Introduction
As one of the most common types of cancer, lung
cancer was the leading cause of cancer-related deaths worldwide
according to the World Health Organization in 2020 (1). Non-small cell lung cancer (NSCLC)
accounts for 85% of lung cancer-associated deaths (2). Available therapies for NSCLC treatment
include surgery, chemotherapy, radiation and targeted therapy. In
order to enhance anticancer effects, there is urgent need for
combination treatments or new agents targeting one or more
molecular pathways.
Targeted treatment, intended to inhibit cancer
malignancies with a focus on critical molecules, has been regarded
as a promising therapy to treat patients with NSCLC (3). Among various targeted therapies,
anti-EGFR therapy has been the one with the most extensive
application in the clinical treatment of NSCLC (4). EGFR is a receptor tyrosine kinase of
the ErbB family which serves a key role in cell proliferation,
survival, differentiation and migration (5). When activated by ligand binding, EGFR
will autophosphorylate and trigger downstream signaling cascades,
including MAPK and PI3K-Akt pathways (6), leading to cell cycle progression and
inhibiting the activation of proapoptotic proteins. Therefore,
several EGFR-inhibiting agents, such as monoclonal antibodies and
tyrosine kinase inhibitors (TKIs) have been developed for
anticancer treatments (7).
Erlotinib (Erl), a first generation EGFR-TKI, has exhibited an
improved survival rate in patients with NSCLC, prolonging the
survival of patients with advanced NSCLC after chemotherapy
(8). However, certain tumors will
develop acquired resistance to Erl over time (9), and prolonged administration of
high-dose Erl can lead to adverse effects, including rashes and
diarrhea (10). Additionally,
certain patients demonstrate intrinsic resistance to Erl, which
further constrains its therapeutic effectiveness (11–13).
Therefore, it is worthwhile to investigate the effect of
combination treatment in potentiating the efficacy of Erl at a low
dose, in place of chemotherapy drugs, such as cisplatin and
pemetrexed, which have been applied as sensitizers to enhance the
efficacy of Erl (14,15), with side effects such as
hypertension and severe diarrhea (16).
On the other hand, hyperthermia has emerged as a
significant therapeutic modality for cancer, recognized not only
for its capability to induce direct cytotoxic effects on cancer
cells, but also for its role in increasing tumor sensitivity when
used in combination with other treatment modalities (17). Additionally, several studies have
reported that hyperthermia may exert a synergistic effect when
paired with chemotherapy or radiotherapy in cancer treatment
(18–20). However, conventional hyperthermia
techniques may result in adverse effects such as pain and
discomfort, as well as non-specific thermal damage to adjacent
healthy tissues, potentially leading to severe complications, such
as pain, unpleasant sensations and burns (18,21).
Therefore, modifying conventional hyperthermia and integrating it
with pharmacological agents or therapies to enhance both anticancer
efficacy and safety represents a promising strategy.
In the present study, a novel thermal therapy,
thermal cycling hyperthermia (TC-HT), was proposed as a substitute
for chemotherapy drugs or agents, to investigate whether TC-HT may
be an effective sensitizer to amplify the anticancer effect of Erl.
Given the safety and effectiveness of TC-HT reported in our
previous studies (22,23), the aim of the present study was to
investigate the role of TC-HT in effectively sensitizing A549 cells
to Erl, thereby improving therapeutic outcomes.
Materials and methods
Cell culture
The human NSCLC cell lines A549 (cat. no. 60074) and
H460 (cat. no. 60373) and the normal lung cell line IMR-90 (cat no.
60204) were purchased from the Bioresource Collection and Research
Center of the Food Industry Research and Development Institute.
Both A549 and IMR-90 cell lines were cultured in DMEM (HyClone;
Cytiva), while the H460 cell line was cultured in RPMI-1640 medium
(Corning, Inc.). All media were supplemented with 10% fetal bovine
serum (HyClone; Cytiva) and 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.), and all cells were maintained in a
humidified incubator with 5% CO2 at 37°C.
Drug treatment and TC-HT exposure
Erl, purchased from MedChemExpress, was dissolved in
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) to a concentration
of 5 mM as the stock solution and stored at −20°C. A549 cells were
seeded in 96-well plates at 3×103 cells/well and
incubated overnight at 37°C before being treated with 0.5, 2.0, 8.0
or 10.0 µM of Erl, TC-HT or a combination treatment. For the
combination treatment, TC-HT was applied for 1 h before Erl
treatment. After single or combination treatment, the cells were
maintained at 37°C in the cell culture humidified incubator for an
additional 24, 48 and 72 h for further analyses. The TC-HT
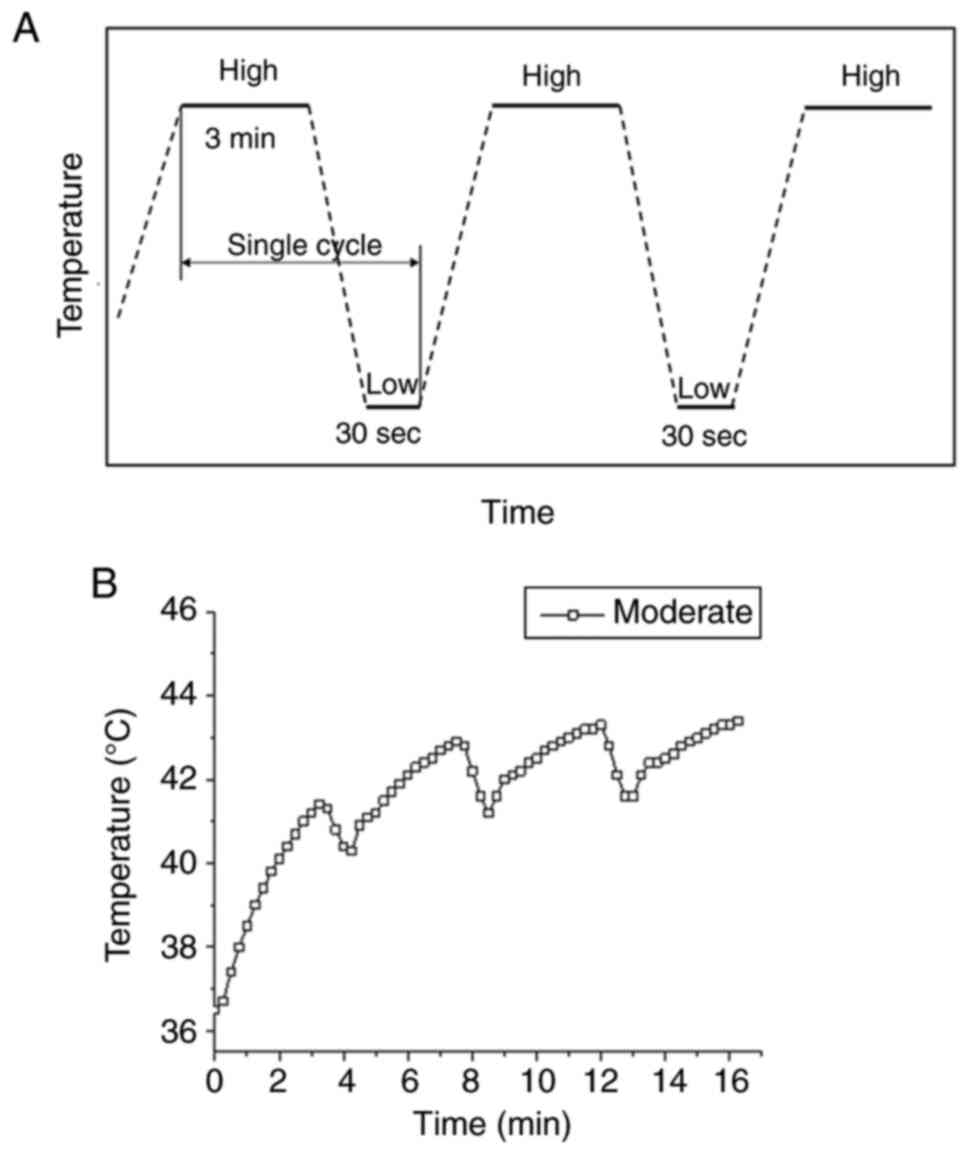
parameters employed in this study were based on our previous study
involving different cell types (22–25),
consisting of a high temperature period for 3 min and a cooling
period of 30 sec, and this protocol was repeated continuously for
10 cycles (Fig. 1A). A modified PCR
system, which featured waterproof capabilities in the heating area,
was utilized to conduct TC-HT (Fig.
S1A). A minimal volume of water served as a thermal conduction
medium, with cell culture dishes placed directly on the heating
area, while the modified PCR apparatus regulated the heating and
cooling processes. The actual temperatures sensed by the cells at
the bottom of the well were measured by a needle thermocouple
(Fig. S1B).
Cell viability assay and cell
morphology
The viability of A549 cells was assessed by MTT
assay (Sigma-Aldrich; Merck KGaA). After drug or TC-HT treatment,
the medium was replaced by DMEM containing 0.5 mg/ml MTT and
incubated for 4 h at 37°C. The formazan crystals were dissolved
using 10% SDS (Bioman Scientific Co., Ltd.) at room temperature
overnight. Thereafter, the optical density of formazan solution was
measured at 570 nm using the Multiskan GO spectrophotometer (Thermo
Fisher Scientific, Inc.). Background absorbance at 690 nm was
subtracted, and the final absorbance value was expressed as a
percentage of the untreated controls to represent the cell
viability. The cell morphology of A549 cells under different
treatments was observed and imaged using the Zyla 5.5 sCMOS camera
(Andor Technology Ltd.).
Synergy quotient (SQ) calculation for
synergism
The SQ was calculated by subtracting the baseline
values from all treatment groups and dividing the resulting net
effect of the combination by the total of the individual effects as
follows: (Erl + TC-HT)/(Erl) + (TC-HT).
