Introduction
It is widely accepted that cholesterol is vital for
survival for due to its role in maintaining the fluidity and
elasticity of cellular membranes (1,2) and
participating the biosynthesis of steroid hormones (3). However, an anomalous increase of
cholesterol in the blood, as well as an imbalance of cholesterol
metabolism, is correlated with an increased risk of atherosclerosis
(4,5). The accumulation of cholesterol promotes
the transformation of macrophages into lipid-laden foam cells,
which is an important hallmark of atherosclerosis. High-density
lipoprotein (HDL) plays a role in preventing the progression of
atherosclerotic plaque through its ability to transport cholesterol
from macrophages in the vessels to the liver, which is known as
reverse cholesterol transport (RCT) (6).
Cholesterol efflux, by which cholesterol-loaded
macrophages within the vessel wall secrete cholesterol outside
cells, is understood to be a major process in RCT (7). Scavenger receptor class B type I
(SR-BI), an 82-kDa glycoprotein comprising 509 amino acids, is a
member of the CD36 superfamily of proteins. SR-BI has been
identified as the HDL receptor which mediates the uptake of
cholesterol from HDL to cells and cholesterol efflux from the cell
to HDL particles (8). HDL, as well
as apolipoprotein A-1 (ApoA-1), the principal apolipoprotein of
HDL, plays an important role in RCT. As extracellular acceptors,
HDL and ApoA-1 determine the efficiencies of RCT and cholesterol
efflux (7).
The peroxisome proliferator-activated receptor-γ
(PPAR-γ) is a member of a group of nuclear receptor proteins, which
is involved in the regulation of cellular differentiation,
development and metabolism (9).
Moreover, recent studies have provided evidence that PPAR-γ is
highly expressed in macrophages, as well as in the foam cells of
atherosclerotic plaques (10) and
mediates the expression of a cascade of genes involved in
cholesterol efflux (9,11).
Baicalin is a flavonoid glycoside extracted from the
dry roots of the traditional Chinese drug Scutellaria
baicalensis, which has been extensively used for thousands of
years. Baicalin has also been purified and used in infectious and
inflammatory diseases for its multiple biological properties,
including anti-inflammatory (12),
antioxidant (13), antimicrobial
(14) and anticancer (15) activities. Furthermore, baicalin has
been demonstrated to function as an activator of PPAR-γ, and thus
modulate the erythroid differentiation of blood hematopoietic stem
cells (16). Therefore, in view of
the effect of baicalin on PPAR-γ, the aim of the present study was
to investigate whether baicalin could also promote cholesterol
efflux in macrophages through the activation of PPAR-γ.
Materials and methods
Reagents
Baicalin, BLT-1, GW9662, rosiglitazone,
geranylgeranyl pyrophosphate (GGPP) and GW3965 were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Rabbit
polyclonal antibodies to GAPDH (cat. no. ab9485), SR-BI (cat. no.
ab106572), PPAR-γ (cat. no. ab45036) and liver X receptor-α (LXRα;
cat. no. ab3585) were purchased from Abcam (Cambridge, UK). SR-BI
small interfering RNA (siRNA) was from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Cell culture
THP-1 cells were purchased from American Type
Culture Collection (Manassas, VA, USA). THP-1 cells were maintained
in RPMI-1640 (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone), streptomycin
(100 mg/ml) and penicillin (100 U/ml) at 37°C in a humidified
atmosphere with 5% CO2. In order to stimulate the
differentiation of cells into macrophages, THP-1 cells in 6-well
culture dishes were treated with 160 nmol/ml phorbol 12-myristate
13-acetate for 72 h.
Cholesterol efflux assay
Following differentiation of the cells into
macrophages, 50 mg/l oxidized low-density lipoprotein (oxLDL;
Biomedical Technologies, Inc., Stoughton, MA, USA) and
(3H)-cholesterol (1.0 µCi/ml; New England Nuclear Corp.,
Boston, MA, USA) were added to the THP-1 cell medium for another 24
h. The cholesterol-loaded macrophages were then exposed to baicalin
of different concentrations (0, 2, 10 and 50 µM) for 48 h, or at a
concentration of 50 µM for different times (0, 6, 12, 24 and 48 h)
in the presence of ApoA-1 (10 µg/ml; Sigma-Aldrich), HDL
subfraction 2 (HDL2; 50 µg/ml) or HDL subfraction 3
(HDL3; 50 µg/ml) (both purchased from USBiological,
Swampscott, MA, USA. To confirm the effect of the PPAR-γ signaling
pathway, the cells were pre-treated with PPAR-γ antagonist GW9662
(20 µM) for 2 h before stimulation with baicalin, or treated with
PPAR-γ agonist rosiglitazone (1 µM) for 12 h. To confirm the effect
of the LXRα signaling pathway, the cells were pre-treated with LXRα
antagonist GGPP (5 µM) for 2 h before stimulation by baicalin, or
treated with LXRα agonist GW3965 (5 µM) for 12 h. For analysis of
cholesterol efflux, the medium and the THP-1 macrophages were
collected, respectively, and the radioactive content was determined
by liquid scintillation (Beckman LS6500; Beckman Coulter, Inc.,
Fullerton, CA, USA). The percentage of cholesterol efflux was
calculated by dividing the radioactive content in the medium by the
sum of the radioactive content in the medium and in the cells
(17).
