Introduction
Biliary tract cancer (BTC), including intrahepatic
and extrahepatic bile duct, gallbladder, and ampullary cancer, is
one of the most aggressive cancer types (1,2).
Surgical resection is currently the only curative treatment, but
the prognosis is usually poor due to difficulties in early
diagnosis and high recurrence rates following resection (3). Therefore, systemic chemotherapy is an
important part of the treatment regimen to improve the prognosis
for patients with advanced BTC.
A combination of gemcitabine (GEM) and cisplatin
(GC) therapy has been considered the first-line standard
chemotherapy regimen for advanced BTC, supported by results from
the previous ABC-02 trial (4).
Another Japanese phase III trial (FUGA-BT) reported that therapy
using GEM plus S-1, an oral fluoropyrimidine prodrug therapy, was
comparable to GC in terms of overall survival (OS). Thus, GEM plus
S-1 (GS) is also considered a standard chemotherapy regimen to
treat advanced BTC (5). As a new
addition, the Kansai Hepatobiliary Oncology Group (KHBO) recently
reported the results of a randomized phase III trial
(KHBO1401-MITSUBA; ClinicalTrials.gov identifier, NCT02182778) that
compared GEM, cisplatin and S-1 therapy (GCS) with GC in patients
with unresectable or recurrent BTC (6). In this trial, GCS demonstrated
superior efficacy compared with GC in terms of OS time (median OS,
13.5 months with GCS and 12.6 months with GC; hazard ratio, 0.79;
90% confidence interval, 0.628-0.996; P=0.046). Therefore, GCS may
soon be a new standard treatment for patients with advanced
BTC.
Despite the advances in chemotherapeutic regimens,
there are no standard second-line treatments for advanced BTC. A
recent phase III trial (ABC-06 study) showed improved patient
survival when patients were treated with folinic acid, fluorouracil
and oxaliplatin (FOLFOX) compared with active symptom control (ASC)
after progression on GC therapy, but the improvement in median OS
time with FOLFOX was modest (6.2 vs. 5.3 months, respectively)
(7). The only trials currently
reporting results are phase II trials with a limited number of
patients (8–10). However, second-line chemotherapies,
such as GC, GS, S-1 and GEM, are widely accepted in daily practice
in Japan. Second-line chemotherapy is decided based on the regimen
received as a first-line therapy. For patients treated with GC,
second-line treatment options include S-1 single-agent chemotherapy
or GS. However, it is difficult to determine the optimal regimens
for patients who receive GCS as a first-line therapy. Thus, the
present study aimed to evaluate the clinical outcomes of patients
receiving second-line chemotherapy after first-line GCS using data
from the KHBO1401 clinical trial.
Patients and methods
Study design and patients
A multicenter, retrospective study was conducted to
examine the clinical outcomes of patients receiving second-line
chemotherapy after first-line GCS treatment using the KHBO1401
study database (6). Between July 9,
2014, and February 4, 2016, 123 patients received GCS as a
first-line treatment in the KHBO1401 trial.
The former KHBO1401 trial was a randomized,
open-label, phase III clinical trial conducted to evaluate the
superiority of GCS compared with GC therapy in patients with
unresectable or recurrent BTC (6).
Written informed consent was obtained from all patients. The study
was initially approved by the Human Ethics Committee at Yamaguchi
University (approval no. H30-199), and it was subsequently approved
by the Institutional Review Boards of all participating centers
(Osaka International Cancer Institute, Osaka; Osaka University
Graduate School of Medicine, Osaka; Hokkaido University Graduate
School of Medicine, Hokkaido; Wakayama Medical University,
Wakayama; Kyoto University Hospital, Kyoto; Tohoku University
Hospital, Miyagi; Kumamoto University, Kumamoto; Nihon University
School of Medicine, Tokyo; Hyogo College of Medicine, Hyogo; Kindai
University Faculty of Medicine, Osaka; Kobe University Hospital and
Graduate School of Medicine, Hyogo; University of Tsukuba, Ibaraki;
Fukushima Medical University, Aizu Medical Center, Fukushima; Saga
University Hospital, Saga; Osaka City General Hospital, Osaka;
Yokohama City University, Graduate School of Medicine, Yokohama;
Toranomon Hospital, Tokyo; Osaka Rosai Hospital, Osaka; Kobe City
Medical Center General Hospital, Kobe; Asahikawa Medical
University, Hokkaido; Kansai Rosai Hospital, Hyogo; Tonan Hospital,
Hokkaido; National Hospital Organization Osaka National Hospital,
Osaka; Nagasaki University Hospital, Nagasaski; Toyonaka Municipal
Hospital, Osaka; Kitano Hospital Medical Research Institute, Osaka,
Japan). The main eligibility criteria were as follows: Unresectable
or recurrent biliary tract cancer, histologically confirmed
adenocarcinoma or adenosquamous carcinoma, no prior chemotherapy,
age ≥20 years, Eastern Cooperative Oncology Group (ECOG)
performance status (PS) of 0–2 (11) and adequate organ function. Exclusion
criteria included an age <20 years, prior chemotherapy or
radiotherapy (except for adjuvant chemotherapy completed at least 6
months before enrolment) and severe or uncontrolled systemic
disease. In the GC group, GEM and cisplatin were administered
intravenously at doses of 1,000 and 25 mg/m2,
respectively, on days 1 and day 8, which was repeated every 3
weeks. The total duration of the treatment period was 24 weeks. In
the GCS group, GEM and cisplatin were administered intravenously at
doses of 1,000 and 25 mg/m2, respectively, on day 1, and
oral S-1 was administered orally twice a day for 7 consecutive
days, repeated every 2 weeks. Doses of S-1 were calculated
according to body surface area (BSA) as follows: BSA <1.25
m2, 80 mg/day; 1.25 m2 ≤ BSA <1.5
m2, 100 mg/day; and BSA ≥1.5 m2, 120 mg/day.
