The importance of preventing lifestyle-related
diseases including heart disease, cerebrovascular disease, diabetes
and cancer has been increasing. In particular, the Global Oral
Health Programme of the World Health Organization (WHO) has
prompted worldwide vigilance of oral health as it is a matter of
considerable importance for general health and quality of life
(1,2). Considerable attention has been given to
the effective use of fluoride (3),
controlling tobacco use (4), oral
health at each age, from children to the elderly (5) and healthy diet and nutrition (6,7).
In order to prevent oral diseases, early detection
and diagnosis is vital. As there is a potential link between poor
oral health and chronic disease, it is important to integrate the
prevention of oral diseases with general health promotion to enable
the prevention of chronic diseases and improve oral health. Oral
diseases affect a large proportion of individuals, for example oral
cancer is the 8th most commonly diagnosed cancer in the world and
its incidence ranges from one to 10 cases per 100,000 people
globally (8). Therefore, a major
priority is to establish innovative health promotion strategies by
developing novel beneficial agents capable of improving the
prevention of oral diseases, for example by integration of oral
health into public health programmes or by supportive school
policies. The physical environment and skills-based health
education are essential in maintaining oral health and reducing
risk factors (2).
The study of associations between diet, health and
the presence of bioactive compounds in foods has previously
received attention. A large number of natural substances, including
curcumin (9), quercetin, genistein
(10) and epigallocatechin-3-gallate
(11) have been identified, which
may provide safe, non-toxic alternative pharmacological treatments
to prevent the onset of oral diseases. Nutraceutical substances may
be used either as individual compounds in their natural form
(isolated from matrixes of plant origin) or obtained from chemical
synthesis and used alone, or in combination with alternative
therapeutic agents, such as phytochemical compounds (10,12).
Traditional Chinese medicine has purported a wide
range of remedies made from herbs rich in polyphenols, including
Radix Curcumae formula to treat cardiovascular disease (13) and Yi Shen Juan Bi Tablets to treat
rheumatoid arthritis (14), which
are responsible not only for providing therapy for specific
diseases but also for improving general health (15). The scientific evidence accumulated
over the past 20 years has suggested that resveratrol (RSV), a
natural polyphenolic compound, exhibits a number of pharmacological
activities (16,17) with beneficial health effects.
Evidence has indicated that RSV is involved in the modulation of
numerous cell-signaling pathways (18,19),
thus exerting a variety of antioxidant (20), anti-inflammatory (21), anti-viral, anti-microbial, estrogenic
(22), anticancer (23,24),
cardioprotective (25),
neuroprotective (26) and
immunomodulatory (27) functions.
The potential beneficial effects of RSV have been linked with a
wide variety of chronic diseases, including cancer and the
analogues of RSV (28–30) are being investigated specifically as
chemopreventive agents in humans (31). RSV has also been linked with
cardiovascular diseases (32), skin
disorders (33), diabetes (34), arthritis (35), neurological diseases (36) and the aging process (37).
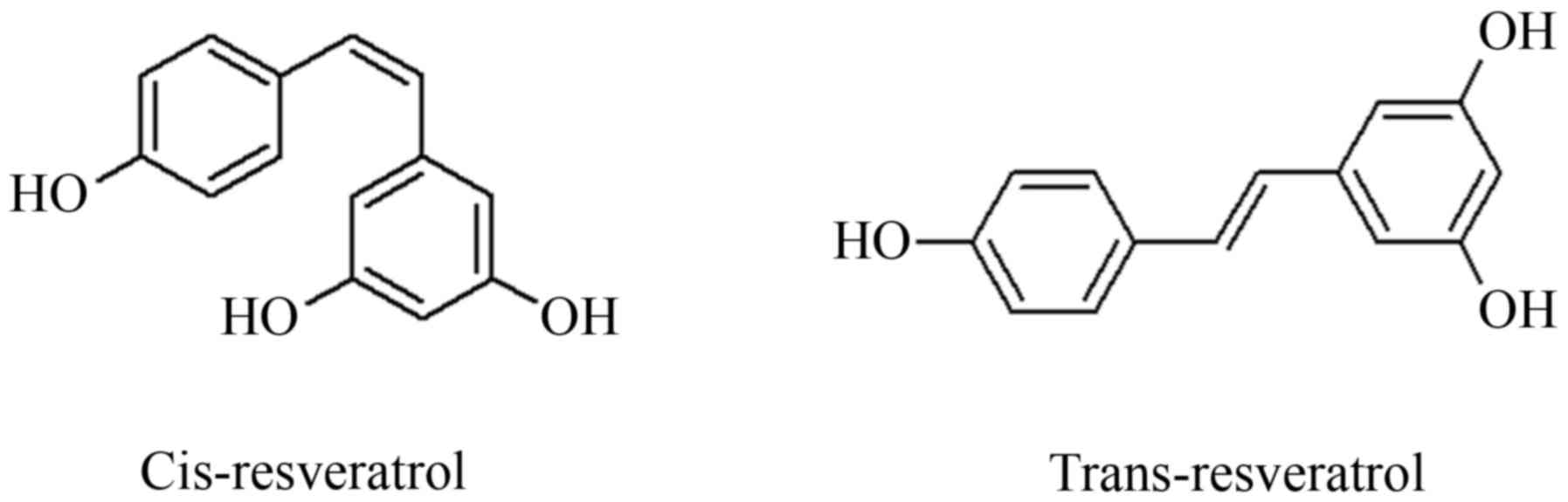
RSV is a polyphenolic compound that functionally
acts as a phytoalexin and is identified in nature as both cis and
trans isomers (Fig. 1). RSV is
synthesized by plants as a defense mechanism in response to
bacterial or fungal infection (17)
and stress factors (such as UV irradiation or ozone exposure
(38,39). RSV is synthesized through stilbene
synthase by three different condensation reactions between three
molecules of malonyl-coenzime A and one molecule of
coumaroyl-coenzyme A (40). RSV is
naturally occurring in >70 different plant species, such as
Polygonum cuspidatum (41),
eucalyptus (42) and Picea
excelsa (43), and fruit
species, including mulberries, raspberries, pines, peanuts,
blueberries and grapes (44).
