Introduction
Gastric cancer (GC) is one of the most prevalent and
lethal malignancies worldwide, with significant variations in age
of onset (1). Population studies
estimate that 30% of newly diagnosed GCs in the USA are early-onset
GC (EO-GC), affecting individuals under 50 years (2). A study has highlighted the critical
distinctions between EO-GC and late-onset GC (L-GC or traditional
GC), emphasizing the need for tailored diagnostic and therapeutic
approaches (3). Approximately 10%
of EO-GC cases have a family history of the disease, primarily
associated with germline mutations in the CDH1 gene, which encodes
the E-cadherin protein (3–5). These and other mutations significantly
contribute to the hereditary diffuse GC risk, such as Lynch
syndrome and Peutz-Jeghers syndrome, among others (3,6). By
contrast, 90% of EO-GC cases lack a family history and are linked
to environmental factors such as obesity, heavy alcohol
consumption, cigarette smoking, Epstein-Barr virus infection and
Helicobacter pylori infection (7,8).
In general, EO-GC exhibits a more aggressive
clinical course than L-GC, with rapid and multifocal disease
progression, poorly differentiated histology with a higher
prevalence of diffuse histologic types and early metastasis
(4,8). Experts agree that these differential
clinical behaviors between EO-GC and L-GC may be due to distinctive
somatic mutations in each type of GC (4,5,9).
A comprehensive genomic analysis by Han et al
(4) revealed distinct mutational
landscapes between EO-GC and L-GC. EO-GC exhibits higher mutation
frequencies in genes such as TP53, CDH1 and MUC6 than L-GC.
Triantafillidis et al (8)
also summarized the existing data supporting the hypothesis of a
series of environmental factors highlighted in recent decades and
which are mainly related to dietary habits, intestinal microbiome
and an increase in the obese population interacting with genetic
factors. All these factors lead to epigenetic changes in DNA and
histones that would ultimately favor carcinogenesis at an early
age.
A study revealed that EO-GC frequently shows a lower
tumor mutation burden (6). However,
it has higher mutation rates of genes related to the regulation of
cell proliferation (PIK3CA, NOTCH1, ERBB4, CDH1, ATM and APC, among
others), which may explain its aggressive nature and poorer
prognosis in younger patients (9).
The present review aligns with that of Machlowska et al
(10), who identified several
candidate genes with high mutation frequencies in EO-GC,
illustrating the unique genetic landscape of EO-GC cases. Despite
these advances, optimal screening and treatment strategies for
EO-GC remain under investigation. The lack of consensus on the age
cutoff for defining EO-GC complicates the establishment of uniform
guidelines, as highlighted by Petrillo et al (5) and Ugai et al (11). Therefore, a differential molecular
evaluation of GC cases based on the age of the patients may be
essential for new therapeutic approaches to improve outcomes for
patients with EO-GC.
A significant limitation currently encountered in
data mining of EO-GC cases is that numerous metadata present in
publicly available transcriptomic databases do not incorporate age
as a variable. Consequently, as an initial approach used in the
present study, the transcriptomic profiles of patients with GC were
analyzed based on their age, encompassing both EO-GC and L-GC.
Using multiple bioinformatics tools, RNA-sequencing (Seq) data from
The Cancer Genome Atlas-Stomach Adenocarcinoma (TCGA-STAD) dataset
were analyzed.
EO-GC exhibits a distinct transcriptomic
expression profile
The study of transcriptomics in EO-GC plays a
crucial role because it allows the exploration and understanding of
the underlying molecular mechanisms and identification of
biomarkers and transcription factors involved in the initiation
and/or progression of the pathology.
Utilizing the ‘TCGAbiolinks’ R package (12), RNA-seq data [transcripts per
million, (TPM)] and corresponding clinical information of n=377
cases were downloaded from the Genomic Data Commons portal. The
data were filtered to include only cases where the stomach was the
primary tumor site with clear and complete clinical information. A
total of n=32 cases were classified as EO-GC (≤50 years) and the
remaining cases (n=345) were classified as L-GC (>50 years). The
clinicopathological characteristics are summarized in Table I.
 | Table I.Clinicopathological characteristics
of The Cancer Genome Atlas-Stomach Adenocarcinoma cases according
to age category. |
Table I.
Clinicopathological characteristics
of The Cancer Genome Atlas-Stomach Adenocarcinoma cases according
to age category.
|
| Early-onset gastric
cancer (≤50 years) | Late-onset gastric
cancer (>50 years) | P-value (EO-GC vs.
L-GC) |
|---|
|
|
|
|
|---|
| Item | 21-40 years | 41-50 years | Total | 51-60 years | 61-80 years | ≥81 years | Total |
|---|
| Age, years | 35.0±3.4 | 46.3±2.7 | 44.9±4.6 | 56.3±2.8 | 70.4±5.2 | 84.3±3.2 | 67.8±8.8 | <0.001 |
| Sex |
|
|
|
|
|
|
| 0.467 |
|
Female | 1 (25.0) | 21 (75.0) | 22 (68.8) | 27 (30.7) | 89 (38.2) | 10 (41.7) | 126 (36.5) |
|
|
Male | 3 (75.0) | 7 (25.0) | 10 (31.2) | 61 (69.3) | 144 (61.8) | 14 (58.3) | 219 (63.5) |
|
| Site of resection
or biopsy |
|
|
|
|
|
|
| 0.911 |
| Body of
stomach | 1 (25) | 3 (10.8) | 4 (12.5) | 21 (23.9) | 59 (25.3) | 6 (25.0) | 86 (24.9) |
|
|
Cardia | 1 (25) | 7 (25.0) | 8 (25.0) | 20 (22.7) | 56 (24.0) | 5 (20.8) | 81 (23.5) |
|
| Fondus
of stomach | - | 2 (7.1) | 2 (6.3) | 14 (15.9) | 28 (12.0) | 4 (16.7) | 46 (13.3) |
|
| Gastric
antrum | 2 (50) | 14 (50.0) | 16 (50.0) | 31 (35.2) | 81 (34.8) | 9 (37.5) | 121 (35.1) |
|
|
Stomach, NOS | - | 2 (7.1) | 2 (6.3) | 2 (2.3) | 9 (3.9) | - | 11 (3.2) |
|
| Stage |
|
|
|
|
|
|
| 0.502 |
| I | - | 4 (14.3) | 4 (12.5) | 8 (9.1) | 35 (15.0) | 8 (33.3) | 51 (14.8) |
|
| II | - | 8 (28.5) | 8 (25.0) | 31 (35.2) | 75 (32.2) | 6 (25.0) | 112 (32.5) |
|
|
III | 4 (100) | 11 (39.3) | 15 (46.9) | 38 (43.2) | 104 (44.6) | 8 (33.3) | 150 (43.5) |
|
| IV | - | 5 (17.9) | 5 (15.6) | 11 (12.5) | 19 (8.2) | 2 (8.4) | 32 (9.2) |
|
| Total | 4 (12.50) | 28 (87.50) | 32 (100) | 88 (25.5) | 233 (67.5) | 24 (7.0) | 345 (100) |
|
As a first approach, it was evaluated whether age
corresponds to a variable associated with changes in TCGA-STAD
transcriptomic expression. To answer this, the EO-GC cases were
categorized into two subgroups (21 to 40 years and 41 to 50 years),
while the L-GC cases were separated into three groups (51 to 60
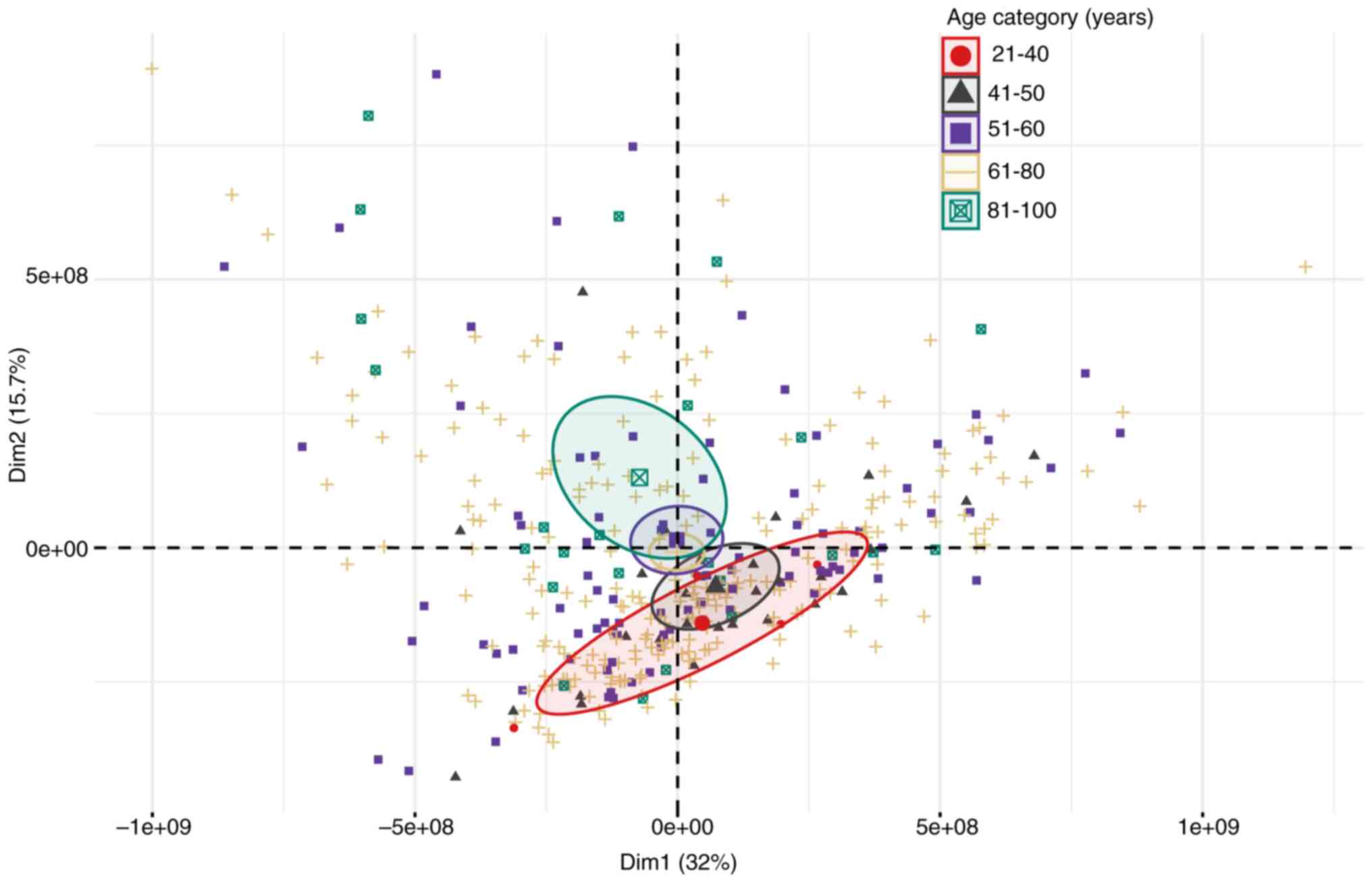
years, 61 to 80 years and >80 years). A principal component
analysis (PCA) was then performed to assess the variability of the
TPM data matrix concerning these age categories using the
‘FactoMineR’ R package (13). Of
note, the PCA results showed that the age groups of 21–40 and
>80 (81–100 years old) exhibited an evident graphic separation,
mainly determined by the second principal component (Fig. 1).
Subsequently, when evaluating the main genes
associated with these differences, a strong influence of the genes
related to cytoskeleton and cell motility (e.g., ACTB, ACTN4, KRT4,
KRT8), immunoglobulins (e.g., IGHA1, IGHA2, IGHG1, IGHG2),
metabolism and energy production (e.g., PKM, MT-ATP6, MT-CO1,
MT-CO2, MT-CO3) and cell adhesion and communication (e.g., CD24,
CD74, EPCAM, CLDN3) was observed, among others. These genes were
selected based on their contribution to the second PC (Dim2) from
the PCA, as shown in Table SI.
