficacy of the novel monocarboxylate transporter 1
inhibitor BAY-8002 and associated markers of resistance. Mol Cancer
Ther. 17:2285–2296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Renner K, Bruss C, Schnell A, Koehl G,
Becker HM, Fante M, Menevse AN, Kauer N, Blazquez R, Hacker L, et
al: Restricting glycolysis preserves T cell effector functions and
augments checkpoint therapy. Cell Rep. 29:135–150.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cho KS, Yamada T, Wynn C, Behanna HA, Hong
IC, Manaves V, Nakanishi T, Hirose J, Abe Y, Jiang H, et al:
Mechanism analysis of long-term graft survival by monocarboxylate
transporter-1 inhibition. Transplantation. 90:1299–1306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kondapalli KC, Prasad H and Rao R: An
inside job: How endosomal Na(+)/H(+) exchangers link to autism and
neurological disease. Front Cell Neurosci. 8:1722014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
De Vito P: The sodium/hydrogen exchanger:
A possible mediator of immunity. Cell Immunol. 240:69–85. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vereninov AA, Vassilieva IO, Yurinskaya
VE, Matveev VV, Glushankova LN, Lang F and Matskevitch JA:
Differential transcription of ion transporters, NHE1, ATP1B1, NKCC1
in human peripheral blood lymphocytes activated to proliferation.
Cell Physiol Biochem. 11:19–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chang CP, Wang SW, Huang ZL, Wang OY,
Huang MI, Lu LM, Tarng DC, Chien CH and Chien EJ: Non-genomic rapid
inhibition of Na+/H+-exchange 1 and apoptotic
immunosuppression in human T cells by glucocorticoids. J Cell
Physiol. 223:679–686. 2010.PubMed/NCBI
|
|
55
|
Chien EJ, Hsu CH, Chang VH, Lin EP, Kuo
TP, Chien CH and Lin HY: In human T cells mifepristone antagonizes
glucocorticoid non-genomic rapid responses in terms of
Na(+)/H(+)-exchange 1 activity, but not ezrin/radixin/moesin
phosphorylation. Steroids. 111:29–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xing K, Gu B, Zhang P and Wu X:
Dexamethasone enhances programmed cell death 1 (PD-1) expression
during T cell activation: An insight into the optimum application
of glucocorticoids in anti-cancer therapy. BMC Immunol. 16:392015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lai JN, Wang OY, Lin VH, Liao CF, Tarng DC
and Chien EJ: The non-genomic rapid acidification in peripheral T
cells by progesterone depends on intracellular calcium increase and
not on Na+/H+-exchange inhibition. Steroids.
77:1017–1024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Singh Y, Zhou Y, Shi X, Zhang S, Umbach
AT, Salker MS, Lang KS and Lang F: Alkaline cytosolic pH and high
sodium hydrogen exchanger 1 (NHE1) activity in Th9 cells. J Biol
Chem. 291:23662–23671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rotte A, Pasham V, Eichenmüller M, Yang W,
Bhandaru M and Lang F: Influence of dexamethasone on
Na+/H+ exchanger activity in dendritic cells.
Cell Physiol Biochem. 28:305–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang W, Bhandaru M, Pasham V, Bobbala D,
Zelenak C, Jilani K, Rotte A and Lang F: Effect of thymoquinone on
cytosolic pH and Na+/H+ exchanger activity in
mouse dendritic cells. Cell Physiol Biochem. 29:21–30. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou Y, Pasham V, Chatterjee S, Rotte A,
Yang W, Bhandaru M, Singh Y and Lang F: Regulation of
Na+/H+ exchanger in dendritic cells by Akt1.
Cell Physiol Biochem. 36:1237–1249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu W, Carney KE, Pigott VM, Falgoust LM,
Clark PA, Kuo JS and Sun D: Glioma-mediated microglial activation
promotes glioma proliferation and migration: Roles of Na+/H+
exchanger isoform 1. Carcinogenesis. 37:839–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Song S, Wang S, Pigott VM, Jiang T, Foley
LM, Mishra A, Nayak R, Zhu W, Begum G, Shi Y, et al: Selective role
of Na+/H+ exchanger in Cx3cr1+
microglial activation, white matter demyelination, and post-stroke
function recovery. Glia. 66:2279–2298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu CL, Zhang X, Liu J, Wang Y, Sukhova
GK, Wojtkiewicz GR, Liu T, Tang R, Achilefu S, Nahrendorf M, et al:
Na+-H+ exchanger 1 determines atherosclerotic
lesion acidification and promotes atherogenesis. Nat Commun.
