Introduction
Lung cancer is one of the prevalent causes of
cancer-related mortality across the world in males and females
(1,2). In the United States alone, lung cancer
accounts for >30% of cancer-related mortalities (3). The total number of mortalities due to
lung cancer exceeds the mortality rate of colon, prostate and
breast cancers combined with a poor 5-year survival rate of ~15%
(4). Among lung cancers, non-small
cell lung cancer (NSCLC) constitutes 80% of cases (5). The present treatment options for lung
cancer include chemotherapy, radiation and surgery. Chemotherapy is
one of the prominent options for the effective treatment of lung
cancers. However, cancer treatment with routine chemotherapeutic
agents results in severe drug-related systemic side effects, which
limits the potential clinical benefits (6–8).
In this regard, natural components from plant
resources offer numerous options for the effective replacement of
routine chemotherapeutic agents. Quercetin (QUR;
3,5,7,3′,4′-pentahydroxyflavone) is a naturally occurring flavonoid
that is widely present in fruits, vegetables and leaves (9,10). QUR
is considered an excellent antioxidant owing to its high number of
hydroxyl groups (11). The numerous
applications of QUR include the treatment of allergy, inflammation,
arteriosclerosis and bleeding (12).
QUR has also received attention as a chemoprotective agent
exhibiting a potent antiproliferative effect on cancer cells
without any effect on normal cells (13,14). QUR
has been indicated to induce the apoptosis of cancer cells by
blocking cell cycle progression at different phases of the cell
cycle and modulating signaling pathways (15). QUR actively suppresses cancer-related
processes such as apoptosis, proliferation and cancer metastasis
(16). Despite its promising
anticancer applications, the therapeutic activity of QUR is
severely hindered owing to its poor aqueous solubility and high
hydrophobicity. In addition, the natural form of QUR has been
reported to possess a short half-life in the systemic circulation
(17). Therefore, efforts are
required to improve the physicochemical characteristics of QUR and
thereby enhance its anticancer effects.
Nanoparticulate drug delivery systems have been
developed to encapsulate hydrophobic small molecules, increase
their solubility in body fluids and improve their anticancer
efficacy. Biodegradable nanocarriers have been reported to improve
