Introduction
Wound healing is a complex and dynamic process that
is essential for tissue homeostasis. In normal adult wound healing,
disruption to skin integrity triggers a series of coordinated
events typically classified into three overlapping phases: The
inflammatory phase entails recruitment of inflammatory cells into
the wound; the proliferative phase involves the formation of
granulation tissue and re-epithelialisation; and during the
remodelling phase, the wound contracts and the scar matures
(1). Chronic wounds may be defined
as those that fail to progress through the reparative processes
required to restore tissue integrity within three months (2). This inability to heal is associated
with cellular and molecular abnormalities within the wound bed and
often involves chronic inflammation (3). Chronic wounds represent a significant
burden to the UK National Health Service, with an estimated cost of
>£1 billion per year (4). One of
the major challenges for clinicians is recognising which wounds
will heal and which will become chronic; therefore, methods for
early diagnosis of the non-healing wound are important for earlier
and more aggressive intervention.
In 1988, a novel growth factor for precursor B cells
was identified and designated lymphopoietin-1 (5). Now more commonly known as interleukin
(IL)-7, its functional significance and therapeutic potential
continue to be investigated. As a pleiotropic cytokine, IL-7 is
involved in early B and T cell development (5–10),
growth, maintenance and differentiation of thymocytes (11–14) and
peripheral T-cell homeostasis (15–17).
IL-7 expression has been detected in numerous types of tissue,
including bone marrow (5), thymus
gland (9,12), liver, kidney, spleen (8), intestine (18,19) and
skin (6,19,20).
The IL-7 receptor (IL-7R) complex is a heterodimer
of transmembrane proteins, the IL-7 specific α chain (also known as
CD127) and the common γ chain (CD132) (21,22).
Neither component is unique to IL-7 signalling, with the α chain
also being utilised by thymic stromal lymphopoietin (23,24) and
the common γ chain by other members of the interleukin family,
including IL-2, IL-4, IL-9 and IL-15 (25–28).
Although it is predominantly expressed by cells of the lymphoid
lineage, IL-7R has also been detected in human endothelial cell
lines (29) and several cancer cell
lines, including lung, central nervous system, renal, colon, breast
and skin cancer, as well haematological malignancies (30). It also exists in a soluble form that
is capable of binding IL-7 in solution (21). IL-7R activation by IL-7 binding leads
to dose-dependent phosphorylation of Janus kinase (JAK)-1 and JAK-3
and subsequent phosphorylation of signal transducer and activator
of transcription 5, which then translocates to the nucleus to
induce gene transcription (31,32), as
reviewed by Mazzucchelli and Durum (33). IL-7 has also been demonstrated to
activate the phosphatidylinositol 3 kinase (PI3K)/protein kinase B
(Akt) signalling pathway in murine and human thymocytes which is
known to influence cell survival (32,34,35);
however, the exact nature of this signalling pathway is not fully
understood.
Although its role in immunological development has
been known for some time, studies with IL-7 transgenic mice
revealed its potential to act as an oncogene in vivo and
promote the malignant transformation of B and T cells (36). IL-7 has been demonstrated to affect
cell growth and survival in certain haematological malignancies,
including acute lymphoblastic leukaemia (37–39),
cutaneous T cell lymphoma (20,40,41),
Hodgkin's disease (42), acute
myeloid leukaemia (43) and chronic
lymphocytic leukaemia (39,43,44).
IL-7 mRNA has also been identified in numerous solid organ tumours,
including Warthin's tumour of the parotid gland (45), head and neck squamous cell carcinomas
(46), renal cell carcinoma
(47), oesophageal carcinoma
(48), colorectal carcinoma
(49) and breast carcinoma (19). The exact role of IL-7 in these
tumours is not fully understood, however it is thought to affect
lymphocytes (49); for instance, in
cutaneous T-cell lymphoma, IL-7 has been shown to support the
growth of malignant T-cells in the skin (21). Increased IL-7 expression in breast
cancer is associated with a higher tumour grade and poorer
prognostic outcome (19). This may
be due to the effects of aberrant IL-7 expression on the
development, growth and differentiation of breast cancer (19), the ability of IL-7 to act as a potent
growth factor for breast cancer and endothelial cells (50) and/or the ability of IL-7 to stimulate
lymphangiogenesis in breast cancer cells in vitro and in a
mouse model (51,52). These findings are concordant with
analyses conducted on non-small cell lung cancer (NSCLC), in which
tumours with high IL-7 expression were more advanced and more
likely to have metastasised to lymph nodes, possibly due to
stimulation of lymphangiogenesis (53). Postoperative survival rates were
shorter in patients with higher levels of IL-7/IL-7R expression
(54). Furthermore, the expression
levels of IL-7 have been reported to correlate with tumour stage
and the presence of lymph node metastases (54). In vitro studies have
demonstrated that IL-7 stimulates lung cancer cell proliferation
and increases cyclin D1 mRNA and protein expression, higher levels
of which correlate with reduced survival rates in patients with
NSCLC (55).
During the study of IL-7 within the fields of
haematology and immunology, it was noted that murine and human
normal keratinocytes express IL-7 mRNA and protein in vitro
(6,20,56). It
appears that one function of IL-7 in skin is to promote the
survival and growth of epidermal T-cells (56,57).
These results prompted further investigation of the role of IL-7 in
inflammatory cutaneous disease, and its involvement has been
suggested in atopic dermatitis (6),
bullous pemphigoid (58) and
cutaneous T-cell lymphoma (20). To
the best of our knowledge, no reports have been published regarding
a correlation between IL-7 and wound healing. Parallels have been
made between the pathophysiological parameters observed in cancer
biology and wound healing (59,60).
Given the involvement of IL-7 in inflammation and immune responses,
its role in tumour development and progression and its expression
by human keratinocytes, the present study investigated the effect
of IL-7 on wound healing.
Materials and methods
Wound tissue cohort
Information regarding the wound tissue cohort and
collection has been previously described (61). Briefly, the tissue cohort consisted
of 71 chronic venous leg ulcer wound edge biopsies. The tissue
samples were collected from patients attending the University of
Wales wound healing clinic, following ethical approval by the South
East Wales Research Ethics Committee (reference no. 09/WSE02/59).
Informed written consent was provided by all patients. Samples were
obtained from 71 patients between 2010 and 2013, 43% of whom were
male and 57% were female. The mean age of these patients was 72.2
years, with a sample range of 34–99 years old. Over a 12 week
follow-up of the subjects, 20 chronic wounds displayed substantial
healing following conventional compression therapy and these
samples were thus defined as ‘healing’ chronic wounds. The
remaining chronic wounds that remained static or were enlarged over
the 12-week period of conventional therapy were termed
‘non-healing’ chronic wounds for the purpose of this study.
