Introduction
Osteonecrosis (ON) of the hip, which predominantly
affects young adults, often induces a series of unrelenting
symptoms that culminate in hip pain and loss of function (1). It is estimated that 20,000 to 30,000
patients are diagnosed with ON annually accounting for 10% of the
25,0000 total hip arthroplasties performed annually in the United
States (2). The disease has also
been termed ‘the coronary disease of the hip’ by Chandler (3) as the disease simulates the ischemic
condition in the heart. Osteonecrosis of the femoral head (ONFH)
frequently progresses to a subchondral fracture of the femoral
head, eventually leading to complete joint destruction.
Joint-preserving surgical interventions are generally more
successful at earlier stages of bone involvement compared with
those after the occurrence of a subchondral fracture. Therefore,
the optimal time to evaluate and treat osteonecrosis of the femoral
head is prior to the occurrence of a subchondral fracture (4). Treatment algorithms for osteonecrosis
of the femoral head are based on the staging of the lesion. Based
on clinical and laboratory observations, there is typically a ≥3
months delay between the occurrence of hormone-related ONFH and
abnormal magnetic resonance imaging (MRI) results (5,6), which
is defined as the 0-stage of the Association Research Circulation
Osseous or Steinberg classification systems (7).
To date, there is no noninvasive technique to
accurately diagnose 0-stage ONFH. If early stage ONFH was diagnosed
with noninvasive techniques, extracorporeal shock wave or
autologous bone marrow stem cell transplantation may be used to
limit the progression of ONFH, which would be of great clinical
value (8). In recent years, MRI
technology has developed rapidly, and the emergence of functional
MRI (fMRI) has facilitated the elucidation of novel information and
an in-depth understanding of various bone diseases (9), including craniofacial fibrous
dysplasia, bone marrow abnormalities in multiple myeloma, systemic
sclerosis, sclerosing osteomyelitis as well as metastasis of
different cancers to the bone (10).
The present study aimed to explore the potential
diagnostic role of diffusion tensor magnetic resonance imaging
(DTI) in the early stages of ONFH in an animal model. The results
of the present study may provide a theoretical basis for the
clinical application of DTI for the early diagnosis of ONFH.
Materials and methods
Animals, grouping and treatment
A total of 20 male beagles (age, 12–14 months;
weight, 5.5–6.5 kg) were obtained from the Animal Center of Capital
Medical University (Beijing, China) and randomly classified into
one of two equal group: Control (CON) group or experimental (LM)
group. Beagles were tagged and housed in cages (n=1/cage) under
standard laboratory conditions at 20°C (humidity, 48%) with a 12-h
dark/light cycle. A standard diet and water were provided ad
libitum. All experimental procedures adhered to the
recommendations outlined by the Chinese Department of Health for
the Care and Use of Laboratory Animals and were approved by the
Ethics Committee of the Chinese Rehabilitation Research Center at
Beijing Charity Hospital (Beijing, China). Beagles in the LM group
were intramuscularly injected with 10 µg/kg lipopolysaccharide
(LPS; Sigma-Aldrich, St. Louis, MO, USA) and 20 mg/kg
methylprednisolone (MPS; Pfizer, Inc., New York, NY, USA) on three
consecutive days. Beagles in the CON group were injected with
normal saline. Following the daily injection, the beagles were
permitted free activity.
MRI protocols
MRI and DTI were performed on the bilateral proximal
femora prior to drug injection and at 8 and 12 weeks following the
last injection using a 1.5 T superconducting magnet system
(Achieva; Philips Healthcare, Best, The Netherlands). Following
anesthetization via initial intramuscular injection of 25 mg/kg
ketamine hydrochloride and intraperitoneal maintenance with 5 mg/kg
sodium pentobarbital and blood pressure monitoring, the beagles
were placed in a supine position with the lower limb flexed and
fixed with adhesive tape. An extremity coil was used on the target
site. MRI investigations were performed using an 1.5 T MRI system.
All magnetic resonance pulse sequences and scan parameters used in
the MRI and DTI protocols are listed in Table I. Two radiologists (independent and
blinded to the investigations) assessed the MRI and DTI scans to
evaluate morphologic and signal alterations in the cartilage and
subchondral bone of the femoral head.
 | Table I.Sequences and scan parameters used for
magnetic resonance imaging and DTI. |
Table I.
