Introduction
Stroke has been recognized as a leading cause of
disability and is the second most common cause of mortality in
adults worldwide (1). Research has
indicated that the use of neuroprotective agents may be a suitable
approach to the treatment of stroke. Although numerous clinical
trials have been conducted with potentially neuroprotective agents,
few have been used clinically (2,3).
During ischemic insult, the production of reactive
oxygen species (ROS) increases. This leads to neuronal death, brain
edema and inflammation (4). ROS
produced at the early phase of neuronal apoptosis act as mediators
of intracellular apoptotic signaling cascades (5), and pathologically accelerate the
release of cytochrome c from mitochondria (6,7). ROS
production evidently increases following ischemia, which leads to
oxidative stress (8–10). Studies have revealed that at least
some of the effects on cells of exposure to ROS are similar to
those of ischemic insult. The brain, which is a major metabolic
organ of oxygen and has relatively weak protective antioxidant
mechanisms, is particularly vulnerable to the insult perpetrated by
ROS. The neuroprotective role of sirtuin 1 (SIRT1) has been
demonstrated in models of various neurodegenerative diseases,
including Alzheimer's disease, Parkinson's disease and Huntington's
disease (11,12).
Resveratrol (RSV;
3,5,4′-trihydroxy-trans-stilbene) is a naturally occurring
phytoalexin that is present in the skin of red grapes and is a
component of red wine (13,14). It mediates a variety of biological
activities that are associated with extension of life span, even in
those with a high caloric diet, and cancer prevention (15,16).
Resveratrol acts as an activator of SIRT1 and has been shown to be
neuroprotective in various models (16–18). The
hippocampus is a region of active proliferation and neurogenesis
within the brain. Ischemia induces apoptosis of neurons in the
subventricular zone of the lateral ventricles and in the
subgranular zone of the hippocampus in the adult brain. Transient
global ischemia induces neuronal damage specifically in the
hippocampus of rats (19,20).
In the current study, the aim was to investigate
whether resveratrol inhibits the caspase cell death pathway to
protect hippocampal neurons from ischemia and evaluate the effects
of SIRT1 activation in the hippocampus. The mechanisms that lead to
this phenomenon within neurons were also investigated.
Materials and methods
Chemicals and animals
Resveratrol was obtained from Sigma Chemicals (St.
Louis, MO, USA). Male Sprague-Dawley rats weighing 200–220 g (n=24)
were obtained from the Experimental Animal Research Center,
Institute of Radiation Medicine, Chinese Academy of Medical
Sciences and Peking Union Medical College, Tianjin, China. The rats
were maintained in a room at 22±2°C, with a relative humidity of
50±10%, on a 12 h light-dark cycle. All animal experiments were
conducted in accordance with a protocol approved by the
Institutional Animal Care and Use Committee (IACUC) of the
Institute of Radiation Medicine, Chinese Academy of Medical
Sciences. (No. 1204). Rats were randomly divided into four groups
(6 rats/group). These were the ischemia group (ISC group), the
ischemia with 5 mg/kg resveratrol group (ISC + RSV-5 group), the
ischemia with 10 mg/kg resveratrol group (ISC + RSV-10 group) and
the control group.
Drug treatments
Rats in the control and ISC groups were given
distilled water, while the rats in the ISC + RSV-5 and ISC + RSV-10
groups were respectively given drinking water plus 5 and 10 mg/kg
resveratrol for 21 days.
Middle cerebral artery occlusion
Rats in the ISC group and the two ISC + RSV groups
were anesthetized with 2% Napental (4 mg/kg, intraperitoneally) and
then underwent surgery to induce middle cerebral artery occlusion.
The middle cerebral artery was ligated with an intraluminal
filament for 1.5 h and then reperfused for 24 h. For the sham
group, the external carotid artery was surgically prepared for
insertion of the filament, but no filament was inserted. During the
experimental procedures, blood pressure, blood gas level and
glucose levels were monitored. Rectal temperature was maintained at
37.0–37.5°C with a heating pad, and body temperature was maintained
at 37°C with a thermostatically controlled infrared lamp. Brain
temperature was maintained at 36–37°C and monitored with a 29-gauge
thermocouple in the right corpus striatum and a
temperature-regulating lamp. An electroencephalogram was taken to
ensure isoelectricity during the ischemic period (19).