10:39782019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Takakuwa S, Mizuno N, Takano T, Asakawa S,
Sato T, Hiratsuka M and Hirasawa N: Down-regulation of
Na+/H+ exchanger 1 by Toll-like receptor
stimulation in macrophages. Immunobiology. 222:176–182. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Provost JJ and Wallert MA: Inside out:
Targeting NHE1 as an intracellular and extracellular regulator of
cancer progression. Chem Biol Drug Des. 81:85–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Asgharzadeh MR, Barar J, Pourseif MM,
Eskandani M, Jafari Niya M, Mashayekhi MR and Omidi Y: Molecular
machineries of pH dysregulation in tumor microenvironment:
Potential targets for cancer therapy. Bioimpacts. 7:115–133. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shi Y, Chanana V, Watters JJ, Ferrazzano P
and Sun D: Role of sodium/hydrogen exchanger isoform 1 in
microglial activation and proinflammatory responses in ischemic
brains. J Neurochem. 119:124–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu CL, Liu X, Wang Y, Deng Z, Liu T,
Sukhova GK, Wojtkiewicz GR, Tang R, Zhang JY, Achilefu S, et al:
Reduced Nhe1 (Na+-H+ Exchanger-1) function
protects ApoE-deficient mice From Ang II (Angiotensin II)-induced
abdominal aortic aneurysms. Hypertension. 76:87–100. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Guan X, Hasan MN, Begum G, Kohanbash G,
Carney KE, Pigott VM, Persson AI, Castro MG, Jia W and Sun D:
Blockade of Na/H exchanger stimulates glioma tumor immunogenicity
and enhances combinatorial TMZ and anti-PD-1 therapy. Cell Death
Dis. 9:10102018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jin W, Li Q, Wang J, Chang G, Lin Y, Li H,
Wang L, Gao W and Pang T: Na+/H+ exchanger 1
inhibition contributes to K562 leukaemic cell differentiation. Cell
Biol Int. 36:739–745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fliegel L: Structural and functional
changes in the Na+/H+ exchanger isoform 1,
induced by Erk1/2 phosphorylation. Int J Mol Sci. 20:23782019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Smith AN, Lovering RC, Futai M, Takeda J,
Brown D and Karet FE: Revised nomenclature for mammalian
vacuolar-type H+ -ATPase subunit genes. Mol Cell.
12:801–803. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Forgac M: Vacuolar ATPases: Rotary proton
pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol.
8:917–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
McGuire C, Stransky L, Cotter K and Forgac
M: Regulation of V-ATPase activity. Front Biosci (Landmark Ed).
22:609–622. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kulshrestha A, Katara GK, Ginter J,
Pamarthy S, Ibrahim SA, Jaiswal MK, Sandulescu C, Periakaruppan R,
Dolan J, Gilman-Sachs A and Beaman KD: Selective inhibition of
tumor cell associated Vacuolar-ATPase ‘a2’ isoform overcomes
cisplatin resistance in ovarian cancer cells. Mol Oncol.
10:789–805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Smith GA, Howell GJ, Phillips C, Muench
SP, Ponnambalam S and Harrison MA: Extracellular and luminal pH
regulation by vacuolar H+-ATPase isoform expression and
targeting to the plasma membrane and endosomes. J Biol Chem.
291:8500–8515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Stransky L, Cotter K and Forgac M: The
function of V-ATPases in cancer. Physiol Rev. 96:1071–1091. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rao Z, Jordan PM, Wang Y, Menche D, Pace
S, Gerstmeier J and Werz O: Differential role of vacuolar
(H+)-ATPase in the expression and activity of
cyclooxygenase-2 in human monocytes. Biochem Pharmacol.
175:1138582020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sahoo M, Katara GK, Bilal MY, Ibrahim SA,
Kulshrestha A, Fleetwood S, Suzue K and Beaman KD: Hematopoietic
stem cell specific V-ATPase controls breast cancer progression and
metastasis via cytotoxic T cells. Oncotarget. 9:33215–33231. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Peterson TV, Jaiswal MK, Beaman KD and
Reynolds JM: Conditional deletion of the V-ATPase a2-subunit
disrupts intrathymic T cell development. Front Immunol.