Sequences and scan parameters used for
magnetic resonance imaging and DTI.
| Purpose | Pulse sequence | TE/TR (mm) | FOV (mm) | Matrix | Thickness (mm) | NEX | Specific
parameters |
|---|
| Anatomical
sequence | TSE/coronal | 35/520 | 160×160 | 256×256 | 4 | 1 |
|
|
| TSE//coronal | 100/2000 | 160×160 | 256×256 | 4 | 1 |
|
| DTI protocol | SE.EPI | 60/2100 | 160×160 | 256×256 | 4 | 4 | VOI: 25×25×2.5 mm
b-value: 500 sec/mm |
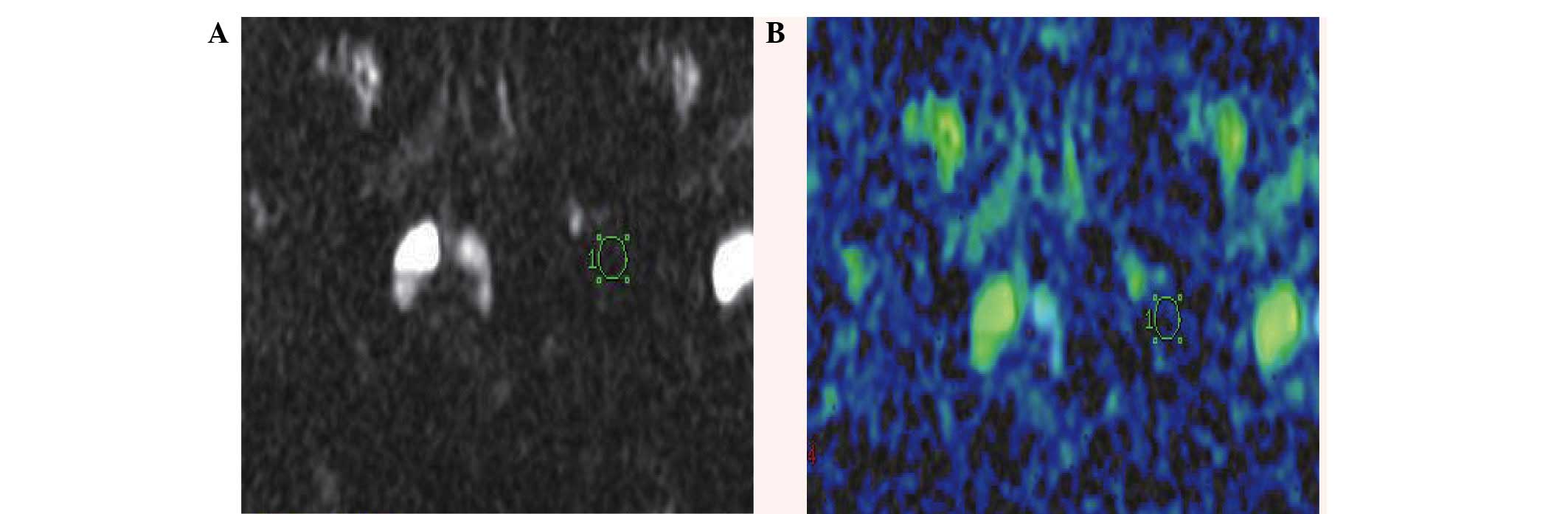
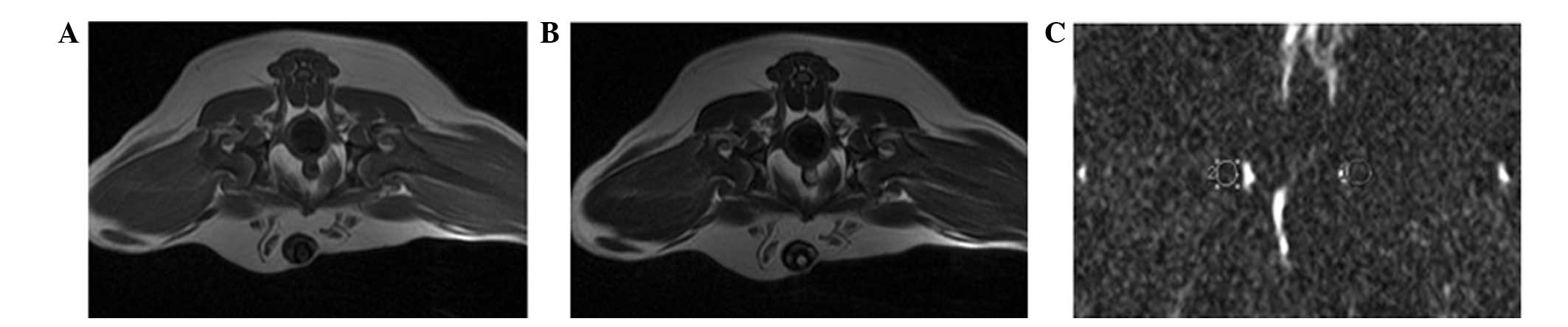
To measure the apparent diffusion coefficient (ADC)
of the femoral head, a circular area (diameter, 5-mm) was selected
to be the region of interest (ROI). The ROI was automatically
transposed onto two maps (ADC values) generated by the image
analysis software (version 17.0 SPSS, Inc., Chicago, IL, USA)
(Fig. 1). ADC values of the femoral
head were obtained by averaging the values obtained from the three
slices selected over the target site beneath the joint space in the
mid-coronal T1-weighted images (Table
II).