Biopsies were initially stored at −80°C prior to immersion in
liquid nitrogen. The tissue biopsies were sectioned using a CM1950
cryostat (Leica Microsystems Ltd., Milton Keynes, UK) at a size of
7 µm for immunohistochemical analysis and 20 µm for use in RNA
extraction and generation of cDNA for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis, where multiple tissue sections were combined and
homogenised using a hand held homogeniser (Cole-Parmer Instrument
Co., Ltd., London, UK) in ice-cold total RNA isolation reagent
(TRI) reagent® (Sigma-Aldrich Co., Ltd., Irvine,
UK).
Reagents, cell lines and culture
conditions
The HaCaT human keratinocyte cell line was purchased
from the German Cancer Research Institute (Heidelberg, Germany).
The cells were grown and maintained at 37°C, 5% CO2 and
95% humidity in Dulbecco's modified Eagle's medium (Sigma-Aldrich
Co., Ltd.) supplemented with 10% foetal calf serum (Sigma-Aldrich
Co., Ltd.) and 100X antibiotic antimycotic solution (Sigma-Aldrich
Co., Ltd.; final concentration 50,000 units penicillin, 50 mg
streptomycin and 125 µg amphotericin B per 500 ml). Recombinant
human IL-7 (rhIL-7) was purchased from R&D Systems (Abingdon,
UK). The neuronal Wiskott-Aldrich syndrome protein (N-WASp)
inhibitor Wiskostatin and the Akt inhibitor were purchased from
Merck Millipore (Calbiochem; Watford, UK).
RNA extraction and reverse
transcription
Total RNA was extracted from the homogenised
sections of wound tissue and human keratinocytes using TRI reagent
as described in the manufacturer's protocol (Sigma-Aldrich Co.,
Ltd.). Once extracted, the RNA was quantified using a
spectrophotometer (WPA UV 1101; Biotech Photometer, Cambridge, UK)
and RNA quantity was standardised to 250 ng prior to reverse
transcription using an iScript cDNA synthesis kit according to the
manufacturer's instructions (Bio-Rad Laboratories Ltd., Hemel
Hempstead, UK). Subsequently, cDNA was diluted 1:16 with
nuclease-free water (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and used for RT-qPCR.
Immunohistochemical staining
Frozen wound tissue sections were fixed in dried
acetone (Thermo Fisher Scientific, Inc.) for 15 min, air dried for
15 min and then hydrated in Tris-buffered saline (TBS). The tissue
sections were incubated in wash buffer containing 10% horse serum
(Sigma-Aldrich Co., Ltd.) for 1 h prior to incubation with
monoclonal mouse anti-IL-7 primary antibody (cat. no. MAB207;
R&D Systems) diluted at a 1:100 concentration (2 µg/ml final
concentration) for 1 h at room temperature. Subsequently, the
tissue sections were washed four times in TBS buffer prior to
identification of primary antibody binding using a Vectastain Elite
ABC (Universal) avidin-biotin peroxidase kit in accordance with the
manufacturers protocol (Vector Laboratories, Ltd., Peterborough,
UK). Visualisation of the staining intensity was obtained through
the addition of 3,3′-diaminobenzidine to the tissue sections.
Finally, tissue sections were counterstained with haematoxylin
(Vector Laboratories, Ltd.), washed thoroughly in tap water and
subjected to dehydration through a graded series (50, 70, 90, 100,
100%) of absolute ethanol (Thermo Fisher Scientific, Inc.), prior
to being cleared in xylene and mounted in DPX mounting medium
(Merck Millipore). Staining intensity and localisation were
visualised under a Leica DM1000 LED microscope (Leica Microsystems
Ltd.). Negative controls were prepared using only the secondary
universal antibody contained within the Vectastain Elite ABC
(Universal) kit, in accordance with the manufacturer's
protocol.
RT-qPCR
IL-7 expression levels were examined within the
wound tissue cohort using RT-qPCR as previously described (61,62).
Briefly, the primer pairs were designed incorporating a
complementary sequence (termed the Z sequence) to the Amplifluor
uniprimer probe (InterGen, New York, NY, USA) into the reverse
primer. RT-qPCR was undertaken using an IQ5 system (Bio-Rad
Laboratories, Ltd.) and transcript copy numbers were calculated
based on the quantification of a defined internal standard run on
the same plate. Sample/standard cDNA was added to iQ supermix
(Bio-Rad Laboratories Ltd.), target specific forward primer (10
pM), reverse primer containing the Z sequence (1 pM) and the
Amplifluor uniprimer probe (10 pM). The reaction conditions were as
follows: 15 min at 95°C followed by 80 cycles of 95°C for 15 sec,
55°C for 60 sec and 72°C for 20 sec. Sample transcript number was
subsequently normalised based on sample total expression levels of
the GAPDH housekeeping gene. Full primer details are presented in
Table I.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Primer | Forward
(5′-3′) | Reverse
(3′-5′) |
|---|
| IL-7 |
ATTGTGATATTGAAGGTAAAGATG |
ACTGAACCTGACCGTACAGCACGGAATAAA |
| GAPDH |
AAGGTCATCCATGACAACTT |
ACTGAACCTGACCGTACAGCCATCCACAGTCTTCTG |
In vitro growth assay
An in vitro cell growth assay was used to
examine the impact of IL-7 on HaCaT cell growth. HaCaT cells were
seeded at a density of 3×103 cells/well into triplicate
96-well plates. Plates were treated, where required, with the
indicated concentration of rhIL-7 (0, 1, 10 and 100 ng/ml) and
incubated overnight for 3 or 5 days. At each incubation point the
appropriate 96-well plate was fixed in 4% formaldehyde (v/v) and
stained with 0.5% (w/v) crystal violet (Sigma-Aldrich Co., Ltd.).
Plates were subsequently treated with 10% acetic acid (v/v) and
placed in an ELx800 spectrophotometer plate reader (Bio-Tek
Instruments Inc., Winooski, VT, USA).
Electric cell-substrate impedance
sensing (ECIS) detection of cell migration
Cell migration was detected using an ECIS Zθ system
(Applied Biophysics Inc., Troy, NY, USA) as described previously
(63). Briefly, cells were added to
96-well electrode arrays (96W1E) in identical numbers (80,000
cells/well) and allowed to form a fully confluent monolayer.
Following confluence, the cells were wounded through the
application of 6 V for 30 sec/well, generating a consistently sized
‘wound’ in the monolayer. The change in resistance was measured in
each well as the cells migrated back to recolonise the electrode.
This process was completed in the presence of varying
concentrations of rhIL-7 (0, 1, 10 and 100 ng/ml) and the rate of
change of resistance was taken as an indication of cellular
migration. Subsequently, 20 ng/ml of rhIL-7 was used in conjunction
with a range of small molecule inhibitors to determine potential
interactions between these signalling pathways on IL-7-regulated
migration.
Statistical analysis
The SigmaPlot 11 statistical software package
(Systat Software Inc., London, UK) was used to identify
statistically significant differences between experimental groups.