the stability of encapsulated compounds and control their release
in the systemic environment (18–20). In
this context, self-assembled polymeric micelles are considered to
be one of the most promising drug delivery systems for small
molecules (21). The core-shell
morphology of micelles protects the drug in the harsh systemic
environment (22). The core material
is used to load the drug while a shell composed of polyethylene
glycol (PEG) provides excellent biocompatibility and protective
effects and prolongs the blood circulation time of the drug
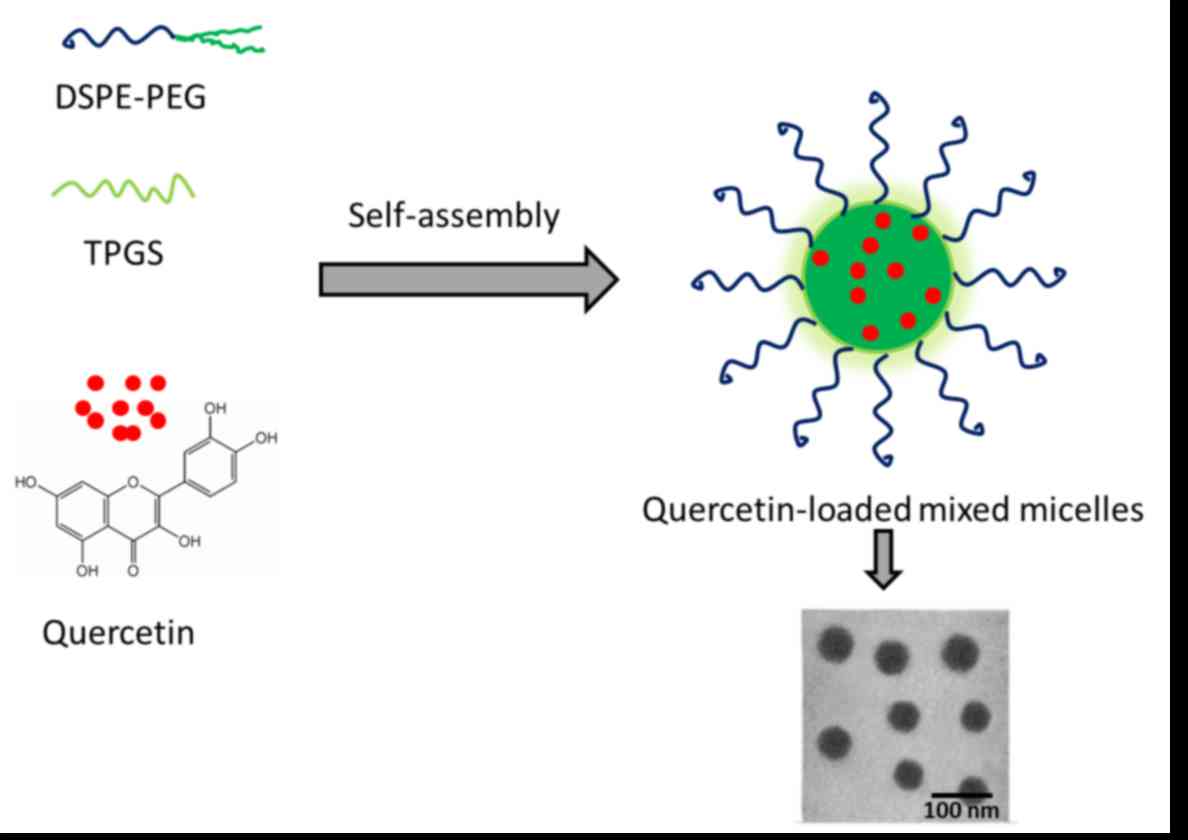
(23). In the present study, mixed
micelles consisting of a
1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine
derivative of PEG (DSPE-PEG) and tocopheryl polyethylene glycol
1000 succinate (TPGS) were employed. The mixed micelles were
designed to act as a protective shield and increase drug delivery
to tumor tissues. TPGS should improve the cell penetration
capacity, and has been reported to increase the toxicity of
docetaxel to cancer cells (24).
TPGS may act synergistically to enhance the therapeutic efficacy of
QUR loaded in the DSPE-PEG micelles. Small-sized, mixed micelles
have the potential to effectively avoid reticuloendothelial system
(RES)-based drug clearance (25).
In the present study, the objective was to improve
the physicochemical and anticancer property of QUR against lung
cancer cells. QUR was encapsulated in DSPE-PEG/TPGS-based mixed
micelles and the physicochemical properties (size, shape and
release kinetics) of the encapsulated QUR were examined. The
anticancer activity of the drug-loaded micelles was evaluated in
A549 lung cancer cells. The cytotoxic potentials of the free drug
and drug-loaded micelles were compared using MTT assays and
assessment of morphological cell density. The cytotoxic potential
of the micelle formulation was further studied using a Hoechst
33342-based apoptosis assay.
Materials and methods
Materials
QUR was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The DSPE-PEG component,
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy
(polyethylene glycol)-2000, was purchased from Corden Pharma
(Cambridge, MA, USA). The specific TPGS used was D-α-tocopheryl
polyethylene glycol 1000 succinate from Eastman Chemical Co.
(Kingsport, TN, USA). All other chemicals were reagent grade and
used without further purification.