Western blot analysis
Radioimmunoprecipitation assay buffer and
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology, Haimen, China) were used to extract the protein from
the THP-1 macrophages following the various treatments. The
bicinchoninic acid method was used to measure the protein
concentration of the lysates. Moderate quantities (5–10 µl) of
protein lysates were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis at 90 V. The proteins
were transferred to nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA), which were then blocked with 5% non-fat milk
for 1 h at room temperature and incubated with primary antibodies
against GAPDH (1:1,000), SR-BI (1:500), PPAR-γ (1:1,000) and LRXα
(1:500) overnight at 4°C. Finally, following incubation with goat
anti-rabbit secondary antibody (cat. no. ZDE-5209; for GAPDH,
1:10,000; for SR-BI, 1:2,000; for PPAR-γ, 1:5,000; for LXR,
1:1,000; ZSGB-Bio, Beijing, China) for an additional 2 h at room
temperature, the antigen-antibody complex was detected using an
electrochemiluminescence detection system (Immobilon Western
Chemiluminescent HRP Substrate; EMD Millipore). The blots were
quantified using Adobe Photoshop CS5 (Adobe Systems, Inc., San
Jose, CA, USA)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following treatment, total RNA was extracted from
the THP-1 macrophages using TranZol Up (Transgen Biotech, Beijing,
China). Then, RNA samples were treated with RNase-free DNase
(Transgen Biotech). Following extraction, 1 µg mRNA was reversely
transcribed using a First Strand cDNA Synthesis kit (Fermentas;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). After that,
total cDNA was amplified using the FastStart Universal SYBR Green
Master (ROX) mix (Roche Diagnostics, Basel, Switzerland) in the
Light Cycler real-time PCR detection system (Roche Diagnostics) for
40 cycles at 95°C for 10 sec, 60°C for 20 sec and 72°C for 30 sec.
18S was chosen as the reference gene and the primer sequences for
qPCR analyses were as follows: 18S, forward primer:
5′-CTTAGTTGGTGGAGCGATTTG-3′, reverse primer:
5′-GCTGAACGCCACTTGTCC-3′ (17).
SR-BI, forward primer 5′-TCCTCACTTCCTCAACGCTG-3′ and reverse primer
5′-TCCCAGTTTGTCCAATGCC-3′ (18).
Melting curves were assessed to confirm the specificity of the
products generated for each set of primers. The 2−ΔΔCq
comparative method was then used to normalize the relative levels
of gene expression (19).
Statistical analysis
SPSS statistical analytical software, version 18.0
(SPSS Inc., Chicago, IL, USA) was used to analyze the data. The
normally distributed data were analyzed by one-way analysis of
variance and the nonparametric variables were analyzed by
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baicalin promotes cholesterol efflux
to ApoA-1, HDL2 and HDL3 in THP-1
macrophages
To determine the effect that baicalin had on
