Introduction
Systemic lupus erythematosus (SLE) is a chronic,
progressive and recurrent autoimmune disease, which affects
multiple systems and organs of the body, including the skin,
serosa, joints, kidneys and the central nervous system (1). It is characterized by the activation,
proliferation and hyperfunction of B lymphocytes, as well as
humoral and cellular immunity functional disorder (2). The majority of cases of SLE are
diagnosed in females of childbearing age (3). SLE is a difficult disease to diagnose
as it present with multiple nonspecific early symptoms, meaning it
is not possible to detect SLE using a single laboratory test
(4). In the majority of cases a
confirmed diagnosis of SLE is only possible following organ damage
(5). Children who are affected by
SLE typically present with severe diseased states requiring special
management (6). Older patients with
SLE may have complicating co-morbid conditions, which makes
treatment difficult (7). Extensive
therapeutic advances have been made, over the last decade, however,
treatment regimens are often long and there have been multiple
previous reports of the use of ineffective drugs that do not target
the desired site (8,9). There are several promising strategies
that are being studied as potential novel treatments for SLE
(10,11).
Animal and clinical studies of SLE pathogenesis have
revealed that B lymphocyte stimulating factor (BLyS) may promote
the activation and proliferation of B-lymphocytes, which leads to
the production of large amounts of immunoglobulins and
autoantibodies (12,13). Immunologic injury is caused by
formation of immune complexes, complement-mediated cytolysis,
opsonophagocytosis and antibody-mediated cell-dependent
cytotoxicity (14). Toll-like
receptors (TLRs) are a family of proteins that recognize an innate
immunity pattern. They are widely expressed in various tissues and
cells of the human body and are able to recognize and bind to
conserved pathogen-associated molecules. This may trigger a series
of signal transduction pathways that lead to the release of
inflammatory mediators, which may then activate acquired immunity.
TLRs are regarded as a bridge between innate and acquired immunity
(15). Furthermore, human B
lymphocytes only express TLR-9 and TLR-10 (16). A study by Medzhitov et al
(17) previously reported that TLRs
regulate the activation of B lymphocytes and antibody production
in vivo. Based on these observations, the aim of the present
study was to investigate the TLR-9 signal transduction pathway in
BLyS-induced SLE in transgenic mice.
Materials and methods
SLE transgenic mice
In total, 21 transgenic female mice (aged 8–10 weeks
and weighing 30–40 g) expressing the Epstein-Barr virus membrane
antigen, BLLF1, were used for experiments. Mice were purchased from
the Laboratory Animal Research Center, Peking University Health
Science Center (Beijing, China). Mice were fed as usual for 1 week,
after which experiments were performed. Mice were kept in standard
air-conditioned rooms, under a 12 h light/dark cycle, maintained at
25°C in 40–60% humidity with food and water available ad
libitum. The present study was approved by the Medical Ethics
Committee of Hainan General Hospital (Haikou, China).
Methods
Mice were randomly divided into the blank control,
BLyS inhibition and TLR-9 inhibition groups, with 7 mice in each
group. The mice in the blank control group received intraperitoneal
injections (0.5 ml) of normal saline (0.90% w/v NaCl in water),
mice in the BLyS inhibition group received intraperitoneal
injections of anti-BR3 monoclonal antibody (cat. no. D201-3; 5,000
ng/day for 10 days; Beijing Hanpu Medical Biology Research
Institute, Beijing, China), and mice in the TLR-9 inhibition group
received intraperitoneal injections (250 ng/day for 10 days) of
anti-human TLR-9 antibody (1:50 dilution; cat. no. IMG-305a;
Imgenex; Novus Biologicals, LLC, Littleton, CO, USA). Peripheral
venous blood was collected prior to intervention and after
maintaining the mice on normal feed for 10 days. The relative
levels of TLR-9 mRNA were measured by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
BLyS protein concentration and IL-10 level were measured by ELISA
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
anti-double stranded (ds)DNA antibody titer was measured using a
dot blot assay, which determines whether an antibody-based
detection system would work effectively. Purified bovine serum
albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and the
test sample were spotted onto the membrane and the membrane was
subsequently incubated with the appropriate primary (25°C for 4 h)
and secondary antibodies (4°C for 24 h), to determine whether a
signal could be detected. The antibodies used were obtained from
the following kits: The complement C3 and C4 levels were estimated
using Abcam Complement ELISA kits (cat. nos. ab157711 and ab108824;
Abcam, Cambridge, UK). The erythrocyte sedimentation rates (ESRs)
were measured using an ESR kit (cat. no. 21200213; Thermo Fisher
Scientific, Inc.) and C-reactive protein (CRP) levels were measured
using an ELISA kit (cat. no. RAB0096; Sigma-Aldrich; Merck
KGaA).
