Introduction
Chronic immune thrombocytopenic purpura (ITP) is an
autoimmune disorder characterized by an elevated rate of platelet
destruction and persistent thrombocytopenia (1,2).
Patients with chronic refractory ITP have the highest risk of death
and disease-related or therapy-related complications (3,4). Despite
intense research efforts and large multicenter clinical trials, the
optimal treatment for patients with chronic ITP in clinical
practice remains to be determined (5).
The number of studies focusing on the therapeutic
potentials of mesenchymal stem cells (MSCs) in experimental models
and clinic trials are growing. One of the reasons for this growing
interest can be explained by the fact that MSCs are assumed to be
effective biological tools to treat degenerative diseases. In
previous studies, we grafted MSCs derived from human umbilical
cord-derived MSCs (hUC-MSCs) to treat non-union in rats and humans
(6–8). Our results demonstrated the safety as
well as the efficiency of osteoblastic differentiation of hUC-MSCs.
In the present study, we describe our experience using hUC-MSCs to
treat patients with chronic refractory ITP.
Materials and methods
Basic principles and ethical
considerations
The protocol of the present study was approved by
the Institutional Review Board and the Ethics Committee of Siping
Hospital of China Medical University (Beijing, China). The trial
was conducted in compliance with current Good Clinical Practice
standards and in accordance with the principles set forth under the
Declaration of Helsinki in 1989.
Confirmation of isolation and
propagation of hUC-MSC
hUC-MSCs used in this trial were derived from two
donated umbilical cords (UC) obtained from healthy mothers during
routine term elective caesarean section births. Fully informed
consent was obtained several weeks prior to delivery. hUC-MSCs were
isolated and propagated, as previously described (6–8).
Patients
ITP was diagnosed in accordance with standard
criteria and other causes of thrombocytopenia were excluded. Three
adult patients with ITP having a platelet count
<30×109/l that persisted for at least 3 months with
an inadequate or transient response to multiple therapies were
treated with hUC-MSCs. The patients were willing to sign an
informed consent form where they agreed to be treated in the Siping
Hospital of China Medical University. The general characteristics
of the patients are presented in Table
I.
 | Table I.Patient characteristics before and
post hUC-MSC transplantation. |
Table I.
Patient characteristics before and
post hUC-MSC transplantation.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|
| Age (years) | 26 | 49 | 54 | 50 |
| Gender | M | F | F | F |
| Duration of disease
(months) | 43 | 71 | 62 | 120 |
| Previous
treatments | P, V, C, IVIg, S | P, V, C, IVIg | P, IVIg, C, De,
S | P, IVIg, V |
| HUC-MSC
transplantation times | 1 | 2 | 2 | 2 |
| Platelet counts
(x109/l)a |
| Before
therapy | 8 | 9 | 5 | 3 |
| After
therapya (2 weeks) | 56 | 94 | 103 | 56 |
| After
therapya (3
months) | 80 | 96 | 105 | 59 |
| After
therapya (6
months) | 82 | 101 | 118 | 61 |
| After
therapya (12
months) | 189 | 84 | 234 | 116 |
| After
therapya (24
months) | 134 |
|
|
|
| Bleedingb |
| Before
therapy bleeding | Skin,
genitourinary | Skin | Epistaxis | Skin, genitourinary
bleeding |
| After
therapy | No | No | Mucosal | Skin |
| Time to
response (days) | 7 | 13 | 16 | 14 |
| Time to
maximum response (days) | 31 | 53 | 42 | 58 |
| Overall
response | Yes | Yes | Yes | Yes |
| Response
duration (months)c | Yes, 24 | Yes, 18 | Yes, 13 | Yes, 13 |
Intravenous infusion of hUC-MSCs
hUC-MSCs (10 ml) with a cell density of
5×106 to 1×107/ml was given intravenously at
a rate up to 12.5×106/min and flushed with 20 ml saline
to ensure full cell dose delivery. Once the needle was fully
withdrawn, the puncture site was wrapped with sterile dressing.
Patients remained in the supine decubitus on the operation bed for
another 30 min before off-bed activities and antibiotics were given
to prevent infection. Patients' conditions were monitored
(temperature, blood pressure, pulse and oxygen saturation) at 15,
30, 45 and 60 min, and then once every hour for a minimum of 4
h.
Measurement of patelet related
parameters
Platelet-related parameters were analyzed before the
operation and at several time points post-transplantation using an
automated blood cell counter model LH-750 (Beckman Coulter, Inc.,
Brea, CA, USA).