Preparation of QUR-loaded mixed
micelles (QUR-M)
The QUR-M was prepared by a solvent evaporation
method. Briefly, QUR (10 mg), DSPE-PEG (90 mg) and TPGS (10 mg)
were dissolved in 2 ml chloroform and stirred for 10 min. The
organic mixture was rotary evaporated for 1 h leaving a thin
polymer-lipid layer. The thin polymer-lipid layer was further
vacuum evaporated for 1 h. Following this, the resulting thin film
was hydrated with phosphate-buffered saline (PBS; pH 7.4) and
vortexed for 10 min. The nanosuspension was immediately sonicated
for 15 min at room temperature. The QUR-M were collected by
centrifugation (500 × g; 10 min at 25°C), washed with water twice
and then lyophilized for use in further experiments. The unloaded
drug in the supernatant was quantified by a HPLC method. HPLC
analysis was performed using a Shimadzu-LC system (Shimadzu, Japan)
equipped with an CBM-20A controller, LC-20AT pump, DGU-20A5
prominence degasser, SIL-20A auto sampler, SPD-20AV detector and
CTO-10ASvp column oven at 25°C. The column (250 mm × 4.6 mm, 5 µm)
packed with silica and was protected with pre-column guard
cartridge RP18 (30 ÿ 4.6 mm, 10 µm; (PerkinElmer, Inc., Waltham,
MA, USA). Acetonitrile and 2% v/v acetic acid (pH 2.60; 40:60 v/v)
was used as a mobile phase and QUR was detected at 370 nm. A 20 µl
sample was injected into the column at a flow rate of 1 ml/min. The
QUR-M were found to have an active drug loading of 8.45% by weight
with a loading efficiency of >90%.
Particle size analysis
The particle size and size distribution of the QUR-M
were evaluated by dynamic light scattering (DLS) analysis using a
Zetasizer Nano ZS system (Malvern Instruments, Ltd., Malvern, UK).
The samples were suitably diluted (1:10) with water prior to
experiments. The experiments were performed in triplicate.
Surface morphology analysis
The surface morphology of the QUR-M was investigated
using a transmission electron microscope (TEM; JEM-2010; JEOL,
Ltd., Japan). The TEM used an accelerating voltage of 120 kV. The
samples were placed in a carbon-coated copper grid, counterstained
with 2% phosphotungistic acid and air dried. Staining was performed
at room temperature for 20 min.
In vitro drug release assay
The in vitro drug release assay was performed
using a dialysis method. In this assay, 2 mg equivalent of QUR-M
was resuspended in 30 ml release medium (PBS, pH 7.4 and
acetate-buffered saline, pH 5.0) and transferred to a dialysis
tube. The sealed dialysis tube was placed in 30 ml release buffer
in a shaking water bath. The samples were withdrawn at specific
time points (1, 2, 4, 8, 12, 24 and 48 h) and the amount of drug
released was determined using HPLC, as described above. The
percentage of QUR released was plotted against time.
Cytotoxicity assay
The cells were incubated in incubator maintained at
37°C and 5% CO2 conditions. In the cytotoxicity assay,
A549 cancer cells (ATCC, Manassas, VA, USA) were seeded in a
96-well plate at a seeding density of 10,000 cells/well and
incubated for 24 h. The following day, the cells were treated with
blank micelles, free QUR and QUR-M in a concentration dependent
manner and incubated for a further 24 h. Untreated cells were
observed as a positive control and DMSO-treated cells served as a
negative control. Following this, the medium was carefully
suctioned off, 10 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT;
5 mg/ml) was added and the cells were incubated for 4 h at 37°C.
After this, 100 µl DMSO was added to dissolve the insoluble
formazan crystals that were produced by the action of mitochondrial
succinate dehydrogenase. The absorbance of each individual well was
quantified using a plate reader at 570 nm. All experiments were
performed in triplicate and results presented as the mean ±
standard deviation (SD). The IC50 value was calculated using
GraphPad Prizm version 17 (GraphPad Software, Inc., La Jolla, CA,
USA).
Apoptosis assay
The apoptosis assay was performed using Hoechst
33342-based nuclear staining and visualization of the cells using a
confocal laser scanning microscope (CLSM). In this assay,
1×105 A549 cells/well were seeded in 12-well plate and
allowed to attach for 24 h. The following day, the cells were
exposed to blank micelles, free QUR and QUR-M (10 µg/ml) and
further incubated for 24 h. The cells were washed twice with PBS
and fixed with 4% paraformaldehyde. The cells were washed again
prior to staining with Hoechst 33342 (10 µg/ml) for 10 min. The
nuclear morphology of the cells was visualized using a CLSM (TCS
SP2; Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Statistical analysis was performed using Student's
t-test and one-way analysis of variance followed up by a post hoc
Tukey's test. All results are expressed as the mean ± SD. P<0.05
was considered to indicate a statistically significant result.