RT-qPCR
The blood was centrifuged at 1,006.2 × g for 20 min
at 4°C (Beijing Liuyi Instrument Factory, Beijing, China), the
serum was isolated and an equal volume of lymphocyte separation
solution (Shanghai Yanjin Biology & Science Co., Ltd.,
Shanghai, China) was added to isolate the mononuclear cells. Total
RNA was extracted with TRIzol (Sigma-Aldrich; Merck KGaA). 1.1%
agarose gel electrophoresis and visualized using ethidium bromide
(Thermo Fisher Scientific, Inc.) and an ultraviolet
spectrophotometer (Thermo Fisher Scientific, Inc.) were used to
measure the quality and concentration of the total RNA,
respectively. Furthermore, RNA was treated with RNAase A
(Sigma-Aldrich; Merck KGaA) and stored at −80°C. Following this,
cDNA was synthesized according to the manufacturer's protocol of
the reverse transcription kit (Fermentas; Thermo Fisher Scientific,
Inc.). Primers were designed by Shanghai Shengong Biology Co.,
Ltd., (Shanghai, China). The PCR machine used was from Shanghai
Sanke Instrument Co., Ltd. (Shanghai, China). The primer sequences
were as follows: TLR-9 forward, 5′-TGGATACGTTTCCTTATAAG-3′ and
reverse, 5′-GAAATGGAGGCACCCCTTC-3′ (418 bp); and β-actin (internal
control) forward, 5′-ATCATGTTTGAGACCTTCAACA-3′ and reverse,
5′-CATCTCTTGCTCGAAGTCCA-3′ (300 bp). For qPCR, the reaction system
included 2 µl cDNA template, 0.5 µl of each primer, 9 µl 2.5X Real
Master mix, 9 µl 20X SYBR solution (Thermo Fisher Scientific, Inc.)
and water to a total volume of 20 µl. The thermal profile was as
follows: Pre-degeneration at 95°C for 2 min, degeneration at 95°C
for 45 sec, 60°C for 20 sec and 75°C for 60 sec for a total of 30
cycles, and with an extension at 72°C for 5 min. Each sample was
detected three times, and the mean values were obtained as the
target gene expression as determined by the relative quantification
method (2−ΔΔCq) (18).
Following the reaction, the specificity of primers was analyzed
using melting curves, and 1.1% agarose gel electrophoresis was
performed to identify the PCR amplification products.
Other indexes
ELISA (cat. no. A20180) and an Immunogold Labeling
kit were purchased from Invitrogen (Thermo Fisher Scientific, Inc.)
and used according to the manufacturer's protocols. The automatic
biochemical analyzer, AU5800, was purchased from Beckman Coulter,
Inc. (Brea, CA, USA).
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. The measurement data were presented
as the mean ± standard deviation. Comparisons between groups were
performed by one-way analysis of variance followed by Fisher's
least significant difference method. Comparisons within groups were
performed using the paired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of the relative expression
of TLR-9 mRNA
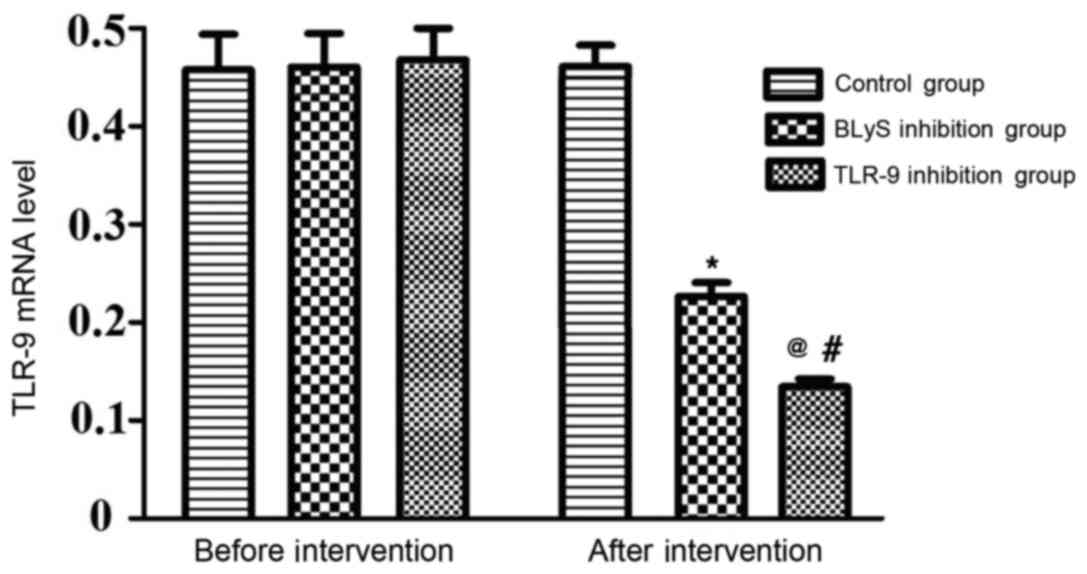
The differences in the levels of TLR-9 mRNA between
the three groups prior to intervention were not statistically
significant. There was no significant alteration to the TLR-9 level
in the control group following intervention. However, the levels of
TLR-9 mRNA in the BLyS and TLR-9 inhibition groups significantly
decreased following intervention compared with their levels prior
to intervention (P<0.05), the levels of TLR-9 mRNA in the TLR-9
inhibition group were significantly lower than those in the BLyS
inhibition group following intervention (P<0.05) (Table I and Fig.