Clinical and functional
assessment
i) Primary safety assessments included monitoring
and recording of all the adverse events as well as the serious
adverse events. The patients were monitored (temperature, blood
pressure, pulse and oxygen saturation) at 15, 30, 45 and 60 min,
and then once every hour for a minimum of 4 h. They were discharged
24 h post-transplantation if they were not febrile and
hemodynamically stable, with no signs of infection or any type of
allergic reaction. Any abnormal reactions within 3 months were
considered to be linked to transplantation.
ii) As exploratory secondary end-points we
investigated the efficacy of hUC-MSC infusion as assessed by
platelet-related parameters, at baseline and at a series of
time-points (2 weeks and 3, 6, 12 and 24 months post-first hUC-MSC
administration). Initial response evaluation was made at the end of
the second week after treatment initiation. Complete remission (CR)
was considered when the platelet count was >1×1011/l,
partial remission (PR) if platelets were >5×1010/l,
and minimal response (MR) if the platelet count was between
3×1010/l and 5×1010/l. No response was
platelet count that remained unchanged. Response was classified as
sustained (SR) when it was stable for a minimum of 6 months.
Relapse was defined as a decline in platelet count to
<30×109/l and/or the need for ITP rescue
treatments.
Pharmacological therapy protocol
Pharmacological therapy consisted of: i) inhaling
high doses of steroids and prednisone (1 mg/kg, p.o., once daily);
ii) vincristine (2 mg, once per month, i.v.); iii) intravenous
immunoglobulins (γ globulin), 0.4 g/kg, once daily, i.p.; and iv)
cyclosporine (3 mg/kg, p.o., once daily).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (Chicago, IL, USA). Safety and exploratory efficacy
secondary endpoints were observed for each patient against the
baseline values. P<0.05 was considered statistically
significant.
Results
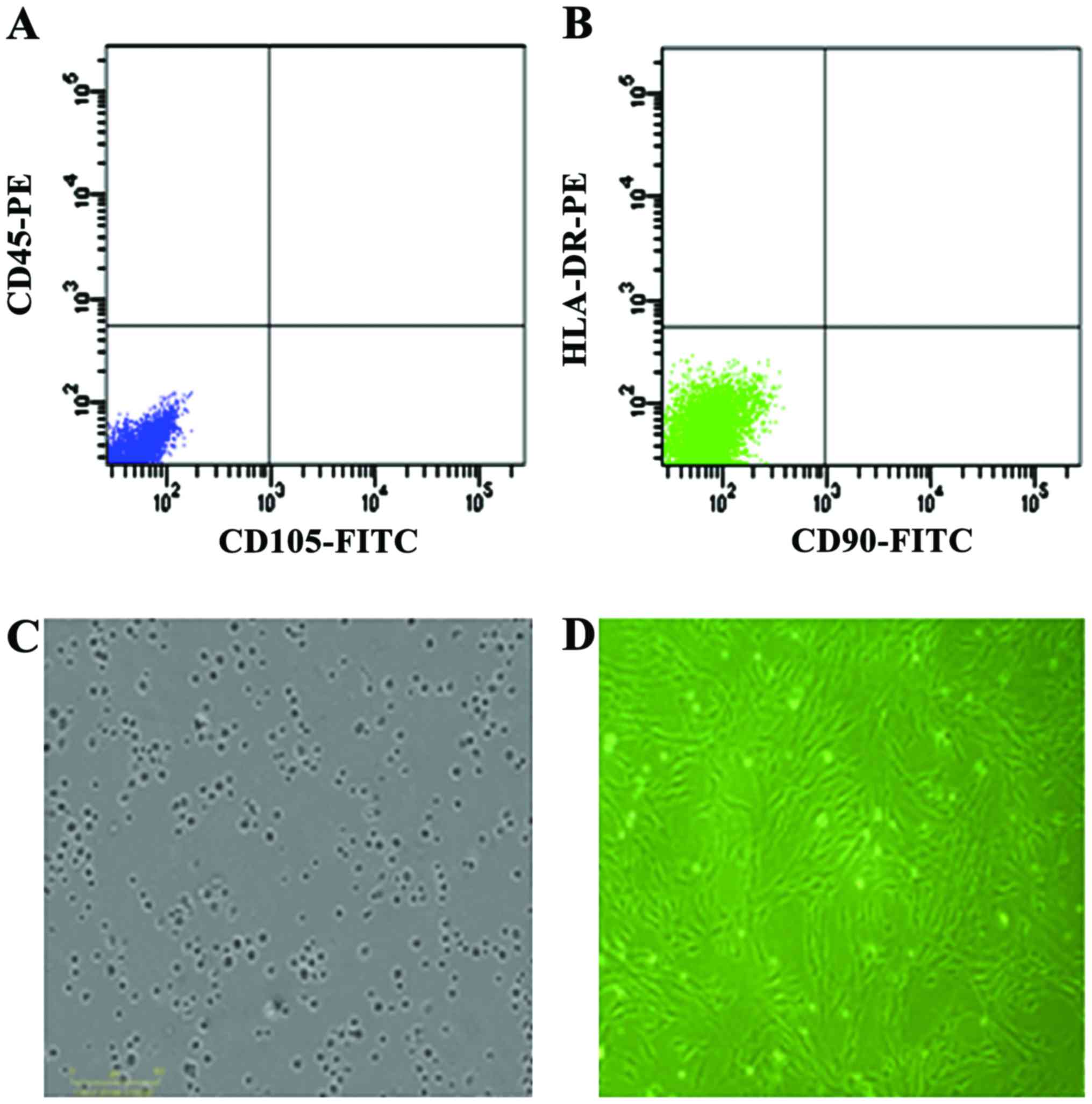
Evaluation of hUC-MSCs
Cells derived from UC were observed 24 h after they
were seeded (Fig. 1A), during the
time that part of the round mononuclear cells was adherent. Three
days after inoculation, small colonies of the adherent cells with
typical fibroblast-shaped morphology were obtained (Fig. 1B). These primary cells reached
monolayer confluence, after planting for 5–6 days, when they were
passaged for the first time. Fifth passage cells were analyzed by
flow cytometry and were strongly positive for CD105 and CD90, but
negative for CD45 and HLA-DR (Fig. 1C
and D).
Patient characteristics
Clinical characteristics of patients who
participated in the present study are summarized in Table I. The patients were 3 females and 1
male, with an age range of 26–54 years (median, 44.75 years).
Median duration of ITP before hUC-MSC transplantation was 74 months
(range, 13–120 months) and the median number of prior treatments
was 2 months (range, 1–3 months), which included splenectomy,