 | Table II.Apparent diffusion coefficient values
in the proximal femur (×10−4 mm2/sec). |
Table II.
Apparent diffusion coefficient values
in the proximal femur (×10−4 mm2/sec).
| Animal group | Prior to
administration | 8 weeks
post-administration | 12 weeks
post-administration | P-value |
|---|
| Beagle with ON of LM
group | 2.7±0.3 | 4.7±0.2a | 4.8±0.3a | <0.05 |
| Beagle without ON
of LM group |
2.5±0.4b |
2.6±0.4a,b |
2.4±0.3a,b | >0.05 |
| Control group |
2.6±0.3b |
2.5±0.3a,b |
2.4±0.3a,b | >0.05 |
Histological examination
Five beagles in each group were sacrificed via an
overdose of sodium pentobarbital at 8 and 12 weeks following the
initiation of the experiment. Tissue samples were harvested from
the femoral head. Specimens (1.0-mm thick) were cut along the
coronal plane and fixed in 10% formalin solution for 2 weeks.
Following treatment with 10% nitric acid and decalcification
overnight, according to conventional methods (11), the specimens were stained with
hematoxylin and eosin (Shanghai Xiangjian Medical Equipment Co.,
Ltd., Shanghai, China). ONFH was considered present when there were
empty lacunae, accumulation of bone marrow cell debris, and an
increase in fat cells in the bone marrow (12). Histological analysis was performed to
observe the osteonecrotic changes and repair processes in the
femoral head 8 and 12 weeks post-treatment. Two pathologists
(independent and blinded to the investigations of the present
study) examined the tissue sections according to the criteria
outlined by Arlet (13). The
presence of degeneration and necrosis, the disappearance of marrow
cells and nuclei and hypochromasia of trabecular osteocytes were