1).
 | Table I.Relative mRNA expression of TLR-9
mRNA in the different groups. |
Table I.
Relative mRNA expression of TLR-9
mRNA in the different groups.
|
| TLR-9 mRNA
expression level |
|
|
|---|
|
|
|
|
|
|---|
| Group | Before
intervention | After
intervention | t-value | P-value |
|---|
| Control | 0.4547±0.0219 | 0.4562±0.0315 | −0.089 | 0.932 |
| BLyS inhibitor | 0.4627±0.0311 | 0.2581±0.0270 | 12.845 | <0.01 |
| TLR-9
inhibitor | 0.4630±0.0248 | 0.1513±0.0311 | 22.792 | <0.01 |
| F-value | 0.226 | 187.009 | – | – |
| P-value | 0.800 | <0.01 | – | – |
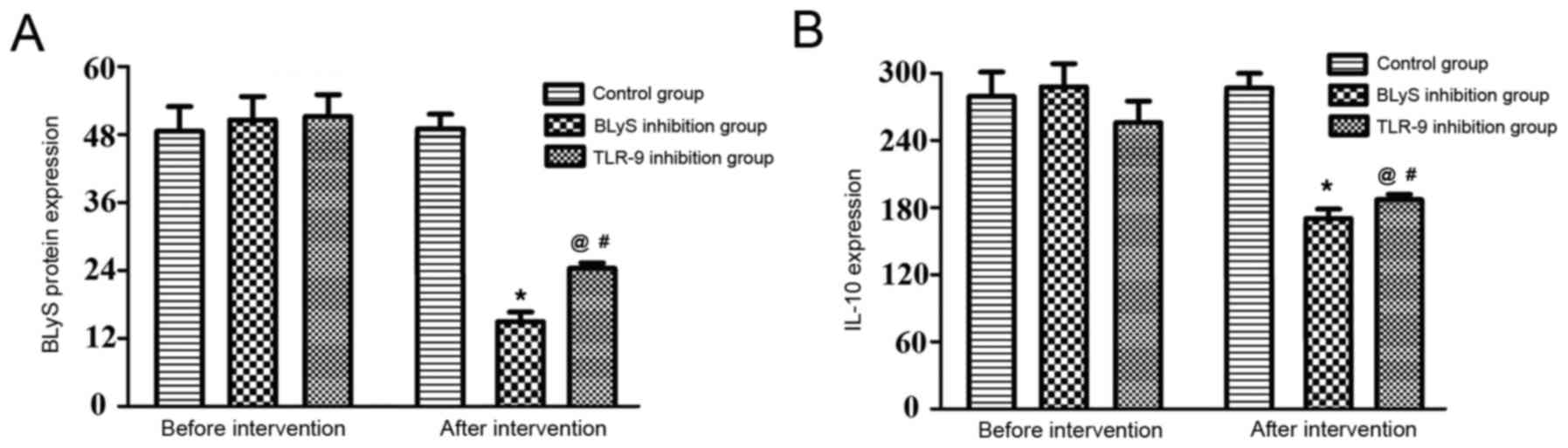
Comparison of the levels of BLyS and
IL-10
The differences in the levels of BLyS and IL-10 in
the three groups prior to intervention were not statistically
significant (P>0.05). Additionally, there was no significant
change in the levels in the control group following intervention.
The BLyS protein concentration and IL-10 level of the BLyS and
TLR-9 inhibition groups significantly decreased following
intervention compared with the levels before intervention
(P<0.05). The differences between the BLyS and TLR-9 inhibition
groups were not statistically significant prior to intervention
(Table II and Fig. 2). However, following intervention
there was a significant difference between the IL-10 expression in
the BLyS and TLR-9 inhibition groups (P<0.05).
 | Table II.Protein expression of BLyS and IL-10
in the different groups. |
Table II.
