Introduction
The kidney undergoes a number of changes in internal
structure and function during pregnancy. During normal pregnancy,
the placenta and mother produce large amounts of hormones including
human chorionic gonadotropin (HCG), human placental lactogen
(h-PL), steroid hormones and estrogen (1). Such changes in hormone levels can lead
to changes in angiotasis and increase water-sodium retention and
the volume load, resulting in changes of maternal hemodynamics and
kidney structure and function (2–6). During
normal pregnancy, 24-h urine protein and urinary albumin excretion
increase significantly after 20 weeks to 200–300 mg and 12–19 mg,
respectively. The maximum urinary protein content can reach 500
mg/24 h (7,8).
The random urine albumin-creatinine ratio (ACR) is a
reliable method for determining the urine protein-creatinine ratio
and monitoring urinary protein excretion. The determination of the
urine protein-creatinine ratio can effectively reflect the 24-h
urine protein content of pregnant women. It is fast, simple,
accurate and has other positive aspects that make it an ideal
clinical indicator for the qualitative and quantitative diagnosis
of proteinuria and for follow-up. It can replace the traditional
24-h urine protein excretion quantification method (5). Clinically, proteinuria is often
determined with 24-h urine protein, and normal urinary protein
content is generally less than 0.15 g/24 h. However, this method is
associated with difficulties such as being greatly influenced by
maternal compliance. This is because of the longer urine sample
collection time, especially when colleting 24-h urine sample from
patients with young children. Therefore, the urine
protein-creatinine ratio can be determined immediately to predict
the 24 h urine protein content (9).
In the present study, random urine ACR was
incorporated into evaluation and the perinatal outcomes of women
were tracked and observed. Various risk factors affecting
gestational hypertension and proteinuria were comprehensively
analyzed to identify the risk factors of proteinuria of pregnant
women with hypertension.
Patients and methods
Inclusion and exclusion criteria
The inclusion criteria of the study were: i) Women
of childbearing age, >18; and ii) those with proteinuria during
pregnancy. The exclusion criteria for the study were: i) Diabetes
before pregnancy and previous history of hypertension; ii) pregnant
women with lung infection, urinary system infection or other
infections, cancers of the reproductive system or breast cancer;
iii) heart, liver, kidney, lung and other organ failure; iv) dying
from any diseases; v) abnormal blood clotting mechanism; vi)
pregnant women or their families who could not provide cooperation;
vii) history of mental illness; viii) not reaching the expected
date of confinement or those whose families required terminating
the pregnancy.
Clinical data
A total of 6,758 pregnant women with
pregnancy-induced hypertension and proteinuria were randomly
selected in the Yantai region from September, 2009 to June, 2011.
The average age of participants was 25.3±12.6 years, with mean
arterial pressure of 118.5±21.3 mmHg and 24-h urine protein content
of 121.7±14.5 mg.
Data collection
An experienced gynecologist collected the detailed
medical history, conducted the physical examination on the
participants and recorded their age, gender, height, weight, body
mass index (BMI), blood triglyceride, blood low-density lipoprotein
cholesterol, blood high-density lipoprotein cholesterol, serum
insulin, fasting blood glucose, glycated hemoglobin, aspartate
transaminase, γ-glutamyl transpeptidase, creatinine, history of
hypertension and history of diabetes.
Diagnostic criteria
The diagnostic criteria were as follows (5): According to the 1999 WHO diagnostic
criteria, ≥2 measurements were carried out where systolic blood
pressure >140 mmHg or diastolic blood pressure >90 mmHg, or
antihypertensive medications were being taken.
According to the 1999 WHO-IDF standards published,
≥2 random measurements were carried out where blood glucose
>11.1 mmol/l or fasting glucose >7.0 mmol/l, there was
definitive history of diabetes, or hypoglycemic agents were being
taken.
Urine ACR ratio was reliably correlated with 24-h
urine protein and the reference value was generally in the range of
0.10–0.20.