The data were analysed using parametric two sample, two-tailed
t-test or analysis of variance (ANOVA), or non-parametric
Mann-Whitney or ANOVA on RANKS depending on normality. All in
vitro assays were repeated a minimum of three times. Data is
presented as mean ± standard deviation or median ± interquartile
range. P<0.05 was considered to indicate a statistically
significant result.
Results
IL-7 expression levels in healing and
non-healing chronic wounds
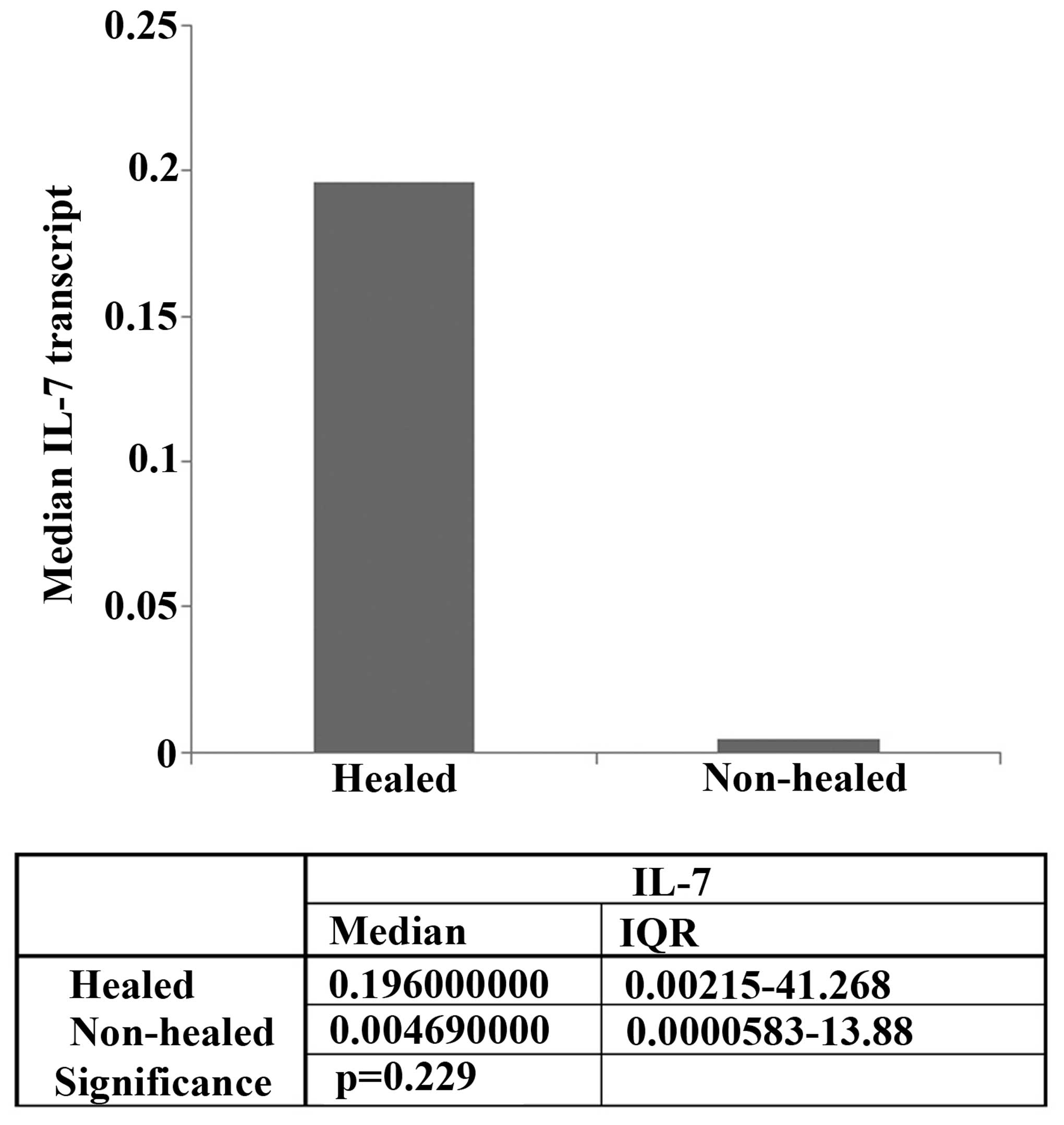
RT-qPCR was used to compare the mRNA levels of IL-7
expression in the healing and non-healing wounds (Fig. 1). IL-7 expression levels were higher
in healing chronic wounds (median expression, 0.196) compared with
non-healing chronic wounds (median expression, 0.00469), although
this difference was not statistically significant (P=0.229).
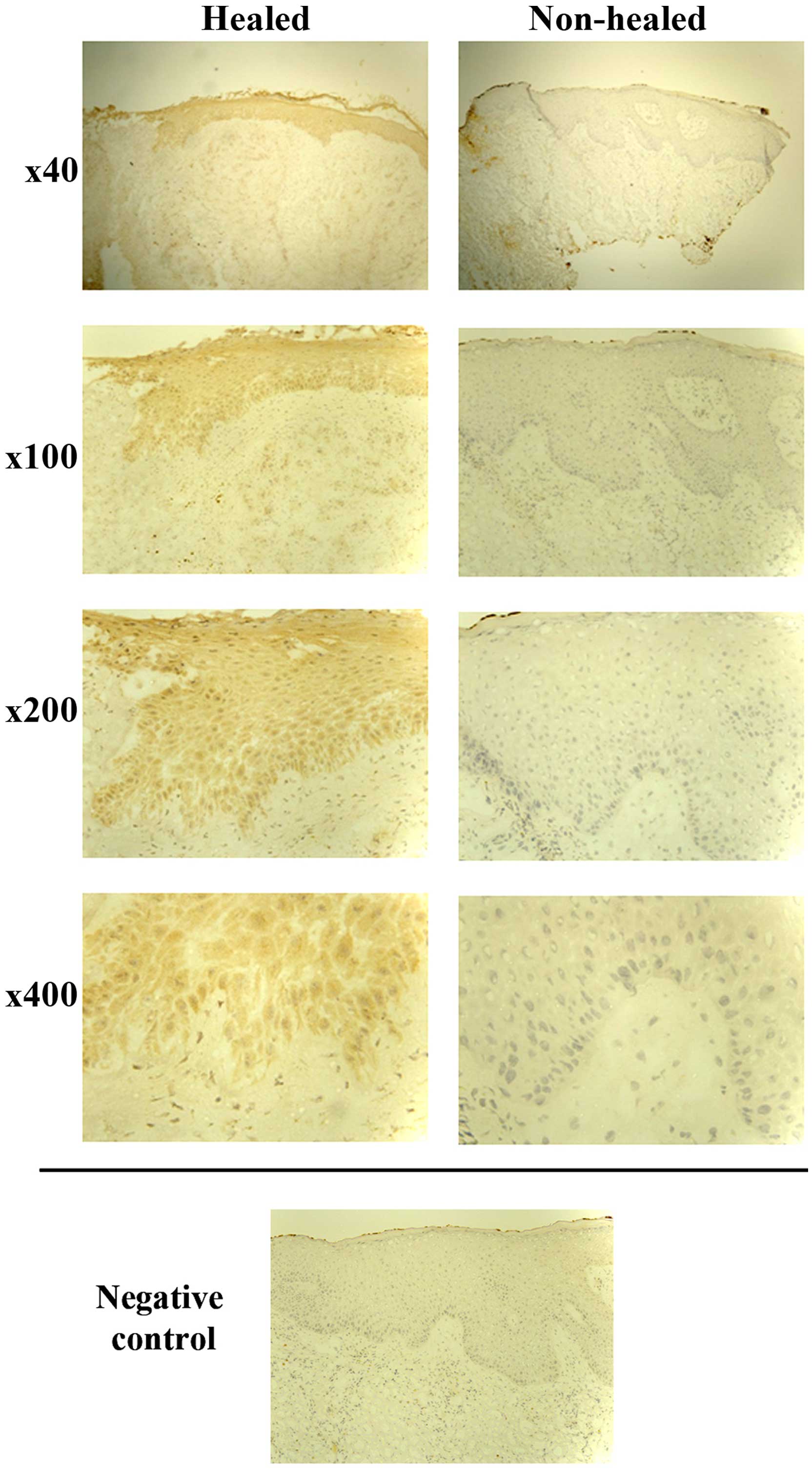
Immunohistochemical analysis was performed on 26
representative chronic wound tissue samples. A total of 13 tissue
samples were defined as non-healing (wound size had increased or
remained the same), and the remaining 13 as healing (wounds had
either completely healed or there was a decrease in size). In line
with the RT-qPCR findings, IL-7 expression was generally enhanced
in healing wound tissue and the majority of healing wounds (8/13)