Protein expression of BLyS and IL-10
in the different groups.
|
| BLyS, µg/l | IL-10, pg/ml |
|---|
|
|
|
|
|---|
| Group | Before
intervention | After
intervention | t-value | P-value | Before
intervention | After
intervention | t-value | P-value |
|---|
| Control | 49.6±3.4 | 47.4±2.8 |
1.630 |
0.154 | 273.1±24.7 | 292.8±32.1 | −1.342 | 0.228 |
| BLyS inhibitor | 52.1±3.7 | 13.1±1.6 | 21.726 | <0.01 | 290.3±35.3 | 171.8±16.5 |
8.355 | <0.01 |
| TLR-9
inhibitor | 53.2±1.9 | 26.5±1.6 | 33.203 | <0.01 | 275.0±31.3 | 182.3±38.7 |
4.983 | 0.002 |
| F-value | 2.451 | 493.896 | – | – | 0.661 | 33.730 | – | – |
| P-value | 0.114 | <0.01 | – | – | 0.528 | <0.01 | – | – |
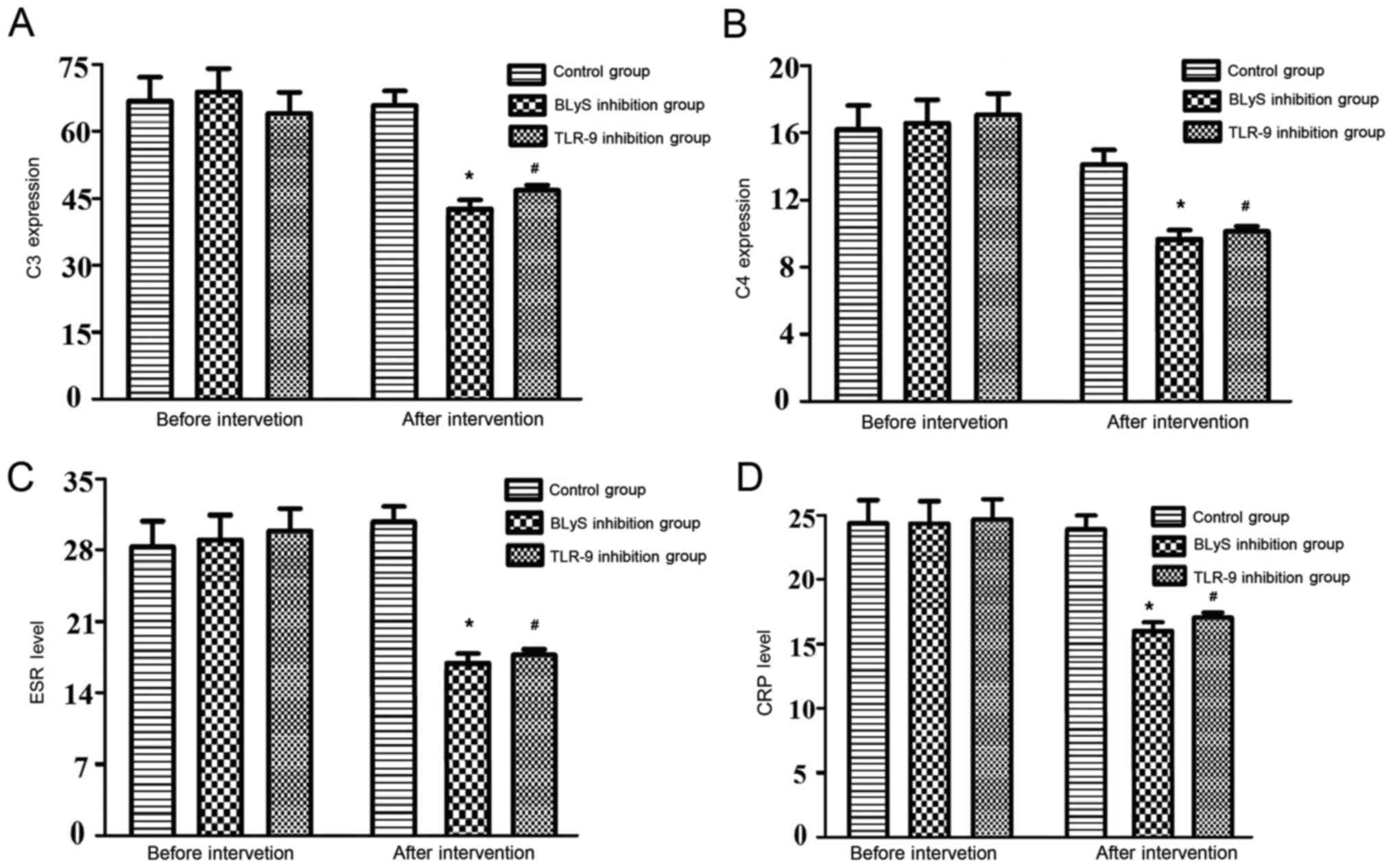
Comparison of anti-dsDNA, C3, C4, ESR
and CRP levels
The differences in the levels of anti-dsDNA
antibody, C3, C4, ESR and CRP levels of the three groups prior to
intervention were not statistically significant. Furthermore, there
were no significant alterations to these levels observed in the
control group following intervention. The above indexes in the BLyS
and TLR-9 inhibition groups decreased significantly following
intervention compared with the levels before intervention
(P<0.05). Furthermore, the differences in the levels of the
above indexes between the BLyS and TLR-9 inhibition groups
following intervention were not statistically significant (Table III and Fig. 3).
 | Table III.Levels of anti-dsDNA antibody, C3,
C4, ESR and CRP in the three groups. |
Table III.