First morning urine was completely drained and urine
was collected from the second urination. The first urine time was
recorded and used as reference for 24 h on the following day. All
urine within 24 h was placed in a container and mixed evenly, then
100–200 ml was extracted. The protein content in the healthy urine
was generally 40–80 mg. Beyond this range, the diagnosis of
proteinuria was made.
Glomerular filtration rate (GFR): The amount of
filtered liquid generated from the two kidneys of normal adults was
80–120 ml/min.
Other biochemical indicators were tested with the
assistance of the clinical laboratory of Yantaishan Hospital
(Shandong, China).
The APGAR score (8)
included skin color, heart rate, reaction of flicking planta pedis
or inserting nasal tube, muscle tension and breathing of the
delivered newborn (Table I).
 | Table I.APGAR score criteria. |
Table I.
APGAR score criteria.
|
| Score criteria |
|---|
|
|
|
|---|
| Physical sign | 0 | 1 | 2 |
|---|
| Skin color | Purple or pale | The body was purplish
red and the limbs were blue and purple | The whole body was
red |
| Heart rate
(beats/min) | None | <100 | >100 |
| Reaction of flicking
planta pedis or inserting nasal tube | No response | Some actions were
made (such as frowning) | Cried and
sneezed |
| Muscle tension | Loose | Limbs were slightly
buckled | The limbs moved
freely |
| Breathing | None | Slow and
irregular | Normal, cried
loudly |
Statistical analysis
