Introduction
Neuropathic pain is a common complication of cancer,
diabetes mellitus, degenerative spine disease, infection with the
human immunodeficiency virus, acquired immunodeficiency syndrome
and various other infectious diseases; it has a profound effect on
an individual's quality of life and health care expenditures
(1). Up to 7–8% of the European
population is affected, and 5% of individuals may experience severe
neuropathic pain (2). Neuropathic
pain may result from disorders of the peripheral nervous system or
the central nervous system (brain and spinal cord). Notably,
neuropathic pain can be particularly difficult to treat with only
40–60% of sufferers achieving partial relief (3). Favored treatments include certain
antidepressants (tricyclic antidepressant and
serotonin-norepinephrine reuptake inhibitors), anticonvulsants
(pregabalin and gabapentin), and topical lidocaine (4). Opioid analgesics are recognized as
useful agents but are not recommended as first line treatments. The
efficacy and safety of pregabalin has been extensively
characterized in previous studies, and the findings have been
positive (3,5). Therefore, pregabalin is currently one
of the recommended first-line treatments for neuropathic pain
(3,5). In particular, its efficacy has been
demonstrated in a number of randomized controlled trials in
patients with neuropathic pain caused by various different events
and diseases (6–9).
The present study compared combination therapy with
pregabalin and morphine with each drug used as a single agent in
320 patients with chronic neuropathic pain.
Materials and methods
Patients
A total of 320 patients (males:females, 1.1:1) with
chronic neuropathic pain, who were at least 18 years old (range,
18–89 years), were enrolled in the present study between November
2009 and October 2010. Pain intensity varied from moderate to
severe. Patients with cancer pain and those who were unsuccessfully
treated with pregabalin were excluded from the present analysis.
The protocol for the present study was approved by the Research
Ethics Board of Shenyang Medical College (Shenyang, China). Written
informed consent was obtained from all patients prior to their
enrollment on the study. All study protocols were in accordance
with the Declaration of Helsinki.
Agents
Patients received oral morphine (MS CONTIN;
Mundipharma International, Ltd., Cambridge, UK) monotherapy, oral
pregabalin (Lyrica; Pfizer, Inc., New York, NY, USA) monotherapy,
or a combination of morphine plus pregabalin for 90 days. These
pharmacological agents were used in line with the manufacturers'
prescribing information and as required by the patient, according
to their condition. The initial mean daily dose of morphine was
36.4 mg in the monotherapy group, and 25.5 mg for the combination
therapy group. The initial mean daily dose of pregabalin was 86.2
mg in the monotherapy group and 106.3 mg in the combination therapy
group. Treatment dosages were altered based on the pain intensity
recorded at visits and telephone interviews on the days of 7, 14,
21, 28, 35, 56, 75 and 90 in order to achieve optimal efficacy and
tolerability based on their response and adverse events. If
necessary, immediate release morphine (oral morphine 5 or 10 mg per
day) was used for breakthrough pain.
Study design
In our single center, an open-label, prospective
comparison of three treatments (morphine, pregabalin and these
drugs in combination) was conducted. Patients receiving pregabalin
who had partial pain control were assigned to the pregabalin
monotherapy group for 90 days (n=102); patients feeling pain which
was not controlled by other drugs were randomized into the morphine
group (n=90) or the morphine and pregabalin combination group for
90 days (n=128). The patient number was not consistent between the
three treatment groups as some patients refused to take morphine in
the combination of morphine plus pregabalin group. Patients in the
morphine groups who refused to take morphine were exempted from the
study.
Efficacy and tolerability were the primary endpoints
evaluated. Pain relief was the primary measure of efficacy and pain
intensity was continually assessed for the eight follow-up visits
(days 7, 14, 21, 28, 35, 56, 75 and 90). Patients were asked to
rate pain during the past 24 h on a numerical rating scale (NRS),
where 0 indicated no pain and 10 was the worst pain imaginable. A
2-point reduction was described as clinically meaningful by Farrar
et al (10) and Rowbotham
(11). Secondary endpoints included
comparisons of daily dosages for monotherapy vs. combination
therapy at the end of treatment, impact on quality of life (QoL),
and patient assessments of treatment efficacy. The interference of
pain with QoL was compared for each of the three treatment
regimens. A Brief Pain Inventory (BPI) questionnaire was utilized
to evaluate how the patient's everyday life was influenced by pain
during the study (12,13). The BPI questionnaire evaluates pain
intensity using an NRS (0, no pain; 10, worst pain imaginable) and
the impact of pain on QoL, by rating how pain interferences with
the following seven domains: General activity, mood, walking,
normal work, sleep, social relations and life enjoyment, using an
NRS (0, does not interfere; 10, completely interferes). In order to
investigate the tolerability of each treatment, adverse events were
recorded at each of the eight follow-up visits. Furthermore,
patients were asked to assess the effectiveness of the treatment by
giving one of the following ratings: Not effective, slightly
effective, effective or very effective.
Statistical analysis
Baseline disease characteristics were compared among
the three different treatment groups. The results were presented as
the mean ± standard deviation or the percentage of patients.
χ2 tests were used to analyze categorical data.