The protocol was halted before the full 24-week term only if any of
the following occurred: Deterioration of general condition due to
disease progression, unacceptable or repeated treatment-related
toxicity, a >6-week delay in the schedule due to
treatment-related toxicity, patient refusal or tumor response
allowing potential curative resection.
For the present subgroup analysis, to investigate
the clinical outcomes of second-line therapy, patients who were
refractory or intolerant to GCS were selected, and the efficacy of
second-line regimens was compared with best supportive care (BSC).
BSC was defined as analgesics, antibiotics, biliary drainage,
transfusions and any other symptomatic treatment. OS time was
measured from the first day of second-line chemotherapy or from the
day of GCS treatment failure (BSC group) to the final follow-up
date or until death from any cause. Progression-free survival (PFS)
time was defined as the time from the first day of second-line
chemotherapy or from the day of first-line GCS treatment failure
(BSC group) to tumor progression or death from any cause.
Second-line regimen
The main regimens were as follows: i) GCS (1,000
mg/m2 GEM and 25 mg/m2 cisplatin on day 1,
and oral S-1 twice a day on days 1–7, every 2 weeks); ii) GC (1,000
mg/m2 GEM and 25 mg/m2 cisplatin on days 1
and 8, every 3 weeks); iii) GS (1,000 mg/m2 GEM on days
1 and 8, and oral S-1 twice a day on days 1–14, every 3 weeks); iv)
GEM (1,000 mg/m2 GEM on days 1 and 8, every 3 weeks);
and v) S-1 (oral S-1 twice a day on days 1–14, every 3 weeks). Dose
reduction and treatment schedule modification for each regimen were
considered in cases of adverse events.
Statistical analysis
Quantitative data are expressed as the median
(range), and qualitative data are expressed as number and
percentage. Categorical variables were compared using the
χ2 and Fisher's exact tests. Mann-Whitney U tests were
used to assess differences between the study groups. Survival
curves were analyzed using the Kaplan-Meier method and log-rank
test or the Gehan-Breslow test. The Benjamini and Hochberg method
was applied for multiple comparisons. Statistical analyses were
performed using GraphPad Prism V8.0 (GraphPad Software, Inc.) and R
version 4.2.1 (The R Foundation for Statistical Computing).
P<0.05 was considered to indicate a statistically significant
difference.
