Introduction
Lyme disease, a widely epidemic tick-borne zoonosis,
is caused by the spirochete Borrelia burgdorferi (B.
burgdorferi) and is spread to humans through the bites of
infected ticks (1). Areas of high
risk include the USA, Northern Europe and parts of Eastern Asia
(1). Arthritis, characterized by
recurrent attacks or persistent swelling in certain large joints,
typically develops during the middle or late stages of Lyme disease
(2). In a number of patients with
Lyme arthritis, the earlier clinical features of Lyme disease,
including skin rash, headache and fever are not evident (2).
B. burgdorferi lacks toxin production and
during infection, the majority of tissue damage results from host
inflammatory reactions rather than from the pathogen itself
(2). Cytokines, including tumor
necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β serve key
functions in the pathogenesis of Lyme arthritis (3). In particular, these cytokines activate
phagocytes in order to eliminate pathogens and attract other immune
cells that aid in the host inflammatory response (3). In a previous study by our group, it was
identified that the B. burgdorferi basic membrane protein A
(BmpA) was involved in the pathogenesis of Lyme arthritis (4). Notably, the BmpA gene of Lyme-causing
spirochete was preferentially upregulated in mouse joints compared
with skin, heart and bladder tissues, and B. burgdorferi
lacking BmpA failed to induce arthritis (4). It has also been reported that
recombinant BmpA (rBmpA) may activate proinflammatory responses in
human synovial cells and induce the expression of TNF-α and IL-1β
(5).
The plant Coleus forskohlii (C.
forskohlii) is primarily distributed in India, Thailand, China,
Egypt and Brazil, and has a history of use in the treatment of
multiple diseases, including heart disease, respiratory disorders,
convulsion, intestinal disturbance and liver fatigue (6). Isoforskolin (ISOF) is the principle
active component of C. forskohlii native to the southwest
region of China, and has previously been studied for its
anti-inflammatory effects (7,8).
Notably, a study performed by Yang et al (9) observed that ISOF reduced the secretion
of lipopolysaccharide (LPS)-induced cytokines, namely TNF-α, IL-1β,
IL-6 and IL-8, in human mononuclear leukocytes. The present study
hypothesized that ISOF suppressed BmpA-induced inflammation. The
results demonstrated the transcription and expression of TNF-α and
IL-6 in vitro and estimated the mean arthritis index (MAI),
X-ray and histopathological examinations in in vivo
experiments in mice.
Materials and methods
Reagents and rBmpA preparation
rBmpA was produced in Escherichia coli BL21
(GE Healthcare, Chicago, IL, USA) using the bacterial expression
vector pGEX-6P1 (GE Healthcare) and the following primers with
EcoRI and XhoI restriction sites: Forward,
5′-ACGAATTCATGAATAAAATATTGTTGTTGA-3′ and reverse,
5′-AGCTCGTAAATAAATTCTTTAAGAAA-3′ (4,5). Pure
ISOF was provided by Dr Weimin Yang [School of Pharmaceutical
Science and Yunnan Key Laboratory of Pharmacology for Natural
Products, Kunming Medical University (KMU), Kunming, China] and
reconstituted at 1×105 µM/ml in dimethyl sulfoxide
(DMSO) for stock solution. LPS and phorbol-12-myristate-13-acetate
(PMA) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany) and ionomycin was purchased from Apollo Scientific Ltd.
(Stockport, UK). Recombinant human (rh)IL-4, rhTNF-α and
rh-granulocyte macrophage colony-stimulating factor (rhGM-CSF) were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Cell culture
The murine macrophage cell line RAW264.7 and human
monocytic leukemia cell line THP-1 were obtained from the Kunming
Institute of Zoology, Chinese Academy of Sciences (Kunming, China).
All cell culture medium, serum and antibiotics were purchased from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). All cells
were cultured at 37°C with a humidified atmosphere of 5%
CO2. Cellular supernatants were collected for ELISA.
Cell lysates were prepared using RNAiso Plus reagent (Takara Bio,
Inc., Otsu, Japan).
For RAW264.7 cells, Dulbecco's modified Eagle's
medium (high glucose) supplemented with 10% FBS and 1%
penicillin-streptomycin was used as culture medium. RAW264.7 cells
were seeded in 96-well microplates at a concentration of
1×105 cells/ml and stimulated with 20 µg/ml rBmpA or 20
µg/ml rBmpA+100 µmol/ml ISOF (n=4 wells per condition) for 24 and
48 h. Additionally, a group of cells was cultured in 10% FBS/DMEM
medium alone as a blank control and subgroups of these cells were
stimulated with 0.1% DMSO (as a solvent control) or 1 µg/ml LPS (as
a positive control).
For THP-1 cells, RPMI-1640 supplemented with 10% FBS
and 1% penicillin-streptomycin was used as cell culture. THP-1
cells at 5×105 cells/ml were differentiated into
macrophages by 100 ng/ml PMA pretreatment for 24 h (10). THP-1 cells at 2×105
cells/ml were pretreated with 200 ng/ml rhIL-4, 100 ng/ml rhGM-CSF,
10 ng/ml rhTNF-α and 200 ng/ml ionomycin for 2 days for
differentiation into mature dendritic cells (11). Subsequently, the macrophages and
dendritic cells were cultured in 10% FBS/RPMI-1640 and stimulated
with 20 µg/ml rBmpA, 20 µg/ml rBmpA + 100 µmol/ml ISOF, 0.1% DMSO
(solvent control) or 1 µg/ml LPS (positive control) (n=4 wells per
condition) for 24, 48 and 72 h. Additionally, a group of cells was
maintained in RPMI 1640 supplemented with 10% FBS medium as a blank
control.
ELISA
The concentrations of TNF-α and IL-6 in the cellular
supernatant samples were measured using Mouse TNF-α ELISA kit (cat.
no. DKW12-2720-096; Dakawe, Shenzhen, China), Mouse IL-6 ELISA kit
(cat. no. DKW12-2720-096; Dakawe), Human TNF-α ELISA kit (cat. no.