P<0.01 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS 15.0
software for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
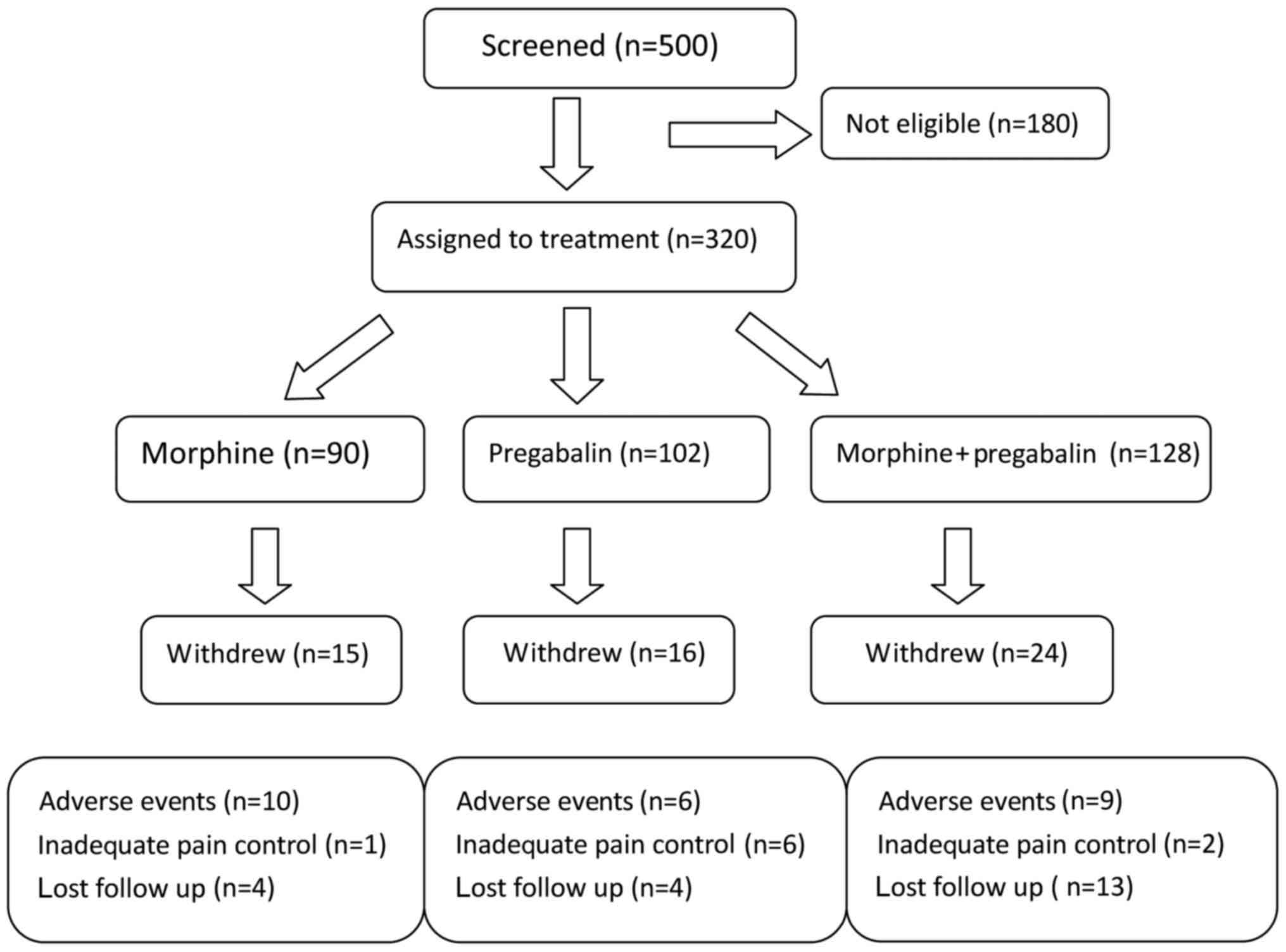
The patient protocol is shown in Fig. 1. A total of 128 patients were
assigned to the combination group of morphine plus pregabalin;
however, 24 were excluded from the study due to adverse events.
Similarly, the number of patients in the morphine monotherapy group
and the pregabalin monotherapy group were reduced from 90 to 15 and
from 102 to 16, respectively, due to adverse events. Adverse events
included blurred vision, abnormal coordination, memory impairment,
dry mouth and constipation, abnormal walking, asthenia and
dysarthria. Details of withdrawal are shown in Fig. 1. Four patients in the morphine
monotherapy group, four patients in the pregabalin monotherapy
group and 13 patients receiving combination therapy were lost to
follow-up.
Patient characteristics are summarized in Table I. There were numerous causes of
neuropathic pain in the present study, including: Failed back
surgery syndrome, post-herpetic neuralgia, radiculopathy, painful
diabetic neuropathy and stenosis of the spinal medullary canal.