Subsequently, differential gene expression analysis
of the EO-GC vs. L-GC groups was conducted utilizing the R package
‘DESeq2’ (14). To evaluate this
parameter, subdivisions by age category were not implemented due to
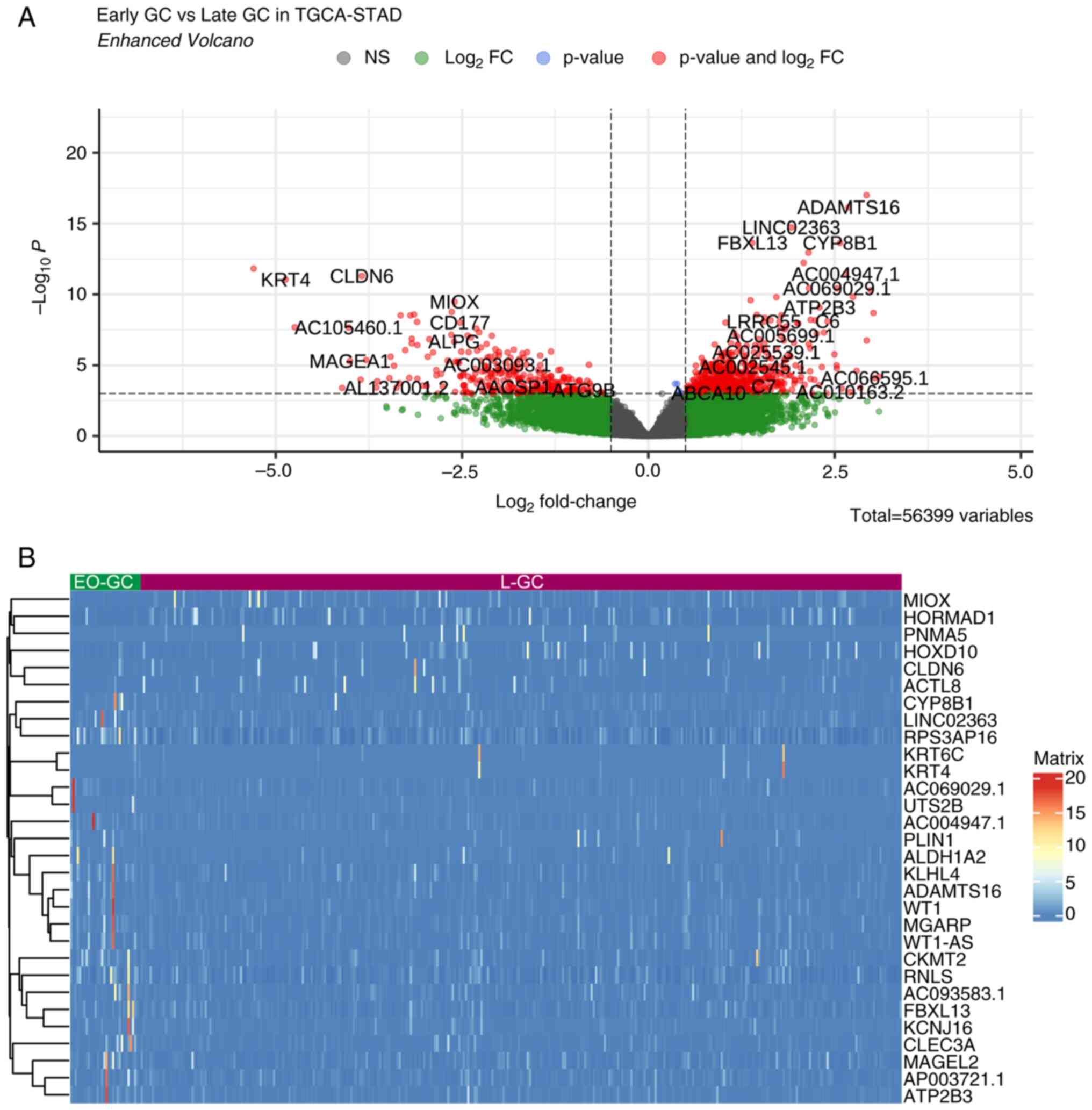
the limited representation of EO-GC in the dataset. Fig. 2 and Table SII illustrate the top 30 genes
exhibiting the highest differential expression between EO-GC and
L-GCs.
Comprehensive transcriptomic profiling elucidated
significant disparities in the gene expression profiles between
EO-GC and L-GC (Fig. 2A). KLHL4,
MAGEL2, RNLS and CYP8B1 demonstrated upregulation in EO-GC.
Concurrently, CLDN6, MIOX, PNMA5 and ACTL8 exhibited downregulation
(Fig. 2B). Subsequently, the
potential predictive value of these selected genes was assessed
utilizing the UALCAN data portal (15). The analysis revealed that elevated
expression levels of KLHL4, MAGEL2 and CLDN6 were associated with
reduced survival rates in the TCGA-STAD platform dataset when age
was not considered as a variable (Fig.
3A). In addition, through the development of receiver operating
characteristic (ROC) curves to depict sensitivity and specificity
and quantify the area under the curve (AUC) using the ‘survivalROC’
R package (16), it was observed
that the EO-GC upregulated genes (KLHL4, MAGEL2, RNLS and CYP8B1)
showed differential survival predictions based on risk scores
(Fig. 3B).
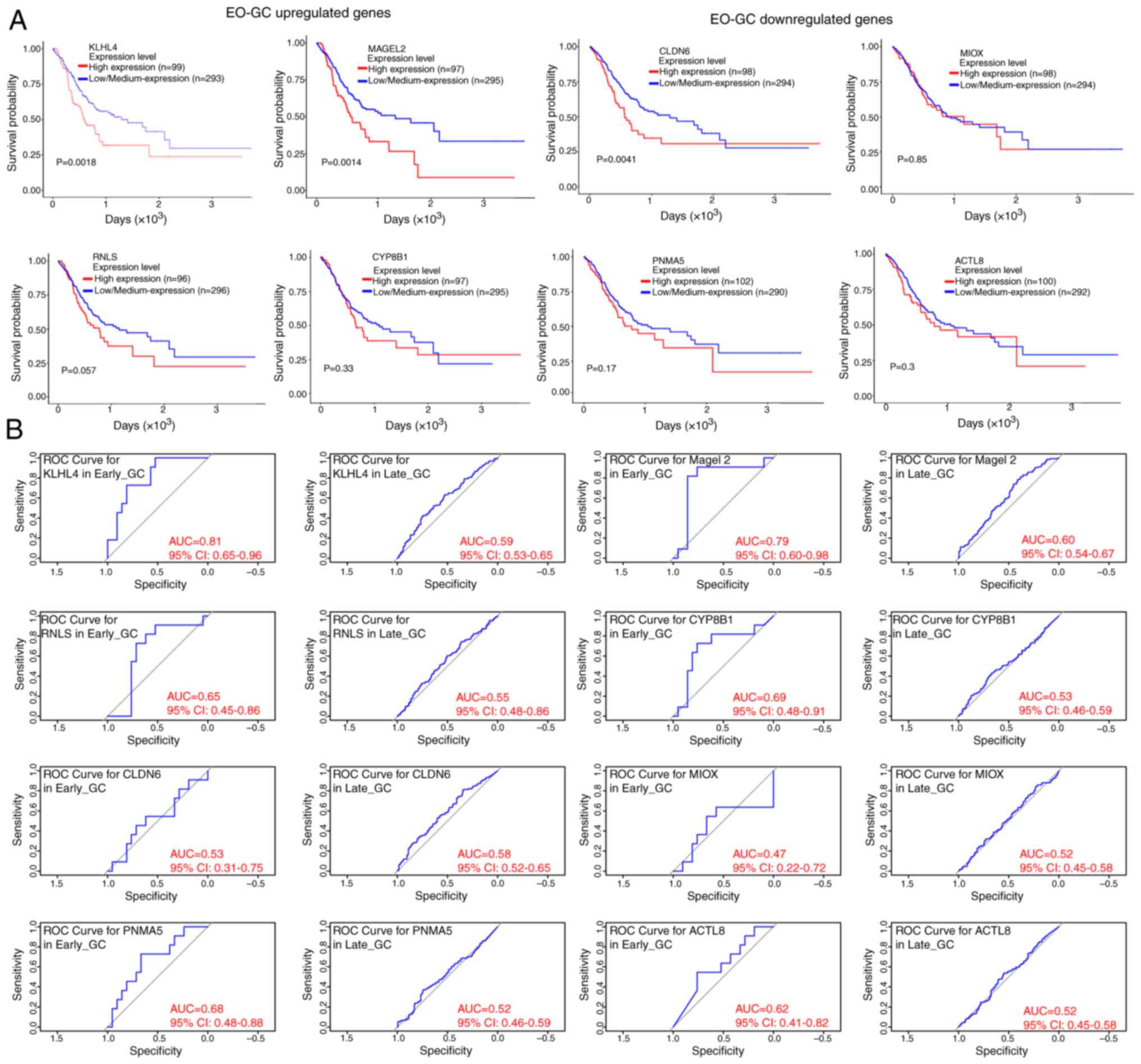 | Figure 3.Prognostic value of differentially
expressed genes in EO-GC in the TCGA-STAD dataset and ROC curve
analysis. (A) Kaplan-Meier survival curves for the TCGA-STAD
dataset based on the expression levels of genes upregulated in
EO-GC, including KLHL4, MAGEL2, RNLS and CYP8B1, and downregulated
genes in EO-GC, namely CLDN6, MIOX, PNMA5 and ACTL8 (based on
UALCAN portal data). P-values were calculated to assess statistical
significance. (B) ROC curves depicting the sensitivity and
specificity of survival predictions based on risk scores for the
upregulated and downregulated EO-GC genes and their comparison with
L-GC. The AUC was quantified using the ‘survivalROC’ R package. The
optimal cutoff risk score was determined at the turning point of
each ROC curve. TCGA-STAD, The Cancer Genome Atlas-Stomach
Adenocarcinoma; ROC, receiver operating characteristic; AUC, area
under the ROC curve; EO-GC, early-onset gastric cancer; L-GC,
late-onset gastric cancer. |
Lastly, considering that aging has been incorporated
as a crucial variable in the understanding of genomic instability
(17) and the multiple efforts to
generate early biomarkers based on DNA methylations in GC (18,19),
the targeted evaluation of the methylation patterns of these genes
according to EO-GC and L-GC classification may be considered. This
highlights the necessity of exploring how age-related genomic
changes contribute to cancer progression.
The following sections will delve into a detailed
analysis of their functional implications and potential
contributions to the pathogenesis and prognosis of EO-GC.
KLHL4, MAGEL2, RNLS and CYP8B1 are
upregulated genes in EO-GC
To obtain a deeper understanding of the molecular
mechanisms underlying EO-GC, a comprehensive transcriptomic
analysis was conducted. This approach aimed to identify
differentially expressed genes and pathways that could distinguish
EO-GC from L-GC, providing insight into potential drivers of the
disease.
KLHL4 gene expression in GC
KLHL4 is part of a family of 42 proteins, each
characterized by a BTB/POZ domain at the N-terminus, a BACK domain
in the middle and 5–6 Kelch domains at the C-terminus. Most KLHL
proteins associate with Cullin 3 to form a Cullin-E3 ubiquitin
ligase complex, acting as adapters that recognize target proteins
via the Kelch domains during ubiquitination. These proteins are
crucial for various cellular processes, including cytoskeletal
organization, ion channel gating, transcriptional suppression and
protein targeting for ubiquitination (20). Furthermore, the KLHL4 gene has also
been linked to the synthesis and transport of long-chain fatty
acids (Fig. 4A; pink cluster), a
process implicated in diabetes and heart diseases. However, the
mechanism of fatty acid entry into cells remains poorly understood
and is thought to involve protein-mediated transport (21). KLHL4 is associated with the kinesin
superfamily proteins (KIFs) family (Fig. 4A; light green cluster), which is
essential for intracellular transport and fundamental for cellular
function, survival and tissue morphogenesis. KIFs act as molecular
motors that directionally transport cargo, including organelles,
protein complexes and mRNAs, and play crucial roles in tumor
suppression (22).
The nuclear factor erythroid 2-related factor 2
(NRF2) is a transcription factor that regulates the cellular
antioxidant response and strongly correlates with KLHL4 expression
(Fig. 4A; yellow cluster). NRF2
regulates genes that protect cells against oxidative stress, which
is significant in cancer. The primary regulator of NRF2 activity is
its interaction with Kelch-like ECH-associated protein 1 (Keap1).
Under normal conditions, Keap1 binds to NRF2, promoting its
degradation, but oxidative stress disrupts this interaction,
allowing NRF2 to activate protective genes (23). For instance, during the
carcinogenesis of GC (24),
oxidative stress promotes the transcription of genes that protect
against oxidative and electrophilic stress (25–27).
In addition, NRF2 expression is associated with tumor-associated
macrophages (TAMs) M2 polarization, which is well-known for
exerting a pro-tumorigenic effect. TAMs are critical in the tumor
microenvironment (TME) for eliminating tumor cells by creating a
toxic environment. Polarization is linked to the NRF2 target
protein Cu/Zn-superoxide dismutase, associated with M2 polarization
through a redox-sensitive mechanism. Oxidative stress and reactive
oxygen species are vital for M2 macrophage activation, promoting an
immunosuppressive TME in GC (28–30).
Therefore, pharmacological activation of NRF2 is a promising
therapeutic approach for chronic diseases underlined by oxidative
stress and inflammation (31)
(Fig. 4A; yellow cluster).
Analysis of the KLHL4 expression in
GC
Based on the TCGA-STAD data, EO-GC cases exhibited a
higher KLHL4 gene expression than L-GC (Fig. 4Ba), principally in the 21–40-year
age range (Fig. 4Bb). Concerning
the methylation KLHL4 score, no differences between EO-GC and L-GC
were observed (Fig. 4Ca), even when
subjects were categorized by age (Fig.
4Cb). Lastly, the Kaplan-Meier analysis, using the median KLHL4
gene expression showed a non-significant tendency where a high
expression of KLHL4 would be associated with lower survival of
patients with EO-GC from the TCGA-STAD dataset (Fig. 4D). The expression data for KLHL4 in
Fig. 4 highlight its significantly
higher expression in EO-GC compared to L-GC. This observation
aligns with the Kaplan-Meier survival analysis, which, although not
statistically significant, suggests a trend where higher KLHL4
expression is associated with poorer survival.
In summary, the upregulation of KLHL4 in EO-GC
suggests its potential involvement in enhancing intracellular
transport and oxidative stress responses, both of which may
contribute to the aggressive clinical phenotype observed in these
patients. These processes likely shape the TME by supporting immune
evasion and promoting tumor cell survival, thereby linking
increased KLHL4 expression to poorer prognosis.