Western blotting
After treatment with Erl and/or TC-HT, cells were
washed with ice-cold PBS and lysed in RIPA lysis buffer
(MilliporeSigma) on ice for 30 min. Cell lysates were clarified by
centrifugation at 23,000 × g for 30 min at 4°C and the protein
concentration in the supernatant fraction was measured using the
Bradford protein assay (cat. no. BRA222; BioShop Canada Inc.).
Proteins (20–40 µg) were subjected to 10% SDS-PAGE and
electrotransferred to polyvinylidene fluoride membranes
(MilliporeSigma). After incubation in a blocking buffer containing
5% bovine serum albumin (BioShop Canada Inc.) in Tris-buffered
saline with 0.1% Tween-20 (TBST) for 1 h at room temperature, the
membranes were immunoblotted with diluted primary antibodies at 4°C
overnight. The specific primary antibodies against phosphorylated
EGFR (p-EGFR; cat. no. 4407; Cell Signaling Technology, Inc.), poly
ADP-ribose polymerase (PARP; cat. no. 9542; Cell Signaling
Technology, Inc.), p-JNK (cat. no. 4668; Cell Signaling Technology,
Inc.), p-Akt (cat. no. 4060; Cell Signaling Technology, Inc.),
total Akt (t-Akt; cat. no. 9272; Cell Signaling Technology, Inc.),
MutT homolog 1 (MTH1; cat. no. 43918; Cell Signaling Technology,
Inc.), p-p38 (cat. no. GTX133460; GeneTex, Inc.), Cdc2 (cat. no.
GTX108120; GeneTex, Inc.) and GAPDH (cat. no. GTX100118; GeneTex,
Inc.) were used. After being washed with TBST, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. no. 111-035-003; Jackson ImmunoResearch
Laboratories, Inc.). All the primary antibodies were diluted at a
1:1,000 concentration and the secondary antibodies were diluted at
a 1:10,000 concentration, according to the manufacturer's
instructions. The membranes were visualized with an enhanced
chemiluminescence substrate (Advansta Inc.) and quantified using an
Amersham Imager 600 imaging system (AI600; GE Healthcare).
Mitochondrial membrane potential (MMP)
measurement
After treatment with Erl and/or TC-HT, cells were
washed and resuspended with PBS followed by staining with 20 nM
3,3′-dihexyloxacarbocyanine iodide (DiOC6(3); Enzo Life Sciences, Inc.) for 30 min at
37°C in the dark. The fraction of mitochondrial depolarization in
cells was indicated by a decrease in fluorescence intensity
measured using a flow cytometer (FACSVerse; BD Biosciences) and
data were analyzed using FlowJo (version 7.6.1; FlowJo LLC).
Wound healing assay
A549 cells were seeded and cultured as a confluent
monolayer in 35 mm Petri dishes. Wounds were made by scratching
straight lines across the cell monolayer using a 10 µl pipette tip.
Non-adherent cells and debris were removed by gently rinsing the
cells with PBS (HyClone; Cytiva). After PBS was discarded and
replaced with fresh medium, the cells were treated with either Erl
and/or TC-HT. Each wound was observed and imaged using the Zyla 5.5
sCMOS camera (Andor Technology Ltd.) at 0 h and 24 h post
treatment. The distances between wound edges were measured and
analyzed using ImageJ (version 1.51j8; National Institutes of
Health).
Colony formation assay
A549 cells were seeded at a density of 300
cells/dish in 35 mm Petri dishes overnight for cell adherence.
Subsequently, cells were treated with Erl and/or TC-HT. The cell
medium was replaced 24 h after treatment, and the cells were
cultured for additional 10 days, with fresh medium replaced every 3
days during the culture period. The cells were fixed with 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature and stained with 0.1% crystal violet (Sigma-Aldrich;
Merck KGaA) at room temperature for 15 min for visualization and
cell counting. A colony was defined as a cluster consisting of ≥50
cells. The colonies were imaged using a light microscope and
manually counted, and the number of colonies in each group was
normalized to the control group.
Cell cycle analysis
After 24 h of treatment with 10 µM Erl and/or TC-HT,
the cells were harvested, rinsed with PBS and fixed with 70%
ethanol at 4°C for 30 min before staining. The cells were stained
for 30 min in the dark at room temperature with propidium iodide
and ribonuclease A (Gibco; Thermo Fisher Scientific, Inc.).
Subsequently, the stained cells were subject to the cell cycle
analysis using a flow cytometer (FACSVerse; BD Biosciences), and
data were analyzed using FlowJo (version 7.6.1; FlowJo LLC).
Statistical analysis
Results were expressed as the mean ± standard
deviation (n=3). Statistical analyses were performed using
OriginPro 2015 (version 92E; OriginLab Corporation). Statistical
significance was determined using one-way ANOVA followed by Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TC-HT for in vitro application
The thermal cycling treatment was applied with a
modified PCR machine as previously described (22,23).
The schematic temperature and duration settings are shown in
Fig. 1A, where the temperature was
increased to the desired high temperature setting and maintained
for 3 min, followed by a natural cooling period for 30 sec. In
practice, the heating device was switched off in the cooling
process, and the low temperature setting was chosen to be 37°C to
mimic human body temperature. A single cycle containing a high
temperature and a low temperature period was repeated 10 times.
Fig. 1B shows the actual
temperature in the culture well measured by a thermocouple at
moderate temperature TC-HT setting. The cycling temperature came to
an equilibrium state after the third heating period, thereafter the
temperature cycling between 41.5–43.0°C for moderate temperature
TC-HT (Fig. 1B). In the current
study, the moderate temperature TC-HT treatment (41.5–43.0°C) was
adopted in the subsequent experiments to study the synergistic
anticancer effect of Erl and TC-HT in A549 cancer cells.
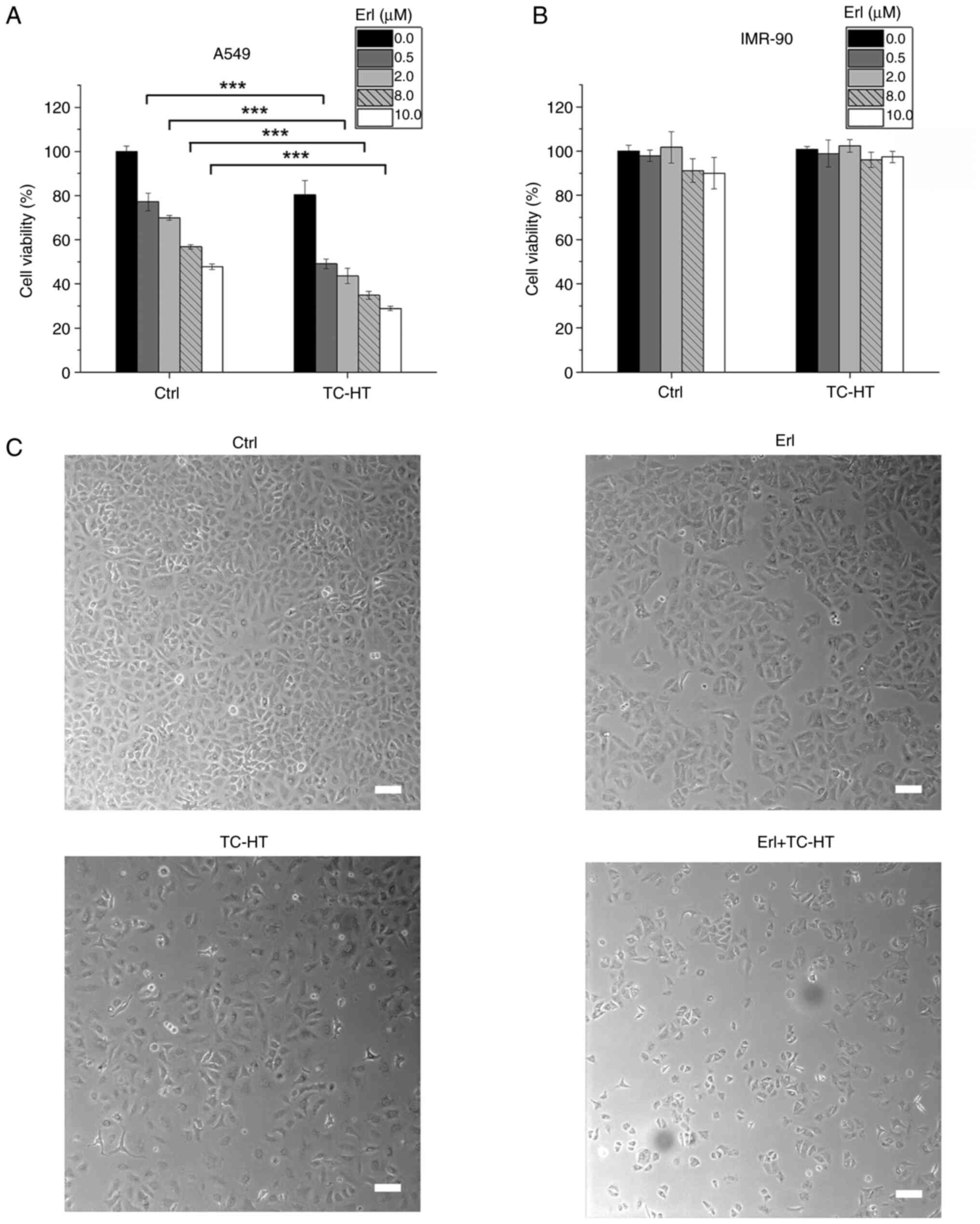
TC-HT potentiates the anticancer
efficacy of Erl in A549 NSCLC cells
To determine the effect of the combination of Erl
and TC-HT treatment on human NSCLC cell viability, A549 cells were
treated with different concentrations of Erl with or without TC-HT.
At 72 h post treatment, cell viability was assessed using an MTT
assay. Erl exhibited a significant antineoplastic effect in a
concentration-dependent manner (Fig.
2A). The IC50 of Erl was ~10 µM in A549 cells after
72 h treatment. Compared with treatment with Erl alone, TC-HT
therapy sensitized A549 cells to Erl and reduced the cell viability
to ~30% of the control group (Fig.