Although these plants and their extracts have been used for various
therapeutic purposes by traditional Chinese and Japanese medicine,
RSV itself was first isolated in 1940 (45) from the roots of the medicinal herb
hellebore (Veratrum grandiflorum) (46) and it was identified as a component of
wine in 1992 (47,48) However, this compound exists in
numerous foods and beverages consumed daily, including cocoa
(49), grapes (50) and red wine (51). Red wine, the most notable dietary
source of RSV (52), has received
special attention and in 1992 an epidemiological study demonstrated
a potential correlation between incidence of cardiovascular disease
and the consumption of red wine, observed in Mediterranean
populations (48).
RSV is composed of two phenol rings, linked by a
styrene double bond to generate 3,4′,5-trihydroxystilbene.
Different conjugate forms of this compound have been detected in
plants and trans-resveratrol exists in glycosylated form;
trans-isomer is sterically a more stable form and for this reason
is considered to be the most abundant form (53). Isomerization is facilitated by UV,
light and pH, therefore trans-resveratrol is rapidly converted into
cis-resveratrol by visible light, high temperatures or low pH
(54).
RSV has a high number of analogues and derivatives,
differing in terms of type, number and position of substituents
(hydroxyl, methoxyl, halogenated, glycosylated, esterified), the
presence of stilbenic double bonds, modified steroisomery and
oxidative dimerizations (to form oligomers) (16). Additionally, a number of studies
indicate that certain resveratrol analogues and derivatives have
pharmacological properties, such as apoptotic and antioxidant
activities (55) and chemopreventive
effects (56).
In grapes and wine, RSV has been identified as a
free-species and in glycosylated form. In particular, piceid
(resveratrol-3-O-beta-D-glucoside, also called polydatin) is a
glucoside of RSV in which the hydroxyl group in the C-3 position is
substituted by a glycoside group, a hydroxyl group substitute
(61). The substitution of the
glycosidic group leads to conformational changes occurring in the
polydatin that are reflected in changes to the biological
properties, greater bioavailability and a higher stability. The
piceid maintains the antioxidant properties and the hydroxyl group
at C-3, which in this compound is replaced by glycosidic group, is
less reactive in regards to the activity of scavenging, undertaken
by the hydroxyl group at C-4 that remains unchanged in polydatin
(62). The piceid retains the
biological activity of resveratrol but has a number of advantages
over RSV in drug research: Polydatins are more resistant to
enzymatic oxidation than RSV, penetrate the cell via an active
carrier mechanism using glucose carrier and due to its solubility
in water, it is absorbed from the intestine with greater efficiency
(63,64).
The beneficial properties of RSV have been
extensively investigated in the literature with studies in
vitro (65) and in vivo
(66); however, there is limited
understanding regarding the pharmacokinetics of RSV. A number of
studies in animals and humans (46)
have demonstrated that unconjugated RSV has a poor in vivo
bioavailability, due to its extremely rapid metabolism to
glucuronide and sulphate derivatives in the liver and intestine
(67). The plasma concentration of
RSV and its metabolites depend on the dose administered. Marier
et al (68) have shown that
trans-resveratrol in in its aglycone and glucuronide forms
exhibited increases in plasma concentrations 4–8 h after oral
administration, with terminal elimination half-life of 1.48 and
1.58 h, respectively. Trans-resveratrol in its aglycone form has
38% bioavailability and its exposure was approximately 46-fold
lower than that of the glucuronide form (The area under the curve
extrapolated to infinity is 7.1 vs. 324.7 µmol·h/l). Due to its
poor water solubility (69) RSV
requires binding with serum proteins (70); however, it is able to passively
diffuse through the plasma membrane (71). Although there is considerable
inter-individual variability, five distinct metabolites may be
detected in the urine following the moderate consumption of red
wine: Resveratrol monosulfate, two isomeric forms of resveratrol
monoglucuronide, dihydroresveratrol monosulfate and
dihydroresveratrol (72). Further
studies of the activity of its metabolites are required to
understand the in vivo concentrations of different
metabolites from ingested RSV that may be much higher than the
concentration of RSV itself.