10:19112019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rothenberg EV, Ungerbäck J and Champhekar
A: Forging T-lymphocyte identity: Intersecting networks of
transcriptional control. Adv Immunol. 129:109–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
McGuire C, Cotter K, Stransky L and Forgac
M: Regulation of V-ATPase assembly and function of V-ATPases in
tumor cell invasiveness. Biochim Biophys Acta. 1857:1213–1218.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ibrahim SA, Kulshrestha A, Katara GK, Amin
MA and Beaman KD: Cancer derived peptide of vacuolar ATPase ‘a2’
isoform promotes neutrophil migration by autocrine secretion of
IL-8. Sci Rep. 6:368652016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Katara GK, Jaiswal MK, Kulshrestha A,
Kolli B, Gilman-Sachs A and Beaman KD: Tumor-associated vacuolar
ATPase subunit promotes tumorigenic characteristics in macrophages.
Oncogene. 33:5649–5654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Katara GK, Kulshrestha A, Mao L, Wang X,
Sahoo M, Ibrahim S, Pamarthy S, Suzue K, Shekhawat GS, Gilman-Sachs
A and Beaman KD: Mammary epithelium-specific inactivation of
V-ATPase reduces stiffness of extracellular matrix and enhances
metastasis of breast cancer. Mol Oncol. 12:208–223. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ibrahim SA, Kulshrestha A, Katara GK,
Riehl V, Sahoo M and Beaman KD: Cancer-associated V-ATPase induces
delayed apoptosis of protumorigenic neutrophils. Mol Oncol.
14:590–610. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gilman-Sachs A, Tikoo A, Akman-Anderson L,
Jaiswal M, Ntrivalas E and Beaman K: Expression and role of a2
vacuolar-ATPase (a2V) in trafficking of human neutrophil granules
and exocytosis. J Leukoc Biol. 97:1121–1131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xia Y, Liu N, Xie X, Bi G, Ba H, Li L,
Zhang J, Deng X, Yao Y, Tang Z, et al: The macrophage-specific
V-ATPase subunit ATP6V0D2 restricts inflammasome activation and
bacterial infection by facilitating autophagosome-lysosome fusion.
Autophagy. 15:960–975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Murase M, Kawasaki T, Hakozaki R, Sueyoshi
T, Putri DD, Kitai Y, Sato S, Ikawa M and Kawai T: Intravesicular
acidification regulates lipopolysaccharide inflammation and
tolerance through TLR4 trafficking. J Immunol. 200:2798–2808. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kimura T, Nada S, Takegahara N, Okuno T,
Nojima S, Kang S, Ito D, Morimoto K, Hosokawa T, Hayama Y, et al:
Polarization of M2 macrophages requires Lamtor1 that integrates
cytokine and amino-acid signals. Nat Commun. 7:131302016.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rao Z, Pace S, Jordan PM, Bilancia R,
Troisi F, Börner F, Andreas N, Kamradt T, Menche D, Rossi A, et al:
Vacuolar (H+)-ATPase critically regulates specialized
proresolving mediator pathways in human M2-like monocyte-derived
macrophages and has a crucial role in resolution of inflammation. J
Immunol. 203:1031–1043. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kuchuk O, Tuccitto A, Citterio D, Huber V,
Camisaschi C, Milione M, Vergani B, Villa A, Alison MR, Carradori
S, et al: pH regulators to target the tumor immune microenvironment
in human hepatocellular carcinoma. OncoImmunology. 7:e14454522018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Thomas L, Rao Z, Gerstmeier J, Raasch M,
Weinigel C, Rummler S, Menche D, Müller R, Pergola C, Mosig A and
Werz O: Selective upregulation of TNFα expression in
classically-activated human monocyte-derived macrophages (M1)
through pharmacological interference with V-ATPase. Biochem
Pharmacol. 130:71–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bowman EJ, Graham LA, Stevens TH and
Bowman BJ: The bafilomycin/concanamycin binding site in subunit c
of the V-ATPases from Neurospora crassa and Saccharomyces
cerevisiae. J Biol Chem. 279:33131–33138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lu X, Chen L, Chen Y, Shao Q and Qin W:
Bafilomycin A1 inhibits the growth and metastatic potential of the
BEL-7402 liver cancer and HO-8910 ovarian cancer cell lines and
induces alterations in their microRNA expression. Exp Ther Med.