Results
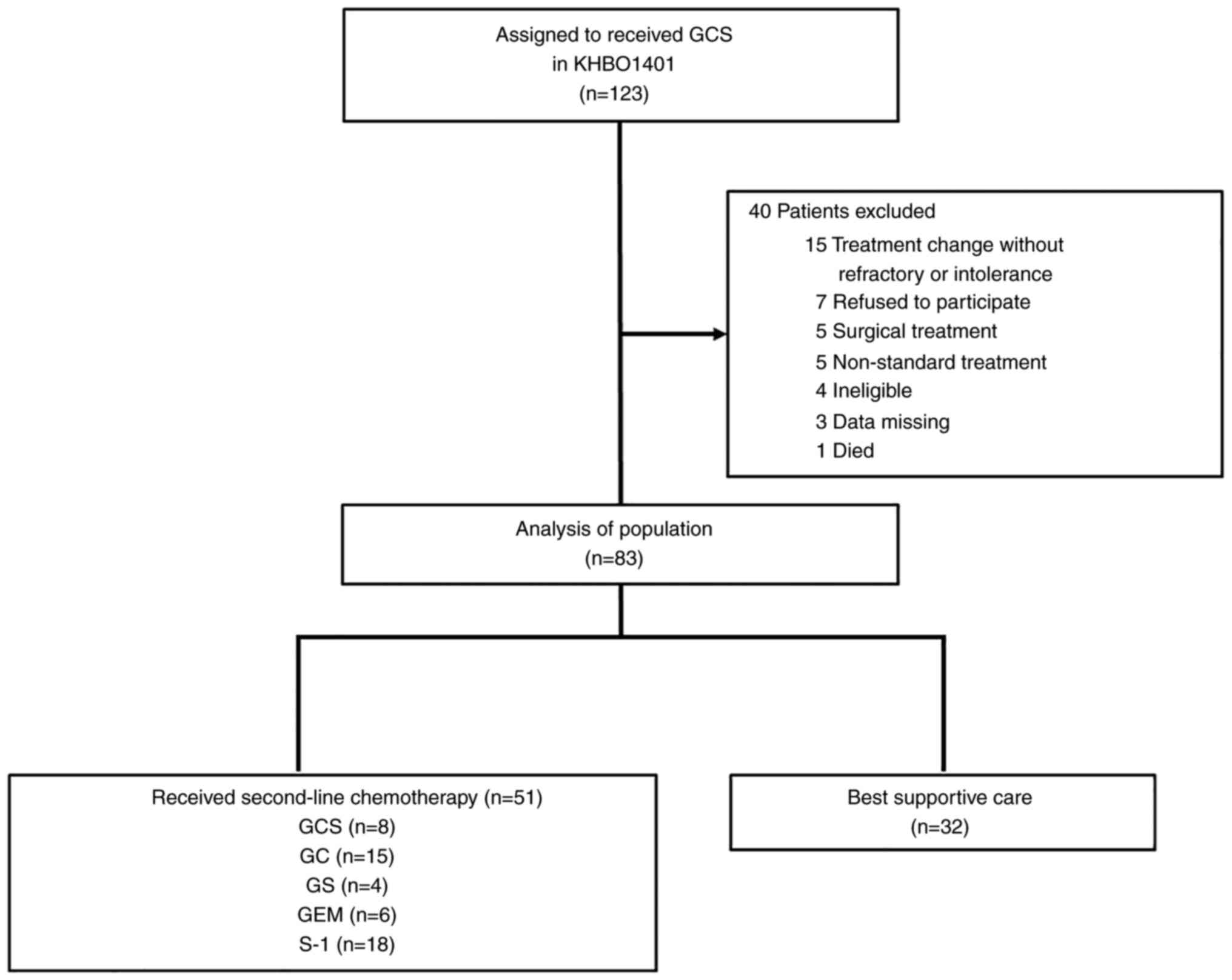
A total of 83 patients who were refractory or
intolerant to first-line GCS treatment, as identified in the trial,
were used in the present study. Of these 83 patients, 51 (61%)
received second-line chemotherapy (Fig.
1); 8 patients received GCS, 15 received GC, 4 received GS, 6
received GEM and 18 received S-1 therapy. The remaining 32 patients
received BSC. The baseline characteristics of the patients are
summarized in Table I. There were
no significant differences between the study groups in terms of
age, sex, primary tumor site, disease stage or GCS duration.
However, the number of patients refractory to first-line GCS
treatment was higher in the BSC group (P=0.0164). The baseline
characteristics of patients who received second-line chemotherapy
are summarized in Table II. The
majority of patients (90%) had an ECOG-PS score of 0 or 1.
 | Table I.Patient characteristics (n=83). |
Table I.
Patient characteristics (n=83).
| Factors | Second-line
chemotherapy (n=51) | Best supportive care
(n=32) | P-value |
|---|
| Median age (range),
years | 67 (39–81) | 69 (56–81) | 0.4572 |
| Sex, n (%) |
|
| >0.9999 |
| Male | 28 (55) | 18 (56) |
|
|
Female | 23 (45) | 14 (44) |
|
| Primary tumor site, n
(%) |
|
| 0.2166 |
| Gall
bladder | 13 (25) | 15 (47) |
|
|
Extrahepatic bile duct | 21 (41) | 10 (31) |
|
|
Intrahepatic bile duct | 16 (31) | 7 (22) |
|
|
Ampullary | 1 (2) | 0 (0) |
|
| Disease stage, n
(%) |
|
| 0.2778 |
|
Unresectable | 35 (69) | 24 (75) |
|
| Locally
advanced | 8 (16) | 2 (6) |
|
|
Metastatic | 27 (53) | 22 (69) |
|
|
Recurrent | 16 (31) | 8 (25) |
|
| Median GCS duration
(range), weeks | 22 (0–84) | 19 (4–79) | 0.4218 |
| GCS duration ≥24
weeks, n (%) | 25 (49) | 14 (44) | 0.6589 |
| Discontinuation of
GCS, n (%) |
|
| 0.0164 |
|
Refractory | 30 (59) | 27 (84) |
|
|
Intolerance | 21 (41) | 5 (16) |
|
 | Table II.Patient characteristics among
second-line regimens. |
Table II.