At present, attention is being paid to novel
techniques and proposals to promote the bioavailability of these
molecules, such as microparticles (77), nanoparticles (78), microsphere (79), microencapsulation (80) and nanoencapsulation (81). Evidence indicates that the reduction
of particle size allows an increase in the contact surface, for
example, trans-resveratrol is the most commonly used isomeric form
due to its numerous health benefits, even though it has poor
bioavailability due to low aqueous solubility and slow dissolution
rate. However, these parameters increase following treatments to
reduce the particle size (82).
With regards to toxicity it has been demonstrated
that, unless extremely high doses are administered, there are no
signs of toxicity following treatment with RSV. Animal models
exhibited no adverse effects following 28 days of RSV
administration at 1,000-fold the levels of RSV present in red wine
(83).
Previous results have indicated that resveratrol is
a free radical scavenger and potent antioxidant, counteracting the
oxidative stress that is considered to be associated with the
etiology and progression of multiple chronic and acute diseases
(84). In clinical studies,
oxidative stress has been associated with a number of degenerative
diseases including atherosclerosis, cancer, asthma, hyperoxia,
arthritis, dermatitis (85,86) and inflammatory conditions (87).
The antioxidant system includes a number of
antioxidant enzymes such as superoxide dismutase and catalase,
non-enzymatic antioxidants such as reduced glutathione (GSH),
protein-sulfhydryls and uric acid. It has been demonstrated that
RSV significantly activates and prevents the oxidation of these
endogenous antioxidant systems. RSV has been demonstrated to reduce
the production of H2O2, and normalize the
level of oxidized glutathione reductase and myeloperoxidase
activities (93).
It is important to note that the protective effects
of RSV against lipids and peroxidation occur over a very short time
frame (16,107–109).
RSV is rapidly absorbed and its peak plasma concentration is
achieved within 15–60 min of its administration (73,110).
Oral submucous fibrosis (OSF) is a precancerous
condition that affects the oral mucosa, which currently cannot be
treated by specific therapeutic drugs. Moderate-to-severe OSF is
irreversible and current treatment strategies include injections or
topical application of steroids, oral subministration of lycopene
(16 mg daily) and pentoxyfilline (400 mg 3 times daily) (16,118,119).
A previous study demonstrated that RSV epigenetically inhibits Zinc
finger E-Box binding homeobox 1 expression to suppress the
myofibroblast activity of fibrotic buccal mucosal fibroblasts, and
may serve as a dietary supplement for OSF patients (120).
Another study demonstrated that RSV inhibited matrix
metalloproteinase-9 expression and metastasis in oral cancer cells
(SCC-9) by downregulating the signaling pathways of c-Jun
N-terminal kinase 1/2 and extra-cellular signal regulated kinase
1/2 signals, thus, exerting beneficial effects in chemoprevention
(123).
Resveratrol is a promising nutraceutical for the
treatment of cancer; however, the molecular mechanisms that explain
the chemopreventive role of RSV remain unknown. Currently, there
are numerous in vitro studies regarding the benefits of RSV
as an anticancer agent (12,117,124,125).
It is necessary to complete and confirm these effects using in
vivo studies and clinical trials.
Finally, in order to resolve the primary problem
associated with the poor bioavailability of RSV and its complicated
pharmacokinetic profile, the development of specific nanotechnology
and controlled and targeted-drug delivery systems are required.
|
1
|
Petersen PE: Oral cancer prevention and
control-the approach of the World Health Organization. Oral Oncol.
45:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petersen PE: World Health Organization
global policy for improvement of oral health-World Health Assembly
2007. Int Dent J. 58:115–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marthaler TM and Petersen PE: Salt
fluoridation-an alternative in automatic prevention of dental
caries. Int Dent J. 55:351–358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petersen PE: Tobacco and oral health-the
role of the World Health Organization. Oral Health Prev Dent.
1:309–315. 2003.PubMed/NCBI
|
|
5
|
Petersen PE, Bourgeois D, Bratthall D and
Ogawa H: Oral health information systems-towards measuring progress
in oral health promotion and disease prevention. Bull World Health
Organ. 83:686–693. 2005.PubMed/NCBI
|
|
6
|
Moynihan P and Petersen PE: Diet,
nutrition and the prevention of dental diseases. Public Health
Nutr. 7:201–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moynihan PJ: The role of diet and
nutrition in the etiology and prevention of oral diseases. Bull
World Health Organ. 83:694–699. 2005.PubMed/NCBI
|
|
8
|
Oral Health Worldwide, . A report by FDI
World Dental Federation. FDI World Dental Federation; Geneva:
2014
|
|
9
|
Nagpal M and Sood S: Role of curcumin in
systemic and oral health: An overview. J Nat Sci Biol Med. 4:3–7.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elattar TM and Virji AS: The inhibitory
effect of curcumin, genistein, quercetin and cisplatin on the
growth of oral cancer cells in vitro. Anticancer Res. 20:1733–1738.