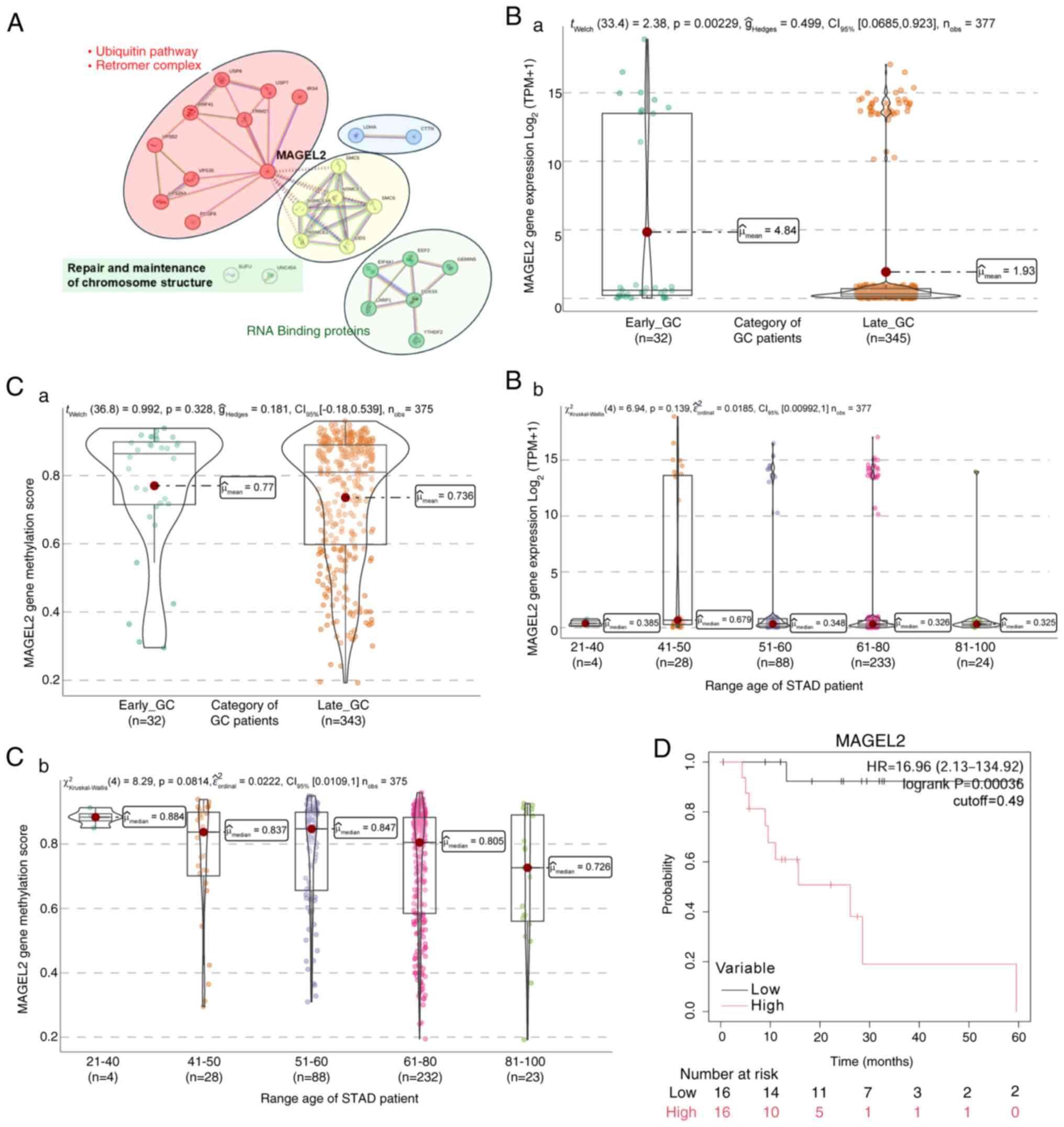
MAGEL2 gene expression in GC
Following the identification of KLHL4, MAGEL2,
another gene found to be upregulated in EO-GC, was next examined.
MAGEL2 acts as a tissue-specific regulator of the
retromer-dependent endosomal protein recycling pathway, important
for secretory granule formation and maturation (32). The retromer complex, composed of
VPS26, VPS29 and VPS35, facilitates the recycling of proteins from
the endocytic pathway back to the plasma membrane and is critical
in secretion regulation (33).
In addition to its role in the endosomal recycling
pathway, MAGEL2 is involved in ubiquitination, interacting with and
stimulating E3 RING ubiquitin ligases (34,35).
This interaction highlights its significant role in ubiquitin
processes.
Data mining of the TCGA-STAD platform revealed an
overexpression of MAGEL2 in GC, with associations to the retromer
multimeric protein complex and ubiquitination system (Fig. 5A; red cluster).
MAGEL2 additionally exhibits interactions with the
structural maintenance of chromosome (SMC) protein complexes, which
play crucial roles in chromatin structure reorganization,
chromosome segregation and DNA repair. The interaction between
SMC5-SMC6 proteins and MAGEL2, identified in the TCGA-STAD
database, suggests a potential role in DNA double-strand break
repair through homologous recombination in patients with GC
(36) (Fig. 5A; yellow cluster). Further
protein-protein association networks and functional enrichment
analyses using the Search Tool for the Retrieval of Interacting
Genes and proteins (STRING; http://string-db.org/) analysis revealed that MAGEL2
interacts with various transcription factors and RNA-binding
proteins, particularly those involved in mRNA metabolic processes.
Notably, MAGEL2 co-immunoprecipitates with YTHDF2, reducing its
nuclear accumulation after heat shock (37) (Fig.
5A; green cluster).
Analysis of MAGEL2 expression in
GC
Based on the TCGA-STAD data, EO-GC cases exhibited
significantly higher MAGEL2 gene expression than L-GC (Fig. 5Ba); this difference was not as
evident when patients were categorized by age (Fig. 5Bb). Concerning the methylation score
of MAGEL2, no statistically significant differences were observed
between EO-GC and L-GC (Fig. 5Ca);
however, a non-significant decreasing trend in methylation levels
was noted with increasing patient age (Fig. 5Cb). Lastly, the Kaplan-Meier
survival analysis demonstrated that higher MAGEL2 gene expression
is associated with low survival in EO-GC (Fig. 5D). This comprehensive analysis
underscores the importance of MAGEL2 in EO-GC, highlighting its
overexpression and involvement in critical cellular pathways and
carcinogenic processes.
The expression patterns of MAGEL2 in Fig. 5 reveal its upregulation in EO-GC,
particularly among younger age groups. The Kaplan-Meier analysis
indicated that elevated MAGEL2 expression is associated with
unfavorable survival outcomes. This suggests that MAGEL2′s role in
protein recycling and chromatin remodeling may contribute to tumor
progression and poor prognosis in patients with EO-GC.
In summary, the overexpression of MAGEL2 in EO-GC
may exacerbate disruptions in protein recycling and chromatin
structure, leading to cellular dysfunctions that support cancer
progression. Its role in endosomal recycling and ubiquitination
highlights its potential as a mediator of poor prognosis through
tumor-promoting pathways.
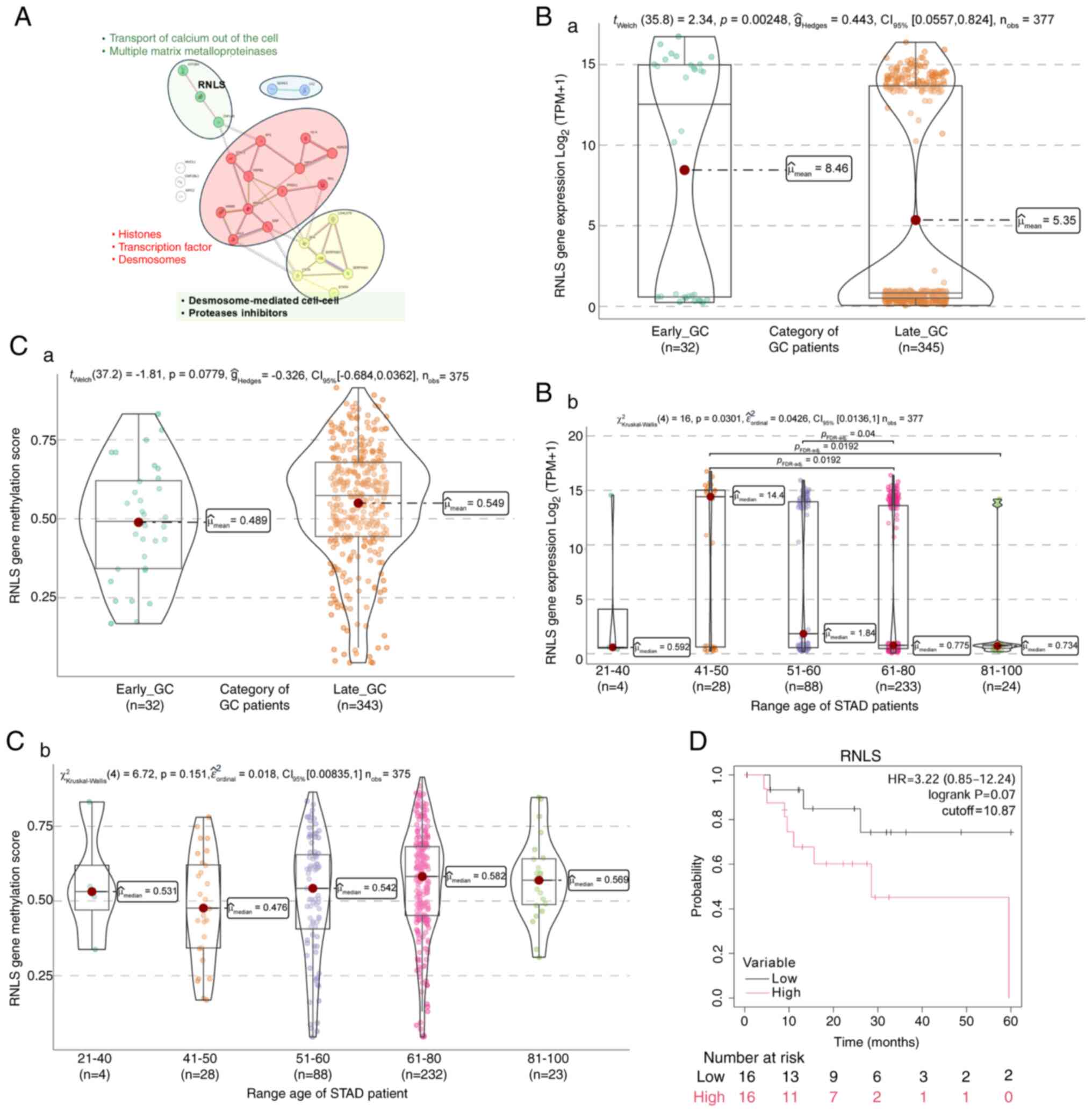
RNLS gene expression in GC
Data mining of KLHL4 and MAGEL2 revealed an
association with RNLS, a gene involved in oxidative stress and
immune regulation. RNLS is an FAD-dependent amine oxidase that
metabolizes water-soluble vitamins and nicotine. This enzymatic
hormone, secreted by the kidneys and circulating in the
bloodstream, oxidizes the less abundant forms of
1,2-dihydro-beta-NAD(P) and 1,6-dihydro-beta-NAD(P) to beta-NAD(P)
(+) (38,39). RNLS impacts various cell types,
suggesting similar roles in cancer. Its transcript levels are
increased in pancreatic cancer, melanoma and other malignancies. In
these cancers, higher RNLS levels are associated with shorter
survival (40). Treatment with
anti-RNLS antibodies at the single-cell level resulted in increased
tumor density of macrophages, neutrophils and lymphocytes and
increased expression of IFN-γ and granzyme B in natural killer
cells and T cells in murine melanoma models (41). The presence of RNLS in both cancer
and immune cells suggests that multiple cell types may contribute
to the effects of RNLS on cancer cell growth (42,43).
STRING analysis, as a functional protein association
network, showed a remarkable association between RNLS and divalent
cation transporting channels, such as ATPase plasma membrane
Ca2+ transporting 4 (ATP2B4) (Fig. 6A; green cluster), which facilitates
divalent cation transport, and ATP4B, which is crucial for gastric
acid secretion (44). RNLS also has
a significant relationship with zinc finger protein 148 (ZNF148), a
member of the Kruppel family of zinc finger DNA-binding proteins
(Fig. 6A; green cluster). Increased
ZNF148 expression has been linked to lower survival in colorectal
cancer. ZNF148 influences the expression of multiple matrix
metalloproteinases, which have protective and damaging effects
during inflammation and are crucial for health maintenance
(45–48).
ZNF148 protein directly engages with two
transcription factors, STAT3 and SP1, which control gene
transcription. It also interacts with several histone-coding genes,
including H1-4, H2AC8 and H4C6. Furthermore, ZNF148 is associated
with genes that contribute to desmosome formation, such as
desmoplakin, filaggrin, hornerin and Annexin A2 (ANXA2). ANXA2
encodes a member of the annexin family, which includes
calcium-dependent phospholipid-binding proteins that play roles in
cellular growth regulation and signal transduction pathways
(Fig. 6A; red cluster).
Another group of genes related to RNLS (Fig. 6A; yellow cluster) includes proteins
like galectin 7B (LGALS7B), which are involved in cell-cell and
cell-matrix interactions necessary for normal growth control.