10:1829–1834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Halcrow P, Khan N, Datta G, Ohm JE, Chen X
and Geiger JD: Importance of measuring endolysosome, cytosolic, and
extracellular pH in understanding the pathogenesis of and possible
treatments for glioblastoma multiforme. Cancer Rep.
2:e11932019.PubMed/NCBI
|
|
98
|
Yuan N, Song L, Zhang S, Lin W, Cao Y, Xu
F, Fang Y, Wang Z, Zhang H, Li X, et al: Bafilomycin A1 targets
both autophagy and apoptosis pathways in pediatric B-cell acute
lymphoblastic leukemia. Haematologica. 100:345–356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Emruli VK, Olsson R, Ek F and Ek S:
Identification of V-ATPase as a molecular sensor of SOX11-levels
and potential therapeutic target for mantle cell lymphoma. BMC
Cancer. 16:4932016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fais S: Proton pump inhibitor-induced
tumour cell death by inhibition of a detoxification mechanism. J
Intern Med. 267:515–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
De Milito A, Canese R, Marino ML, Borghi
M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et
al: pH-dependent antitumor activity of proton pump inhibitors
against human melanoma is mediated by inhibition of tumor acidity.
Int J Cancer. 127:207–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Denny WA and Wilson WR: Considerations for
the design of nitrophenyl mustards as agents with selective
toxicity for hypoxic tumor cells. J Med Chem. 29:879–887. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Luciani F, Spada M, De Milito A, Molinari
A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone
F, et al: Effect of proton pump inhibitor pretreatment on
resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst.
96:1702–1713. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Corbet C and Feron O: Tumour acidosis:
From the passenger to the driver's seat. Nat Rev Cancer.
17:577–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Neri D and Supuran CT: Interfering with pH
regulation in tumours as a therapeutic strategy. Nat Rev Drug
Discov. 10:767–777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fan J, Kamphorst JJ, Mathew R, Chung MK,
White E, Shlomi T and Rabinowitz JD: Glutamine-driven oxidative
phosphorylation is a major ATP source in transformed mammalian
cells in both normoxia and hypoxia. Mol Syst Biol. 9:7122013.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sedlakova O, Svastova E, Takacova M,
Kopacek J, Pastorek J and Pastorekova S: Carbonic anhydrase IX, a
hypoxia-induced catalytic component of the pH regulating machinery
in tumors. Front Physiol. 4:4002014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hunt JF, Fang K, Malik R, Snyder A,
Malhotra N, Platts-Mills TA and Gaston B: Endogenous airway
acidification. Implications for asthma pathophysiology. Am J Respir
Crit Care Med. 161((3 Pt 1)): 694–699. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wen T, Mingler MK, Wahl B, Khorki ME,
Pabst O, Zimmermann N and Rothenberg ME: Carbonic anhydrase IV is
expressed on IL-5-activated murine eosinophils. J Immunol.
192:5481–5489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Pulendran B and Artis D: New paradigms in
type 2 immunity. Science. 337:431–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Henry EK, Sy CB, Inclan-Rico JM, Espinosa
V, Ghanny SS, Dwyer DF, Soteropoulos P, Rivera A and Siracusa MC:
Carbonic anhydrase enzymes regulate mast cell-mediated
inflammation. J Exp Med. 213:1663–1673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Noti M, Kim BS, Siracusa MC, Rak GD, Kubo
M, Moghaddam AE, Sattentau QA, Comeau MR, Spergel JM and Artis D:
Exposure to food allergens through inflamed skin promotes
intestinal food allergy through the thymic stromal
lymphopoietin-basophil axis. J Allergy Clin Immunol. 133:1390–1399,
1399.e1-6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Winum JY: Carbonic anhydrase enzymes for
regulating mast cell hematopoiesis and type-2 inflammation: A
patent evaluation (WO2017/058370). Expert Opin Ther Pat.
28:741–743. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Supuran CT: Carbonic anhydrase inhibitors
and their potential in a range of therapeutic areas. Expert Opin
Ther Pat. 28:709–712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Supuran CT, Altamimi ASA and Carta F:
Carbonic anhydrase inhibition and the management of glaucoma: A
literature and patent review 2013–2019. Expert Opin Ther Pat.