2000.PubMed/NCBI
|
|
11
|
Ramshankar V and Krishnamurthy A:
Chemoprevention of oral cancer: Green tea experience. J Nat Sci
Biol Med. 5:3–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan J, Yue W, E J, Malhotra J, Lu SE, Gu
J, Xu F and Tan XL: In vitro comparative studies of resveratrol and
triacetylresveratrol on cell proliferation, apoptosis, and STAT3
and NFκB signaling in pancreatic cancer cells. Sci Rep.
6:316722016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao W, Xu X, Wang X, Li B, Wang Y, Li Y
and Yang L: Network pharmacology-based prediction of the active
ingredients and potential targets of Chinese herbal Radix Curcumae
formula for application to cardiovascular disease. J
Ethnopharmacol. 145:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang M, Lu C, Chen G, Xiao C, Zha Q, Niu
X, Chen S and Lu A: Understanding the molecular mechanism of
interventions in treating rheumatoid arthritis patients with
corresponding traditional Chinese medicine patterns based on
bioinformatics approach. Evid Based Complement Alternat Med.
2012:1294522012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S and Zhang B: Traditional Chinese
medicine network pharmacology: Theory, methodology and application.
Chin J Nat Med. 11:110–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
17
|
Pervaiz S and Holme AL: Resveratrol: Its
biologic targets and functional activity. Antioxid Redox Signal.
11:2851–2897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harikumar KB and Aggarwal BB: Resveratrol:
A multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Athar M, Back JH, Kopelovich L, Bickers DR
and Kim AL: Multiple molecular targets of resveratrol:
Anti-carcinogenic mechanisms. Arch Biochem Biophys. 486:95–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kerem Z, Chetrit D, Shoseyov O and
Regev-Shoshani G: Protection of lipids from oxidation by
epicatechin, trans-resveratrol, and gallic and caffeic acids in
intestinal model systems. J Agric Food Chem. 54:10288–10293. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murias M, Handler N, Erker T, Pleban K,
Ecker G, Saiko P, Szekeres T and Jäger W: Resveratrol analogues as
selective cyclooxygenase-2 inhibitors: Synthesis and
structure-activity relationship. Bioorg Med Chem. 12:5571–5578.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gehm BD, McAndrews JM, Chien PY and
Jameson JL: Resveratrol, a polyphenolic compound found in grapes
and wine, is an agonist for the estrogen receptor. Proc Natl Acad
Sci USA. 94:pp. 14138–14143. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kraft TE, Parisotto D, Schempp C and
Efferth T: Fighting cancer with red wine? Molecular mechanisms of
resveratrol. Crit Rev Food Sci Nutr. 49:782–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benová B, Adam M, Onderková K, Královsky J
and Krajicek M: Analysis of selected stilbenes in Polygonum
cuspidatum by HPLC coupled with CoulArray detection. J Sep Sci.
31:2404–2409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou
JG and Huang YZ: Mechanism of cardioprotection by resveratrol, a
phenolic antioxidant present in red wine (Review). Int J Mol Med.
8:3–17. 2001.PubMed/NCBI
|
|
26
|
Jung JC, Lim E, Lee Y, Kang JM, Kim H,
Jang S, Oh S and Jung M: Synthesis of novel trans-stilbene
derivatives and evaluation of their potent antioxidant and
neuroprotective effects. Eur J Med Chem. 44:3166–3174. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
King RE, Kent KD and Bomser JA:
Resveratrol reduces oxidation and proliferation of human retinal
pigment epithelial cells via extracellular signal-regulated kinase
inhibition. Chem Biol Interact. 151:143–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saiko P, Szakmary A, Jaeger W and Szekeres
T: Resveratrol and its analogs: Defense against cancer, coronary
disease and neurodegenerative maladies or just a fad? Mutat Res.
658:68–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szekeres T, Fritzer-Szekeres M, Saiko P
and Jäger W: Resveratrol and resveratrol
analogues-structure-activity relationship. Pharm Res. 27:1042–1048.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szekeres T, Saiko P, Fritzer-Szekeres M,
Djavan B and Jäger W: Chemopreventive effects of resveratrol and
resveratrol derivatives. Ann N Y Acad Sci. 1215:89–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piotrowska H, Kucinska M and Murias M:
Biological activity of piceatannol: Leaving the shadow of
resveratrol. Mutat Res. 750:60–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petrovski G, Gurusamy N and Das DK:
Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci.
