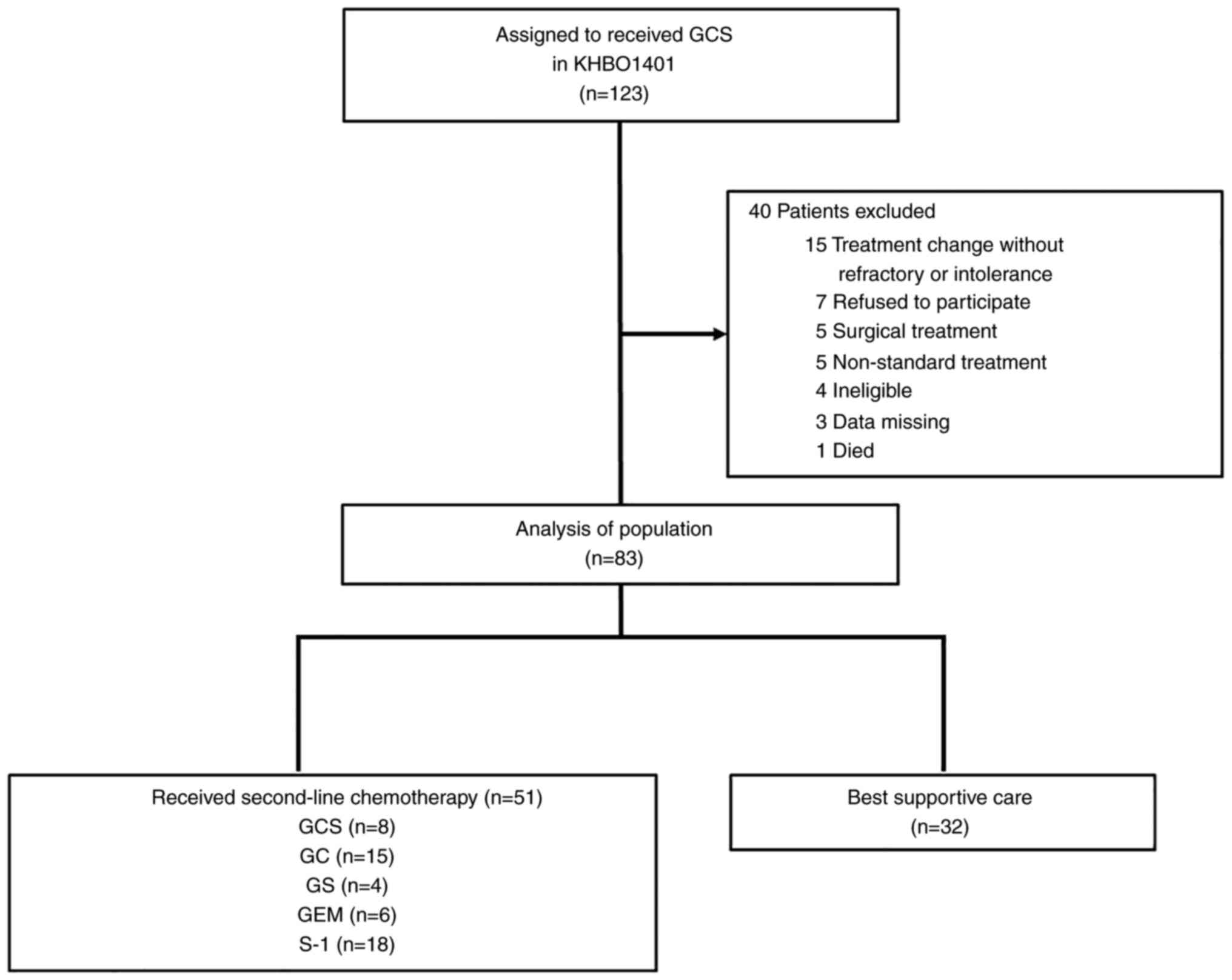
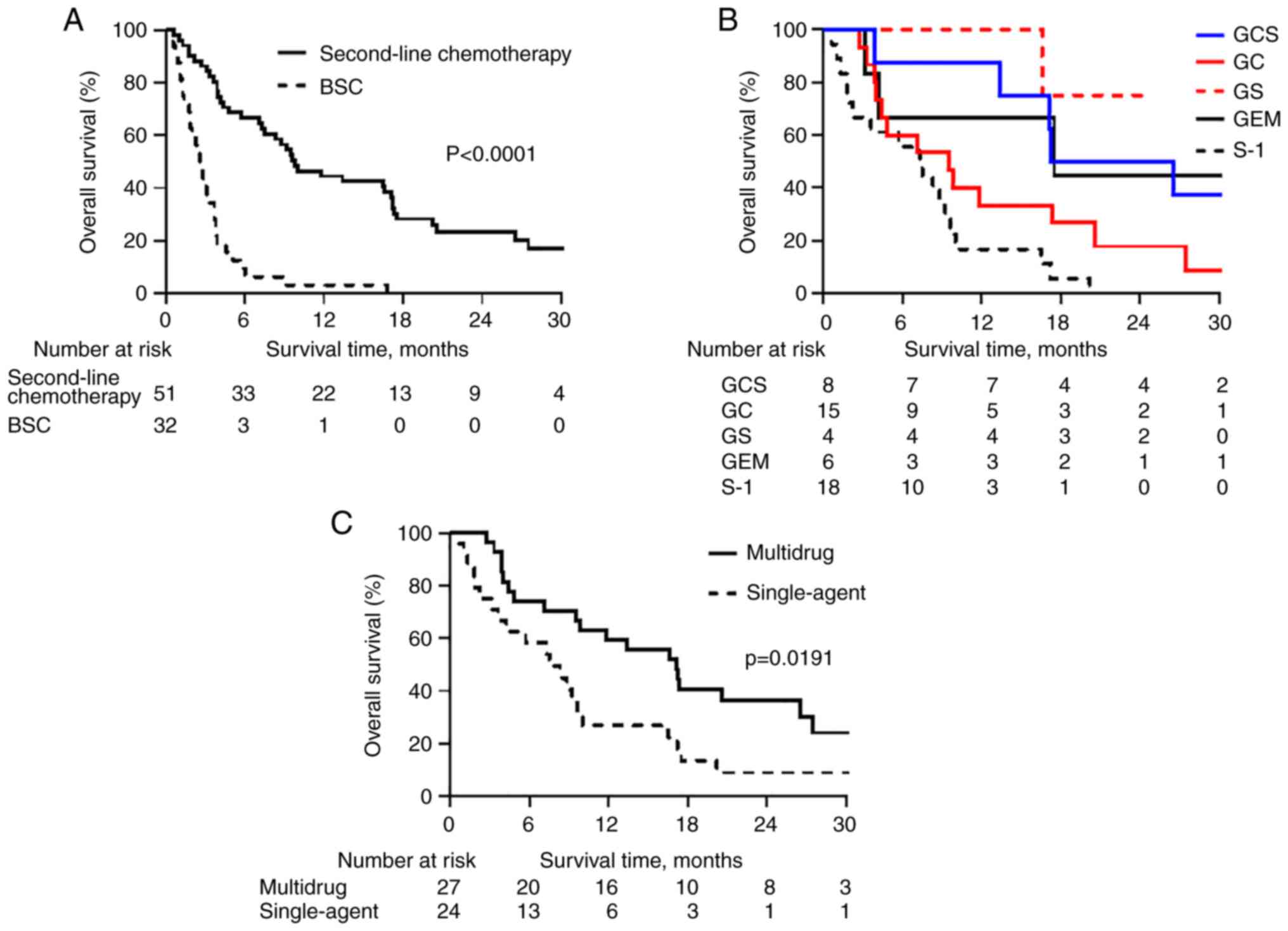
|
1
|
Matsukuma S, Tokumitsu Y, Shindo Y, Matsui
H and Nagano H: Essential updates to the surgical treatment of
biliary tract cancer. Ann Gastroenterol Surg. 3:378–389. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuyama R, Yabusita Y, Homma Y, Kumamoto
T and Endo I: Essential updates 2019/2020: Surgical treatment of
gallbladder cancer. Ann Gastroenterol Surg. 5:152–161. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sutherland M, Ahmed O, Zaidi A and Ahmed
S: Current progress in systemic therapy for biliary tract cancers.
J Hepatobiliary Pancreat Sci. 29:1094–1107. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morizane C, Okusaka T, Mizusawa J,
Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi
A, et al: Combination gemcitabine plus S-1 versus gemcitabine plus
cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT
(JCOG1113) randomized phase III clinical trial. Ann Oncol.