Concerning patients with post-herpetic neuropathy, there were fewer
patients in the morphine group compared with the other two
treatment groups, and other diseases were of a similar incidence
among the three groups. Also, the mean score of pain was similar
between the groups receiving monotherapy and those exposed to
morphine and pregabalin combination therapy, both of which were
higher than the mean score in the pregabalin monotherapy group.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Morphine | Pregabalin | Morphine +
Pregabalin |
|---|
| Patients | 90 | 102 | 128 |
| Male | 42 | 59 | 60 |
|
Female | 48 | 43 | 68 |
| Disease (%) |
|
|
|
| Stenosis
MSC | 20.8 | 19.1 | 21.3 |
| FBSS | 23.1 | 20.2 | 19.8 |
| PHN | 15.5 | 22.2 | 23.6 |
| DPN | 16.3 | 17.8 | 16.7 |
|
Radiculopathy | 24.3 | 20.7 | 18.6 |
| NRS (mean
score) | 7.5 | 5.6 | 7.2 |
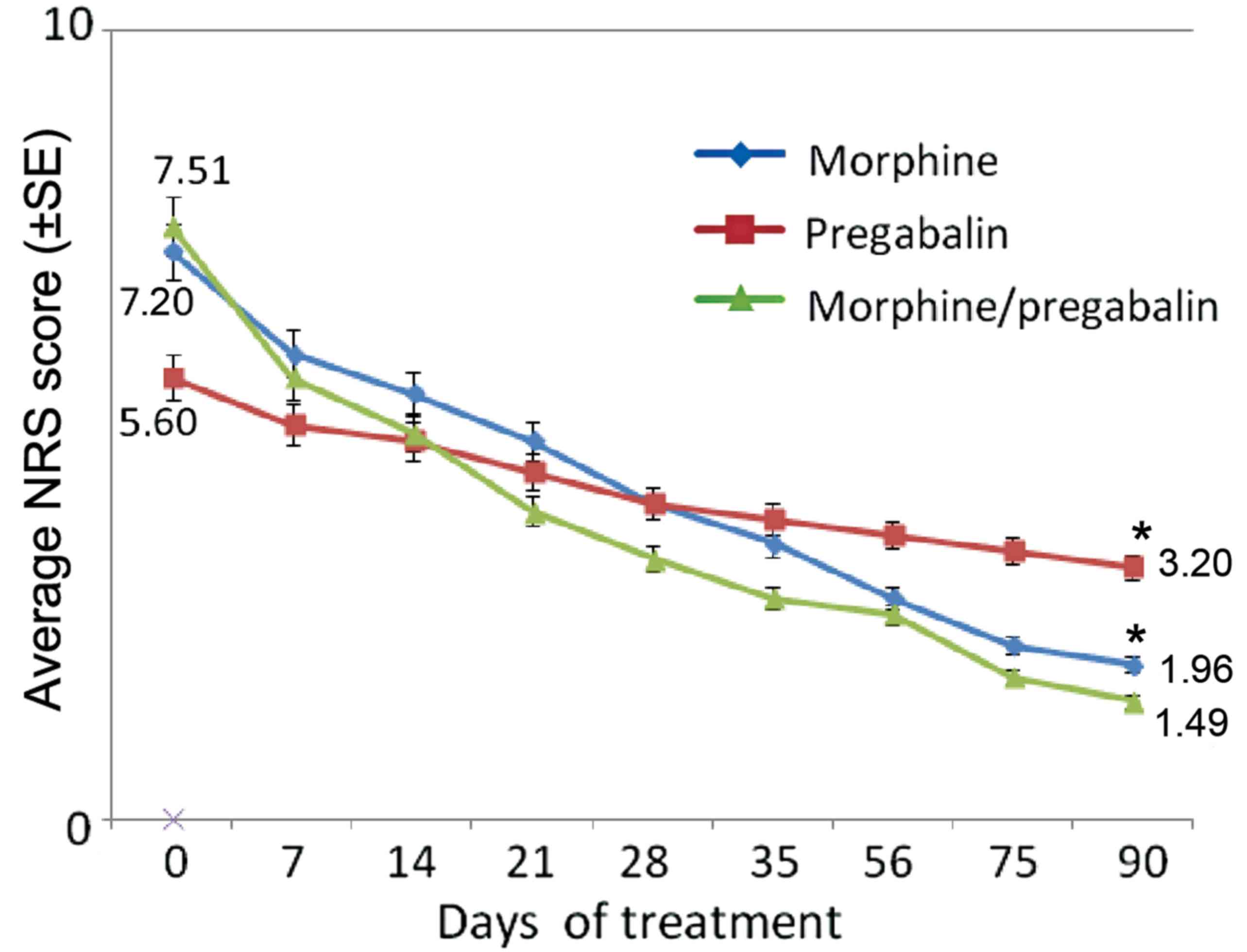
Pain intensity
Morphine plus pregabalin combination therapy and
morphine monotherapy resulted in much faster pain relief than
pregabalin monotherapy (Fig. 2).
After 90 days, compared with the baseline, the mean NRS score of
the combination therapy group had decreased by 80.2%. This value
decrease was significantly greater than that of the two monotherapy
groups. The mean NRS score of the pregabalin monotherapy group had
only decreased by 42.9% (P<0.01 vs. combination therapy);
whereas that score in the morphine monotherapy group had decreased
by 72.8% (P<0.01 vs. combination therapy).