1215:22–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ndiaye M, Philippe C, Mukhtar H and Ahmad
N: The grape antioxidant resveratrol for skin disorders: Promise,
prospects, and challenges. Arch Biochem Biophys. 508:164–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szkudelska K and Szkudelski T:
Resveratrol, obesity and diabetes. Eur J Pharmacol. 635:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wahba MG, Messiha BA and Abo-Saif AA:
Protective effects of fenofibrate and resveratrol in an aggressive
model of rheumatoid arthritis in rats. Pharm Biol. 54:1705–1715.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bastianetto S, Ménard C and Quirion R:
Neuroprotective action of resveratrol. Biochim Biophys Acta.
1852:1195–1201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Markus MA and Morris BJ: Resveratrol in
prevention and treatment of common clinical conditions of aging.
Clin Interv Aging. 3:331–339. 2008.PubMed/NCBI
|
|
38
|
Frémont L: Biological effects of
resveratrol. Life Sci. 66:663–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang W, Tang K, Yang HR, Wen PF, Zhang P,
Wang HL and Huang WD: Distribution of resveratrol and stilbene
synthase in young grape plants (Vitis vinifera L. cv. Cabernet
Sauvignon) and the effect of UV-C on its accumulation. Plant
Physiol Biochem. 48:142–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soleas GJ, Diamandis EP and Goldberg DM:
Resveratrol: A molecule whose time has come? And gone? Clin
Biochem. 30:91–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z
and Rosen RT: Isolation and identification of stilbenes in two
varieties of Polygonum cuspidatum. J Agric Food Chem. 48:253–256.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hathway DE and Seakins JW:
Hydroxystilbenes of Eucalyptus wandoo. Biochem J. 72:369–374. 1959.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rolfs CH and Kindl H: Stilbene synthase
and chalcone synthase: Two different constitutive enzymes in
cultured cells of Picea excelsa. Plant Physiol. 75:489–492. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
King RE, Bomser JA and Min DB: Bioactivity
of resveratrol. Comprehensive Reviews in Food Science and Food
Safety. 5:65–70. 2006. View Article : Google Scholar
|
|
45
|
Takaoka MJ: The phenolic substances of
white hellebore (Veratrum grandiflorum Loes. fil.). J Faculty Sci.
3:1–16. 1940.
|
|
46
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Langcake P and Pryce RJ: A new class of
phytoalexins from grapevines. Experientia. 33:151–152. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Renaud S and de Lorgeril M: Wine, alcohol,
platelets, and the French paradox for coronary heart disease.
Lancet. 339:1523–1526. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Counet C, Callemien D and Collin S:
Chocolate and cocoa: New sources of trans-resveratrol and
trans-piceid. Food Chemistry. 98:649–657. 2006. View Article : Google Scholar
|
|
50
|
Wang Y, Catana F, Yang Y, Roderick R and
van Breemen RB: An LC-MS method for analyzing total resveratrol in
grape juice, cranberry juice, and in wine. J Agric Food Chem.
50:431–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Siemann EH and Creasy LL: Concentration of
the Phytoalexin resveratrol in wine. Am J Enol Vitic. 43:49–52.
1992.
|
|
52
|
Guerrero RF, Garcia-Parrilla MC, Puertas B
and Cantos-Villar E: Wine, resveratrol and health: A review. Nat
Prod Commun. 4:635–658. 2009.PubMed/NCBI
|
|
53
|
Stervbo U, Vang O and Bonnesen C: A review
of the content of the putative chemopreventive phytoalexin
resveratrol in red wine. Food Chemistry. 101:449–457. 2007.
View Article : Google Scholar
|
|
54
|
Figueiras TS, Neves-Petersen MT and
Petersen SB: Activation energy of light induced isomerization of
resveratrol. J Fluoresc. 21:1897–1906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cai YJ, Wei QY, Fang JG, Yang L, Liu ZL,
Wyche JH and Han Z: The 3,4-dihydroxyl groups are important for
trans-resveratrol analogs to exhibit enhanced antioxidant and
apoptotic activities. Anticancer Res. 24:999–1002. 2004.PubMed/NCBI
|
|
56
|
Wieder T, Prokop A, Bagci B, Essmann F,
Bernicke D, Schulze-Osthoff K, Dörken B, Schmalz HG, Daniel PT and
Henze G: Piceatannol, a hydroxylated analog of the chemopreventive
agent resveratrol, is a potent inducer of apoptosis in the lymphoma
cell line BJAB and in primary, leukemic lymphoblasts. Leukemia.
15:1735–1742. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang T, Wang L, Zhu M, Zhang L and Yan L:
Properties and molecular mechanisms of resveratrol: A review.