Data were presented as mean ± standard error. The
Pearson correlation analysis was performed to investigate
associations between various indicators. In addition, multivariate
and logistic regression analyses were carried out to identify the
variables predictive of APGAR scores. P<0.05 was consideted to
indicate a statistically significant difference.
Results
General clinical parameters of the
enrolled participants
A total of 6,758 pregnant women with combined
gestational hypertension and proteinuria diagnosed at the
Department of Obstetrics and Gynecology in Yantaishan Hospital from
September, 2009 to June, 2011 were randomly selected for the study.
Kidney function, blood pressure, history of gravidity and parity,
embryo number and birth weight of the enrolled participants were
determined. The average age of participants was 25.3±12.6 years,
with mean arterial pressure of 118.5±21.3 mmHg and 24-h urine
protein content of 121.7±14.5 mg. According to the medical
examination information of pregnant women, various indicators were
recorded before and after pregnancy and statistical analysis was
performed (Table II).
 | Table II.Summary of baseline clinical data of
participants. |
Table II.
Summary of baseline clinical data of
participants.
| Data | Age, years | HCa, month | SBP, mmHg | DBP, mmHg | MAP, mmHg | BMIa, (kg/m2) | AST, U/l | ALT, U/l | GGT, U/l | FPG, mmol/l | HbA1C, % | TC, mmol/l | HDL-C, mmol/l | LDL-C, mmol/l | GWa | Urine ACR | GFR, (ml/min) | Cr, mmol/l |
|---|