ELH-TNFα-001; Ray Biotech, Inc., Norcross, GA, USA) and Human IL-6
ELISA kit (cat. no. ELH-IL6-001; Ray Biotech, Inc.) were used
according to the manufacturers' protocols.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
TNF-α and IL-6 mRNA expression was assessed by
RT-qPCR. Total RNA was extracted from cells using the RNAiso Plus
reagent, and a PrimeScript Reverse Transcription reagent kit with
gDNA Eraser (Takara Bio, Inc.) was used to synthesize cDNA,
according to the manufacturer's protocol. mRNA expression was
detected using the standard SYBR-Green RT-PCR kit (Takara Bio,
Inc.) according to the manufacturer's protocol. The sequences of
the specific primers used to amplify murine and human TNF-α, IL-6
and β-actin were as follows: For murine TNF-α, forward,
5′-GGTCCCCAAAGGGATGAGAA-3′ and reverse, 5′-TGAGGGTCTGGGCCATAGAA-3′;
for murine IL-6, forward, 5′-TCGGAGGCTTAATTACACATGTTC-3′ and
reverse, 5′-CATACAATCAGAATTGCCATTGC-3′; for murine β-actin,
forward, 5′-TGCCGCATCCTCTTCCTC-3′ and reverse,
5′-CGCCTTCACCGTTCCAGT-3′; for human TNF-α, forward,
5′-CCTCTCTCTAATCAGCCCTCTG-3′ and reverse,
5′-GAGGACCTGGGAGTAGATGAG-3′; for human IL-6, forward,
5′-ACTCACCTCTTCAGAACGAATTG-3′ and reverse,
5′-CCATCTTTGGAAGGTTCAGGTTG-3′; and for human β-actin, forward,
5′-CAAGGCCAACCGCGAGAAGA-3′ and reverse, 5′-GGATAGCACAGCCTGGATAG-3′
(12). qPCR was performed using SYBR
Premix Ex Taq (Takara Bio, Inc.), according to the manufacturer's
protocol. The reaction conditions were 95°C for 10 min, and 40
cycles of denaturation at 95°C for 15 sec and annealing/elongation
at 60°C for 30 sec. The relative expression was analyzed by the
2−ΔΔCq method (12).
Animals
A total of 45 female Kunming mice (age, 6–8 weeks;
weight, 18–22 g) were acquired from the Experimental Animal Center
of KMU, Kunming, China. All animals were cared for according to the
Guide for the Care and Use of Experimental Animals formulated by
the Chinese Council on Animal Care and the Principles of Laboratory
Animal Care formulated by Yunnan Provincial Council. The
experimental protocol received approval from the Animal Use and
Care Committee of KMU and was performed according to Public Health
Service Policy on Humane Care and Use of Laboratory Animals by the
National Institute of Health (13).
The mice were kept in standardized units with filtered air at a
controlled temperature of 18–22°C and humidity of 50–60% with a l2
h light/dark cycle and free access to food and water. Prior to
experimental procedures, the mice were adapted to the environment
for 1 week. The surgical procedures performed in animals were under
general anesthesia using 360 mg/kg chloral hydrate [intraperitoneal
(i.p.)].
Reagents
The preparation of ISOF stock and purified rBmpA was
performed using the aforementioned methods. Phosphate-buffered
saline (PBS) was purchased from Thermo Fisher Scientific, Inc., and
diluted to 0.01 M with ultrapure water. The 0.01 M PBS was then
used to dilute ISOF stock (1×105 Mm/ml) and purified
rBmpA to the concentration of 0.05 mg/ml.
Grouping of mice and treatment
protocol
Mice were randomly divided into a PBS control group
(n=15), Lyme arthritis group (n=15) and ISOF group (n=15). The Lyme
arthritis model was established in the Lyme arthritis and ISOF
groups as follows: 50 µl rBmpA (0.05 mg/ml) was injected into the
tibiotarsal joint cavity of both hind legs once every 3 days for a
total of four injections. PBS (0.01 M) or ISOF solution (1 mg/kg
weight, i.p.) was then administered to the mice in the Lyme
arthritis and ISOF groups, respectively. In the control group, 0.01
M PBS was administered instead of rBmpA and ISOF.
Evaluation of mice
To assess the extent of arthritis, two researchers
blinded to the experimental conditions scored the tibiotarsal
joints twice a week following the initial injection. The joints
were evaluated according to the following arthritis scoring scale:
0, no joint swelling or redness; 1, slight reddening of the joint
but no swelling; 2, slight reddening and swelling of the joint; 3,
moderate reddening and swelling of the joint; 4, notable swelling
and dysfunction of the joint. The MAI in each group was equal to
the sum of arthritis scores divided by the number of mice hind
legs. At 4 weeks after discontinuation of injections, dental
radiography units were used to obtain X-ray images.
Histopathological examination
All 45 mice were anaesthetized with 360 mg/kg
chloral hydrate (i.p.) and euthanized 4 weeks following the last
injection in order to collect the bilateral knee joint, the
tibiotarsal joint and the entire paw. The joint tissue was fixed in
4% paraformaldehyde at room temperature for 24 h. Following
decalcification with EDTA, all samples were cut into sections 5 µm
thick and stained using hematoxylin and eosin at room temperature.