Pharmazie. 70:501–506. 2015.PubMed/NCBI
|
|
58
|
Biasutto L, Marotta E, Bradaschia A,
Fallica M, Mattarei A, Garbisa S, Zoratti M and Paradisi C: Soluble
polyphenols: Synthesis and bioavailability of
3,4′,5-tri(alpha-D-glucose-3-O-succinyl) resveratrol. Bioorg Med
Chem Lett. 19:6721–6724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ratnam DV, Ankola DD, Bhardwaj V, Sahana
DK and Kumar MN: Role of antioxidants in prophylaxis and therapy: A
pharmaceutical perspective. J Control Release. 113:189–207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin YL, Chang HC, Chen TL, Chang JH, Chiu
WT, Lin JW and Chen RM: Resveratrol protects against oxidized
LDL-induced breakage of the blood-brain barrier by lessening
disruption of tight junctions and apoptotic insults to mouse
cerebrovascular endothelial cells. J Nutr. 140:2187–2192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Du QH, Peng C and Zhang H: Polydatin: A
review of pharmacology and pharmacokinetics. Pharm Biol.
51:1347–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Plou FJ, Segura AGd and Ballesteros A:
Application of Glycosidases and Transglycosidases in the synthesis
of oligosaccharidesIndustrial enzymes: Structure, function and
applications. Polaina J and MacCabe AP: Springer; Netherlands,
Dordrecht: pp. 141–157. 2007, View Article : Google Scholar
|
|
63
|
Bertrand A, Morel S, Lefoulon F, Rolland
Y, Monsan P and Remaud-Simeon M: Leuconostoc mesenteroides
glucansucrase synthesis of flavonoid glucosides by acceptor
reactions in aqueous-organic solvents. Carbohydr Res. 341:855–863.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cichewicz RH and Kouzi SA:
Biotransformation of resveratrol to piceid by Bacillus cereus. J
Nat Prod. 61:1313–1314. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Baek SJ, Wilson LC and Eling TE:
Resveratrol enhances the expression of non-steroidal
anti-inflammatory drug-activated gene (NAG-1) by increasing the
expression of p53. Carcinogenesis. 23:425–434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Aziz MH, Kumar R and Ahmad N: Cancer
chemoprevention by resveratrol: In vitro and in vivo studies and
the underlying mechanisms (review). Int J Oncol. 23:17–28.
2003.PubMed/NCBI
|
|
67
|
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr
and Walle UK: High absorption but very low bioavailability of oral
resveratrol in humans. Drug Metab Dispos. 32:1377–1382. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Marier JF, Vachon P, Gritsas A, Zhang J,
Moreau JP and Ducharme MP: Metabolism and disposition of
resveratrol in rats: Extent of absorption, glucuronidation, and
enterohepatic recirculation evidenced by a linked-rat model. J
Pharmacol Exp Ther. 302:369–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Belguendouz L, Fremont L and Linard A:
Resveratrol inhibits metal ion-dependent and independent
peroxidation of porcine low-density lipoproteins. Biochem
Pharmacol. 53:1347–1355. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jannin B, Menzel M, Berlot JP, Delmas D,
Lancon A and Latruffe N: Transport of resveratrol, a cancer
chemopreventive agent, to cellular targets: Plasmatic protein
binding and cell uptake. Biochem Pharmacol. 68:1113–1118. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lançon A, Delmas D, Osman H, Thénot JP,
Jannin B and Latruffe N: Human hepatic cell uptake of resveratrol:
Involvement of both passive diffusion and carrier-mediated process.
Biochem Biophys Res Commun. 316:1132–1137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lucas R, Alcantara D and Morales JC: A
concise synthesis of glucuronide metabolites of urolithin-B,
resveratrol, and hydroxytyrosol. Carbohydr Res. 344:1340–1346.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bertelli AA, Giovannini L, Stradi R,
Bertelli A and Tillement JP: Plasma, urine and tissue levels of
trans- and cis-resveratrol (3,4′,5-trihydroxystilbene) after
short-term or prolonged administration of red wine to rats. Int J
Tissue React. 18:67–71. 1996.PubMed/NCBI
|
|
74
|
El Mohsen MA, Marks J, Kuhnle G, Moore K,
Debnam E, Srai S Kaila, Rice-Evans C and Spencer JP: Absorption,
tissue distribution and excretion of pelargonidin and its
metabolites following oral administration to rats. Br J Nutr.
95:51–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Vitrac X, Desmoulière A, Brouillaud B,
Krisa S, Deffieux G, Barthe N, Rosenbaum J and Mérillon JM:
Distribution of [14C]-trans-resveratrol, a cancer chemopreventive
polyphenol, in mouse tissues after oral administration. Life Sci.
72:2219–2233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
de Santi C, Pietrabissa A, Spisni R, Mosca
F and Pacifici GM: Sulphation of resveratrol, a natural product
present in grapes and wine, in the human liver and duodenum.
Xenobiotica. 30:609–617. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Koga CC, Andrade JE, Ferruzzi MG and Lee
Y: Stability of trans-resveratrol encapsulated in a protein matrix
produced using spray drying to UV light stress and simulated
gastro-intestinal digestion. J Food Sci. 81:C292–C300. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Teskac K and Kristl J: The evidence for
solid lipid nanoparticles mediated cell uptake of resveratrol. Int
J Pharm. 390:61–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nam JB, Ryu JH, Kim JW, Chang IS and Suh
KD: Stabilization of resveratrol immobilized in monodisperse
cyano-functionalized porous polymeric microspheres. Polymer.