| After pregnancy | 24.3±16.4 | – | 102.4±27.5 | 66.8±12.7 |
87.3±12.6 | 24.31±1.8 | 17.83±2.4 | 13.25±4.2 | 18.12±1.4 | 3.25±0.27 | 4.82±0.13 | 178.6±22.3 | 68.4±10.1 | 98.6±11.3 | – | 0.13±0.06 | 92.8±12.5 | 58.2±12.7 |
| Perinatal period | – | 3.27±3.27 | 138.5±30.5 | 82.4±22.8 | 118.5±21.3 |
19.7±2.4 |
17.2±2.1 |
12.7±3.6 |
17.6±2.2 | 4.27±0.85 | 4.70±0.43 | 185.4±20.6 | 67.5±13.5 | 98.5±10.2 | 36.4±1.8 | 0.87±0.23 | 67.8±24.2 | 263.7±80.38 |
| T-value | – | – | 22.5 | 21.7 | 24.8 | 12.83 | 0.24 | 0.22 |
| 6.31 | 0.37 | 0.82 | 0.25 | 0.37 | – | 28.9 | 28.7 | 23.6 |
| P-value | – | – | 0.002 | 0.002 | 0.001 | 0.017 | 0.79 | 0.78 |
| 0.06 | 0.62 | 0.25 | 0.31 | 0.66 | – | 0.001 | 0.001 | 0.001 |
Pearson correlation analysis between
perinatal outcomes and other factors
We recorded and statistically analyzed the APGAR
scores of newborns delivered by the participants. According to a
literature review, the indicators affecting APGAR score during
pregnancy were screened as independent variables and a correlation
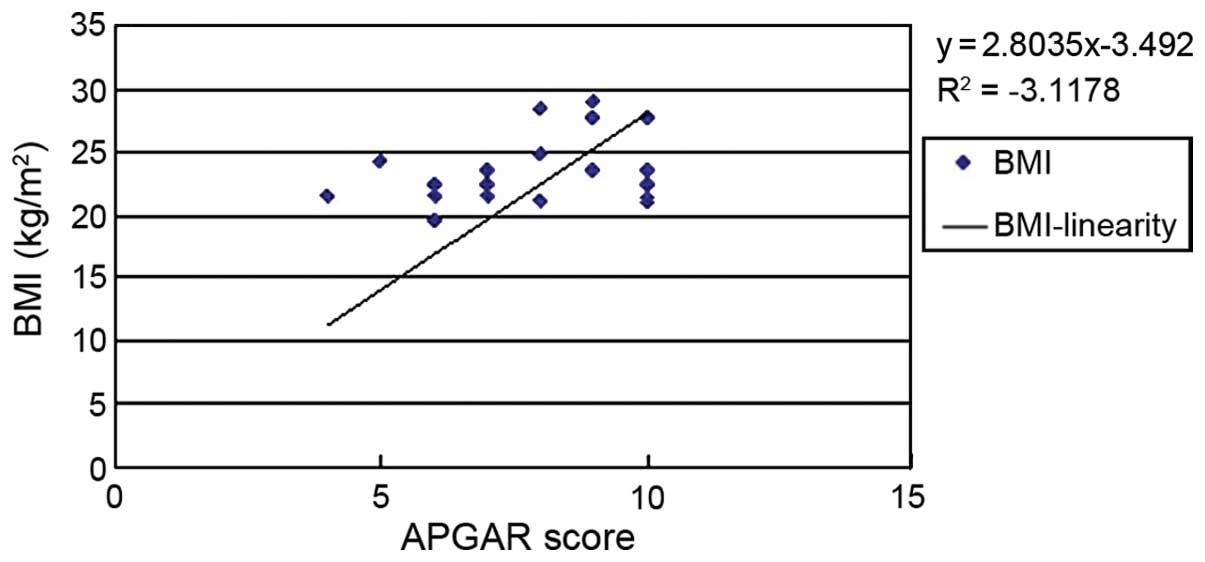
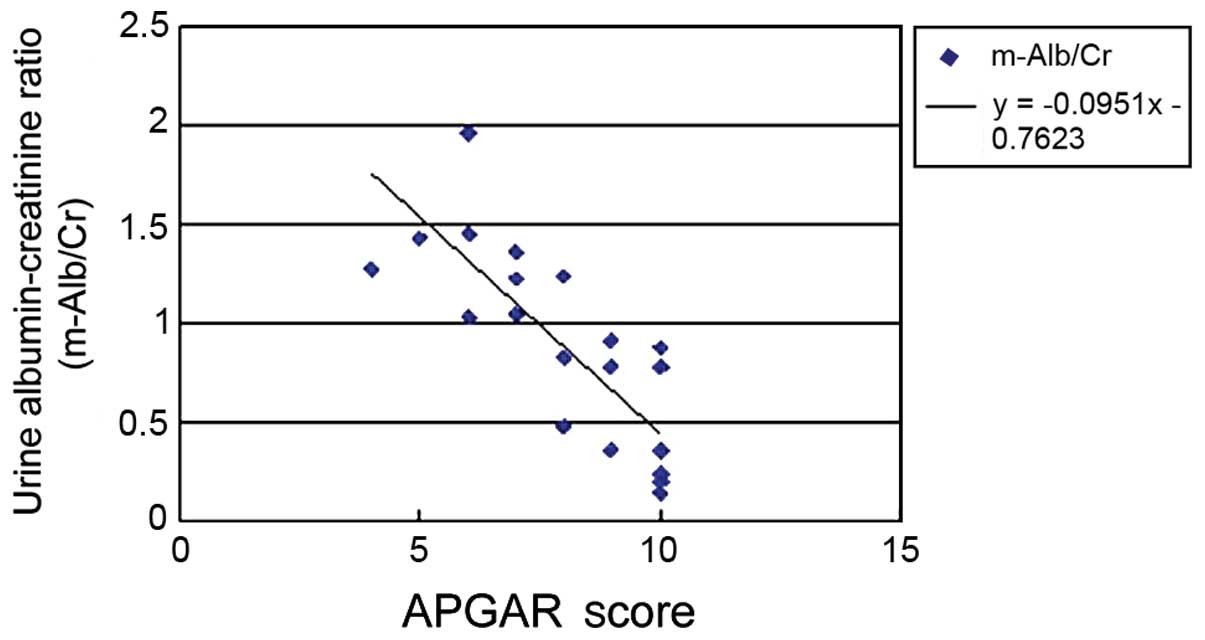
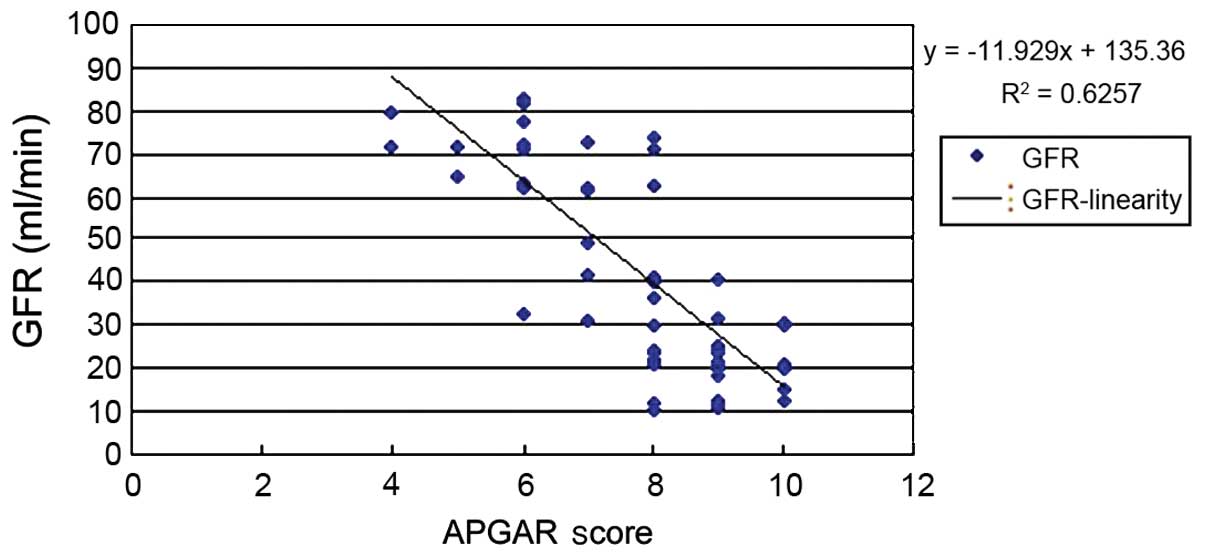
analysis was performed. The results showed that BMI (Fig. 1), urinary ACR (Fig. 2) and GFR (Fig. 3) of pregnant women were related to
the APGAR score and the differences were statistically significant
(P<0.05; Table III).
 | Table III.Correlation analysis between the APGAR
score and the detection indicators of pregnant women. |
Table III.