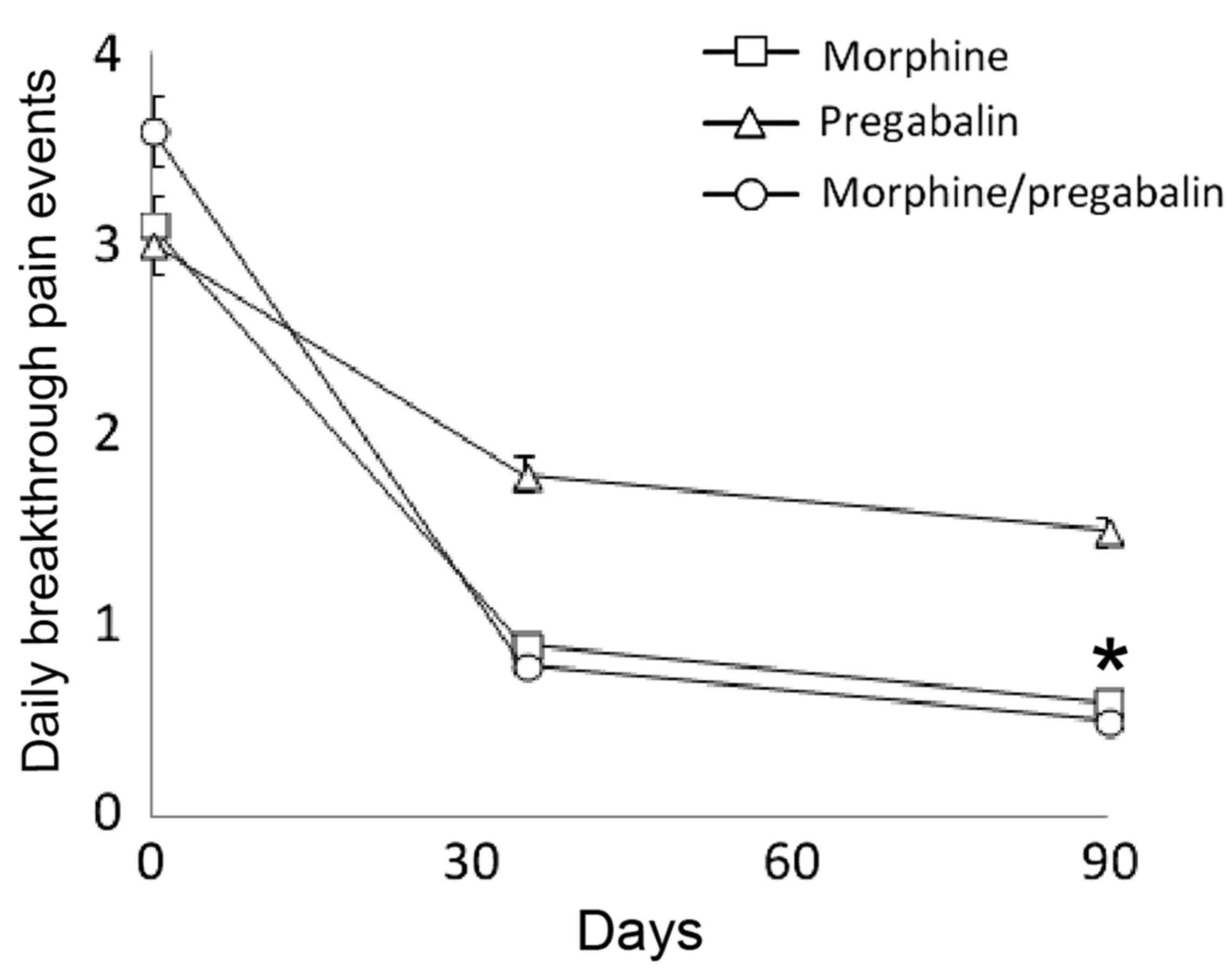
Daily pain crises
At baseline, the three groups had a similar mean
number of breakthrough pain events per day (morphine plus
pregabalin, 3.63; morphine, 3.15; pregabalin, 3.05). At the end of
the study, patients that had received morphine plus pregabalin
combination therapy and those taking morphine monotherapy showed a
significant decrease in the mean number of breakthrough pain events
per day compared with the pregabalin monotherapy group (P<0.01;
Fig. 3).
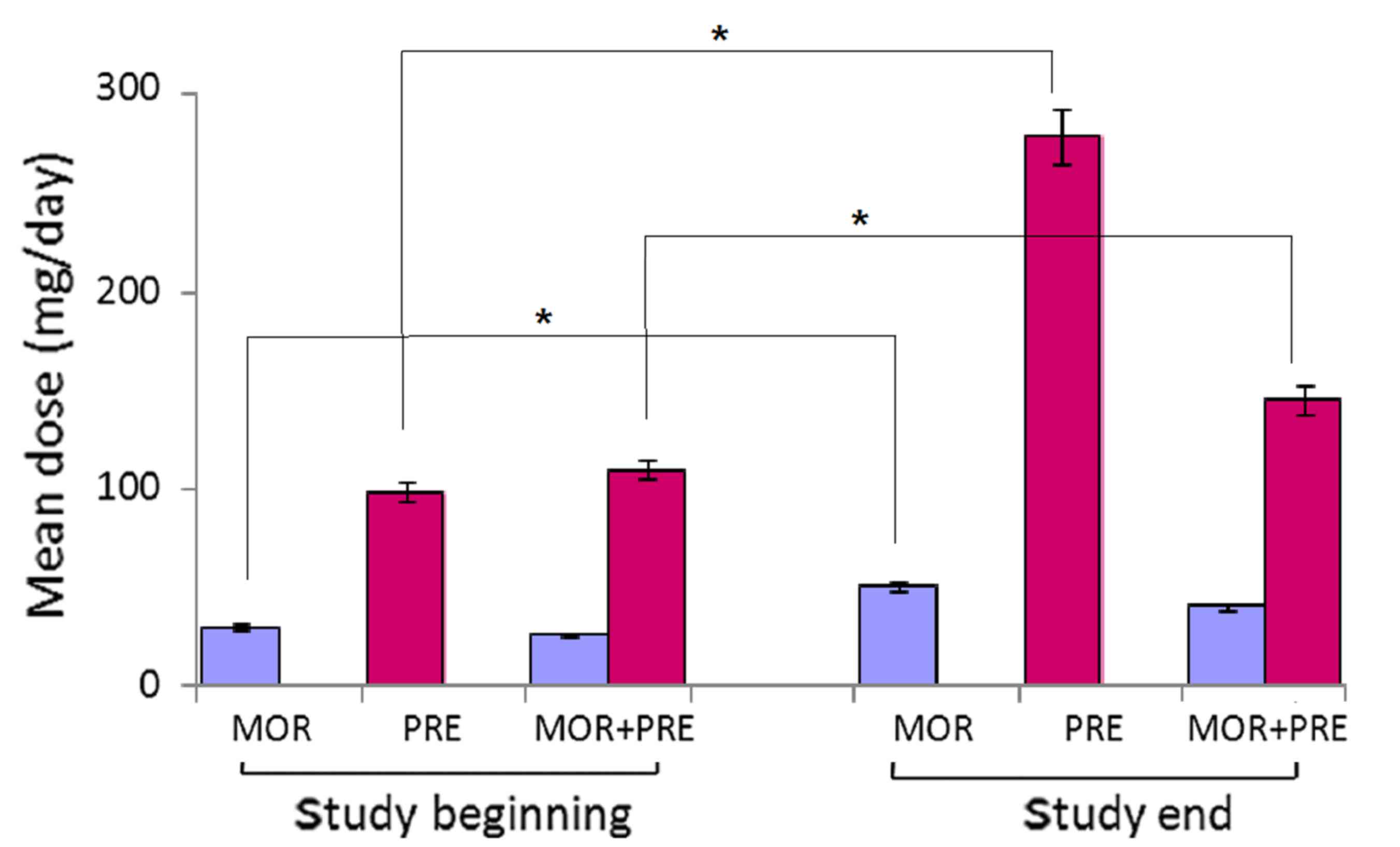
Mean daily medication doses
Combination treatment was effective at lower mean
doses of morphine and pregabalin compared with either morphine or
pregabalin monotherapy, respectively (Fig. 4). In the morphine monotherapy group,
the mean daily doses were 31.2 and 52.8 mg at the beginning and the
end of the study, respectively. In the combination group of
morphine plus pregabalin, the mean daily doses were 26.4 and 110.3
mg at the beginning, respectively, and 41.8 and 142.5 mg at the
end, respectively,. In the pregabalin monotherapy group, the mean
daily doses were 99.6 and 286.3 mg at the beginning and the end of
the study, respectively.