Results and Discussion
Characterization of QUR-M
The severe toxicity and insufficient therapeutic
efficacy of chemotherapeutic drugs in cancer treatment necessitates
the development of new therapeutic agents without toxic effects and
with improved anticancer effects. Natural components from plant
resources offer numerous options for the effective replacement of
routine chemotherapeutic agents. QUR is a naturally occurring
flavonoid that has exhibited potent antiproliferative effects on
cancer cells without any effect on normal cells (16). QUR induces cancer cell apoptosis by
blocking the cell cycle progression of cancer cells and modulating
signaling pathways (20). However,
the high hydrophobicity and short half-life span of QUR in the
systemic circulation limit its therapeutic activity (17). Therefore, efforts are required to
improve the physicochemical characteristics and anticancer effects
of QUR. Thus, in the present study, QUR was encapsulated in
DSPE-PEG/TPGS-based mixed micelles in order to improve its
physicochemical and anticancer properties. PEG is likely to
minimize the RES-based systemic clearance and increase the blood
circulation time (26), while TPGS
should improve the cell penetration capacity and increase the
toxicity to cancer cells (27). TPGS
may act synergistically with DSPE-PEG to increase the therapeutic
efficacy of QUR loaded in the micelles (Fig. 1).
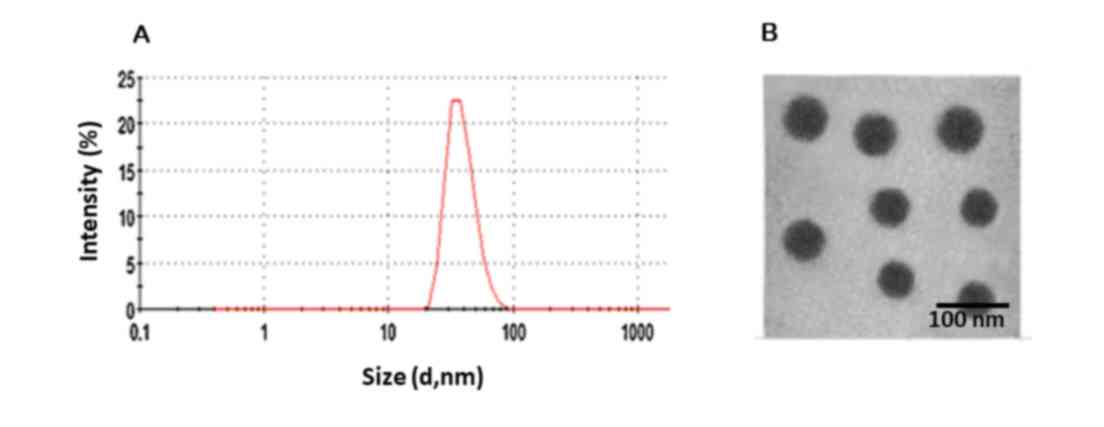
DLS analysis
The average particle size and particle size
distribution were evaluated using DLS. The average particle size of
the QUR-M was observed to be 70±1.5 nm with a polydispersity index
of 0.142, indicating that the particles were monodisperse (Fig. 2). A particle diameter of <200 nm
has been reported to be favorable for cancer-targeting applications
(10). The QUR-M particle size of
<100 nm is likely to allow preferential accumulation in cancer
tissues via an enhanced permeation and retention effect (15). The particle size will allow cellular
uptake after intravenous administration (18). Moreover, the zeta potential was
−8.25±1.26 mV, which indicates that the QUR-M may avoid RES-based
systemic clearance.
Morphological analysis
The size and surface morphology of QUR-M were
investigated by analysis using a TEM (Fig. 1). The particles were clearly
spherical and appeared uniform in the copper grid. The particle
size (55 nm) was slightly smaller when compared with that indicated
by the DLS analysis, and the particles were observed to be
monodisperse. The difference in particle size may be due to the
folding of the PEG chain and aggregation of the micelles during the
drying process.