LGALS7B has a tumor-suppressive function, with gene down-regulation
in GC (49). Stratifin is an
adapter protein regulating general and specialized signaling
pathways, playing a significant role in cell proliferation and
metastasis in GC (50,51).
Analysis of RNLS expression in GC
Based on the TCGA-STAD data, EO-GC cases exhibited a
higher RNLS gene expression than L-GC (Fig. 6Ba), principally in the 41–50-year
age range (Fig. 6Bb). Concerning
the methylation score of RNLS, an insignificant increase in the
methylation score was found in L-GC (Fig. 6Ca), but certain changes in
methylation levels were observed in the 41–50-year age range
(Fig. 6Cb). Lastly, the
Kaplan-Meier survival analysis demonstrated that higher RNLS gene
expression showed an insignificant trend toward lower overall
survival in patients with EO-GC (Fig.
6D).
Fig. 6 demonstrates
the increased expression of RNLS in EO-GC, particularly in the
41–50-year age group. The Kaplan-Meier analysis further shows a
trend where higher RNLS expression is linked to reduced survival,
emphasizing its potential involvement in oxidative stress
regulation and immune evasion, key factors in EO-GC
progression.
In summary, the increased expression of RNLS in
EO-GC highlights its role in oxidative stress regulation and immune
modulation. These functions may contribute to the establishment of
an immunosuppressive TME, promoting cancer cell survival and
explaining its association with poor prognosis.
CYP8B1 gene expression in GC
In addition to KLHL4, MAGEL2 and RNLS, cytochrome
P450 family 8 subfamily B member 1 (CYP8B1) was also identified to
be upregulated in EO-GC. This gene's role in bile acid metabolism
and steroid hormone synthesis suggests a potential connection
between metabolic reprogramming and EO-GC progression.
CYP8B1 plays a crucial role in metabolic pathways
such as steroid hormone synthesis, bile acid metabolism,
cholesterol metabolism and lipid homeostasis. In steroid hormone
synthesis, CYP8B1 collaborates with enzymes such as
hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid
delta-isomerase 1,2,7 (HSD3B1, HSB3B2 and HSB3B7) (52,53)
(Fig. 7A; yellow cluster). L-GC has
been linked to androgens, estrogens, progesterone, their receptors
and related signals (54). Cellular
responses to steroid hormones, including ESR1, ESR2 and AR, are
facilitated by hormone-receptor binding. ESR1 has been implicated
in the cancer-promoting effects of estrogen in various cancers,
including breast, colon, prostate and gastric cells. However, the
association between these receptors and GC has yielded inconsistent
results in several studies (55–57).
Additionally, the CYP8B1 pathway produces bile
acids, which serve as potent signaling molecules that influence
various metabolic processes, such as lipid homeostasis, glucose
regulation and microbiota composition (Fig. 7A; red cluster). These bile acids are
synthesized from cholesterol in the liver through the action of key
enzymes, including CYP8B1 and CYP7A1, which are transcriptionally
regulated by NR1H4 (nuclear receptor subfamily 1 group H member 4),
a nuclear receptor also known as the bile acid receptor. Lastly,
research has demonstrated a correlation between the presence of
gastric intestinal metaplasia and an increased risk of gastric
cancer, particularly for the intestinal subtype, which follows a
well-established carcinogenic cascade (58). A retrospective study showed that
high levels of bile acids in the stomach were associated with a
higher incidence of GC (59,60).
Analysis with STRING revealed a direct relationship
between CYP8B1 and hepatocyte nuclear factor 4α (HNF4α) involving
bile acid homeostasis (Fig. 7A;
blue circle). HNF4α is a transcription factor that binds DNA as a
homodimer and regulates genes preferentially expressed in the
liver. It plays a central role in bile acid homeostasis by
controlling genes involved in bile acid biosynthesis, including
hydroxylation and beta-oxidation of the cholesterol side chain
in vivo (61).
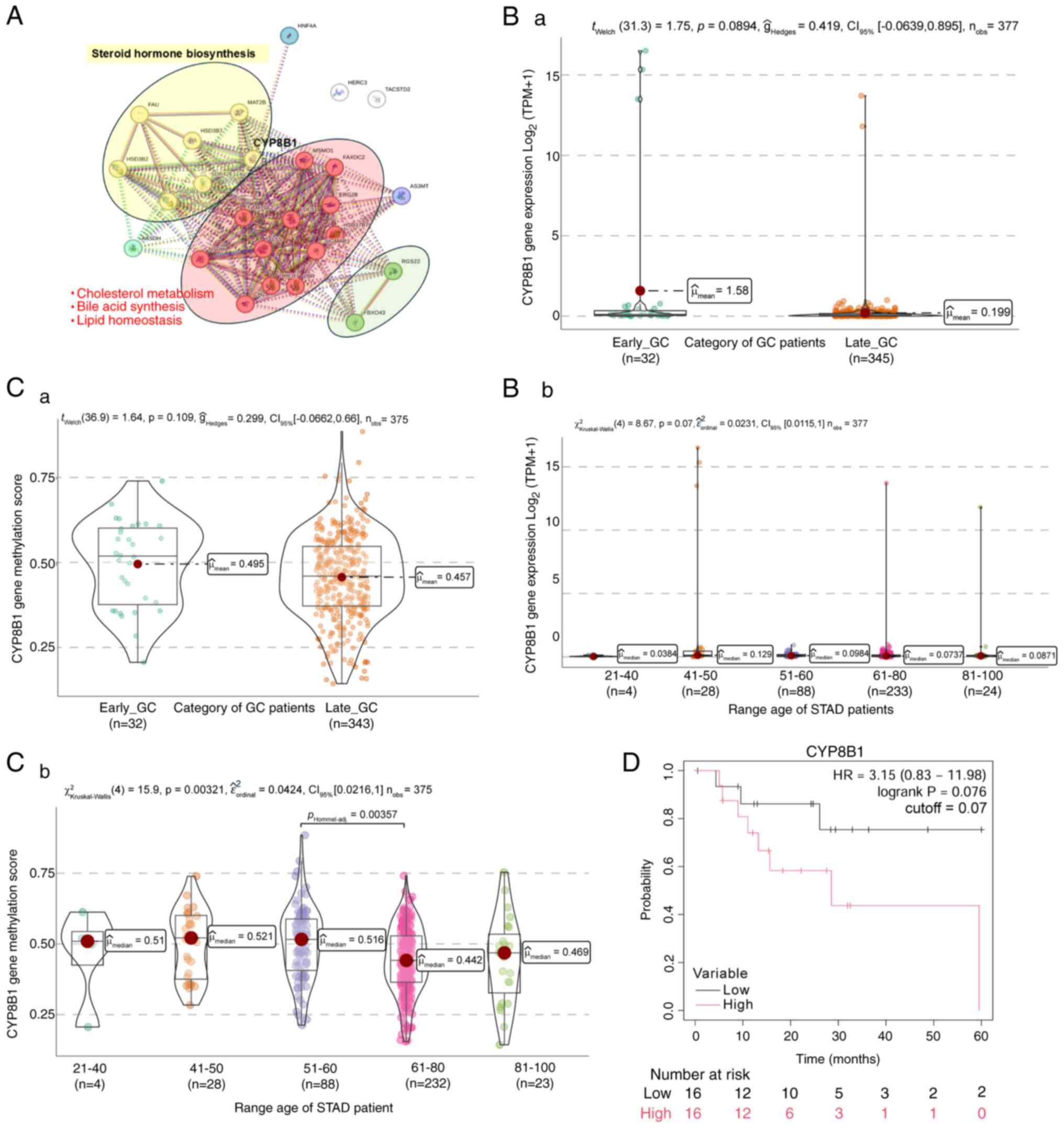
Analysis of CYP8B1 expression in
GC
Using TCGA-STAD data, it was verified that CYP8B1
transcript expression in EO-GC and L-GC individuals did not exhibit
any statistically significant differences (Fig. 7Ba), nor was any change observed when
patients were categorized by age (Fig.
7Bb). The methylation levels of the gene did not show any
significant differences between both groups of patients (Fig. 7Ca). Subsequently, when subjects were
categorized into different age groups, a statistically significant
difference in the CYP8B1 methylation score between the 51–60-year
age range and the 61–80-year age range was found (Fig. 7Cb). Lastly, Kaplan-Meier survival
analysis revealed a non-significant trend toward better overall
survival in patients with EO-GC with lower CYP8B1 gene expression
(Fig. 7D).
The data in Fig. 7
show no significant differences in CYP8B1 expression between EO-GC
and L-GC. However, the Kaplan-Meier analysis indicated a trend
where lower CYP8B1 expression is associated with better survival
outcomes. This finding highlights its potential role in EO-GC
pathogenesis.
In summary, the role of CYP8B1 in bile acid
metabolism and lipid homeostasis suggests that its upregulation in
EO-GC could contribute to metabolic reprogramming in tumor cells.
By influencing bile acid signaling and microbiota composition,
CYP8B1 may drive gastric carcinogenesis and affect patient
outcomes.
CLDN6, MIOX, PNAM5 and ACTL8 are
downregulated genes in EO-GC
CLDN6 gene expression in GC
Tight junctions (TJ) are critical for the
functioning of epithelial and endothelial cells, maintaining cell
polarity, adhesion and permeability. Reduced TJ integrity leads to
increased tissue permeability, a characteristic of tumors and
inflamed tissues. During the initial stage of tumor metastasis, the
disconnection between tumor and endothelial cells makes the TJ the
first barrier cancer cells must overcome in metastasis (62).
TJs comprise three essential membrane proteins:
Occludin, claudin and junctional adhesion molecules. The CLDN
family is vital for TJ functions, including regulating defense and
barrier functions, differentiation and polarity in epithelial and
endothelial cells (Fig. 8A; blue
cluster). The loss of CLDNs contributes to the disruption of cell
junctions in a tissue-dependent manner and plays an essential role
in cancer cell migration, invasion and metastasis (63). The distribution patterns of various
claudins in GC differ between tumor tissue and adjacent tissue
(64–66). Specifically, CLDN6 expression is
higher in GC tissues than in adjacent tissues (67). However, certain studies suggest that
lower levels of CLDN6 expression in GC tissues compared to adjacent
tissues are associated with factors such as age, lymph node
metastasis, pathological stage and tumor metastasis. Furthermore,
several studies have reported that the upregulation of CLDN6
expression is associated with decreased survival rates in GC
(68–71). Known for its role in TJ integrity,
its reduced expression may contribute to increased tissue
permeability and metastasis in EO-GC.
Analysis of protein interactions using STRING
revealed an association between CLDN6 and proteins implicated in
mesenchymal cell proliferation (72) (Fig.
8A; green cluster). Another group of proteins interacting with
CLDN6 includes mitofusin proteins and mitochondrial outer membrane
GTPases mediating mitochondrial clustering and fusion (Fig. 8A; red cluster). CLDN6 interacted
with dynamins (DNMs), which catalyze the hydrolysis of GTP and
utilize this energy to mediate vesicle scission (Fig. 8A; green cluster). These proteins
participate in various forms of endocytosis, including
clathrin-mediated synaptic vesicle and rapid endocytosis (73). Also, DNM2 is part of the machinery
responsible for vesicle formation and regulates the cytoskeleton,
facilitating intracellular vesicle transport (74).