regarded as early signs of ON (13).
Sporadic empty lacunae, which may have been induced by sectioning
through the edge of a lacuna, were not considered as a sign of
ON.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. Differences between the LM and CON
groups were compared using two-sided Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Histopathological findings
No changes in form, weight, abdominal distension or
tapering limbs were observed in the LM or CON groups, and no
mortality occurred during the experimental period. In the CON
group, no pathological alterations were observed at 8 or 12 weeks
post-treatment. The articular cartilage was rich in blood vessels
and intramedullary hematopoietic cells, and the trabeculae were
arranged neatly and clearly. Trabecular bone cells, which were
surrounded by many osteoblasts, were clearly visible, whereas empty
lacunae were rare (Fig. 1). In the
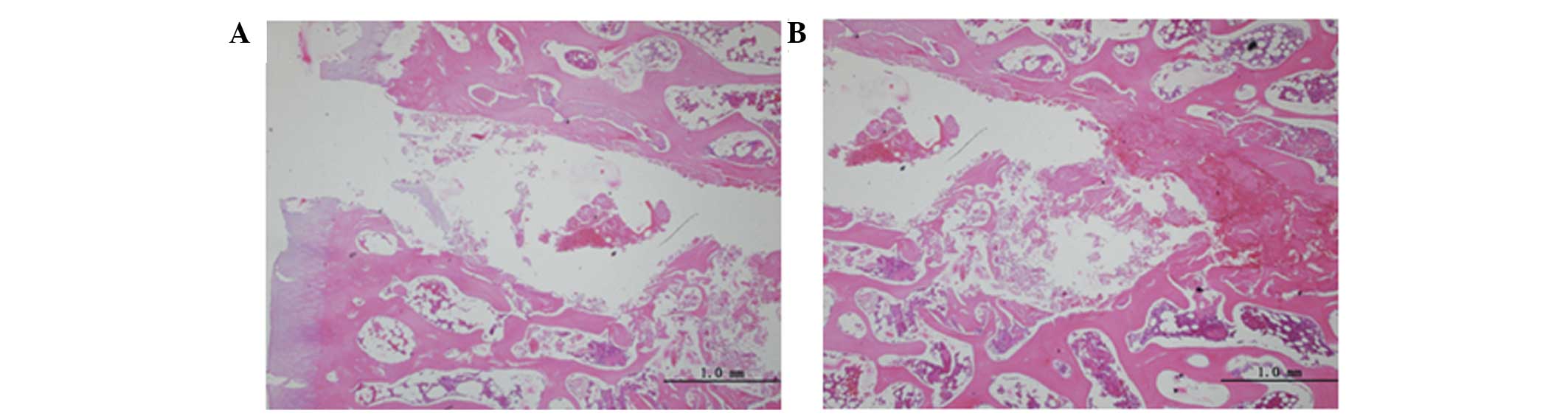
LM group, ONFH occurred in four beagles 8 weeks after treatment,
which was detected via fat cell hypertrophy, decreased marrow
hematopoietic cells, trabecular bone thinning and increasing
spaces, structural disorder, partial trabecular breakage and
trabecular karyopyknosis of the bone. The nuclei were small, with
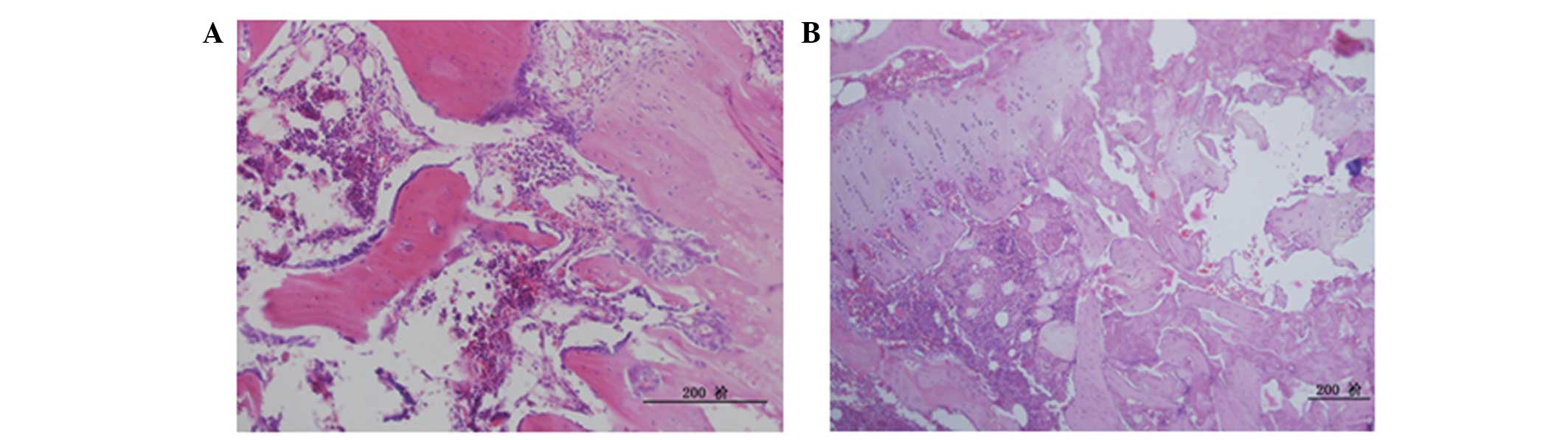
increasing numbers of empty lacunae detected (Fig. 2). At 12 weeks post-treatment, ONFH
was detected in six animals in the LM group. The bone marrow was
saturated with fat cells, bone trabecular interruption and empty
lacunae; increased trabecular bone necrosis was detected around
small osteoblasts, and necrotic inflammatory cells were visible.