46:8956–8963. 2005. View Article : Google Scholar
|
|
80
|
Shi G, Rao L, Yu H, Xiang H, Yang H and Ji
R: Stabilization and encapsulation of photosensitive resveratrol
within yeast cell. Int J Pharm. 349:83–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kesisoglou F, Panmai S and Wu Y:
Nanosizing-oral formulation development and biopharmaceutical
evaluation. Adv Drug Deliv Rev. 59:631–644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rasenack N and Müller BW: Crystal habit
and tableting behavior. Int J Pharm. 244:45–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Juan ME, Vinardell MP and Planas JM: The
daily oral administration of high doses of trans-resveratrol to
rats for 28 days is not harmful. J Nutr. 132:257–260.
2002.PubMed/NCBI
|
|
84
|
Leonard SS, Xia C, Jiang BH, Stinefelt B,
Klandorf H, Harris GK and Shi X: Resveratrol scavenges reactive
oxygen species and effects radical-induced cellular responses.
Biochem Biophys Res Commun. 309:1017–1026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Halliwell B and Gutteridge JM: Role of
free radicals and catalytic metal ions in human disease: An
overview. Methods Enzymol. 186:1–85. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rizvi SI and Pandey KB: Activation of the
erythrocyte plasma membrane redox system by resveratrol: A possible
mechanism for antioxidant properties. Pharmacol Rep. 62:726–732.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Das S and Das DK: Anti-inflammatory
responses of resveratrol. Inflamm Allergy Drug Targets. 6:168–173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kim CH, Jeon HM, Lee SY, Jeong EK, Ju MK,
Park BJ, Park HG, Lim SC, Han SI and Kang HS: Role of reactive
oxygen species-dependent protein aggregation in metabolic
stress-induced necrosis. Int J Oncol. 37:97–102. 2010.PubMed/NCBI
|
|
91
|
la Lastra CA and Villegas I: Resveratrol
as an antioxidant and pro-oxidant agent: Mechanisms and clinical
implications. Biochem Soc Trans. 35:1156–1160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Azmi AS, Bhat SH, Hanif S and Hadi SM:
Plant polyphenols mobilize endogenous copper in human peripheral
lymphocytes leading to oxidative DNA breakage: A putative mechanism
for anticancer properties. FEBS Lett. 580:533–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jang M and Pezzuto JM: Cancer
chemopreventive activity of resveratrol. Drugs Exp Clin Res.
25:65–77. 1999.PubMed/NCBI
|
|
94
|
Cao Z and Li Y: Potent induction of
cellular antioxidants and phase 2 enzymes by resveratrol in
cardiomyocytes: Protection against oxidative and electrophilic
injury. Eur J Pharmacol. 489:39–48. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yen GC, Duh PD and Lin CW: Effects of
resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced
oxidative DNA damage in human lymphocytes. Free Radic Res.
37:509–514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Melov S: Therapeutics against
mitochondrial oxidative stress in animal models of aging. Ann N Y
Acad Sci. 959:330–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ates O, Cayli SR, Yucel N, Altinoz E,
Kocak A, Durak MA, Turkoz Y and Yologlu S: Central nervous system
protection by resveratrol in streptozotocin-induced diabetic rats.
J Clin Neurosci. 14:256–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Belguendouz L, Frémont L and Gozzelino MT:
Interaction of transresveratrol with plasma lipoproteins. Biochem
Pharmacol. 55:811–816. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Rotondo S, Rajtar G, Manarini S, Celardo
A, Rotillo D, De Gaetano G, Evangelista V and Cerletti C: Effect of
trans-resveratrol, a natural polyphenolic compound, on human
polymorphonuclear leukocyte function. Br J Pharmacol.
123:1691–1699. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
West JD and Marnett LJ: Endogenous
reactive intermediates as modulators of cell signaling and cell
death. Chem Res Toxicol. 19:173–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lee HS, Kim HN, Jung MH and Choi JY:
Electrically evoked potentials recorded in a patient with auditory
neuropathy. Cochlear Implants Int. 5 Suppl 1:S2312004. View Article : Google Scholar
|
|
102
|
Rizvi SI and Maurya PK: Alterations in
antioxidant enzymes during aging in humans. Mol Biotechnol.