cholesterol efflux in THP-1 macrophages, THP-1 cells were first
exposed to oxLDL and (3H) cholesterol for 24 h, and then
the cholesterol-loaded THP-1 macrophages were cultured with
different concentrations of baicalin (0, 2, 10 and 50 µM) for 48 h
in the presence of ApoA-1, HDL2 or HDL3 in
the medium (Fig. 1). As shown in
Fig. 1A-C, baicalin at
concentrations of 2 and 10 µM failed to promote cholesterol efflux
to ApoA-1, HDL2 and HDL3. However, 50 µM
baicalin significantly promoted cholesterol efflux to
HDL2 and HDL3 by ~3.4- and 2.2-fold
respectively compared with the control group (P<0.05), while
this concentration of baicalin failed to promote cholesterol efflux
to ApoA-1 (P>0.05). The results demonstrated that baicalin
accelerated cholesterol efflux to HDL2 and
HDL3, but not ApoA-1, when its concentration reached 50
µM.
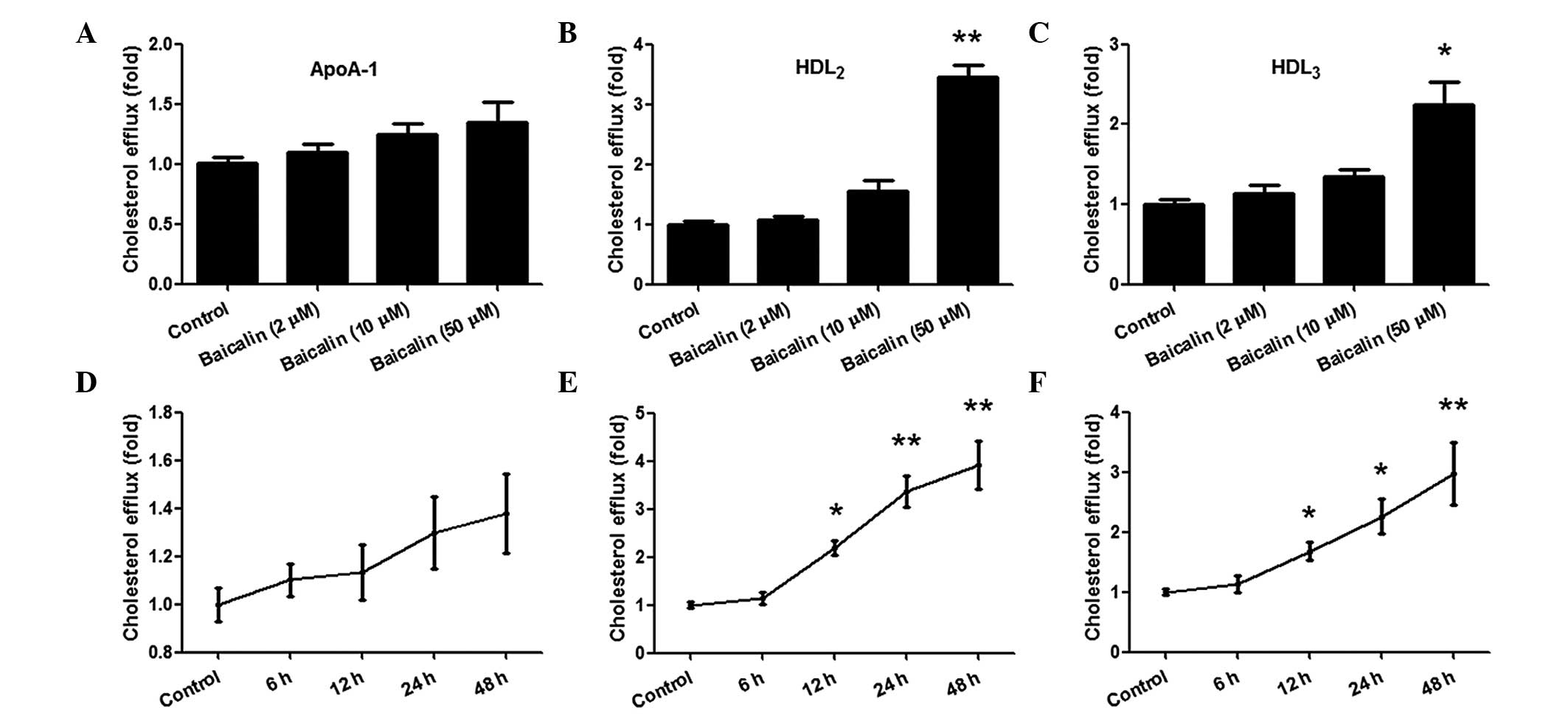 | Figure 1.Effects of baicalin on cholesterol
efflux in THP-1 macrophages. Following stimulation with phorbol
12-myristate 13-acetate, THP-1 macrophages were exposed to oxidised
low-density lipoprotein and (3H)-cholesterol for 24 h,
and the effect of baicalin on cholesterol efflux was evaluated in
the presence of ApoA-1, HDL2 or HDL3. The
cells were treated with different concentrations of baicalin for 48
h, and cholesterol efflux was (A) not significantly changed in the
presence of Apo-A1, but was significantly increased in a
concentration-dependent manner in the presence of (B)
HDL2 or (C) HDL3, Cells were treated with 50
µM baicalin for different time periods, and cholesterol efflux was
(D) not significantly increased by ApoA-1, but was increased in a
time-dependent manner in the presence of (E) HDL2 and
(F) HDL3. *P<0.05 vs. control group; **P<0.01 vs.
control group. Data shown are means ± standard error of the mean
from three independent experiments in duplicate. ApoA-1,
apolipoprotein A-1; HDL2, high-density lipoprotein
subfraction 2; HDL3, high-density lipoprotein
subfraction 3. |
Subsequently, 50 µM baicalin was used to stimulate
the cholesterol-loaded THP-1 macrophages for various times (0, 6,
12, 24 and 48 h). As shown in Fig. 1E
and F, incubation with baicalin for 6 h did not enhance
cholesterol efflux to HDL2 and HDL3, while
≥12 h incubation significantly increased cholesterol efflux
(P<0.05), which peaked at 48 h (P<0.01). However, as shown in
Fig. 1D, incubation with baicalin
did not increase cholesterol efflux to ApoA-1. These results
indicate that baicalin is able to accelerate cholesterol efflux to
HDL2 and HDL3, but not ApoA-1, in THP-1
macrophages in a concentration- and time-dependent manner.
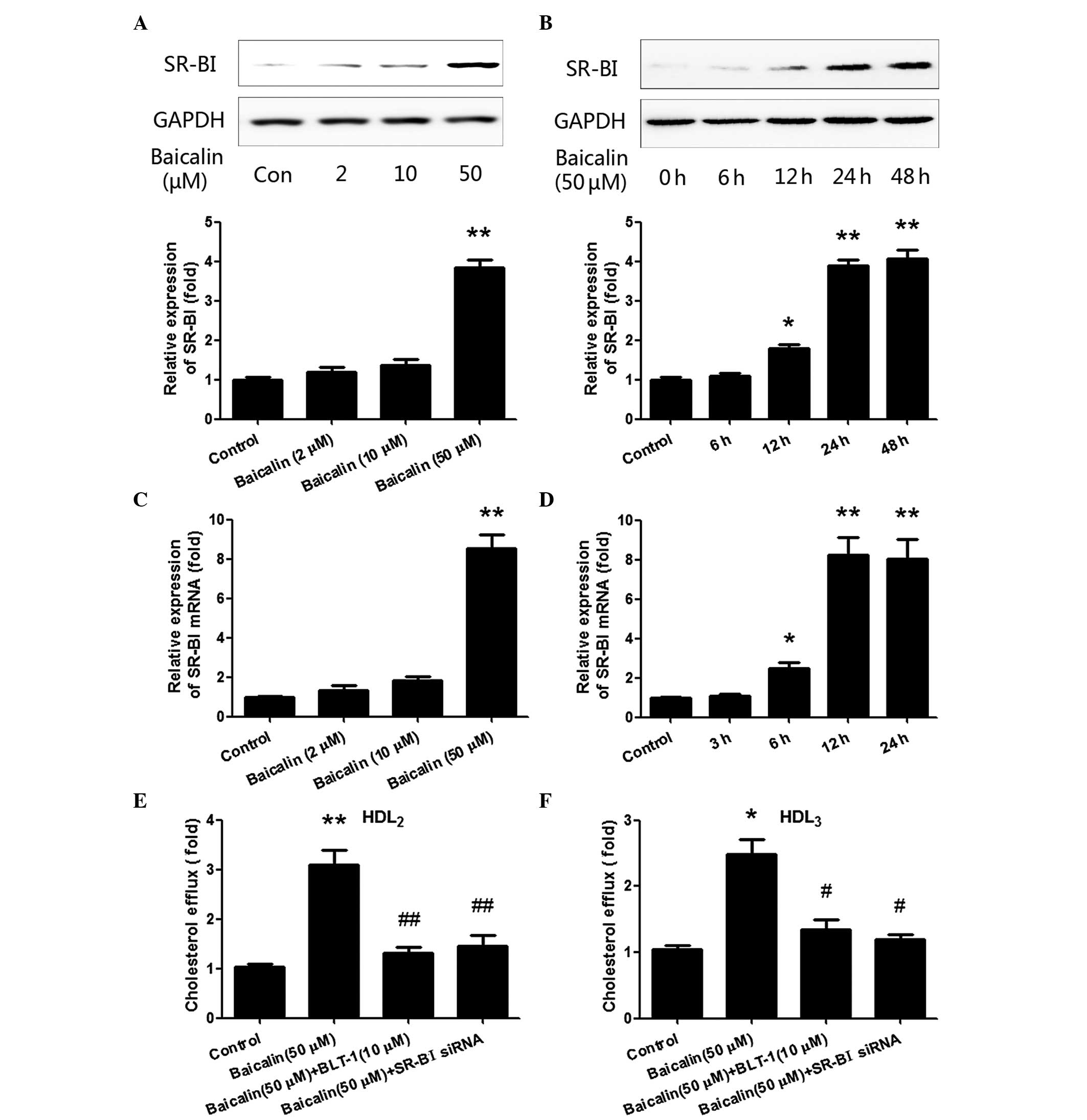
Baicalin enhances the expression of
SR-BI and thereby mediates cholesterol efflux in THP-1
macrophages
SR-BI has been reported to be the major mediator of
cellular cholesterol efflux (20).