Quality of life
The interference of pain with activities of daily
life was substantially reduced after 90 days treatment in patients
taking morphine plus pregabalin compared with the monotherapy
groups. After 90 days of treatment, patients receiving combination
therapy also exhibit improvements in other OoL terms, including
walking, normal work and social relations. Furthermore, combination
therapy exhibited lower scores than the other two monotherapy
groups, respectively. (Table
II).
 | Table II.Impact of three different therapies on
quality of life. |
Table II.
Impact of three different therapies on
quality of life.
|
| Pregabalin | Morphine | Pregabalin +
Morphine |
|---|
|
|
|
|
|
|---|
| Variable | Begin | End | Begin | End | Begin | End |
|---|
| General activity | 6.31 | 3.24 | 7.54 | 2.75 | 7.58 | 2.34 |
| Mood | 5.16 | 2.81 | 6.77 | 2.30 | 6.21 | 1.67 |
| Walking | 6.21 | 3.45 | 8.11 | 3.13 | 7.64 | 2.33 |
| Normal work | 6.09 | 3.59 | 7.14 | 2.44 | 7.98 | 2.09 |
| Social relations | 4.65 | 2.24 | 6.05 | 3.11 | 6.97 | 2.41 |
| Sleep | 4.12 | 2.32 | 6.87 | 2.57 | 6.64 | 2.44 |
| Life enjoyment | 4.20 | 2.42 | 6.22 | 2.73 | 6.81 | 2.05 |
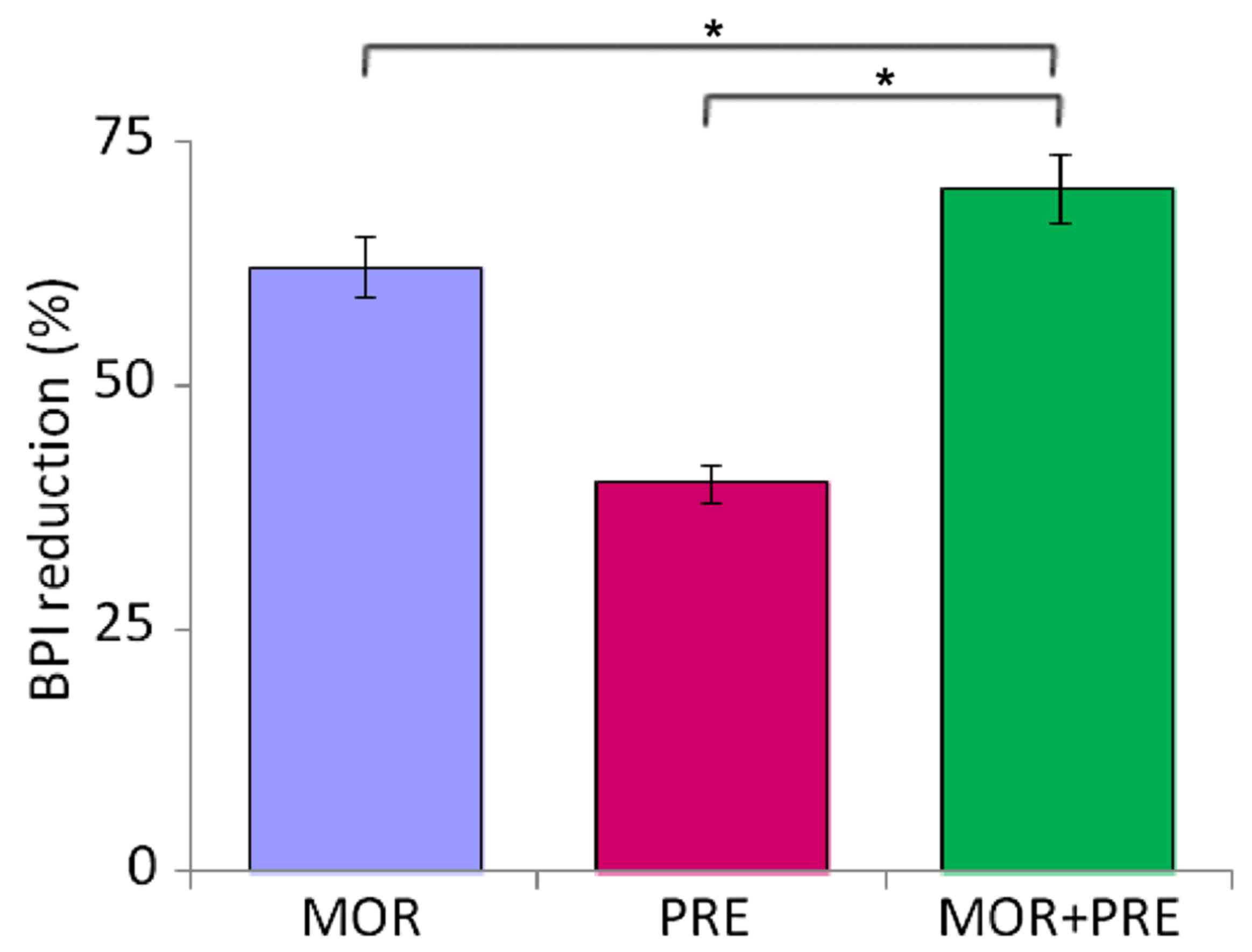
The overall decrease in BPI scores at the end of
study compared with the baseline was 72.1% for combination
treatment (P<0.01 vs. either monotherapy), 61.8% for morphine
monotherapy, and 39.8% for pregabalin monotherapy (Fig. 5).