There were minimal nascent blood vessels and necrosis was detected
around the areas of fibrovascular tissue repair (Fig. 3). These results demonstrate that
corticosteroid treatment results in the development of ONFH in 60%
of the animals.
Radiographical findings
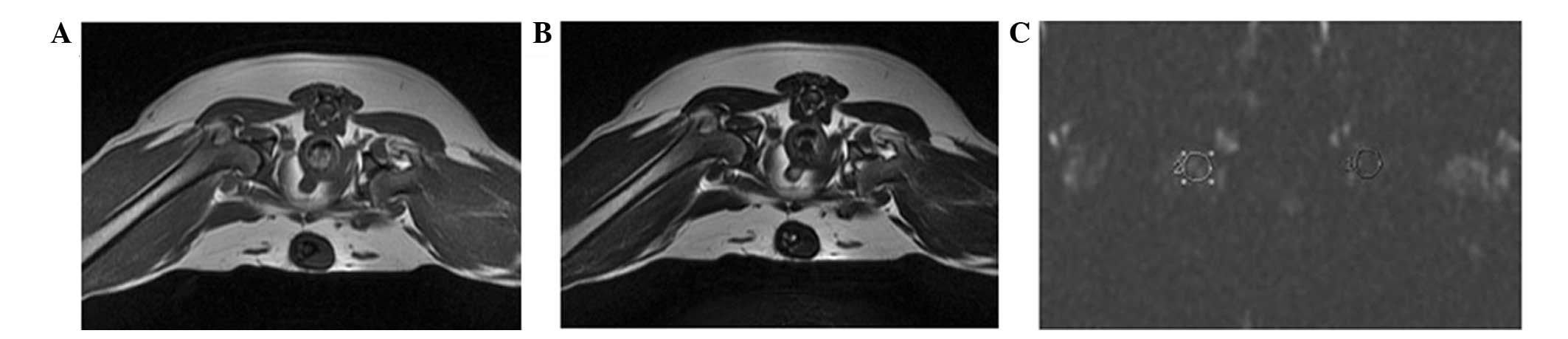
T1-weighted images showed a high signal and
T2-weighted images showed a low or intermediate signal for beagles
in the CON group. No abnormal signal alterations were observed in
the femoral head via MRI or DTI in any of the animals in the CON
group at 8 and 12 weeks post-treatment (Fig. 4). In the LM group, the results of MRI
and DTI were consistent with the CON group (Fig. 5). In the LM group, mean ADC values in
the ROI of beagles with ONFH were significantly increased, as
compared with the LM group without ONFH or the CON group
(P<0.05; Table II). These
results further confirm that corticosteroid treatment leads to
ONFH.
Discussion
Since the etiology of ON remains unknown, there is
no standard method for the establishment of an animal model for
experimental studies. Corticosteroid medication is a pivotal risk
factor for the development of ON (14). Previous clinical and experimental
studies have confirmed that long- or short-term intermittent use of
high-dose corticosteroids may induce ON (15), and the response of both humans and
animals to glucocorticoids is consistent (16).
Climcher and Kenzora (17) determined that steroids do not cause
ONFH unless the patient has a disease that predisposes them to
ONFH. The characteristics of ON induced by horse serum or endotoxin
combined with steroids are similar to those of clinical ON, and are
more stable than those induced by corticosteroids alone. In
previous studies, a combination of low-dose LPS and high-dose MPS
was used to induce ON, resulting in a high rate of ON and low
animal mortality (6.2%) (18–23).
Steroid injection combined with intravenous injection of LPS 24 h
later has been demonstrated to induce a local and systemic
Shwartzman reaction (shw-r), which is characterized by disseminated
intravascular coagulation and results in blood coagulation
disorders, blood clotting and microthrombosis formation (24).
It has previously been demonstrated that shw-r is
capable of causing vasculitis, endothelial cell necrosis,
hemorrhage and thrombosis, which result in tissue damage (25). As the mortality of animals with shw-r
is high, a modified shw-r was elicited with a single injection of
LPS combined with MPS in the large animals used in the present
study. Histopathological analysis was performed 8 and 12 weeks
post-treatment, which demonstrated that 4 and 6 beagles,
respectively, developed ONFH. No mortality occurred. These results
showed that the experimental protocol of low-dose LPS and
subsequent pulsed high-dose MPS injections used in the present
study is an effective method for inducing ON in beagles, with a
high incidence of ONFH and low mortality. This animal model may be
useful for subsequent studies investigating imaging alterations in
the early stages of ONFH.