In the present study, in order to determine whether the effect of
baicalin on cholesterol efflux in THP-1 macrophages was mediated by
SR-BI, the expression of SR-BI was estimated. The cells were first
treated with different concentrations (0, 2, 10 and 50 µM) of
baicalin for 48 h. SR-BI was detected by western blotting and
RT-qPCR at the protein and mRNA levels, respectively. As shown in
Fig. 2A, following stimulation with
50 µM baicalin for 48 h, the expression of SR-BI was significantly
increased compared with that in the control group (P<0.01).
However, although 2 and 10 µM baicalin also slightly increased the
expression of SR-BI, the increases were not statistically
significant. Subsequently, baicalin (50 µM) was cultured with the
cells for different time periods (0, 6, 12, 24 and 48 h). As shown
in Fig. 2B, the relative expression
of SR-BI was significantly increased when the stimulation time
reached 12 h (P<0.05), and peaked at 24–48 h (P<0.01).
RT-qPCR was used to examine the mRNA level of SR-BI after the
treatment, and similar results were obtained; the expression of
SR-BI at the mRNA level exhibited changes similar to those of the
protein with different concentrations of baicalin (Fig. 2C) and different treatment durations
(Fig. 2D). These data showed that
baicalin induced SR-BI production at the protein and mRNA levels in
a concentration- and time-dependent manner.
To further confirm the pivotal role of SR-BI in
baicalin-induced cholesterol efflux, BLT-1 (a specific inhibitor of
SR-BI) and SR-BI siRNA were each used to pre-treat the cells for 2
h before baicalin was added into the cells. As shown in Fig. 2E and F, BLT-1 and SR-BI siRNA
significantly inhibited baicalin-induced cholesterol efflux
compared with that in the baicalin group (P<0.05). Thus, all the
results above suggest that baicalin enhanced cholesterol efflux in
THP-1 macrophages via SR-BI.
Baicalin-induced cholesterol efflux in
THP-1 macrophages is mediated by PPAR-γ
PPAR-γ has been reported to promote cholesterol
efflux and regulate the expression of SR-BI (19,21). In
order to determine whether PPAR-γ is involved in the regulation of
cholesterol efflux by baicalin, the expression of PPAR-γ in THP-1
macrophages following treatment with baicalin was examined. As
shown in Fig. 3A, following
stimulation with baicalin (50 µM) for 3 h, the expression of PPAR-γ
significantly increased (P<0.05), and a peak was reached at the
12-h time point (P<0.01). An agonist and antagonist of PPAR-γ
were then respectively applied to further clarify the role of
PPAR-γ. As shown in Fig. 3B,
pre-treatment with PPAR-γ antagonist GW9662 (20 µM) significantly
inhibited the upregulated expression of SR-BI (P<0.01)
stimulated by baicalin, while treatment with the PPAR-γ agonist
rosiglitazone (1 µM) significantly increased the expression of
SR-BI (P<0.01). Cholesterol efflux was then estimated following
the treatment with GW9662 and rosiglitazone. As shown in Fig. 3C and D, cholesterol efflux was
significantly inhibited following pre-incubation with PPAR-γ
antagonist GW9662, but increased after incubation with PPAR-γ
agonist rosiglitazone. Thus, these results suggest that
baicalin-induced cholesterol efflux was mediated by PPAR-γ.