Tissue preparation
At 24 h after the experimental interventions, the
rats were deeply anesthetized, sacrificed by an overdose of
anesthesia and quickly treated with a cardiac perfusion of 4%
paraformaldehyde. The hippocampi from 3 rats per group were
extracted and cut into 12-µm coronal sections with a cryostat (CM
3000; Leica, Manheim, Germany) then placed on glass slides and
stored at −80°C (21,22).
Immunohistology, terminal
deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL)
staining and cresyl violet (CV) staining
The avidin-biotin-peroxidase complex method of
immunohistochemical staining was conducted as previously described
(23). DNA fragmentation was
detected using a TUNEL kit (Roche Diagnostics Corporation,
Indianapolis, IN, USA) according to the manufacturer's
instructions. The sections were stained with cresyl violet (CV)
using the conventional method and mounted (24).
Western blot analysis
The total protein and nuclear protein were isolated
from hippocampi using RIPA buffer (Beyotime, Jiangsu, China)
according to the manufacturer's instructions. The protein
concentration of the supernatant homogenate was determined using a
Bio-Rad kit (Bio-Rad, Hercules, CA, USA) at an absorbance of 595
nm. The samples (80 µg) were transferred to polyvinylidene
difluoride membranes and incubated with the following primary
antibodies: Rabbit monoclonal anti-SIRT1 antibodies obtained from
Cell Signaling Technology (dilution 1:500; cat. no. 9475P, Beverly,
MA, USA); rabbit monoclonal anti-β-actin (dilution 1:1,500; Sangon
Biotech, Shanghai, China) was used for loading control and the
secondary antibody was goat anti-rabbit IgG conjugated to
horseradish peroxidase (dilution 1:500; ZSGB-Bio, Beijing,
China).