Stained sections were observed and photographed with a light
inverted microscope.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean. Differences between the mean values of the groups were
evaluated by one-way analysis of variance followed by a
Student-Newman-Keuls test using the statistical analysis software
GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
TNF-α concentrations in cellular
supernatants from murine macrophages, human macrophages and human
dendritic cells
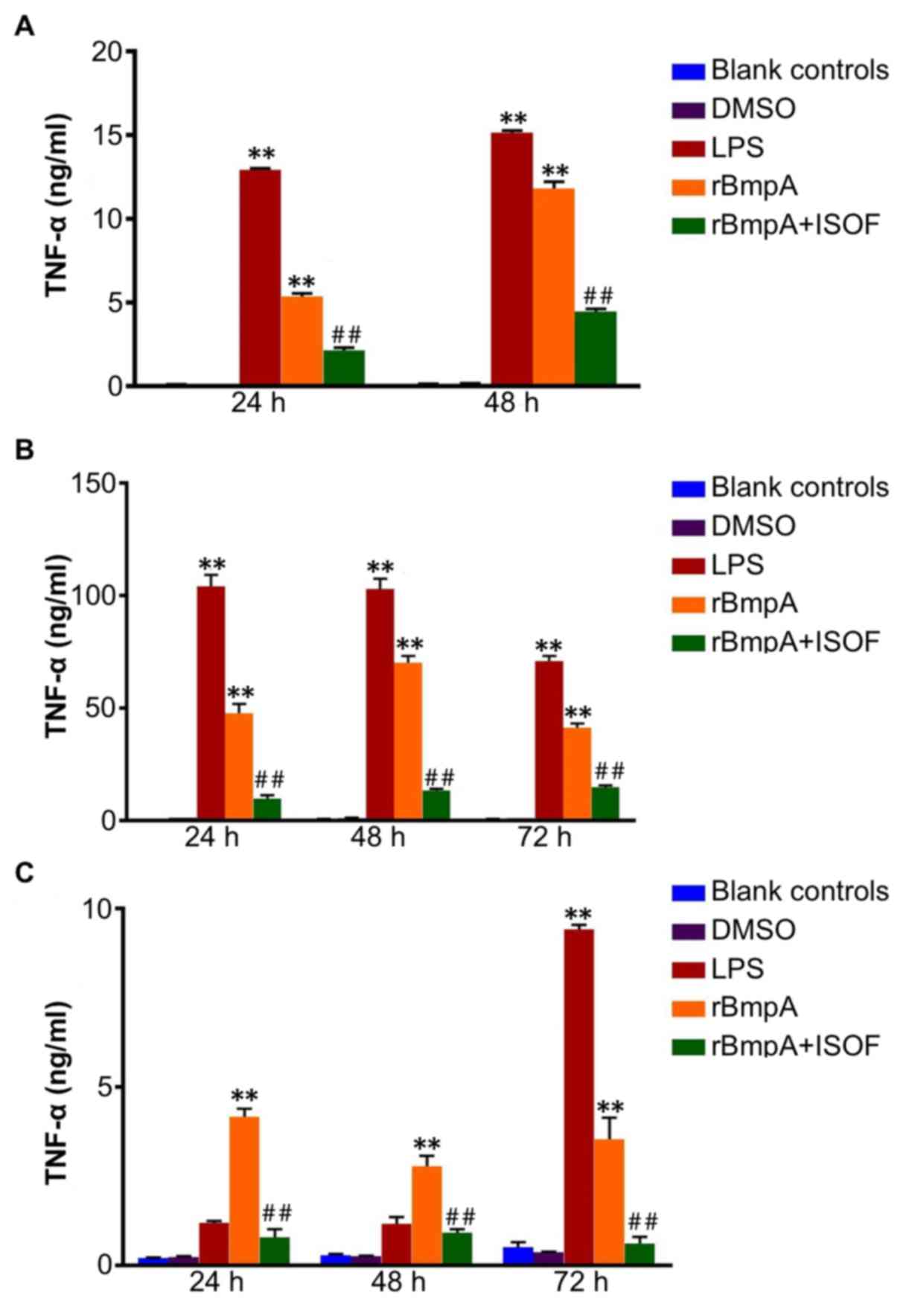
The TNF-α concentrations in the cellular
supernatants of the different cell groups were detected. TNF-α was
quantified at different culture time points using specific ELISA
kits. In the supernatant from murine macrophages at 24 and 48 h, it
was observed that treatment with LPS or rBmpA significantly
increased the level of TNF-α when compared with the blank controls
(all P<0.01; Fig. 1A). In turn,
ISOF treatment significantly reduced the secretion of TNF-α induced
by rBmpA (all P<0.01; Fig.
1A).
Similarly, LPS or rBmpA treatment induced a
significant increase in TNF-α in the supernatant of human
macrophages at 24, 48 and 72 h compared with the blank control
group (all P<0.01; Fig. 1B).
Additionally, treatment with ISOF significantly reduced the
secretion of TNF-α induced by rBmpA at 24, 48 and 72 h compared
with the rBmpA group (all P<0.01; Fig. 1B).
Furthermore, as depicted in Fig. 1C, rBmpA upregulated the levels of
TNF-α in the supernatants of human dendritic cells after 24, 48 and
72 h, and LPS after 72 h compared with the blank controls (all
P<0.01). Meanwhile, ISOF treatment significantly suppressed the
rBmpA-induced secretion of TNF-α at 24, 48 and 72 h
(P<0.01).