MRI signal intensity is predominantly correlated
with the hydrogen density of the scanned organ (26); however, the pathological changes
detected in ON are mainly due to alterations in fat and water
content. MRI, which has a high sensitivity and specificity in the
diagnosis of ON, began to be used in clinical exploration of
avascular necrosis in 1983 (27). In
recent years, technology has advanced to the point that MRI can be
used for the early diagnosis of ONFH and the determination of
pathophysiological changes in the femoral head according to imaging
alterations (28). Different
pathological stages of avascular necrosis exhibit differing MRI
variations (29). Nevertheless, it
is important to note that MRI can only detect alterations after the
ON has progressed to a certain degree; therefore, even if the
results of MRI are normal, ON cannot be completely ruled out.
Notably, Beltran et al (30)
have reported on cases of biopsy-proven ON in which the MRI scans
of five patients appeared normal.
fMRI, which is an imaging technology based on
conventional MRI, is a novel approach for examining patients at
risk of developing ONFH. Exploratory research into the early
diagnosis of ONFH with fMRI, including magnetic resonance
spectroscopy, controlled perfusion MRI and diffusion MRI, have
previously been reported (31–33).
DTI is an MRI technique that has been employed to
microimage cartilage and skeletal muscle (34). DTI has been demonstrated to be
extremely sensitive to the morphology of the tissue examined and
precise to the scale of tens of microns (35). DTI has also been used to study the
changes in collagen fiber orientation under mechanical
compression.
DTI facilitates the noninvasive microarchitectural
characterization of heterogeneous tissue. DTI parameters of ADC of
water in the bone marrow can be quantified, which provides a useful
tool for the noninvasive investigation of human tissues (36,37). ADC
values reflect the association between the direction of the
diffusion of water molecules and the organizational structure
(38). These results may have been
induced by increased free water in the surrounding tissue caused by
acute ischemia of the femoral head and cell necrosis. MRI and DTI
analysis demonstrated that the local signal strength was not
significantly altered in the experimental beagles with ONFH, as
compared with the beagles without. This may be due to the fact that
the spatial resolution of DTI remains low, and the osteonecrotic
lesions and water content of bone tissue are limited.
The results of the present study had certain
limitations. Firstly, the statistical analyses are limited by the
relatively small sample size. Secondly, the observation period was
relatively short, which may have limited the observation of
consecutive pathological and radiological changes that may occur in
ON. Prolonging the observation period and establishing various time
points would be of value in future studies. Furthermore, DTI also
has limitations, including low resolution.
In conclusion, the results of the present study
demonstrated a good correlation between the histological
alterations and the DTI findings. These data may enable future
studies to explore the role of fMRI in the early diagnosis of ONFH.
Improvements in the hardware, image signal-to-noise ratio, spatial
resolution and image post-processing software of DTI may enable the
application of this technique to clinical screening, particularly
in patients who exhibit at high risk for ONFH and whom conventional
MRI does not show any abnormalities. As negative MRI scan cannot
completely rule out ONFH, a method of diagnosing early-stage ONFH
may enable the prevention or reversal of the progression of ON.
Diffusion analysis with ADC may allow physicians to obtain more
detailed information on the structure of cancellous tissue, changes
in bone marrow composition and bone metabolism. Further
investigation and hardware improvements are required to investigate
the advantages of DTI for the diagnosis of ONFH.
Acknowledgements
The present study was supported by the Basic
Scientific Research Foundation of China Rehabilitation Research
Center (grant no. 2013C2-19).
References
|
1
|
Petrigliano FA and Lieberman JR:
Osteonecrosis of the hip: Novel approach to evaluation and
treatment. Clin Orthop Relat Res. 465:53–62. 2007.PubMed/NCBI
|
|
2
|
Mankin HJ: Nontraumatic necrosis of bone
(osteonecrosis). N Engl J Med. 326:1473–1479. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chandler FA: Coronary disease of the hip.
J Int Coll Surg. 1:34–36. 1948.
|
|
4
|
Ficat RP: Idiopathic bone necrosis of the
femoral head. Early diagnosis and treatment. J Bone Joint Surg Br.