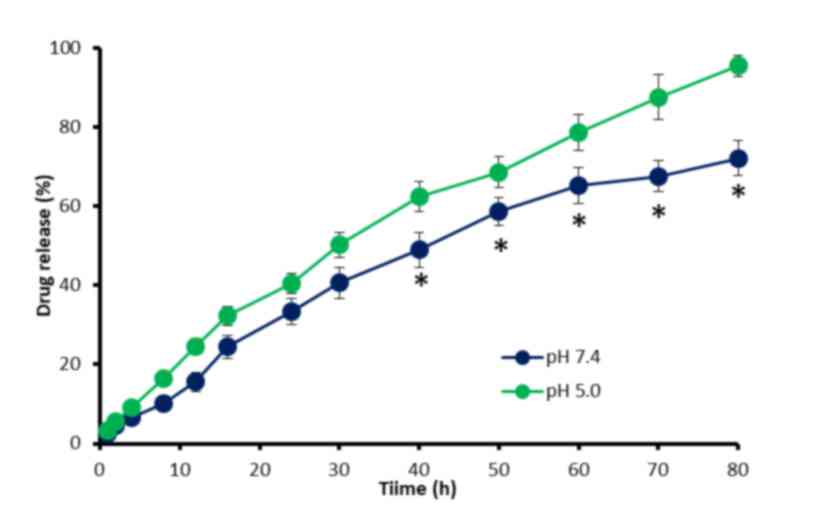
In vitro drug release
The in vitro release of QUR from the QUR-M
was investigated as pH 7.4 and 5.0 (Fig.
3). QUR was released in a controlled manner under the two pH
conditions, indicating the stability of the mixed micelles. It was
observed that 35–40% of the encapsulated QUR was released within
the first 24 h of the release experiment. It may be noted that a
slightly higher release rate was observed in acidic conditions. For
example, ~65% of the QUR was released in pH 7.4 conditions after 80
h while ~90% of QUR was released in pH 5.0 conditions in the same
time period. The increased release of QUR in acidic conditions is
likely to be favorable to cancer-targeting applications (22). Overall, QUR-M exhibited a
sustained-release profile, due to the stable incorporation of
hydrophobic QUR in the core of the mixed micelles, which increased
the path length. The sustained release of QUR may provide a stable
concentration of the drug and prolong its therapeutic effects.
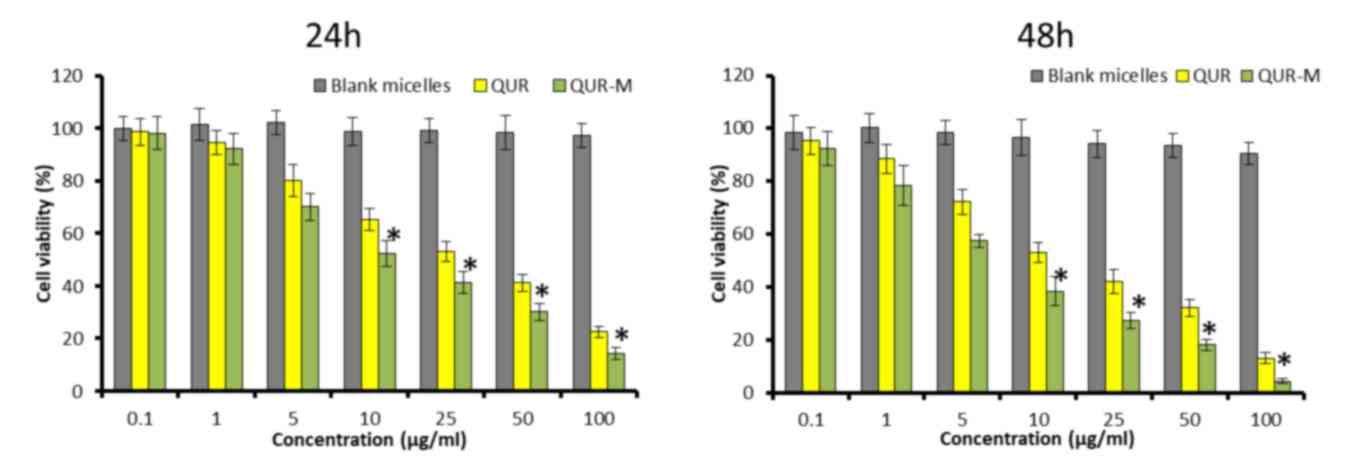
Anticancer effect of QUR-M in lung
cancer cells
The anticancer effect of blank micelles and
drug-loaded micelles in A549 lung cancer cells was evaluated by MTT
assay. As shown in Fig. 4, free QUR
and QUR-M exhibited a typical dose-dependent and time-dependent
cytotoxic effect in A549 cancer cells. Notably, drug-loaded
micelles exhibited a more potent anticancer effect (P<0.05)
compared with that of free QUR at the two time points. The results
demonstrated that the micellar system not only maintained the
pharmacological action of QUR but also enhanced its anticancer
effect. IC50 values were calculated to quantify the
effects of the different formulations. The IC50 values
of QUR and QUR-M were observed to be 12.45 and 6.42 µg/ml,
respectively. The superior cytotoxic effect of QUR-M may be
attributed to the preferentially higher cellular uptake and
controlled release of QUR in the intracellular environment.