showed cytoplasmic IL-7 expression in all the layers of the
epidermis. By contrast, the majority of the non-healing wounds did
not show IL-7 expression (9/13), and in cases where faint staining
was observed, it was localised in the basal layer (Fig. 2).
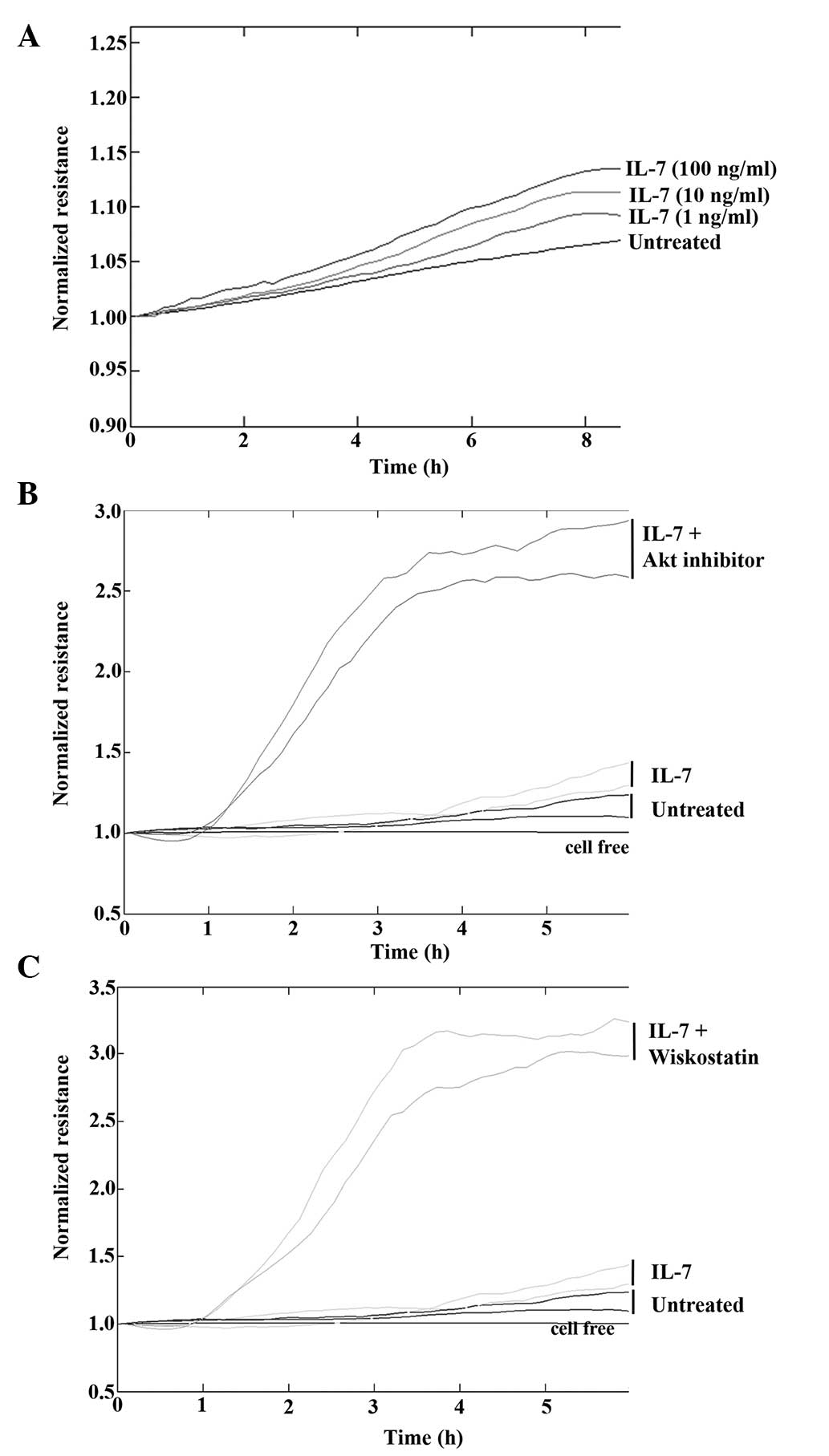
IL-7 enhances keratinocyte migration
in vitro
To determine the effect of IL-7 on keratinocyte
migration in vitro, HaCaT cells were treated with various
concentrations of rhIL-7 and cell migration was analysed using the
ECIS system. Following electrical wounding, HaCaT cells treated
with rhIL-7 migrated at a faster rate compared with untreated cells
(Fig. 3A) and there appeared to be a
concentration-dependent gradient, with the maximum effect observed
following treatment with 100 ng/ml rhIL-7. To further investigate
this effect, cell migration was examined in the presence of IL-7
and numerous small molecule signalling pathway inhibitors.
Concordant with the original observations, treatment of HaCaT cells
with IL-7 (20 ng/ml) induced an increase in cell migration rates
compared with the untreated controls. However, marked effects on
cell migration were observed when rhIL-7 was combined with an Akt
inhibitor (Fig. 3B) or N-WASp
inhibitor (Fig. 3C). In both cases,
the combination of rhIL-7 with the inhibitor induced an evident
increase in cellular migration rate.