Correlation analysis between the APGAR
score and the detection indicators of pregnant women.
| Indicator | Gender | Age | SBP, mmHg | DBP, mmHg | AST, U/l | ALT, U/l | logGGT, U/l | FPG, mmol/l | BMIa, kg/m2 | HbA1C, % | TC, mmol/l | HDL-C, mmol/l | LDL-C, mmol/l | GWa | Urine ACR | GFR, ml/min | Cr, mmol/l |
|---|
| APGAR |
|
|
|
|
|
|
|
|
| 4.70±0.43 | 185.4±20.6 | 67.5±13.5 | 98.5±10.2 | 36.4±1.8 | 0.87±0.23 | 67.8±24.2 | 263.7±80.38 |
| r | 0.02 | 0.35 | 0.16 |
0.28 |
0.24 |
0.15 |
0.06 |
0.38 |
2.80 |
0.37 |
0.823 | 0.252 |
0.371 |
0.243 | −0.095 | −11.93 | 0.164 |
| P-value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | <0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
0.003 |
0.001 | 0.365 |
Multivariate and logistic regression
analysis on perinatal outcomes and other factors
A logistic regression analysis on all risk factors
was performed, and we found that urine ACR and the APGAR score were
positively correlated and the correlation coefficient was −0.0951.
The difference was statistically significant (P=0.001; Table IV).
 | Table IV.Logistic regression analysis on the
newborn APGAR score and influencing factors. |
Table IV.
Logistic regression analysis on the
newborn APGAR score and influencing factors.
|
| (95% CI) |
|---|
|
|
|
|---|
| Variables | β | SE | β' | t | P-value | Upper limit | Lower limit |
|---|
| GFR | 0.581 | 0.10 | 0.642 | 0.652 | 0.362 | 0.39 | 0.78 |
| m-Alb/Cr | 0.243 | 0.01 | 0.352 | 0.431 | 0.001 | 0.21 | 0.80 |
| BMI | 0.768 | 0.08 | 0.871 | 0.981 | 0.325 | 0.61 | 0.92 |
Discussion
Proteinuria during pregnancy severely affects the
health of the fetus and mother (2).
In normal delivery or after termination of pregnancy, these changes
may gradually be restored. Under the physiological environment of
pregnancy, previous kidney diseases may become worse and severe
cases may cause new kidney damage such as acute renal failure
(5,7,9–11). From the classification of
pregnancy-related kidney diseases, kidney diseases caused by
pregnancy do not include pre-eclampsia and eclampsia-induced renal
lesions, or diseases occurring before pregnancy such as
preeclampsia-associated focal segmental glomerulosclerosis. Some
kidney diseases before pregnancy such as focal segmental
glomerulosclerosis, IgA nephropathy, membranous nephropathy, reflux
nephropathy and even lupus nephritis may be triggered by pregnancy.
In addition, hemolytic uremic syndrome and renal cortical necrosis
after pregnancy is not uncommon (12–16). In
the present study, the average age of enrolled women was 25.3±12.6
years, with mean arterial pressure of 118.5±21.3 mmHg and 24 h
urine protein content of 121.7±14.5 mg. None of the pregnant women
had significant hypertension or proteinuria prior to pregnancy.