RNA extraction, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was purified and extracted as described
previously by Chen et al (25). Equal concentrations of total RNA were
reverse-transcribed using Prime Script RT reagent kit (Takara Bio
Inc., Shiga, Japan) according to the manufacturer's instructions.
cDNA samples were blended with primers and SYBR Master Mix
(Invitrogen Life Technologies, Carlsbad, CA, USA) in a total volume
of 25 ml. The samples were assayed in triplicate using an ABI
PRISM® 7500 Sequence Detection system (Applied Biosystems-Life
Technologies, Foster City, CA, USA). The cycle threshold (CT)
values for each reaction were determined and the mean was
calculated using TaqMan SDS analysis software (Applied
Biosystems-Life Technologies). The expression levels of target
genes were determined by the comparative CT method (fold change =
2−ΔΔCT). PCR primers for SIRT1 were obtained from Sangon
Biotech (Shanghai, China). The primer pairs for SIRT1 were as
follows: forward, 5′-CCAGATCCTCAAGCCATGT-3′ and reverse,
5′-TTGGATTCCTGCAACCTG-3′ (26).
Analysis of reactive oxygen species
and SIRT1 activity
The fluorogenic probe
2′,7′-dichlorodihydrofluorescein diacetate was used to assess the
production of ROS, as previously described (22). The activity of SIRT1 was analyzed
using a fluorogenic assay according to the technique previously
described by Li et al (22).
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed using one-way analysis of variance with a post
hoc test (multiple comparison test), which determined the
significant differences among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Immunohistology and TUNEL-positive
cells within the hippocampi
CV staining was conducted to stain Nissl bodies a
purple-blue color (Fig. 1A, D and
G). This revealed that distinct damage of neurons occurred in