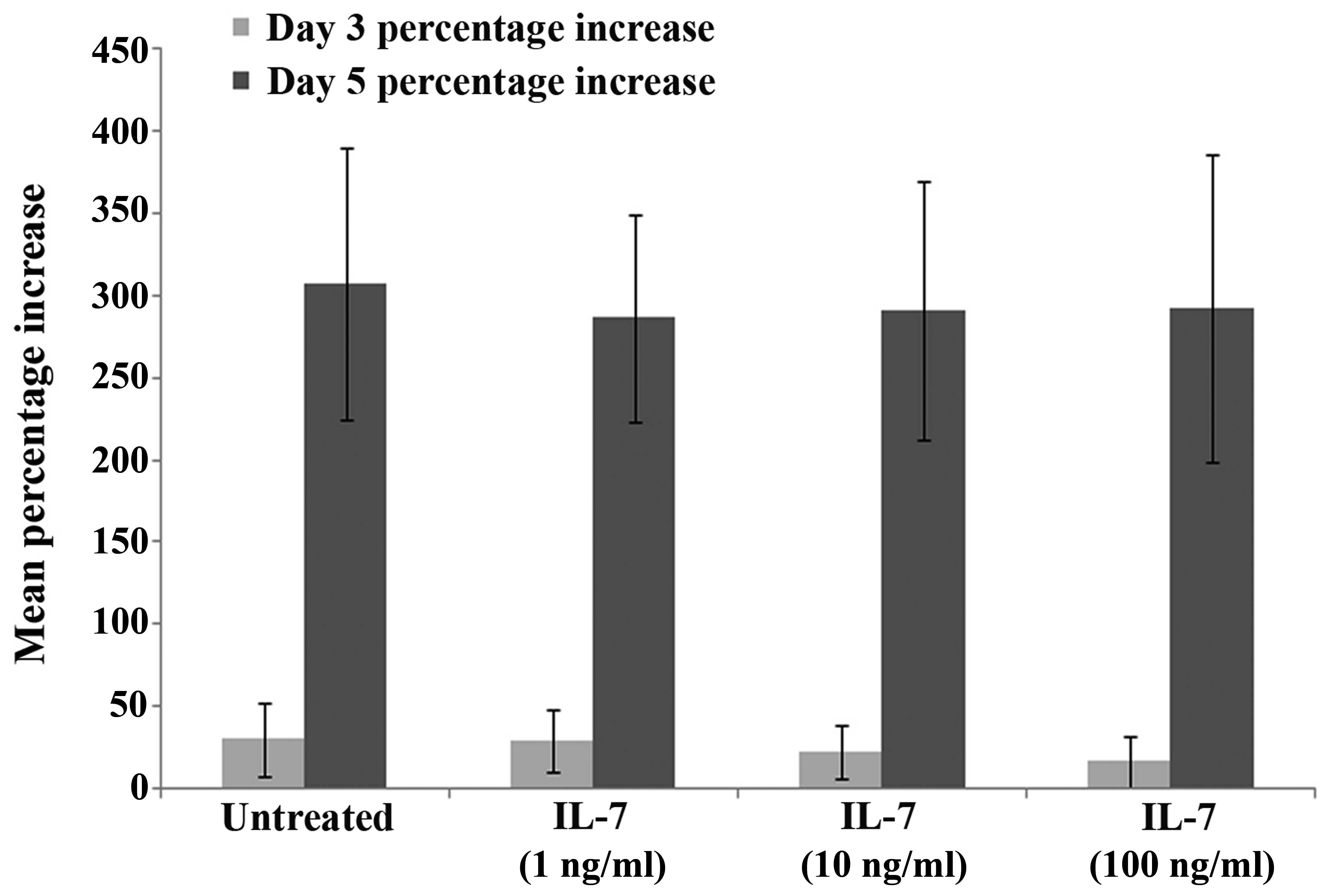
IL-7 does not affect keratinocyte
growth in vitro
To determine the effect of IL-7 on keratinocyte
growth, HaCaT cells were treated with various concentrations of
rhIL-7 and cell growth was analysed over a 3-day or 5-day
incubation period. No difference in cell growth was observed
between the untreated HaCaT controls and the cells treated with
various concentrations of rhIL-7 at day 3 or 5, and there were no
statistically significant differences observed between the groups
at days 3 or 5 (P>0.05; Fig.
4).
Discussion
Wound healing is a complex process that requires
interaction between numerous cytokines and growth factors (1). A greater understanding of these factors
and interactions may provide an insight into possible treatments of
chronic wounds. Therefore, the present study investigated the
presence of IL-7 in healing and non-healing wounds, and its effect
on keratinocytes in vitro.
IL-7 is a pleomorphic cytokine expressed in normal
human keratinocytes (6), where it
has been previously shown to support epidermal T-cell growth and
survival (56,57). It has an important role in
immunological development, including involvement in early B- and
T-cell development (5–10) and peripheral T-cell homeostasis
(15–17). IL-7 may also act as an oncogene,
stimulating malignant transformation, and cell growth and survival
in haematological cancers (36–43).
The results of the present study demonstrated that
IL-7 expression was higher in healing chronic wounds compared with
non-healing chronic wounds, although the difference was not
statistically significant. This was supported by
immunohistochemical analysis, which also demonstrated that IL-7
expression was enhanced in healing chronic wounds, being expressed
in all layers of the epidermis. To investigate the potential
underlying causes for this, the effects of rhIL-7 on human
keratinocytes were analysed in vitro. Although rhIL-7
treatment led to faster keratinocyte migration, it did not affect
the cell growth. Therefore, if IL-7 is affecting wound healing, it
appears that the mechanism underlying its effects may occur via
enhanced keratinocyte migration. Given that previous studies have
demonstrated the role of IL-7 as a growth factor for epidermal
T-cell growth and survival (56,57), it
is possible that IL-7 may be involved in the inflammatory phase of
wound healing. However, a study of mRNA expression in human dermal
wounds demonstrated that IL-7 mRNA expression levels initially
decreased then increased in the middle and late phases of wound
healing (64). These results are
concordant with the data of the present study, which indicated that
rhIL-7 enhanced keratinocyte migration, a process that occurs
during re-epithelialisation. IL-7 was shown to be a growth factor
for endothelial cells in breast cancer studies (51,52);
therefore, it may influence neoangiogenesis and re-vascularisation,
another essential element of wound healing that occurs following
the initial inflammatory phase. Since IL-7 supports cancer cell
proliferation and growth (20,50,54), it
was initially hypothesised that it may enhance keratinocyte growth
in vitro. However, no significant impact of rhIL-7 on HaCaT
cell growth was observed following in vitro assays, despite
the addition of high concentrations of rhIL-7.