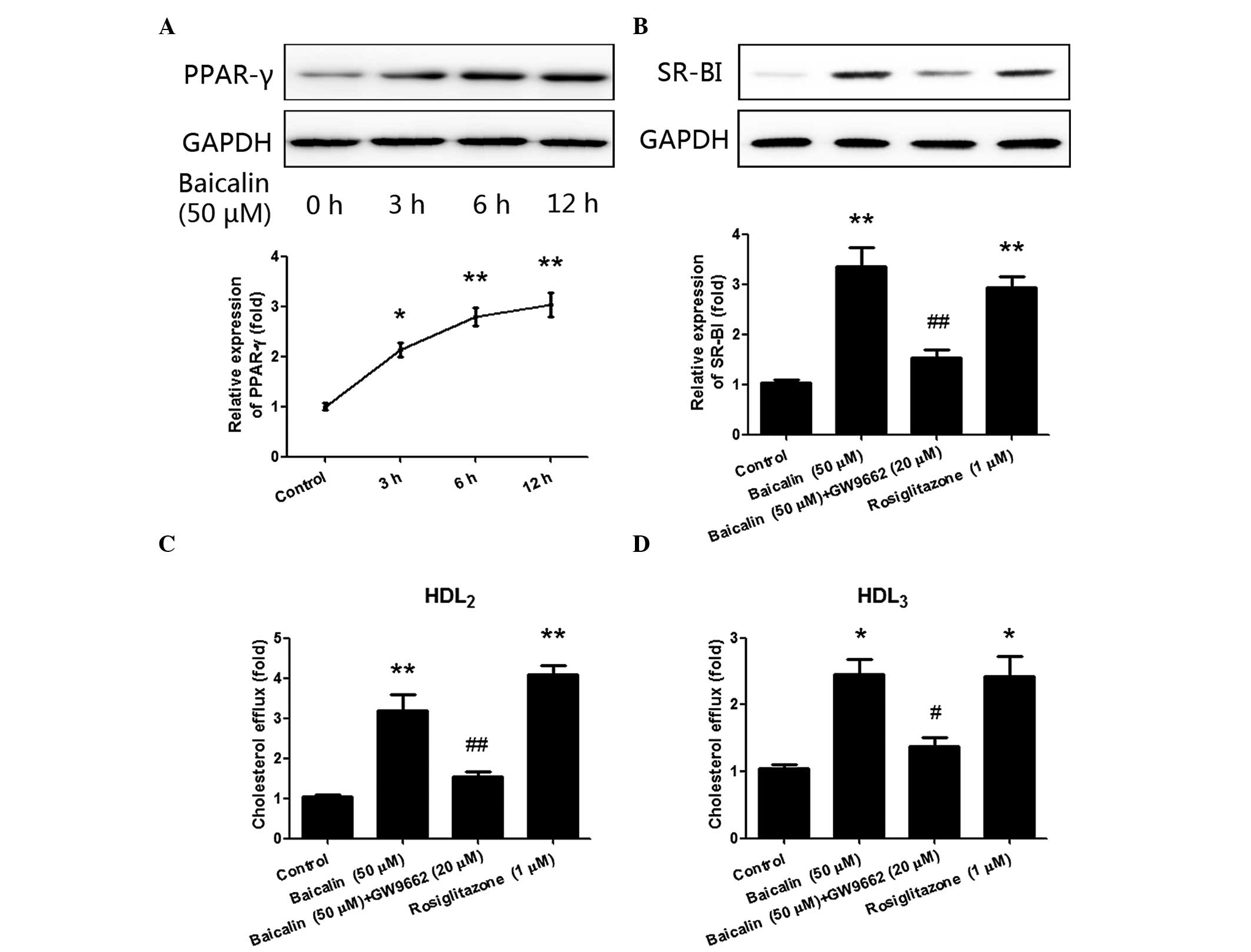 | Figure 3.PPAR-γ mediated the regulation of
SR-BI by baicalin. (A) THP-1 macrophages were stimulated with 50 µM
baicalin for 0, 3, 6 and 12 h, and the expression of PPAR-γ was
significantly increased in a time-dependent manner. (B)
Pre-incubation with PPAR-γ antagonist GW9662 (20 µM) significantly
inhibited the upregulating effect of baicalin on the expression of
SR-BI, while PPAR-γ agonist rosiglitazone (1 µM) significantly
enhanced the expression of SR-BI. (C and D) Pre-incubation with
GW9662 (20 µM) significantly inhibited the upregulating effect of
baicalin on cholesterol efflux, while PPAR-γ agonist rosiglitazone
(1 µM) significantly increased cholesterol efflux, in the presence
of (C) HDL2 or (D) HDL3. *P<0.05 vs.
control group; **P<0.01 vs. control group; #P<0.05
vs. the baicalin group; ##P<0.01 vs. the baicalin
group. Data shown are means ± standard error of the mean from three
independent experiments in duplicate. PPAR, peroxisome
proliferator-activated receptor; SR-BI, scavenger receptor class B
type I; HDL2, high-density lipoprotein subfraction 2;
HDL3, high-density lipoprotein subfraction 3. |
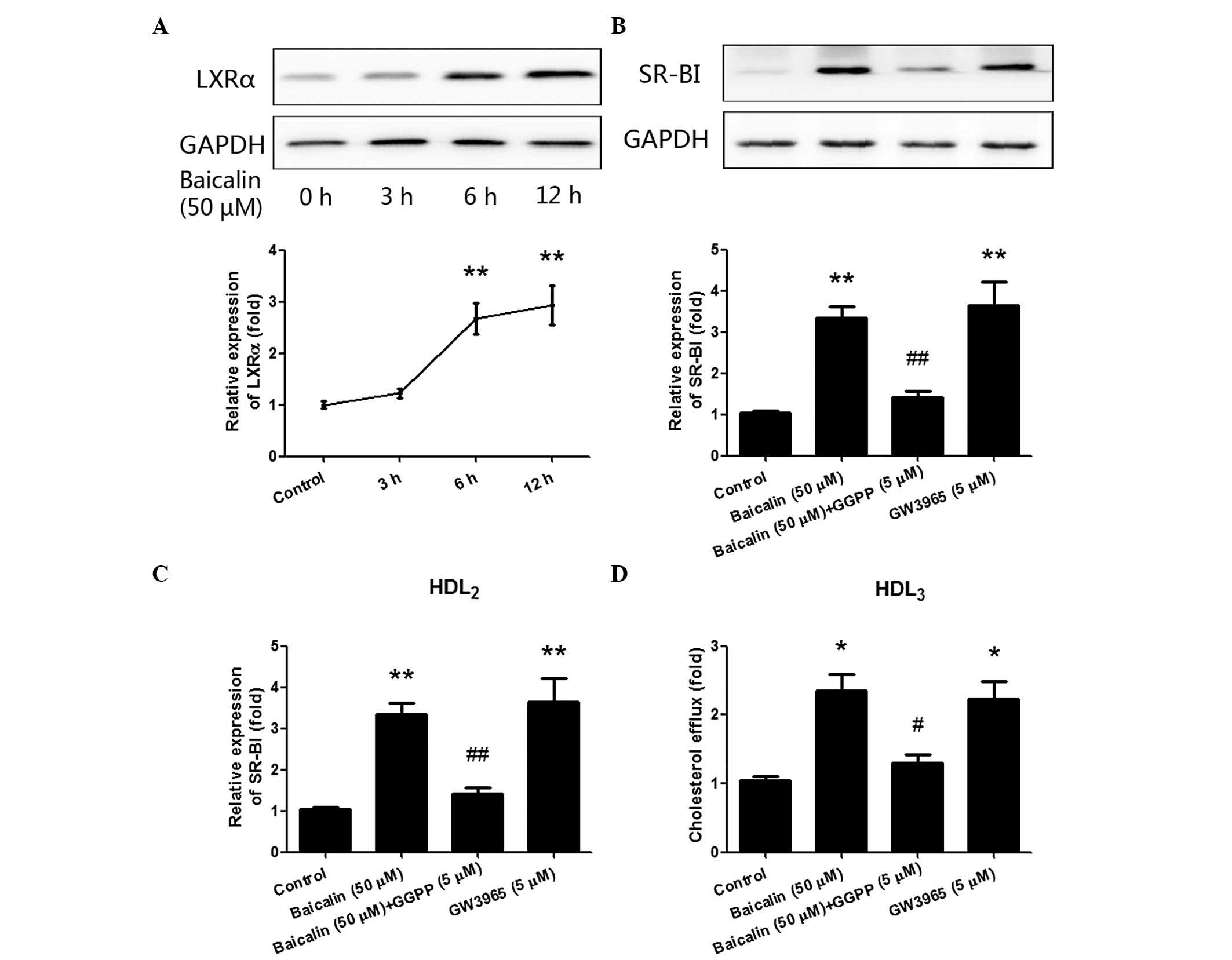
Baicalin-induced cholesterol efflux in
THP-1 macrophages is mediated by LXRα
LXRα has been identified as a target gene of PPAR-γ.
The binding of PPAR-γ with the peroxisome proliferator hormone
response element (PPRE), which is located in the promoter region of
LXRα, is reported to directly upregulate the expression of LXRα
(11). PPAR-γ and LXRα are involved
in a metabolic cascade that regulates the expression of downstream
genes and increases cholesterol efflux (9,22). Thus,
in the present study, the expression of LXRα in THP-1 macrophages
was detected following the treatment with baicalin.
As shown in Fig. 4A,
although the expression of LXRα only increased slightly after 3 h
stimulation with baicalin (P>0.05), it significantly increased
after stimulation with baicalin (50 µM) for 6 h (P<0.05), and
reached a peak at the 12-h time point (P<0.01). Subsequently, an
agonist and antagonist of LXRα were also respectively applied to
further confirm the role of LXRα. As shown in Fig. 4B, pre-treatment with the LXRα
antagonist GGPP (5 µM) significantly inhibited the upregulating
effect of baicalin on the expression of SR-BI (P<0.01), while
LXRα agonist GW3965 (5 µM) significantly increased the expression
of SR-BI (P<0.01). Finally, the effects of GGPP pre-treatment
and GW3965 treatment on cholesterol efflux were also determined. As