the ISC and ISC + RSV-10 groups (Fig. 1D
and G) when compared with the control group (Fig. 1A). Immunohistochemical staining of
SIRT1 in the hippocampi of the ISC group (Fig. 1H) was more intense than in the ISC +
RSV group (Fig. 1E), which in turn
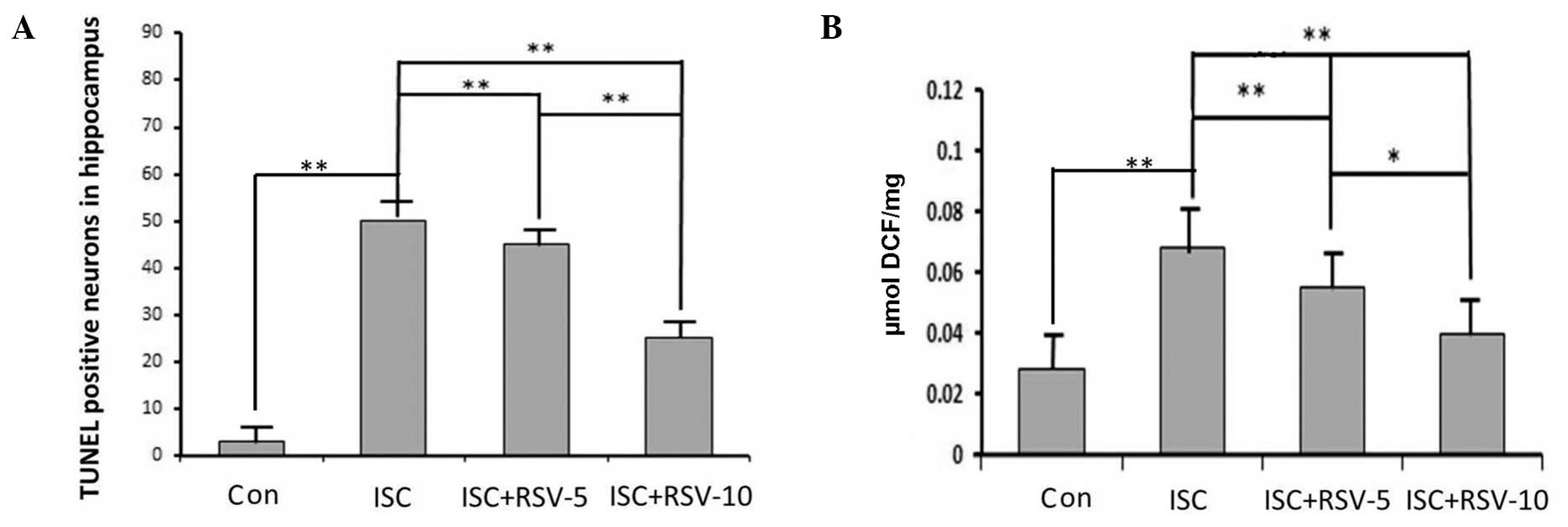
was more intense than that in the control group (Fig. 1B). TUNEL-positive cells were most
visible in the hippocampi of the ISC group (Fig. 1F), and visible at lower levels in the
ISC + RSV group (Fig. 1I). The
number of TUNEL-positive cells detected in the control rats was low
(Fig. 1C) compared with that in the
other two groups. These results indicate that RSV attenuated the
ischemia-induced damage. The neurons in the hippocampi from control
rats displayed few positive neurons. Rats in the ISC group notably
became evident by a prominent growth in the number of TUNEL
positive cells. TUNEL positive neurons in the ISC+RSV group
markedly decreased compared with ischemia only (Fig. 2A).
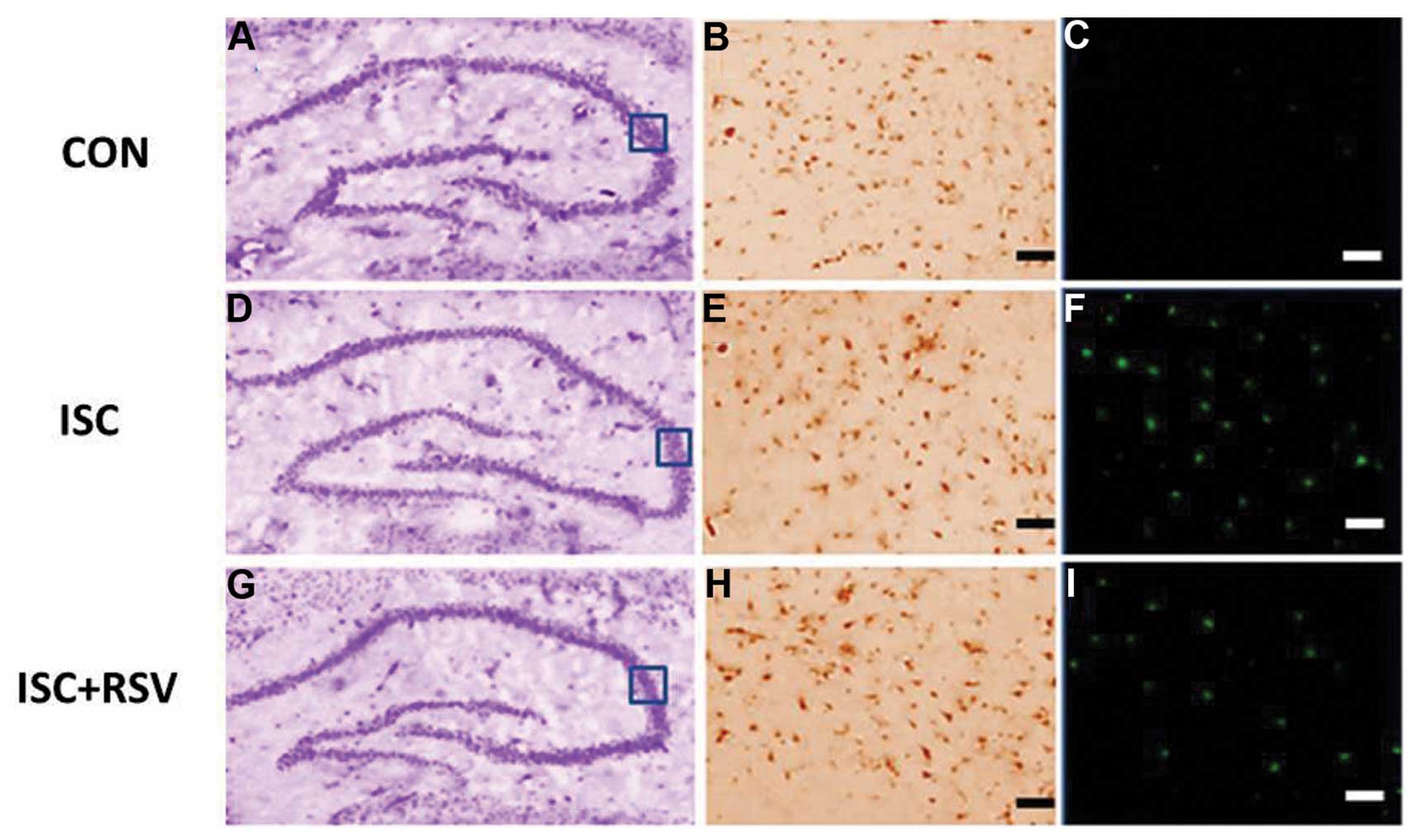 | Figure 1.Representative images for (A, D, G)
CV, (B, E, H) SIRT1 and (C, F, I) TUNEL staining in the CA3
hippocampal region. Scale bars: (B, C, E, F, H, I) 50 µm. The box
indicates the image positioning of SIRT1 and TUNEL. CV, cresyl
violet; TUNEL, terminal deoxynucleotidyl transferase dUTP nick
end-labeling; SIRT1, sirtuin 1; CON, control; ISC, ischemia; RSV,
resveratrol-10. |
ROS accumulation
As shown in Fig. 2B,
ischemia increased the levels of ROS that were detected in the rat
hippocampi. In addition, RSV effectively attenuated the
ischemia-induced ROS production in the RSV-treated groups in a
dose-dependent manner.
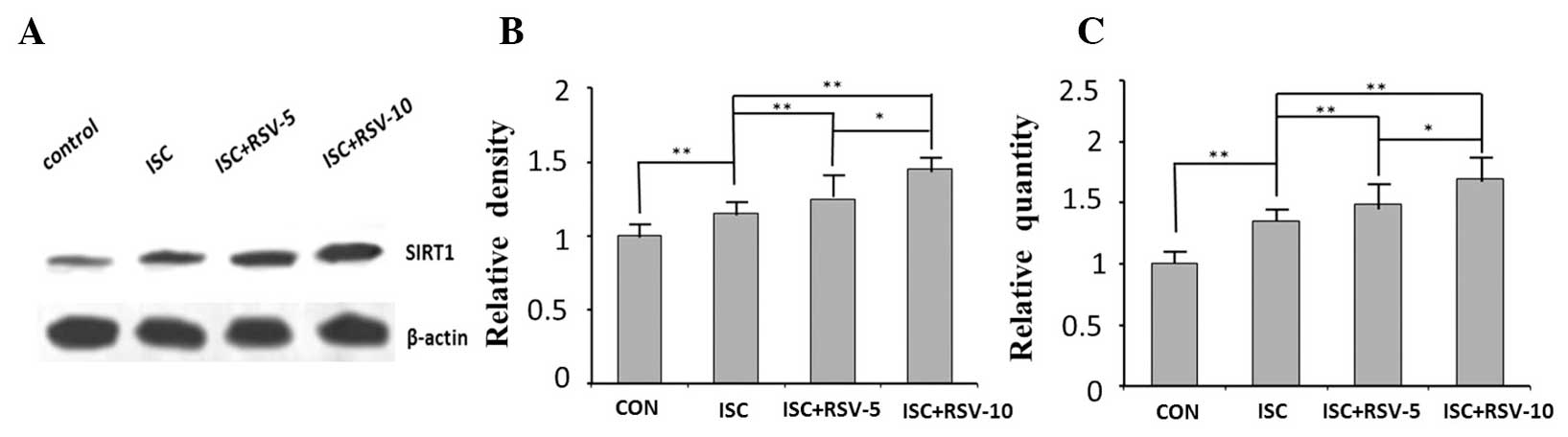
SIRT1 expression determined by western