Akt is a serine/threonine kinase involved in
numerous cellular signalling pathways. It is an important mediator
of numerous functions initiated by growth factor receptors that
activate PI3K (65). Akt is
oncogenic and contributes to the malignant behaviour of cells,
promoting cell survival, enhancing tumour cell invasion, and
stimulating motility (66). IL-7 has
been shown to activate the PI3K/Akt signalling pathway, promoting
the survival of certain immune and cancer cells (32,34,35,67). In
order to evaluate the potential signalling pathways involved in the
pro-migratory effect of rhIL-7 on HaCaT cells, two small inhibitors
(Akt and N-WASp) were used to target the possible downstream
signalling pathways of IL-7. Since previous studies suggested that
Akt stimulates cell motility, a reasonable hypothesis appears to be
that inhibiting Akt may negate the pro-migratory effect of IL-7.
However, the opposite was shown, since a marked increase in cell
migration was observed upon addition of the Akt inhibitor. A
previous study demonstrated that IL-24 inhibited keratinocyte
migration in vitro via an Akt-dependent signalling pathway,
where addition of an Akt inhibitor reversed the inhibition of
migration caused by IL-24 (61).
However, the results of the current study suggested that inhibition
of Akt, through small molecular inhibitors, had a pro-migratory
effect on HaCaT cells when combined with rhIL-7 treatment. The
precise mechanism underlying this phenomenon is not fully
known.
Wiskott-Aldrich syndrome (WAS) is a primary
immunodeficiency disorder, in which lymphocytes exhibit
cytoskeletal abnormalities and a reduced response to proliferative
stimuli (68). The WAS gene encodes
a prolene-rich protein, termed WASp. Neural WASp (N-WASp) is a 65
kDa protein with a 50% homology to WASp that was first identified
in the bovine brain (69). Unlike
WASp, which is only expressed in haematopoietic cells, N-WASp is
ubiquitous (68). N-WASp is required
for stimulation of actin polymerization, a process necessary for
cell movement and division (70). It
has also been shown to stabilise intracellular adherens junctions,
which maintain the endothelial barrier (71). The results of the present study
demonstrated that inhibition of N-WASp caused a marked increase in
the cellular migration rate with the addition of rhIL-7 in
vitro. This suggested that N-WASp may have been acting to
prevent migration, possibly replicating its activity in endothelial
cells.
In conclusion, as chronic wounds continue to pose a
significant health problem, investigations to uncover the complex
processes required for prompt wound healing are ongoing. The
results of the present study demonstrated that IL-7 may have a role
in keratinocyte migration and may be differentially expressed
between healing and non-healing chronic wounds. Further studies are
required to fully establish this association and the significance
of IL-7 in chronic wound healing and in the wound healing process
as a whole, using different clinical cohorts with acute and chronic
wound tissues. The data also suggested a potential association
between IL-7 and N-WASp and Akt signalling, although additional
investigation isrequired to fully understand this association and
its significance to clinical wound healing.
Acknowledgements
The present study was supported by the A4B Scheme of
the Welsh Government Ser Cymru, the NRN Life Sciences Research
Network, and Cancer Research Wales.
References
|
1
|
Behm B, Babilas P, Landthaler M and
Schreml S: Cytokines, chemokines and growth factors in wound
healing. J Eur Acad Dermatol Venereol. 26:812–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Werdin F, Tennenhaus M, Schaller HE and
Rennekampff HO: Evidence-based management strategies for treatment
of chronic wounds. Eplasty. 9:e192009.PubMed/NCBI
|
|
3
|
Demidova-Rice TN, Hamblin MR and Herman
IM: Acute and impaired wound healing: Pathophysiology and current
methods for drug delivery, part 2: Role of growth factors in normal
and pathological wound healing: Therapeutic potential and methods
of delivery. Adv Skin Wound Care. 25:349–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas DW and Harding KG: Wound healing.
Br J Surg. 89:1203–1205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Namen AE, Schmierer AE, March CJ, Overell
RW, Park LS, Urdal DL and Mochizuki DY: B cell precursor
growth-promoting activity. Purification and characterization of a
growth factor active on lymphocyte precursors. J Exp Med.
167:988–1002. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heufler C, Topar G, Grasseger A, Stanzl U,
Koch F, Romani N, Namen AE and Schuler G: Interleukin 7 is produced
by murine and human keratinocytes. J Exp Med. 178:1109–1114. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goodwin RG, Lupton S, Schmierer A,
Hjerrild KJ, Jerzy R, Clevenger W, Gillis S, Cosman D and Namen AE:
Human interleukin 7: Molecular cloning and growth factor activity
on human and murine B-lineage cells. Proc Natl Acad Sci USA.
86:302–306. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Namen AE, Lupton S, Hjerrild K, Wignall J,
Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D and Gillis
S: Stimulation of B-cell progenitors by cloned murine
interleukin-7. Nature. 333:571–573. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakata T, Iwagami S, Tsuruta Y, Teraoka H,
Tatsumi Y, Kita Y, Nishikawa S, Takai Y and Fujiwara H:
Constitutive expression of interleukin-7 mRNA and production of
IL-7 by a cloned murine thymic stromal cell line. J Leukoc Biol.
48:205–212. 1990.PubMed/NCBI
|
|
10
|
Watson JD, Morrissey PJ, Namen AE, Conlon
PJ and Widmer MB: Effect of IL-7 on the growth of fetal thymocytes
in culture. J Immunol. 143:1215–1222. 1989.PubMed/NCBI
|
|
11
|
Murray R, Suda T, Wrighton N, Lee F and
Zlotnik A: IL-7 is a growth and maintenance factor for mature and
immature thymocyte subsets. Int Immunol. 1:526–531. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolf SS and Cohen A: Expression of
cytokines and their receptors by human thymocytes and thymic
stromal cells. Immunology. 77:362–368. 1992.PubMed/NCBI
|
|
13
|
Grabstein KH, Namen AE, Shanebeck K, Voice
RF, Reed SG and Widmer MB: Regulation of T cell proliferation by
IL-7. J Immunol. 144:3015–3020. 1990.PubMed/NCBI
|
|
14
|
Uckun FM, Tuel-Ahlgren L, Obuz V, Smith R,
Dibirdik I, Hanson M, Langlie MC and Ledbetter JA: Interleukin 7
receptor engagement stimulates tyrosine phosphorylation, inositol
phospholipid turnover, proliferation and selective differentiation
to the CD4 lineage by human fetal thymocytes. Proc Natl Acad Sci
USA. 88:6323–6327. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chazen GD, Pereira GM, LeGros G, Gillis S
and Shevach EM: Interleukin 7 is a T-cell growth factor. Proc Natl
Acad Sci USA. 86:5923–5927. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Londei M, Verhoef A, Hawrylowicz C, Groves
J, De Berardinis P and Feldmann M: Interleukin 7 is a growth factor
for mature human T cells. Eur J Immunol. 20:425–428. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simonetta F, Gestermann N, Martinet KZ,
Boniotto M, Tissières P, Seddon B and Bourgeois C: Interleukin-7
influences FOXP3+CD4+ regulatory T cells peripheral homeostasis.