37:58–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wenzel U, Nickel A and Daniel H: Increased
mitochondrial palmitoylcarnitine/carnitine countertransport by
flavone causes oxidative stress and apoptosis in colon cancer
cells. Cell Mol Life Sci. 62:3100–3105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Guo R, Su Y, Liu B, Li S, Zhou S and Xu Y:
Resveratrol suppresses oxidised low-density lipoprotein-induced
macrophage apoptosis through inhibition of intracellular reactive
oxygen species generation, LOX-1, and the p38 MAPK pathway. Cell
Physiol Biochem. 34:603–616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dani C, Bonatto D, Salvador M, Pereira MD,
Henriques JA and Eleutherio E: Antioxidant protection of
resveratrol and catechin in Saccharomyces cerevisiae. J Agric Food
Chem. 56:4268–4272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Naylor AJ Dirks: Cellular effects of
resveratrol in skeletal muscle. Life Sci. 84:637–640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pandey KB and Rizvi SI: Protective effect
of resveratrol on formation of membrane protein carbonyls and lipid
peroxidation in erythrocytes subjected to oxidative stress. Appl
Physiol Nutr Metab. 34:1093–1097. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Pandey KB and Rizvi SI: Plant polyphenols
as dietary antioxidants in human health and disease. Oxid Med Cell
Longev. 2:270–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rizvi SI, Jha R and Pandey KB: Activation
of erythrocyte plasma membrane redox system provides a useful
method to evaluate antioxidant potential of plant polyphenols.
Methods Mol Biol. 594:341–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Goldberg DM, Yan J and Soleas GJ:
Absorption of three wine-related polyphenols in three different
matrices by healthy subjects. Clin Biochem. 36:79–87. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Perrone D, Ardito F, Giannatempo G,
Dioguardi M, Troiano G, Lo Russo L, De Lillo A, Laino L and Lo
Muzio L: Biological and therapeutic activities, and anticancer
properties of curcumin. Exp Ther Med. 10:1615–1623. 2015.PubMed/NCBI
|
|
112
|
Maioli E, Torricelli C and Valacchi G:
Rottlerin and cancer: Novel evidence and mechanisms. Scientific
World Journal. 2012:3508262012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Russo M, Russo GL, Daglia M, Kasi PD, Ravi
S, Nabavi SF and Nabavi SM: Understanding genistein in cancer: The
‘good’ and the ‘bad’ effects: A review. Food Chem. 196:589–600.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Brito AF, Ribeiro M, Abrantes AM, Pires
AS, Teixo RJ, Tralhão JG and Botelho MF: Quercetin in cancer
treatment, alone or in combination with conventional therapeutics?
Curr Med Chem. 22:3025–3039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhao X and Zhang J: Mechanisms for
quercetin in prevention of lung cancer cell growth and metastasis.
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 40:592–597. 2015.(In Chinese).
PubMed/NCBI
|
|
116
|
Feitelson MA, Arzumanyan A, Kulathinal RJ,
Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML,
Nawroth R, Sanchez-Garcia I, et al: Sustained proliferation in
cancer: Mechanisms and novel therapeutic targets. Semin Cancer
Biol. 35 Suppl:S25–S54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yu XD, Yang JL, Zhang WL and Liu DX:
Resveratrol inhibits oral squamous cell carcinoma through induction
of apoptosis and G2/M phase cell cycle arrest. Tumour Biol.
37:2871–2877. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ray JG, Ranganathan K and Chattopadhyay A:
Malignant transformation of oral submucous fibrosis: Overview of
histopathological aspects. Oral Surg Oral Med Oral Pathol Oral
Radiol. 122:200–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sharma M and Radhakrishnan R: Limited
mouth opening in oral submucous fibrosis: Reasons, ramifications,
and remedies. J Oral Pathol Med. Oct 15–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
120
|
Chang YC, Lin CW, Yu CC, Wang BY, Huang
YH, Hsieh YC, Kuo YL and Chang WW: Resveratrol suppresses
myofibroblast activity of human buccal mucosal fibroblasts through
the epigenetic inhibition of ZEB1 expression. Oncotarget.
7:12137–12149. 2016.PubMed/NCBI
|
|
121
|
Mohan A, Narayanan S, Balasubramanian G,
Sethuraman S and Krishnan UM: Dual drug loaded nanoliposomal
chemotherapy: A promising strategy for treatment of head and neck
squamous cell carcinoma. Eur J Pharm Biopharm. 99:73–83. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
ElAttar TM and Virji AS: Modulating effect
of resveratrol and quercetin on oral cancer cell growth and
proliferation. Anticancer Drugs. 10:187–193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lin FY, Hsieh YH, Yang SF, Chen CT, Tang
CH, Chou MY, Chuang YT, Lin CW and Chen MK: Resveratrol suppresses
TPA-induced matrix metalloproteinase-9 expression through the
inhibition of MAPK pathways in oral cancer cells. J Oral Pathol
Med. 44:699–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Masuelli L, Di Stefano E, Fantini M,
Mattera R, Benvenuto M, Marzocchella L, Sacchetti P, Focaccetti C,
Bernardini R, Tresoldi I, et al: Resveratrol potentiates the in
vitro and in vivo anti-tumoral effects of curcumin in head and neck
carcinomas. Oncotarget. 5:10745–10762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shan Z, Yang G, Xiang W, Pei-Jun W and Bin
Z: Effects of resveratrol on oral squamous cell carcinoma (OSCC)
cells in vitro. J Cancer Res Clin Oncol. 140:371–374. 2014.
View Article : Google Scholar : PubMed/NCBI
|