2A). It is noteworthy that the combination of Erl and TC-HT
demonstrated synergistic ability compared with Erl monotherapy,
which reduced the IC50 to 0.5 µM. To determine the
synergistic effect of TC-HT and Erl, SQ calculations were
conducted, where an SQ value >1.0 indicated a synergistic effect
(25–27). The SQ calculations of cell viability
indicated a synergistic effect when TC-HT was combined with Erl at
concentrations of 0.5–8.0 µM (Table
SI). The highest SQ value was observed with the combination
treatment using 0.5 µM Erl, suggesting that the synergistic effects
at lower concentrations of Erl were more pronounced compared with
those at higher concentrations. To enhance understanding of the
temporal effects on cell viability, the viability of A549 cells was
evaluated at 24 and 48 h (Fig.
S2A). Additionally, the investigation was expanded to include
the H460, another NSCLC cell line, assessing the impact of the
combination of Erl and TC-HT on NSCLC (Fig. S2B). These results demonstrated that
the combination of Erl and TC-HT led to a significant time- and
dose-dependent decrease in cell viability in both A549 and H460
cell lines. To verify the effect of this combined treatment in
normal cells, the normal lung cell line IMR-90 was used and the
same treatment conditions were applied. The cell viability of
IMR-90 normal lung cells decreased to ~90% when treated with 8 or
10 µM Erl, while it remained unaffected by doses <2 µM Erl
and/or TC-HT (Fig. 2B).
Additionally, Erl and TC-HT combination treatment resulted in
notable morphological changes in A549 cells, including shrinkage
and fragmentation of cells as well as a decreased number of cells
(Fig. 2C). These result suggested
that TC-HT may serve as a sensitizer with minimal observed adverse
effects to increase the anticancer effect of targeted therapy drugs
(22,23).
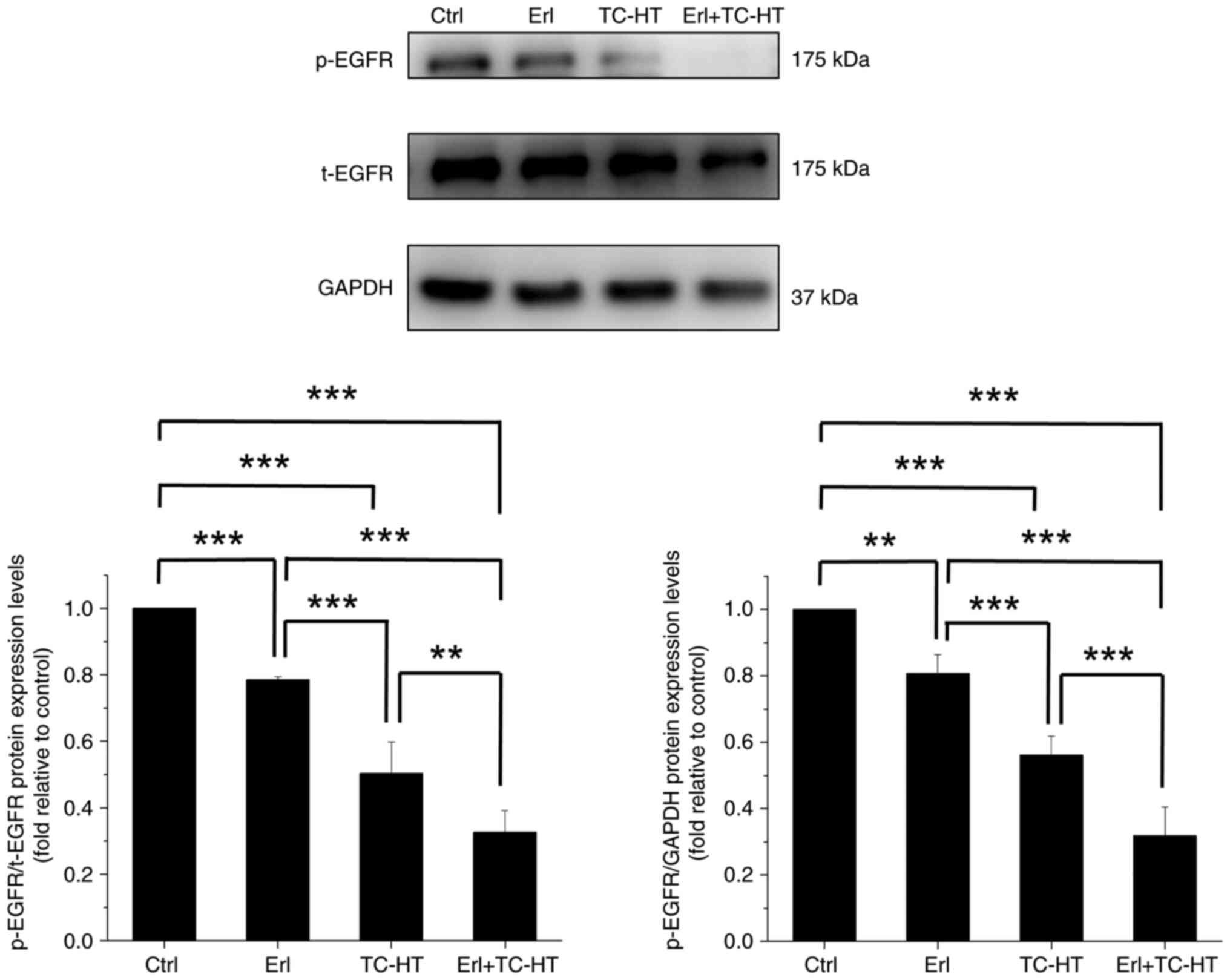
Effect of Erl combined with TC-HT on
EGFR protein expression in A549 cells
The signaling pathways involved in the anticancer
mechanism of Erl and TC-HT combination treatment were investigated.
Despite the application of targeted therapy agents aimed at
inhibiting EGFR activity, limited efficacy remains a challenge in
certain NSCLC cells with intrinsic resistance to Erl (11–13).
In vitro studies have identified A549 cells as resistant to
the EGFR-TKI Erl (15,28,29).
Consequently, it is imperative to explore innovative strategies to
augment the anticancer effects of Erl (14,30–32).
In the present study, Erl alone caused a marked decrease in EGFR
phosphorylation compared with the control cells (Fig. 3). Moreover, while TC-HT alone was
capable of significantly reducing p-EGFR expression compared with
the control cells, the combination of Erl and TC-HT resulted in a
more substantial inhibitory effect on p-EGFR expression compared
with the single treatment and control cells. Additionally, a
comparable decrease in p-EGFR expression was also observed in the
combination of low-dose Erl and TC-HT, compared with both low-dose
Erl alone and the control cells (Fig.
S3). Although this combination was less effective in inhibiting
p-EGFR compared with higher concentrations of Erl in conjunction
with TC-HT, the lower doses of Erl still exhibited significant
inhibitory effects when combined with TC-HT. These results
suggested that TC-HT may modulate the resistance of A549 cells to
Erl.
Effect of Erl combined with TC-HT on
EGFR signaling pathways in A549 cells
In the present study, the expression levels of JNK
and Akt proteins (33) were
examined in A549 cells. JNK, an EGFR downstream protein belonging
to the MAPK family, is responsive to stress stimuli and heat shock,
with its phosphorylation capable of altering the activities of
several proteins that reside in mitochondria or act in the nucleus
to trigger apoptosis (34). TC-HT
treatment significantly increased the expression levels of the
p-JNK protein compared with the control or Erl-treated A549 cells
(Fig. 4A). Furthermore, it was
demonstrated that the treatment combining Erl and TC-HT was more
effective in increasing p-JNK protein expression levels compared
with TC-HT alone. On the other hand, another EGFR downstream
protein, Akt, is involved in cellular survival pathways by
inhibiting the apoptotic process (35). Once activated by phosphorylation,
Akt actively regulates several transcription factors facilitating
the expression levels of survival proteins. It was shown that the
protein expression levels of p-Akt in Erl-treated A549 cells were
significantly lower than that compared with untreated cells, while
the inhibitory effect on phosphorylation of Akt was further
enhanced upon TC-HT treatment and was more pronounced in the
combination Erl and TC-HT treatment group (Fig. 4B). Meanwhile, t-Akt exhibited a
trend similar to that of the p-Akt protein, suggesting that
decrease in the phosphorylation of Akt by these treatments was
caused by a reduction in the amount of Akt present, leading to a
weakened survival signal. Moreover, it has been reported that both
JNK and Akt signaling pathways are important regulators in
influencing mitochondrial function. In addition,
mitochondria-dependent apoptosis is associated with a loss in MMP,
causing the release of cytochrome c and other apoptotic
factors (36). To confirm that the
apoptosis caused by Erl and TC-HT could be related to mitochondrial
disruption, MMP was assessed with DiOC6(3) fluorescence staining using flow
cytometry. It was confirmed that Erl and TC-HT individually had no
effect on MMP level compared with the control, but the combination
treatment caused significant MMP depolarization in A549 cells,
indicating mitochondrial dysfunction in their apoptosis mechanism
(Fig. S4).