Patient characteristics among
second-line regimens.
| Factors | GCS (n=8) | GC (n=15) | GS (n=4) | GEM (n=6) | S-1 (n=18) |
|---|
| Median age (range),
years | 69 (60–78) | 67 (42–78) | 67 (64–69) | 67 (44–81) | 67 (39–78) |
| Sex, n (%) |
|
|
|
|
|
|
Male | 6 (75) | 7 (47) | 3 (75) | 1 (17) | 11 (61) |
|
Female | 2 (25) | 8 (53) | 1 (25) | 5 (83) | 7 (39) |
| ECOG performance
status, n (%) |
|
|
|
|
|
| 0 | 4 (50) | 5 (33) | 4 (100) | 2 (33) | 5 (28) |
| 1 | 4 (50) | 8 (53) | 0 (0) | 4 (67) | 10 (56) |
| 2 | 0 (0) | 2 (13) | 0 (0) | 0 (0) | 2 (11) |
|
Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Primary tumor site,
n (%) |
|
|
|
|
|
| Gall
bladder | 2 (25) | 2 (13) | 0 (0) | 2 (33) | 7 (39) |
|
Extrahepatic bile duct | 6 (75) | 7 (47) | 3 (75) | 1 (17) | 4 (22) |
|
Intrahepatic bile duct | 0 (0) | 6 (40) | 1 (25) | 2 (33) | 7 (39) |
|
Ampullary | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 0 (0) |
| Disease stage, n
(%) |
|
|
|
|
|
|
Unresectable | 7 (88) | 11 (73) | 1 (25) | 3 (50) | 13 (72) |
| Locally
advanced | 3 (38) | 2 (13) | 1 (25) | 0 (0) | 3 (17) |
|
Metastatic | 4 (50) | 9 (60) | 0 (0) | 3 (50) | 10 (56) |
|
Recurrent | 1 (13) | 4 (27) | 3 (75) | 3 (50) | 5 (28) |
| Median GCS duration
(range), weeks | 12 (0–26) | 20 (4–50) | 40 (24–63) | 26 (17–84) | 40 (2–55) |
| GCS duration ≥24
weeks, n (%) | 1 (13) | 6 (40) | 4 (100) | 4 (67) | 10 (56) |
| Discontinuation of
GCS, n (%) |
|
|
|
|
|
|
Refractory | 4 (50) | 7 (47) | 0 (0) | 4 (67) | 15 (83) |
|
Intolerance | 4 (50) | 8 (53) | 4 (100) | 2 (33) | 3 (17) |
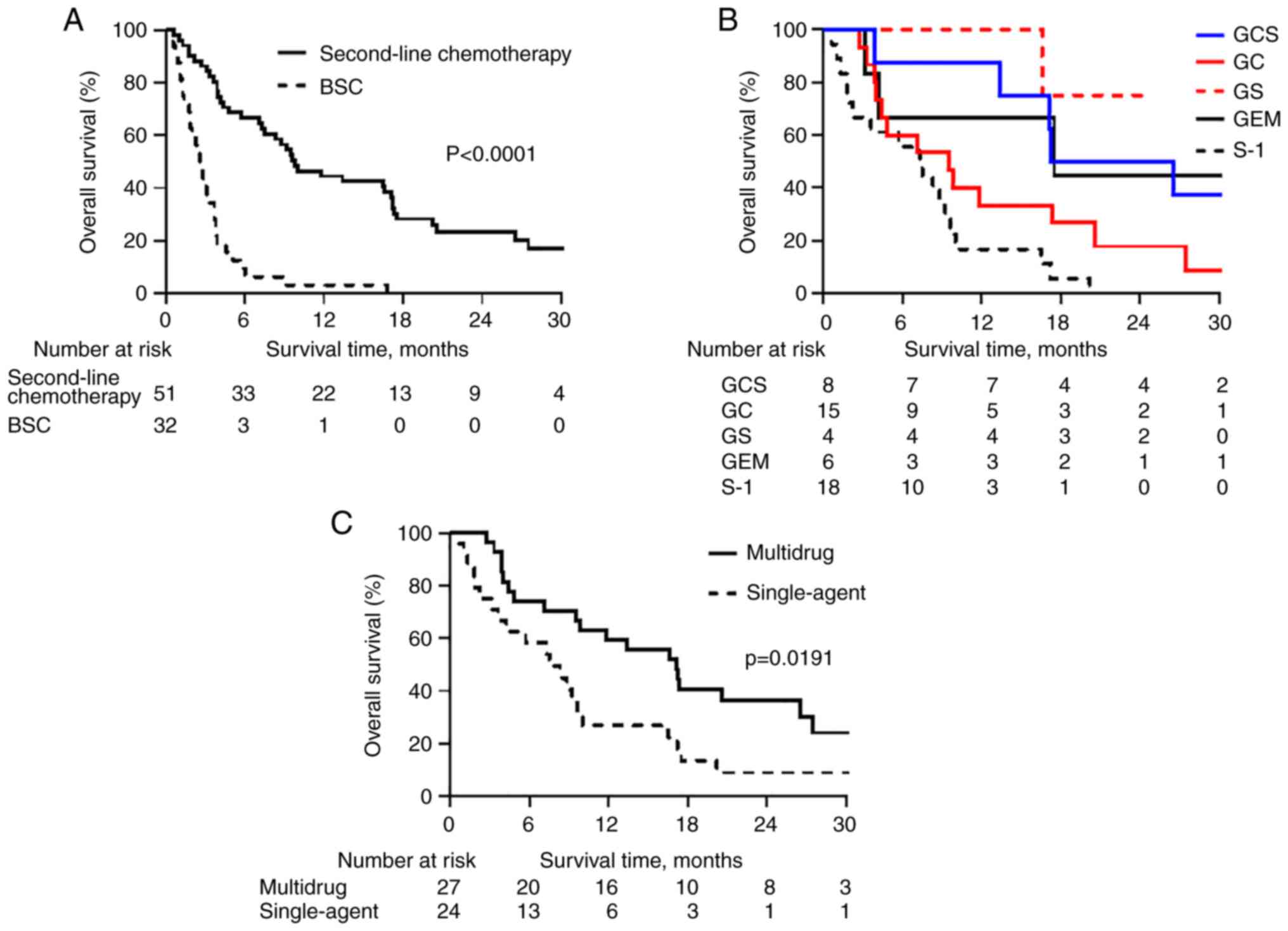
The 6- and 12-month OS rates were 66.7 and 44.4%,
respectively, following second-line chemotherapy, and 6.3 and 3.1%,