Levels of anti-dsDNA antibody, C3,
C4, ESR and CRP in the three groups.
|
| Level of anti-ds
DNA | C3, mg/ml | C4, mg/ml | ESR, mm/h | CRP, mg/l |
|---|
|
|
|
|
|
|
|
|---|
| Group | Before
intervention | After
intervention | Before
intervention | After
intervention | Before
intervention | After
intervention | Before
intervention | After
intervention | Before
intervention | After
intervention |
|---|
| Control | 1:16 | 1:14 | 69.3±4.7 | 69.6±2.7 | 16.1±1.7 | 14.7±1.2 | 27.7±3.2 | 29.1±2.5 | 24.1±3.6 | 24.1±2.3 |
| BLyS inhibitor | 1:18 | 1:7 | 70.6±3.8 | 45.7±4.0 | 16.7±2.2 | 8.6±0.6 | 30.8±2.8 | 16.6±3.2 | 23.6±2.2 | 16.2±2.4 |
| TLR-9
inhibitor | 1:15 | 1:6 | 67.5±1.8 | 48.6±1.4 | 17.6±1.1 | 8.7±0.5 | 29.5±2.6 | 18.9±2.0 | 24.1±2.2 | 17.1±2.0 |
| F-value | 0.865 | 56.324 | 1.245 | 143.790 | 1.334 | 129.512 | 2.014 | 45.910 | 0.086 | 25.893 |
| P-value | 0.423 | <0.01 | 0.312 | <0.01 | 0.288 | <0.01 | 0.162 | <0.01 | 0.918 | <0.01 |
Discussion
Mice with congenital deficiency of BLyS exhibit a
reduced number of B lymphocytes and decreased levels of
immunoglobulin (19). By contrast, B
lymphocytes in transgenic mice with overexpression of BLyS increase
in number and may lead to hyperimmunoglobulinemia (20). In patients with SLE, various
high-titer autoantibodies, such as anti-dsDNA, have been detected
in circulation, and the level of immune complexes has been
demonstrated to increase, as well as the immunoglobulin deposited
in the kidney (21). BLyS
antagonists may be used to inhibit progression and improve the
survival rate of SLE (22). In the
present study, it was revealed that the levels of BLyS in the
plasma or serum of patients with SLE were significantly higher than
those of the control subjects. Additionally, the biological
activity of BLyS in circulation was significantly higher compared
with the control group, and was closely associated with anti-dsDNA
antibody titer, disease activity, ESR, CRP levels, and serum
immunoglobulin G (23). A previous
study indicated that BLyS may block the expression of
apoptosis-related genes in B lymphocytes downstream of signals from
the B cell receptor (BCR) (24).
Furthermore, TLR signaling was demonstrated to serve an
indispensable role in BLyS transgenic mice by upregulating the
expression of anti-apoptotic genes, including cluster of
differentiation (CD)40 (25).
A total of 13 members of the TLR family have been
identified (26). Lipopolysaccharide
(LPS) from the wall of Gram-negative bacteria is the main ligand of
TLR-9 (27). Additionally, TLR-9,
CD14, myeloid differentiation protein 2 and LPS binding protein
combine to form the LPS recognition receptor complex, with a high
affinity and signal transduction function (28). The LPS recognition receptor may cause
the translocation of nuclear factor-κB (NF-κB) from the cytoplasm
to the nucleus by myeloid differentiation protein 88
(MyD88)-dependent or independent signaling pathways and bind with
the NF-site in the promoter region of inflammatory response
regulator genes, promoting the initiation of transcription and
translation of genes encoding inflammatory cytokines, as well as
the large release of cytokines (29). The immune response of the organism is
thereby initiated (30). When BCR
and CD40 are combined or triggered by CpG DNA, TLR expression
increases (31). Furthermore, the
induced expression of TLRs on B lymphocytes may serve a role in the
pathological process of autoimmune diseases (32). A study by Marshak-Rothstein et
al (33) reported that B
lymphocytes that express membrane-bound immunoglobulin M rheumatoid
factor may be activated by chromosome-chromosome antibody immune
complexes through the TLR9-MyD88 dependent pathway. The
chromosome-chromosome antibody immune complex may then be
endocytosed into the endoplasmic reticulum through BCR-mediated
mechanisms, which then transmit signals by TLR9 that is expressed
in the endoplast. Therefore, TLRs on B lymphocytes connect innate
immunity with autoimmunity (34).