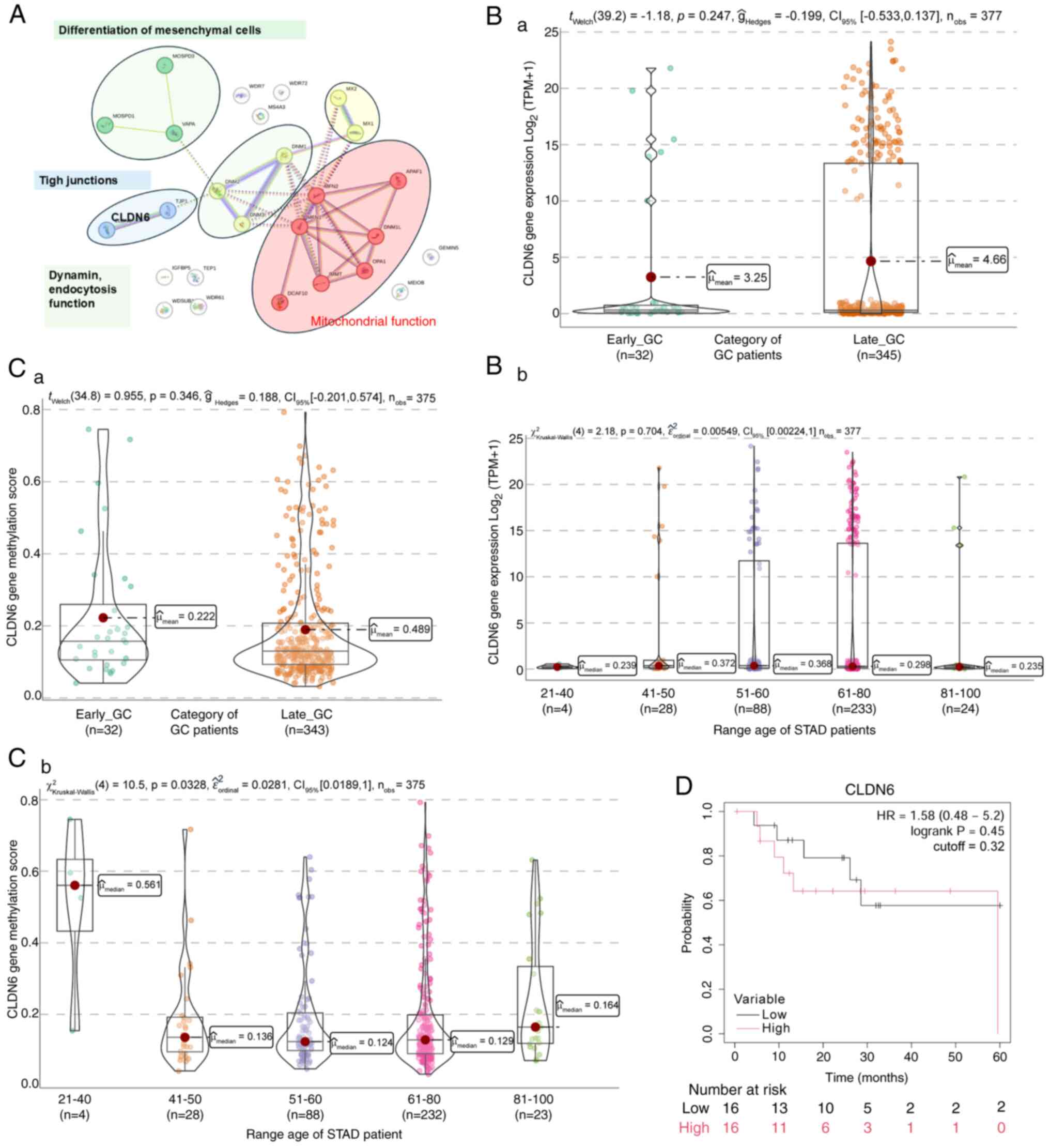
Analysis of CLDN6 expression in
GC
Based on the TCGA-STAD data, no general differences
in CLDN6 gene expression were seen between EO-GC and L-GC (Fig. 8Ba); there was also no significant
difference in protein levels when patients were categorized by age
(Fig. 8Bb). In terms of the gene
methylation levels, no significant changes were observed when
comparing both groups of patients (Fig.
8Ca). However, the 21–40-year age range exhibited a
significantly higher methylation score than the other age ranges
(Fig. 8Cb). Kaplan-Meier survival
analysis did not indicate any survival differences between EO-GC
and L-GC according to the median CLDN6 expression (Fig. 8D).
In summary, the downregulation of CLDN6 in EO-GC
suggests a weakening of TJ integrity, potentially facilitating
cancer cell invasion and metastasis. This highlights the biological
importance of CLDN6 in maintaining epithelial barriers and its
potential role as a prognostic biomarker in EO-GC.
MIOX gene expression in GC
Following the identification of CLDN6, MIOX gene
expression was next examined. The initial committed step in
mammalian inositol catabolism is catalyzed by MIOX, which performs
the unique four-electron dioxygen-dependent ring cleavage of
myo-inositol to D-glucuronate. This enzyme facilitates the binding
of ferric iron and inositol oxygenase activity, playing a
significant role in the inositol catabolic process, primarily
located in the cytoplasm and inclusion bodies (75).
STRING analysis involving the MIOX gene revealed a
direct relationship with uridine
5′-diphosphate-glucuronosyltransferase (UGT) genes (Fig. 9A; red cluster), which are membrane
proteins of the endoplasmic reticulum expressed in a
tissue-specific manner. MIOX has been identified as a regulatory
gene of tumor ferroptosis in several cancer types, such as clear
cell renal cell carcinoma (ccRCC). In ccRCC, a significant
downregulation of MIOX in tumor tissues relative to adjacent renal
tissues has been observed, with a negative correlation between MIOX
expression levels in ccRCC tissues and the malignant behavior, as
well as poor prognosis of ccRCC (76).
Additionally, MIOX has been implicated in bladder
(77) and prostate cancer
progression (78) and lung squamous
cell carcinoma, where it is part of a gene signature indicative of
the connection with glycolysis (79). Furthermore, it has been established
that certain gene isotypes of the UGT family have differential
expressions between normal and tumor stomach tissue, whose
expression changes would affect the progression of GC (80). However, no relationship between MIOX
and GC, particularly EO-GC, has been reported.
STRING analysis identified a gene cluster, including
the ABCG2 transporter and two UGT genes (Fig. 9A; green cluster). Additionally,
relationships were observed with genes involved in ATP-dependent
ABC-type transporters (Fig. 9A;
yellow cluster). A connection was also established between two
genes related to E3 ubiquitin ligases (Fig. 9A; blue cluster).
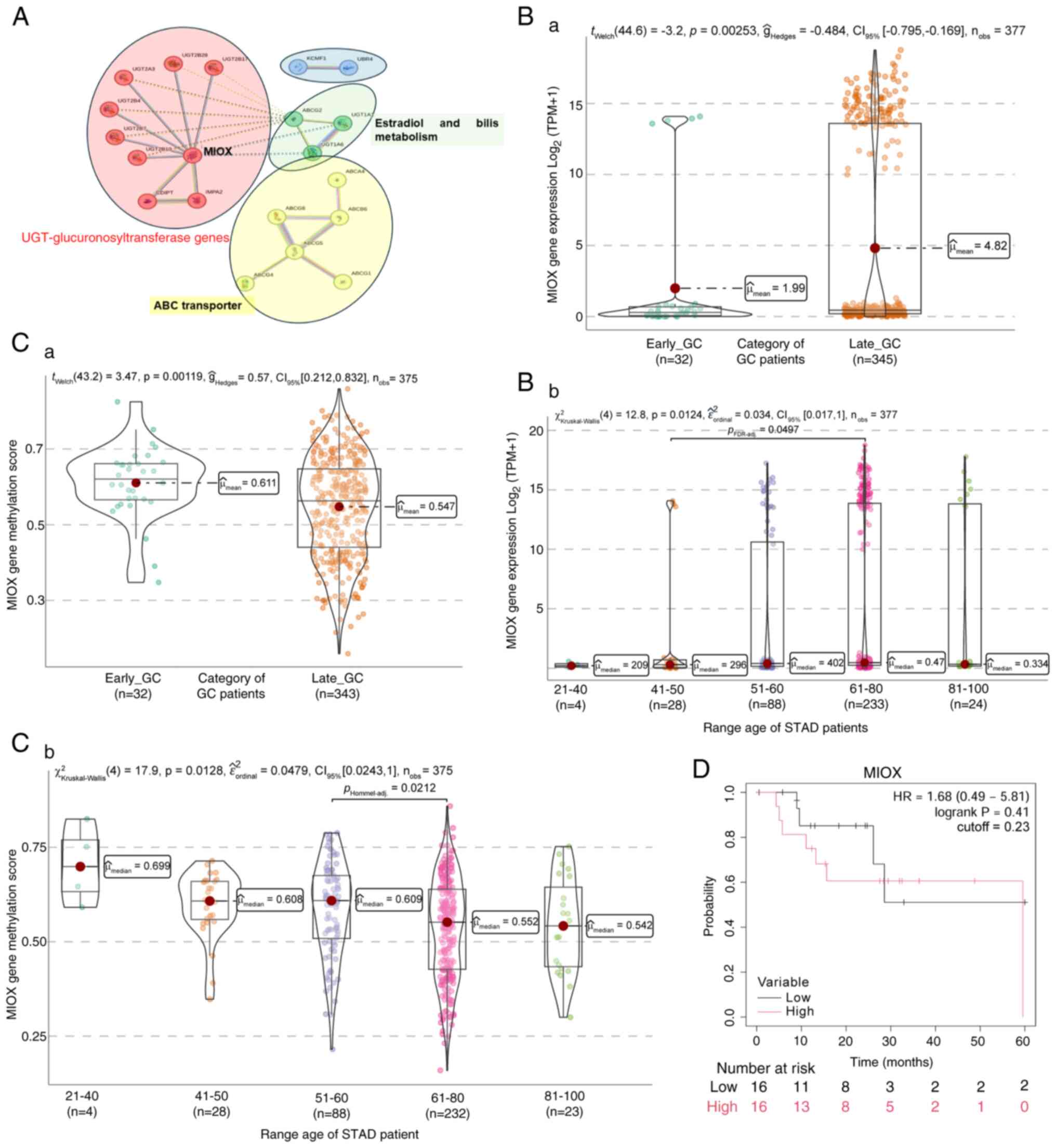
Analysis of MIOX expression in GC
Based on the TCGA-STAD dataset, it was found that
EO-GC exhibited a significantly lower MIOX gene expression and a
relative increase in gene expression in L-GC (Fig. 9Ba); this was confirmed by an
increase in transcripts in older age groups, between 51 and 100
years (Fig. 9Bb). Furthermore,
EO-GC displayed a higher methylation score than L-GC (Fig. 9Ca), particularly in the 21–40-year
age range (Fig. 9Cb). Kaplan-Meier
survival analysis did not indicate any association of MIOX
expression with survival in patients with EO-GC using the median
MIOX expression level as a cut-off (Fig. 9D).
The lower expression of MIOX in EO-GC (Fig. 9) suggests that an alteration in
inositol metabolism may contribute to tumor progression. While
Kaplan-Meier analysis did not show a significant difference in
survival, the methylation changes observed in EO-GC warrant further
studies on the role of MIOX in metabolic reprogramming and the
regulation of ferroptosis. In summary, the significant
downregulation of MIOX in EO-GC suggests a disruption in inositol
metabolism, potentially impairing ferroptosis-a cell death pathway
crucial for tumor suppression. This alteration may create metabolic
vulnerabilities that cancer cells exploit for progression.
PNMA5 gene expression in GC
PNMA5 gene, also downregulated in EO-GC, has been
implicated in apoptosis and cancer metastasis. The PNMA family
members have been identified as onconeural antigens exhibiting
aberrant expression in cancer cells in patients with paraneoplastic
disorders. This protein family is closely associated with
autoimmunity, neurodegeneration and cancer, with several PNMA
family members characterized by their involvement in apoptosis and
cancer-related signaling pathways (81). Studies have shown that PNMA5 is
deregulated in patients with CRC; it contributes to CRC metastasis
by potentially facilitating cancer cell migration and invasion.
Cellular markers related to epithelial-mesenchymal transition (EMT)
revealed that PNMA5 promotes EMT in CRC, promoting cell migration
and invasion (82,83). Given that metastases are more
detrimental to cancer-associated mortality than primary tumors,
understanding the role of PNMA5 in EMT and metastasis in GC is
crucial for developing targeted therapies.
In particular, PNMA5 is associated with ZBTB8A,
which facilitates DNA-binding activity specific to RNA polymerase
II transcription regulatory regions, potentially playing a role in
transcriptional regulation. Another noteworthy gene is ARPC4,
identified as a potential biomarker or drug target in metastatic GC
(Fig. 10A; green cluster). In
addition, a group of PNMA5-related genes is involved in DNA
replication, single-strand DNA binding, repair and homologous
recombination (Fig. 10A; red
cluster). Two smaller clusters relate to genes regulating
transcription (Fig. 10A; blue
cluster). Furthermore, PNMA5 is related to genes that initiate
transcription, such as GTF3C1 (Fig.
10A; yellow cluster), which activates polymerases to initiate
gene transcription (84–86).
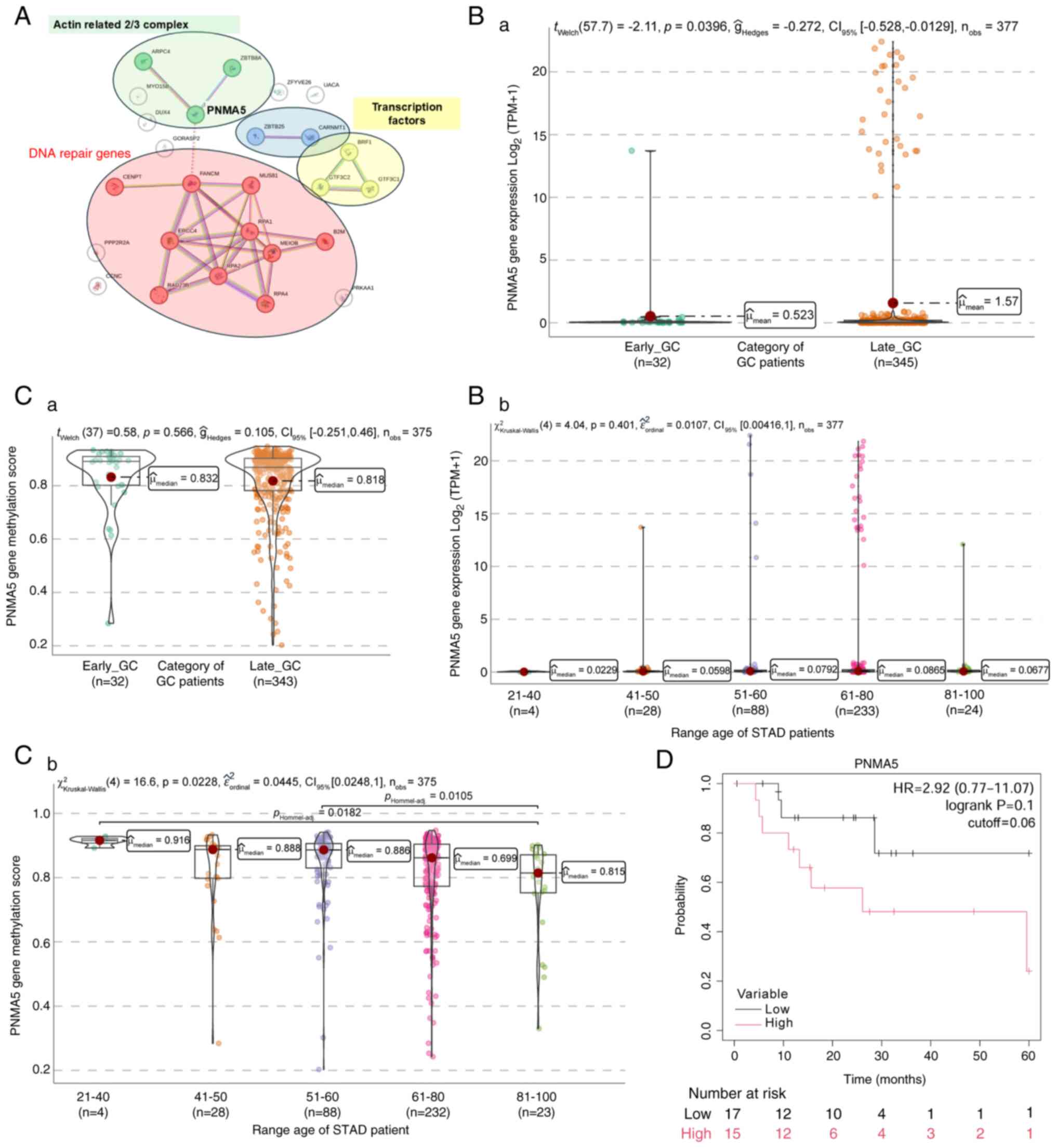
Analysis of PNMA5 expression in
GC
Based on the TCGA-STAD dataset, it was found that
EO-GC exhibited a lower PNMA5 gene expression than L-GC (Fig. 10Ba). A tendency toward increased
transcript levels was observed in older age groups, particularly
between 51 and 80 years of age, although no significant differences
were detected across groups (Fig.