67:3–9. 1985.PubMed/NCBI
|
|
5
|
Chen XC, Weng J, Chen XQ, Du JZ, Zhu MP,
Pan YQ and Liu M: Relationships among magnetic resonance imaging,
histological findings, and IGF-I in steroid-induced osteonecrosis
of the femoral head in rabbits. J Zhejiang Univ Sci B. 9:739–746.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berg BC Vande, Gilon R, Malghem J,
Lecouvet F, Depresseux G and Houssiau FA: Correlation between
baseline femoral neck marrow status and the development of femoral
head osteonecrosis in corticosteroid-treated patients: A
longitudinal study by MR imaging. Eur J Radiol. 58:444–449. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitt-Sody M, Kirchhoff C, Mayer W,
Goebel M and Jansson V: Avascular necrosis of the femoral head:
Inter- and intraobserver variations of Ficat and ARCO
classifications. Int Orthop. 32:283–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang C, Peng J and Lu S: Summary of the
various treatments for osteonecrosis of the femoral head by
mechanism: A review. Exp Ther Med. 8:700–706. 2014.PubMed/NCBI
|
|
9
|
Dickerson BC: Advances in functional
magnetic resonance imaging: Technology and clinical applications.
Neurotherapeutics. 4:360–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhutani M, Turkbey B, Tan E, Korde N, Kwok
M, Manasanch EE, Tageja N, Mailankody S, Roschewski M, Mulquin M,
et al: Bone marrow abnormalities and early bone lesions in multiple
myeloma and its precursor disease: A prospective study using
functional and morphologic imaging. Leuk Lymphoma. 57:1114–1121.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sangeetha R, Uma K and Chandavarkar V:
Comparison of routine decalcification methods with microwave
decalcification of bone and teeth. J Oral Maxillofac Pathol.
17:386–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wada M, Kumagai K, Murata M, S-Yamashita Y
and Shindo H: Warfarin reduces the incidence of osteonecrosis of
the femoral head in spontaneously hypertensive rats. J Orthop Sci.
9:585–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arlet J: Atraumatic necrosis of the
femoral head: General reportBone Circulation and Vascularization in
Normal and Pathological Conditions. Schoutens A, Arlet J,
Gardeniers JWM and Hughes SPF: Springer US; New York, NY: pp.
235–240. 1993, View Article : Google Scholar
|
|
14
|
Mont MA, Jones JC and Hungerford DS:
Nontraumatic osteonecrosis of the femoral head: Ten years later. J
Bone Joint Surg Am. 88:1117–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan KL and Mok CC: Glucocorticoid-induced
avascular bone necrosis: Diagnosis and management. Open Orthop J.
6:449–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cress RL: Osteonecrosis of bone. Current
concept as to etiology and pathogenesis. Clin Orthop Relat Res.
208:30–39. 1986.PubMed/NCBI
|
|
17
|
Glimcher MJ and Kenzora JE: The biology of
osteonecrosis of the human femoral head and its clinical
implications. III. Discussion of the etiology and genesis of the
pathological sequelae; commments on treatment. Clin Orthop Relat
Res. 140:273–312. 1979.PubMed/NCBI
|
|
18
|
Sheng HH, Zhang GG, Cheung WH, Chan CW,
Wang YX, Lee KM, Wang HF, Leung KS and Qin LL: Elevated
adipogenesis of marrow mesenchymal stem cells during early
steroid-associated osteonecrosis development. J Orthop Surg Res.
2:152007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang G, Sheng H, He YX, Xie XH, Wang YX,
Lee KM, Yeung KW, Li ZR, He W, Griffith JF, et al: Continuous
occurrence of both insufficient neovascularization and elevated
vascular permeability in rabbit proximal femur during inadequate
repair of steroid-associated osteonecrotic lesions. Arthritis
Rheum. 60:2966–2977. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang G, Qin L, Sheng H, Yeung KW, Yeung
HY, Cheung WH, Griffith J, Chan CW, Lee KM and Leung KS:
Epimedium-derived phytoestrogen exert beneficial effect on
preventing steroid-associated osteonecrosis in rabbits with
inhibition of both thrombosis and lipid-deposition. Bone.
40:685–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Yang S, Duan D, Zhang Y and Wang J:
Experimental osteonecrosis induced by a combination of low-dose
lipopolysaccharide and high-dose methylprednisolone in rabbits.