PloS One. 7:e365962012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe M, Ueno Y, Yajima T, Iwao Y,
Tsuchiya M, Ishikawa H, Aiso S, Hibi T and Ishii H: Interleukin 7
is produced by human intestinal epithelial cells and regulates the
proliferation of intestinal mucosal lymphocytes. J Clin Invest.
95:2945–2953. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Rawi MA, Rmali K, Watkins G, Mansel RE
and Jiang WG: Aberrant expression of interleukin-7 (IL-7) and its
signalling complex in human breast cancer. Eur J Cancer.
40:494–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dalloul A, Laroche L, Bagot M, Mossalayi
MD, Fourcade C, Thacker DJ, Hogge DE, Merle-Béral H, Debré P and
Schmitt C: Interleukin-7 is a growth factor for Sézary lymphoma
cells. J Clin Invest. 90:1054–1060. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goodwin RG, Friend D, Ziegler SF, Jerzy R,
Falk BA, Gimpel S, Cosman D, Dower SK, March CJ and Namen AE:
Cloning of the human and murine interleukin-7 receptors:
Demonstration of a soluble form and homology to a new receptor
superfamily. Cell. 60:941–951. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ziegler SE, Morella KK, Anderson D, Kumaki
N, Leonard WJ, Cosman D and Baumann H: Reconstitution of a
functional interleukin (IL)-7 receptor demonstrates that the IL-2
receptor gamma chain is required for IL-7 signal transduction. Eur
J Immunol. 25:399–404. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quentmeier H, Drexler HG, Fleckenstein D,
Zaborski M, Armstrong A, Sims JE and Lyman SD: Cloning of human
thymic stromal lymphopoietin (TSLP) and signaling mechanisms
leading to proliferation. Leukemia. 15:1286–1292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pandey A, Ozaki K, Baumann H, Levin SD,
Puel A, Farr AG, Ziegler SF, Leonard WJ and Lodish HF: Cloning of a
receptor subunit required for signaling by thymic stromal
lymphopoietin. Nat Immunol. 1:59–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kondo M, Takeshita T, Higuchi M, Nakamura
M, Sudo T, Nishikawa S and Sugamura K: Functional participation of
the IL-2 receptor gamma chain in IL-7 receptor complexes. Science.
263:1453–1454. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Russell SM, Keegan AD, Harada N, Nakamura
Y, Noguchi M, Leland P, Friedmann MC, Miyajima A, Puri RK and Paul
WE: Interleukin-2 receptor gamma chain: A functional component of
the interleukin-4 receptor. Science. 262:1880–1883. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimura Y, Takeshita T, Kondo M, Ishii N,
Nakamura M, Van Snick J and Sugamura K: Sharing of the IL-2
receptor gamma chain with the functional IL-9 receptor complex. Int
Immunol. 7:115–120. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson DM, Kumaki S, Ahdieh M, Bertles
J, Tometsko M, Loomis A, Giri J, Copeland NG, Gilbert DJ and
Jenkins NA: Functional characterization of the human interleukin-15
receptor alpha chain and close linkage of IL15RA and IL2RA genes. J
Biol Chem. 270:29862–29869. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dus D, Krawczenko A, Załecki P, Paprocka
M, Wiedłocha A, Goupille C and Kieda C: IL-7 receptor is present on
human microvascular endothelial cells. Immunol Lett. 86:163–168.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cosenza L, Gorgun G, Urbano A and Foss F:
Interleukin-7 receptor expression and activation in
nonhaematopoietic neoplastic cell lines. Cell Signal. 14:317–325.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Foxwell BM, Beadling C, Guschin D, Kerr I
and Cantrell D: Interleukin-7 can induce the activation of Jak 1,
Jak 3 and STAT 5 proteins in murine T cells. Eur J Immunol.
25:3041–3046. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pallard C, Stegmann AP, van Kleffens T,
Smart F, Venkitaraman A and Spits H: Distinct roles of the
phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated
development of human thymocyte precursors. Immunity. 10:525–535.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mazzucchelli R and Durum SK: Interleukin-7
receptor expression: Intelligent design. Nat Rev Immunol.
7:144–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li WQ, Jiang Q, Khaled AR, Keller JR and
Durum SK: Interleukin-7 inactivates the pro-apoptotic protein Bad
promoting T cell survival. J Biol Chem. 279:29160–29166. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dadi HK and Roifman CM: Activation of
phosphatidylinositol-3 kinase by ligation of the interleukin-7
receptor on human thymocytes. J Clin Invest. 92:1559–1563. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rich BE, Campos-Torres J, Tepper RI,
Moreadith RW and Leder P: Cutaneous lymphoproliferation and
lymphomas in interleukin 7 transgenic mice. J Exp Med. 177:305–316.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Touw I, Pouwels K, van Agthoven T, van
Gurp R, Budel L, Hoogerbrugge H, Delwel R, Goodwin R, Namen A and
Löwenberg B: Interleukin-7 is a growth factor of precursor B and T
acute lymphoblastic leukemia. Blood. 75:2097–2101. 1990.PubMed/NCBI
|
|
38
|
Eder M, Ottmann OG, Hansen-Hagge TE,
Bartram CR, Gillis S, Hoelzer D and Ganser A: Effects of
recombinant human IL-7 on blast cell proliferation in acute
lymphoblastic leukemia. Leukemia. 4:533–540. 1990.PubMed/NCBI
|
|
39
|
Sasson SC, Smith S, Seddiki N, Zaunders
JJ, Bryant A, Koelsch KK, Weatherall C, Munier ML, McGinley C,
Yeung J, et al: IL-7 receptor is expressed on adult pre-B-cell
acute lymphoblastic leukemia and other B-cell derived neoplasms and
correlates with expression of proliferation and survival markers.
Cytokine. 50:58–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foss FM, Koc Y, Stetler-Stevenson MA,
Nguyen DT, O'Brien MC, Turner R and Sausville EA: Costimulation of
cutaneous T-cell lymphoma cells by interleukin-7 and interleukin-2:
Potential autocrine or paracrine effectors in the Sézary syndrome.