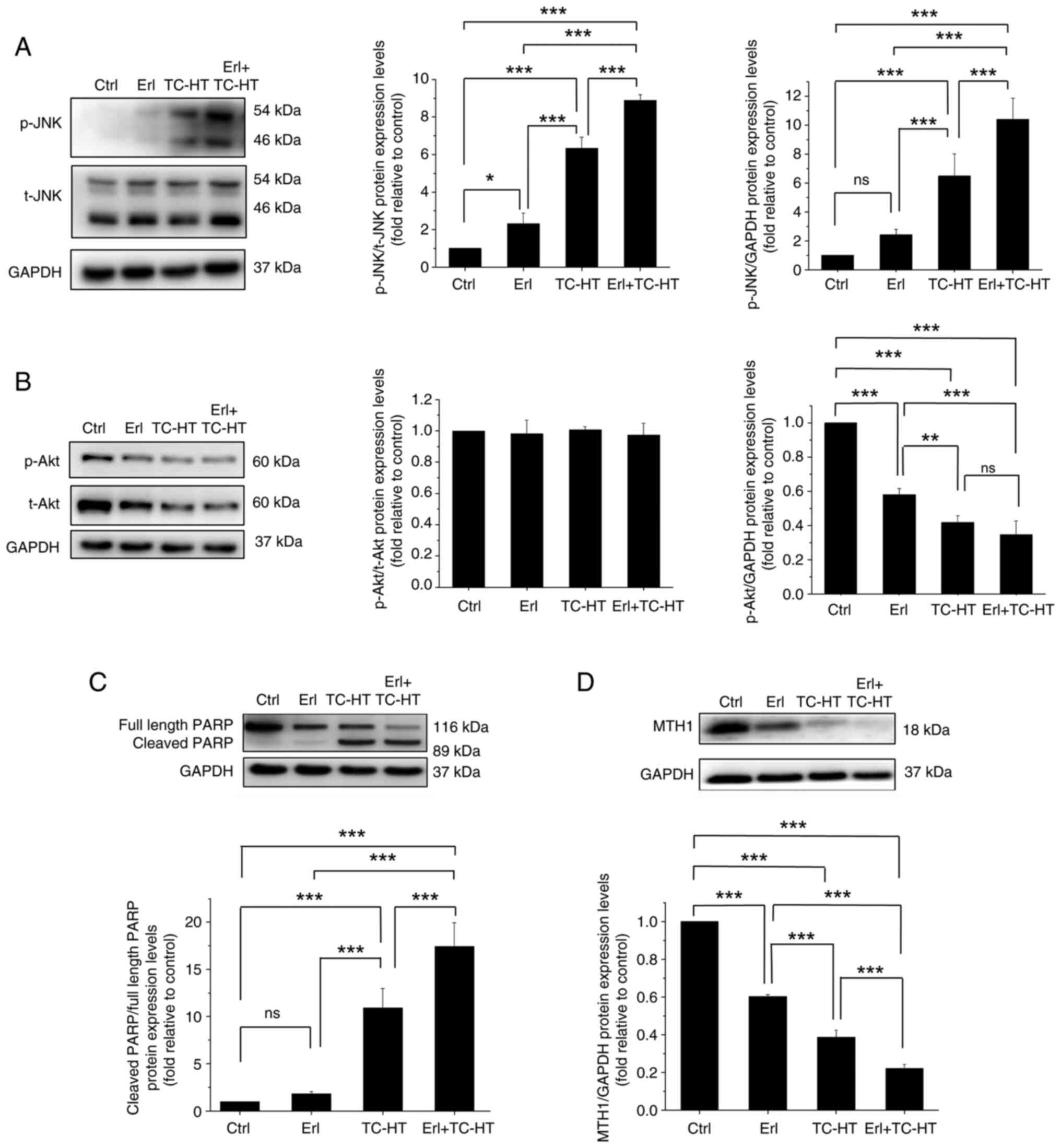 | Figure 4.Effect of Erl combined with TC-HT
treatment on survival- and apoptosis-related protein expression in
A549 cells. The anticancer experiments were conducted in A549
cancer cells treated with 10 µM Erl, moderate temperature TC-HT and
the combination treatment. Western blotting of (A) p-JNK, (B) p-Akt
and t-Akt, (C) cleaved PARP and full-length PARP (D) MTH1. The
protein expression levels of p-JNK, p-Akt, t-Akt and MTH1 were
normalized to GAPDH, and cleaved PARP was normalized to full-length
PARP. Each relative expression level was compared with the control
and represented as fold relative to the control. Data are shown as
mean ± standard deviation (n=3). Statistical significance was
determined using one-way ANOVA followed by Tukey's post-hoc test.
*P<0.05, **P<0.01, ***P<0.001. Erl, erlotinib; TC-HT,
thermal cycling-hyperthermia; p, phosphorylated; t, total; PARP,
poly ADP-ribose polymerase; MTH1, MutT homolog 1; ctrl, control;
ns, not significant. |
The injured mitochondria release cytochrome c
into the cytoplasm, cleaving caspase 9 and thus activating caspase
3 downstream (37), which enters
further the nucleus and cleaved PARP. It should be noted that PARP
serves an important role in mitochondria-mediated apoptosis, in
addition to being a key enzyme for DNA repair. In apoptosis, the
PARP protein is typically cleaved and inactivated, thereby
suppressing DNA repair and causing programmed cell death (38). To understand the apoptosis mechanism
in A549 cells triggered by the combination Erl and TC-HT treatment,
the present study evaluated the expression levels of
apoptosis-related proteins using western blotting. It was shown
that the ratio of cleaved PARP to full length PARP in TC-HT-treated
cells was significantly higher compared with that in Erl-treated
cells (Fig. 4C). Moreover, it was
demonstrated that the ratio of cleaved PARP to full length PARP in
combination Erl and TC-HT treatment was significantly higher
compared with that in the TC-HT treatment alone, indicating that
the combination treatment enhanced apoptosis of A549 cells via the
mitochondrial pathway. Furthermore, the MTH1 protein has received
increasing attention in cancer treatment, due to its key role in
DNA repair (39). High expression
level of MTH1 is deemed to be a sign of NSCLC metastasis (40). Therefore, the present study examined
MTH1 protein expression levels via western blotting. MTH1 protein
expression levels were found to significantly decrease in
Erl-treated cells compared with controls, while the inhibitive
effect on MTH1 protein expression levels was significantly higher
in the TC-HT treatment group, which significantly increased further
in the combination Erl and TC-HT treatment group (Fig. 4D). These results suggested that the
combination of Erl and TC-HT may prevent MTH1-related DNA repair
and induce the death of cancer cells via apoptosis.
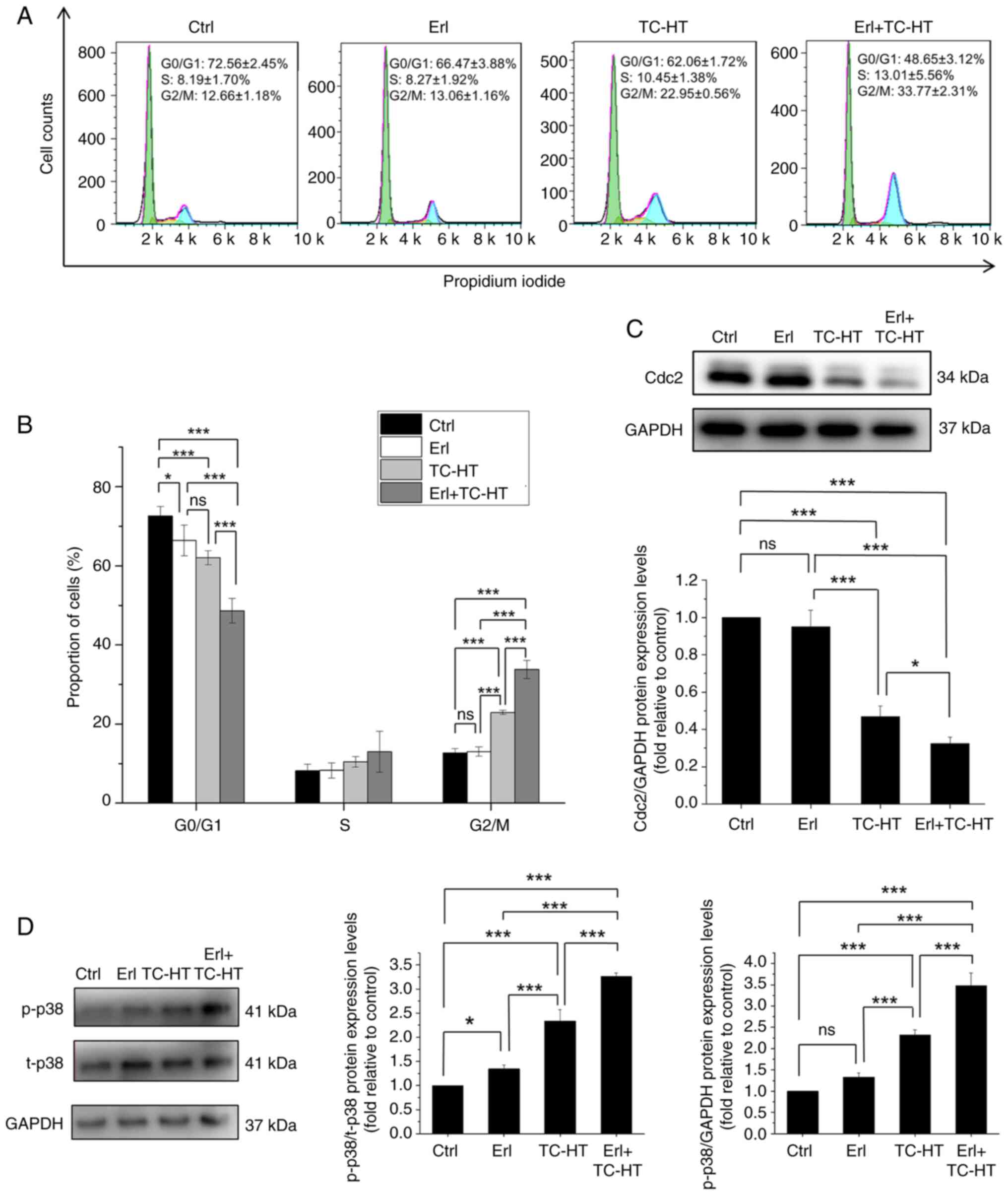
Combination treatment of Erl and TC-HT
causes G2/M cell cycle arrest in A549 cells
To further evaluate the anticancer effects of the
combination of Erl and TC-HT treatment on human NSCLC, the cell
cycle progression in A549 cells was examined by flow cytometry.