shown in Fig. 4C and D, cholesterol
efflux was also significantly inhibited following pre-incubation
with GGPP, but elevated following incubation with GW3965. The
results above indicate that the PPAR-γ/LXRα pathway plays a pivotal
role in cholesterol efflux, and baicalin-accelerated cholesterol
efflux is mediated by the PPAR-γ/LXRα pathway in THP-1
macrophages.
Discussion
Baicalin has been extensively studied and used for
its various therapeutic potencies in inflammation (12), infections (14) and cancers (15). A recent study has shown that baicalin
attenuates high-fat diet-induced obesity, liver weight, as well as
hyperlipidemia and liver dysfunction (23). Intake of a high dosage of baicalin
was found to significantly restore high-fat diet-induced increases
of triglyceride, cholesterol and LDL levels and reduction of HDL
levels (24). In addition, treatment
with baicalin was also shown to decrease the atherosclerotic lesion
area in the aortic sinus of ApoE−/− mice (25). Previous studies have discovered that
PPAR-γ not only regulates the expression of its target gene LXRα,
but also participates in the control of cholesterol efflux from
macrophages together with LXRα. According to the study of Abbasi
et al (16), baicalin is an
activator of PPAR-γ. Thus, based on the results above, it may be
speculated that baicalin could affect cholesterol metabolism
through the PPAR-γ pathway.
RCT has been proposed to transport cholesterol from
vessel walls to the liver and intestine for excretion or recycling,
by which RCT prevents the development of atherosclerosis (26). RCT is a HDL- and ApoA-1-mediated
process that involves complicated steps, beginning with the efflux
of free cholesterol from peripheral tissues to the extracellular
lipoprotein acceptors (17).
Cholesterol efflux from cells to HDL and ApoA-1 has been widely
accepted to be mediated by members of the ATP-binding cassette
(ABC) transporter family, particularly ABC transporter A1 (ABCA1)
(17). Studies have also
demonstrated that SR-BI plays a critical role in promoting
cholesterol efflux and preventing cholesterol accumulation in
macrophages (27,28). A study by Jian et al suggested
that SR-BI in cultured cells increased the rate of cholesterol
efflux from the cell to HDL particles, but not to lipid-free ApoA-1
(29). Liadaki et al found
that the high affinity binding of rHDL to SR-BI was due to the
direct association of ApoA-1 with SR-BI; however, the authors
failed to establish direct binding of SR-BI to the lipid-free
ApoA-1 (30).