10Bb). Gene methylation levels did not show any significant
differences when only the two groups of EO-GC vs. L-GC were
compared (Fig. 10Ca). Regarding
methylation, EO-GC displayed a higher PNMA5 methylation score,
especially in the 21–40-year age group. While differences between
several age groups were observed (Fig.
10Cb). Kaplan-Meier survival analysis indicated a
non-significant trend toward lower survival among EO-GC patients
with higher PNMA5 gene expression (Fig. 10D).
Based on the TCGA-STAD dataset, PNMA5 gene
expression was significantly lower in EO-GC compared to L-GC.
However, when patients were stratified into age groups, this
difference was not statistically significant, indicating that the
observed expression difference is more clearly captured when using
a binary classification (EO-GC vs. L-GC) rather than categorical
age groupings. Kaplan-Meier survival analysis showed a tendency for
poorer outcomes in patients with higher PNMA5 expression,
suggesting its potential involvement in EMT and cancer
metastasis.
In summary, the downregulation of PNMA5 in EO-GC may
reflect alterations in apoptosis and EMT, processes critical for
cancer metastasis. Its association with EMT markers highlights its
potential role in driving invasive behavior and poor prognosis in
patients with EO-GC.
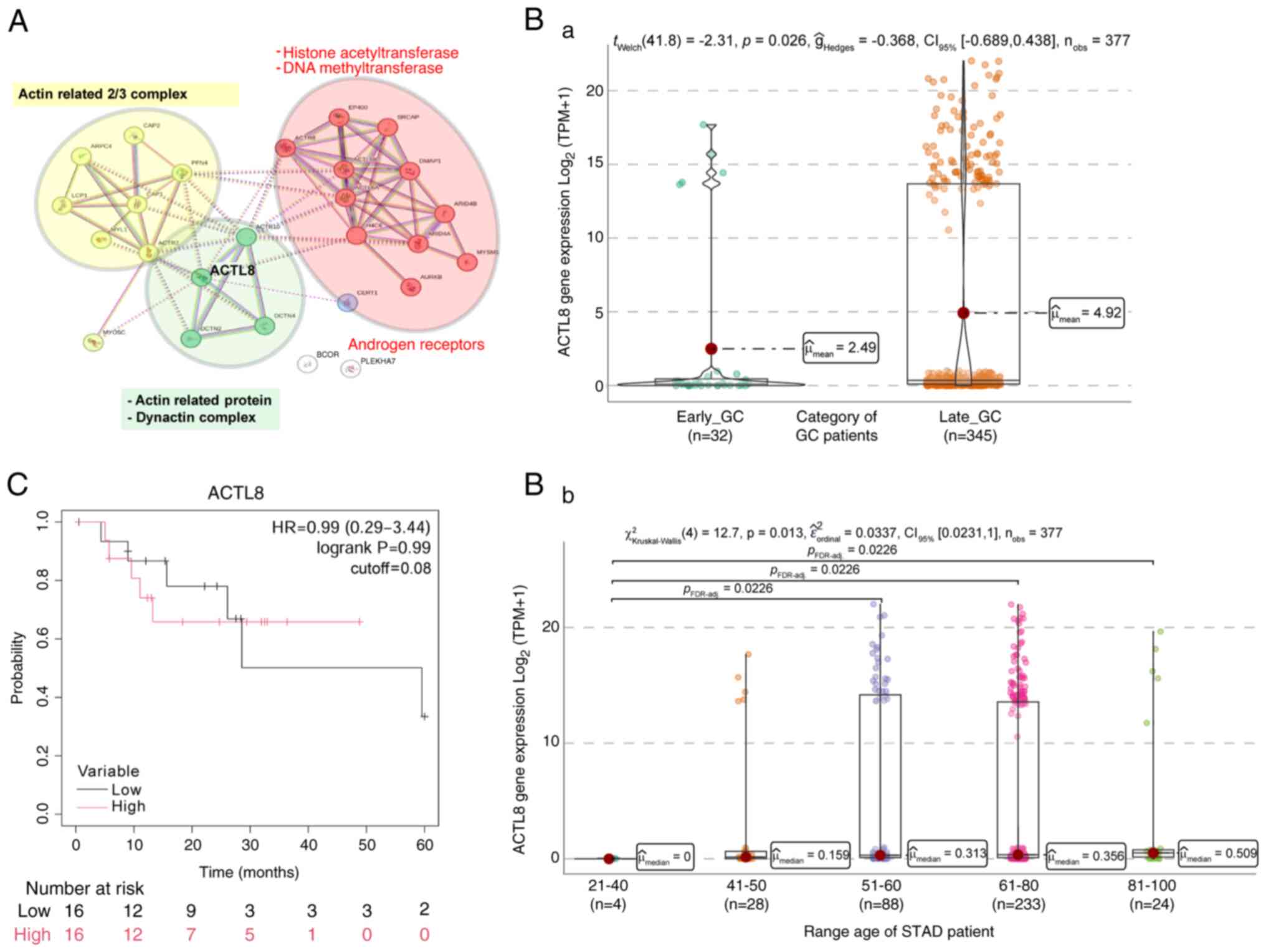
ACTL8 gene expression in GC
Lastly, ACTL8 was also down-regulated in EO-GC
compared to L-GC cases. ACTL8 has been implicated in the
differentiation of epithelial cells and is thought to be located
within the cytoplasm of the dynactin complex, facilitating the
activation of the dynein molecular motor for ultra-processive
transport along microtubules (Fig.
11A; green cluster). The dynactin complex, a critical component
of the ARP2/3 complex, is crucial for cell shape and movement by
forming actin filaments on the lamellipodial cell surface (87). Furthermore, the ARP2/3 complex plays
a role in the cytoplasmic cytoskeleton by promoting actin
polymerization in the nucleus, which regulates gene transcription
and DNA repair (88).
The relationship between this cluster network and
histone-related genes, including EP400, H4C6 and MYSM1, is
noteworthy (Fig. 11A; red
cluster). These genes involve essential processes, such as
chromatin remodeling (89).
Furthermore, high ACTL8 expression has been associated with poor
prognosis in head and neck cancer (90) and metastasis in CRC (91).
Analysis of ACTL8 expression in
GC
Based on the TCGA-STAD data, it was found that
EO-GC exhibited a lower PNMA5 gene expression as compared with L-GC
(Fig. 11Ba), particularly in the
21–40-year age range (Fig. 11Bb).
Unfortunately, no methylation data for ACTL8 in GC were found.
Lastly, Kaplan-Meier survival analysis based on the median ACTL8
gene expression did not indicate any significant influence of ACTL8
on survival outcomes in EO-GC cases (Fig. 11C).
The expression data for ACTL8 in Fig. 11 highlight its downregulation in
EO-GC compared to L-GC, particularly in younger patients. Although
the Kaplan-Meier analysis did not reveal any significant survival
association, ACTL8′s role in cytoskeletal organization and
chromatin remodeling suggests its potential importance in EO-GC
pathogenesis.
In summary, the downregulation of ACTL8 in EO-GC
suggests impaired cytoskeletal dynamics and chromatin remodeling.
These disruptions may hinder normal cell differentiation while
facilitating cancer cell motility, contributing to EO-GC
progression.
Discussion
Various attempts have been made to characterize the
pathological characteristics of EO-GC in different regions,
including Colombia (92), China
(93) and Japan (94). Additionally, studies have reported
on the treatment patterns of patients with EO-GC based on the
Surveillance, Epidemiology and End Results database and their
impact on patient survival (1,95,96).
Proteogenomics analyses have been published to
provide additional information beyond genomic analyses, thereby
improving the understanding of cancer biology in patients with
EO-GC (97). More epidemiological
research and knowledge of the clinicopathological characteristics
and mechanisms are urgently required to better understand this
emerging situation affecting the young population (5,98).
This understanding is crucial for developing preventive strategies
and early detection methods tailored to this emerging group.
Considering the context, identifying key genes and
pathways involved in EO-GC remains a complex and challenging task.
Numerous potential modifications may trigger carcinogenic activity
in these genes, with many overlapping pathways and unclear mutation
patterns. Consequently, the current scientific priority is to
pinpoint the essential genes and pathways, comprehend the interplay
between these modifications and devise strategies to prevent their
occurrence (6,99).
The intensive data mining performed in the present
study identified KLHL4, MAGEL2, RNLS and CYP8B1 as upregulated
genes. These genes are involved in metabolic pathways that pertain
to protein ubiquitination and histone regulation. Of note, the
particularly significant pathways are very long fatty acid
synthesis, cholesterol metabolism, steroid hormone production and
their receptors. Furthermore, these pathways contribute to the
production of bile acids, which have been implicated in promoting
intestinal metaplasia and gastric carcinogenesis (60).
Our comprehensive search for gene expression and
methylation data in patients with EO-GC and L-GC revealed several
notable findings concerning upregulated genes in EO-GC; first,
KLHL4 expression may be particularly high in younger individuals
with EO-GC. High KLHL4 expression may also be associated with lower
survival in patients with EO-GC. Second, high MAGEL2 expression was
found mainly in the 41–50-year age range and was linked to lower
survival in patients with EO-GC. Third, RNLS expression was higher
in EO-G, particularly in the 41–50-year age range, like MAGEL2.
Furthermore, Kaplan-Meier analysis indicated a tendency for higher
survival in patients with low RNLS expression. Fourth, CYP8B1
expression showed no significant differences between patients with
EO-GC and L-GC. Nevertheless, Kaplan-Meier analysis suggested that
lower CYP8B1 expression may be associated with better survival
outcomes.
In terms of downregulated genes in EO-GC, the
following may be summarized: First, the 21–40-year age range
featured significantly lower CLDN6 expression and a tendency
towards higher methylation scores. Kaplan-Meier analysis did not
indicate any influence of CLDN6 expression on the survival of
patients with EO-GC. Studies that have explored the relationship
between CLDN6 and prognosis in GC show diverse results. For
instance, a study indicated that high transcriptomic expression of
CLDN6 was associated with a better survival rate (100), but according to another study,
high protein expression was associated with lower survival
(70). Furthermore, the CLDN6 gene
has even been proposed as a TME prognostic marker in GC (101). Second, EO-GC exhibited
significantly lower MIOX expression and higher methylation scores,
particularly in the 21–40-year age range. Kaplan-Meier analysis did
not correlate MIOX expression with survival outcomes in patients
with EO-GC. Third, EO-GC cases had lower PNMA5 expression,
particularly in the 21–40-year age range, which also showed higher
methylation scores. Kaplan-Meier analysis indicated a trend where
high PNMA5 expression may be associated with lower survival in
EO-GC cases. Fourth, EO-GC exhibited lower ACTL8 expression than
L-GC, particularly in the 21–40-year age range. No methylation data
was available for ACTL8. Kaplan-Meier analysis did not link median
ACTL8 expression with survival outcomes in EO-GC cases.
A detailed description of the gene expression and
methylation status of genes involved in GC highlights the complex
interplay between genetic and epigenetic factors and the effects on
the onset and progression of EO-GC. Overall, it was verified that
genes overexpressed in EO-GC exhibit bimodal expression patterns
and can be overexpressed even in young individuals (aged 21–40
years). In addition, it was discovered that the influences of
mutations in EO-GC are mainly described in downregulated genes.
Understanding these genetic landscapes is vital for developing
targeted therapies and enhancing patient prognosis in GC.