Treatment with moderate TC-HT led to a significant increased in
cell cycle arrest at the G2/M phase (22.95±0.56%) compared with the
group treated with Erl (13.06±1.16%) and the untreated cells
(12.66±1.18%; Fig. 5A and B).
Meanwhile, the combination of Erl and moderate TC-HT caused
significant accumulation of cells in the G2/M phase (33.77±2.31%)
compared with the single treatments and the untreated cells, with a
concomitant reduction of cells in the G0/G1 phase. Besides, no
significant differences in the S phase were observed among the
various treatments tested. Next, relevant proteins involved in the
G2/M progression were examined to investigate the mechanism of the
combined treatment effect on cell cycle distribution. Cdc2 is a
core regulator in the cell cycle and it binds to the cyclin B
complex guiding G2/M cell cycle transition (41,42).
It has been previously reported that the inhibition of Cdc2
expression resulted in G2/M cell cycle arrest (43–45).
In the present study, the protein expression levels of Cdc2 were
reduced significantly upon treatment with the combination of Erl
and TC-HT compared with the single Erl and TC-HT treatments and the
control group (Fig. 5C). Meanwhile,
as a well-known MAPK member, p38 serves an important role in cell
cycle regulation, and numerous studies have reported that
activation of the p38 pathway can induce G2/M cell cycle arrest via
Cdc2 suppression in human NSCLC cells (46–48).
Consistently, it was shown that the protein expression levels of
p-p38 were significantly increased by the combination treatment of
Erl and moderate TC-HT (Fig. 5D).
Taken together, these results suggested that the combined treatment
could synergistically induce cell cycle arrest in the G2/M phase by
increasing the activation of p38 while reducing Cdc2 expression in
cells, thereby suppressing cell cycle progression in human lung
cancer A549 cells.
Anti-proliferation and anti-migration
effects of the combination treatment (Erl + moderate temperature
TC-HT) in A549 NSCLC cells
Cancer mortality is associated with cancer
recurrence and metastasis, both of which are common clinical issues
in NSCLC (49,50). Therefore, it is important to reduce
the proliferation and migration ability of cancer cells. To further
confirm the anti-proliferative and anti-migration activities of the
Erl and TC-HT combination treatment in NSCLC, wound healing
(Fig. 6A) and colony formation
assays (Fig. 6B) were performed
using A549 cells. Erl-treated cells showed a significantly
decreased migration capacity compared with the control cells, while
cells treated with TC-HT alone exhibited a significant reduction in
migration compared with single Erl treatment and the control cells
(Fig. 6C). Furthermore, the Erl and
TC-HT combination treatment caused a more significant decrease in
A549 cell migration compared with that of the single treatments.
Compared with the control group, the wound closure percentage in
the Erl-treated group and the TC-HT-treated group was 83.8 and
59.2%, respectively, while the wound closure percentage in the
combined treatment group was only 35.9%. The results indicated that
the migration ability of A549 cells was significantly suppressed
after the application of the Erl and TC-HT combination treatment.
On the other hand, it was demonstrated that colony formation
ability of A549 cells treated with either Erl or TC-HT was
significantly decreased compared with the control cells, whereas
the colony formation ability of cells treated with the combination
treatment was further significantly reduced compared with that of
the single treatments (Fig. 6D).
Overall, the combination treatment was shown to impede A549 cell
proliferation and migration, indicating the potential anticancer
efficacy of the Erl and TC-HT combination treatment.
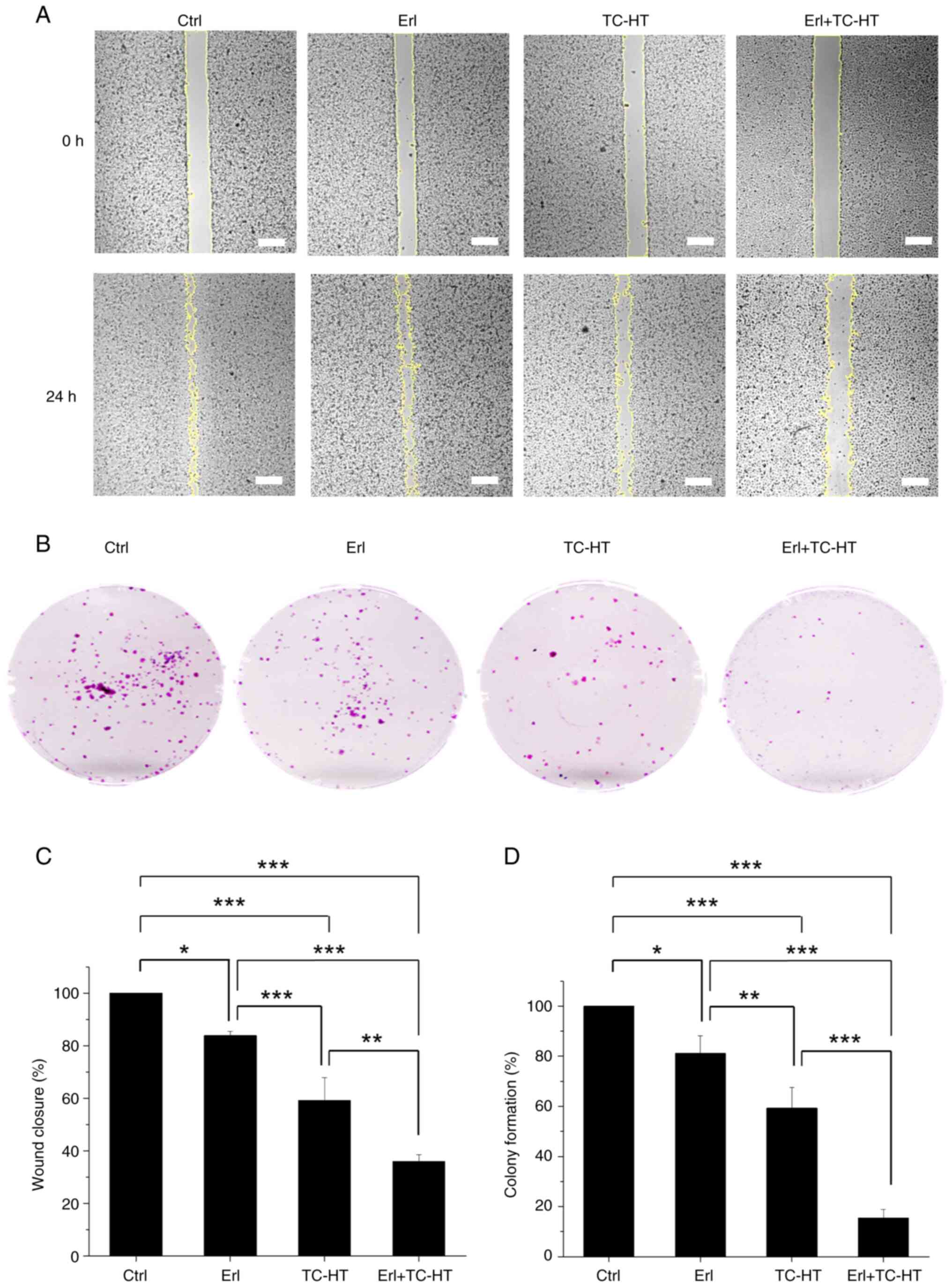 | Figure 6.Effect of Erl or TC-HT or in
combination on the inhibition of A549 cell colony formation and
migration. (A) Wound healing assay to determine the effect of Erl,
TC-HT or combination treatment on the migration ability of A549
cells. After scratch gaps were made, A549 cells were treated with
10 µM Erl, moderate temperature TC-HT or the combination treatment.
Yellow lines indicate wound edges detected by ImageJ (version
1.51j8; National Institutes of Health). Magnification, ×40. (B)
Colony formation assay for A549 cells treated with 10 µM Erl,
moderate temperature TC-HT or the combination treatment. (C) Wound
closure rate for A549 cells was determined as the percentage of the
area closed after 24 h from the initial wound area, and each group
was normalized to the control group and expressed as a fraction of
100. The areas of cell-free gaps were measured and quantified using
ImageJ (version 1.51j8; National Institutes of Health). (D) Colony
formation rate for A549 cells at 10 days after treatment with 10 µM
Erl, moderate temperature TC-HT or the combination treatment. Each
group was normalized to the control group and represented as a
percentage. The colony counting was performed using ImageJ (version
1.51j8; National Institutes of Health). Statistical significance
was determined by one-way ANOVA followed by Tukey's post-hoc test.
*P<0.05, **P<0.01, ***P<0.001. Erl, erlotinib; TC-HT,
thermal cycling-hyperthermia; ctrl, control. |
Discussion
The present study demonstrated the synergistic
anticancer effect of the combination of Erl, an EGFR-TKI, and TC-HT
on A549 NSCLC cells. EGFR is a receptor tyrosine kinase critical
for the initiation and development of malignant tumors via the MAPK
and PI3K/Akt pathways (6). Although
EGFR-TKIs, such as Erl, can target and inhibit the EGFR pathways
(7), their clinical benefit has
been limited, due to the resistance to TKIs among a proportion of
patients with NSCLC (9). In certain
NSCLC tumors, the development of acquired resistance to Erl occurs
following prolonged treatment periods (9). Additionally, the effectiveness of Erl
is constrained by the intrinsic resistance present in specific
patient populations (11–13), underscoring the need to improve the
sensitivity of these patients to Erl. Amid the efforts to combat
the resistance to EGFR-TKIs (51),
combination treatment has been deemed as a promising approach to
overcome the resistance problem (52). Several studies have investigated the
effect of combining Erl and other anticancer agents such as
cisplatin, monoclonal antibodies, aspirin and capsaicin (14,53–55),
aiming to enhance the therapeutic effect of this treatment.
However, the combined use of multiple drugs in clinical
applications is often hampered by unpredictable drug interactions
or side effects. Therefore, alternate methods are required to
enhance the anticancer effect of these drugs by combining physical
stimulation with the use of a drug treatment. It has been
previously reported that mild hyperthermia, when combined with
other therapeutic approaches, has resulted in enhanced anticancer
effects in various clinical trials, with treatment durations
ranging from 10–90 min (56).