Following cellular internalization, QUR stored in the micelles may
be gradually released, thereby exposing the cells to the drug for a
prolonged time period. In addition, cells treated with blank
micelles exhibited a high viability at all the concentrations
tested, indicating the lack of toxicity and excellent
biocompatibility profile of the micelles. A potent anticancer and
time-dependent effect may be expected when a nanocarrier is
internalized (28).
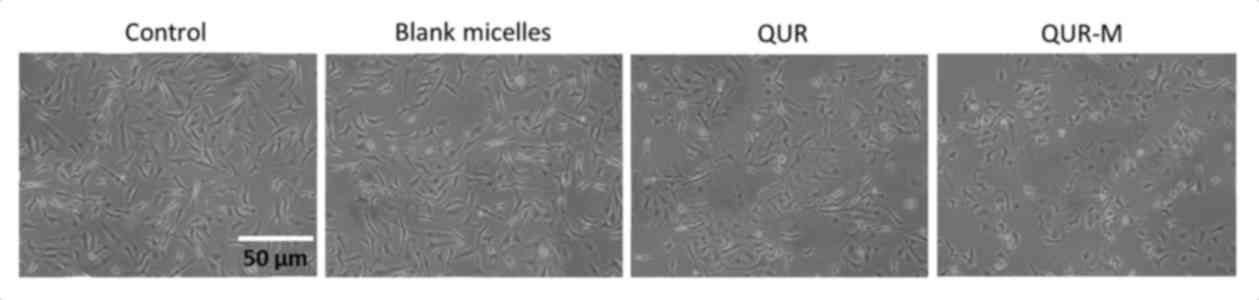
The cytotoxic effects of free QUR and QUR-M were
further compared by morphological cellular imaging (Fig. 5). It was observed that control and
blank micelle-treated cells were almost intact and maintained their
typical morphology. The QUR-M induced a higher anticancer effect,
compared with that of free QUR indicated by severe apoptotic body
formation and a reduction in the number of cells present on the
cover glass. Moreover, cells were rounded, which was indicative of
cell death.
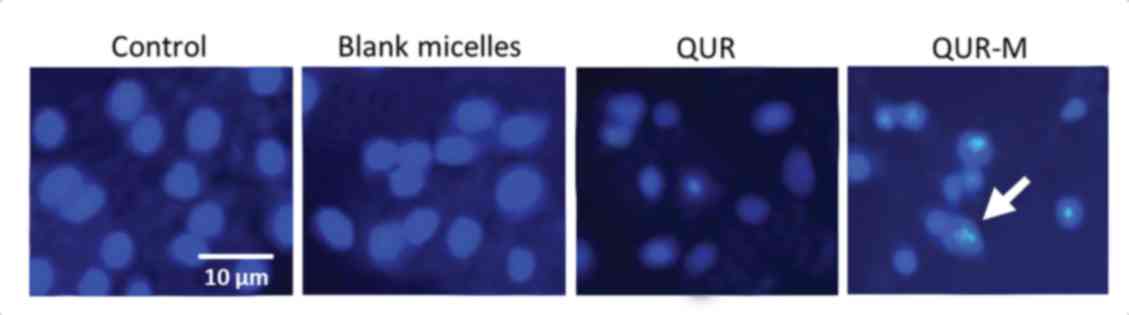
Apoptosis assay
The apoptosis potential of the formulation was
tested using Hoechst 33342 staining of the lung cancer cells. The
untreated and blank micelle-treated groups maintained a homogenous
cellular morphology and no changes were observed (Fig. 6). The cells treated with free QUR or
QUR-M, however, exhibited severe chromatin condensation and
apoptotic body formation of the nuclei. The higher apoptosis
potential of QUR-M may be attributed to its higher cellular uptake
and the controlled release of the encapsulated QUR.