Although numerous unfilled gaps are inherent in
data mining approaches, it raises some important questions and
sheds light on previously unexplored areas of GC. It is essential
to acknowledge certain limitations of the present study, such as
the reliance on a single database (TCGA-STAD) and the relatively
low representation of EO-GC cases compared to L-GC. Nonetheless,
this type of data mining may provide a fertile field for future
studies on tumor progression and survival associations of EO-GC in
relation to these genes. Furthermore, no in vitro assays or
preclinical models are currently available that have evaluated the
expression of these genes and their methylation profiles in GC.
This study highlights the need for a deeper
understanding of the molecular pathways involved in EO-GC to
identify novel therapeutic targets and strategies. While the
identification of molecular targets, such as KLHL4, MAGEL2 and
RNLS, offers exciting prospects, the translational pathway from
molecular discovery to therapeutic application requires additional
investigation. Existing therapeutic approaches for GC focus on
chemotherapeutic agents, immunotherapies and molecularly targeted
treatments, such as trastuzumab and immune checkpoint inhibitors,
which have shown promise in advanced cases (102,103). In parallel, there is growing
interest in exploring alternative therapeutic approaches, including
natural compounds with anti-tumor properties (104,105). For instance, Rabdosia
rubescens has demonstrated potential anti-cancer effects
through its phytochemical constituents, offering a complementary
avenue for therapy (104).
Although the current study does not focus on
therapeutic interventions, the identified genes and pathways
provide a foundation for exploring novel therapeutic targets.
Integrating these molecular insights with established and emerging
treatments, including natural compounds, may lead to more
personalized and effective strategies for EO-GC management.
The integration of bioinformatics tools in cancer
research allows for the identification of potential biomarkers and
therapeutic targets (106).
Therefore, based on the present data mining, the development of a
gene panel that includes the identified upregulated and
downregulated genes may be proposed, along with their methylation
profiles focused on the TME of GC categorized according to the age
of the patients. This comprehensive panel could enhance EO-GC
diagnosis, facilitating prompt and timely clinical management. By
integrating methylation data, particularly for the downregulated
genes, a more precise and effective approach to understanding and
treating EO-GC may be provided.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by Fondo Nacional de Desarrollo Científico
y Tecnológico (FONDECYT; grant nos. 1221499 to Marcelo Garrido and
11220563 to Ignacio N Retamal).
Availability of data and materials
Raw counts, upper-quartile normalized fragments per
kilo base per million mapped reads and TPM RNA-seq expression, and
clinical data related to the STAD-TCGA project can be accessed and
downloaded from Genomic Data Commons through the TCGA-biolinks R
package.
Authors' contributions
FGV, IS, BGB, JAG and MG were involved in the
conceptualization of the study. TdMG and CS were responsible for
the methodology. INR, CSM, MMM, MGV, FSC, PAM, IC, HB, FP, JME, ACS
and AG performed investigations and data adquisition. FSC, PA and
JAG were involved in the study's conceptualization, wrote the
original draft, and reviewed and edited the manuscript to produce
the final version. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
MG has been involved as a principal investigator in
clinical trials from Merck Sharp & Dohme, Bristol Myers Squibb,
Novartis, Roche, Astellas, Deciphera, Thermo Fisher Scientific, IMS
Health and Quintiles (IQVIA), Bayer, Principia, Covance,
Daiichi-Sankyo, Basilea, PRA-Exelisis, Syneos and Zimeworks. All
other authors declare that they have no competing interests.
References
|
1
|
Zhang C, Tang R, Zhu H, Ge X, Wang Y, Wang
X and Miao L: Comparison of treatment strategies and survival of
early-onset gastric cancer: A population-based study. Sci Rep.
12:62882022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergquist JR, Leiting JL, Habermann EB,
Cleary SP, Kendrick ML, Smoot RL, Nagorney DM, Truty MJ and Grotz
TE: Early-onset gastric cancer is a distinct disease with worrisome
trends and oncogenic features. Surgery. 166:547–555. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vishwanath A, Krishna S, Manudhane AP,
Hart PA and Krishna SG: Early-onset gastrointestinal malignancies:
An investigation into a rising concern. Cancers (Basel).
16:15532024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han X, Jia X, Sheng C, Li M, Han J, Duan F
and Wang K: A comparison analysis of the somatic mutations in
early-onset gastric cancer and traditional gastric cancer. Clin Res
Hepatol Gastroenterol. 48:1022872024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrillo A, Federico P, Marte G, Liguori
C, Seeber A, Ottaviano M, Tufo A and Daniele B: Non-hereditary
early onset gastric cancer: An unmet medical need. Curr Opin
Pharmacol. 68:1023442023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben-Aharon I, van Laarhoven HWM, Fontana
E, Obermannova R, Nilsson M and Lordick F: Early-onset cancer in
the gastrointestinal tract is on the rise-evidence and
implications. Cancer Discov. 13:538–551. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Milne AN and Offerhaus GJ: Early-onset
gastric cancer: Learning lessons from the young. World J
Gastrointest Oncol. 2:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Triantafillidis JK, Georgiou K,
Konstadoulakis MM and Papalois AE: Early-onset gastrointestinal
cancer: An epidemiological reality with great significance and
implications. World J Gastrointest Oncol. 16:583–597. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Q, Tao F, Qiu L, Chen H, Bao H, Wu X,
Shao Y, Chi L and Song H: Somatic alteration characteristics of
early-onset gastric cancer. J Oncol. 2022:14980532022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:40122020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ugai T, Sasamoto N, Lee HY, Ando M, Song
M, Tamimi RM, Kawachi I, Campbell PT, Giovannucci EL, Weiderpass E,
et al: Is early-onset cancer an emerging global epidemic? Current
evidence and future implications. Nat Rev Clin Oncol. 19:656–673.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colaprico A, Silva TC, Olsen C, Garofano
L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM,
Castiglioni I, et al: TCGAbiolinks: An R/bioconductor package for
integrative analysis of TCGA data. Nucleic Acids Res. 44:e712016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lê S, Josse J and Husson F: FactoMineR: An
R package for multivariate analysis. J Stat Softw. 25:1–18. 2008.
View Article : Google Scholar
|
|
14
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heagerty PJ and Saha P: SurvivalROC:
Time-dependent ROC curve estimation from censored survival data.
Biometrics. 2000.https://doi.org/10.32614/CRAN.package.survivalROC
View Article : Google Scholar
|
|
17
|
Wang X, Dong Y, Zhang H, Zhao Y, Miao T,
Mohseni G, Du L and Wang C: DNA methylation drives a new path in
gastric cancer early detection: Current impact and prospects. Genes
Dis. 11:847–860. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao X, Liu H, Yu J and Nie Y: DNA
methylation biomarkers for early detection of gastric and
colorectal cancers. Cancer Biol Med. 20:955–962. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Necula L, Matei L, Dragu D, Neagu AI,
Mambet C, Nedeianu S, Bleotu C, Diaconu CC and Chivu-Economescu M:
Recent advances in gastric cancer early diagnosis. World J
Gastroenterol. 25:2029–2044. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi SH, Cho SY, Song J and Hur MW: KLHL4,
a novel p53 target gene, inhibits cell proliferation by activating
p21WAF/CDKN1A. Biochem Biophys Res Commun. 530:588–596.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gimeno RE, Ortegon AM, Patel S, Punreddy
S, Ge P, Sun Y, Lodish HF and Stahl A: Characterization of a
heart-specific fatty acid transport protein. J Biol Chem.
278:16039–16044. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirokawa N, Noda Y, Tanaka Y and Niwa S:
Kinesin superfamily motor proteins and intracellular transport. Nat
Rev Mol Cell Biol. 10:682–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Shi Y, Han R, Liu C, Qin X, Li P
and Gu R: Signaling pathways of oxidative stress response: The
potential therapeutic targets in gastric cancer. Front Immunol.
14:11395892023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baird L and Yamamoto M: The molecular
mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol.
40:e00099–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi A, Kang MI, Watai Y, Tong KI,
Shibata T, Uchida K and Yamamoto M: Oxidative and electrophilic
stresses activate Nrf2 through inhibition of ubiquitination
activity of Keap1. Mol Cell Biol. 26:221–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ulasov AV, Rosenkranz AA, Georgiev GP and
Sobolev AS: Nrf2/Keap1/ARE signaling: Towards specific regulation.
Life Sci. 291:1201112022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freigang S, Ampenberger F, Spohn G, Heer
S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF
and Kopf M: Nrf2 is essential for cholesterol crystal-induced
inflammasome activation and exacerbation of atherosclerosis. Eur J
Immunol. 41:2040–1051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuhn AM, Tzieply N, Schmidt MV, von
Knethen A, Namgaladze D, Yamamoto M and Brüne B: Antioxidant
signaling via Nrf2 counteracts lipopolysaccharide-mediated
inflammatory responses in foam cell macrophages. Free Radic Biol
Med. 50:1382–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Choksi S, Chen K, Pobezinskaya Y,
Linnoila I and Liu ZG: ROS play a critical role in the
differentiation of alternatively activated macrophages and the
occurrence of tumor-associated macrophages. Cell Res. 23:898–914.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robledinos-Antón N, Fernández-Ginés R,
Manda G and Cuadrado A: Activators and inhibitors of NRF2: A review
of their potential for clinical development. Oxid Med Cell Longev.
2019:93721822019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tooze SA: Biogenesis of secretory granules
in the trans-Golgi network of neuroendocrine and endocrine cells.
Biochim Biophys Acta. 1404:231–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Štepihar D, Florke Gee RR, Hoyos Sanchez
MC and Fon Tacer K: Cell-specific secretory granule sorting
mechanisms: The role of MAGEL2 and retromer in hypothalamic
regulated secretion. Front Cell Dev Biol. 11:12430382023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chomez P, De Backer O, Bertrand M, De
Plaen E, Boon T and Lucas S: An overview of the MAGE gene family
with the identification of all human members of the family. Cancer
Res. 61:5544–5551. 2001.PubMed/NCBI
|
|
35
|
Hao YH, Doyle JM, Ramanathan S, Gomez TS,
Jia D, Xu M, Chen ZJ, Billadeau DD, Rosen MK and Potts PR:
Regulation of WASH-dependent actin polymerization and protein
trafficking by ubiquitination. Cell. 152:1051–1064. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoencamp C and Rowland BD: Genome control
by SMC complexes. Nat Rev Mol Cell Biol. 24:633–650. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sanderson MR, Fahlman RP and Wevrick R:
The N-terminal domain of the Schaaf-Yang syndrome protein MAGEL2
likely has a role in RNA metabolism. J Biol Chem. 297:1009592021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beaupre BA, Hoag MR, Roman J, Försterling
FH and Moran GR: Metabolic function for human renalase: Oxidation
of isomeric forms of β-NAD(P)H that are inhibitory to primary
metabolism. Biochemistry. 54:795–806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Beaupre BA, Carmichael BR, Hoag MR, Shah
DD and Moran GR: Renalase is an α-NAD(P)H oxidase/anomerase. J Am
Chem Soc. 135:13980–13987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pointer TC, Gorelick FS and Desir GV:
Renalase: A multi-functional signaling molecule with roles in
gastrointestinal disease. Cells. 10:20062021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo X, Jessel S, Qu R, Kluger Y, Chen TM,
Hollander L, Safirstein R, Nelson B, Cha C, Bosenberg M, et al:
Inhibition of renalase drives tumour rejection by promoting T cell
activation. Eur J Cancer. 165:81–96. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo X, Hollander L, MacPherson D, Wang L,
Velazquez H, Chang J, Safirstein R, Cha C, Gorelick F and Desir GV:
Inhibition of renalase expression and signaling has antitumor
activity in pancreatic cancer. Sci Rep. 6:229962016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hollander L, Guo X, Velazquez H, Chang J,
Safirstein R, Kluger H, Cha C and Desir GV: Renalase expression by
melanoma and tumor-associated macrophages promotes tumor growth
through a STAT3-mediated mechanism. Cancer Res. 76:3884–3894. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan Y, Wang X, He Y, Lin S, Zhu M, Li Y,
Wang J, Wang J, Ma X, Xu J, et al: Tumor suppressor ATP4B serve as
a promising biomarker for worsening of gastric atrophy and poor
differentiation. Gastric Cancer. 24:314–326. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Borghaei RC, Gorski G, Seutter S, Chun J,
Khaselov N and Scianni S: Zinc-binding protein-89 (ZBP-89)
cooperates with NF-κB to regulate expression of matrix
metalloproteinases (MMPs) in response to inflammatory cytokines.
Biochem Biophys Res Commun. 471:503–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Borghaei RC, Gorski G and Javadi M; Mariah
Chambers, : NF-kappaB and ZBP-89 regulate MMP-3 expression via a
polymorphic site in the promoter. Biochem Biophys Res Commun.
382:269–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Borghaei RC, Rawlings PL Jr, Javadi M and
Woloshin J: NF-kappaB binds to a polymorphic repressor element in
the MMP-3 promoter. Biochem Biophys Res Commun. 316:182–188. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morán A, Iniesta P, de Juan C,
García-Aranda C, Díaz-López A and Benito M: Impairment of
stromelysin-1 transcriptional activity by promoter mutations in
high microsatellite instability colorectal tumors. Cancer Res.