Additionally, studies focusing on NSCLC have implemented
hyperthermia therapies with durations of ~30 min (57–59).
In accordance with these established research protocols and
recognizing that an appropriate duration of thermal exposure may
reduce damage to normal cells, the present study demonstrated the
efficacy of TC-HT physical stimulation in augmenting the anticancer
effects of Erl. Compared with Erl or TC-HT mono treatment, the
combination treatment produced an increased inhibitory effect on
A549 and H460 NSCLC cells, without damaging the IMR-90 normal lung
cells, thereby circumventing drug interaction issues and
potentially minimizing the risk of side effects by reducing the
required dosage of Erl. To elucidate the molecular mechanisms
underlying the anticancer effect of the combination treatment on
A549 NSCLC cells, the expression levels of certain proteins in the
apoptotic pathway were evaluated. It has been previously reported
that the relative expression levels of phosphorylated proteins
normalized to an internal control are associated with cellular
survival or apoptosis (60–63). The focus of the present study was to
elucidate the relative changes in signaling intensity and, to the
best of our knowledge, the present study represented the first
report into the enhanced apoptosis resulting from the combination
of TC-HT and Erl in A549 cells. It was demonstrated that TC-HT
significantly potentiated the efficacy of Erl in inhibiting the
phosphorylation of EGFR, which amplified the inhibitory effect of
Erl on A549 NSCLC cells. Subsequently, the expression levels of JNK
and Akt proteins were investigated in the EGFR downstream pathway.
The activation of JNK is involved in the induction of apoptosis
(34), while the activation of Akt
inhibits apoptosis (35).
Additionally, both JNK and Akt proteins serve a crucial role in the
regulation of mitochondrial function (36), and thus investigating the activation
status of these proteins contributes to a better understanding of
cellular apoptotic tendency. The results of the current study
demonstrated that the combination of TC-HT and Erl increased p-JNK
expression levels while decreasing p-Akt expression levels.
Concurrently, the combination treatment of Erl and TC-HT
significantly increased MMP depolarization in A549 cells,
indicating that apoptosis induced by Erl and TC-HT may be partly
associated with mitochondrial apoptosis pathways. In addition, the
cleavage of the PARP protein and the downregulation of MTH1
expression have been reported to contribute to the initiation
apoptosis in cancer cells (38,39).
The present study demonstrated that a significant increase in the
expression level of cleaved PARP was accompanied by a decrease in
MTH1 expression levels. These findings suggested that the
combination treatment of Erl and TC-HT effectively enhanced the
apoptosis of A549 NSCLC cells.
Cell cycle dysregulation, a common feature of
cancer, could be ameliorated through the modulation of cell cycle
regulatory proteins, offering the potential to attenuate the
uncontrolled proliferation of cancer cells (44,46–48).
The present study demonstrated that the combination treatment of
Erl and TC-HT resulted in G2/M phase arrest. The Cdc2 protein is
considered to be a core regulator involved in the G2/M cell cycle
transition (41,42). Significant downregulation of Cdc2
expression levels was demonstrated in the Erl and TC-HT combination
treatment group. Furthermore, the expression levels of p38 were
investigated, as previous studies in A549 NSCLC cells demonstrated
that increased p38 phosphorylation resulted in a reduction of the
level of Cdc2 protein expression, eventually leading to G2/M cell
cycle arrest (46–48). In the current study, it was also
demonstrated that the combined treatment of Erl and TC-HT
significantly increased the protein expression levels of p-p38.
These results indicated that the combination treatment of Erl and
TC-HT effectively reduced the occurrence of mitosis in A549 NSCLC
cells, inhibiting of cancer cell proliferation. It has been
previously reported that Erl treatment alone has a mild or
negligible effect on G2/M arrest, while combining Erl with other
approaches, such as protein kinase C-β inhibitor enzastaurin and
small molecule drug a-tocopheryl succinate hold promise for
improving its efficacy (64,65).
Similarly, the present study also demonstrated a negligible
influence of Erl on G2/M arrest when Erl was administered alone,
whereas a notable increase in G2/M arrest was observed when Erl was
combined with TC-HT. It is noteworthy that TC-HT, as a method of
physical stimulation, offers a novel method to avoid potential
drug-drug interactions, meriting further investigation in
anticancer applications.
Inhibiting cancer cell proliferation, recurrence and
migration is an important part of cancer treatment, and high rates
of recurrence and metastasis are concerns for patients with NSCLC
(49,50). The present study demonstrated that
the combined treatment of Erl and TC-HT reduced the viability of
A549 NSCLC cells and induced G2/M cell cycle arrest. Additionally,
the proliferation and migration of A549 cancer cells under the
combined treatment of Erl and TC-HT was investigated. These results
demonstrated significant findings concerning the migratory behavior
of A549 cells under different treatments. It was shown that single
Erl or TC-HT treatment reduced the migration area of treated cells
in comparison with control cells. Notably, the combination
treatment of Erl and TC-HT was more effective in inhibiting A549
NSCLC cell migration compared with either the single Erl or TC-HT
treatment. Moreover, A549 NSCLC cells treated with Erl or TC-HT
displayed reduced colony formation, whereas the combination
treatment of Erl and TC-HT exerted an increased inhibitory effect
on A549 cells compared with the single treatments. Collectively,
these findings highlighted the combined treatment with Erl and
TC-HT as a promising anticancer strategy as it significantly
impeded both A549 NSCLC cell migration and colony formation.
HT has long been a promising cancer treatment
method, to be applied alone or in combination with other
conventional therapies (66).
However, this treatment can cause cellular damage, due to an
overdose of HT, a practical problem which can now be overcome by
TC-HT due to the ability to control the thermal dosage applied, as
the intermittent cooling process can avoid excessive thermal dosage
accumulation and subsequent cytotoxic cell damage. Excessive
thermal dosage may induce mitochondrial damage and oxidative stress
not only in cancer cells, but also in normal cells (67). However, a previous study reported
that cancer cells have higher levels of oxidative stress and thus
they are more sensitive to thermal stress (68). Additionally, cancer cells are more
thermosensitive compared with normal cells (69). In the current study, the results
demonstrated that moderate temperature TC-HT treatment
(41.5–43.0°C) alone inhibited the viability of A549 NSCLC cells,
reducing their viability to 80% compared with the control group,
without damaging IMR-90 normal lung cells. To further examine the
effect of TC-HT alone on cancer cells at a higher temperature, the
temperature setting of TC-HT application was raised to the range of
a high temperature TC-HT treatment (42.5–45.6°C). It was
demonstrated that the high temperature TC-HT treatment alone
resulted in significant inhibition in the viability of A549 NSCLC
cells, with their viability dropping to only 17.9% of that of the
control group. It is noteworthy that under high temperature TC-HT
treatment, the additional incorporation of Erl in the range of 0–10
µM did not cause a significant additional decrease in cell
viability. Moreover, the TC-HT treatment at high temperature alone
did not have an effect on the viability of IMR-90 normal lung
cells, which remained at 80% of that of the control group, even in
the case of combined treatment with 10 µM Erl. Additionally, a
similar effect was observed in H460 NSCLC cells (Fig. S5). These results suggested that
TC-HT may be a potential future anticancer treatment for NSCLC and
reduce the current reliance on drug treatments. However, further
research to explore its efficacy is required.
Hypoxia is also a common feature of NSCLC and can
contribute to drug resistance (70). Hypoxic cancer cells exhibit an
increased susceptibility to hyperthermia in both in vitro
and in vivo models (71–74).
However, certain challenges remain, as hypoxic conditions can lead
to elevated expression of heat shock factor 1 (HSF1) and heat shock
proteins (HSPs), which may confer protective effects (75,76).
Additionally, HT has been associated with the upregulation of HSF1
and HSP expression, further enhancing thermotolerance in cancer
cells (77,78). Studies have reported that EGFR-TKIs
can mitigate hypoxia (70,79,80),
underscoring the potential of the Erl and TC-HT treatment
combination. Furthermore, it has been reported that the concurrent
application of HT with HSF1 and HSP inhibitors may represent a
promising anticancer strategy (78). Therefore, exploring the combination
of HSF1 and HSP inhibitors, or other EGFR-TKIs, with TC-HT could
significantly enhance therapeutic outcomes for anticancer treatment
and merits further investigation. In practice, non-contact thermal
modalities, such as radiofrequency (RF) and focused ultrasound
(FUS), present promising localized heating techniques for cancer
treatment. These approaches may be applicable for the treatment of
NSCLC (20,81–83).
Specifically, RF and multi-focal FUS can effectively regulate the
heating area and temperature used, achieving precise thermal
dissipation while maintaining the desired temperature. Although
additional research is necessary to substantiate these
possibilities, these features render these techniques suitable for
the implementation of TC-HT in anticancer treatments.
In conclusion, the present study reported that
TC-HT, a novel thermal treatment, is capable of sensitizing A549
cells to the EGFR-TKI Erl. The anticancer effect of the combination
of TC-HT and Erl occurred through the downstream of EGFR signaling
cascades, including the MAPK and PI3K-Akt pathways. These results
showed that TC-HT could enhance the anticancer efficacy of Erl on
A549 cells, thereby reducing Erl drug dosage and associated side
effects. Furthermore, TC-HT may have the potential to be used as a
drug-free cancer treatment method by raising the high temperature
of TC-HT in its application. These findings highlighted the
potential for TC-HT in combination therapy with other chemotherapy
or targeted therapy drugs, and further studies are needed to
examine specific TC-HT parameters in treating different types of
cancers.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to acknowledge the service
provided by the Research Core Facilities 3 Laboratory of the
Department of Medical Research at National Taiwan University
hospital for the use of the flow cytometry system.