We determined the indicators associated with
proteinuria during pregnancy from the relevant literature and
statistically analyzed them in the study participants. The results
showed that the BMI, urine ACR and GFR of pregnant woman were
associated with the APGAR score and the differences were
statistically significant (P<0.05). The Pearson correlation
analysis diagram for BMI and APGAR score was y=2.8035x-3.492, but
in multivariate analysis (P>0.05). During pregnancy, change in
weight is highly related to gestational week and fetal weight,
although we found there was a linear relationship between weight
and the APGAR score. However, there were many confounding factors
in the linear relationship, and after the exclusion of these
factors, no significant correlation between BMI and APGAR score was
found (P>0.05).
We also analyzed the relationship between GFR and
the APGAR score and found that y=−11.929x+135.36 (P>0.05).
According to previous literature, an increase in GFR mainly occured
because of increased space between glomerular podocytes, caused by
renal lesions. Among women with hypertensive proteinuria, the
hypertension-induced glomerular perfusion pressure was
significantly increased because membrane filtration permeability
was increased. In addition, HCG, h-PL, steroid hormones and
estrogen during pregnancy may have increased GFR. Decreased
negatively charged salivary proteins on the filtration membrane
attenuated the repulsion effect on albumin which was also
negatively charged, and proteinuria occurred. However, it was not
considered the influencing factor on perinatal outcome.
The determination of urine protein-creatinine ratio
can effectively reflect the maternal 24-h urine protein content and
excludes the result bias caused by poor compliance and many other
factors. In the present study, we found a linear relationship
between maternal ACR and APGAR score, y=−0.0951x-0.7623
(P<0.05), suggesting that proteinuria may affect the fetus in
utero, thereby affecting perinatal outcomes. Previous studies
showed that if early proteinuria during pregnancy was >300 mg/24
h, careful clinical attention should be given. These patients were
frequently combined with gestational hypertension, leading to
reduced fetal survival rate, growth retardation and premature birth
(13,15,16–18).
Proteinuria during pregnancy can manifest as mild proteinuria,
combined proteinuria and hypertension, severe pre-eclampsia, severe
eclampsia and other serious obstetric complications that
potentially cause hypoxia, acidosis, bleeding, microcirculation,
multiple organ failure and other serious complications (19–22).
In summary, there is an important correlation
between perinatal maternal ACR and perinatal outcome. The increase
in random urine ACR may predict postpartum outcome. Intervention in
early pregnancy or before pregnancy has important clinical
significance in reducing adverse complications for infants and
mothers such as hypertension in pregnancy and improving the outcome
of pregnancy.
References
|
1
|
Puri CP and Garfield RE: Changes in
hormone levels and gap junctions in the rat uterus during pregnancy
and parturition. Biol Reprod. 27:967–975. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duvekot JJ, Cheriex EC, Pieters FA,
Menheere PP and Peeters LH: Early pregnancy changes in hemodynamics
and volume homeostasis are consecutive adjustments triggered by a
primary fall in systemic vascular tone. Am J Obstet Gynecol.
169:1382–1392. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holobotovskyy V, Chong YS, Burchell J, He
B, Phillips M, Leader L, Murphy TV, Sandow SL, McKitrick DJ,
Charles AK, et al: Regulator of G protein signaling 5 is a
determinant of gestational hypertension and preeclampsia. Sci
Transl Med. 7:290ra882015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi WY and Lin XJ: Clinical analysis of 43
cases of nephrotic syndrome with pregnancy induced hypertension.
Chin Birth Health Hered. 6:76–78. 2007.(In Chinese).
|
|
5
|
Fraser A, Nelson SM, Macdonald-Wallis C,
Sattar N and Lawlor DA: Hypertensive disorders of pregnancy and
cardiometabolic health in adolescent offspring. Hypertension.
62:614–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thoulass JC, Robertson L, Denadai L, Black
C, Crilly M, Iversen L, Scott NW and Hannaford PC: Hypertensive
disorders of pregnancy and adult offspring cardiometabolic
outcomes: A systematic review of the literature and meta-analysis.