30:1950–1958. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ioka T, Kanai M, Kobayashi S, Sakai D,
Eguchi H, Baba H, Seo S, Taketomi A, Takayama T, Yamaue H, et al:
Randomized phase III study of gemcitabine, cisplatin plus S-1
versus gemcitabine, cisplatin for advanced biliary tract cancer
(KHBO1401-MITSUBA). J Hepatobiliary Pancreat Sci. Jul 28–2022.(Epub
ahead of print). PubMed/NCBI
|
|
7
|
Lamarca A, Palmer DH, Wasan HS, Ross PJ,
Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, et al:
Second-line FOLFOX chemotherapy versus active symptom control for
advanced biliary tract cancer (ABC-06): A phase 3, open-label,
randomised, controlled trial. Lancet Oncol. 22:690–701. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng Y, Tu X, Zhao P, Jiang W, Liu L,
Tong Z, Zhang H, Yan C, Fang W and Wang W: A randomised phase II
study of second-line XELIRI regimen versus irinotecan monotherapy
in advanced biliary tract cancer patients progressed on gemcitabine
and cisplatin. Br J Cancer. 119:291–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ying J and Chen J: Combination versus
mono-therapy as salvage treatment for advanced biliary tract
cancer: A comprehensive meta-analysis of published data. Crit Rev
Oncol Hematol. 139:134–142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demols A, Borbath I, Van den Eynde M,
Houbiers G, Peeters M, Marechal R, Delaunoit T, Goemine JC, Laurent
S, Holbrechts S, et al: Regorafenib after failure of gemcitabine
and platinum-based chemotherapy for locally advanced/metastatic
biliary tumors: REACHIN, a randomized, double-blind, phase II
trial. Ann Oncol. 31:1169–1177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bridgewater J, Palmer D, Cunningham D,
Iveson T, Gillmore R, Waters J, Harrison M, Wasan H, Corrie P and
Valle J: Outcome of second-line chemotherapy for biliary tract
cancer. Eur J Cancer. 49:15112013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walter T, Horgan AM, McNamara M, McKeever
L, Min T, Hedley D, Serra S, Krzyzanowska MK, Chen E, Mackay H, et
al: Feasibility and benefits of second-line chemotherapy in
advanced biliary tract cancer: A large retrospective study. Eur J
Cancer. 49:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi S, Ueno M, Ohkawa S, Andou T,
Kameda R, Yamamoto N and Morinaga S: A retrospective study of S-1
monotherapy as second-line treatment for patients with advanced
biliary tract cancer. Jpn J Clin Oncol. 42:800–806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki E, Ikeda M, Okusaka T, Nakamori S,
Ohkawa S, Nagakawa T, Boku N, Yanagimoto H, Sato T and Furuse J: A
multicenter phase II study of S-1 for gemcitabine-refractory
biliary tract cancer. Cancer Chemother Pharmacol. 71:1141–1146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inoue H, Todaka A, Yamazaki K, Fushiki K,
Shirasu H, Kawakami T, Tsushima T, Hamauchi S, Yokota T, Machida N,
et al: Efficacy and safety of S-1 following gemcitabine with
cisplatin for advanced biliary tract cancer. Invest New Drugs.
39:1399–1404. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abou-Alfa GK, Sahai V, Hollebecque A,
Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson
D, Murphy AG, et al: Pemigatinib for previously treated, locally
advanced or metastatic cholangiocarcinoma: A multicentre,
open-label, phase 2 study. Lancet Oncol. 21:671–684. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abou-Alfa GK, Macarulla T, Javle MM,
Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ,
Bridgewater J, et al: Ivosidenib in IDH1-mutant,
chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A
multicentre, randomised, double-blind, placebo-controlled, phase 3
study. Lancet Oncol. 21:796–807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wakai T, Nagahashi M, Shimada Y, Prasoon P
and Sakata J: Genetic analysis in the clinical management of
biliary tract cancer. Ann Gastroenterol Surg. 4:316–323. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
Study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ewalt MD, West H and Aisner DL: Next
generation sequencing-testing multiple genetic markers at once.
JAMA Oncol. 5:10762019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arrichiello G, Nacca V, Paragliola F and
Giunta EF: Liquid biopsy in biliary tract cancer from blood and
bile samples: Current knowledge and future perspectives. Explor
Target Antitumor Ther. 3:362–374. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Normanno N, Martinelli E, Melisi D, Pinto
C, Rimassa L, Santini D and Scarpa A: Role of molecular genetics in
the clinical management of cholangiocarcinoma. ESMO Open.
7:1005052022. View Article : Google Scholar : PubMed/NCBI
|