Joint Bone Spine. 75:573–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okazaki S, Nishitani Y, Nagoya S, Kaya M,
Yamashita T and Matsumoto H: Femoral head osteonecrosis can be
caused by disruption of the systemic immune response via the
toll-like receptor 4 signalling pathway. Rheumatology (Oxford).
48:227–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Y, Feng Y and Zhang C: The effect of
bone marrow mononuclear cells on vascularization and bone
regeneration in steroid-induced osteonecrosis of the femoral head.
Joint Bone Spine. 76:685–690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brozma JP: Schwartzman reaction. Semin
Thromb Hemost. 16:326–332. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scholzen TE, Sunderkotter C, Kalden DH,
Brzoska T, Fastrich M, Fisbeck T, Armstrong CA, Ansel JC and Luger
TA: Alpha-melanocyte stimulating hormone prevents
lipopolysaccharide-induced vasculitis by down-regulating
endothelial cell adhesion molecule expression. Endocrinology.
144:360–370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milne S and King GG: Advanced imaging in
COPD: Insights into pulmonary pathophysiology. J Thorac Dis.
6:1570–1585. 2014.PubMed/NCBI
|
|
27
|
Plenk H Jr, Gstettner M, Grosschmidt K,
Breitenseher M, Urban M and Hofmann S: Magnetic resonance imaging
and histology of repair in femoral head osteonecrosis. Clin Orthop
Relat Res. 386:42–53. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fordyce MJ and Solomon L: Early detection
of avascular necrosis of the femoral head by MRI. J Bone Joint Surg
Br. 75:365–367. 1993.PubMed/NCBI
|
|
29
|
Hauzeur JP, Sintzoff S Jr, Applelboom T,
De Maertelaer V, Bentin J and Pasteels JL: Relationship between
magnetic resonance imaging and histologic findings by bone biopsy
in nontraumatic osteonecrosis of the femoral head. J Rheumatol.
19:385–392. 1992.PubMed/NCBI
|
|
30
|
Beltran J, Herman LJ, Burk JM, Zuelzer WA,
Clark RN, Lucas JG, Weiss LD and Yang A: Femoral head avascular
necrosis: MR imaging with clinical-pathologic and radionuclide
correlation. Radiology. 166:215–220. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hou CH, Shih TT, Liu CY, Li YD and Enright
T: Proton MR spectroscopy of the femoral head-evaluation of
patients at risk for avascular necrosis. J Magn Reson Imaging.
24:409–417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du J, Lu A, Dempsey M, Herring JA and Kim
HK: MR perfusion index as a quantitative method of evaluating
epiphyseal perfusion in legg-calve-perthes disease and correlation
with short-term radiographic outcome: A preliminary study. J
Pediatr Orthop. 33:707–713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoo WJ, Kim YJ, Menezes NM, Cheon JE and
Jaramillo D: Diffusion-weighted MRI reveals epiphyseal and
metaphyseal abnormalities in legg-calvé-perthes disease: A pilot
study. Clin Orthop Relat Res. 469:2881–2888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Visser SK, Crawford RW and Pope JM:
Structural adaptations in compressed articular cartilage measured
by diffusion tensor imaging. Osteoarthritis Cartilage. 16:83–89.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hagmann P, Thiran JP, Jonasson L,
Vandergheynst P, Clarke S, Maeder P and Meuli R: DTI mapping of
human brain connectivity: Statistical fibre tracking and virtual
dissection. Neuroimage. 19:545–554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Liu W, Zhang H, Lacy L, Yang X,
Song SK, Wickline SA and Yu X: Regional ventricular wall thickening
reflects changes in cardiac fiber and sheet structure during
contraction: Quantification with diffusion tensor MRI. Am J Physiol
Heart Circ Physiol. 289:H1898–H1907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Raya JG, Horng A, Dietrich O, Krasnokutsky
S, Beltran LS, Storey P, Reiser MF, Recht MP, Sodickson DK and
Glaser C: Articular cartilage: In vivo diffusion-tensor imaging.
Radiology. 262:550–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Provenzale JM, Isaacson J, Chen S,
Stinnett S and Liu C: Correlation of apparent diffusion coefficient
and fractional anisotropy values in the developing infant brain.
AJR Am J Roentgenol. 195:W456–62. 2010. View Article : Google Scholar : PubMed/NCBI
|