Funding
The present study was supported by research grants from the
Ministry of Science and Technology (grant nos. MOST
110-2112-M-002-004 and MOST 109-2112-M-002-004 to CYC) and the
National Science and Technology Council (grant no. NSTC
112-2112-M-002-033 to CYC) of the Republic of China.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CYC conceived and supervised the study. GBL, WTC and
CYC designed the study. GBL and WTC performed the experiments and
collected the data. GBL and WTC confirmed the authenticity of all
the raw data. GBL, WTC, YYK, HHL, YMC, SJL and CYC contributed to
data analysis. GBL, WTC and CYC wrote the manuscript. All authors
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization, . WHO report on
cancer: Setting priorities, investing wisely and providing care for
all. World Health Organization; 2020
|
|
2
|
Balani C, Goss G and Blumenschein G Jr:
Recent clinical developments and rationale for combining targeted
agents in non-small cell lung cancer (NSCLC). Cancer Treat Rev.
38:174–184. 2012.
|
|
3
|
Imyanitov EN, Iyevleva AG and Levchenko
EV: Molecular testing and targeted therapy for non-small cell lung
cancer: Current status and perspectives. Crit Rev Oncol Hemat.
157:1031942021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wieduwilt MJ and Moasser MM: The epidermal
growth factor receptor family: Biology driving targeted
therapeutics. Cell Mol Life Sci. 65:1566–1584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yewale C, Baradia D, Vhora I, Patil S and
Misra A: Epidermal growth factor receptor targeting in cancer: A
review of trends and strategies. Biomaterials. 34:8690–8707. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
29:522017. View Article : Google Scholar
|
|
7
|
Ciardiello F, De Vita F, Orditura M and
Tortora G: The role of EGFR inhibitors in nonsmall cell lung
cancer. Curr Opin Oncol. 16:130–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gridelli C, Maione P, Bareschino MA,
Schettino C, Sacco PC, Ambrosio R, Barbato V, Falanga M and Rossi
A: Erlotinib in the treatment of non-small cell lung cancer:
Current status and future developments. Anticancer Res.
30:1301–1310. 2010.PubMed/NCBI
|
|
9
|
Lin Y, Wang X and Jin H: EGFR-TKI
resistance in NSCLC patients: Mechanisms and strategies. Am J
Cancer Res. 4:411–435. 2014.PubMed/NCBI
|
|
10
|
Melosky B: Supportive care treatments for
toxicities of anti-EGFR and other targeted agents. Curr Oncol.
19:59–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu CQ, da Cunha Santos G, Ding K,
Sakurada A, Cutz JC, Liu N, Zhang T, Marrano P, Whitehead M, Squire
JA, et al: Role of KRAS and EGFR as biomarkers of response to
erlotinib in National Cancer Institute of Canada Clinical Trials
Group Study BR.21. J Clin Oncol. 26:4268–4275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calvo E and Baselga J: Ethnic differences
in response to epidermal growth factor receptor tyrosine kinase
inhibitors. J Clin Oncol. 24:2158–2163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garassino MC, Martelli O, Broggini M,
Farina G, Veronese S, Rulli E, Bianchi F, Bettini A, Longo F,
Moscetti L, et al: Erlotinib versus docetaxel as second-line
treatment of patients with advanced non-small-cell lung cancer and
wild-type EGFR tumours (TAILOR): A randomised controlled trial.
Lancet Oncol. 14:981–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raimbourg J, Joalland MP, Cabart M, de
Plater L, Bouquet F, Savina A, Decaudin D, Bennouna J, Vallette FM
and Lalier L: Sensitization of EGFR wild-type non-small cell lung
cancer cells to EGFR-tyrosine kinase inhibitor erlotinib. Mol
Cancer Ther. 16:1634–1644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Ling YH, Goldman ID and Perez-Soler
R: Schedule-dependent cytotoxic synergism of pemetrexed and
erlotinib in human non-small cell lung cancer cells. Clin Cancer
Res. 13:3413–3422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Almanric K, Marceau N, Cantin A and Bertin
É: Risk factors for nephrotoxicity associated with cisplatin. Can J
Hosp Pharm. 70:99–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oei AL, Vriend LE, Crezee J, Franken NA
and Krawczyk PM: Effects of hyperthermia on DNA repair pathways:
One treatment to inhibit them all. Radiat Oncol. 10:1652015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaur P, Hurwitz MD, Krishnan S and Asea A:
Combined hyperthermia and radiotherapy for the treatment of cancer.
Cancers (Basel). 3:3799–3823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon S, Jung S and Baek SH: Combination
therapy of radiation and hyperthermia, focusing on the synergistic
Anti-cancer effects and research trends. Antioxidants. 12:9242023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang WH, Xie J, Lai ZY, Yang MD, Zhang GH,
Li Y, Mu JB and Xu J: Radiofrequency deep hyperthermia combined
with chemotherapy in the treatment of advanced Non-small cell lung
cancer. Chin Med J. 132:922–927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beik J, Abed Z, Ghoreishi FS,
Hosseini-Nami S, Mehrzadi S, Shakeri-Zadeh A and Kamrava SK:
Nanotechnology in hyperthermia cancer therapy: From fundamental
principles to advanced applications. J Control Release.
235:205–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen WT, Sun YK, Lu CH and Chao CY:
Thermal cycling as a novel thermal therapy to synergistically
enhance the anticancer effect of propolis on PANC-1 cells. Int J
Oncol. 55:617–628. 2019.PubMed/NCBI
|
|
23
|
Lu CH, Chen WT, Hsieh CH, Kuo YY and Chao
CY: Thermal cycling-hyperthermia in combination with polyphenols,
epigallocatechin gallate and chlorogenic acid, exerts synergistic
anticancer effect against human pancreatic cancer PANC-1 cells.
PLoS One. 14:e02176762019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuo YY, Chen WT, Lin GB, Lu CH and Chao
CY: Study on the effect of a triple cancer treatment of propolis,
thermal cycling-hyperthermia, and low-intensity ultrasound on
PANC-1 cells. Aging. 15:7496–7512. 2023.PubMed/NCBI
|
|
25
|
Lu CH, Kuo YY, Lin GB, Chen WT and Chao
CY: Application of non-invasive low-intensity pulsed electric field
with thermal cycling-hyperthermia for synergistically enhanced
anticancer effect of chlorogenic acid on PANC-1 cells. PLoS One.
15:e02221262020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh CH, Lu CH, Chen WT, Ma BL and Chao
CY: Application of non-invasive low strength pulsed electric field
to EGCG treatment synergistically enhanced the inhibition effect on
PANC-1 cells. PLoS One. 12:e01888852017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruttanapattanakul J, Wikan N, Potikanond S
and Nimlamool W: Combination of pinocembrin and epidermal growth
factor enhances the proliferation and survival of human
keratinocytes. Int J Mol Sci. 24:124502023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou Y, Ling YH, Sironi J, Schwartz EL,
Perez-Soler R and Piperdi B: The autophagy inhibitor chloroquine
overcomes the innate resistance of Wild-type EGFR Non-small-cell
lung cancer cells to erlotinib. J Thorac Oncol. 8:693–702. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Otahal A, Aydemir D, Tomasich E and
Minichsdorfer C: Delineation of cell death mechanisms induced by
synergistic effects of statins and erlotinib in non-small cell lung
cancer cell (NSCLC) lines. Sci Rep. 10:9592020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li YL, Hu X, Li QY, Wang F, Zhang B, Ding
K, Tan BQ, Lin NM and Zhang C: Shikonin sensitizes wild type EGFR
NSCLC cells to erlotinib and gefitinib therapy. Mol Med Rep.
18:3882–3890. 2018.PubMed/NCBI
|
|
31
|
Howe GA, Xiao B, Zhao H, Al-Zahrani KN,
Hasim MS, Villeneuve J, Sekhon HS, Goss GD, Sabourin LA,
Dimitroulakos J and Addison CL: Focal adhesion kinase inhibitors in
combination with erlotinib demonstrate enhanced Anti-tumor activity
in Non-small cell lung cancer. PLoS One. 11:e01505672016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greve G, Schiffmann I, Pfeifer D, Pantic
M, Schüler J and Lübbert M: The pan-HDAC inhibitor panobinostat
acts as a sensitizer for erlotinib activity in EGFR-mutated and
-wildtype non-small cell lung cancer cells. BMC Cancer. 15:9472015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Atalay G, Cardoso F, Awada A and Piccart
MJ: Novel therapeutic strategies targeting the epidermal growth
factor receptor (EGFR) family and its downstream effectors in
breast cancer. Ann Oncol. 14:1346–1363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeuchi K and Ito F: EGF receptor in
relation to tumor development: Molecular basis of responsiveness of
cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J.
277:316–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akca H, Tani M, Hishida T, Matsumoto S and
Yokota J: Activation of the AKT and STAT3 pathways and prolonged
survival by a mutant EGFR in human lung cancer cells. Lung Cancer.
54:25–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsuyama S and Reed JC:
Mitochondria-dependent apoptosis and cellular pH regulation. Cell
Death Differ. 7:1155–1165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yi M, Dong B, Qin S, Chu Q, Wu K and Luo
S: Advances and perspectives of PARP inhibitors. Exp Hematol Oncol.
8:445732019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gad H, Koolmeister T, Jemth AS, Eshtad S,
Jacques SA, Ström CE, Svensson LM, Schultz N, Lundbäck T,
Einarsdottir BO, et al: MTH1 inhibition eradicates cancer by
preventing sanitation of the dNTP pool. Nature. 508:215–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li DN, Yang CC, Li J, Ou Yang QG, Zeng LT,
Fan GQ, Liu TH, Tian XY, Wang JJ, Zhang H, et al: The high
expression of MTH1 and NUDT5 promotes tumor metastasis and
indicates a poor prognosis in patients with non-small-cell lung
cancer. Biochim Biophys Acta Mol Cell Res. 1868:1188952021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dorée M and Hunt T: From Cdc2 to Cdk1:
When did the cell cycle kinase join its cyclin partner? J Cell Sci.