respectively, in the BSC group (P<0.0001) (Fig. 2A). The 6- and 12-month PFS rates
were 35.0 and 17.9%, respectively, following second-line
chemotherapy, and 6.3 and 3.1%, respectively, in the BSC group
(P=0.0363) (Fig. S1).
Next, the outcomes of second-line chemotherapy and
BSC in first-line GCS treatment refractory and intolerance groups,
respectively, were compared. There were no differences in patient
background in the refractory and intolerance groups (Tables SI and SII). In patients refractory to first-line
GCS treatment, the 6- and 12-month OS rates were 53.3 and 32.0%,
respectively, following second-line chemotherapy, and 3.7 and 0.0%,
respectively, in the BSC group (P<0.0001) (Fig. S2A). In patients with intolerance to
first-line GCS treatment, the 6- and 12-month OS rates were 85.7
and 61.9%, respectively, following second-line chemotherapy, and
20.0 and 20.0%, respectively, in the BSC group, respectively
(P=0.0011) (Fig. S2B).
Following second-line chemotherapy regimens, the 12-
and 24-month OS rates were 87.5 and 50.0%, respectively, in the GCS
group, 33.3 and 17.8%, respectively, in the GC group, 100.0 and
75.0%, respectively, in the GS group, 66.7 and 44.4%, respectively,
in the GEM group, and 16.7 and 0.0%, respectively, in the S-1 group
(Fig. 2B). The GCS and GS groups
had significantly improved OS rates compared with the S-1 group
(P=0.0069 and P=0.0069, respectively) (Fig. 2B). In addition, the 12- and 24-month
OS rates were 59.3 and 36.2%, respectively, for the 27 patients in
the multidrug chemotherapy group (combinations such as GCS, GC and
GS), and 26.9 and 9.0%, respectively, for the 24 patients within
the single-agent chemotherapy group (P=0.0191) (Fig. 2C).