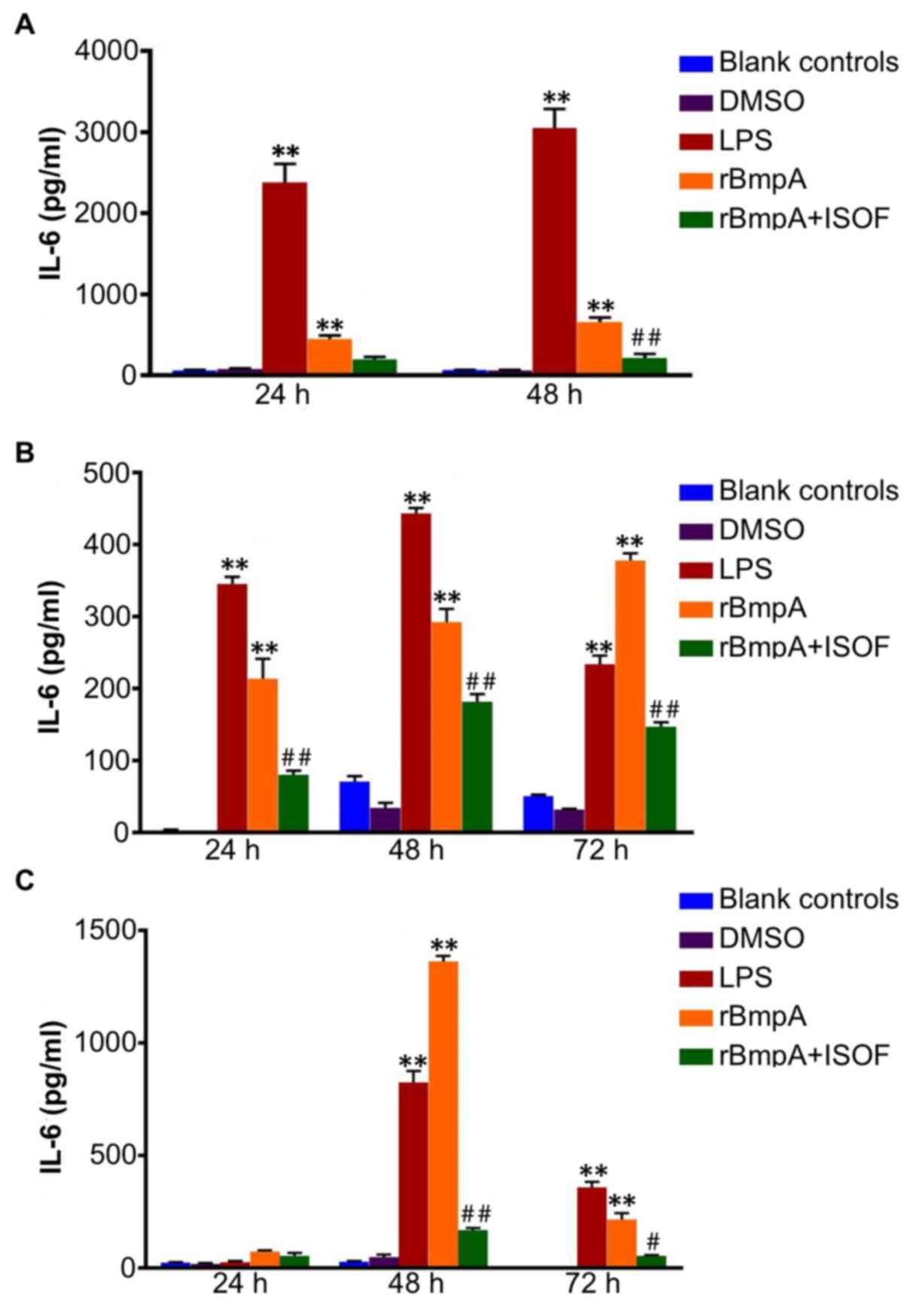
IL-6 concentrations in cellular
supernatants from murine macrophages, human macrophages and human
dendritic cells
As for TNF-α, ELISA was used to quantify the
supernatant concentrations of IL-6 at different culture time
points. For murine macrophages, compared with the blank control
group, the LPS or rBmpA groups exhibited significantly increased
supernatant levels of IL-6 at 24 and 48 h (P<0.01; Fig. 2A); In turn, ISOF treatment reduced
the secretion of IL-6 induced by rBmpA, which was deemed to be
significant at 48 h (P<0.01) but not at 24 h (Fig. 2A).
Accordingly, IL-6 levels were significantly
increased in the supernatants of human macrophages by LPS or rBmpA
compared with the blank controls at 24, 48 and 72 h (P<0.01).
Meanwhile, ISOF treatment significantly reduced the secretion of
IL-6 induced by rBmpA at 24, 48 and 72 h (P<0.01; Fig. 2B).
For human dendritic cells, the supernatant levels of
IL-6were significantly increased by the LPS and rBmpA at 48 and 72
h, compared with the blank controls (P<0.01). Treatment with
ISOF significantly reduced the rBmpA-induced increase in IL-6 at 48