115:2461–2464. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang CC, Heller JD, Kuo J and Huang RC:
Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and
cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc
Natl Acad USA Sci. 101:13239–13244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Senju M, Sueoka N, Sato A, Iwanaga K,
Sakao Y, Tomimitsu S, Tominaga M, Irie K, Hayashi S and Sueoka E:
Hsp90 inhibitors cause G2/M arrest associated with the reduction of
Cdc25C and Cdc2 in lung cancer cell lines. J Cancer Res Clin Oncol.
132:150–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoshida M, Matsui Y, Iizuka A and Ikarashi
Y: G2-phase arrest through p21(WAF1/Cip1) induction and cdc2
repression by gnidimacrin in human hepatoma HLE cells. Anticancer
Res. 29:1349–1354. 2009.PubMed/NCBI
|
|
46
|
Su JC, Lin KL, Chien CM, Lu CM, Chen YL,
Chang LS and Lin SR: Novel indoloquinoline derivative, IQDMA,
induces G(2)/M phase arrest and apoptosis in A549 cells through
JNK/p38 MAPK signaling activation. Life Sci. 85:505–516. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pai JT, Hsu MW, Leu YL, Chang KT and Weng
MS: Induction of G2/M cell cycle arrest via
p38/p21Waf1/Cip1-dependent signaling pathway activation by
bavachinin in non-small-cell lung cancer cells. Molecules.
26:51612021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo YH, Wang C, Xu WT, Zhang Y, Zhang T,
Xue H, Li YN, Fu ZR, Wang Y and Jin CH: 18β-Glycyrrhetinic acid has
Anti-cancer effects via inducing apoptosis and G2/M cell cycle
arrest, and inhibiting migration of A549 lung cancer cells. Onco
Targets Ther. 14:5131–5144. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tamura T, Kurishima K, Nakazawa K,
Kagohashi K, Ishikawa H, Satoh H and Hizawa N: Specific organ
metastases and survival in metastatic non-small-cell lung cancer.
Mol Clin Oncol. 3:217–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Uramoto H and Tanaka F: Recurrence after
surgery in patients with NSCLC. Transl Lung Cancer Res.
4:2422014.PubMed/NCBI
|
|
51
|
Passaro A, Jänne PA, Mok T and Peters S:
Overcoming therapy resistance in EGFR-mutant lung cancer. Nat
Cancer. 2:377–391. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tong CW, Wu WK, Loong HH, Cho WC and To
KK: Drug combination approach to overcome resistance to EGFR
tyrosine kinase inhibitors in lung cancer. Cancer Lett.
405:100–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cavazzoni A, Alfieri RR, Cretella D,
Saccani F, Ampollini L, Galetti M, Quaini F, Graiani G, Madeddu D,
Mozzoni P, et al: Combined use of anti-ErbB monoclonal antibodies
and erlotinib enhances antibody-dependent cellular cytotoxicity of
wild-type erlotinib-sensitive NSCLC cell lines. Mol Cancer.
11:912012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu X, Wu LW, Weng X, Lin NM and Zhang C:
Synergistic antitumor activity of aspirin and erlotinib: Inhibition
of p38 enhanced aspirin plus Erlotinib-induced suppression of
metastasis and promoted cancer cell apoptosis. Oncol Lett.
16:2715–2724. 2018.PubMed/NCBI
|
|
55
|
Chen JC, Ko JC, Yen TC, Chen TY, Lin YC,
Ma PF and Lin YW: Capsaicin enhances erlotinib-induced cytotoxicity
via AKT inactivation and excision repair cross-complementary 1
(ERCC1) down-regulation in human lung cancer cells. Toxicol Res.
8:459–470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bing C, Cheng B, Staruch RM, Nofiele J,
Wodzak Staruch M, Szczepanski D, Farrow-Gillespie A, Yang A,
Laetsch TW and Chopra R: Breath-hold MR-HIFU hyperthermia: Phantom
and in vivo feasibility. Int J Hyperthermia. 36:1084–1097. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sadhukha T, Wiedmann TS and Panyam J:
Inhalable magnetic nanoparticles for targeted hyperthermia in lung
cancer therapy. Biomaterials. 34:5163–5171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park J and Baek SH: Combination therapy
with cinnamaldehyde and hyperthermia induces apoptosis of A549
Non-small cell lung carcinoma cells via regulation of reactive
oxygen species and mitogen-Activated protein kinase family. Int J
Mol Sci. 21:62292020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Heo J, Jo Y and Yoon M: Synergistic
effects of combined hyperthermia and electric fields treatment in
non-small cell lung-cancer (NSCLC) cell lines. Clin Transl Oncol.
Oct 22–2024.(Epub ahead of print). doi: 10.1007/s12094-024-03760-6.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cheng H, An SJ, Dong S, Zhang YF, Zhang
XC, Chen ZH, Jian-Su and Wu YL: Molecular mechanism of the
schedule-dependent synergistic interaction in EGFR-mutant non-small
cell lung cancer cell lines treated with paclitaxel and gefitinib.
J Hematol Oncol. 4:52011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Volman Y, Hefetz R, Galun E and
Rachmilewitz J: DNA damage alters EGFR signaling and reprograms
cellular response via Mre-11. Sci Rep. 12:57602022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Feng YB, Chen L, Chen FX, Yang Y, Chen GH,
Zhou ZH and Xu CF: Immunopotentiation effects of apigenin on NK
cell proliferation and killing pancreatic cancer cells. Int J
Immunopathol Pharmacol. 37:39463202311611742023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu J, Jiao W, Wu DB, Yu JH, Liu LJ, Zhang
MY and Chen GX: Yishen Tongbi decoction attenuates inflammation and
bone destruction in rheumatoid arthritis by regulating
JAK/STAT3/SOCS3 pathway. Front Immunol. 15:13818022024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Steen NV, Potze L, Giovannetti E,
Cavazzoni A, Ruijtenbeek R, Rolfo C, Pauwels P and Peters GJ:
Molecular mechanism underlying the pharmacological interactions of
the protein kinase C-β inhibitor enzastaurin and erlotinib in
non-small cell lung cancer cells. Am J Cancer Res. 7:816–830.
2017.PubMed/NCBI
|
|
65
|
Cheng F, Peng X, Meng G, Pu Y, Luo K and
He B: Poly(ester-thioether) microspheres co-loaded with erlotinib
and α-tocopheryl succinate for combinational therapy of non-small
cell lung cancer. J Mater Chem B. 8:1728–1738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Belhadj Slimen I, Najar T, Ghram A,
Dabbebi H, Ben Mrad M and Abdrabbah M: Reactive oxygen species,
heat stress and oxidative-induced mitochondrial damage. A review.
Int J Hyperthermia. 30:513–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Panieri E and Santoro M: ROS homeostasis
and metabolism: A dangerous liason in cancer cells. Cell Death Dis.
7:e22532016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Van der Zee J: Heating the patient: A
promising approach? Ann Oncol. 13:1173–1184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Salem A, Asselin MC, Reymen B, Jackson A,
Lambin P, West CML, O'Connor JPB and Faivre-Finn C: Targeting
hypoxia to improve non-Small cell lung cancer outcome. J Natl
Cancer Inst. 110:14–30. 2018. View Article : Google Scholar
|
|
71
|
Gerweck LE, Nygaard TG and Burlett M:
Response of cells to hyperthermia under acute and chronic hypoxic
conditions. Cancer Res. 39:966–972. 1979.PubMed/NCBI
|
|
72
|
Bicher HI, Hetzel FW, Sandhu TS, Frinak S,
Vaupel P, O'Hara MD and O'Brien T: Effects of hyperthermia on
normal and tumor microenvironment. Radiology. 137:523–530. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Overgaard J: Effect of hyperthermia on the
hypoxic fraction in an experimental mammary carcinoma in vivo. Br J
Radiol. 54:245–249. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Elming PB, Sørensen BS, Oei AL, Franken
NAP, Crezee J, Overgaard J and Horsman MR: Hyperthermia: The
optimal treatment to overcome radiation resistant hypoxia. Cancers.
11:602019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kabakov AE and Yakimova AO:
Hypoxia-induced cancer cell responses driving radioresistance of
hypoxic tumors: Approaches to targeting and radiosensitizing.
Cancers. 13:11022021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F,
Mei H, Liu J, Wang W and Liu Q: Heat shock proteins: Biological
functions, pathological roles, and therapeutic opportunities.
MedComm. 3:e1612022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ahmed K, Zaidi SF, Mati-Ur-Rehman Rehman R
and Kondo T: Hyperthermia and protein homeostasis: Cytoprotection
and cell death. J Therm Biol. 91:1026152020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Scutigliani EM, Liang Y, Crezee H, Kanaar
R and Krawczyk PM: Modulating the heat stress response to improve
Hyperthermia-Based anticancer treatments. Cancers. 13:12432021.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Karar J and Maity A: Modulating the tumor
microenvironment to increase radiation responsiveness. Cancer Biol
Ther. 8:1994–2001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nijkamp MM, Span PN, Bussink J and
Kaanders JH: Interaction of EGFR with the tumour microenvironment:
Implications for radiation treatment. Radiother Oncol. 108:17–23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang T, Chen L, Zhang S, Xu Y, Fan Y and
Zhang L: Effects of high-intensity focused ultrasound on
Cisplatin-resistant human lung adenocarcinoma in vitro and in vivo.
Acta Biochim Biophys Sin. 49:1092–1098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Qin Y, Sun Y, Liu Y, Luo Y and Zhu J:
Pilot study of radiofrequency hyperthermia in combination with
gefitinib in gefitinib-effective patients with advanced NSCLC.
Thorac Cancer. 7:422–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sekins KM, Leeper DB, Hoffman JK, Keilman
GW, Ziskin MC, Wolfson MR and Shaffer TH: Feasibility of lung
cancer hyperthermia using breathable perfluorochemical (PFC)
liquids. Part II: Ultrasound hyperthermia. Int J Hyperthermia.
20:278–299. 2004. View Article : Google Scholar : PubMed/NCBI
|