Discussion
GC is the global standard for first-line
chemotherapy in patients with advanced BTC. In Japan, GS is also
considered an alternative first-line chemotherapy. Due to the
results of the recent KHBO1401 clinical study, a new standard
treatment using a triplet GCS regimen could represent a replacement
therapy. However, the prognosis for patients with advanced BTC
remains poor, with a median OS time of only 11–15 months (4–6).
Therefore, it is critically important to establish effective
second-line therapy options after first-line chemotherapy.
The present study is the first to assess the
efficacy of second-line chemotherapy in patients with advanced BTC
who received first-line GCS chemotherapy. The administration of
second-line chemotherapies after GCS therapy has been shown to
significantly improve OS compared with BSC. Previous studies have
also suggested that 15–25% of patients with BTC may be eligible for
second-line chemotherapy despite there being no established
second-line treatments (4,12,13).
In the present study, 51 out of 83 (61%) patients with BTC who were
refractory or intolerant to first-line GCS treatment received
second-line chemotherapy. Almost all patients (90%) with an ECOG PS
performance status of 0 or 1 were selected for second-line
chemotherapy. Therefore, second-line chemotherapy may be most
effective in patients with a good ECOG PS score.
Fluoropyrimidine-based regimens are the most common
second-line regimens after GEM-based first-line therapy. However,
few therapeutics have been considered as second-line therapies to
be combined with fluoropyrimidines, and thus, none are currently
recommended. Although the recent ABC-06 study demonstrated that
second-line treatment with FOLFOX after GC resulted in improved
patient survival compared with ASC for advanced BTC, it is unclear
whether a doublet regimen that included FOLFOX would be superior to
fluoropyrimidine alone (7).
In the present study, there was no significant
difference in survival between single and doublet drug regimens
(Fig. S3). However, significantly
improved OS was observed with doublet and triplet regimens compared
with single drug regimens, suggesting that multidrug chemotherapy
after GCS may be more effective than single-agent chemotherapy. A
total of 8 patients received a triplet GCS regimen as second-line
chemotherapy. Second-line chemotherapy regimens were based on
clinician discretion and patient preference. Therefore, it is
assumed that patients in good condition would have preferred
triplet GCS as second-line chemotherapy. Second-line chemotherapy
using the same regimen as the first-line chemotherapy might be
considered a re-challenge chemotherapy. Re-challenge chemotherapy
may be performed in clinical practice for patients with advanced
BTC, as second-line regimens are limited. However, there are no
studies about the efficacy of re-challenge chemotherapy for BTC.
Therefore, the efficacy and safety of GCS re-challenge chemotherapy
needs to be well evaluated in future studies.
S-1 is one of the most commonly used second-line
chemotherapeutics. Several studies showed that S-1 second-line
chemotherapy could be well tolerated by patients with advanced BTC
after receiving first-line GEM or GC, but its efficacy was modest
(14–16). Although S-1 seems to have some
degree of antitumor activity in patients with GEM-refractory
advanced BTC, S-1 single-agent chemotherapy may be insufficient to
improve the prognosis (15,16). In the present study, the GS regimen
had a high OS rate compared with other regimens; all patients who
received GS had a PS of 0 and were intolerant to GCS. In addition,
the total duration of the GCS treatment period was >24 weeks.
Therefore, selection bias might affect OS. Furthermore, it may be
difficult to compare the efficacy of each regimen accurately due to
the small sample size. Expanded prospective studies are required to
further explore the efficacy of these regimens.
Recently, the development of genomic sequencing in
BTC has rapidly progressed, and favorable treatment effects of
molecular targeting agents, such as isocitrate dehydrogenase-1
inhibitors and fibroblast growth factor receptor inhibitors, in
BTC, have been reported (17–19).
Furthermore, the development of immune checkpoint inhibitors is
promising, and for tumors with high microsatellite instability,
pembrolizumab can be administered for multiple cancer types in
various organs, including BTC (20). With these new developments, progress
continues to be made toward effective systemic therapies for
advanced BTC. However, there remain some issues to be resolved,
such as the amount of time it takes for the results of genomic
testing to be obtained, the lack of methods to obtain a sufficient
sample volume in unresectable cases, the lack of effective
therapeutic agents for genetic alterations and the cost of genomic
testing (19,21–23).
The present exploratory study had several
limitations. First, it included only Asian patients and
non-randomized second-line chemotherapy regimens. Second, the
number of patients treated with each regimen was small, so there
was a potential selection bias for patients who received
second-line chemotherapy. Finally, there were insufficient data to
assess dose reduction, treatment interruption and toxicity profiles
of the second-line chemotherapy regimens.