29:781–792. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Borenshtein D, Schlieper KA, Rickman BH,
Chapman JM, Schweinfest CW, Fox JG and Schauer DB: Decreased
expression of colonic Slc26a3 and carbonic anhydrase iv as a cause
of fatal infectious diarrhea in mice. Infect Immun. 77:3639–3650.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mori K, Yamanishi H, Ikeda Y, Kumagi T,
Hiasa Y, Matsuura B, Abe M and Onji M: Oral administration of
carbonic anhydrase I ameliorates murine experimental colitis
induced by Foxp3-CD4+CD25-T cells. J Leukoc Biol. 93:963–972. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lavin Y, Winter D, Blecher-Gonen R, David
E, Keren-Shaul H, Merad M, Jung S and Amit I: Tissue-resident
macrophage enhancer landscapes are shaped by the local
microenvironment. Cell. 159:1312–1326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lounnas N, Rosilio C, Nebout M, Mary D,
Griessinger E, Neffati Z, Chiche J, Spits H, Hagenbeek TJ, Asnafi
V, et al: Pharmacological inhibition of carbonic anhydrase XII
interferes with cell proliferation and induces cell apoptosis in
T-cell lymphomas. Cancer Lett. 333:76–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ibrahim-Hashim A and Estrella V: Acidosis
and cancer: From mechanism to neutralization. Cancer Metastasis
Rev. 38:149–155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chafe SC, McDonald PC, Saberi S,
Nemirovsky O, Venkateswaran G, Burugu S, Gao D, Delaidelli A, Kyle
AH, Baker JHE, et al: Targeting hypoxia-induced carbonic anhydrase
IX enhances immune-checkpoint blockade locally and systemically.
Cancer Immunol Res. 7:1064–1078. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
McDonald PC, Chia S, Bedard PL, Chu Q,
Lyle M, Tang L, Singh M, Zhang Z, Supuran CT, Renouf DJ and Dedhar
S: A phase 1 study of SLC-0111, a novel inhibitor of carbonic
anhydrase IX, in patients with advanced solid tumors. Am J Clin
Oncol. 43:484–490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Nasu K, Yamaguchi K, Takanashi T, Tamai K,
Sato I, Ine S, Sasaki O, Satoh K, Tanaka N, Tanaka Y, et al:
Crucial role of carbonic anhydrase IX in tumorigenicity of
xenotransplanted adult T-cell leukemia-derived cells. Cancer Sci.
108:435–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chen LQ, Howison CM, Spier C, Stopeck AT,
Malm SW, Pagel MD and Baker AF: Assessment of carbonic anhydrase IX
expression and extracellular pH in B-cell lymphoma cell line
models. Leuk Lymphoma. 56:1432–1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Mei Y, Zhao L, Liu Y, Gong H, Song Y, Lei
L, Zhu Y, Jin Z, Ma S, Hu B, et al: Combining DNA vaccine and
AIDA-1 in attenuated Salmonella activates tumor-specific
CD4+ and CD8+ T-cell responses. Cancer
Immunol Res. 5:503–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sun YY, Peng S, Han L, Qiu J, Song L, Tsai
Y, Yang B, Roden RB, Trimble CL, Hung CF and Wu TC: Local HPV
Recombinant vaccinia boost following priming with an HPV DNA
vaccine enhances local HPV-specific CD8+ T-cell-mediated
tumor control in the genital tract. Clin Cancer Res. 22:657–669.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Duan Y, Yang C, Zhang Z, Liu J, Zheng J
and Kong D: Poly(ethylene glycol)-grafted polyethylenimine modified
with G250 monoclonal antibody for tumor gene therapy. Hum Gene
Ther. 21:191–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhao Y, Wei Z, Yang H, Li X, Wang Q, Wang
L and Li S: Enhance the anti-renca carcinoma effect of a DNA
vaccine targeting G250 gene by co-expression with cytotoxic
T-lymphocyte associated antigen-4(CTLA-4). Biomed Pharmacother.