In the present study, the effects of baicalin on the
cholesterol efflux of macrophages in the presence of different
mediators were investigated. The results demonstrated that baicalin
accelerated cholesterol efflux from THP-1 macrophages in a
concentration- and time-dependent manner when the mediator was
HDL2 or HDL3. However, when the mediator was
changed to ApoA-1, the cholesterol efflux-promoting effect of
baicalin disappeared. Therefore, it was speculated that the
promoting effect of baicalin on cholesterol efflux might depend on
the activation of SR-BI. Then, in order to confirm this hypothesis,
macrophages were treated with different concentrations of baicalin
for different time periods. SR-BI was detected by western blot
analysis and RT-qPCR at protein and mRNA levels, respectively, and
the results showed that baicalin induced SR-BI production in a
concentration- and time-dependent manner. Following that, to
further confirm the role of SR-BI, BLT-1 and SR-BI siRNA were used
to pre-treat the cells. The results again demonstrated that both
agents significantly inhibited baicalin-induced cholesterol efflux
compared with that in the baicalin group. Thus, these results
suggest that baicalin increased cholesterol efflux via SR-BI.
PPAR-γ and LXRα are considered to be two key nuclear
receptors that are crucial in the process of RCT. PPAR-γ
upregulates the expression of LXRα, either by directly binding to
the PPRE within the promoter region of LXRα (31), or by other indirect pathways
(11). Previous studies have
demonstrated that the PPAR-γ/LXRα pathway is not only upstream of
ABCA1, but also has the ability to regulate the expression of
SR-BI. In a study by Tang et al, it was found that PPAR-A
downregulated the expression of ABCA1, ABCG1 and SR-B1, but
specific activation of LXRα with its agonist significantly
attenuated these reductions in expression levels (32). In a study by Ma et al
(33), it was demonstrated that
treatment with LXR agonist T0901317 substantially increased the
mRNA and protein expression levels of ABCA1, ABCG1 and SR-BI, and
further regulated HDL- and ApoA-1-mediated cholesterol efflux.
However, silencing of LXRα significantly reduced the protein levels
of ABCA1, ABCG1 and SR-BI in human macrophages. A study by Kämmerer
et al demonstrated that 13-hydroxy linoleic acid increased
the expression levels of ABCA1, ABCG1 and SR-BI and stimulated
cholesterol efflux in macrophages via the PPAR-γ/LXRα pathway
(34). In the present study, using
antagonists and agonists of PPAR-γ and LXRα, the pivotal role of
the PPAR-γ/LXRα pathway in cholesterol efflux was confirmed, and it
was clarified that baicalin-accelerated cholesterol efflux was
mediated by PPAR-γ/LXRα pathway in THP-1 macrophages.
In conclusion, the results of the present study
indicated that baicalin promotes the expression of SR-BI, and
induces cholesterol efflux in macrophages through a
PPAR-γ/LXRα/SR-BI signaling pathway. These results provide new
insight into the effect of baicalin on cholesterol efflux, which
might be a new approach for use in the management of cardiovascular
diseases.
Acknowledgements
The present study was supported by the Science
Foundation of Shandong Province (grant no. ZR2014HP005).
References
|
1
|
Cooper RA: Influence of increased membrane
cholesterol on membrane fluidity and cell function in human red
blood cells. J Supramol Struct. 8:413–430. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Needham D and Nunn RS: Elastic deformation
and failure of lipid bilayer membranes containing cholesterol.
Biophys J. 58:997–10094. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu J, Zhang Z, Shen WJ and Azhar S:
Cellular cholesterol delivery, intracellular processing and
utilization for biosynthesis of steroid hormones. Nutr Metab
(Lond). 7:472010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta A and Smith DA: The 2013 American
college of cardiology/American heart association guidelines on
treating blood cholesterol and assessing cardiovascular risk: A
busy practitioner's guide. Endocrinol Metab Clin North Am.
43:869–892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo HS and Choi MH: Cholesterol
homeostasis in cardiovascular disease and recent advances in
measuring cholesterol signatures. J Steroid Biochem Mol Biol.
153:72–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hovingh GK, Van Wijland MJ, Brownlie A,
Bisoendial RJ, Hayden MR, Kastelein JJ and Groen AK: The role of
the ABCA1 transporter and cholesterol efflux in familial
hypoalphalipoproteinemia. J Lipid Res. 44:1251–1255. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohashi R, Mu H, Wang X, Yao Q and Chen C:
Reverse cholesterol transport and cholesterol efflux in
atherosclerosis. QJM. 98:845–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thuahnai ST, Lund-Katz S, Dhanasekaran P,
de la Llera-Moya M, Connelly MA, Williams DL, Rothblat GH and
Phillips MC: Scavenger receptor class B type I-mediated cholesteryl
ester-selective uptake and efflux of unesterified cholesterol.
Influence of high density lipoprotein size and structure. J Biol
Chem. 279:12448–12455. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chawla A, Boisvert WA, Lee CH, Laffitte
BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, et
al: A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in
cholesterol efflux and atherogenesis. Mol Cell. 7:161–171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tontonoz P, Nagy L, Alvarez JG, Thomazy VA
and Evans RM: PPARgamma promotes monocyte/macrophage
differentiation and uptake of oxidized LDL. Cell. 93:241–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao R, Feng J and He G: miR-613 regulates
cholesterol efflux by targeting LXRα and ABCA1 in PPARγ activated
THP-1 macrophages. Biochem Biophys Res Commun. 448:329–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krakauer T, Li BQ and Young HA: The
flavonoid baicalin inhibits superantigen-induced inflammatory
cytokines and chemokines. FEBS Lett. 500:52–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Li H, Gao Z and Xu H: Effects of
dietary baicalin supplementation on iron overload-induced mouse
liver oxidative injury. Eur J Pharmacol. 509:195–200. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li BQ, Fu T, Dongyan Y, Mikovits JA,
Ruscetti FW and Wang JM: Flavonoid baicalin inhibits HIV-1
infection at the level of viral entry. Biochem Biophys Res Commun.