The differentiation and activation of helper T cells
is not sufficient for T cell-dependent activation of B lymphocytes.
In addition to the assistance of CD4+ T cells,
antigen-specific T cell-dependent antibody responses require the
activation of TLRs on B lymphocytes (35). The function of TLRs on B lymphocytes
may assist BCR to identify antigens of microbial origin, and assist
with the anti-infection response (36).
Cytokines serve a critical role in regulating
disease activity and organ injury in SLE. Of these cytokines, IL-10
is predominantly produced by mononuclear macrophages, fibroblasts
and endothelial cells and functions to stimulate the maturation of
B-lymphocytes and the secretion of immunoglobulins (37). A study in New Zealand Black and New
Zealand White mice revealed that IL-10 directly caused the
pathogenesis of SLE (38),
indicating that the rise of exogenous IL-10 in vitro may
lead to increased levels of immunoglobulin G and anti-dsDNA
antibodies that are produced by B lymphocytes of old B/W mice, and
may reduce albuminuria as well as the fatality rate. A previous
study reported that IL-10 is highly and spontaneously expressed in
the peripheral blood of patients with lupus, and is associated with
disease activity (39). Lymphocytes
isolated from patients with SLE may spontaneously increase IL-10
production in vitro, and anti-IL-10 may reduce the
anti-ds-DNA level (40).
Furthermore, multiple models of lupus have demonstrated the
positive therapeutic effects of IL-10 and IL-10 receptor
antagonists (41). In addition to
inhibiting the ultimate IL-10 output, inhibition of the source of
IL-10 production is an attractive concept.
At present, there are three types of murine model of
SLE: Spontaneous, artificial induction and gene regulation types
(42). The spontaneous type has a
specific genetic background and good genetic stability, which is of
great significance in the studies of genetic factors that affect
SLE (43). The artificial induction
type is suitable for short-term studies, and the majority of the
mice succumb to the disease ~5 months after induction of SLE. Mice
of the gene regulation type, including transgenic and knockout
mice, may be used to perform genetic level analyses for studies on
the mechanism of SLE (44).
In the present study, it was concluded that TLR-9
mRNA, BLyS, IL-10, anti-dsDNA antibody titer, C3, C4, ESR and CRP
levels of the blank control group were significantly higher than
those of the other two groups. These results are consistent with
the findings of previous studies (45–48).
Additionally, the difference in comparison of the above indexes
between the BLyS and the TLR-9 inhibition groups were not
statistically significant, with the exception of TLR-9 mRNA and
BLyS. This implied that TLR-9 represents an important signaling
pathway that may regulate the inflammatory immune level for
BLyS-induced SLE. Therefore, inhibiting TLR-9 or BLyS expression
may inhibit the process of autoimmune injury in SLE.
References
|
1
|
Thong B and Olsen NJ: Systemic lupus
erythematosus diagnosis and management. Rheumatology (Oxford).
56(suppl_1): i3–i13. 2017.PubMed/NCBI
|
|
2
|
Golder V and Hoi A: Systemic lupus
erythematosus: An update. Med J Aust. 206:215–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murphy G and Isenberg D: Effect of gender
on clinical presentation in systemic lupus erythematosus.
Rheumatology (Oxford). 52:2108–2115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sebastiani GD, Prevete I, Iuliano A and
Minisola G: The importance of an early diagnosis in systemic lupus
erythematosus. Isr Med Assoc J. 18:212–215. 2016.PubMed/NCBI
|
|
5
|
Lu A, Li H, Niu J, Wu S, Xue G, Yao X, Guo
Q, Wan N, Abliz P, Yang G, et al: Hyperactivation of the NLRP3
inflammasome in myeloid cells leads to severe organ damage in
experimental lupus. J Immunol. 198:1119–1129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zahran AM, Elsayh KI, Saad K, Eloseily EM,
Osman NS, Alblihed MA, Badr G and Mahmoud MH: Effects of royal
jelly supplementation on regulatory T cells in children with SLE.
Food Nutr Res. 60:329632016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambrose N, Morgan TA, Galloway J, Ionnoau
Y, Beresford MW and Isenberg DA; UK JSLE Study Group, : Differences
in disease phenotype and severity in SLE across age groups. Lupus.
25:1542–1550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Narang T, Sharma M, Gulati N and Kaur A:
Extensive hypertrophic lupus erythematosus: Atypical presentatio.