and 72 h (P<0.01 and P<0.05, respectively; Fig. 2C).
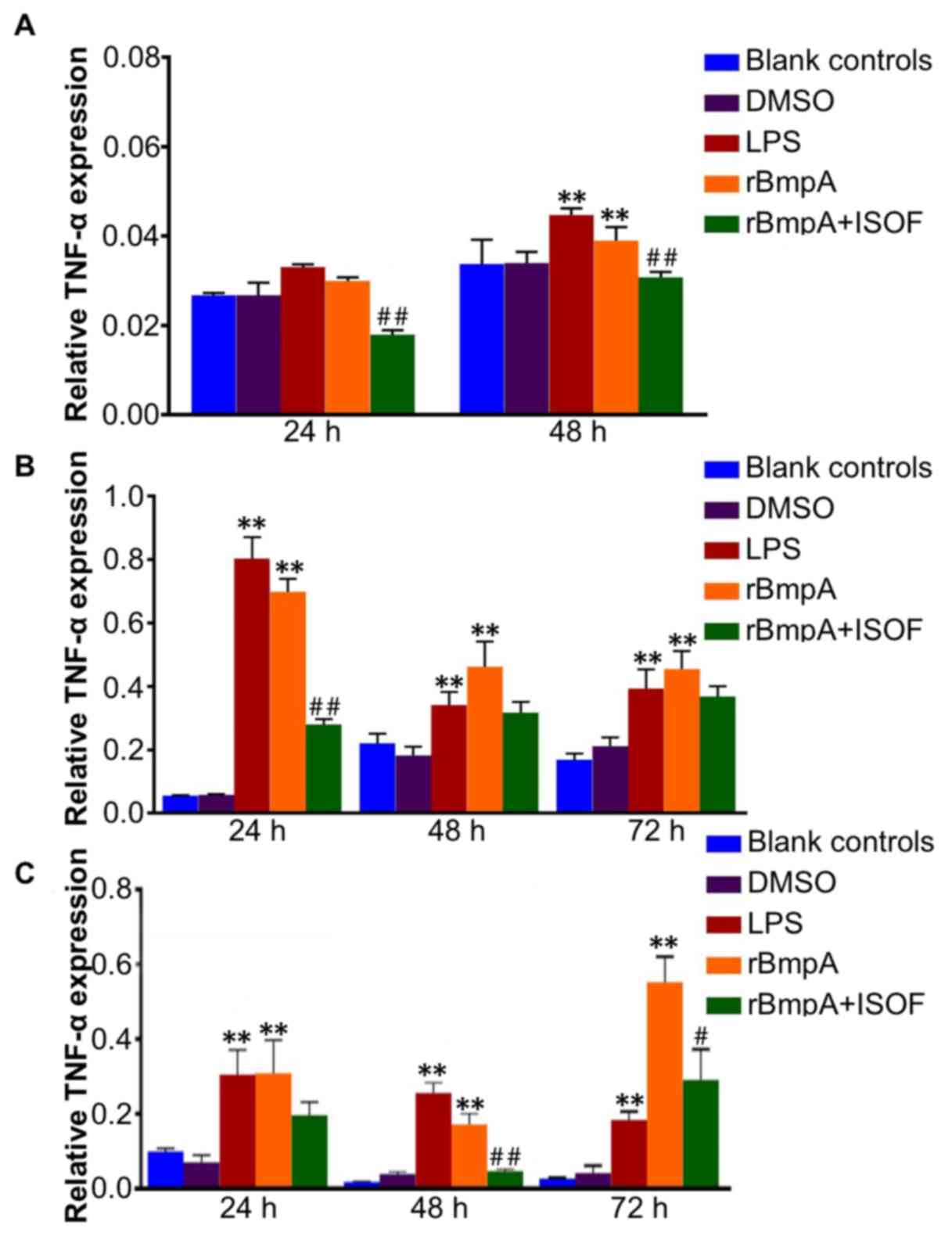
TNF-α mRNA levels in murine
macrophages, human macrophages and human dendritic cells
TNF-α mRNA levels in murine macrophages, human
macrophages and human dendritic cells were determined by RT-qPCR.
Murine TNF-α expression was significantly increased by LPS or rBmpA
at 48 h compared with the blank control group (P<0.01; Fig. 3A). ISOF downregulates murine TNF-α
expression induced by the rBmpA at 24 and 48 h (P<0.01). For
human macrophages and human dendritic cells, the expression levels
of TNF-α were significantly elevated in the LPS and rBmpA groups
compared with the blank controls at 24, 48 and 72 h (all P<0.01;
Fig. 3B and C). While ISOF treatment
significantly reduced the rBmpA-induced upregulation of TNF-α in in
human macrophages at 24 h (P<0.01; Fig. 3B), and in human dendritic cells at 48
and 72 h (P<0.01 and P<0.05, respectively).
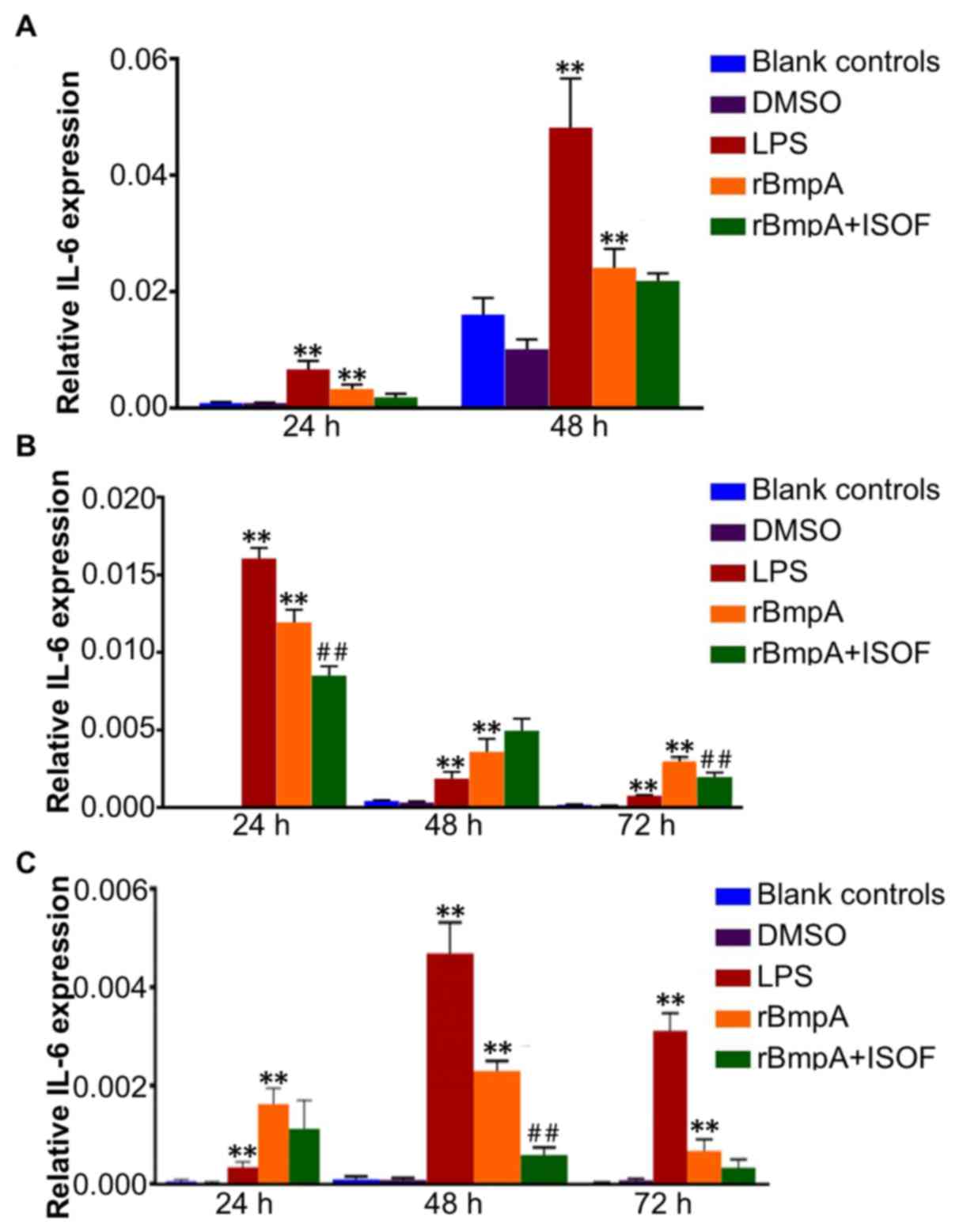
IL-6 mRNA expression levels in murine
macrophages, human macrophages and human dendritic cells
The IL-6 mRNA levels in murine macrophages, human
macrophages and human dendritic cells were determined by RT-qPCR.