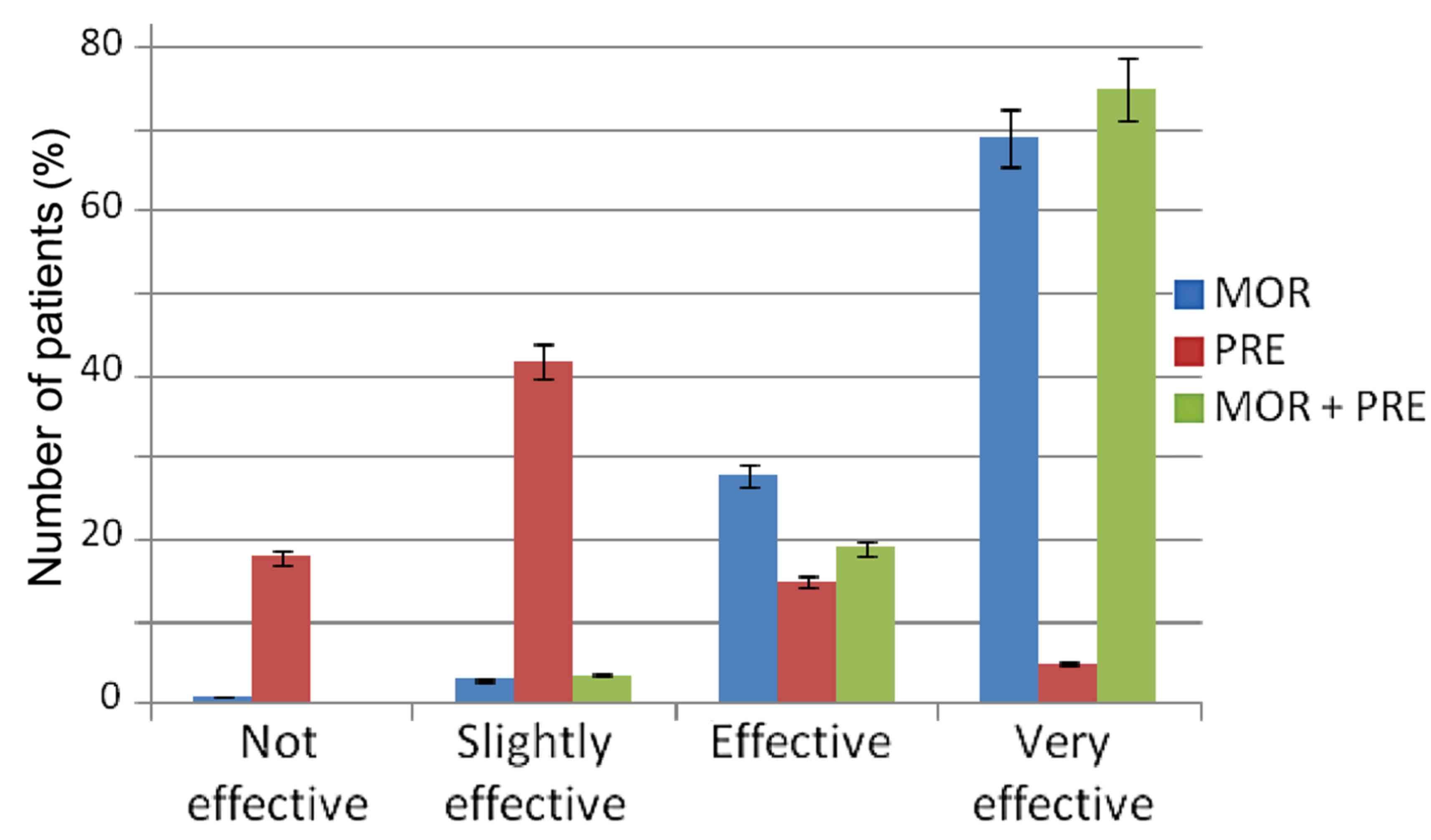
Patient evaluation of efficacy
After 90 days of treatment, 92.2% of patients taking
combination therapy and 93.9% of patients receiving morphine
described the therapy as ‘effective’ or ‘very effective’. In
contrast, in the group taking pregabalin monotherapy, just 18.8% of
patients found the therapy to be ‘effective’ or ‘very effective’
(Fig. 6).