In conclusion, second-line chemotherapies could
provide a new treatment option for patients with advanced BTC who
become refractory or intolerant to first-line GCS therapy. In the
present study, multidrug chemotherapy regimens tended to be more
effective than single-agent regimens at improving patient survival.
Prospective studies are needed to further explore the efficacy of
second-line chemotherapy regimens in BTC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors are also grateful to Ms. Masami Kashibo
(Osaka International Cancer Institute, Osaka, Japan) and Ms. Hiromi
Oura (Osaka International Cancer Institute), members of the KHBO
data center, for their support in data management. This manuscript,
including the concept, analyses, statistics, interpretation and
conclusion, was reviewed by other members of the KHBO group, in
addition to the authors: Dr Takamichi Ishii at the Division of
Hepato-Biliary-Pancreatic Surgery and Transplantation, Department
of Surgery, Graduate School of Medicine, Kyoto University, Kyoto,
Japan; Dr Atsushi Miyamoto at the Department of Surgery, Sakai City
Medical Center, Osaka, Japan; Dr Taishi Ota at the Department of
Gastroenterology and Hepatology, Kansai Rosai Hospital, Hyogo,
Japan; Dr Yasuyuki Kawamoto at the Department of Gastroenterology
and Hepatology, Hokkaido University Graduate School of Medicine,
Hokkaido, Japan; and Dr Toru Otsuru at the Department of National
Hospital Organization Shikoku Cancer Center, Ehime, Japan.
Funding
The present study was funded by Taiho Pharmaceutical Co., Ltd.
(Tokyo, Japan).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YS, HN and TI were responsible for the conception
and design of the study. All the authors were involved in the
study. All authors had full access to all the data reported in the
study and had final responsibility for the decision to submit for
publication. MK, SK, HW, DS, HE, HB, HK, TT, MU, MT, YN and EH
collected and interpreted the data. YS, YN, KY and HN had full
access to the raw data, analyzed the data and wrote the manuscript.
TI and HN confirm the authenticity of all the raw data. All authors
have read and approved the manuscript.
Ethics approval and consent to
participate
The study was initially approved by the Human Ethics
Committee at Yamaguchi University (approval no. H30-199), and it
was subsequently approved by the Institutional Review Boards of all
participating centers(Osaka International Cancer Institute, Osaka;
Osaka University Graduate School of Medicine, Osaka; Hokkaido
University Graduate School of Medicine, Hokkaido; Wakayama Medical
University, Wakayama; Kyoto University Hospital, Kyoto; Tohoku
University Hospital, Miyagi; Kumamoto University, Kumamoto; Nihon
University School of Medicine, Tokyo; Hyogo College of Medicine,
Hyogo; Kindai University Faculty of Medicine, Osaka; Kobe
University Hospital and Graduate School of Medicine, Hyogo;
University of Tsukuba, Tsukuba; Fukushima Medical University, Aizu
Medical Center, Fukushima; Saga University Hospital, Saga; Osaka
City General Hospital, Osaka; Yokohama City University, Graduate
School of Medicine, Yokohama; Toranomon Hospital, Tokyo; Osaka
Rosai Hospital, Osaka; Kobe City Medical Center General Hospital,
Kobe; Asahikawa Medical University, Hokkaido; Kansai Rosai
Hospital, Hyogo; Tonan Hospital, Hokkaido; National Hospital
Organization Osaka National Hospital, Osaka; Nagasaki University
Hospital, Nagasaski; Toyonaka Municipal Hospital, Toyonaka; Kitano
Hospital Medical Research Institute, Osaka). All participants
provided written informed consent for participation in the study,
which was conducted in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsukuma S, Tokumitsu Y, Shindo Y, Matsui
H and Nagano H: Essential updates to the surgical treatment of
biliary tract cancer. Ann Gastroenterol Surg. 3:378–389. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuyama R, Yabusita Y, Homma Y, Kumamoto
T and Endo I: Essential updates 2019/2020: Surgical treatment of
gallbladder cancer. Ann Gastroenterol Surg. 5:152–161. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sutherland M, Ahmed O, Zaidi A and Ahmed
S: Current progress in systemic therapy for biliary tract cancers.
J Hepatobiliary Pancreat Sci. 29:1094–1107. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morizane C, Okusaka T, Mizusawa J,
Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi
A, et al: Combination gemcitabine plus S-1 versus gemcitabine plus
cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT
(JCOG1113) randomized phase III clinical trial. Ann Oncol.