90:147–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chai D, Shan H, Wang G, Zhang Q, Li H,
Fang L, Song J, Liu N, Zhang Q, Yao H and Zheng J: Combining DNA
vaccine and AIM2 in H1 nanoparticles exert anti-renal carcinoma
effects via enhancing tumor-specific multi-functional
CD8+ T-cell responses. Mol Cancer Ther. 18:323–334.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang Q, Xu J, Ding J, Liu H, Li H, Li H,
Lu M, Miao Y, Wang Z, Fu Q and Zheng J: Bortezomib improves
adoptive carbonic anhydrase IX-specific chimeric antigen
receptor-modified NK92 cell therapy in mouse models of human renal
cell carcinoma. Oncol Rep. 40:3714–3724. 2018.PubMed/NCBI
|
|
131
|
Li H, Ding J, Lu M, Liu H, Miao Y, Li L,
Wang G, Zheng J, Pei D and Zhang Q: CAIX-specific CAR-T cells and
sunitinib show synergistic effects against metastatic renal cancer
models. J Immunother. 43:16–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lau J, Liu Z, Lin KS, Pan J, Zhang Z,
Vullo D, Supuran CT, Perrin DM and Bénard F: Trimeric
radiofluorinated sulfonamide derivatives to achieve in vivo
selectivity for carbonic anhydrase IX-targeted PET imaging. J Nucl
Med. 56:1434–1440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Dubois LJ, Niemans R, van Kuijk SJ, Panth
KM, Parvathaneni NK, Peeters SG, Zegers CM, Rekers NH, van
Gisbergen MW, Biemans R, et al: New ways to image and target tumour
hypoxia and its molecular responses. Radiother Oncol. 116:352–357.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Justus CR, Dong L and Yang LV: Acidic
tumor microenvironment and pH-sensing G protein-coupled receptors.
Front Physiol. 4:3542013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Liu JP, Nakakura T, Tomura H, Tobo M, Mogi
C, Wang JQ, He XD, Takano M, Damirin A, Komachi M, et al: Each one
of certain histidine residues in G-protein-coupled receptor GPR4 is
critical for extracellular proton-induced stimulation of multiple
G-protein-signaling pathways. Pharmacol Res. 61:499–505. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ludwig MG, Vanek M, Guerini D, Gasser JA,
Jones CE, Junker U, Hofstetter H, Wolf RM and Seuwen K:
Proton-sensing G-protein-coupled receptors. Nature. 425:93–98.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Damaghi M, Wojtkowiak JW and Gillies RJ:
pH sensing and regulation in cancer. Front Physiol. 4:3702013.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Pilon-Thomas S, Kodumudi KN, El-Kenawi AE,
Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mulé JJ,
Ibrahim-Hashim A and Gillies RJ: Neutralization of tumor acidity
improves antitumor responses to immunotherapy. Cancer Res.
76:1381–1390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wiley SZ, Sriram K, Salmerón C and Insel
PA: GPR68: An emerging drug target in cancer. Int J Mol Sci.
20:5592019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lardner A: The effects of extracellular pH
on immune function. J Leukoc Biol. 69:522–530. 2001.PubMed/NCBI
|
|
141
|
de Vallière C, Wang Y, Eloranta JJ, Vidal
S, Clay I, Spalinger MR, Tcymbarevich I, Terhalle A, Ludwig MG,
Suply T, et al: G Protein-coupled pH-sensing receptor OGR1 is a
regulator of intestinal inflammation. Inflamm Bowel Dis.
21:1269–1281. 2015.PubMed/NCBI
|
|
142
|
Wang Y, de Vallière C, Imenez Silva PH,
Leonardi I, Gruber S, Gerstgrasser A, Melhem H, Weber A, Leucht K,
Wolfram L, et al: The proton-activated receptor GPR4 modulates
intestinal inflammation. J Crohn's Colitis. 12:355–368. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Frasch SC, McNamee EN, Kominsky D,
Jedlicka P, Jakubzick C, Zemski Berry K, Mack M, Furuta GT, Lee JJ,
Henson PM, et al: G2A signaling dampens colitic inflammation via
production of IFN-γ. J Immunol. 197:1425–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li HM, Jang JH, Jung JS, Shin J, Park CO,
Kim YJ, Ahn WG, Nam JS, Hong CW, Lee J, et al: G2A protects mice
against sepsis by modulating kupffer cell activation: Cooperativity
with adenosine receptor 2b. J Immunol. 202:527–538. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Su YS, Huang YF, Wong J, Lee CW, Hsieh WS
and Sun WH: G2A as a Threshold regulator of inflammatory
hyperalgesia modulates chronic hyperalgesia. J Mol Neurosci.