prednisone, intravenous immune globulin, cyclosporine and
vincristine. All the patients had a history of major bleeding and
those episodes were often transient but recurrent. Major
hemorrhagic events included genitourinary bleeding, diffuse
ecchymosis and prolonged epistaxis.
Clinical therapeutic effect of
hUC-MSCs
Results of hUC-MSC treatment are shown in Table I. Overall responses were reached in
all the patients at the end of the second week after the hUC-MSCs
had been administered. The patients achieved a platelet count of
>50×109/l and 2 patients achieved a platelet count of
>90×109/l. The median platelet count on treatment was
77.25×109/l (range, 56×109 to
10.3×1010/l). The median time to response and the median
time to maximum response were 12.5 days (range, 7–16 days) and 46
days (range, 31–53 days), respectively. One patient sustained
response after a single course of hUC-MSCs without any further
therapy during the follow-up. Major bleeding episodes did not
occur. The remaining 3 patients (patients 2–4) had a relapse within
12 months after the first hUC-MSC administration but responded to
the second hUC-MSC treatment. The time to the second response for
patients 2, 3 and 4, was 13, 16 and 18 days, respectively, whereas
the time to the second maximum response was 34, 38 and 43 days,
respectively. All the patients achieved a sustained response of
>10 months.
Safety outcomes
No serious or clinically significant side effects
were observed during the entire study period. During the whole
follow-up period, neither ectopic tissue formation nor other
illnesses related to the hUC-MSCs treatment were recorded in the
patients.
Discussion
In the present study, we evaluated the response rate
achieved in 4 patients with chronic and refractory ITP after
hUC-MSC intravenous infusion in 24 months. The clinical median
relieved time of symptoms after one transplant was 17 months. To
the best of our knowledge, this is the first study on the
efficiency of hUC-MSC transplantation treatment for ITP patients.
Our experimental results supported cell therapy for ITP.
The hUC-MSCs used in the present study met the
criteria of the International Society for Cell Therapy (9–11). In
this experiment, we observed three favorable responses (100%) in 12
months after one hUC-MSC transplantation. Patient 1 recurrence
occurred in 29 months, while the other three patients had a
recurrence in almost 13 months. Our data suggested that symptom
alleviation was not complete. Based on the characteristics of the
evolution of our group of patients, we may emphasize a few
important points: i) hUC-MSC transplantation is beneficial for the
recovery of bone marrow megakaryocytes, in order that patients can
achieve long-term relief; and ii) some cytokines are reduced in
this reaction, and the effect of hUC-MSC transplantation is
decreased. Thus, the underlying mechanisms of hUC-MSC therapy on
ITP need further exploration.