30:1950–1958. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ioka T, Kanai M, Kobayashi S, Sakai D,
Eguchi H, Baba H, Seo S, Taketomi A, Takayama T, Yamaue H, et al:
Randomized phase III study of gemcitabine, cisplatin plus S-1
versus gemcitabine, cisplatin for advanced biliary tract cancer
(KHBO1401-MITSUBA). J Hepatobiliary Pancreat Sci. Jul 28–2022.(Epub
ahead of print). PubMed/NCBI
|
|
7
|
Lamarca A, Palmer DH, Wasan HS, Ross PJ,
Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, et al:
Second-line FOLFOX chemotherapy versus active symptom control for
advanced biliary tract cancer (ABC-06): A phase 3, open-label,
randomised, controlled trial. Lancet Oncol. 22:690–701. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng Y, Tu X, Zhao P, Jiang W, Liu L,
Tong Z, Zhang H, Yan C, Fang W and Wang W: A randomised phase II
study of second-line XELIRI regimen versus irinotecan monotherapy
in advanced biliary tract cancer patients progressed on gemcitabine
and cisplatin. Br J Cancer. 119:291–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ying J and Chen J: Combination versus
mono-therapy as salvage treatment for advanced biliary tract
cancer: A comprehensive meta-analysis of published data. Crit Rev
Oncol Hematol. 139:134–142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demols A, Borbath I, Van den Eynde M,
Houbiers G, Peeters M, Marechal R, Delaunoit T, Goemine JC, Laurent
S, Holbrechts S, et al: Regorafenib after failure of gemcitabine
and platinum-based chemotherapy for locally advanced/metastatic
biliary tumors: REACHIN, a randomized, double-blind, phase II
trial. Ann Oncol. 31:1169–1177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bridgewater J, Palmer D, Cunningham D,
Iveson T, Gillmore R, Waters J, Harrison M, Wasan H, Corrie P and
Valle J: Outcome of second-line chemotherapy for biliary tract
cancer. Eur J Cancer. 49:15112013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walter T, Horgan AM, McNamara M, McKeever
L, Min T, Hedley D, Serra S, Krzyzanowska MK, Chen E, Mackay H, et
al: Feasibility and benefits of second-line chemotherapy in
advanced biliary tract cancer: A large retrospective study. Eur J
Cancer. 49:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi S, Ueno M, Ohkawa S, Andou T,
Kameda R, Yamamoto N and Morinaga S: A retrospective study of S-1
monotherapy as second-line treatment for patients with advanced
biliary tract cancer. Jpn J Clin Oncol. 42:800–806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki E, Ikeda M, Okusaka T, Nakamori S,
Ohkawa S, Nagakawa T, Boku N, Yanagimoto H, Sato T and Furuse J: A
multicenter phase II study of S-1 for gemcitabine-refractory
biliary tract cancer. Cancer Chemother Pharmacol. 71:1141–1146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inoue H, Todaka A, Yamazaki K, Fushiki K,
Shirasu H, Kawakami T, Tsushima T, Hamauchi S, Yokota T, Machida N,
et al: Efficacy and safety of S-1 following gemcitabine with
cisplatin for advanced biliary tract cancer. Invest New Drugs.
39:1399–1404. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abou-Alfa GK, Sahai V, Hollebecque A,
Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson
D, Murphy AG, et al: Pemigatinib for previously treated, locally
advanced or metastatic cholangiocarcinoma: A multicentre,
open-label, phase 2 study. Lancet Oncol. 21:671–684. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abou-Alfa GK, Macarulla T, Javle MM,
Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ,
Bridgewater J, et al: Ivosidenib in IDH1-mutant,
chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A
multicentre, randomised, double-blind, placebo-controlled, phase 3
study. Lancet Oncol. 21:796–807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wakai T, Nagahashi M, Shimada Y, Prasoon P
and Sakata J: Genetic analysis in the clinical management of
biliary tract cancer. Ann Gastroenterol Surg. 4:316–323. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
Study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ewalt MD, West H and Aisner DL: Next
generation sequencing-testing multiple genetic markers at once.
JAMA Oncol. 5:10762019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arrichiello G, Nacca V, Paragliola F and
Giunta EF: Liquid biopsy in biliary tract cancer from blood and
bile samples: Current knowledge and future perspectives. Explor
Target Antitumor Ther. 3:362–374. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Normanno N, Martinelli E, Melisi D, Pinto
C, Rimassa L, Santini D and Scarpa A: Role of molecular genetics in
the clinical management of cholangiocarcinoma. ESMO Open.
7:1005052022. View Article : Google Scholar : PubMed/NCBI
|