64:39–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Osthues T, Zimmer B, Rimola V, Klann K,
Schilling K, Mathoor P, Angioni C, Weigert A, Geisslinger G, Münch
C, et al: The lipid receptor G2A (GPR132) mediates macrophage
migration in nerve injury-induced neuropathic pain. Cells.
9:17402020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Kern K, Schäfer SMG, Cohnen J, Pierre S,
Osthues T, Tarighi N, Hohmann S, Ferreiros N, Brüne B, Weigert A,
et al: The G2A receptor controls polarization of macrophage by
determining their localization within the inflamed tissue. Front
Immunol. 9:22612018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kung CC, Dai SP, Chiang H, Huang HS and
Sun WH: Temporal expression patterns of distinct cytokines and
M1/M2 macrophage polarization regulate rheumatoid arthritis
progression. Mol Biol Rep. 47:3423–3437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Dai SP, Hsieh WS, Chen CH, Lu YH, Huang
HS, Chang DM, Huang SL and Sun WH: TDAG8 deficiency reduces
satellite glial number and pro-inflammatory macrophage number to
relieve rheumatoid arthritis disease severity and chronic pain. J
Neuroinflammation. 17:1702020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Tsurumaki H, Mogi C, Aoki-Saito H, Tobo M,
Kamide Y, Yatomi M, Sato K, Dobashi K, Ishizuka T, Hisada T, et al:
Protective role of proton-sensing TDAG8 in
lipopolysaccharide-induced acute lung injury. Int J Mol Sci.
16:28931–28942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Tcymbarevich I, Richards SM, Russo G,
Kühn-Georgijevic J, Cosin-Roger J, Baebler K, Lang S, Bengs S,
Atrott K, Bettoni C, et al: Lack of the pH-sensing receptor TDAG8
[GPR65] in macrophages plays a detrimental role in murine models of
inflammatory bowel disease. J Crohns Colitis. 13:245–258. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q,
Saghatelian A, Siegwart DJ and Wan Y: Gpr132 sensing of lactate
mediates tumor-macrophage interplay to promote breast cancer
metastasis. Proc Natl Acad Sci USA. 114:580–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Yan L, Singh LS, Zhang L and Xu Y: Role of
OGR1 in myeloid-derived cells in prostate cancer. Oncogene.
33:157–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Holgate ST: Innate and adaptive immune
responses in asthma. Nat Med. 18:673–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Aoki H, Mogi C, Hisada T, Nakakura T,
Kamide Y, Ichimonji I, Tomura H, Tobo M, Sato K, Tsurumaki H, et
al: Proton-sensing ovarian cancer G protein-coupled receptor 1 on
dendritic cells is required for airway responses in a murine asthma
model. PLoS One. 8:e799852013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kottyan LC, Collier AR, Cao KH, Niese KA,
Hedgebeth M, Radu CG, Witte ON, Khurana Hershey GK, Rothenberg ME
and Zimmermann N: Eosinophil viability is increased by acidic pH in
a cAMP- and GPR65-dependent manner. Blood. 114:2774–2782. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Sanderlin EJ, Marie M, Velcicky J,
Loetscher P and Yang LV: Pharmacological inhibition of GPR4
remediates intestinal inflammation in a mouse colitis model. Eur J
Pharmacol. 852:218–230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Velcicky J, Miltz W, Oberhauser B, Orain
D, Vaupel A, Weigand K, Dawson King J, Littlewood-Evans A, Nash M,
Feifel R and Loetscher P: Development of selective, orally active
GPR4 antagonists with modulatory effects on nociception,
inflammation, and angiogenesis. J Med Chem. 60:3672–3683. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Miltz W, Velcicky J, Dawson J,
Littlewood-Evans A, Ludwig MG, Seuwen K, Feifel R, Oberhauser B,
Meyer A, Gabriel D, et al: Design and synthesis of potent and
orally active GPR4 antagonists with modulatory effects on
nociception, inflammation, and angiogenesis. Bioorg Med Chem.