J Epidemiol Community Health. 70:414–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen TK, Gelber AC, Witter FR, Petri M and
Fine DM: Renal biopsy in the management of lupus nephritis during
pregnancy. Lupus. 24:147–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCarthy EA, Carins TA, Hannigan Y,
Bardien N, Shub A and Walker SP: Effectiveness and safety of 1 vs 4
h blood pressure profile with clinical and laboratory assessment
for the exclusion of gestational hypertension and pre-eclampsia: A
retrospective study in a university affiliated maternity hospital.
BMJ Open. 5:e0094922015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pahwa MB, Seth S and Khosla A:
Significance of urine protein/creatinine ratio in pregnancy-induced
hypertension. Clin Chimacta. 382:145–147. 2007. View Article : Google Scholar
|
|
10
|
Van Lente F and Suit P: Assessment of
renal function by serum creatinine and creatinine clearance:
Glomerular filtration rate estimated by four procedures. Clin Chem.
35:2326–2330. 1989.PubMed/NCBI
|
|
11
|
Milnerowicz-Nabzdyk E, Zimmer M, Tlolka J,
Michniewicz J, Pomorski M and Wiatrowski A: Umbilical cord
morphology in pregnancies complicated by IUGR in cases of tobacco
smoking and pregnancy-induced hypertension. Neuro Endocrinol Lett.
31:842–847. 2010.PubMed/NCBI
|
|
12
|
Dong XD and Peng J: Pregnancy combined
with chronic glomerulonephritis. Chin Obstetric Emergency. 2:96–98.
2012.(In Chinese).
|
|
13
|
Zhou JF, Wang XY, Shangguan XJ, Gao ZM,
Zhang SM, Xiao WQ and Chen CG: Increased oxidative stress in women
with pregnancy-induced hypertension. Biomed Environ Sci.
18:419–426. 2005.PubMed/NCBI
|
|
14
|
Egerman RS, Witlin AG, Friedman SA and
Sibai BM: Thrombotic thrombocytopenic purpura and hemolytic uremic
syndrome in pregnancy: Review of 11 cases. Am J Obstet Gynecol.
175:950–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YQ, Wu YY, Gong X, Wu XF, Liu HY and
Qiao FY: The study about the relationship between the kidney injury
in gestational hypertension and the pregnancy outcomes. Chin Birth
Health Hered. 3:71–72. 2013.(In Chinese).
|
|
16
|
Morikawa M, Yamada T, Yamada T, Shimada S,
Koyama T, Cho K and Minakami H: Pregnancy-induced antithrombin
deficiency. J Perinat Med. 38:379–385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugisaki T: Mechanism by which proteinuria
exacerbates kidney diseases. Nippon Naika Gakkai Zasshi.
90:1292–1298. 2001.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thornton CE, Makris A, Ogle RF, Tooher JM
and Hennessy A: Role of proteinuria in defining pre-eclampsia:
Clinical outcomes for women and babies. Clin Exp Pharmacol Physiol.
37:466–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bramham K, Briley AL, Seed P, Poston L,
Shennan AH and Chappell LC: Adverse maternal and perinatal outcomes
in women with previous preeclampsia: A prospective study. Am J
Obstet Gynecol. 204:512.e1–512.e9. 2011. View Article : Google Scholar
|
|
20
|
Craici IM, Wagner SJ, Weissgerber TL,
Grande JP and Garovic VD: Advances in the pathophysiology of
pre-eclampsia and related podocyte injury. Kidney Int. 86:275–285.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmed A and Ramma W: Unravelling the
theories of pre-eclampsia: Are the protective pathways the new
paradigm? Br J Pharmacol. 172:1574–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Law KP, Han TL, Tong C and Baker PN: Mass
spectrometry-based proteomics for pre-eclampsia and preterm birth.
Int J Mol Sci. 16:10952–10985. 2015. View Article : Google Scholar : PubMed/NCBI
|