65:3811–3814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim SJ, Hwang JA, Ro JY, Lee YS and Chun
KH: Galectin-7 is epigenetically-regulated tumor suppressor in
gastric cancer. Oncotarget. 4:1461–1471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hou W, Pan M, Xiao Y and Ge W: Serum
extracellular vesicle stratifin is a biomarker of perineural
invasion in patients with colorectal cancer and predicts worse
prognosis. Front Oncol. 12:9125842022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jung JY, Koh SA, Lee KH and Kim JR: 14-3-3
Sigma protein contributes to hepatocyte growth factor-mediated cell
proliferation and invasion via matrix metalloproteinase-1
regulation in human gastric cancer. Anticancer Res. 42:519–530.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chang WC, Huang SF, Lee YM, Lai HC, Cheng
BH, Cheng WC, Ho JY, Jeng LB and Ma WL: Cholesterol import and
steroidogenesis are biosignatures for gastric cancer patient
survival. Oncotarget. 8:692–704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cho LY, Yang JJ, Ko KP, Ma SH, Shin A,
Choi BY, Han DS, Song KS, Kim YS, Chang SH, et al: Genetic
susceptibility factors on genes involved in the steroid hormone
biosynthesis pathway and progesterone receptor for gastric cancer
risk. PLoS One. 7:e476032012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG,
Shen JY and Wang LB: Prognostic role of estrogen receptor alpha and
estrogen receptor beta in gastric cancer. Ann Surg Oncol.
17:2503–2509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chandanos E, Rubio CA, Lindblad M, Jia C,
Tsolakis AV, Warner M, Gustafsson JA and Lagergren J: Endogenous
estrogen exposure in relation to distribution of histological type
and estrogen receptors in gastric adenocarcinoma. Gastric Cancer.
11:168–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Frycz BA, Murawa D, Borejsza-Wysocki M,
Wichtowski M, Spychała A, Marciniak R, Murawa P, Drews M and
Jagodziński PP: mRNA expression of steroidogenic enzymes, steroid
hormone receptors and their coregulators in gastric cancer. Oncol
Lett. 13:3369–3378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kameda C, Nakamura M, Tanaka H, Yamasaki
A, Kubo M, Tanaka M, Onishi H and Katano M: Oestrogen
receptor-alpha contributes to the regulation of the hedgehog
signalling pathway in ERalpha-positive gastric cancer. Br J Cancer.
102:738–747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Correa P and Piazuelo MB: The gastric
precancerous cascade. J Dig Dis. 13:2–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
He Q, Liu L, Wei J, Jiang J, Rong Z, Chen
X, Zhao J and Jiang K: Roles and action mechanisms of bile
acid-induced gastric intestinal metaplasia: A review. Cell Death
Discov. 8:1582022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tatsugami M, Ito M, Tanaka S, Yoshihara M,
Matsui H, Haruma K and Chayama K: Bile acid promotes intestinal
metaplasia and gastric carcinogenesis. Cancer Epidemiol Biomarkers
Prev. 21:2101–2107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Inoue Y, Yu AM, Yim SH, Ma X, Krausz KW,
Inoue J, Xiang CC, Brownstein MJ, Eggertsen G, Björkhem I and
Gonzalez FJ: Regulation of bile acid biosynthesis by hepatocyte
nuclear factor 4alpha. J Lipid Res. 47:215–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tsukita S, Tanaka H and Tamura A: The
claudins: From tight junctions to biological systems. Trends
Biochem Sci. 44:141–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Singh AB, Uppada SB and Dhawan P: Claudin
proteins, outside-in signaling, and carcinogenesis. Pflugers Arch.
469:69–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gao M, Li W, Wang H and Wang G: The
distinct expression patterns of claudin-10, −14, −17 and E-cadherin
between adjacent non-neoplastic tissues and gastric cancer tissues.
Diagn Pathol. 8:2052013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang H and Yang X: The expression patterns
of tight junction protein claudin-1, −3, and −4 in human gastric
neoplasms and adjacent non-neoplastic tissues. Int J Clin Exp
Pathol. 8:881–887. 2015.PubMed/NCBI
|
|
66
|
Zhu J and Wang R, Cao H, Zhang H, Xu S,
Wang A, Liu B, Wang Y and Wang R: Expression of claudin-5, −7, −8
and −9 in cervical carcinoma tissues and adjacent non-neoplastic
tissues. Int J Clin Exp Pathol. 8:9479–9486. 2015.PubMed/NCBI
|
|
67
|
Lu YZ, Li Y, Zhang T and Han ST: Claudin-6
is down-regulated in gastric cancer and its potential pathway.
Cancer Biomark. 28:329–340. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kohmoto T, Masuda K, Shoda K, Takahashi R,
Ujiro S, Tange S, Ichikawa D, Otsuji E and Imoto I: Claudin-6 is a
single prognostic marker and functions as a tumor-promoting gene in
a subgroup of intestinal type gastric cancer. Gastric Cancer.
23:403–417. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Łukaszewicz-Zając M and Mroczko B:
Claudins-promising biomarkers for selected gastrointestinal (GI)
malignancies? Cancers (Basel). 16:1522023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Simon AG, Lyu SI, Laible M, Wöll S, Türeci
Ö, Şahin U, Alakus H, Fahrig L, Zander T, Buettner R, et al: The
tight junction protein claudin 6 is a potential target for
patient-individualized treatment in esophageal and gastric
adenocarcinoma and is associated with poor prognosis. J Transl Med.
21:5522023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Torres-Martínez AC, Gallardo-Vera JF,
Lara-Holguin AN, Montaño LF and Rendón-Huerta EP: Claudin-6
enhances cell invasiveness through claudin-1 in AGS human
adenocarcinoma gastric cancer cells. Exp Cell Res. 350:226–235.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Thaler R, Rumpler M, Spitzer S, Klaushofer
K and Varga F: Mospd1, a new player in mesenchymal versus epidermal
cell differentiation. J Cell Physiol. 226:2505–2515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Imoto Y, Raychaudhuri S, Ma Y, Fenske P,
Sandoval E, Itoh K, Blumrich EM, Matsubayashi HT, Mamer L,
Zarebidaki F, et al: Dynamin is primed at endocytic sites for
ultrafast endocytosis. Neuron. 110:2815–2835.e13. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Meng J: Distinct functions of dynamin
isoforms in tumorigenesis and their potential as therapeutic
targets in cancer. Oncotarget. 8:41701–41716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Thorsell AG, Persson C, Voevodskaya N,
Busam RD, Hammarström M, Gräslund S, Gräslund A and Hallberg BM:
Structural and biophysical characterization of human myo-inositol
oxygenase. J Biol Chem. 283:15209–15216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Meng L, Gao J, Mo W, Wang B, Shen H, Cao
W, Ding M, Diao W, Chen W, Zhang Q, et al: MIOX inhibits autophagy
to regulate the ROS-driven inhibition of STAT3/c-Myc-mediated
epithelial-mesenchymal transition in clear cell renal cell
carcinoma. Redox Biol. 68:1029562023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang L, Li C, Qin Y, Zhang G, Zhao B, Wang
Z, Huang Y and Yang Y: A Novel Prognostic model based on
ferroptosis-related gene signature for bladder cancer. Front Oncol.
11:6860442021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu W, Xiang J, Wu X, Wei S, Huang H, Xiao
Y, Zhai B and Wang T: Transcriptome profiles reveal a 12-signature
metabolic prediction model and a novel role of myo-inositol
oxygenase in the progression of prostate cancer. Front Oncol.
12:8998612022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xu Z, Zhang S, Nian F and Xu S:
Identification of a glycolysis-related gene signature associated
with clinical outcome for patients with lung squamous cell
carcinoma. Cancer Med. 10:4017–4029. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cengiz B, Yumrutas O, Bozgeyik E, Borazan
E, Igci YZ, Bozgeyik I and Oztuzcu S: Differential expression of
the UGT1A family of genes in stomach cancer tissues. Tumor Biol.
36:5831–5837. 2015. View Article : Google Scholar
|
|
81
|
Pang SW, Lahiri C, Poh CL and Tan KO: PNMA
family: Protein interaction network and cell signalling pathways
implicated in cancer and apoptosis. Cell Signal. 45:54–62. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lee YH, Pang SW, Poh CL and Tan KO:
Distinct functional domains of PNMA5 mediate protein-protein
interaction, nuclear localization, and apoptosis signaling in human
cancer cells. J Cancer Res Clin Oncol. 142:1967–1977. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lin J, Zhang X, Meng F, Zeng F, Liu W and
He X: PNMA5 accelerated cellular proliferation, invasion and
migration in colorectal cancer. Am J Transl Res. 4:2231–2243.
2022.PubMed/NCBI
|
|
84
|
Cabarcas S and Schramm L: RNA polymerase
III trans-cription in cancer: The BRF2 connection. Mol Cancer.
10:472011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kang M, Lu S, Chong PK, Yeoh KG and Lim
YP: Comparative proteomic profiling of extracellular proteins
between normal and gastric cancer cells. Curr Cancer Drug Targets.
16:442–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang Y, Wu H, Yang F, Ning J, Li M, Zhao
C, Zhong S, Gu K and Wang H: Prognostic value of the expression of
DNA repair-related biomarkers mediated by alcohol in gastric cancer
patients. Am J Pathol. 188:367–377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Welch MD, DePace AH, Verma S, Iwamatsu A
and Mitchison TJ: The human Arp2/3 complex is composed of
evolutionarily conserved subunits and is localized to cellular
regions of dynamic actin filament assembly. J Cell Biol.
138:375–384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yoo Y, Wu X and Guan JL: A novel role of
the actin-nucleating Arp2/3 complex in the regulation of RNA
polymerase II-dependent transcription. J Biol Chem. 282:7616–7623.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee GE, Kim JH, Taylor M and Muller MT:
DNA methyltransferase 1-associated protein (DMAP1) is a
co-repressor that stimulates DNA methylation globally and locally
at sites of double strand break repair. J Biol Chem.
285:37630–37640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li B, Zhu J and Meng L: High expression of
ACTL8 is poor prognosis and accelerates cell progression in head
and neck squamous cell carcinoma. Mol Med Rep. 19:877–884.
2019.PubMed/NCBI
|
|
91
|
Han Q, Sun ML, Liu WS, Zhao HS, Jiang LY,
Yu ZJ and Wei MJ: Upregulated expression of ACTL8 contributes to
invasion and metastasis and indicates poor prognosis in colorectal
cancer. Onco Targets Ther. 12:1749–1763. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mantilla MJ, Chaves JJ, Ochoa-Vera M,
Africano F, Parra-Medina R and Tovar-Fierro G: Clinical
characteristics of early-onset gastric cancer. A study in a
Colombian population. Rev Gastroenterol Peru. 43:236–241. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu H, Li Z, Zhang Q, Li Q, Zhong H, Wang
Y, Yang H, Li H, Wang X, Li K, et al: Multi-institutional
development and validation of a nomogram to predict prognosis of
early-onset gastric cancer patients. Front Immunol. 13:10071762022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Umeyama K, Sowa M, Kamino K, Kato Y and
Satake K: Gastric carcinoma in young adults in Japan. Anticancer
Res. 2:283–286. 1982.PubMed/NCBI
|
|
95
|
LaPelusa M, Shen C, Gillaspie EA, Cann C,
Lambright E, Chakravarthy AB, Gibson MK and Eng C: Variation in
treatment patterns of patients with early-onset gastric cancer.
Cancers (Basel). 14:36332022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Setia N, Wang CX, Lager A, Maron S, Shroff
S, Arndt N, Peterson B, Kupfer SS, Ma C, Misdraji J, et al:
Morphologic and molecular analysis of early-onset gastric cancer.
Cancer. 127:103–114. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mun DG, Bhin J, Kim S, Kim H, Jung JH,
Jung Y, Jang YE, Park JM, Kim H, Jung Y, et al: Proteogenomic
characterization of human early-onset gastric cancer. Cancer Cell.
35:111–124.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ma Z, Liu X, Paul ME, Chen M, Zheng P and
Chen H: Comparative investigation of early-onset gastric cancer.
Oncol Lett. 21:3742021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Skierucha M, Milne AN, Offerhaus GJ,
Polkowski WP, Maciejewski R and Sitarz R: Molecular alterations in
gastric cancer with special reference to the early-onset subtype.
World J Gastroenterol. 22:2460–2474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Gao F, Li M, Xiang R, Zhou X, Zhu L and
Zhai Y: Expression of CLDN6 in tissues of gastric cancer patients:
Association with clinical pathology and prognosis. Oncol Lett.
17:4621–4625. 2019.PubMed/NCBI
|
|
101
|
Wu LH, Wang XX, Wang Y, Wei J, Liang ZR,
Yan X and Wang J: Construction and validation of a prognosis
signature based on the immune microenvironment in gastric cancer.
Front Surg. 10:10882922023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gao S, Li J, Wang W, Wang Y, Shan Y and
Tan H: Rabdosia rubescens (Hemsl.) H. Hara: A potent
anti-tumor herbal remedy-Botany, phytochemistry, and clinical
applications and insights. J Ethnopharmacol. 340:1192002025.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gao S, Shan Y, Wang Y, Wang W, Li J and
Tan H: Polysaccharides from Lonicera japonica Thunb.: Extraction,
purification, structural features and biological activities-A
review. Int J Biol Macromol. 281:1364722024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gao S, Gang J, Yu M, Xin G and Tan H:
Computational analysis for identification of early diagnostic
biomarkers and prognostic biomarkers of liver cancer based on GEO
and TCGA databases and studies on pathways and biological functions
affecting the survival time of liver cancer. BMC Cancer.
21:7912021. View Article : Google Scholar : PubMed/NCBI
|