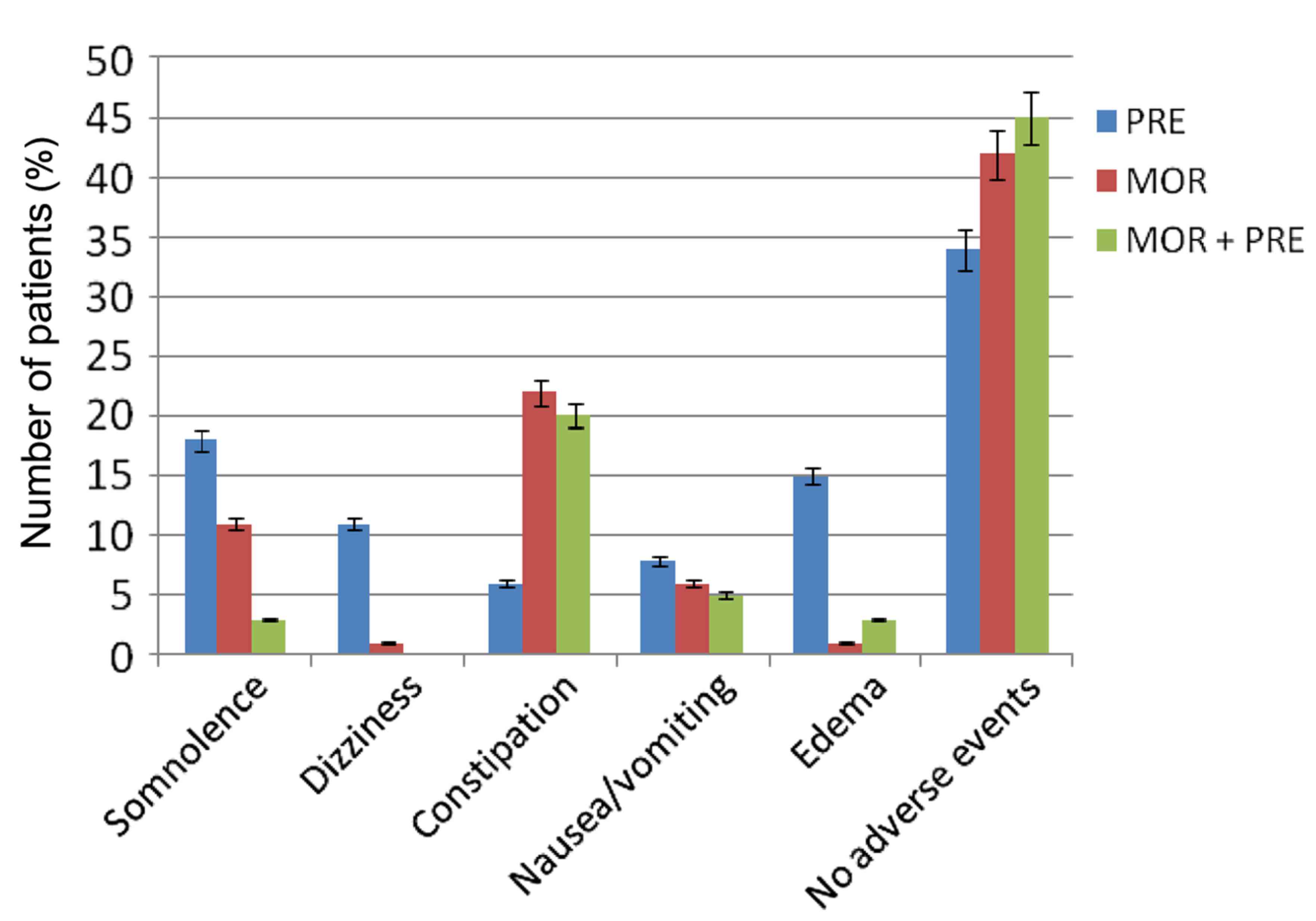
Safety analysis
In sum, patients receiving combination therapy had a
better safety profile than patients receiving either morphine or
pregabalin monotherapy. The most frequent adverse events were
somnolence and peripheral edema with pregabalin, and constipation
with morphine (Fig. 7). Constipation
was most associated with combination therapy. Notably, the
frequency of adverse events tended to decrease with the duration of
the study. After 90 days of therapy, 57.3% of patients receiving
combination therapy, 51.8% of patients receiving morphine and 36.9%
of patients receiving pregabalin monotherapy exhibited no adverse
events.
Combination therapy markedly reduced adverse events
compared with the two monotherapy groups (Fig. 7). The incidence of peripheral edema
was significantly improved in the combination therapy group
compared with the pregabalin monotherapy group (P<0.01). Nausea
and vomiting were also improved in the combination treatment
compared with other two monotherapy groups.
Discussion
The present results suggest that treatment of
neuropathic pain with pregabalin and morphine combination therapy
results in less pain than treatment with either pregabalin or
morphine as a single agent, as indicated by patients' pain
intensity and responses to pain questionnaire in the present
study.
In terms of pain relief, the efficacy of combined
morphine and pregabalin was significantly better than that of
morphine monotherapy, and was similar to pregabalin monotherapy.
The pain intensities exhibited by patients in the combination group
and the morphine monotherapy group were markedly decreased compared
with pregabalin monotherapy (80.2 and 72.8 vs. 42.9%). In the two
groups, this finding was considered to be clinically relevant
because the decrease in pain scores from baseline to the end of the
study was >2 points (10,11). Our study is in agreement with the
substantial opioid responsiveness for neuropathic pain reported by
another study, which focused on opioid based therapies (14). Other aspects of pain relief were also
improved by combination treatment with morphine plus pregabalin and
morphine monotherapy compared with pregabalin treatment. The number
of breakthrough pain events per day was decreased in the
combination and morphine therapy groups, compared with the
pregabalin group.