Indian J Dermatol. 57:5042012. View Article : Google Scholar
|
|
9
|
Tunnicliffe DJ, Singh-Grewal D, Kim S,
Craig JC and Tong A: Diagnosis, monitoring, and treatment of
systemic lupus erythematosus: A systematic review of clinical
practice guidelines. Arthritis Care Res (Hoboken). 67:1440–1452.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaul A, Gordon C, Crow MK, Touma Z,
Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G and Hughes G:
Systemic lupus erythematosus. Nat Rev Dis Primers. 2:160392016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aringer M and Voll RE: Lupus
erythematosus-Update 2016. Dtsch Med Wochenschr. 141:1785–1788.
2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin WY, Gong Q, Seshasayee D, Lin Z, Ou Q,
Ye S, Suto E, Shu J, Lee WP, Lee CW, et al: Anti-BR3 antibodies: A
new class of B-cell immunotherapy combining cellular depletion and
survival blockade. Blood. 110:3959–3967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furie R, Petri M, Zamani O, Cervera R,
Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill
JT, Chatham WW, et al: A phase III, randonlized, placebo-controlled
study of belimumab, a monoclonal antibody that inhibits B
lymphocyte stimulator, in patients with systemic lupus
erythematosus. Arthritis Rheum. 63:3918–3930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko GJ, Zakaria A, Womer KL and Rabb H:
Immunologic research in kidney ischemia/reperfusion injury at johns
hopkins university. Immunol Res. 47:78–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Y, Chai Q, Zhao Y, Li P, Qiao J and
Huang J: Increased activation of toll-like receptors-7 and −8 of
peripheral blood mononuclear cells and upregulated serum cytokines
in patients with pediatric systemic lupus erythematosus. Int J Clin
Exp Med. 8:20472–20480. 2015.PubMed/NCBI
|
|
16
|
Khoryati L, Augusto JF, Shipley E,
Contin-Bordes C, Douchet I, Mitrovic S, Truchetet ME, Lazaro E,
Duffau P, Couzi L, Jacquemin C, et al: IgE inhibits Toll-like
receptor 7- and Toll-like receptor 9 mediated expression of
interferon-α by plasmacytoid dendritic cells in systemic lupus
patients. Arthritis Rheumatol. 68:2221–2231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Medzhitov R, Kashgarian M, Shlomchik MJ,
Teichmann LL and Schenten D: Signals via the adaptor MyD88 in B
cells and DCs make distinct and synergistic contributions to immune
activation and tissue damage in lupus. Immunity. 38:528–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cancro MP, D'Cruz DP and Khamashta MA: The
role of B lymphocyte stimulator (BLyS) in systemic lupus
erythematosus. J Clin Invest. 119:1066–1073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramanujam M and Davidson A: BAFF blockade
for systemic lupus erythematosus: Will the promise be fulfilled?
Immunol Rev. 223:156–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ritterhouse LL, Crown SR, Niewold TB,
Merrill JT, Roberts VC, Dedeke AB, Neas BR, Thompson LF, Guthridge
JM and James JA: B lymphocyte stimulator levels in systemic lupus
erythematosus: Higher circulating levels in African American
patients and increased production after influenza vaccination in
patients with low baseline levels. Arthritis Rheum. 63:3931–3941.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stohl W: Biologic differences between
various inhibitors of the BLyS/BAFF pathway: Should we expect
differences between belimumab and other inhibitors in development?
Curr Rheumatol Rep. 14:303–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ju S, Zhang D, Wang Y, Ni H, Kong X and
Zhong R: Correlation of the expression levels of BLyS and its
receptors mRNA in patients with systemic lupus erythematosus. Clin
Biochem. 39:1131–1137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scholz JL, Oropallo MA, Sindhava V, Goenka
R and Cancro MP: The role of B lymphocyte stimulator in B cell
biology: Implications for the treatment of lupus. Lupus.
22:350–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Treml LS, Carlesso G, Hoek KL, Stadanlick
JE, Kambayashi T, Bram RJ, Cancro MP and Khan WN: TLR stimulation
modifies BLyS receptor expression in follicular and marginal zone B
cells. J Immunol. 178:7531–7539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cargill EJ and Womack JE: Detection of
polymorphisms in bovine toll-like receptors 3, 7, 8 and 9.
Genomics. 89:745–755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park BS and Lee JO: Recognition of
lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med.
45:e662013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castellano G, Stasi A, Intini A, Gigante
M, Di Palma AM, Divella C, Netti GS, Prattichizzo C, Pontrelli P,
Crovace A, et al: Endothelial dysfunction and renal fibrosis in
endotoxemia-induced oliguric kidney injury: Possible role of
LPS-binding protein. Crit Care. 18:5202014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim BM, Jeong CB, Rhee JS and Lee JS:
Transcriptional profiles of Rel/NF-κB, inhibitor of NF-κB (IκB) and
lipopolysaccharide-induced TNF-α factor (LITAF) in the
lipopolysaccharide (LPS) and two Vibrio sp.-exposed intertidal
copepod, Tigriopus japonicus. Dev Comp Immunol. 42:229–239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson JS, Bixler SA, Qian F, Vora K,
Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C,
et al: BAFF-R, a newly identified TNF receptor that specifically
interacts with BAFF. Science. 293:2108–2111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pone EJ, Lou Z, Lam T, Greenberg ML, Wang
R, Xu Z and Casali P: B cell TLR1/2, TLR4, TLR7 and TLR9 interact
in induction of class switch DNA recombination: Modulation by BCR
and CD40 and relevance to T-independent antibody responses.