The results indicated that IL-6 expression levels in murine
macrophages, human macrophages and human dendritic cells were
significantly increased by LPS or rBmpA treatment when compared
with the blank controls at all time points (all P<0.01; Fig. 4). While ISOF treatment reduced the
expression of IL-6 induced by rBmpA in human macrophages at 24 and
72 h (both P<0.01; Fig. 4B) but
not at 48 h. ISOF treatment also significantly decreased the mRNA
expression of IL-6 in rBmpA-stimulated human dendritic cells, which
was deemed to be significant at 48 h (P<0.01) but not at 24 or
72 h (Fig. 4C).
ISOF effects in the mouse model of
Lyme arthritis in vivo
On initiation of animal experimentation, mice in the
control and ISOF groups exhibited similar feeding behavior and
activity levels. At 1–2 days following the initial injection, the
mice in the ISOF group presented with slight redness and swelling
at the injection site, though this diminished after 3–4 days. The
groups administered with rBmpA (Lyme arthritis group and ISOF
group) presented slight redness and swelling in the tibiotarsal
joints at day 4 after receiving the initial rBmpA injection. The
symptoms peaked at days 14–18 and gradually resolved thereafter. A
total of 3 mice in rBmpA group with severe inflammation exhibited
deformities in the tibiotarsal joint and limited bending ability.
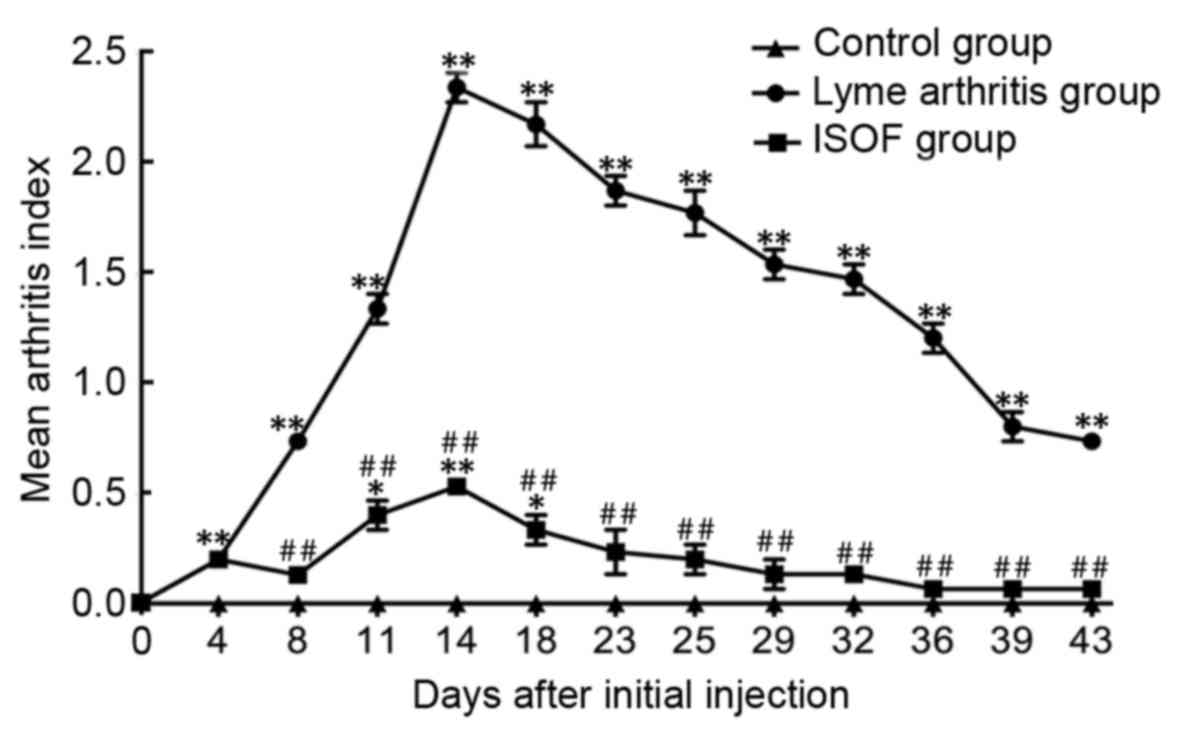
The injection of rBmpA caused a significant increase in the MAI
compared to that in the control group (all P<0.01; Fig. 5). The treatment of ISOF significantly
decreased the MAI compared to rBmpA group (all P<0.01; Fig. 5).