In addition, the pathophysiology of ITP is complex
and abnormalities of the B- and T-cell compartments have been
identified. Furthermore, MSCs in ITP patients have a reduced
proliferative capacity and a lower inhibitory effect on activated
T-cell proliferation compared with MSC from healthy donors
(12). These abnormalities indicate
a possible role for MSC malfunction in the physiopathology of the
disease and may have therapeutic implications. In the present
study, we did not monitor changes in B-cell counts and platelet
autoantibodies, which may influence the response as observed in
other trials (13,14), therefore we cannot confirm the effect
of hUC-MSC transplantation on the immune regulation. Future
large-scale trials are likely to be designed to investigate the
immunomodulatory characteristics of hUC-MSCs on treating ITP.
In conclusion, hUC-MSC transplantation is safe and
effective for treating ITP. Our data suggest that MSC therapy may
be a reasonable salvage treatment in severe, potentially
life-threatening, refractory ITP. The optimal period of hUC-MSC
transplantation for treating ITP is once a year. Prospective
randomized clinical trials are needed to elucidate the efficacy of
hUC-MSC transplantation therapy on ITP in the future.
Acknowledgements
The present study has been supported by the National
High Technology Research and Development Program, 863 Program, nos.
2011AA020101 and 2012A020905.
References
|
1
|
Berchtold P and McMillan R: Therapy of
chronic idiopathic thrombocytopenic purpura in adults. Blood.
74:2309–2317. 1989.PubMed/NCBI
|
|
2
|
Imbach P: Treatment of immune
thrombocytopenia with intravenous immunoglobulin and insights for
other diseases. A historical review. Swiss Med Wkly.
142:w135932012.PubMed/NCBI
|
|
3
|
McMillan R and Durette C: Long-term
outcomes in adults with chronic ITP after splenectomy failure.
Blood. 104:956–960. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Portielje JE, Westendorp RG,
Kluin-Nelemans HC and Brand A: Morbidity and mortality in adults
with idiopathic thrombocytopenic purpura. Blood. 97:2549–2554.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saeidi S, Jaseb K, Asnafi AA, Rahim F,
Pourmotahari F, Mardaniyan S, Yousefi H, Alghasi A, Shahjahani M
and Saki N: Immune thrombocytopenic purpura in children and adults:
a comparative retrospective study in IRAN. Int J Hematol Oncol Stem
Cell Res. 8:30–36. 2014.PubMed/NCBI
|
|
6
|
Qu Z, Guo S, Fang G, Cui Z and Liu Y: AKT
pathway affects bone regeneration in nonunion treated with
umbilical cord-derived mesenchymal stem cells. Cell Biochem
Biophys. 71:1543–1551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Z, Guo L, Fang G, Cui Z, Guo S and Liu
Y: Biological characteristics and effect of human umbilical cord
mesenchymal stem cells (hUC-MSCs) grafting with blood plasma on
bone regeneration in rats. Cell Biochem Biophys. 63:171–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu Z, Fang G, Cui Z and Liu Y: Cell
therapy for bone nonunion: a retrospective study. Minerva Med.
106:315–321. 2015.PubMed/NCBI
|
|
9
|
Fang B, Mai L, Li N and Song Y: Favorable
response of chronic refractory immune thrombocytopenic purpura to
mesenchymal stem cells. Stem Cells Dev. 21:497–502. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodeghiero F, Stasi R, Gernsheimer T,
Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH,
Cooper N, et al: Standardization of terminology, definitions and
outcome criteria in immune thrombocytopenic purpura of adults and
children: report from an international working group. Blood.
113:2386–2393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pérez-Simón JA, Tabera S, Sarasquete ME,
Díez-Campelo M, Canchado J, Sánchez-Abarca LI, Blanco B, Alberca I,
Herrero-Sánchez C, Cañizo C, et al: Mesenchymal stem cells are
functionally abnormal in patients with immune thrombocytopenic
purpura. Cytotherapy. 11:698–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Zhong H, Bao W, Boulad N,
Evangelista J, Haider MA, Bussel J and Yazdanbakhsh K: Defective
regulatory B-cell compartment in patients with immune
thrombocytopenia. Blood. 120:3318–3325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cines DB, Bussel JB, Liebman HA and Prak
Luning ET: The ITP syndrome: pathogenic and clinical diversity.
Blood. 113:6511–6521. 2009. View Article : Google Scholar : PubMed/NCBI
|