276:534–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiu YW, Lin TH, Huang WS, Teng CY, Liou
YS, Kuo WH, Lin WL, Huang HI, Tung JN, Huang CY, et al: Baicalein
inhibits the migration and invasive properties of human hepatoma
cells. Toxicol Appl Pharmacol. 255:316–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abbasi P, Shamsasenjan K, Akbari AA
Movassaghpour, Akbarzadehlaleh P, Dehdilani N and Ejtehadifar M:
The effect of Baicalin as A PPAR activator on erythroid
differentiation of CD133 (+)hematopoietic stem cells in umbilical
cord blood. Cell J. 17:15–26. 2015.PubMed/NCBI
|
|
17
|
Yue J, Li B, Jing Q and Guan Q:
Salvianolic acid B accelerated ABCA1-dependent cholesterol efflux
by targeting PPAR-γ and LXRα. Biochem Biophys Res Commun.
462:233–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng Y, Liu Y, Jin H, Pan S, Qian Y,
Huang C, Zeng Y, Luo Q, Zeng M and Zhang Z: Scavenger receptor B1
is a potential biomarker of human nasopharyngeal carcinoma and its
growth is inhibited by HDL-mimetic nanoparticles. Theranostics.
3:477–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yancey PG, Bortnick AE, Kellner-Weibel G,
de la Llera-Moya M, Phillips MC and Rothblat GH: Importance of
different pathways of cellular cholesterol efflux. Arterioscler
Thromb Vasc Biol. 23:712–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinetti G, Gbaguidi FG, Griglio S, Mallat
Z, Antonucci M, Poulain P, Chapman J, Fruchart JC, Tedgui A,
Najib-Fruchart J, et al: CLA-1/SR-BI is expressed in
atherosclerotic lesion macrophages and regulated by activators of
peroxisome proliferator-activated receptors. Circulation.
101:2411–2417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakaya K, Ayaori M, Hisada T, Sawada S,
Tanaka N, Iwamoto N, Ogura M, Yakushiji E, Kusuhara M, Nakamura H
and Ohsuzu F: Telmisartan enhances cholesterol efflux from THP-1
macrophages by activating PPARgamma. J Atheroscler Thromb.
14:133–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voloshyna I, Hai O, Littlefield MJ,
Carsons S and Reiss AB: Resveratrol mediates anti-atherogenic
effects on cholesterol flux in human macrophages and endothelium
via PPARγ and adenosine. Eur J Pharmacol. 698:299–309. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xi Y, Wu M, Li H, Dong S, Luo E, Gu M,
Shen X, Jiang Y, Liu Y and Liu H: Baicalin attenuates high fat
diet-induced obesity and liver dysfunction: Dose-response and
potential role of CaMKKβ/AMPK/ACC pathway. Cell Physiol Biochem.
35:2349–2359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao P, Liu L, Wang B, Li W, Fang X and
Guan S: Baicalin and geniposide attenuate atherosclerosis involving
lipids regulation and immunoregulation in ApoE−/- mice.
Eur J Pharmacol. 740:488–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosenson RS, Brewer HB Jr, Davidson WS,
Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips
MC, Rader DJ, et al: Cholesterol efflux and atheroprotection:
Advancing the concept of reverse cholesterol transport.
Circulation. 125:1905–1919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trigatti B, Covey S and Rizvi A: Scavenger
receptor class B type I in high-density lipoprotein metabolism,
atherosclerosis and heart disease: Lessons from gene-targeted mice.
Biochem Soc Trans. 32:116–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu X, Kozarsky K and Krieger M: Scavenger
receptor class B, type I-mediated [3H]cholesterol efflux to high
and low density lipoproteins is dependent on lipoprotein binding to
the receptor. J Biol Chem. 275:29993–30001. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jian B, de la Llera-Moya M, Ji Y, Wang N,
Phillips MC, Swaney JB, Tall AR and Rothblat GH: Scavenger receptor
class B type I as a mediator of cellular cholesterol efflux to
lipoproteins and phospholipid acceptors. J Biol Chem.
273:5599–5606. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liadaki KN, Liu T, Xu S, Ishida BY,
Duchateaux PN, Krieger JP, Kane J, Krieger M and Zannis VI: Binding
of high density lipoprotein (HDL) and discoidal reconstituted HDL
to the HDL receptor scavenger receptor class B type I. Effect of
lipid association and APOA-I mutations on receptor binding. J Biol
Chem. 275:21262–21471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Majdalawieh A and Ro HS: PPARgamma1 and
LXRalpha face a new regulator of macrophage cholesterol homeostasis
and inflammatory responsiveness, AEBP1. Nucl Recept Signal.
8:e0042010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC,
Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS, et al: PAPP-A
negatively regulates ABCA1, ABCG1 and SR-B1 expression by
inhibiting LXRα through the IGF-I-mediated signaling pathway.
Atherosclerosis. 222:344–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma AZ, Song ZY and Zhang Q: Cholesterol
efflux is LXRalpha isoform-dependent in human macrophages. BMC
Cardiovasc Disord. 14:802014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kämmerer I, Ringseis R, Biemann R, Wen G
and Eder K: 13-hydroxy linoleic acid increases expression of the
cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates
apoA-I-dependent cholesterol efflux in RAW264. 7 macrophages.
Lipids Health Dis. 10:2222011. View Article : Google Scholar : PubMed/NCBI
|