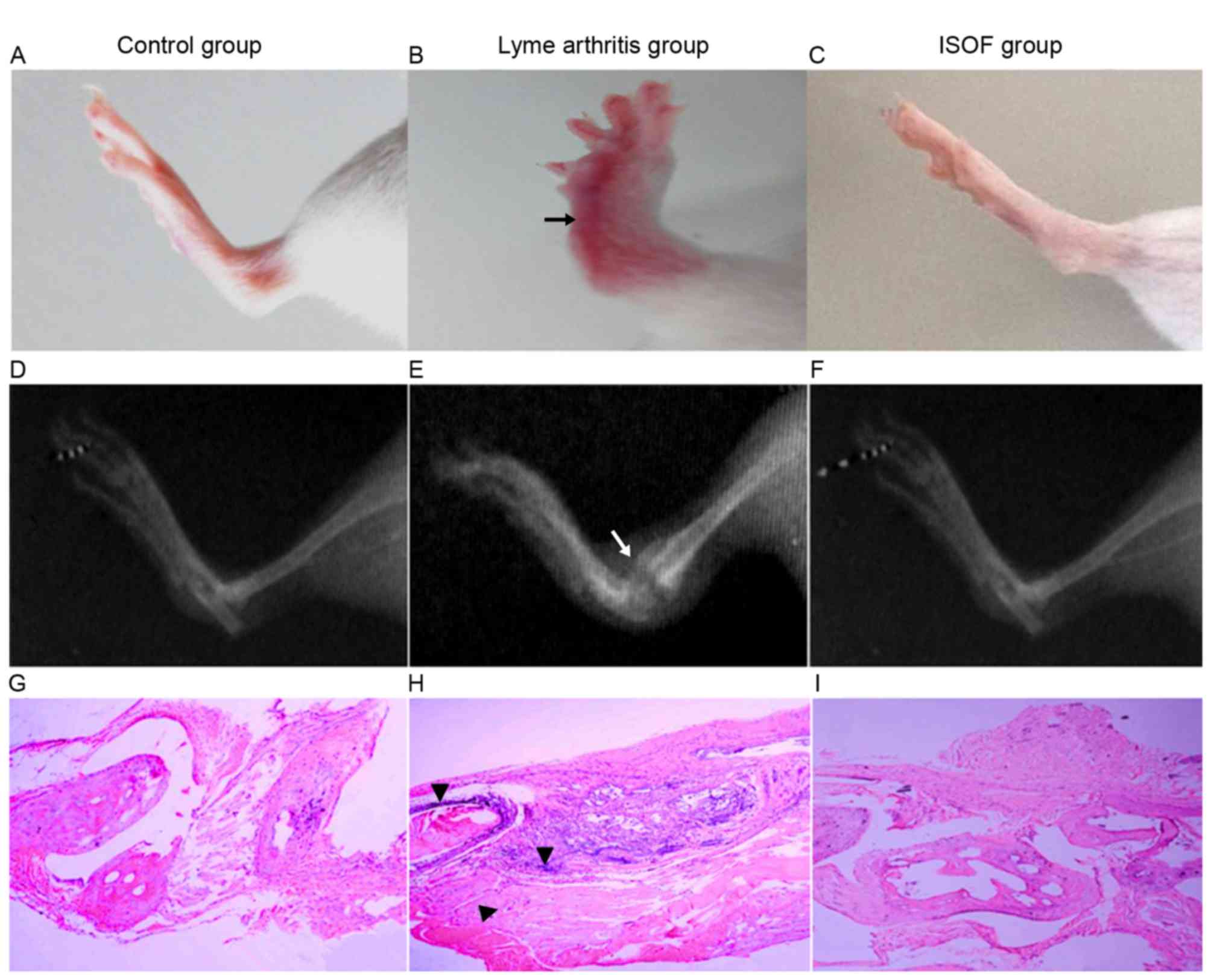
In X-ray images (Fig.
6), the mice with experimental arthritis exhibited swelling of
the soft tissue and joint, joint deformity, stiffness and narrow
joint spaces. Meanwhile, the mice administered with ISOF exhibited
no stiffness and less swelling in soft tissue.
On histopathological examination of control group
tissues (Fig. 6G), a regular joint
space and orderly arrangement of cells in the synovial membrane
were observed. Additionally, the articular synovial surface was
neat and there was no infiltration of inflammatory cells. For the
arthritis group (Fig. 6H), the joint
tissue sections exhibited narrowing of the joint space, increased
synovial cells, thickened synovial tissues and obvious fibrous
tissue hyperplasia when compared with the control tissues.
Furthermore, a high number of inflammatory cells (including
polymorphonuclear leukocytes, monocytes-macrophages and
lymphocytes) had infiltrated and the synovial membrane and pannus
had invaded the joint, causing joint structural damage between
bones. For the ISOF group (Fig. 6I),
the joint tissue sections exhibited normalization of the joint
space and neater rows of joint synovial cells compared with the
arthritis group tissues. While the synovial cells remained slightly
thickened compared with control tissues, the articular surface
appeared neat and no notable inflammatory cell infiltration was
observed.
Discussion
B. burgdorferi infection in joints has been
correlated with Lyme arthritis (2).
In infected joints, recognition by the innate immune system is key
to the host response (14).
Bacterial lipoproteins are recognized by toll-like receptor 2
(TLR2), which induces macrophages and dendritic cells to secrete
cytokines (14). By interacting with
co-receptors, TLR2 represents a key recognition receptor on immune
cells (3). Following immune
recognition, the phagocytosis of B. burgdorferi by
macrophages results in increased transcription of IL-1β, IL-6 and
TNF-α, which activates dendritic cells by inducing the expression
of CD83, and leads to increased transcription of IL-1β, IL-6 and
TNF-α in dendritic cells (15).
In contrast to other gram-negative bacteria, B.
burgdorferi does not express LPS, as the relevant genes are
absent (3). The organism responds to
different environments through specific gene expression and by
altering the composition of its membrane proteins, which are
involved in the pathogenesis of Lyme arthritis (16). The pathogen expresses numerous
proteins on its outer surface, which are able to modulate the
immune system of the host (3). BmpA
is an immuno-dominant protein of B. burgdorferi as well as
an arthritogenic factor (4,17). BmpA activates proinflammatory
responses in human synovial cells and induces the expression of
TNF-α and IL-1β (5). The current
study implicated rBmpA as a key activating factor of
proinflammatory responses in murine macrophages, human macrophages
and dendritic cells through the transcription and expression of
TNF-α and IL-6.