blotting and RT-qPCR analysis and SIRT1 activity
The expression levels of SIRT1 in the ISC and ISC +
RSV groups exhibited significant differences compared with those in
the control group at the protein and mRNA levels (Fig. 3). Treatment with resveratrol
increased the expression of SIRT1 in a concentration-dependent
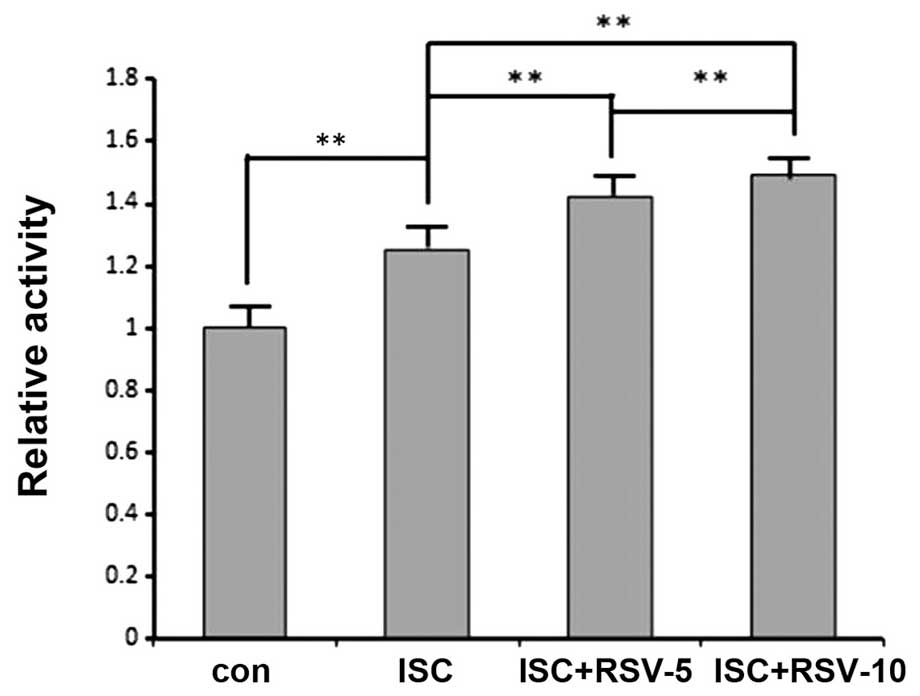
manner (Fig. 3). SIRT1 activity was
significantly increased following ischemia, and was particularly
high in the ISC + RSV-5 and ISC + RSV-10 groups, where resveratrol
increased the SIRT1 activity in a concentration-dependent manner
(Fig. 4).
Discussion
The results of the present study revealed that SIRT1
was highly expressed in rat hippocampal neurons following ischemia
and that resveratrol effectively antagonized the oxidation induced
by ischemia. SIRT1 provides neurons with tolerance against
oxidative stress. It also plays an important role in the regulation
of cellular functions. It can act on numerous non-histone proteins,
including transcription factors and transcriptional coregulatory
proteins (11,13). SIRT1 participates in the control of
systemic metabolism via the regulation of glucose and lipid
homeostasis by deacetylating various targets, and also increases
chromatin silencing. Various target proteins are substrates for
SIRT1 (27). Histone acetylation is
closely associated with active transcription, and histone
methylation is often associated with transcriptional repression
(28). SIRT1 suppresses the
expression of inflammatory genes by enhancing the activities of
histone methyl transferases. For example, SIRT1 deacetylates and
activates histone methyl transferase, resulting in increased levels
of trimethyl-substituted histone, which suppresses the expression
of inducible inflammatory genes (29).