Compared with monotherapy, combination therapy
required lower mean dosages of morphine and pregabalin to induce
pain relief. This is particularly relevant to the management of
chronic conditions that require long-term treatment, due to the
potential for adverse events and addiction with opiod analgesics
(15).
QoL is a useful measure of the influence of chronic
conditions on the daily activities of patients. In the present
study, patients' total scores for the intensity of pain were
significantly lower in the combination therapy group, as compared
with the two monotherapy groups. Furthermore, combination treatment
was accompanied with less interference of pain with activity, mood,
walking, normal work, social relations, sleep and enjoyment of
life.
The present study showed that morphine-based therapy
may greatly improve treatment compliance. This is based on evidence
that patients receiving morphine-based treatment were more
satisfied with their condition, when compared with pregabalin
monotherapy. At the end of the study, >90% of patients who
received morphine monotherapy or combination therapy gave positive
ratings (‘effective’ and ‘very effective’), whereas <20% of
patients receiving pregabalin reported that treatment was
effective.
According to published data, adverse events in line
with opioid treatment were more common and persistent, particularly
constipation. In the present study, compared with patients who
received monotherapy, only a small proportion of patients had their
daily lives interrupted because of adverse events. Nevertheless, in
the combination therapy group, adverse events tended to decline in
frequency as the study duration increased.
The results of the present study indicate the
excellent efficacy of therapy combination with pregabalin and
morphine in the treatment of neuropathic pain. Given the potential
benefits and drawbacks of drug combinations, a randomized
controlled study is required to investigate whether dosages could
be further reduced to improve safety, and how the combination of
the two drugs may be optimized to achieve long-term pain relief. In
conclusion, combination treatment with morphine and pregabalin is
effective and may be adopted as a therapeutic option for the
treatment of patients suffering from neuropathic pain.
References
|
1
|
Schmader KE: Epidemiology and impacton
quality of life of postherpetic neuralgiaand painful diabetic
neuropathy. Clin J Pain. 18:350–354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouhassira D, Lantéri-Minet M, Attal N,
Laurent B and Touboul C: Prevalence of chronic pain with
neuropathic characteristics in the general population. Pain.
136:380–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dworkin RH, O'Connor AB, Backonja M,
Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski
C, Nurmikko TJ, et al: Phgroupacologic management of neuropathic
pain: Evidence based recommendations. Pain. 132:237–251. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dworkin RH, O'Connor AB, Audette J, Baron
R, Gourlay GK, Haanpää ML, Kent JL, Krane EJ, Lebel AA, Levy RM, et
al: Recommendations for the pharmacological management of
neuropathic pain: an overview and literature update. Mayo Clin
Proc. 85:(Suppl 3). S3–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Attal N, Cruccu G, Haanpää M, Hansson P,
Jensen TS, Nurmikko T, Sampaio C, Sindrup S and Wiffen P: EFNS Task
Force: EFNS guidelineson phgroupacological treatment of neuropathic
pain. Eur J Neurol. 13:1153–1189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenstock J, Tuchman M, LaMoreaux L and
Shgroupa U: Pregabalin for the treatment of painful diabetic
peripheral neuropathy: A double-blind, placebo-controlled trial.
Pain. 110:628–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lesser H, Shgroupa U, LaMoreaux L and
Poole RM: Pregabalin relieves symptoms of painful diabetic
neuropathy: A randomized controlled trial. Neurology. 63:2104–2110.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richter RW, Portenoy R, Shgroupa U,
Lamoreaux L, Bockbrader H and Knapp LE: Relief of painful diabetic
peripheral neuropathy with pregabalin: A randomized,
placebo-controlled trial. J Pain. 6:253–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freynhagen R, Strojek K, Griesing T,
Whalen E and Balkenohl M: Efficacy of pregabalin inneuropathic pain
evaluated in a 12-week, randomized, double-blind, multicentre,
placebo-controlled trial of flexible- and fixed-dose regimens.
Pain. 115:254–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farrar JT, Young JP Jr, LaMoreaux L, Werth
JL and Poole RM: Clinical importance of changes in chronic pain
intensity measured on an 11-point numerical pain rating scale.
Pain. 94:149–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rowbotham MC: What is a ‘clinically
meaningful’ reduction in pain? Pain. 94:131–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cleeland CS and Ryan KM: Pain assessment:
Global use of the Brief pain inventory. Ann Acad Med Singapore.
23:129–138. 1994.PubMed/NCBI
|
|
13
|
Bonezzi C, Nava A, Barbieri M, Bettaglio
R, Demartini L, Miotti D and Paulin L: Validazione della versione
italiana del Brief Pain inventory nei pazienti con dolore cronico.
Minerva Anestesiol. 68:607–611. 2002.(In Italian). PubMed/NCBI
|
|
14
|
Watson CP, Moulin D, Watt-Watson J, Gordon
A and Eisenhoffer J: Controlled-release oxycodone relieves
neuropathic pain: A randomized controlled trial in painful diabetic
neuropathy. Pain. 105:71–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenberg E, McNicol ED and Carr DB:
Efficacy and safety of opioid agonists in the treatment of
neuropathic pain of nonmalignant origin: Systematic review and
meta-analysis of randomized controlled trials. JAMA. 293:3043–3052.
2005. View Article : Google Scholar : PubMed/NCBI
|