Autoimmunity. 48:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu K and Mohan C: Altered B-cell
signaling in lupus. Autoimmun Rev. 8:214–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marshak-Rothstein A, Viglianti GA, Moody
KL, Uccellini MB and Avalos AM: Toll-like receptor-dependent immune
complex activation of B cells and dendritic cells. Methods Mol
Biol. 1390:249–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morimoto S, Nakano S, Watanabe T, Tamayama
Y, Mitsuo A, Nakiri Y, Suzuki J, Nozawa K, Amano H, Tokano Y, et
al: Expression of B-cell activating factor of the tumour necrosis
factor family (BAFF) in T cells in active systemic lupus
erythematosus: The role of BAFF inT cell-dependent B cell
pathogenic autoantibody production. Rheumatology. 46:1083–1086.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hua Z and Hou B: TLR signaling in B-cell
development and activation. Cell Mol Immunol. 10:103–106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YH, Choi SJ, Ji JD and Song GG:
Association between toll-like receptor polymorphisms and systemic
lupus erythematosus: A meta-analysis update. Lupus. 25:593–601.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao Y and Cao X: The immune potential and
immunopathology of cytokine-producing B cell subsets: A
comprehensive review. J Autoimmun. 55:10–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abdallah E, Waked E and Abdelwahab MA:
Evaluating the association of interleukin-10 gene promoter-592 A/C
polymorphism with lupus nephritis susceptibility. Kidney Res Clin
Pract. 35:29–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Timóteo RP, Micheli DC, Teodoro RB, Freire
M, Bertoncello D, Murta EF and Tavares-Murta BM: Characterization
of inflammatory markers associated with systemic lupus
erythematosus patients undergoing treatment. Rev Bras Reumatol Engl
Ed. 56:497–503. 2016.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heinemann K, Wilde B, Hoerning A, Tebbe B,
Kribben A, Witzke O and Dolff S: Decreased IL-10(+) regulatory B
cells (Bregs) in lupus nephritis patients. Scand J Rheumatol.
45:312–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Steiman AJ, Gladman DD, Ibañez D, Noamani
B, Landolt-Marticorena C, Urowitz MB and Wither JE: Lack of
interferon and proinflammatory cyto/chemokines in serologically
active clinically quiescent systemic lupus erythematosus. J
Rheumatol. 42:2318–2326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu Y, Zeumer L, Reeves WH and Morel L:
Induced murine models of systemic lupus erythematosus. Methods Mol
Biol. 1134:103–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Davison LM and Jørgensen TN: Sialic
acid-binding immunoglobulin-type lectin H-positive plasmacytoid
dendritic cells drive spontaneous lupus-like disease development in
B6. Nba2 mice. Arthritis Rheumatol. 67:1012–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jain S, Park G, Sproule TJ, Christianson
GJ, Leeth CM, Wang H, Roopenian DC and Morse HC III: Interleukin 6
accelerates mortality by promoting the progression of the systemic
lupus erythematosus-like disease of BXSB. Yaa Mice. PLoS One.
11:e01530592016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee HY, Kim YI, Lee JW, Byun JY, Park MS
and Yeo SG: Decreased expression of TLR-9 and cytokines in the
presence of bacteria in patients with otitis media with effusion.
Clin Exp Otorhinolaryngol. 6:195–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Capobianco MP, Cassiano GC, Furini AA,
Storti-Melo LM, Pavarino EC, Galbiatti AL, Fraga VD, Conceição LM,
Couto VS, Couto AA and Machado RL: No evidence for association of
the CD40, CD40L and BLYS polymorphisms, B-cell co-stimulatory
molecules, with Brazilian endemic Plasmodium vivax malaria. Trans R
Soc Trop Med Hyg. 107:377–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tan X, Fujiu K, Manabe I, Nishida J,
Yamagishi R, Nagai R and Yanagi Y: Choroidal neovascularization is
inhibited via an intraocular decrease of inflammatory cells in mice
lacking complement component C3. Sci Rep. 5:157022015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taylor SH, Ripley BS, Martin T, De-Wet LA,
Woodward FI and Osborne CP: Physiological advantages of C4 grasses
in the field: A comparative experiment demonstrating the importance
of drought. Glob Chang Biol. 20:1992–2003. 2014. View Article : Google Scholar : PubMed/NCBI
|