Previous studies have suggested that SIRT1
activation elicits resistance to oxidative stress through the
regulation of transcription factors and coactivators such as Hif-2a
and NF-κB (30). P53 is an important
factor involved in myocardial apoptosis, whose effects are achieved
through the activation of the renin-angiotensin system. A study has
indicated that when myocardial ischemia occurs, SIRT1 reduces the
activity of P53 through deacetylation (31). SIRT1 is ubiquitously expressed in all
tissues, particularly in the brain, and has been demonstrated to be
a nuclear protein (32). However,
SIRT1 has both nuclear import and export sequences and has been
found to be present in the cytosolic fraction of mouse brains
(33). Oxidative stress occurs as
the result of a shift in balance that favors the generation of
oxygen-derived ROS over certain antioxidant defense mechanisms. ROS
can cause lipid peroxidation and lead to a loss of membrane
integrity, reduction of mitochondrial membrane potential and
increased permeability to Ca2+ in the plasma membrane
(34). In the central nervous
system, neurons are particularly vulnerable to assault by
neurotoxins. Ischemia and oxidative stress are common underlying
factors in this type of damage. In addition to exerting direct
effects on vascular tone, ROS also impair vasomotor responses to
other stimuli. Resveratrol has been identified to have notable
antiinflammatory activity. It downregulates the activation of
immune cells and the subsequent synthesis and release of
pro-inflammatory mediators through the inhibition of
transcriptional factors such as NF-κB. Resveratrol has also been
shown to inhibit the activation of microglia (35,36).
In a number of experimental paradigms, resveratrol
is used to increase SIRT1 activity. However, recent studies
(26,37,38) have
demonstrated that resveratrol does not directly activate SIRT1,
which indicates that mediators may be involved in the activation
process. Certain studies, discussed in a review (38), support the hypothesis that mechanisms
other than direct activation bring about P53 acetylation by
resveratrol. Another study demonstrated that SIRT1-dependent
pathways, in addition to nitric oxide (NO)-dependent mechanisms,
contribute to the beneficial mitochondrial and cellular effects of
dietary restriction (39). Many of
the effects that have been observed in resveratrol-treated animals
are consistent with the modulation of SIRT1 targets (40). SIRT1 may regulate a number of
pathways involved in mitochondrial biogenesis. The pathways
controlled by endothelium-derived NO are suggested to be the most
important (41). Resveratrol
improves NO production by upregulating endothelial NO synthase at
the level of transcription (42).
However, whether resveratrol modulates other critical genes remains
unclear. Although SIRT1 levels have been observed to increase
following the administration of resveratrol, the protective effect
of resveratrol may be SIRT1-independent. Future studies to clarify
the understanding of the cellular mechanisms involved in the
neuroprotective effects of resveratrol may provide new avenues for
the treatment of ischemia-induced disorders and experiments using
SIRT1-knockout rats may reveal the true correlation between SIRT1
and resveratrol.
In conclusion, in the present study, it was observed
that resveratrol significantly decreased the number of
TUNEL-positive cells, and increased SIRT1 mRNA expression levels,
in addition to increasing the expression levels of SIRT1 protein
and SIRT1 activity. The results indicate the neuroprotective and
antioxidant effects of resveratrol against ischemia-induced
apoptosis in the rat hippocampus.
Acknowledgements
The authors are grateful to the National Science
& Technology Pillar Program during the 12th Five-year Plan
Period (No. 2012BA127B02) and the PUMC graduate student innovation
fund.
References
|
1
|
Feigin VL, Lawes CM, Bennett DA and
Anderson CS: Stroke epidemiology: A review of population-based
studies of incidence, prevalence and case-fatality in the late 20th
century. Lancet Neurol. 2:43–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Green AR, Odergren T and Ashwood T: Animal
models of stroke: Do they have value for discovering
neuroprotective agents. Trend Pharmacol Sci. 24:402–408. 2003.