25:4512–4525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Fukuda H, Ito S, Watari K, Mogi C, Arisawa
M, Okajima F, Kurose H and Shuto S: Identification of a potent and
selective GPR4 antagonist as a drug lead for the treatment of
myocardial infarction. ACS Med Chem Lett. 7:493–497. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lengacher S, Nehiri-Sitayeb T, Steiner N,
Carneiro L, Favrod C, Preitner F, Thorens B, Stehle JC, Dix L,
Pralong F, et al: Resistance to diet-induced obesity and associated
metabolic perturbations in haploinsufficient monocarboxylate
transporter 1 mice. PLoS One. 8:e825052013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Draoui N, Schicke O, Fernandes A, Drozak
X, Nahra F, Dumont A, Douxfils J, Hermans E, Dogné JM, Corbau R, et
al: Synthesis and pharmacological evaluation of carboxycoumarins as
a new antitumor treatment targeting lactate transport in cancer
cells. Bioorg Med Chem. 21:7107–7117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Reshkin SJ, Cardone RA and Harguindey S:
Na+-H+ exchanger, pH regulation and cancer.
Recent Patents Anticancer Drug Discov. 8:85–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ferrari S, Perut F, Fagioli F, Brach Del
Prever A, Meazza C, Parafioriti A, Picci P, Gambarotti M, Avnet S,
Baldini N and Fais S: Proton pump inhibitor chemosensitization in
human osteosarcoma: From the bench to the patients' bed. J Transl
Med. 11:2682013. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Mentzer RM Jr, Bartels C, Bolli R, Boyce
S, Buckberg GD, Chaitman B, Haverich A, Knight J, Menasché P, Myers
ML, et al: Sodium-hydrogen exchange inhibition by cariporide to
reduce the risk of ischemic cardiac events in patients undergoing
coronary artery bypass grafting: Results of the EXPEDITION study.
Ann Thorac Surg. 85:1261–1270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zeymer U, Suryapranata H, Monassier JP,
Opolski G, Davies J, Rasmanis G, Linssen G, Tebbe U, Schröder R,
Tiemann R, et al: The Na(+)/H(+) exchange inhibitor eniporide as an
adjunct to early reperfusion therapy for acute myocardial
infarction. Results of the evaluation of the safety and
cardioprotective effects of eniporide in acute myocardial
infarction (ESCAMI) trial. J Am Coll Cardiol. 38:1644–1650. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Théroux P, Chaitman BR, Danchin N, Erhardt
L, Meinertz T, Schroeder JS, Tognoni G, White HD, Willerson JT and
Jessel A: Inhibition of the sodium-hydrogen exchanger with
cariporide to prevent myocardial infarction in high-risk ischemic
situations. Main results of the GUARDIAN trial. Guard during
ischemia against necrosis (GUARDIAN) Investigators. Circulation.
102:3032–3038. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Atwal KS, O'Neil SV, Ahmad S, Doweyko L,
Kirby M, Dorso CR, Chandrasena G, Chen BC, Zhao R and Zahler R:
Synthesis and biological activity of
5-aryl-4-(4-(5-methyl-1H-imidazol-4-yl)piperidin-1-yl)pyrimidine
analogs as potent, highly selective, and orally bioavailable NHE-1
inhibitors. Bioorg Med Chem Lett. 16:4796–4799. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Harguindey S, Arranz JL, Polo Orozco JD,
Rauch C, Fais S, Cardone RA and Reshkin SJ: Cariporide and other
new and powerful NHE1 inhibitors as potentially selective
anticancer drugs-an integral
molecular/biochemical/metabolic/clinical approach after one hundred
years of cancer research. J Transl Med. 11:2822013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Huber V, De Milito A, Harguindey S,
Reshkin SJ, Wahl ML, Rauch C, Chiesi A, Pouysségur J, Gatenby RA,
Rivoltini L and Fais S: Proton dynamics in cancer. J Transl Med.
8:572010. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Spugnini EP, Sonveaux P, Stock C,
Perez-Sayans M, De Milito A, Avnet S, Garcìa AG, Harguindey S and
Fais S: Proton channels and exchangers in cancer. Biochim Biophys
Acta. 1848:2715–2726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Chiche J, Ilc K, Laferrière J, Trottier E,
Dayan F, Mazure NM, Brahimi-Horn MC and Pouysségur J:
Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell
growth by counteracting acidosis through the regulation of the
intracellular pH. Cancer Res. 69:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Salmon H, Remark R, Gnjatic S and Merad M:
Host tissue determinants of tumour immunity. Nat Rev Cancer.
19:215–227. 2019.PubMed/NCBI
|