Conclusion
In summary, QUR-loaded mixed micelles were
successfully prepared in order to improve the physicochemical
properties and anticancer efficacy of QUR in lung cancer cells. The
nanosized QUR-M exhibited pH-responsive and controlled-release
properties that may be beneficial in cancer treatment. The results
of the present study clearly demonstrate that the QUR-M exhibited a
superior anticancer effect compared with that of free QUR at 24 and
48 h time points in A549 cancer cells. The IC50 values
of QUR and QUR-M were observed to be 12.45 and 6.42 µg/ml,
respectively. Consistent with this, cancer cells treated with QUR-M
exhibited severe chromatin condensation and apoptotic body
formation of the nuclei. Overall, the higher intracellular uptake,
sustained drug release and presence of TPGS in the mixed micelles
may inhibit cell proliferation and improve the therapeutic efficacy
in lung cancers.
Acknowledgements
This study was funded by a research grant from the
Third People's Hospital of Yancheng (Yancheng, China).
References
|
1
|
American Cancer Society, . Cancer Facts
& Figures 2013. American Cancer Society; Atlanta: 2013,
http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf
|
|
2
|
Zarogoulidis K, Zarogoulidis P, Darwiche
K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N,
Kougioumtzi I, Karapantzos I, Huang H and Spyratos D: Treatment of
non-small cell lung cancer (NSCLC). J Thorac Dis. 5 Suppl
4:S389–S396. 2013.PubMed/NCBI
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vineis P and Wild CP: Global cancer
patterns: Causes and prevention. Lancet. 383:549–557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sridhar SS, Seymour L and Shepherd FA:
Inhibitors of epidermal growth-factor receptors: A review of
clinical research with a focus on non-small-cell lung cancer.
Lancet Oncol. 4:397–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grossi F, Gridelli C, Aita M and De
Marinis F: Identifying an optimum treatment strategy for patients
with advanced non-small cell lung cancer. Crit Rev Oncol Hematol.
67:16–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otterson GA, Villalona-Calero MA, Hicks W,
Pan X, Ellerton JA, Gettinger SN and Murren JR: Phase I/II study of
inhaled doxorubicin combined with platinum-based therapy for
advanced non-small cell lung cancer. Clin Cancer Res. 16:2466–2473.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pilkington G, Boland A, Brown T, Oyee J,
Bagust A and Dickson R: A systematic review of the clinical
effectiveness of first-line chemotherapy for adult patients with
locally advanced or metastatic non-small cell lung cancer. Thorax.
70:359–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beecher GR, Warden BA and Merken H:
Analysis of tea polyphenols. Proc Soc Exp Biol Med. 220:pp.
267–270. 1999, View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Formica JV and Regelson W: Review of the
biology of Quercetin and related bioflavonoids. Food Chem Toxicol.
33:1061–1080. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hollman PC and Katan MB: Dietary
flavonoids: Intake, health effects and bioavailability. Food Chem
Toxicol. 37:937–942. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A, Birhman K, Raheja I, Sharma SK
and Kar HK: Quercetin: A wonder bioflavonoid with therapeutic
potential in disease management. Asian Pac J Trop Dis. 6:248–252.
2016. View Article : Google Scholar
|
|
13
|
Park MH and Min do S: Quercetin-induced
downregulation of phospholipase D1 inhibits proliferation and
invasion in U87 glioma cells. Biochem Biophys Res Commun.
412:710–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng SY, Li Y, Jiang D, Zhao J and Ge JF:
Anticancer effect and apoptosis induction by quercetin in the human
lung cancer cell line A-549. Mol Med Rep. 5:822–826.