View Article : Google Scholar
|
|
3
|
Xu SY and Pan SY: The failure of animal
models of neuroprotection in acute ischemic stroke to translate to
clinical efficacy. Med Sci Monit Basic Res. 19:37–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moro MA, Almeida A, Bolanos JP and
Lizasoain I: Mitochondrial respiratory chain and free radical
generation in stroke. Free Radic Biol Med. 39:1291–1304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin-Romero FJ, Garcia-Martin E and
Gutierrez-Merino C: Inhibition of oxidative stress produced by
plasma membrane NADH oxidase delays low-potassium-induced apoptosis
of cerebellar granule cells. J Neurochem. 82:705–715. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Atlante A, Bobba A, Calissano P,
Passarella S and Marra E: The apoptosis/necrosis transition in
cerebellar granule cells depends on the mutual relationship of the
antioxidant and the proteolytic systems which regulate ROS
production and cytochrome c release enroute to death. J
Neurochem. 84:960–971. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Wang Y, Du L, Xu C, Cao J, Wang Q,
Liu Q and Fan F: Radiation-induced cytochrome c release and
the neuroprotective effects of the pan-caspase inhibitor z-VAD-fmk
in the hypoglossal nucleus. Exp Ther Med. 7:383–388.
2014.PubMed/NCBI
|
|
8
|
Kleikers PW, Wingler K, Hermans JJ,
Diebold I, Altenhöfer S, Radermacher KA, Janssen B, Görlach A and
Schmidt HH: NADPH oxidases as a source of oxidative stress and
molecular target in ischemia/reperfusion injury. J Mol Med (Berl).
90:1391–1406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuo L and Motherwell MS: The impact of
reactive oxygen species and genetic mitochondrial mutations in
Parkinson's disease. Gene. 532:18–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sochocka M, Koutsouraki ES, Gasiorowski K
and Leszek J: Vascular oxidative stress and mitochondrial failure
in the pathobiology of Alzheimer's disease: A new approach to
therapy. CNS Neurol Disord Drug Targets. 12:870–881. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong H, Cohen DE, Cui L, Supinski A,
Savas JN, Mazzulli JR, Yates JR III, Bordone L, Guarente L and
Krainc D: SIRT1 mediates neuroprotection from mutant huntingtin by
activation of the TORC1 and CREB transcriptional pathway. Nat Med.
18:159–165. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donmez G, Arun A, Chung CY, McLean PJ,
Lindquist S and Guarente L: SIRT1 protects against alpha-synuclein
aggregation by activating molecular chaperones. J Neurosci.
32:124–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chong ZZ, Shang YC, Wang S and Maiese K:
SIRT1: New avenues of discovery for disorders of oxidative stress.
Expert Opin Ther Targets. 16:167–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakata R, Takahashi S and Inoue H: Recent
advances in the study on resveratrol. Biol Pharm Bull. 35:273–279.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gruber J, Tang SY and Halliwell B:
Evidence for a trade-off between survival and fitness caused by
resveratrol treatment of Caenorhabditis elegans. Ann NY Acad
Sci. 1100:530–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pasinetti GM, Wang J, Marambaud P,
Ferruzzi M, Gregor P, Knable LA and Ho L: Neuroprotective and
metabolic effects of resveratrol: Therapeutic implications for
Huntington's disease and other neurodegenerative disorders. Exp
Neurol. 232:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brasnyó P, Molnár GA, Mohás M, Markó L,
Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R,
Mészáros LG, et al: Resveratrol improves insulin sensitivity,
reduces oxidative stress and activates the Akt pathway in type 2
diabetic patients. Br J Nutr. 106:383–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bradamante S, Barenghi L and Villa A:
Cardiovascular protective effects of resveratrol. Cardiovasc Drug
Rev. 22:169–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pulsinelli WA, Brierley JB and Plum F:
Temporal profile of neuronal damage in a model of transient
forebrain ischemia. Ann Neurol. 11:491–498. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nikonenko AG, Radenovic L, Andjus PR and
Skibo GG: Structural features of ischemic damage in the
hippocampus. Anat Rec (Hoboken). 292:1914–1921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Zhang Y, Yu Y, Li B, Chen Y, Wu H,
Wang J, Li J, Xiong X, He Q, Tian J, et al: Systemic revealing
pharmacological signalling pathway networks in the hippocampus of
ischaemia-reperfusion rats treated with baicalin. Evid Based
Complement Alternat Med. 2013:6307232013.PubMed/NCBI
|
|
22
|
Li J, Feng L, Xing Y, Wang Y, Du L, Xu C,
Cao J, Wang Q, Fan S, Liu Q and Fan F: Radioprotective and
Antioxidant effect of Resveratrol in Hippocampus by activating
SIRT1. Int J Mol Sci. 15:5928–5939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Y, Jung WY, Lee H, Lee E, Kim A and
Kim BH: Expression of SIRT1 and DBC1 in gastric adenocarcinoma.