J Clin Oncol. 12:326–335. 1994.PubMed/NCBI
|
|
41
|
Qin JZ, Zhang CL, Kamarashev J, Dummer R,
Burg G and Dobbeling U: Interleukin-7 and interleukin-15 regulate
the expression of the bcl-2 and c-myb genes in cutaneous T-cell
lymphoma cells. Blood. 98:2778–2783. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Foss HD, Hummel M, Gottstein S, Ziemann K,
Falini B, Herbst H and Stein H: Frequent expression of IL-7 gene
transcripts in tumor cells of classical Hodgkin's disease. Am J
Pathol. 146:33–39. 1995.PubMed/NCBI
|
|
43
|
Digel W, Schmid M, Heil G, Conrad P,
Gillis S and Porzsolt F: Human interleukin-7 induces proliferation
of neoplastic cells from chronic lymphocytic leukemia and acute
leukemias. Blood. 78:753–759. 1991.PubMed/NCBI
|
|
44
|
Yoshioka R, Shimizu S, Tachibana J, Hirose
Y, Fukutoku M, Takeuchi Y, Sugai S, Takiguchi T and Konda S:
Interleukin-7 (IL-7)-induced proliferation of CD8+ T-chronic
lymphocytic leukemia cells. J Clin Immunol. 12:101–106. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Takeuchi T, Yamanouchi H, Yue Q and
Ohtsuki Y: Epithelial component of lymphoid stroma-rich Warthin's
tumour expresses interleukin (IL)-7. Histopathology. 32:383–384.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Paleri V, Pulimood A, Davies GR and
Birchall MA: Interleukins 7 and 12 are expressed in head and neck
squamous cancer. Clin Otolaryngol Allied Sci. 26:302–306. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Trinder P, Seitzer U, Gerdes J, Seliger B
and Maeurer M: Constitutive and IFN-gamma regulated expression of
IL-7 and IL-15 in human renal cell cancer. Int J Oncol. 14:23–31.
1999.PubMed/NCBI
|
|
48
|
Oka M, Hirose K, Iizuka N, Aoyagi K,
Yamamoto K, Abe T, Hazama S and Suzuki T: Cytokine mRNA expression
patterns in human esophageal cancer cell lines. J Interferon
Cytokine Res. 15:1005–1009. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maeurer MJ, Walter W, Martin D, Zitvogel
L, Elder E, Storkus W and Lotze MT: Interleukin-7 (IL-7) in
colorectal cancer: IL-7 is produced by tissues from colorectal
cancer and promotes preferential expansion of tumour infiltrating
lymphocytes. Scand J Immunol. 45:182–192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Al-Rawi MA, Rmali K, Mansel RE and Jiang
WG: Interleukin 7 induces the growth of breast cancer cells through
a wortmannin-sensitive pathway. Br J Surg. 91:61–68. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Al-Rawi MA, Watkins G, Mansel RE and Jiang
WG: The effects of interleukin-7 on the lymphangiogenic properties
of human endothelial cells. Int J Oncol. 27:721–730.
2005.PubMed/NCBI
|
|
52
|
Al-Rawi MA, Watkins G, Mansel RE and Jiang
WG: Interleukin 7 upregulates vascular endothelial growth factor D
in breast cancer cells and induces lymphangiogenesis in vivo. Br J
Surg. 92:305–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ming J, Zhang Q, Qiu X and Wang E:
Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent
vascular endothelial growth factor-D up-regulation: A mechanism of
lymphangiogenesis in lung cancer. Eur J Cancer. 45:866–873. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ming J, Zhang Q, Jiang Y, Qiu X and Bai X:
The expressions of IL-7 and IL-7R and the relationship between them
with lymph node metastasis and prognosis in non-small cell lung
cancer. Zhongguo Fei Ai Za Zhi. 13:1101–1106. 2010.(In Chinese).
PubMed/NCBI
|
|
55
|
Ming J, Jiang G, Zhang Q, Qiu X and Wang
E: Interleukin-7 up-regulates cyclin D1 via activator protein-1 to
promote proliferation of cell in lung cancer. Cancer Immunol
Immunother. 61:79–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Matsue H, Bergstresser PR and Takashima A:
Keratinocyte-derived IL-7 serves as a growth factor for dendritic
epidermal T cells in mice. J Immunol. 151:6012–6019.
1993.PubMed/NCBI
|
|
57
|
Takashima A, Matsue H, Bergstresser PR and
Ariizumi K: Interleukin-7-dependent interaction of dendritic
epidermal T cells with keratinocytes. J Invest Dermatol. 105(1
Suppl): 50S–53S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Giacalone B, D'Auria L, Bonifati C,
Ferraro C, Riccardi E, Mussi A, D'Agosto G, Cordiali-Fei P and
Ameglio F: Decreased interleukin-7 and transforming growth
factor-beta1 levels in blister fluids as compared to the respective
serum levels in patients with bullous pemphigoid. Opposite behavior
of TNF-alpha, interleukin-4 and interleukin-10. Exp Dermatol.
7:157–161. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bosanquet DC, Harding KG, Ruge F, Sanders
AJ and Jiang WG: Expression of IL-24 and IL-24 receptors in human
wound tissues and the biological implications of IL-24 on
keratinocytes. Wound Repair Regen. 20:896–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang WG, Sanders AJ, Ruge F and Harding
KG: Influence of interleukin-8 (IL-8) and IL-8 receptors on the
migration of human keratinocytes, the role of PLC-γ and potential
clinical implications. Exp Ther Med. 3:231–236. 2012.PubMed/NCBI
|
|
63
|
Jiang WG, Martin TA, Lewis-Russell JM,
Douglas-Jones A, Ye L and Mansel RE: Eplin-alpha expression in
human breast cancer, the impact on cellular migration and clinical
outcome. Mol Cancer. 7:712008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Palagummi S, Harbison S and Fleming R: A
time-course analysis of mRNA expression during injury healing in
human dermal injuries. Int J Legal Med. 128:403–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kandel ES and Hay N: The regulation and
activities of the multifunctional serine/threonine kinase Akt/PKB.
Exp Cell Res. 253:210–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
67
|
Barata JT, Silva A, Brandao JG, Nadler LM,
Cardoso AA and Boussiotis VA: Activation of PI3K is indispensable
for interleukin 7-mediated viability, proliferation, glucose use
and growth of T cell acute lymphoblastic leukemia cells. J Exp Med.
200:659–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ramesh N, Antón IM, Martínez-Quiles N and
Geha RS: Waltzing with WASp. Trends Cell Biol. 9:15–19. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Miki H, Miura K and Takenawa T: N-WASp, a
novel actin-depolymerizing protein, regulates the cortical
cytoskeletal rearrangement in a PIP2-dependent manner downstream of
tyrosine kinases. EMBO J. 15:5326–5335. 1996.PubMed/NCBI
|
|
70
|
Rohatgi R, Ma L, Miki H, Lopez M,
Kirchhausen T, Takenawa T and Kirschner MW: The interaction between
N-WASp and the Arp2/3 complex links Cdc42-dependent signals to
actin assembly. Cell. 97:221–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rajput C, Kini V, Smith M, Yazbeck P,
Chavez A, Schmidt T, Zhang W, Knezevic N, Komarova Y and Mehta D:
Neural Wiskott-Aldrich syndrome protein (N-WASp)-mediated
p120-catenin interaction with Arp2-Actin complex stabilizes
endothelial adherens junctions. J Biol Chem. 288:4241–4250. 2013.
View Article : Google Scholar : PubMed/NCBI
|