2012.PubMed/NCBI
|
|
15
|
David Anand AV, Arulmoli R and Parasuraman
S: Overviews of biological importance of quercetin: A bioactive
flavonoid. Pharmacogn Rev. 10:84–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lugli E, Ferraresi R, Roat E, Troiano L,
Pinti M, Nasi M, Nemes E, Bertoncelli L, Gibellini L, Salomoni P,
et al: Quercetin inhibits lymphocyte activation and proliferation
without inducing apoptosis in peripheral mononuclear cells. Leuk
Res. 33:140–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gibellini L, Pinti M, Nasi M, Montagna JP,
Biasi SD, Roat E, Bertoncelli E, Cooper EL and Cossarizza A:
Quercetin and cancer chemoprevention. Evid Based Compl Altern Med.
1011:5913562011.
|
|
18
|
Puoci F, Morelli C, Cirillo G, Curcio M,
Parisi OI, Maris P, Sisci D and Picci N: Anticancer activity of a
quercetin-based polymer towards HeLa cancer cells. Anticancer Res.
32:2843–2847. 2012.PubMed/NCBI
|
|
19
|
Tran TH, Ramasamy T, Truong DH, Shin BS,
Choi HG, Yong CS and Kim JO: Development of vorinostat-loaded solid
lipid nanoparticles to enhance pharmacokinetics and efficacy
against multidrug-resistant cancer cells. Pharm Res. 31:1978–1988.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramasamy T, Choi JY, Cho HJ, Umadevi SK,
Shin BS, Choi HG, Yong CS and Kim JO: Polypeptide-based Micelles
for Delivery of Irinotecan: Physicochemical and in vivo
characterization. Pharm Res. 32:1947–1956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramasamy T, Ruttala HB, Gupta B, Poudel
BK, Choi HG, Yong CS and Kim JO: Smart chemistry-based nanosized
drug delivery systems for systemic applications: A comprehensive
review. J Control Release. 258:226–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi JY, Ramasamy T, Kim SY, Kim J, Ku SK,
Youn YS, Kim JR, Jeong JH, Choi HG, Yong CS and Kim JO: PEGylated
lipid bilayer-supported mesoporous silica nanoparticle composite
for synergistic co-delivery of axitinib and celastrol in
multi-targeted cancer therapy. Acta Biomater. 39:94–105. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramasamy T, Kim J, Choi HG, Yong CS and
Kim JO: Novel dual drug-loaded block ionomer complex micelles for
enhancing the efficacy of chemotherapy treatments. J Biomed
Nanotechnol. 10:1304–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tran TH, Ramasamy T, Choi JY, Nguyen HT,
Pham TT, Jeong JH, Ku SK, Choi HG, Yong CS and Kim JO:
Tumor-targeting, pH-sensitive nanoparticles for docetaxel delivery
to drug-resistant cancer cells. Int J Nanomedicine. 10:5249–5262.
2015.PubMed/NCBI
|
|
25
|
Zhou X, Luo S, Tang R, Wang R and Wang J:
Diblock copolymers of polyethylene glycol and a polymethacrylamide
with side-chains containing twin ortho ester rings: Synthesis,
characterization, and evaluation as potential pH-responsive
micelles. Macromol Biosci. 15:385–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Corvazier E and Maclouf J: Interference of
some flavonoids and non-steroidal anti-inflammatory drugs with
oxidative metabolism of arachidonic acid by human platelets and
neutrophils. Biochim Biophys Acta. 835:315–321. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan G, Lemmouchi Y, Akala EO and Bakare O:
Studies on PEGylated and drug-loaded PAMAM dendrimers. J Bioact
Compat Polym. 20:113–128. 2005. View Article : Google Scholar
|
|
28
|
Yamazaki M and Ito T: Deformation and
instability in membrane structure of phospholipid vesicles caused
by osmophobic association: Mechanical stress model for the
mechanism of poly(ethylene glycol)-induced membrane fusion.
Biochemistry. 29:1309–1314. 1990. View Article : Google Scholar : PubMed/NCBI
|