Korean J Pathol. 46:523–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsushita T, Sasaki H, Takayama K, Ishida
K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M and
Kuroda R: The overexpression of SIRT1 inhibited osteoarthritic gene
expression changes induced by interleukin-1β in human chondrocytes.
J Orthop Res. 31:531–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen F, Xu C, Du L, Wang Y, Cao J, Fu Y,
Guo Y, Liu Q and Fan F: Tat-SmacN7 induces radiosensitization in
cancer cells through the activation of caspases and induction of
apoptosis. Int J Oncol. 42:985–992. 2013.PubMed/NCBI
|
|
26
|
Fu Y, Wang Y, Du L, Xu C, Cao J, Fan T,
Liu J, Su F, Fan S, Liu Q and Fan F: Resveratrol inhibits ionising
irradiation-induced inflammation in MSCs by activating SIRT1 and
limiting NLRP-3 inflammasome activation. Int J Mol Sci.
14:14105–14118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kitada M and Koya D: SIRT1 in Type 2
Diabetes: Mechanisms and therapeutic potential. Diabetes Metab J.
37:315–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bernstein BE, Meissner A and Lander ES:
The mammalian epigenome. Cell. 128:669–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vaquero A, Scher M, Erdjument-Bromage H,
Tempst P, Serrano L and Reinberg D: SIRT1 regulates the histone
methyl-transferase SUV39H1 during heterochromatin formation.
Nature. 450:440–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nadtochiy SM, Redman E, Rahman I and
Brookes PS: Lysine deacetylation in ischaemic preconditioning: The
role of SIRT1. Cardiovasc Res. 89:643–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin SM, Cho IJ and Kim SG: Resveratrol
protects mitochondria against oxidative stress through
AMP-activated protein kinase-mediated glycogen synthase
kinase-3beta inhibition downstream of poly (ADP-ribose)
polymerase-LKB1 pathway. Mol Pharmacol. 76:884–895. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramadori G, Lee CE, Bookout AL, Lee S,
Williams KW, Anderson J, Elmquist JK and Coppari R: Brain SIRT1:
anatomical distribution and regulation byenergy availability. J
Neurosci. 28:9989–9996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanno M, Sakamoto J, Miura T, Shimamoto K
and Horio Y: Nucleocytoplasmic shuttling of the
NAD+-dependent histone deacetylase SIRT1. J Biol Chem.
282:6823–6832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wassmann S, Wassmann K and Nickenig G:
Modulation of oxidant and antioxidant enzyme expression and
function in vascular cells. Hypertension. 44:381–386. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang
Z, Wang Z, Wang JM and Le Y: Resveratrol differentially modulates
inflammatory responses of microglia and astrocytes. J
Neuroinflammation. 7:462010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Terashvili M, Pratt PF, Gebremedhin D,
Narayanan J and Harder DR: Reactive oxygen species cerebral
autoregulation in health and disease. Pediatr Clin North Am.
53:1029–1037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang F, Liu J and Shi JS:
Anti-inflammatory activities of resveratrol in the brain: Role of
resveratrol in microglial activation. Eur J Pharmacol. 636:1–7.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu Y, Liu J, Wang J and Liu Q: The
controversial links among calorie restriction, SIRT1 and
resveratrol. Free Radic Biol Med. 51:250–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nisoli E, Tonello C, Cardile A, Cozzi V,
Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E,
Moncada S, et al: Calorie restriction promotes mitochondrial
biogenesis byinducing the expression of eNOS. Science. 310:314–317.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, Geny B, et al: Resveratrol improves mitochondrial
function and protects against metabolic disease by activating SIRT1
and PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Csiszar A, Labinskyy N, Pinto JT, Ballabh
P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, et
al: Resveratrol induces mitochondrial biogenesis in endothelial
cells. Am J Physiol Heart Circ Physiol. 297:H13–H20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pearson KJ, Baur JA, Lewis KN, Peshkin L,
Price NL, Labinskyy N, et al: Resveratrol delays age-related
deterioration and mimics transcriptional aspects of dietary
restriction without extending life span. Cell Metab. 8:157–168.
2008.
Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang
Z, Wang Z, Wang JM and Le Y: Resveratrol differentially modulates
inflammatory responses of microglia and astrocytes. J
Neuroinflammation. 7:462010. View Article : Google Scholar : PubMed/NCBI
|