The present study builds on previous findings
regarding the role of ISOF in downregulating inflammatory
responses. In animal models of LPS-induced acute lung injury, ISOF
increased cyclic adenosine monophosphate levels and activated
adenylyl cyclase isoforms 1, 2 and 5 (9). ISOF was also demonstrated to suppress
LPS-induced secretion of cytokines, including TNF-α, IL-1β, IL-6
and IL-8, in human mononuclear leukocytes (9). LPS, as a component of the outer
membrane of gram-negative bacteria, induces the activation of
macrophages and dendritic cells, which leads to the release of
cytokines, including TNF-α, IL-1β and IL-6 (18,19). The
present study demonstrated that ISOF exerted general downregulatory
effects on the transcription and expression of TNF-α and IL-6
induced by rBmpA in murine macrophages, human macrophages and human
dendritic cells, and suppressed the inflammatory response and
rBmpA-induced arthritis in a mice model.
In conclusion, the present study indicated that
rBmpA, which induced the transcription and expression of TNF-α and
IL-6, may activate proinflammatory responses in murine macrophages,
human macrophages and dendritic cells and cause Lyme arthritis in
mice. ISOF downregulated the transcription and expression of TNF-α
and IL-6 induced by rBmpA, and therefore may inhibit Lyme arthritis
induced by rBmpA. Thus, ISOF may have a potential clinical
application in the treatment of Lyme arthritis.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31560051,
81560596 and 81173110) and the Natural Foundation of Yunnan
Province (grant nos. 2012FB011, 2014FA011, 2014FB001 and
2014BC012).
References
|
1
|
Gerstenblith TA and Stern TA: Lyme
disease: A review of its epidemiology, evaluation, and treatment.
Psychosomatics. 55:421–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stanek G, Wormser GP, Gray J and Strle F:
Lyme borreliosis. Lancet. 379:461–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oosting M, Buffen K, van der Meer JW,
Netea MG and Joosten LA: Innate immunity networks during infection
with Borrelia burgdorferi. Crit Rev Microbiol. 42:233–244.
2016.PubMed/NCBI
|
|
4
|
Pal U, Wang P, Bao F, Yang X, Samanta S,
Schoen R, Wormser GP, Schwartz I and Fikrig E: Borrelia
burgdorferi basic membrane proteins A and B participate in the
genesis of Lyme arthritis. J Exp Med. 205:133–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Izadi H, Coleman AS, Wang P, Ma Y,
Fikrig E, Anguita J and Pal U: Borrelia burgdorferi
lipoprotein BmpA activates proinflammatory responses in human
synovial cells through a protein way. Microbes Infect.
10:1300–1308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin J, Wang Y, Tan B, Kang Y, Xie D, Tian
L and Huang J: Matrix solid-phase dispersion extraction for
chromatographic analysis of labdane diterpenoids in Coleus
forskohlii. Phytochem Anal. 24:117–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian L, Wang Y, Ling Y, Yin J, Chen J and
Huang J: A sensitive and specific HPLC-MS/MS analysis and
preliminary pharmacokinetic characterization of isoforskolin in
beagle dogs. J Chromatogr B Analyt Technol Biomed Life Sci.
879:3688–3693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng S, Wang LP, Voravuthikunchai S, Liang
Z, Liu AH and Bao FK: Isoforskolin from native plant Coleus
forskohlii of Yunnan, China plays multiple biological roles.
Open J Immunol. 6:63–66. 2016. View Article : Google Scholar
|
|
9
|
Yang WM, Qiang DQ, Zhang M, Ma L, Zhang Y,
Qing C, Xu Y, Zhen C, Liu J and Chen YH: Isoforskolin pretreatment
attenuates lipopolysaccharide-induced acute lung injury in animal
models. Int Immunopharmacol. 11:683–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ringseis R, Schulz N, Saal D and Eder K:
Troglitazone but not conjugated linoleic acid reduces gene
expression and activity of matrix-metalloproteinases-2 and −9 in
PMA-differentiated THP-1 macrophages. J Nutr Biochem. 19:594–603.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berges C, Naujokat C, Tinapp S, Wieczorek
H, Höh A, Sadeghi M, Opelz G and Daniel V: A cell line model for
the differentiation of human dendritic cells. Biochem Bioph Res
Comm. 333:896–907. 2005. View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
U.S. Department of Health And Human
Services, National Institutes of Health, Office of Laboratory
Animal Welfare: Public Health Service Policy on Humane Care and Use
of Laboratory Animals, Revised 2015, NIH Publication No.
15–8013Office of Laboratory Animal Welfare, National Institutes of
Health. U.S. Department of Health and Human Services; Bethesda, MD,
USA:
|
|
14
|
Nowalk AJ, Gilmore RD Jr and Carroll JA:
Serologic proteome analysis of Borrelia burgdorferi
membrane-associated proteins. Infect Immun. 74:3864–3873. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bryksin AV, Tomova A, Godfrey HP and
Cabello FC: BmpA is a surface-exposed outer membrane protein of
Borrelia burgdorferi. FEMS Microbiol Lett. 309:77–83.
2010.PubMed/NCBI
|
|
16
|
Hu L: Lyme Arthritis. Infect Dis Clin N
Am. 19:947–961. 2005. View Article : Google Scholar
|
|
17
|
Cervantes JL, Hawley KL, Benjamin SJ,
Weinerman B, Luu SM and Salazar JC: Phagosomal TLR signaling upon
Borrelia burgdorferi infection. Front Cell Infect Microbiol.
4:552014.PubMed/NCBI
|
|
18
|
Zanoni I and Granucci F: Differences in
lipopolysaccharide-induced signaling between conventional dendritic
cells and macrophages. Immunobiology. 215:709–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chow JC, Young DW, Golenbock DT, Christ WJ
and Gusovsky F: Toll-like receptor-4 mediates
lipopolysaccharide-induced signal transduction. J Biol Chem.
274:10689–10692. 1999. View Article : Google Scholar : PubMed/NCBI
|